Abstract
Objectives:
To investigate whether Forkhead family transcription factors are responsive to mechanical force and the resulting influence on the osteoclast differentiation mediated by human periodontal ligament cells (PDLCs).
Materials and Methods:
A high-throughput RNA sequencing assay was performed in compressive force–stimulated and control human PDLCs. Alteration of FOXM1, a member of the Forkhead family transcription factors, was further confirmed by Western blotting and quantitative reverse-transcription polymerase chain reaction. Expression of FOXM1 was inhibited by either small interfering RNA (siRNA) transfection or addition of its specific inhibitor Siomycin A. Then, cells were exposed to compressive force and co-cultured with the murine macrophage cell line Raw264.7, followed by tartrate-resistant acid phosphatase staining assay. Expression changes of receptor activator of nuclear factor κB ligand (RANKL) and osteoprotegetin (OPG) caused by FOXM1 suppression were measured. Alkaline phosphatase (ALP) staining, ALP activity assay, and crystal violet staining assay were performed after FOXM1 inhibition.
Results:
FOXM1 transcription decreased after mechanical stimulation in PDLCs. Inhibition of FOXM1 promoted force-induced osteoclast differentiation of RAW264.7 and upregulated the RANKL/OPG ratio in PDLCs. Interference of FOXM1 led to promoted osteogenic differentiation but decreased proliferation of PDLCs.
Conclusions:
FOXM1 is a novel mechano-responsive gene in human PDLCs. Suppressing FOXM1 expression could promote osteoclast differentiation as well as RANKL/OPG in human PDLCs. FOXM1 also plays a role in controlling PDLC differentiation and proliferation capacity.
Keywords: Compressive force, FOXM1, RANKL/OPG, Osteoclast differentiation
INTRODUCTION
Orthodontic tooth movement (OTM) involves alveolar bone remodeling triggered by mechanical force stimuli.1 During OTM, the periodontal ligament (PDL) on the compression side displays bone resorption, while the tension side is characterized by osteoblast differentiation.1,2 Bone resorption on the compression side can be attributed to a series of proinflammatory cytokines secreted by periodontal ligament cells (PDLCs), such as macrophage colony-stimulating factor, interleukin-6, interleukin-8, tumor necrosis factor–α, and others.3 Among them, the receptor activator of nuclear factor κB (RANK)/receptor activator of nuclear factor κ-B ligand (RANKL)/osteoprotegetin (OPG) axis plays a pivotal role and is considered to be a rate-limiting determinant of OTM.4,5 OPG, a decoy receptor of RANKL, competes with RANK for the binding and therefore functions as a negative regulator of osteoclastogenesis.6 Studies have demonstrated the reduced expression of OPG during OTM.7–11 Local OPG gene transfer to periodontal tissue was demonstrated to inhibit OTM,12 suggesting the critical role of OPG downregulation in OTM. However, little is known about the mechanisms governing the declined expression of OPG. In addition, force-induced changes at the genetic level and the mechano-transduction mechanisms in PDLCs are not fully understood.
The Forkhead family of transcriptional factors (TFs) are characterized by a Forkhead DNA-binding domain with approximately 100 amino acids, which are capable of binding on the target genes' promoter or enhancer elements with the consensus DNA sequence.13 Their target genes are involved in a wide range of biological processes, including embryonic organogenesis, carcinogenesis, and cellular senescence.13–16 However, whether this family of TFs could respond to mechanical stimuli and mediate downstream biological events in PDLCs is still unknown.
To screen for mechano-responsive genes among the FOX family in human PDLCs, a high-throughput RNA sequencing assay was performed. The results indicated a significant decrease of FOXM1, a pro-proliferative transcription factor with diverse functions in vivo such as proliferation, differentiation, migration, DNA repair, surfactant production, and formation of cellular junctions.13,17 Because of such critical roles of FOXM1 in cell functional maintenance, it is plausible to speculate that force-induced reduction of FOXM1 might lead to expression changes of its downstream target genes, thereby resulting in corresponding alterations in cellular behaviors such as osteoclastogenesis, proliferation, and differentiation capacity of PDLCs. Therefore, this study aimed to elucidate the functional changes in human PDLCs caused by force-induced downregulation of FOXM1, with emphasis on the induction of osteoclast differentiation and RANKL/OPG changes of PDLCs.
MATERIALS AND METHODS
Cell Culture and Force Static Compressive Application
This study was conducted with approval from the Human Research Ethics Committee of Peking University (PKUSSIRB-201630098). Human PDLCs were isolated from normal premolars extracted for orthodontic purposes, as previously reported.18 Teeth were extracted from four donors with their written informed consent. Cells were used at three to six passages.
For the application of static compressive force, PDLCs were seeded into the six-well plate (1 × 105 cells/well). After reaching 80% confluence, a cover glass and a glass bottle with steel granules were placed over the monolayer PDLCs. The weight of the steel granules was adjusted to reach the indicated force intensity (Figure 1A–D).
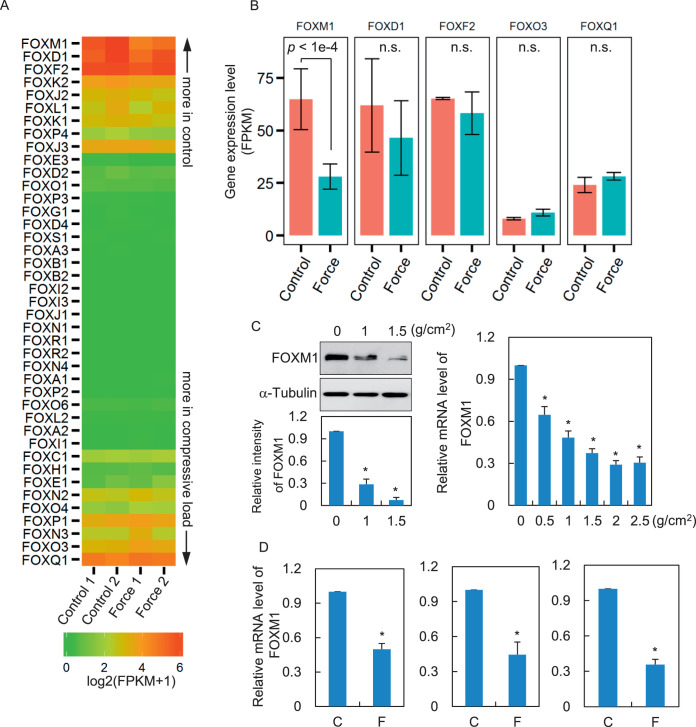
Figure 1.
FOXM1 decreases in response to compressive force stimuli. (A) PDLCs were exposed to compression stress (1 g/cm2) for 24 hours. The total mRNA from two biological replicates of stressed or control PDLCs was then isolated and subjected to RNA-seq. The gene expression level was measured as fragments per kilobase of transcript sequence per millions base (FPKM). The heatmap shows expression levels of Forkhead family TFs. This map was sorted by the mean FPKM difference in stressed vs control cells. (B) Bar plot shows FPKM for indicated genes. n.s., no statistical significance (Student's t-test). (C) Protein and mRNA levels of FOXM1 in stressed or control PDSCs were determined by Western blotting (left panel) and qRT-PCR (right panel). α-tubulin served as a loading control. (D) Freshly isolated PDLCs from three donors were subjected to static compression force. FOXM1 expression was examined by RT-qPCR. C indicates control; F, force. Data represent the mean ± SD for triplicate experiments. *P < .05.
RNA Sequencing Assay
In-depth whole transcriptomic sequencing was performed by the Beijing Genome Institute in PDLCs subjected to static compressive force of 1 g/cm2 for 24 hours and the control cells. The reads from RNA-seq were aligned to the human genome with TopHat.19 Cufflinks20 was used to normalize and call mechanical stress–induced differentially expressed genes.
Western Blotting Analyses
Western blotting analyses were performed according to protocols described previously.15,16 Antibodies used in this study were as follows: anti-FOXM1 (C15410232, Diagenode, San Diego, Calif), anti-RANKL (ab45039, Abcam, Cambridge, UK), anti-OPG (ab11994, Abcam), anti-α-tubulin (2125s, CST, Danvers, Mass), anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH; sc-47724, Santa Cruz, Dallas, Tex). Each experiment was repeated three times. The relative intensities of the results were measured by Image J 1.37v software.
RNA Isolation and Reverse-Transcription Polymerase-Chain Reaction
Total RNAs were isolated from cultured PDLCs using Trizol reagent (Invitrogen, Waltham, Mass) according to the manufacturer's instructions. Reverse-transcription and real-time polymerase chain reaction (PCR) were performed following protocols described elsewhere.15 All quantitative reverse-transcription PCR (qRT-PCR) processes were performed three times using GAPDH as the internal control. The primer sequences of each gene are listed in Table 1.
Table 1.
Primer Sequences for Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
| Target |
Sense Primer (5′-3′) |
Antisense Primer (3′-5′) |
| GAPDH | CAATGACCCCTTCATTGACC | ATGACAAGCTTCCCGTTCTC |
| RANKL | ATCACAGCACATCAGAGCAGAGA | AGGACAGACTCACTTTATGGGAAC |
| OPG | GAGGCATTCTTCAGGTTTGC | GCTGTGTTGCCGTTTTATCC |
| FOXM1 | TGCCAAGGGAAAAGAGAGTG | TAGCTGGTTTGGGTTTGAGG |
Small-Interfering RNA Transfection
Small-interfering RNAs (siRNAs) against FOXM1 and the scrambled control siRNA were chemically synthesized (GenePharma, Shanghai, China). Cells were transfected with 50-nm siRNA oligonucleotides for 72 hours using lipofectamine RNAiMAX (Invitrogen). The siRNA sequences were as follows: siFOXM1, CUCUUCUCCCUCAGAUAUA, and siNC, UUCUCCGAACGUGUCACGU.
Co-culture of PDLC and RAW 264.7 and Tartrate-Resistant Acid Phosphatase Staining
The murine monocytic cell line RAW264.7 was cultured in α-MEM containing 10% fetal bovine serum. PDLCs were seeded into six-well plates, and then FOXM1 was suppressed by either siRNA transfection (Figure 2A) or addition of Siomycin A (15378, Cayman, Ann Arbor, Mich) into the medium with increasing concentrations (as indicated in Figures 2B, 3A,B, and 4A,B). Then, the cells were subjected to a compressive force of 1 g/cm2 for 24 hours followed by supernatant collection. RAW264.7 cells were seeded into six-well plates and cultured with supernatant from the PDLCs. After 5 days, the cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP) using an acid phosphatase kit (387A-1KT, Sigma, St Louis, Mo). TRAP-positive multinucleated osteoclasts were counted in each visual field (n = 3). The average value of three experiments was calculated.
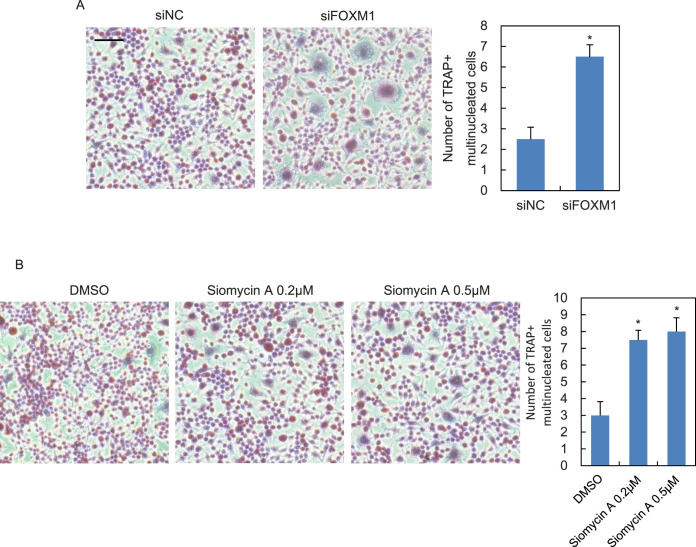
Figure 2.
Blockade of FOXM1 promotes force-induced osteoclast differentiation of co-cultured RAW264.7 cells. PDLCs were transfected with siRNA against FOXM1 (siFOXM1) or none-sense control (siNC; A) or pretreated with increasing dosage of Siomycin A (B) followed by compressive force application (1 g/cm2, 24 hours). TRAP staining images of RAW264.7 co-cultured with supernatants from PDLCs are shown. The statistical analyses are shown on the right. Scale bar: 200 μm. Data represent mean ± SD for triplicate experiments. *P < .05.
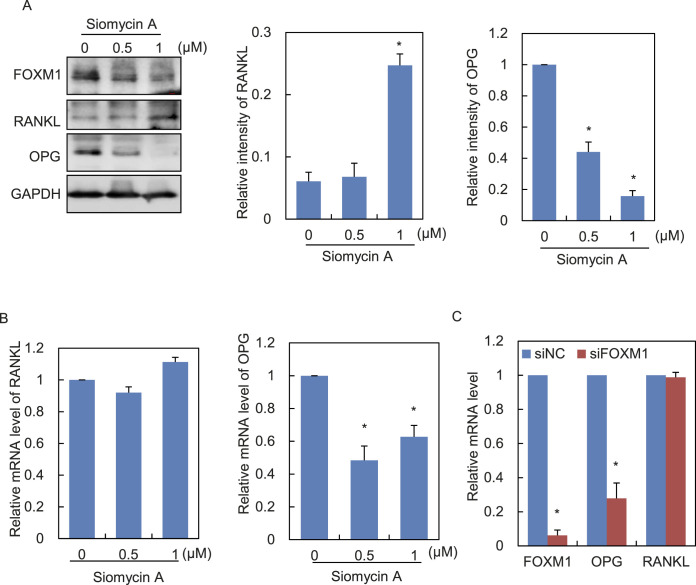
Figure 3.
Blockade of FOXM1 increases the RANKL/OPG ratio in human PDLCs. (A,B) The protein and mRNA levels of RANKL and OPG in PDLCs treated with increasing dosage of Siomycin A were determined by Western blotting (A) and qRT-PCR (B), respectively. GAPDH served as a loading control. (C) PDLCs were transfected with siRNA against FOXM1 (siFOXM1) or the none-sense control (siNC). The mRNA levels of indicated genes were determined by qRT-PCR. Data represent the mean ± SD for triplicate experiments. *P < .05.
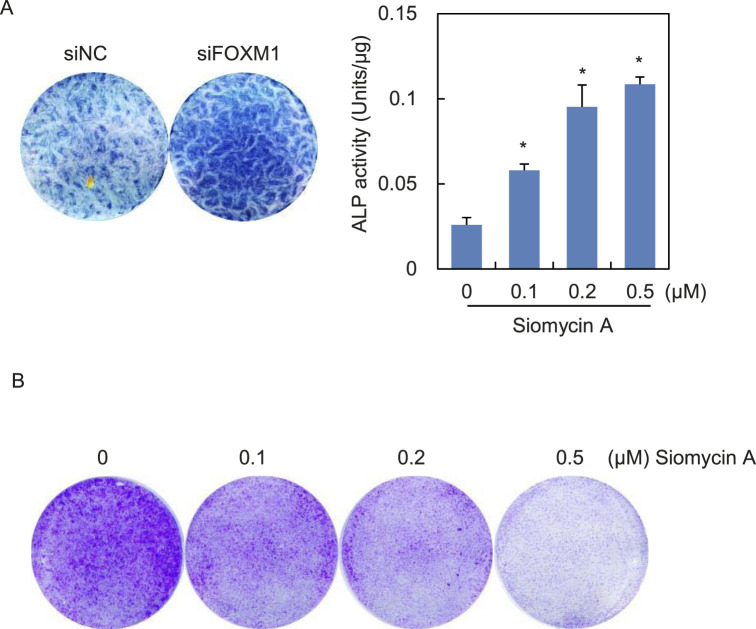
Figure 4.
Influence of FOXM1 inhibition on the proliferation and osteogenic differentiation potential of PDLCs. (A) Human PDLCs were transfected with siRNA against FOXM1 (siFOXM1) or none-sense control (siNC; left panel) or pretreated with increasing dosage of Siomycin A (right panel). Then, cells were induced toward osteogenic differentiation and subjected to ALP staining (left panel) or ALP activity assay (right panel). Data represent mean ± SD for triplicate experiments. *P < .05. (B) PDLCs were cultured in medium containing indicated concentrations of Siomycin A for 2 weeks. Results of the crystal violet staining are shown.
Alkaline Phosphatase Staining, Alkaline Phosphatase Activity Assay, and Crystal Violet Staining Assay
For alkaline phosphatase (ALP) staining and ALP activity assay, PDLCs were cultured in 24-well plates and transfected with siRNA against FOXM1 or the none-sense control or subjected to Siomycin A treatment. Then, the medium was replaced by the osteogenic medium. After 2 weeks' incubation, an ALP staining kit or ALP activity assay kit (Jiancheng Bioengineering Institute, Nanjing, China) was applied to perform ALP staining or ALP activity assay. Siomycin A–treated PDLCs cultured in normal medium were subjected to crystal violet staining as previously described.16
Statistical Analyses
Statistical analysis was performed using Student's t-test. P values are specified in the relevant figure legends. All data are expressed as mean ± standard deviation. Statistical significance was considered at P < .05.
RESULTS
Compressive Force Decreases the Expression of FOXM1 in Human PDLCs
To understand mechanical loading–induced global gene transcriptional changes, high-throughput RNA sequencing (RNA-seq) was performed in human PDLCs. Human PDLCs were exposed to a compressive force of 1 g/cm2. After 24 hours of strain, total mRNA from the stressed or control PDLCs were collected and subjected to RNA-seq. Surprisingly, of the 41 factors of the Forkhead family, FOXM1 was the only member showing significant expression changes. Mean expression levels measured by fragments per kilobase of transcript sequence per millions base (FPKM) were downregulated more than twofold responding to mechanical force (Figure 1A,B; Student's t-test, P < 1e-4). The following members with the most decreased FPKM, including FOXD1 and FOXF2, or the members with the most increased FPKM, including FOXQ1 and FOXO3, showed no significant changes in their mRNA levels (Figure 1B). Western blotting and qRT-PCR analyses confirmed the downregulation of FOXM1 after stress loading (Figure 1C). This point was further consolidated by qRT-PCR using PDLCs isolated from the other three donors (Figure 1D).
Inhibition of FOXM1 in PDLCs Promotes Osteoclast Differentiation of Co-cultured RAW264.7 Cells
Force application was able to induce osteoclast differentiation both in vitro and in vivo, as demonstrated by previous studies.4,21,22 Therefore, this study set out to investigate whether force-induced decline of FOXM1 could influence osteoclastogenesis of RAW264.7 cells. RAW264.7 cells were co-cultured with supernatant from PDLCs transfected with FOXM1 or none-sense control siRNA followed by force application. TRAP staining indicated elevated numbers of multinucleated osteoclasts in FOXM1-knocked down cells, as compared with the control group (Figure 2A). Consistent results were obtained with RAW264.7 cells co-cultured with PDLCs pretreated with Siomycin A, an inhibitor specific for FOXM123 (Figure 2B).
Inhibition of FOXM1 Promotes the RANKL/OPG Ratio
Next, the molecular mechanism by which FOXM1 influences the pro-osteoclast differentiation capacity of PDLCs was explored. Because of the pivotal role of RANKL/OPG in osteoclast differentiation, the expression changes of RANKL and OPG in PDLCs treated with increasing dosage of FOXM1 inhibitor Siomycin A were examined. Western blot analysis revealed an upregulation of RANKL and downregulation of OPG expression, along with the gradual reduction of FOXM1 protein level (Figure 3A). A drop was also observed in OPG expression at the mRNA level, while alteration of RANKL was not significant as shown by qRT-PCR (Figure 3B). Knockdown of FOXM1 by siRNA transfection also revealed the remarkable decrease of OPG at the mRNA level (Figure 3C). These data collectively demonstrated that loss of FOXM1 could upregulate the RANKL/OPG ratio.
FOXM1 Affects Osteogenic Differentiation Potential and Proliferation Ability of Human PDLCs
Since PDLCs contain osteoblast progenitors possessing osteogenic differentiation potential, the last question was whether a force-induced decrease of FOXM1 could affect PDLC differentiation potential, as well as proliferation capacity. Depletion of FOXM1 through siRNA transfection or Siomycin A treatment led to enhanced osteogenic differentiation, as revealed by ALP staining and ALP activity assay (Figure 4A). Crystal violet staining indicated that proliferation of PDLCs was repressed upon Siomycin A treatment (Figure 4B).
DISCUSSION
Although OTM-associated compressive force was discovered to be able to induce alteration of large amounts of genes,24 the molecular mechanisms accounting for alteration of these genes are still not clear enough. Forkhead TFs are well known to play pivotal roles in regulating various cellular behaviors.13,25 For the first time, the Forkhead family member, FOXM1, was identified as a novel mechanosensitive gene in human PDLCs.
This study demonstrated that suppression of FOXM1 in PDLCs promoted the osteoclastogenesis of co-cultured RAW264.7 cells, implying that force-induced FOXM1 reduction contributed to osteoclast differentiation (Figure 2). It was further demonstrated that this effect could be attributed to regulation of RANKL/OPG ratio by FOXM1, since FOXM1 suppression led to a transcriptional decrease of OPG (Figure 3). This study proposed FOXM1 as a regulator of OPG, accounting for force-induced reduction of OPG, which further contributes to osteoclast differentiation and OTM rate. FOXM1 as a TF can not only occupy its cognate-cis elements in the promoter region but can also bind at distal regulatory enhancers to create chromatin competency, allowing efficient recruitment of downstream TFs. Thus, future research should include probing the precise binding site of FOXM1 on the regulatory elements of OPG. In addition, the protein level of RANKL was upregulated with force application, but its mRNA level remained unchanged. It is likely that RANKL is not a direct transcriptional target of FOXM1 but, rather, there exist some unknown factors that are transcriptionally regulated by FOXM1 and mediate the posttranscriptional regulation of RANKL. Given the contributing effect of FOXM1 inhibition on RANKL/OPG upregulation and osteoclastogenesis, this study indicated FOXM1 as a novel molecular therapeutic target whose pharmacological inhibitors might be used to accelerate the clinical OTM process and perhaps reduce the adverse effects caused by OTM in the future.
Another interesting finding of this work was the influence of FOXM1 on PDLC proliferation and osteogenesis. It was reported that PDLC proliferation was repressed after compressive force stimulation.8 Given the finding that FOXM1 depletion suppressed proliferation of PDLCs (Figure 4B), it is possible that compressive force–induced growth retardation might be mediated at least in part by the reduced expression of FOXM1. In addition, increased osteogenic differentiation was found after FOXM1 inhibition (Figure 4A). The expression of osteoblastic marker genes was shown in previous reports to be elevated in PDLCs under pressure.26 It may be inferred that the force-induced decrease of FOXM1 might be a player in mediating osteogenesis in response to compressive force. The underlying mechanisms through which FOXM1 exerts its influence on the osteogenesis of PDLCs remain to be clarified by future work.
CONCLUSIONS
Expression of FOXM1 decreased in response to static compressive force in human PDLCs, which enhanced force-induced osteoclast differentiation and the RANKL/OPG ratio.
FOXM1 inhibition also affected proliferation and osteogenic differentiation potential of human PDLCs.
These findings shed new light on molecular mechanisms governing force-induced osteoclastogenesis during OTM.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (#81502345). The authors report no conflicts of interest related to this study.
REFERENCES
- 1.Li Y, Jacox LA, Little SH, Ko CC. Orthodontic tooth movement: the biology and clinical implications. Kaohsiung J Med Sci. 2018;34:207–214. doi: 10.1016/j.kjms.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baloul SS. Osteoclastogenesis and osteogenesis during tooth movement. Front Oral Biol. 2016;18:75–79. doi: 10.1159/000351901. [DOI] [PubMed] [Google Scholar]
- 3.Kitaura H, Kimura K, Ishida M, et al. Effect of cytokines on osteoclast formation and bone resorption during mechanical force loading of the periodontal membrane. ScientificWorldJournal. 2014;2014:617032. doi: 10.1155/2014/617032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res. 2002;17:210–220. doi: 10.1359/jbmr.2002.17.2.210. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi M. RANK/RANKL/OPG during orthodontic tooth movement. Orthod Craniofac Res. 2009;12:113–119. doi: 10.1111/j.1601-6343.2009.01444.x. [DOI] [PubMed] [Google Scholar]
- 6.Sokos D, Everts V, de Vries TJ. Role of periodontal ligament fibroblasts in osteoclastogenesis: a review. J Periodontal Res. 2015;50:152–159. doi: 10.1111/jre.12197. [DOI] [PubMed] [Google Scholar]
- 7.Li B, Zhang YH, Wang LX, Li X, Zhang XD. Expression of OPG, RANKL, and RUNX2 in rabbit periodontium under orthodontic force. Genet Mol Res. 2015;14:19382–19388. doi: 10.4238/2015.December.29.48. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Li M, Tan L, et al. Analysis of time-course gene expression profiles of a periodontal ligament tissue model under compression. Arch Oral Biol. 2013;58:511–522. doi: 10.1016/j.archoralbio.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Zheng W, Liu JS, et al. Expression of osteoclastogenesis inducers in a tissue model of periodontal ligament under compression. J Dent Res. 2011;90:115–120. doi: 10.1177/0022034510385237. [DOI] [PubMed] [Google Scholar]
- 10.Nishijima Y, Yamaguchi M, Kojima T, Aihara N, Nakajima R, Kasai K. Levels of RANKL and OPG in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthod Craniofac Res. 2006;9:63–70. doi: 10.1111/j.1601-6343.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- 11.Toygar HU, Kircelli BH, Bulut S, Sezgin N, Tasdelen B. Osteoprotegerin in gingival crevicular fluid under long-term continuous orthodontic force application. Angle Orthod. 2008;78:988–993. doi: 10.2319/100507-483.1. [DOI] [PubMed] [Google Scholar]
- 12.Kanzaki H, Chiba M, Takahashi I, Haruyama N, Nishimura M, Mitani H. Local OPG gene transfer to periodontal tissue inhibits orthodontic tooth movement. J Dent Res. 2004;83:920–925. doi: 10.1177/154405910408301206. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 14.Katoh M. Human FOX gene family (review) Int J Oncol. 2004;25:1495–1500. [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang D, Li Q, et al. Nucleation of DNA repair factors by FOXA1 links DNA demethylation to transcriptional pioneering. Nat Genet. 2016;48:1003–1013. doi: 10.1038/ng.3635. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Zhang Y, Fu J, et al. FOXA1 mediates p16(INK4a) activation during cellular senescence. EMBO J. 2013;32:858–873. doi: 10.1038/emboj.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Regan RM, Nahta R. Targeting forkhead box M1 transcription factor in breast cancer. Biochem Pharmacol. 2018;154:407–413. doi: 10.1016/j.bcp.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Ma Y, Zhu Y, Zhang T, Zhou Y. Declined expression of histone deacetylase 6 contributes to periodontal ligament stem cell aging. J Periodontol. 2017;88:e12–e23. doi: 10.1902/jop.2016.160338. [DOI] [PubMed] [Google Scholar]
- 19.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trapnell C, Williams BA, Pertea G, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanuki R, Shionome C, Kuwabara A, et al. Compressive force induces osteoclast differentiation via prostaglandin E(2) production in MC3T3-E1 cells. Connect Tissue Res. 2010;51:150–158. doi: 10.3109/03008200903168484. [DOI] [PubMed] [Google Scholar]
- 22.Jin Y, Li J, Wang Y, et al. Functional role of mechanosensitive ion channel Piezo1 in human periodontal ligament cells. Angle Orthod. 2015;85:87–94. doi: 10.2319/123113-955.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radhakrishnan SK, Bhat UG, Hughes DE, Wang IC, Costa RH, Gartel AL. Identification of a chemical inhibitor of the oncogenic transcription factor forkhead box M1. Cancer Res. 2006;66:9731–9735. doi: 10.1158/0008-5472.CAN-06-1576. [DOI] [PubMed] [Google Scholar]
- 24.Lee YH, Nahm DS, Jung YK, et al. Differential gene expression of periodontal ligament cells after loading of static compressive force. J Periodontol. 2007;78:446–452. doi: 10.1902/jop.2007.060240. [DOI] [PubMed] [Google Scholar]
- 25.Link W, Fernandez-Marcos PJ. FOXO transcription factors at the interface of metabolism and cancer. Int J Cancer. 2017;141:2379–2391. doi: 10.1002/ijc.30840. [DOI] [PubMed] [Google Scholar]
- 26.Yang YQ, Li XT, Rabie AB, Fu MK, Zhang D. Human periodontal ligament cells express osteoblastic phenotypes under intermittent force loading in vitro. Front Biosci. 2006;11:776–781. doi: 10.2741/1835. [DOI] [PubMed] [Google Scholar]