Abstract
Helicobacter pylori (HP) infection is one of the most frequent bacterial infections in humans and is associated with the pathogenesis of gastric motility disorders such as delayed gastric emptying (DGE). Although HP infection is considered to delay gastric emptying, there has been little research on the underlying mechanism. Gastric motility involves interactions among gastrointestinal hormones, smooth muscle, enteric and extrinsic autonomic nerves and interstitial cells of Cajal (ICCs), and ICCs play an important role in gastrointestinal motility. Mutation or loss of stem cell factor (SCF) expression is known to reduce the number of ICCs or alter the integrity of the ICC network, contributing to gastrointestinal dysmotility. The aim of the present study was to investigate whether a reduction in ICCs contributes to the DGE caused by HP. A mouse model of HP infection was established and gastric emptying was compared between HP-infected and uninfected mice using the bead method. In addition, ICC counts and SCF expression levels in gastric tissue were evaluated using immunohistochemistry and western blotting, respectively. The results revealed that gastric emptying was significantly slower, the number of ICCs in gastric tissue was significantly reduced and the protein level of SCF in gastric tissue was significantly decreased in HP-infected mice compared with uninfected mice. Therefore, it may be concluded that HP reduced the number of ICCs by decreasing the expression of SCF protein in gastric tissue, thereby causing DGE.
Keywords: Helicobacter pylori, interstitial cells of Cajal, stem cell factor, delayed gastric emptying
Introduction
Helicobacter pylori (HP) infection is one of the most frequent bacterial infections in humans, and approximately half of the global population are infected with HP (1,2). HP is associated with the pathogenesis of chronic gastritis, peptic ulcers and gastric cancer (3), and also with gastric motility disorders such as functional dyspepsia and delayed gastric emptying (DGE) (4,5). The effect of HP infection on gastric emptying has been an enduring topic of study and most researchers consider that HP infection delays gastric emptying, but there has been little research on the associated mechanism (5-8).
Gastric motility involves interactions among gastrointestinal hormones, smooth muscle, enteric and extrinsic autonomic nerves and interstitial cells of Cajal (ICCs) (9-11). ICCs are a specific type of interstitial cells in the gastrointestinal tract (12); they play an important role in gastrointestinal motility and are acknowledged to act as physiological pacemakers for the gastrointestinal tract (13-16). The proliferation, differentiation and function of ICCs is associated with activation of c-kit receptors on their surfaces (17,18). The activation of c-kit is dependent on the natural ligand stem cell factor (SCF) (19,20). Previous studies have shown that a mutation or loss of expression of SCF leads to a reduction in the number of ICCs or alters the integrity of the ICC network, which contributes to gastrointestinal dysmotility, including chronic idiopathic intestinal pseudo-obstruction, achalasia, afferent loop syndrome and chronic constipation (21-23). Therefore, the SCF/c-kit pathway is important for the development and maintenance of ICC phenotype and function (24,25).
In the present study, an HP-infected C57BL/6 mouse model was established with the aim of studying the effect of HP infection on gastric emptying function and the underlying mechanism.
Materials and methods
Animals and HP infection
All experiments and procedures in the present study were approved by the Ethics Committee of Shandong Cancer Hospital. A total of 24 female specific pathogen-free (SPF) C57BL/6 mice, aged 6-8 weeks and weighing 16-18 g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. To establish a model of HP infection, 12 mice (the HP+ group) were infected with HP Sydney strain 1 (SS1) at a concentration of 1x109 colony-forming units/ml by oral gavage. Another 12 mice remained uninfected and were used as controls (the HP- group). All the mice were housed at constant temperature (23±2˚C) and humidity (50±10%) in a SPF facility with a 12-h light/dark cycle and unconditional access to water and food. The mice were monitored twice each day to evaluate their health. If they were extremely weak, unable to drink and eat by themselves, or suffered from cachexia, they would be euthanized by an intraperitoneal injection of barbiturate (150 mg/kg). After 6 weeks, all 24 mice were sacrificed using cervical dislocation. The criteria used to confirm death included immobility, respiratory arrest and pupil dilation. The stomachs were dissected from the mice and subjected to histological examination and the extraction of protein. The gastric emptying of the HP-infected mice (n=12) and control mice (n=12) was evaluated prior to sacrifice as described below. HP infection was confirmed in the stomachs of all the HP-infected mice by the microaerobic bacterial culture of stomach homogenates on tryptic soy agar medium (cat. no. T8650; Beijing Solarbio Science & Technology Co., Ltd.) supplemented with 5% sheep blood (cat. no. TX0030; Beijing Solarbio Science & Technology Co., Ltd.) and HP selective supplement (cat. no. HB8646a; Qingdao Hope Bio-Technology Co., Ltd.) at 37˚C in an atmosphere containing 5% O2, 10% CO2 and 85% N2 and by immunohistochemical (IHC) examination.
Evaluation of gastric emptying and specimen collection
Distilled water (0.5 ml/mouse) containing 40 resin beads (diameter, 0.4 mm) was administered to each mouse via oral gavage. The stomach was removed 1 h after the gavage of beads and cut along the greater curvature. The contents were washed out into a Petri dish and the number of beads in the dish was counted. Gastric emptying was calculated using the following formula: Gastric emptying (%)= (40-number of beads in the stomach at 1 h)/40 x100 (26,27).
Each gastric tissue specimen was divided into two pieces: One piece was washed with PBS and fixed with 4% paraformaldehyde at room temperature for 48 h for immunohistochemistry, while the other piece was frozen quickly with liquid nitrogen and placed in a refrigerator at -70˚C until required for protein extraction.
Confirmation of HP infection by immunohistochemistry
Each gastric tissue specimen in 4% paraformaldehyde was encased in a paraffin block and sectioned. The sections (~5 µm) were mounted on slides and baked at 70˚C for 10 min. Xylene solution was used for dewaxing.
The gastric tissue specimens were also examined by hematoxylin and eosin (H&E) staining as follows. The sections were hydrated for 15 min in anhydrous ethanol, 95% ethanol, 80% ethanol and 70% ethanol and then washed with water for 2 min. Hematoxylin staining was performed at room temperature for 3 min, after which the sections were washed with flowing water for 5 min, 1% hydrochloric acid-ethanol for 5 sec, flowing water for 5 min and 0.5% aqueous ammonia for 20 sec. The sections were then stained with 1% eosin at room temperature for 2 min and washed with water for 2 min. After this, they were dehydrated in an ethanol gradient, made transparent in xylene for 20 min, washed with PBS three times and sealed with neutral gum.
The procedures used for the IHC staining of HP were as follows. The sections were dewaxed, hydrated and then washed with PBS for 5 min. After this, they were incubated with 3% hydrogen peroxide solution at room temperature for 15 min to block endogenous peroxidase activity and washed with PBS three times for 5 min each. Sodium citrate was used for antigen retrieval at 100˚C for 20 min, and the sections were allowed to cool naturally. The sections were washed with PBS three times for 3 min each and then incubated in 5% BSA blocking solution (cat. no. SW3015; Beijing Solarbio Science & Technology Co., Ltd.) at room temperature for 20 min. The excess liquid was removed without washing. Anti-HP antibody (dilution 1:500; ab140128; Abcam) was added and the sections were incubated at 4˚C overnight and at room temperature for 45 min. After washing the sections with PBS for 5 min four times each, the sections were incubated with biotin-labeled goat anti-rabbit secondary antibody (dilution 1:200; cat. no. BA1003; Wuhan Boster Biological Technology, Ltd.) at room temperature for 30 min and then washed with PBS for 5 min three times each. The sections were subsequently incubated with streptavidin-biotin-peroxidase complex (cat. no. SA1029; Wuhan Boster Biological Technology, Ltd.) at room temperature for 20 min and washed with PBS for 5 min four times each. They were then incubated with diaminobenzidine detection reagent solution at room temperature for 3 min, washed with distilled water, counterstained with hematoxylin at room temperature for 2 min and washed with flowing water for 5 min. The stained sections were immersed in 1% hydrochloric acid-ethanol for 10 sec, washed with flowing water for 5 min, immersed in 0.5% aqueous ammonia for 20 sec and washed with flowing water for 5 min (all at room temperature). Finally, the sections were dehydrated in an ethanol gradient, made transparent in xylene for 20 min, washed with PBS three times and sealed with neutral gum.
Specimens were observed under a light microscope (magnification, x400). A yellow or brown bacterial colony visible on the surface of the gastric mucosa was considered positive, which indicated the success of the model.
Measurement of c-kit-positive ICCs and c-kit expression in the gastric mucosa
The procedures for c-kit protein IHC staining were the same as those used for HP. The primary antibody used was a rabbit anti-mouse c-kit monoclonal antibody (dilution 1:200; sc-365504; Santa Cruz Biotechnology, Inc.).
Specimens were observed under a light microscope (magnification, x400). The c-kit-positive cells, which were indicated to be ICCs, exhibited blue nuclei and brown cytoplasm. Five high-magnification views (x400) were randomly selected in each section and the number of ICCs was calculated in each field; the sections were collected from different groups in a blinded manner by different examiners (9,28). The number of ICCs was calculated in the intramuscular layer (ICC-IM subtype) and submucosal layer (ICC-SM subtype).
Western blotting of SCF
The protein expression levels of SCF in gastric samples were evaluated by western blotting. Each sample (~50 mg) was harvested and homogenized. The homogenate was added to lysis buffer (cat. no. AR0101; Wuhan Boster Biological Technology, Ltd.) for 30 min at 4˚C and then subjected to centrifugation at 10,000 x g for 10 min at 4˚C. The supernatants were collected and the protein concentrations determined using a BCA protein concentration determination kit. Total protein samples (~40 µg/lane) were separated by SDS-PAGE (12% gel by weight) and transferred to PVDF membranes. Following blocking with 5% skimmed milk on a shaker at room temperature for 1 h, the membranes were incubated with primary antibodies against SCF (dilution 1:200; rabbit polyclonal antibody; ab83866; Abcam) and glyceraldehyde 3 phosphate dehydrogenase (GAPDH; dilution 1:500; rabbit polyclonal antibody; BA2913; Wuhan Boster Biological Technology, Ltd.) at 4˚C overnight. The membranes were then washed with TBS containing 0.05% Tween-20 three times prior to incubation with the HRP-conjugated secondary antibody (dilution 1:5,000; goat anti-rabbit IgG; BA1054; Wuhan Boster Biological Technology, Ltd.) at room temperature for 1 h. Hypersensitive ECL Chemiluminescence Ready-to-Use Substrate (cat. no. AR1170; Wuhan Boster Biological Technology, Ltd.) was used to reveal the bands and the Odyssey Infrared Imaging System (LI-COR Biosciences) was used for chemiluminescence detection. The reference protein GAPDH was used as a loading control. The amount of protein expression relative to that of GAPDH was calculated using ImageJ software version 1.8.0 (National Institutes of Health).
Statistical analysis
SPSS software version 20.0 (IBM Corp.) was used for data analysis. Measurement data are presented as the mean ± SD. For the normally distributed data, differences between two groups were evaluated using a Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Confirmation of HP infection by histological staining and IHC analysis
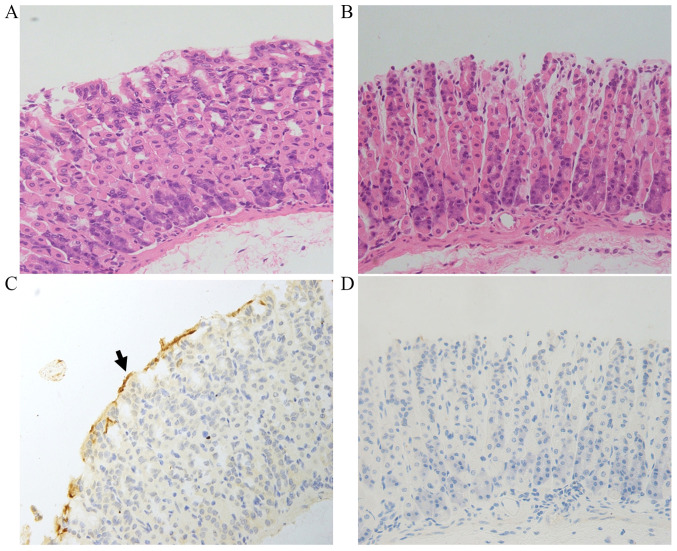
Tissue sections were examined to confirm the HP infection in the HP-treated mice. As shown in Fig. 1, no visible differences between groups were detected by H&E staining. IHC analysis revealed that in the HP+ group, yellow or brown HP colonies were present on the surface of the tissue, while no such HP colonies were detected in the HP- group (Fig. 1).
Figure 1.
HP bacterial colonization in gastric tissue. Hematoxylin and eosin staining of gastric tissue sections from the (A) HP+ and (B) HP- groups. Immunohistochemical staining of HP in gastric tissue sections from the (C) HP+ and (D) HP- groups. The arrow indicates the HP bacterial colony. Magnification, x400. HP, Helicobacter pylori.
Influence of HP infection on gastric emptying
The gastric emptying rate in the HP+ group was 65.8±5.6%, which was significantly lower compared with that in the HP- group (83.8±6.4%) (P<0.001).
Number of ICCs is decreased in the gastric tissues of C57BL/6 mice with HP infection
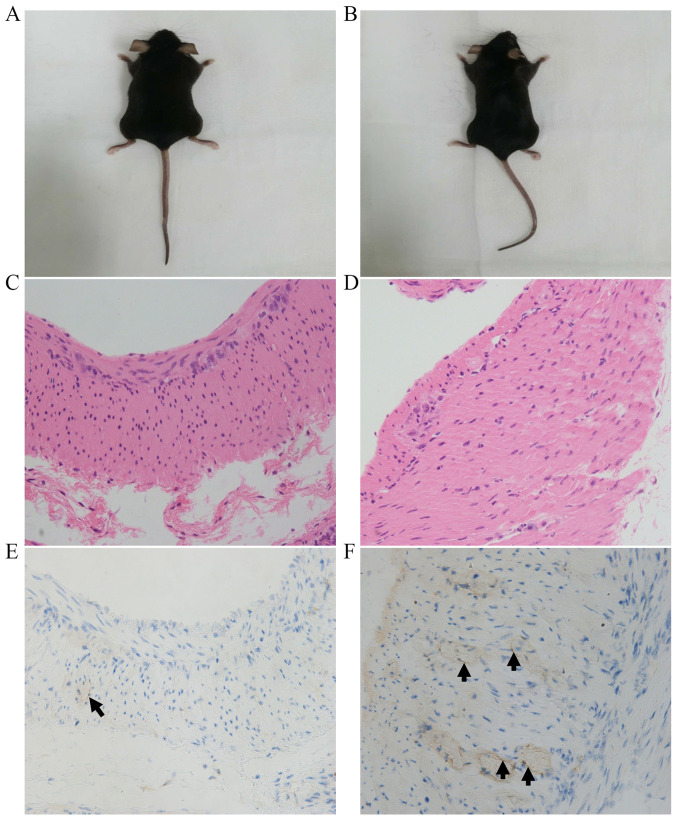
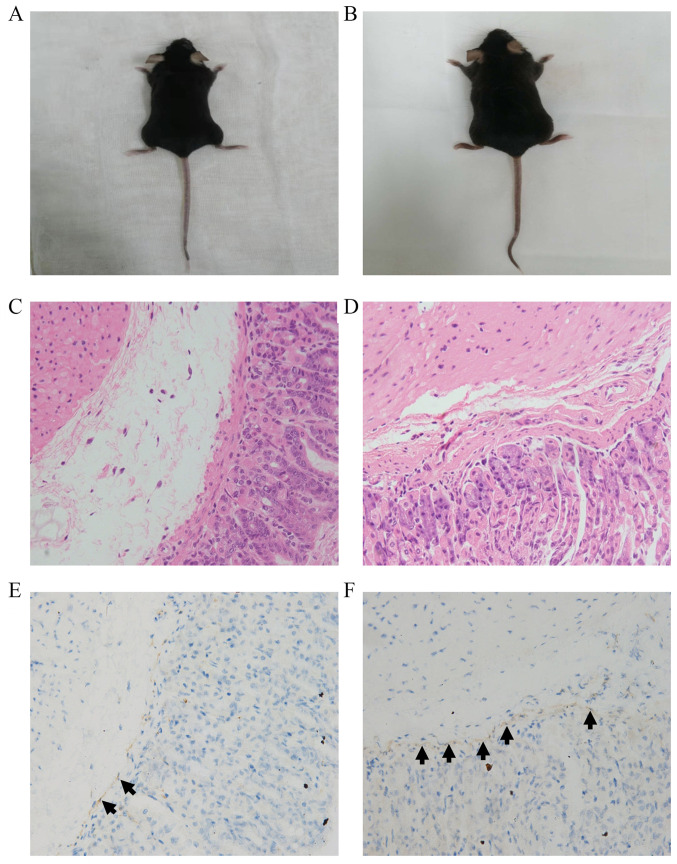
As shown in Figs. 2 and 3, there was no significant difference in the appearance between the two groups of mice, and no visible differences between groups were detected by H&E staining. As shown in Fig. 2 and Table I, the number of ICCs in the intramuscular layer in the HP+ group was significantly lower compared with that in the HP- group (3.32±1.60 vs. 5.52±2.17, respectively; P=0.011). Furthermore, as shown in Fig. 3 and Table II, the number of ICCs in the submucosal layer in the HP+ group was also significantly lower compared with that in the HP- group (6.29±2.46 vs. 14.00±5.18, respectively; P<0.001).
Figure 2.
Interstitial cells of Cajal in the gastric intramuscular layers of mice with and without HP infection. Representative images of mice from the (A) HP+ and (B) HP- groups. Hematoxylin and eosin staining of gastric intramuscular layers from the (C) HP+ and (D) HP- groups. Immunohistochemical staining of c-kit in gastric intramuscular layers from the (E) HP+ and (F) HP- groups. The arrows indicate the interstitial cells of Cajal. Magnification, x400. HP, Helicobacter pylori.
Figure 3.
Interstitial cells of Cajal in the gastric submucosal layers of mice with and without HP infection. Representative images of mice from the (A) HP+ and (B) HP- groups. Hematoxylin and eosin staining of gastric submucosal layers from the (C) HP+ and (D) HP- groups. Immunohistochemical staining of c-kit in gastric submucosal layers from the (E) HP+ and (F) HP- groups. The arrows indicate the interstitial cells of Cajal. Magnification, x400. HP, Helicobacter pylori.
Table I.
ICC count in the gastric intramuscular layer.
| Groups | Number of ICCs | P-value |
|---|---|---|
| HP+ group | 3.32±1.60 | 0.011 |
| HP- group | 5.52±2.17 |
Date are presented as the mean ± SD. The between-group comparison was performed using a Student's t-test. ICCs, interstitial cells of Cajal; HP, Helicobacter pylori.
Table II.
ICC count in the gastric submucosal layer.
| Groups | Number of ICCs | P-value |
|---|---|---|
| HP+ group | 6.29±2.46 | <0.001 |
| HP- group | 14.00±5.18 |
Data are presented as the mean ± SD. The between-group comparison was performed using a Student's t-test. ICCs, interstitial cells of Cajal; HP, Helicobacter pylori.
Protein expression of SCF in the gastric tissues of C57BL/6 mice after HP infection
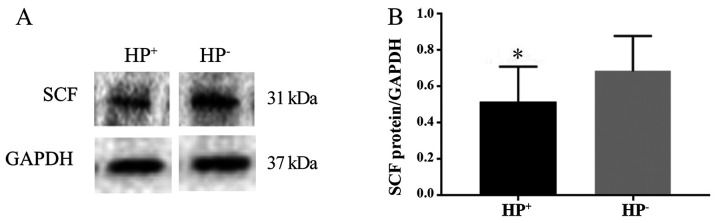
The protein expression level of SCF was determined by western blotting and normalized to that of the internal control GAPDH. The presence of the 31-kDa SCF protein was revealed by western blot analysis (Fig. 4A). The expression level of SCF in the HP+ group was significantly lower compared with that in the HP- group (P<0.05). The SCF/GAPDH ratio was 0.52±0.19 in the HP+ group and 0.69±0.19 in the HP- group (Fig. 4B).
Figure 4.
Expression of SCF in the gastric tissues of mice with and without HP infection. (A) Representative western blots show that the protein expression of SCF was downregulated in the HP+ group compared with the HP- group. GAPDH was used as internal control. (B) Quantification of SCF protein expression. Each bar represents the mean ± SD (n=12). *P<0.05.
Discussion
HP infection is a highly prevalent bacterial infection in humans worldwide (1,2), and is a cause of organic diseases, including gastritis, gastric ulcer and gastric cancer, and also gastrointestinal functional diseases such as functional dyspepsia (3,4).
DGE is defined as the delayed emptying of gastric contents in the absence of mechanical obstruction. The major symptoms of DGE include nausea, vomiting, bloating, early satiety and abdominal pain (29-31). DGE is a common complication following gastrointestinal surgery, particularly pancreatoduodenectomy (32,33). Among patients with DGE who have not undergone surgery, diabetes is the most common cause of the condition (11,34). Patients with DGE suffer from nutritional deficiencies and metabolic consequences as well as impairment of social activities and quality of life (18,35). There have been a number of studies on the relationship between HP infection and DGE, and it has been reported that HP infection can retard the gastric emptying function and induce DGE, but the specific mechanism has rarely been studied (5,6).
The present study was conducted to investigate the mechanism underlying the effect of HP infection on DGE in mice. The SS1 strain of HP was selected because it is the most widely used strain for establishing the mouse model of HP infection. Furthermore, the use of this strain is convenient for comparing the results of the present study with those of other studies using the same strain.
The results of the present study showed that the gastric emptying rate of the mice infected with HP was significantly lower than that of noninfected mice, which is consistent with the results of previous studies (5,6).
Gastric motility involves interactions between smooth muscle, enteric and extrinsic autonomic nerves and ICCs, with the role of ICC considered to be critical for proper gastrointestinal motility; several gastrointestinal motility disorders have been confirmed to be caused by ICC damage (21-23). ICCs are mesenchymal cells that act as pacemakers for the generation of slow waves in the gastrointestinal tract. The electrical activity of ICCs defines the frequency of the rhythmic contraction of smooth muscles in the tract (13-16). ICCs are distributed throughout the gastrointestinal tract from the esophagus to the internal anal sphincter (36). They are involved in motor activities as conduits for muscular innervation and may also provide sensory innervation to the gastrointestinal tract (37). It has been identified that subtypes of ICC exist according to their location in the body and certain morphological and functional criteria; these include ICCs located in muscle bundles and between muscle cells (the ICC-IM subtype) and ICCs located in submucosal layers (the ICC-SM subtype) (38-40). Smooth muscle cells (SMCs), ICC-IMs and platelet-derived growth factor α-positive cells form the SIP syncytium, and ICC-IMs are considered to integrate neuronal signals in the SMC syncytium to ensure functional gastrointestinal motility (41). ICC-SMs are able to generate slow waves and induce gastrointestinal activity (42). ICCs express the proto-oncogene c-kit and its natural ligand SCF, which are associated with the proliferation, differentiation and function of ICCs (17-20,43). Changes in SCF levels have been shown to lead to changes in the numbers of ICCs, which contributes to gastrointestinal dysmotility (12).
In the present study, western blot analysis revealed that the expression levels of SCF were significantly lower in gastric tissue from the HP-infected group in comparison with that from the HP-uninfected group. The numbers of ICC-SMs and ICC-IMs were also significantly reduced in the HP-infected group. From these results, it may be concluded that HP infection caused a reduction in the SCF level, leading to a reduction in the number of ICCs in gastric tissue and resulting in DGE. Specifically, this suggests that HP infection causes DGE by reducing the number of ICCs in gastric tissue. At present, there is no literature describing the mechanism by which HP causes SCF protein expression to be reduced. As protein expression requires transcription and translation processes, any factors that affect the process of transcription and translation could potentially affect protein expression. Therefore, it may be inferred that HP infection changed the environment in the stomach so that it was less suitable for SCF protein expression, which caused the reduction in SCF protein expression.
At present, the rate of antibiotic resistance to HP is worrying and continues to increase each year, leading to difficulties in eradicating the infection (44,45). The rates of resistance of HP to various antibiotics are rising (44). Therefore, it may become increasingly difficult to eradicate HP and thereby resolve associated diseases, including DGE caused by HP. Thus, it is necessary to explore novel methods for treating DGE, such as methods for increasing the number of ICCs in gastric tissue.
Previous studies have shown that electroacupuncture at ST36(25), aqueous extracts of Herba Cistanche (13) and Yangyin Runchang decoction (14) can increase the number of ICCs by increasing the expression of the SCF protein. Therefore, we hypothesize that the reduction in the number of ICCs caused by HP infection may be rescued by the overexpression of SCF. However, this additional animal experiment requires ethical approval and fund applications to acquire financing. Therefore, this experiment was not performed as part of the present study, which is a limitation of the study; however, it will be conducted in the future.
Although the effect of HP infection on solid gastric emptying in mice was studied in the present study, its effect of liquid gastric emptying was not investigated, which is another limitation of the study. The gastric emptying of liquid is physiologically different from that of a solid meal; therefore, the effect of HP infection on liquid gastric emptying requires investigation in another study.
In summary, the present study investigated whether HP causes DGE by reducing the number of ICCs. However, whether the increased expression of ICCs can be targeted for the treatment of DGE remains to be explored, and other mechanisms of HP that affect gastric emptying require further study.
Acknowledgements
The authors would like to thank Professor Wenjuan Li at Shandong University for her gift of HP SS1.
Funding Statement
Funding: Funding was received from the National Natural Science Fund of China (grant nos. 81272375 and 81872400) and from the Shandong Provincial Health and Family Planning Commission (grant no. 2018WS218).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BL analyzed the data and drafted the manuscript. JD established the model and collected samples from the mice. SW and HY performed IHC staining. ZL and PS performed western blotting. LZ designed the study and revised the paper. BL and LZ confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experiments and procedures in the present study were approved by the Ethics Committee of Shandong Cancer Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Polyzos SA, Zeglinas C, Artemaki F, Doulberis M, Kazakos E, Katsinelos P, Kountouras J. Helicobacter pylori infection and esophageal adenocarcinoma: A review and a personal view. Ann Gastroenterol. 2018;31:8–13. doi: 10.20524/aog.2017.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolak W, Bilgilier C, Stadlmann A, Leiner J, Püspök A, Plieschnegger W, Siebert F, Wewalka F, Schöfl R, Huber-Schönauer U, et al. A multicenter prospective study on the diagnostic performance of a new liquid rapid urease test for the diagnosis of Helicobacter pylori infection. Gut Pathog. 2017;9(78) doi: 10.1186/s13099-017-0226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gressot P, Frossard JL, Grosgurin O, Marti C. First line eradication treatment of Helicobacter pylori in 2019. Rev Med Suisse. 2019;15:1854–1858. doi: 10.15403/jgld.2014.1121.281.hpy. (In French) [DOI] [PubMed] [Google Scholar]
- 4.Saito Y, Suzuki H, Tsugawa H, Suzuki S, Matsuzaki J, Hirata K, Hibi T. Dysfunctional gastric emptying with down-regulation of muscle-specific microRNAs in Helicobacter pylori-infected mice. Gastroenterology. 2011;140:189–198. doi: 10.1053/j.gastro.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Huang J. Analysis of the relationship between Helicobacter pylori infection and diabetic gastroparesis. Chin Med J (Engl) 2017;130:2680–2685. doi: 10.4103/0366-6999.218012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang CL, Geng CH, Yang ZW, Li YL, Tong LQ, Gao P, Gao YQ. Changes in patients' symptoms and gastric emptying after Helicobacter pylori treatment. World J Gastroenterol. 2016;22:4585–4593. doi: 10.3748/wjg.v22.i18.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verdu EF, Bercik P, Huang XX, Lu J, Al-Mutawaly N, Sakai H, Tompkins TA, Croitoru K, Tsuchida E, Perdue M, Collins SM. The role of luminal factors in the recovery of gastric function and behavioral changes after chronic Helicobacter pylori infection. Am J Physiol Gastrointest Liver Physiol. 2008;295:G664–G670. doi: 10.1152/ajpgi.90316.2008. [DOI] [PubMed] [Google Scholar]
- 8.Leontiadis GI, Minopoulos GI, Maltezos E, Kotsiou S, Manolas KI, Simopoulos K, Hatseras D. Effects of Helicobacter pylori infection on gastric emptying rate in patients with non-ulcer dyspepsia. World J Gastroenterol. 2004;10:1750–1754. doi: 10.3748/wjg.v10.i12.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park KS, Cho KB, Hwang IS, Park JH, Jang BI, Kim KO, Jeon SW, Kim ES, Park CS, Kwon JG. Characterization of smooth muscle, enteric nerve, interstitial cells of Cajal, and fibroblast-like cells in the gastric musculature of patients with diabetes mellitus. World J Gastroenterol. 2016;22:10131–10139. doi: 10.3748/wjg.v22.i46.10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong L, Ao JP, Lu HL, Huang X, Zang JY, Liu SH, Song NN, Huang SQ, Lu C, Chen J, Xu WX. Tyrosine kinase Pyk2 is involved in colonic smooth muscle contraction via the RhoA/ROCK pathway. Physiol Res. 2019;68:89–98. doi: 10.33549/physiolres.933857. [DOI] [PubMed] [Google Scholar]
- 11.Neshatian L, Gibbons SJ, Farrugia G. Macrophages in diabetic gastroparesis-the missing link? Neurogastroenterol Motil. 2015;27:7–18. doi: 10.1111/nmo.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng J, Gao J, Zhou S, Liu Y, Zhong Y, Shu Y, Meng MS, Yan J, Sun D, Fang Q, Sun D. Role of stem cell factor in the regulation of ICC proliferation and detrusor contraction in rats with an underactive bladder. Mol Med Rep. 2017;16:1516–1522. doi: 10.3892/mmr.2017.6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan S, Yue YZ, Wang XP, Dong HL, Zhen SG, Wu BS, Qian HH. Aqueous extracts of Herba Cistanche promoted intestinal motility in loperamide-induced constipation rats by ameliorating the interstitial cells of cajal. Evid Based Complement Alternat Med. 2017;2017(6236904) doi: 10.1155/2017/6236904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang F, Zhou JY, Wu J, Tian F, Zhu XX, Zhu CL, Yang BL, Chen YG. Yangyin Runchang Decoction improves intestinal motility in mice with atropine/diphenoxylate-induced slow-transit constipation. Evid Based Complement Alternat Med. 2017;2017(4249016) doi: 10.1155/2017/4249016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang SJ, Pardo DM, Zheng H, Bayguinov Y, Blair PJ, Fortune-Grant R, Cook RS, Hennig GW, Shonnard MC, Grainger N, et al. Differential sensitivity of gastric and small intestinal muscles to inducible knockdown of anoctamin 1 and the effects on gastrointestinal motility. J Physiol. 2019;597:2337–2360. doi: 10.1113/JP277335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blair PJ, Hwang SJ, Shonnard MC, Peri LE, Bayguinov Y, Sanders KM, Ward SM. The role of prostaglandins in disrupted gastric motor activity associated with type 2 diabetes. Diabetes. 2019;68:637–647. doi: 10.2337/db18-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S, Wu B, Sun H, Sun T, Han K, Li D, Ji F, Zhang G, Zhou D. Impaired insulin/IGF-1 is responsible for diabetic gastroparesis by damaging myenteric cholinergic neurones and interstitial cells of Cajal. Biosci Rep. 2017;37(BSR20170776) doi: 10.1042/BSR20170776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, An J, Liu S. Electroacupuncture at ST36 increases bone Marrow-Derived interstitial cells of Cajal via the SDF-1/CXCR4 and mSCF/Kit-ETV1 pathways in the stomach of diabetic mice. Evid Based Complement Alternat Med. 2018;2018(7878053) doi: 10.1155/2018/7878053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muangchan N, Kooptiwut S, Tapechum S, Akarasereenont P, Vongsopanagul N, Pongwattanapakin K, Chaikomin R. 13C-Acetic Acid Breath test monitoring of gastric emptying during disease progression in diabetic rats. Biol Pharm Bull. 2017;40:1506–1514. doi: 10.1248/bpb.b17-00320. [DOI] [PubMed] [Google Scholar]
- 20.Jin QH, Shen HX, Wang H, Shou QY, Liu Q. Curcumin improves expression of SCF/c-kit through attenuating oxidative stress and NF-κB activation in gastric tissues of diabetic gastroparesis rats. Diabetol Metab Syndr. 2013;5(12) doi: 10.1186/1758-5996-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, O'Connor MD, Ho V. The potential for Gut Organoid derived interstitial cells of Cajal in replacement therapy. Int J Mol Sci. 2017;18(2059) doi: 10.3390/ijms18102059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan YY, Ji ZL, Zhao G, Jiang JR, Wang D, Wang JM. Decreased SCF/c-kit signaling pathway contributes to loss of interstitial cells of Cajal in gallstone disease. Int J Clin Exp Med. 2014;7:4099–4106. eCollection 2014. [PMC free article] [PubMed] [Google Scholar]
- 23.Bekkelund M, Sangnes DA, Gunnar Hatlebakk J, Aabakken L. Pathophysiology of idiopathic gastroparesis and implications for therapy. Scand J Gastroenterol. 2019;54:8–17. doi: 10.1080/00365521.2018.1558280. [DOI] [PubMed] [Google Scholar]
- 24.Yadak R, Breur M, Bugiani M. Gastrointestinal Dysmotility in MNGIE: From thymidine phosphorylase enzyme deficiency to altered interstitial cells of Cajal. Orphanet J Rare Dis. 2019;14(33) doi: 10.1186/s13023-019-1016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian L, Zhu B, Liu S. Electroacupuncture at ST36 Protects ICC Networks via mSCF/Kit-ETV1 Signaling in the stomach of diabetic mice. Evid Based Complement Alternat Med. 2017;2017(3980870) doi: 10.1155/2017/3980870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ando K, Takagi K. Solid gastric emptying mediated by the serotonin (5-HT)3 receptor in mice is a simple marker to predict emesis. J Toxicol Sci. 2011;36:23–29. doi: 10.2131/jts.36.23. [DOI] [PubMed] [Google Scholar]
- 27.Ando K, Takagi K, Tsubone H. Enhanced gastric retention of solid resin beads as a marker for emetic potential of agents in rats. J Toxicol Sci. 2012;37:549–553. doi: 10.2131/jts.37.549. [DOI] [PubMed] [Google Scholar]
- 28.Bhatia Y, Singh S, Rattan KN, Parmar P, Sahni D, Sen R. Anorectal malformations: Histomorphological and immunohistochemical evaluation of neuronal dysfunction. J Neonatal Surg. 2017;6(29) doi: 10.21699/jns.v6i2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim BJ, Kuo B. Gastroparesis and functional dyspepsia: A blurring distinction of pathophysiology and treatment. J Neurogastroenterol Motil. 2019;25:27–35. doi: 10.5056/jnm18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanger GJ, Pasricha PJ. Investigational drug therapies for the treatment of gastroparesis. Expert Opin Investig Drugs. 2017;26:331–342. doi: 10.1080/13543784.2017.1288214. [DOI] [PubMed] [Google Scholar]
- 31.Choi KM, Gibbons SJ, Sha L, Beyder A, Verhulst PJ, Cipriani G, Phillips JE, Bauer AJ, Ordog T, Camp JJ, et al. Interleukin 10 restores gastric emptying, electrical activity, and interstitial cells of Cajal networks in diabetic mice. Cell Mol Gastroenterol Hepatol. 2016;2:454–467. doi: 10.1016/j.jcmgh.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan AS, Williams G, Woolsey C, Liu J, Fields RC, Doyle MMB, Hawkins WG, Strasberg SM. Flange gastroenterostomy results in reduction in delayed gastric emptying after standard pancreaticoduodenectomy: A prospective cohort study. J Am Coll Surg. 2017;225:498–507. doi: 10.1016/j.jamcollsurg.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn JY, Jung HY, Bae SE, Jung JH, Choi JY, Kim MY, Lee JH, Choi KS, Kim DH, Choi KD, et al. Proper preparation to reduce endoscopic reexamination due to food residue after distal gastrectomy for gastric cancer. Surg Endosc. 2013;27:910–917. doi: 10.1007/s00464-012-2532-9. [DOI] [PubMed] [Google Scholar]
- 34.Othman MO, Davis B, Saroseik I, Torabi A, McCallum RW. EUS-guided FNA biopsy of the muscularis propria of the antrum in patients with gastroparesis is feasible and safe. Gastrointest Endosc. 2016;83:327–333. doi: 10.1016/j.gie.2015.06.056. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi Y, Toyomasu Y, Saravanaperumal SA, Bardsley MR, Smestad JA, Lorincz A, Eisenman ST, Cipriani G, Nelson Holte MH, Al Khazal FJ, et al. Hyperglycemia increases interstitial cells of Cajal via MAPK1 and MAPK3 Signaling to ETV1 and KIT, leading to rapid gastric emptying. Gastroenterology. 2017;153:521–535.e20. doi: 10.1053/j.gastro.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J, Du L, Xiao YT, Cai W. Disruption of interstitial cells of Cajal networks after massive small bowel resection. World J Gastroenterol. 2013;19:3415–3422. doi: 10.3748/wjg.v19.i22.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu F, Xu S, Zhang Y, Chen F, Ji J, Xie G. Total Glucosides of Paeony promote intestinal motility in slow transit constipation rats through amelioration of interstitial cells of Cajal. PLoS One. 2016;11(e0160398) doi: 10.1371/journal.pone.0160398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian L, Song S, Zhu B, Liu S. Electroacupuncture at ST-36 protects interstitial cells of Cajal via Sustaining Heme Oxygenase-1 Positive M2 Macrophages in the stomach of diabetic mice. Oxid Med Cell Longev. 2018;2018(3987134) doi: 10.1155/2018/3987134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bashashati M, McCallum RW. Is interstitial cells of Cajal-opathy present in gastroparesis? J Neurogastroenterol Motil. 2015;21:486–493. doi: 10.5056/jnm15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Xu JJ, Liu S, Hou XH. Electroacupuncture at ST36 ameliorates gastric emptying and rescues networks of interstitial cells of Cajal in the stomach of diabetic rats. PLoS One. 2013;8(e83904) doi: 10.1371/journal.pone.0083904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu C, Lu H, Huang X, Liu S, Zang J, Li Y, Chen J, Xu W. Colonic transit disorder mediated by downregulation of interstitial cells of Cajal/Anoctamin-1 in dextran sodium sulfate-induced colitis mice. J Neurogastroenterol Motil. 2019;25:316–331. doi: 10.5056/jnm18173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Liang Y, Chen Q, Ahmed N, Wang F, Hu B, Yang P. Identification and distribution of the interstitial cells of Cajal in the abomasum of goats. Cell Transplant. 2018;27:335–344. doi: 10.1177/0963689717722561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon YH, Kim N, Nam RH, Park JH, Lee SM, Kim SK, Lee HS, Kim YS, Lee DH. Change in the interstitial cells of Cajal and nNOS positive neuronal cells with aging in the stomach of F344 Rats. PLoS One. 2017;12(e0169113) doi: 10.1371/journal.pone.0169113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.An B, Moon BS, Kim H, Lim HC, Lee YC, Lee G, Kim SH, Park M, Kim JB. Antibiotic resistance in Helicobacter pylori strains and its effect on H. pylori eradication rates in a single center in Korea. Ann Lab Med. 2013;33:415–419. doi: 10.3343/alm.2013.33.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diaconu S, Predescu A, Moldoveanu A, Pop CS, Fierbinteanu-Braticevici C. Helicobacter pylori infection: Old and new. J Med Life. 2017;10:112–117. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.