Abstract
Objectives:
To assess alterations in respiratory muscle strength and inspiratory and expiratory peak flow, as well as skeletal and dental changes in patients diagnosed with transverse maxillary deficiency before and after microimplant-assisted rapid maxillary expansion (MARPE).
Materials and Methods:
Twenty patients (13 female and 7 male) were assessed by respiratory tests in three different periods: T0 initial, T1 immediately after expansion, and T2 after 5 months. Tests included: maximum inspiratory pressure (MIP) and maximum expiratory pressure (MEP), oral expiratory peak flow, and inspiratory nasal flow. Cone-beam computed tomography measurements were performed in the maxillary arch, nasal cavity, and airway before and immediately after expansion.
Results:
There was a significant increase in MIP between T0 and T2 and MEP between T0 and T1 (P<.05). Oral and nasal peak flow increased immediately after and 5 months later, especially in patients with initial signs of airway obstruction (P<.05). In addition, after expansion there was a significant enlargement of the nasal cavity, alveolar bone, and interdental widths at the premolar and molar region. Molars tipped buccally (P<.05) but no difference was found in premolar inclination. MARPE increased airway volume significantly.
Conclusions:
Skeletal changes promoted by MARPE directly affected airway volume, resulting in a significant improvement in muscle strength and nasal and oral peak flow.
Keywords: Nasal cavity, Nasal obstruction, MARPE, Airway volume
INTRODUCTION
Transverse maxillary deficiency is characterized by lateral growth failure resulting in a narrow maxilla, narrow palatal vault and, often, posterior crossbite.1 Patients with maxillary constriction tend to have airway problems. Mouth breathing can also cause muscle imbalance, postural axis alteration, disorganization of muscle groups, inhibition of nasal afferent nerves, decreased pulmonary compliance, and restricted chest expansion as well as alveolar ventilation.2
Once the nasal airway is obstructed, reducing airflow, the patient starts breathing through the mouth, causing air to arrive faster to the lungs. With less effort to breathe, the entire ventilatory mechanism is compromised, with reduced diaphragm action and less strength of the respiratory muscles.3,4
The most common methods used for nasal flow studies are rhinomanometry, acoustic rhinomanometry, and nasal inspiratory peak flow (NIPF). All of them offer accurate results to measure airflow throughout the nasal cavity. The NIPF is used to detect clinical changes caused by respiratory problems. It is a low-cost, and portable device, with good reproducibility, and is not dependent upon computer systems or expertise to be operate it.5,6
Maximum inspiratory pressure and maximum expiratory pressure (MIP and MEP) are used to assess respiratory muscle strength, indicating the strength of the inspiratory and expiratory muscle groups. MIP reflects the strength of the diaphragm and other inspiratory muscles whereas MEP measures intercostal and abdominal muscle strength.7–9
Rapid palatal expansion is the treatment choice to correct maxillary transverse skeletal deficiency and is recommended in growing patients. Due to higher interdigitation of the midpalatal suture found in adult patients, surgically-assisted rapid palatal expansion (SARPE) is indicated.10 Recently, micro-implant assisted rapid palatal expansion (MARPE), using a bone-borne expander in mature patients, has been found to provide skeletal expansion, reducing adverse dental effects and, thus protecting the periodontium. MARPE may represent an alternative to SARPE, which is often refused by patients.11–13 Although there are studies evaluating MARPE effects on airway using cone-beam computed tomography (CBCT) measurements,14–16 only a few studies correlated MARPE with airflow. The aim of this study was to evaluate respiratory muscle strength as well as inspiratory nasal and expiratory oral peak flows before and after MARPE.
MATERIALS AND METHODS
The sample of this study consisted of 20 patients, with a mean age of 17.1 years (13 female and 7 male) with the following inclusion criteria: patients with maxillary transverse deficiency, permanent dentition, CS6 skeletal maturation stage,17 and mouth breathers. The exclusion criteria were: failure to split midpalatal suture, systemic diseases, craniofacial anomalies, obesity, and patients with cold or flu symptoms.
The study was conducted in the Department of Postgraduate Orthodontics and was approved by the Research Ethics Committee (No. 1.745.233) at São Leopoldo Mandic Institute and Research Center (Campinas, SP/Brazil).
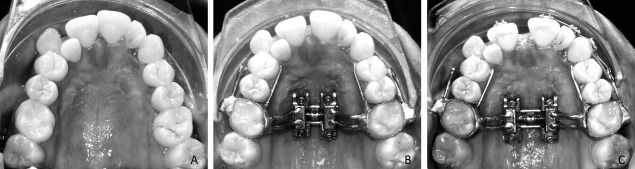
MARPE appliances (Figure 1A–C) used in this study consisted of orthodontic bands on the upper first molars, the expander, and four mini-implants 1.8 diameter × 11 mm length (Peclab, Belo Horizonte, Brazil) and Maxillary Skeletal Expander (Biomaterials Korea, Seoul, South Korea).12,18 Maxillary expansion was initiated immediately after placement. Activation was performed twice a day, one turn in the morning (0.25 mm) and one turn at night (0.25 mm), until the necessary expansion was achieved.19
Figure 1.
Micro-implant rapid palatal expansion (Peclab, Belo Horizonte, Brazil) (A) Initial. (B) Beginning of activation. (C) After expansion.
Respiratory tests were performed at three timepoints: before expansion (T0), immediately after expansion (T1), and after 5 months when MARPE was removed (T2). All tests were done three times at each time point, and the highest value was recorded.9,20
To measure respiratory muscle strength, an analogue manometer (Instrumentation Industries, Bethel Park, PA, USA) with a numerical scale of 0 to 120 cmH2O was used, and muscle strength was evaluated by 2 measurements: MIP and MEP. Normative values were calculated using an equation considering age and gender for the Brazilian population.9 The collected data were then compared to predicted values and converted into a percentage21 (Figure 2A).
Figure 2.
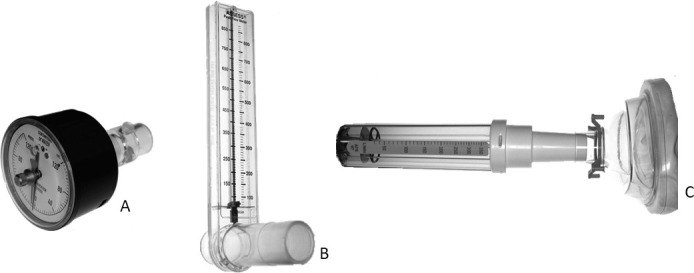
(A) Analog manometer. (B) Expiratory peak flow meter. (C) Nasal inspiratory peak flow meter.
An ASSESS expiratory peak flow meter device (Respironics HealthScan, Cedar Grove, NJ, USA) with a numerical range of 60–900 l/min was used to measure maximum airflow achieved during a forceful expiratory maneuver starting with the lungs fully inflated (Figure 2B). All data were compared to a reference table and chart considering age, height, and gender for a healthy Brazilian population.20,22 After converting data to percentage based on the predicted value as recommended in the guidelines published by the National Institutes of Health,23 subjects were divided into two groups according to the peak flow results: values above 100%, meaning patients with no signs of lower airway obstruction, and below 100%, suggesting some degree of airway obstruction. To evaluate nasal inspiratory peak flow, a measuring device of nasal inspiratory flow (InCheck Nasal; Clement Clarke, Harlow, UK) with a numerical range of 30–370l/min24 was used to quantify maximum inspiratory flow (Figure 2C). Raw data were compared before and after expansion.
During oral and nasal exams, patients remained seated in a 90° upright position at room temperature. They were instructed regarding the use of the nasal clip and position of the mouthpiece to obtain the best seal for the expiratory and inspiratory tests.9,20 Before starting the nasal exam, the patient was instructed to clear the airway by blowing their nose. The device was placed horizontally so that the mask could be fitted and adapted around the nose and sealed lips with the correct pressure to avoid air leaks.24
CBCT acquisition was performed using the I-CAT Scanner machine (Imaging Sciences International, Hatfield, PA) before (T0) and immediately after (T1) expansion, using the following parameters: 16 cm diameter × 13 cm height volume size; 0.25 mm voxel resolution; 36mAs and 120kVp exposure conditions. Images were generated in digital imaging and communications in medicine format. Visualization and measurements were performed using OnDemand3D software (Cybermed, Seoul, South Korea). To standardize software measurements (Figure 3), images were previously aligned using the following reference lines: in the coronal view, N-ANS point (Nasion-Anterior Nasal Spine) and for the sagittal and axial views, ANS-PNS (Anterior Nasal Spine-Posterior Nasal Spine).16
Figure 3.
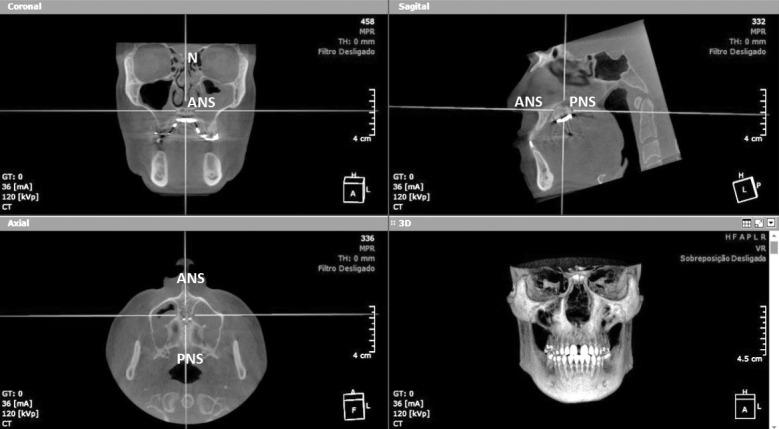
OnDemand3D software images in the first molar region.
Linear and angular measurements were performed in a coronal view at the furcations of the first premolar and first molar before and after expansion. They are described in Table 1.14,25,26 Airway volume was measured using the three-dimensional module to create a threshold of -4000 to -600 Hounsfield units (HU) representing air. The nasopharynx was sculpted and cut based on the C3 plane as the inferior border and the Choanae plane as the superior border (Figure 4).
Table 1.
Definition of Landmarks and Reference Planes Used to Assess Changes in CBCT Images Before and After Expansion
| Term |
Definition |
| Nasal cavity width (linear distance) | Distance between the most lateral point of each side of the nasal cavity |
| Midpalatal suture opening (linear distance) | Distance between the two halves of the ANS and PNS after split |
| Alveolar bone width (linear distance) | From the lowest point of the alveolar bone crest to the opposite side |
| Interdental distance (linear distance) | Distance between the middle fossa of right and left upper first premolars and molars |
| Tooth inclination (degrees) | Long axis of the first premolar and molar to the palatal base of the maxilla |
ANS indicates anterior nasal spine; PNS, posterior nasal spine.
Figure 4.
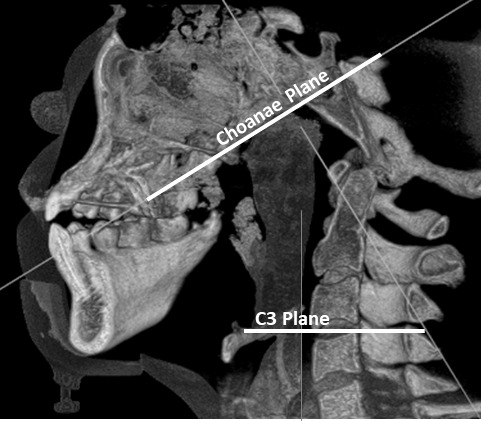
Limits to the airway volume measurement.
For the statistical analysis, data normality was determined using the Shapiro-Wilk test. To statistically evaluate MEP, MIP, inspiratory, and oral expiratory nasal Peak flow, one-way analysis of variance test followed by the Tukey test were used. To compare CBCT data before and after expansion, Student's t-test was performed using GraphPad Prism 8 software (GraphPad Software, San Diego, CA) using a significance level of P less than .05. Pearson correlation analysis was performed to verify correlations between variables.
RESULTS
Respiratory muscle strength was assessed by MIP and MEP mean values. MIP showed a slight but not significant increase after expansion. A significant increase was found between T0 and T2 (P < .05), showing an improvement of 20% after 5 months of expansion (Figure 5A). MEP showed a significant increase of 10% between before (T0) and immediately after expansion (T1) but no change was observed after 5 months (Figure 5B).
Figure 5.
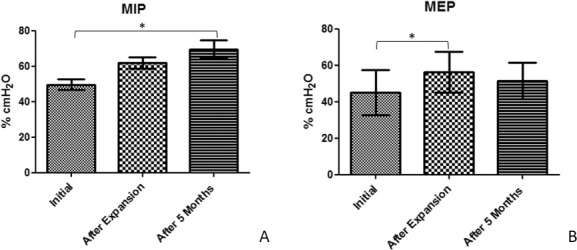
Mean values and standard deviation over time for (A) Maximum inspiratory pressure; (B) Maximum expiratory pressure (*P < .05).
A significant increase of 30.45% was observed in nasal inspiratory peak flow between T0 and T1 (P < .05), as well as a significant increase between T0 and T2 of 30.28% (P < .05) (Figure 6).
Figure 6.
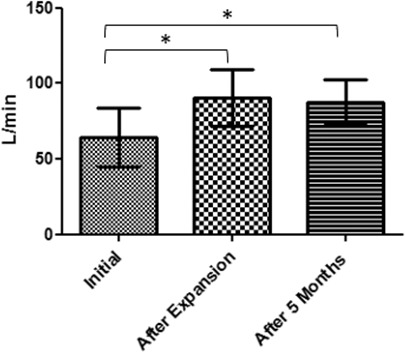
Mean values and standard deviation over time for nasal peak flow (*P < .05).
Oral expiratory peak flow results were divided between patients presenting with values greater than 100% (no lower airway obstruction) and lower values than 100% (sign of airway constriction20,22). A significant increase was found between T0 and T1 (25%) and T0–T2 (40%) in patients who presented initially low airflow values (P < .05). Patients with a satisfactory initial oral expiratory flow also showed a significant increase between T0 and T2 (20%) (Figure 7).
Figure 7.
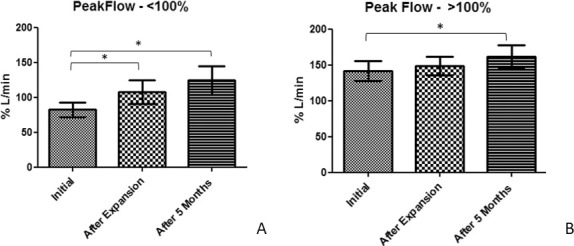
Representative mean values and standard deviation for (A) Patients with initial low oral expiratory peak flow; (B) Patients with initial good oral expiratory peak flow (*P < .05).
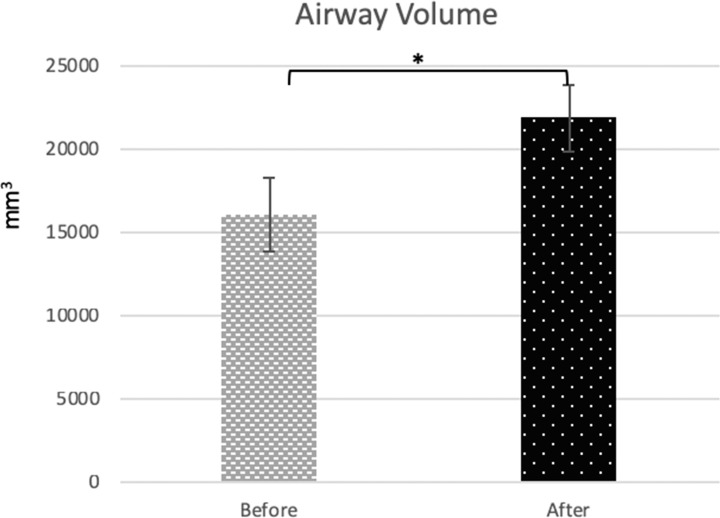
Regarding CBCT measurements, most linear and angular measurements showed significant increases from before to after expansion except for premolar inclination (P = .173) (Table 2). Total nasopharynx volume showed a significant increase from 16,058 (±2171.98) to 21,835.55 (±1937.64) mm3 (P < .05) (Figure 8 and 9).
Table 2.
Means and Standard Deviation for CBCT Measurements Between T0 and T1 (*P < .05).
| Region |
T0 |
T1 |
△T1–T0 |
P Value |
| Nasal cavity width (mm) | ||||
| 1° PM | 24.70 (2.15) | 28.17 (2.80) | 3.47 | .001* |
| 1° M | 26.22 (2.64) | 28.4 (2.59) | 2.2 | .003* |
| Midpalatal suture opening (mm) | ||||
| 1° PM | 0 (0) | 4.7 (1.49) | 4.7 | <.001* |
| 1° M | 0 (0) | 4 (1.17) | 4 | <.001* |
| Interdental distance (mm) | ||||
| 1° PM | 36.48 (3.3) | 42.38 (3.82) | 3.59 | <.001* |
| 1° M | 47.33 (3.45) | 52.67 (3.50) | 5.34 | <.001* |
| Tooth inclination (degrees) | ||||
| 1° PM | 98.66 (6.00) | 100.49 (7.43) | 1.83 | .173 |
| 1° M | 98.23 (6.29) | 101.85 (5.61) | 3.61 | .011* |
| Alveolar bone width (mm) | ||||
| 1° PM | 46.38 (2.99) | 49.97 (2.5) | 3.59 | <.001* |
| 1° M | 57.67 (2.29) | 61.55 (2.84) | 3.88 | <.001* |
CBCT indicates cone-beam computed tomography.
Figure 8.
Mean values and standard deviation over time for airway volume (*P < .05).
Figure 9.
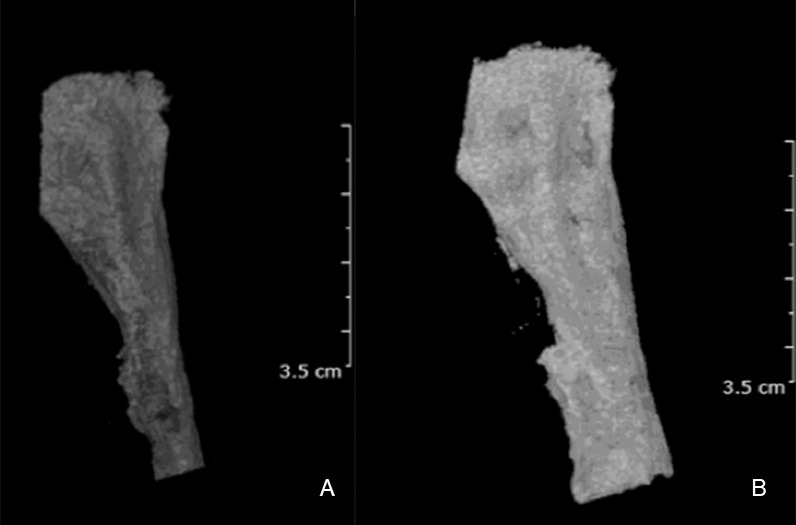
Airway volume increased significantly after expansion, (A) Initial (T0); (B) After expansion (T1).
Pearson correlation and linear regression analyses of delta values (post-pre expansion) showed a strong positive correlation between airway volume and nasal (r2 = 0.9804; P < .01) oral peak flow (r2 = 0.9364; P < .01) and MIP (r2 = 0.9482; P < .01), which meant that an increase in the airway volume had a positive effect in airflow and muscular strength during maximum inspiratory pressure. There was no correlation between the airway volume and MEP (r2 = 0.0016; P > .05).
DISCUSSION
The CBCT results of this study regarding the suture's split (premolar and molar areas) were similar to those obtained by Christie et al.27 using the Haas appliance in growing patients (mean age: 10.3 years old) and Cantarella et al.16 using maxillary skeletal expansion (MSE) in older patients with a mean age of 17.2 years old. Nasal cavity width showed a greater increase in the current study compared with another with a different MARPE design14 and Haas appliance.27 A recent study showed a 3.94 mm increase of the nasal cavity width using the MSE appliance compared with 1.95 mm for the Hyrax appliance.28 Alveolar bone width increased significantly compared to the results of another study using a different bone-borne rapid palatal expander in patients with a mean age of 18.1 years.29 No difference was found in premolar inclination. Although there was significant molar inclination observed in the current study, it was less than that found by Park et al.14 using a different design of mini-screw assisted expander. The current results were more similar to the results found by Yilmaz et al.30
Airway volume increased significantly in the current study after expansion (26%). The increase was greater than found by conventional rapid maxillary expansion in younger patients,31 surgically assisted rapid palatal expansion in adult patients,32 and the change of nasopharynx volume found by Kim et al.33 using a different design for MARPE.
Peak flow methods are important to measure oral and nasal obstruction.34 Nasal inspiratory peak flow increased immediately after expansion and this result was maintained after 5 months. This finding was in agreement with Zambon et al. who found that patients undergoing a SARPE procedure showed respiratory improvement documented as an increase in nasal respiratory flow.35 More recently, Bazargani et al., comparing two types of expanders (tooth-borne and tooth-bone-borne) for RPE, found that tooth-bone-borne appliances using two miniscrews induced significantly higher nasal airway flow in young patients aged between 8 and 13 years old.36 The current results showed a significant increase in more mature patients after less invasive expansion, using a MARPE appliance.
Oral peak expiratory flow is one technique to assess lung function.37 The results of this study showed that expiratory peak flow increased significantly after expansion in patients with initial values lower than 100%, indicating that enlargement of the nasal cavity may facilitate airflow. It may be hypothesized that, by increasing nasal airway with MARPE, patients may tend to breathe more through their nose and, therefore, probably alter tongue posture and muscular dynamics, indirectly increasing the nasopharyngeal airway. In consequence, the overall airflow and respiratory function is improved. Also, enhancing respiratory muscle strength may have led to an increase in oral expiratory peak flow.
Okuro et al.,38 assessing the strength developed by respiratory muscles, showed lower values of MEP and MIP in the mouth-breathing group of individuals, compared with nasal breathers. The authors observed that mouth breathing included altered respiratory mechanics since an anterior head position may affect the contraction of diaphragmatic and abdominal muscles. Additionally, mouth breathing requires less muscle effort and, along with inhibition of afferent nasal nerves, results in poorer use of respiratory muscles and progressive muscle weakening.39 It is natural to expect improvement in respiratory tests after MARPE, since a larger dimension of the airway would improve nasal airflow, leading to better ventilatory muscle function.
This study showed that MARPE not only affects naso-maxillary bone structures but also has a direct effect on airway flow and muscle strength, consequently improving respiratory function. However, further studies are needed to demonstrate how MARPE treatment may influence respiratory functions in a larger number of patients and on long-term evaluation.
CONCLUSIONS
Skeletal results promoted by micro-implant assisted rapid maxillary expansion (MARPE) therapy resulted in an enlargement of airway volume and a significant positive impact on respiratory functions evaluated by airflow and muscle strength.
REFERENCES
- 1.Kurol J, Berglund L. Longitudinal study and cost-benefit analysis of the effect of early treatment of posterior cross-bites in the primary dentition. Eur J Orthod. 1992;14(3):173–179. doi: 10.1093/ejo/14.3.173. [DOI] [PubMed] [Google Scholar]
- 2.Lombardo L, Carlucci A, Maino BG, Colonna A, Paoletto E, Siciliani G. Class III malocclusion and bilateral cross-bite in an adult patient treated with miniscrew-assisted rapid palatal expander and aligners. Angle Orthod. 2018;88:649–664. doi: 10.2319/111617-790.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunha RA, Cunha DA, Bezerra LA, et al. Nasal aeration and respiratory muscle strength in mouth breathers'' children. Rev. CEFAC. 2015;17(5):1432–1440. [Google Scholar]
- 4.Felcar JM, Bueno IR, Massan ACS, Torezan RP, Cardoso JR. Prevalência de respiradores bucais em crianças de idade escolar. Cien Saude Colet. 2010;15(2):437–444. doi: 10.1590/S1413-81232010000200020. [DOI] [PubMed] [Google Scholar]
- 5.Teixeira RUF, Zappelini CEM, Alves FS, Costa EA. Peak nasal inspiratory flow evaluation as an objective method of measuring nasal airflow. Braz J Otorhinolaryngol. 2011;77(4):473–480. doi: 10.1590/S1808-86942011000400011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kjærgaard T, Cvancarova M, Steinsvåg SK. Relation of nasal air flow to nasal cavity dimensions. Arch Otolaryngol Head Neck Surg. 2009;135(6):565–570. doi: 10.1001/archoto.2009.50. [DOI] [PubMed] [Google Scholar]
- 7.Evans JA, Whitelaw WA. The assessment of maximal respiratory mouth pressures in adults. Respir Care. 2009;54(10):1348–1359. [PubMed] [Google Scholar]
- 8.Giuliani BB, Olavo GC, Machado KS, Abreu LC, Valenti VE, Raimundo RD. Evaluation of the effect of learning on the full extent of inspiratory and expiratory pressure in healthy adults. MedicalExpress. 2016;3(1):1–5. [Google Scholar]
- 9.Costa D, Gonçalves HA, Lima LP, Ike D, Cancelliero KM, Montebelo MIL. Novos valores de referência para pressões respiratórias máximas na população brasileira. J Bras Pneumol. 2010;36(3):306–312. doi: 10.1590/s1806-37132010000300007. [DOI] [PubMed] [Google Scholar]
- 10.Machado AW, Briss B, Huang G, Kulbersh R, Caldas SR. An interview with Won Moon. Dental Press J Orthod. 2013;18(3):12–28. doi: 10.1590/s2176-94512013000300005. [DOI] [PubMed] [Google Scholar]
- 11.Choi SH, Shi KK, Cha JY, Park YC, Lee KJ. Nonsurgical miniscrew-assisted rapid maxillary expansion results in acceptable stability in young adults. Angle Orthod. 2016;86(5):713–720. doi: 10.2319/101415-689.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki H, Moon W, Previdente LH, Suzuki SS, Garcez AS, Consolaro A. Miniscrew-assisted rapid palatal expander (MARPE): the quest for pure orthopedic movement. Dental Press J Orthod. 2016;21(4):17–23. doi: 10.1590/2177-6709.21.4.017-023.oin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacGinnis M, Chu H, Youssef G, Wu KW, Machado AW, Moon W. The effects of micro-implant assisted rapid palatal expansion (MARPE) on the nasomaxillary complex–a finite element method (FEM) analysis. Prog Orthod. 2014;15(52):1–15. doi: 10.1186/s40510-014-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JJ, Park YC, Lee KJ, Cha JY, Tahk JH, Choi YJ. Skeletal and dentoalveolar changes after miniscrew-assisted rapid palatal expansion in young adults: A cone-beam computed tomography study. Korean J Orthod. 2017;47(2):77–86. doi: 10.4041/kjod.2017.47.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SY, Park YC, Lee KJ, et al. Assessment of changes in the nasal airway after nonsurgical miniscrew-assisted rapid maxillary expansion in young adults. Angle Orthod. 2018;88(4):435–441. doi: 10.2319/092917-656.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantarella D, Dominguez-Mompell R, Mallya SM, et al. Changes in the midpalatal and pterygopalatine sutures induced by micro-implant-supported skeletal expander, analyzed with a novel 3D method based on CBCT imaging. Prog Orthod. 2017;18(34):1–12. doi: 10.1186/s40510-017-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baccetti T, Franchi L, Mcnamara J., Jr An improved version of the cervical vertebral maturation (CVM) method for the assessment of mandibular growth. Angle Orthod. 2002;72(4):316–323. doi: 10.1043/0003-3219(2002)072<0316:AIVOTC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Carlson C, Sung J, McComb RW, MacHado AW, Moon W. Microimplant-assisted rapid palatal expansion appliance to orthopedically correct transverse maxillary deficiency in an adult. Am J Orthod Dentofac Orthop. 2016;149(5):716–728. doi: 10.1016/j.ajodo.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 19.Brunetto DP, Sant'Anna EF, Machado AW, Moon W. Non-surgical treatment of transverse deficiency in adults using microimplant-assisted rapid palatal expansion (MARPE) Dental Press J Orthod. 2017;22(1):110–125. doi: 10.1590/2177-6709.22.1.110-125.sar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boaventura CM, Amuy FF, Franco JH, Sgarbi ME, Matos LB, Matos LB. Valores de referência de medidas de pico de fluxo expiratório máximo em escolares. Arq Med ABC. 2007;32(Supl.2):30–34. [Google Scholar]
- 21.Borja RDO, Campos TF, Freitas DA, Macêdo TMF, Mendonça WCM, Mendonça KMPP. Predicted normal values for maximal respiratory pressures in children. ConScientiae Saúde. 2015;14(2):535–538. [Google Scholar]
- 22.Nunn AJ, Gregg I. New regression equations for predicting peak expiratory flow in adults. BMJ. 1989;298:1068–1070. doi: 10.1136/bmj.298.6680.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guidelines for the diagnosis and management of Asthma. 4051. Vol. 97. Bethesda, MD: National Institutes of Health Publication; (1997). National Asthma Education Program Expert Panel Report II; pp. 1–153. [Google Scholar]
- 24.Tantilipikorn P, Meekul N, Suwanwech T, Assanasen P, Bunnag C, Thinkhamrop B. Peak nasal inspiratory flow: reference values for thais. Siriraj Med J. 2015;67(6):267–272. [Google Scholar]
- 25.Garrett BJ, Caruso JM, Rungcharassaeng K, Farrage JR, Kim JS, Taylor GD. Skeletal effects to the maxilla after rapid maxillary expansion assessed with cone-beam computed tomography. Am J Orthod Dentofac Orthop. 2008;134(1):16–21. doi: 10.1016/j.ajodo.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Almuzian M, Ju X, Almukhtar A, Ayoub A, Al-Muzian L, McDonald JP. Does rapid maxillary expansion affect nasopharyngeal airway? A prospective Cone Beam Computerised Tomography (CBCT) based study. Surgeon. 2018;16(1):1–11. doi: 10.1016/j.surge.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Christie KF, Boucher N, Chung CH. Effects of bonded rapid palatal expansion on the transverse dimensions of the maxilla: A cone-beam computed tomography study. Am J Orthod Dentofac Orthop. 2010;137:79–85. doi: 10.1016/j.ajodo.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Moschik CE. Morphometric Analysis of Maxillary Skeletal Expansion Effects on the Nasal Cavity [thesis] Los Angeles, Calif: University of California, Los Angeles; 2018. [Google Scholar]
- 29.Lin L, Ahn HW, Kim SJ, Moon SC, Kim SH, Nelson G. Tooth-borne vs bone-borne rapid maxillary expanders in late adolescence. Angle Orthod. 2015;85(2):253–262. doi: 10.2319/030514-156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yilmaz A, Arman-Özçlrplcl A, Erken S, Polat-Özsoy Ö. Comparison of short-term effects of mini-implant-supported maxillary expansion appliance with two conventional expansion protocols. Eur J Orthod. 2015;37(5):556–564. doi: 10.1093/ejo/cju094. [DOI] [PubMed] [Google Scholar]
- 31.Hakan EI, Palomo JM. Three-dimensional evaluation of upper airway following rapid maxillary expansion A CBCT study. Angle Orthod. 2014;84(2):265–273. doi: 10.2319/012313-71.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira-Filho VA, Monnazzi MS, Gabrielli MAC, et al. Volumetric upper airway assessment in patients with transverse maxillary deficiency after surgically assisted rapid maxillary expansion. Int J Oral Maxillofac Surg. 2014;43(5):681–686. doi: 10.1016/j.ijom.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Baratieri CL, Alves M, Jr, Mattos CT, Lau GWT, Nojima LI, Souza MMG. Transverse effects on the nasomaxillary complex one year after rapid maxillary expansion as the only intervention: A controlled study. Dental Press J Orthod. 2014;19(5):79–87. doi: 10.1590/2176-9451.19.5.079-087.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bathala S, Eccles R. Assessment of upper airway obstruction by measuring peak oral and nasal inspiratory flow. J Laryngol Otol. 2015;129(5):473–477. doi: 10.1017/S0022215115000523. [DOI] [PubMed] [Google Scholar]
- 35.Zambon CE, Ceccheti MM, Utumi ER, et al. Orthodontic measurements and nasal respiratory function after surgically assisted rapid maxillary expansion: An acoustic rhinometry and rhinomanometry study. Int J Oral Maxillofac Surg. 2012;41(9):1120–1126. doi: 10.1016/j.ijom.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 36.Bazargani F, Magnuson A, Ludwig B. Effects on nasal airflow and resistance using two different RME appliances: a randomized controlled trial. Eur J Orthod. 2017;40(3):281–284. doi: 10.1093/ejo/cjx081. [DOI] [PubMed] [Google Scholar]
- 37.Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can peak expiratory flow predict airflow obstruction in children with asthma? Pediatrics. 2000;105(2):354–358. doi: 10.1542/peds.105.2.354. [DOI] [PubMed] [Google Scholar]
- 38.Okuro RT, Morcillo AM, Sakano E, Schivinski CI, Ribeiro MA, Ribeiro JD. Exercise capacity, respiratory mechanics and posture in mouth breathers. Braz J Otorhinolaryngol. 2011;77(5):656–662. doi: 10.1590/S1808-86942011000500020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trevisan ME, Boufleur J, Soares JC, Haygert CJ, Ries LG, Corrêa EC. Diaphragmatic amplitude and accessory inspiratory muscle activity in nasal and mouth-breathing adults: A cross-sectional study. J Electromyogr Kinesiol. 2015;25(3):463–468. doi: 10.1016/j.jelekin.2015.03.006. [DOI] [PubMed] [Google Scholar]