Abstract
We reviewed surgical cases from 4 Thoracic Surgery departments in the Lombardia region of Italy, the area mostly affected by Coronavirus pandemic in Europe, with the aim to describe the impact of COVID-19 on the treatment of thoracic surgical patients. Clinical, radiological and laboratory data from patients who underwent lung resection from December 2019 to March 2020 were retrospectively collected until June 2020. Univariable Cox regression models were estimated to evaluate potential prognostic factors for developing COVID-19 and to investigate postoperative mortality among patients who developed symptomatic COVID-19 infection. We examined data from 107 patients. (74 lobectomies, 32 wedge/segmentectomies and 1 pneumonectomy). Twelve patients developed COVID-19 (Group 1), whereas 95 patients were not infected (Group 2). In Group 1, 6 patients (50%) died from complications related to infection; in Group 2, one patient (1%) died because of non-COVID-19-related causes. Median days from surgery to first symptoms, CT confirmation, clinical confirmation and PCR positivity was 48.1, 54.3, 55.1, and 55.2 respectively. At univariable analysis, DLCO/VA% (P = 0.008), duration of the surgery (P = 0.009), smoking history (pack/year) (P < 0.001), BMI (P< 0.001) and number of segments resected (P = 0.010) were associated with COVID-19 onset. Moreover, CCI (P < 0.001), DLCO/VA% (P = 0.002), cigarette pack/year (P < 0.001), BMI (P < 0.001) and COVID-19 (P < 0.001) were associated with death. Patients who undergo lung resection and then develop symptomatic COVID-19 infection are at higher risk of developing severe respiratory complications and postoperative death. Insidious symptoms’ onset may lead to a delay in diagnosis. We suggest two mitigating strategies: (1) Improve symptoms surveillance and isolation during recovery period, (2) Be aware of a potential greater risk of developing symptomatic COVID-19 and death correlated with elevated CCI, BMI, smoking history, DLCO/VA%, number of resected segments and duration of surgery.
KEYWORDS: COVID-19, Lung resection, Thoracic surgery, Northern Italy
Abbreviations: COVID-19, COronaVIrus Disease 19; DLCO/VA, Diffusing capacity divided by the alveolar volume; BMI, Body Mass Index; CCI, Charlson Comorbidity Index; rt-PCR, Real-time polymerase chain reaction; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; ALI, Acute Lung Injury; GGO, Ground Glass Opacity
Graphical Abstract

Coronavirus corpses loaded in army trucks since cemeteries are full (Bergamo 19/03/2020).
Alt-text: Unlabelled box
Central Message.
Patients who develop symptomatic COVID-19 infection during the 90 days after lung resection have a high risk of death. Postoperative surveillance and risk factors monitoring are mandatory.
Alt-text: Unlabelled box
Perspective Statement.
Our findings, that come from the area that was most heavily impacted by the Coronavirus pandemic in Europe, reveal that patients who undergo a lung resection and then developed symptomatic COVID-19 have very high risk of perioperative death. According to our results, we propose some mitigating perioperative strategies to improve outcomes after surgery during the pandemic.
Alt-text: Unlabelled box
INTRODUCTION
The first cases of Coronavirus disease 2019 (COVID-19) were registered in Wuhan, capital city of the Hubei province, China, in December 2019.1 Since then the infection has spread globally and was declared a Public Health Emergency of International Concern on 30 January 2020 by World Health Organization.2
It is with the first case of secondary transmission which occurred in Codogno, Municipality of Lombardy in the province of Lodi, on February 18, 2020 that the epidemic spread throughout Italy. During the initial period, as well as the peak of the pandemic, the North of the Country, and, in particular the Lombardy region, has been the hardest affected area with 86,384 infected and 15,784 deaths (data updated on May 21 2020).3 In particular, the most affected provinces were Brescia, Bergamo, Milano and Monza Brianza (with 14,345, 12,733, 22,534 and 5,412 cases),3 whereas overall positive and fatal cases in Italy were 228,658 and 32,616, on the 21th May 2020.4 This data also made the Lombardy region the most distressed area in Europe for highest number of deaths per 100,000 inhabitants on May 15.5 , 6
In comparison with the general population, patients with cancer in the active phase of the disease have a higher risk of death in the weeks after infection and poorer outcomes than non-cancer patients, as shown in some preliminary studies.7 Therefore, many thoracic oncology patients, considering their multiple co-morbidities (advanced age, smoking history, emphysema, heart disease), are classified in the “high risk” group.
Some of the most important institutions such as the Royal College of Surgeons, The Royal College of Physicians and Surgeons of Canada and the American College of Surgeons, quickly provided expert level surgical recommendations for patients with thoracic malignancies;8 , 9 however, there is still a lack of studies investigating outcomes and risk factors among patients who underwent thoracic surgery and developed COVID-19.
We reviewed data of patients who underwent lung resection in some of the most affected areas in Lombardy, with the aim to determine which variables are clinical or surgical prognostic factors for developing COVID-19 and for mortality, during postoperative recovery period of 90 days, among patients who developed symptomatic COVID-19.
METHODS
We retrospectively reviewed and prospectively collected clinical data of all patients who underwent lung resection from December 2019 through March 2020 at four different high-volume Thoracic Surgery Departments from Northern Italy (Humanitas Gavazzeni Bergamo, San Gerardo Monza, Spedali Civili Brescia, ASST Santi Paolo e Carlo Milano) irrespective of whether they contracted the disease.
The timeline of cases has been set with the aim to investigate COVID-19 impact on postoperative and recovery time (conventionally equal to about 90 days) outcomes; therefore, we collected cases from December 1, 2019.
Consent to data collection and use was included as part of the hospital surgical consent form that comprises also infective complication (Mod.3864 ASST-MA-008 rev. 0 15.05.17). Hospital approval was granted and IRB waived the need to review this case series. Patients operated on in December and January did not receive any preoperative COVID-19 investigation. Swab real-time polymerase chain reaction (rt-PCR) became readily available in February and patients were, thereafter, universally tested before surgery. In case of positive swab, surgery was postponed until complete recovery and two consecutive negative nasal swabs.
Clinical variables included age, sex, length of stay in days, comorbidities (Charlson Comorbidity Index (CCI), smoking history, oncological history, BMI, arterial hypertension) and pulmonary function (FEV1%, FVC%, DLCO/VA%, V02 max). The surgical variables considered were type of resection, number of resected segments, duration of surgery and surgical approach (multiportal VATS, uniportal VATS or open thoracotomy). This data was obtained by review of medical records. Follow-up data was recorded until the beginning of June by reviewing outpatient clinic records, telephone follow-up calls or hospital records in case of readmission. Patients who developed the disease after discharge were identified by phone-administered survey, due to the inability to visit hospitals because of the quarantine, or through access to the emergency room. We focused our attention on the initial signs and symptoms of COVID-19 such as fever (defined as body temperature > 37.3°C with remittent or intermittent pattern), shortness of breath, chest tightness, fatigue, dry cough, loss of appetite, nausea, headache, diarrhea, rhinorrhea, dizziness and newly developed pleural effusion.
Postoperative CT scan findings have been recorded to support diagnoses of COVID-19 both in case of positive rt-PCR and in the absence of a swab test. Patients who developed the disease were classified as Group 1, while the others as Group 2. Patients in Group 1 were then further classified in 3 subgroups based on the clinical pattern of disease extrapolated from the guidelines (version 7.0) of China's National Health Commission:10 (1) critical, in case of respiratory failure requiring mechanical ventilation, (2) severe, in case of respiratory rate > 30/minutes, PaO2/Fi02 ratio < 300 mm Hg or Sp02 < 93%, lung involvement > 50%, (3) mild, in all remaining cases.
We have also investigated how many days elapsed since the onset of COVID-19 signs and definitive diagnosis by reviewing medical records and by obtaining patients or relatives’ statements, in order to determine a timeline in disease management.
Statistics
Our dataset consisted of 30 clinical variables from 107 patients who underwent lung resection. Associations between categorical variables were assessed by the Fisher's exact test; for continuous variables, differences between means from two separate groups of subjects were evaluated using the nonparametric Wilcoxon test. To evaluate the impact of patient's characteristics on the risk of developing COVID-19 and on mortality, we estimate a univariable Cox proportional hazards regression model. Statistical significance of the regression coefficient was assumed if the p-value was less than 0.05. All analysis was computed by the R software (version 3.6.3)
RESULTS
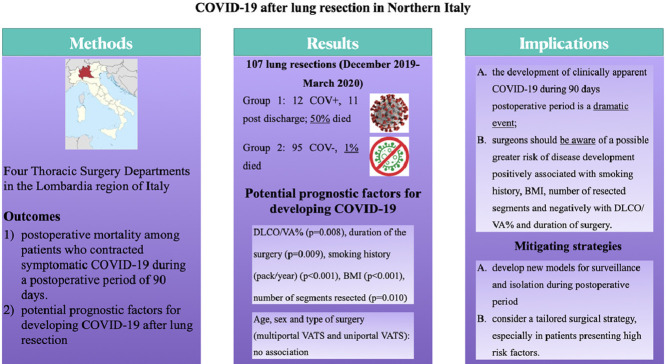
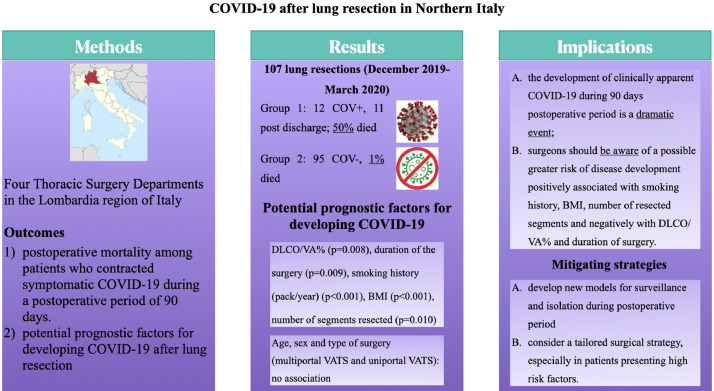
From December 2019 to March 2020, 107 patients underwent lung resection in four thoracic surgery centers in Northern Italy. Twelve developed COVID-19 (Group 1), whereas 95 patients were not infected (Group 2). In Group 1, 6 patients (50%) died from respiratory failure or others complication related to infection, whereas in Group 2, one patient (1%) died because of non-COVID-19-related cause.
No signs and symptoms of Coronavirus-19 infection were retrospectively recorded before surgery. Preoperative swab real-time polymerase chain reaction (rt-PCR), when introduced at the end of February 2020, was always negative, and surgery proceeded as scheduled.
Overall median age of patients was 66.5 years (17-84 years), 70 were male and 37 female. All patients but 16 were current or past smokers. Median BMI was 27.24. Median CCI was 10.30. The majority of patients underwent surgery for oncologic disease (primary or metastatic disease) except 7 who presented persistent air leaks after spontaneous pneumothorax or complicated infectious disease. In these patients, 74 lobectomies, 32 segmentectomies and/or wedge resections and 1 pneumonectomy were successfully performed without intra-operative adverse event. There were 45 open procedures, whereas all other resections were performed by VATS. Among discharged patients, no one was discharged to rehab.
In Group 1, median age was 65.9 years (21-82 years), 9 were male and 3 female. All patients but 3 were current or past heavy smokers. Median BMI was 34.9. Median CCI was 16.8 . All patients underwent surgery for cancer but 1 who presented with relapsing spontaneous pneumothorax. Surgeries were successfully performed in all cases without intra-operative event, including 5 lobectomies and 7 segmentectomies/wedge resections There were 3 open thoracotomy lobectomies, whereas all other resections were performed by VATS.
In Group 2, median age was 66.5 years (17-84 years), 61 were male and 34 female. All patients but 13 were current or past heavy smokers. Median BMI was 25.27. All patients underwent surgery for oncologic disease (primary or metastatic disease) but 4 who presented with complicated infectious disease (aspergilloma and tuberculosis) and 2 with relapsing persisting spontaneous pneumothorax. Surgeries were successfully performed in all cases without event, including 1 pneumonectomy, 69 lobectomies, and 25 segmentectomies and/or wedge resections. There were 42 open thoracotomies, whereas all other resections were performed by VATS.
Contact tracing allowed identification of viral exposure in only 2 out of 12 patients in group I. Therefore, we decided to base our time-line on postoperative days count. Furthermore, this choice seemed appropriate to visualize the relationship between date of surgery, onset of the disease and time spent for diagnosis. Median days from surgery to first symptoms, CT confirmation, clinical confirmation and PCR positivity was 48.1, 54.3, 55.1 and 55.2 respectively.
Eleven of 12 patients in Group 1 presented first signs and symptoms of infection after discharge. Diagnostic suspicion occurred secondary to telephone follow-up or spontaneous presentation to the emergency room. At hospital readmission, viral pneumonia was confirmed in all cases. Only 1 patient developed the disease at the 9th postoperative day during hospital stay.
Six cases presented with critical COVID-19 syndrome whereas 2 and 4 had a severe and mild form respectively. Seven and two patients presented intermittent and remittent fever. No patient had fever immediately after surgery; six patients had body temperature ranging between 38 and 39°C; the others inferior to 38.
Symptoms at onset included dyspnea,7 chest tightness,4 fatigue,6 dry cough,6 loss of appetite,4 headache,1 dizziness,1 and pleural effusion.3
Preoperative chest CT scan images have been re-analyzed and no signs of viral pneumonia were found. Every patient had a new CT scan after postoperative onset of one or more symptoms of COVID-19. In 8 cases chest tomography immediately detected radiological signs of viral pneumonia such as multifocal GGOs, whereas 4 patients presented these typical signs at second scan. At last follow-up, one patient was cured and discharged in good general conditions in Group 1; 5 were still hospitalized in stable condition and 6 patients received mechanical ventilation and eventually died from respiratory failure or others complication related to infection. One fatal case, not attributable to COVID-19, was recorded in Group 2.
At univariable analysis, DLCO/VA% (P = 0.008) and duration of the surgery (P = 0.009) were negatively associated with postoperative COVID-19 onset. Smoking history (pack/year) (P = <0.001), BMI (P< 0.001) and number of segments resected (P = 0.010) were positively associated with COVID-19. Age, sex and type of surgery (multiportal VATS and uniportal VATS) were not significant (Table 1 ).
Table 1.
Mean demographics and preoperative characteristics for patients who developed COVID-19 (Group 1) and patients that were not infected (Group 2)
| Characteristics | COVID-19 positive (n = 12) | COVID-19 negative (n = 95) | HR | 95% CI | p-value |
|---|---|---|---|---|---|
| Age, years | 65.92 | 66.54 | 0.99 | 0.94 - 1.04 | 0.639 |
| Cigarettes smoked, pack/year | 45.75 | 18.42 | 1.03 | 1.01 - 1.05 | <0.001* |
| BMI | 34.73 | 26.00 | 1.09 | 1.05 - 1.14 | <0.001* |
| Resected lung segments | 3.25 | 2.08 | 1.56 | 1.36 - 1.87 | 0.010* |
| Charlson Comorbidity Index | 16.09 | 11.43 | 1.08 | 1.01 - 1.15 | 0.033* |
| FEV1% | 91.00 | 95.12 | 0.99 | 0.97 - 1.02 | 0.464 |
| DLCO/VA% | 77.00 | 90.88 | 0.95 | 0.92 - 0.99 | 0.008* |
| Duration of surgery | 111.25 | 150.24 | 0.98 | 0.97 - 1.01 | 0.009* |
| Sex | |||||
| Female | 3 | 34 | |||
| Male | 9 | 61 | 1.85 | 0.51 - 2.85 | 0.356 |
Hazard ratios (HR) and 95% Confidence intervals (CI) are obtained from a univariable Cox regression model to assess the impact of patient's characteristics on the risk of being infected by COVID-19.
Significant p-value.
The same univariable Cox regression model has been adopted to investigate prognostic factors for death. At univariable analysis CCI (p < 0.001), cigarette pack/year (P < 0.001), BMI (P < 0.001) and COVID-19 (P < 0.001) were positively associated with death, whereas DLCO/VA% (P = 0.002) was negatively associated (Table 2 ).
Table 2.
Mean demographics and preoperative characteristics for dead and alive patients
| Characteristics | Dead (n = 7) | Alive (n = 100) | HR | 95% CI | p-value |
|---|---|---|---|---|---|
| Age, years | 70.43 | 66.19 | 1.04 | 0.95 - 1.14 | 0.394 |
| Cigarettes smoked, pack/year | 62.00 | 18.65 | 1.05 | 1.02 - 1.07 | <0.001* |
| BMI | 41.13 | 25.99 | 1.15 | 1.08 - 1.23 | <0.001* |
| Resected lung segments | 3.14 | 2.86 | 1.39 | 0.87 - 1.54 | 0.566 |
| Charlson Comorbidity Index | 24.13 | 11.10 | 1.21 | 1.11 - 1.33 | <0.001* |
| FEV1% | 90.29 | 94.96 | 0.99 | 0.96 - 1.02 | 0.516 |
| DLCO/VA% | 71.29 | 90.57 | 0.93 | 0.88 - 0.97 | 0.002* |
| Duration of surgery | 129.29 | 147.03 | 0.99 | 0.98 - 1.01 | 0.405 |
| COVID-19 | 6 | 6 | 4.49 | 2.71 - 5.82 | <0.001* |
Hazard ratios (HR) and 95% Confidence intervals (CI) are obtained from a univariable Cox regression model to assess the impact of patient's characteristics on the mortality rate.
Significant p-value.
DISCUSSION
Few articles have focused on the clinical course of patients with severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection during perioperative period,11, 12, 13 and even fewer studies specifically focus on patients who have underwent thoracic surgery.
In that arena a very interesting article has been published by Peng and coworkers14 from China. They reported the postoperative clinical events involving a series of 11 patients without control-cohort.
Of 11 patients, they reported 3 fatal cases after pulmonary or esophageal resection and percentages of severe and critical respiratory pattern of 27.3% and 36.4% respectively, versus 6.1% and 13.8% among overall COVID-19 population in China.15 , 16 Their results seem to correlate COVID-19 and outcomes in thoracic surgery patients.
Cai and Coworkers, in their series of 7 cases after thoracic surgery, also concluded that lung resection surgery might be a risk factor for death in patients with COVID-19 in the perioperative period.17
Our data shows a dramatic pattern as well, since 16% and 50% of COVID-19 patients developed severe and critical respiratory pattern respectively and we registered 50% fatal cases among symptomatic COVID-19 patients.
Secondly, their results highlighted a substantial delay from symptom onset to diagnosis, probably due to a confusing overlap between COVID-19 and thoracic surgery postoperative signs and symptoms. We instead observed a smaller delay (about 7 days) that could be explained because all but 1 patient developed symptoms during recovery when postoperative symptoms should have disappeared or decreased. Indeed, most of patients were readmitted to hospital as soon as symptoms occurred. Their detection was probably facilitated by careful phone follow-up but also by the state of national alert promoted by the media. That suggests the need for a tighter and more rigorous early follow-up during the pandemic period, supported by adequate information on signs and symptoms that patients or relatives must be able to check daily.
Our data also showed that smoking history, BMI, number of resected segments, DLCO/VA%, and duration of surgery are potential prognostic factors for onset of COVID-19.
As concerns smoking history, we already know that smoking impairs lung immune functions and damages upper airways, increasing risks of contracting a severity of infectious diseases. Therefore, it is not surprising that smoking may be an independent risk for severe COVID-19 progression, including mortality.18
BMI prognostic value is consistent with recent papers showing that adiposity is strongly linked to COVID-19.19 In particular it has been showed that obesity may be a stronger risk factor for susceptibility to SARS-CoV-2 infection and severity of consequent disease, including death.20 Indeed, excess fat can impair lung and metabolic function, stresses inflammatory pathways and cardiorenal systems, and promotes thrombotic responses.
Although our is a small case series, we suggest that, (1) the development of clinically apparent COVID-19 during 90 days postoperative period is a dramatic event, (2) surgeons should be aware of a possible greater risk of disease onset and critical pattern development positively associated with smoking history, BMI, number of resected segments and negatively with DLCO/VA% and duration of surgery.
A very interesting point to address is that the most of patients who got clinically apparent disease presented with symptoms after almost one month from surgery. Even considering an incubation period of 2 weeks,21 , 22 we are unable to demonstrate whether the infection occurred during the hospital stay or at home, but what matters is that also the period outside the strict perioperative time seems to be dangerous.
Therefore, we suggest a rigorous postdischarge follow-up should be established, covering a recovery phase of almost 3 months, to early identify any possible signs and symptoms, as long as the pandemic lasts. Regulated Outpatient Clinics, phone interview or survey administered by digital application should be encouraged. Moreover, patients should also observe equally rigorous isolation standards, with particular attention to cases who present prognostic factors for COVID-19 development.
Taipe and Coworkers, in their case report, also stressed this point suggesting that early diagnosis and use of antibiotics at doses of sepsis, associated with corticosteroid pulses and respiratory physiotherapy improve COVID-19 pneumonia in postoperative lung surgery.23
Stoleriu and Huang, in their series of 2 and 3 cases respectively, found that mortality may be very high in patients who contract SARS-CoV-2 pneumonia after lung lobectomy and underline that lung surgery should be performed with extreme caution in SARS-CoV-2 epidemic outbreak areas and underline the vigilance required in the perioperative setting.24 , 25
In addition, we recommend consideration of a tailored surgical strategy, especially in patients presenting high risk factors. Despite this, we showed only a correlation between the number of resected segments and COVID-19, parenchymal sparing procedures could be preferred to standard lobectomy, when tumor stage is permissive, even if respiratory and cardiac function are good for larger resections, as long as respecting current recommendations on margins.
As concerning association between duration of surgery and COVID-19 onset, we underline that, regardless of the pandemic, several studies have already reported ventilation-induced lung injury among adverse complications after thoracic surgery.26, 27, 28 It is well-known that acute lung injury (ALI) is an acute inflammatory disorder, characterized by disrupted vascular integrity but increased permeability of epithelium and endothelium.29 , 30 Pulmonary damage in the form of acute lung injury and adult respiratory distress syndrome is a major cause of morbidity and mortality after thoracic surgery. Its incidence is about 2%-5% of all cases and correlates with duration of surgery.26
Our data revealed an association between duration of surgery with COVID-19 onset, as well.
Based on these results, strategies to reduce surgical time may be considered, such as encouraging preoperative diagnosis, to avoid frozen section or avoid nodal dissection, when possible as in the case of pure GGO. Moreover, since no association emerged between open surgery and VATS, it could be suggested that each surgeon considers the most time saving approach with a view to personal expertise in the field of minimal invasive surgery.
Some authors even proposed to delay surgery; however, this proposal may be weakened since the end of the pandemic is unknown. It could instead be more feasible using alternative therapies (i.e., SBRT).31 , 32
Unfortunately, this is a retrospective study, affected by many limitations. The main ones are the small size of the COVID-19 population and incomplete follow-up of patients operated on in March, therefore, we can only draw initial suggestions but further data is definitively needed to strengthen our findings. This bias could be overcome by the use of a web global registry, as TERAVOLT introduced in March 2020.33
Another important bias is represented by the testing strategy, which did not included postoperative swab in asymptomatic patients. Moreover, also COVID-19 diagnosis was sometimes obtained only on CT findings without swab confirmation. This meant that, Group 2 may have comprised patients who acquired the infection without developing the disease. That is why, it is worth emphasizing that, in this study, we always refer to the confirmed disease and not to mere infection. However, that could be a minor bias considering our aim to understand the impact of the disease after surgery rather than the risk of contracting the virus.
The final bias is the heterogeneity of the sample determined by multicentric study design. In fact, results could have been influenced by different geographical incidence of the infection but also by the absence of a common protocol in patients management.
CONCLUSIONS
The outbreak of Coronavirus 2019 is a new challenge in the management of thoracic surgery patients who are exposed to high risk of death if they develop clinically apparent COVID-19. Our results could be potentially useful in patients’ triage and postoperative management during the coming months until a vaccine is developed and widely administered.
Our suggestions for mitigating risk in surgical patients during the COVID-19 pandemic include:
-
1.
Improve and develop new models for symptoms surveillance and isolation during postoperative and recovery period;
-
2.
Be aware of a possible greater risk correlated with BMI, smoking history, DLCO/VA%, number of resected segments and duration of surgery.
Although univariable analysis showed an association between operative duration and development of symptomatic postoperative Covid 19, further data is necessary to define the relationship between a surgical strategy based on parenchymal sparing and minimization of procedure time, and risk to contract the disease (Fig. 1 ).
Figure 1.
(graphical abstract): the paper entitled COVID-19 after lung resection in Northern Italy is a retrospective study from four Thoracic Surgery Departments in the Lombardia region. Univariable Cox regression models were estimated to investigated postoperative mortality among patients who contracted symptomatic COVID-19 and to evaluate potential prognostic factors for developing COVID-19.
Our data showed that symptomatic COVID-19 severely increases risk of death after surgery. So, we suggest to consider a patient tailored surgical programme based on after discharge surveillance or isolation and to be aware of a possible greater risk correlated with BMI, smoking history, DLCO/VA%, number of resected segments and duration of surgery.
Acknowledgments
A special thanks for the collaboration go to Gabrile Maffeis MD1, Alessandro Baisi MD2, Giovanna Rizzardi MD3, Diego Gavello MD4, Francesco Carleo MD7.
Footnotes
IRB waived the need to review this retrospective case series. Patients’ informed written consent for data publication was always collected
Conflict of Interest Statement and Sources of Funding: there is no conflict and no funding
References
- 1.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohrabi C., Alsafi Z., O'Neill N., et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020 76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regione Lombardia, Coronavirus updated epidemiological data.Available at: https://experience.arcgis.com/experience/0a5dfcc103d0468bbb6b14e713ec1e30/. Accessed May 15, 2020
- 4.Dipartimento della Protezione Civile. Coranavirus, the state of the infection in Italy. Available at: http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1. Accessed May 15, 2020
- 5.Mapped out: which European regions have been most severely affected by the coronavirus? Available at: https://innovationorigins.com/mapped-out-which-european-regions-have-been-most-severely-affected-by-the-coronavirus/. Accessed May 15, 2020
- 6.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 7.Dai M., Liu D., Liu M., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: A multicenter study during the COVID-19 outbreak. Cancer Discov. 2020 Jun;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finley C., Prashad A., Camuso N. Guidance for management of cancer surgery during the COVID-19 pandemic. Can J Surg. 2020;63(22):S2–S4. doi: 10.1503/cjs.005620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thoracic Surgery Outcomes Research Network, Inc COVID-19 guidance for triage of operations for thoracic malignancies: A consensus statement from thoracic surgery outcomes research network. Ann Thorac Surg. 2020;110(2):692–696. doi: 10.1016/j.athoracsur.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7) (Released by National Health Commission & State Administration of Traditional Chinese Medicine on March 3, 2020)
- 11.Iacobucci G. Covid-19: all non-urgent elective surgery is suspended for at least three months in England. BMJ. 2020;368:m1106. doi: 10.1136/bmj.m1106. [DOI] [PubMed] [Google Scholar]
- 12.Li X., Liu M., Zhao Q., et al. [Preliminary recommendations for lung surgery during 2019 novel coronavirus disease (COVID-19) epidemic period] Zhongguo Fei Ai Za Zhi. 2020;23:133–135. doi: 10.3779/j.issn.1009-3419.2020.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lei S., Jiang F., Su W., et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng Shu, Huang Liu, Zhao Bo, et al. Clinical course of coronavirus disease 2019 in 11 patients after thoracic surgery and challenges in diagnosis. The Journal of Thoracic and Cardiovascular Surgery. 2020;160(2):585–592. doi: 10.1016/j.jtcvs.2020.04.005. Published online: April 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z., Li S., Tian S., et al. Full spectrum of COVID-19 severity still being depicted. Lancet. 2020;395:947–948. doi: 10.1016/S0140-6736(20)30308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 17.Cai Yixin, Hao Zhipeng, Gao Yi. Coronavirus Disease 2019 in the Perioperative Period of Lung Resection: A Brief Report From a Single Thoracic Surgery Department in Wuhan, People's Republic of China. J Thorac Oncol. 2020;15:1065–1072. doi: 10.1016/j.jtho.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patanavanich R, Glantz SA. Smoking is associated with worse outcomes of COVID-19 particularly among younger adults: A systematic review and meta-analysis. medRxiv. 2020 Sep 23;2020.09.22.20199803 preprint [DOI] [PMC free article] [PubMed]
- 19.Sattar N., Ho F.K., Gill J.M., Ghouri N., Gray S.R., Celis-Morales C.A., Katikireddi S.V., Berry C., Pell J.P., McMurray J.J., Welsh P. BMI and future risk for COVID-19 infection and death across sex, age and ethnicity: Preliminary findings from UK biobank. Diabetes Metab Syndr. 2020;14:1149–1151. doi: 10.1016/j.dsx.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Ping, Chen Lulu, Zheming Liu, et al. Clinical features and short-term outcomes of elderly patients with COVID-19. Int J Infect Dis. 2020;97:245–250. doi: 10.1016/j.ijid.2020.05.107. S1201-9712(20)30415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Chaomin, Chen Xiaoyan, Cai Yanping. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taipe Ricardo, Euscatigue Mardonio, Valdivia Fernando, et al. SARS-COV-2 infection in the perioperative of pulmonary lobectomy. About a case. Int J Surg Case Rep. 2020;77:719–725. doi: 10.1016/j.ijscr.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Jingyu, Wang Aifen, Kang Ganjun. Clinical course of patients infected with severe acute respiratory syndrome coronavirus 2 soon after thoracoscopic lung surgery. J Thorac Cardiovasc Surg. 2020;160:e91–e93. doi: 10.1016/j.jtcvs.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoleriu Mircea Gabriel, Gerckens Michael, Hetrodt Justin. Clinical course of three postoperative symptomatic COVID-19 cases in patients after lung lobectomy. Ann Thoard Surg. 2020;110:e461–e463. doi: 10.1016/j.athoracsur.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gothard J. Lung injury after thoracic surgery and one-lung ventilation. Curr Opin Anaesthesiol. 2006;19:5–10. doi: 10.1097/01.aco.0000192783.40021.c1. Ann Thorac Surg 2020 Dec;110(6):e461-e463. [DOI] [PubMed] [Google Scholar]
- 27.Licker M., Fauconnet P., Villiger Y., et al. Acute lung injury and outcomes after thoracic surgery. Curr Opin Anaesthesiol. 2009;22:61–67. doi: 10.1097/ACO.0b013e32831b466c. [DOI] [PubMed] [Google Scholar]
- 28.Della Rocca G., Coccia C. Acute lung injury in thoracic surgery. Curr Opin Anaesthesiol. 2013;26:40–46. doi: 10.1097/ACO.0b013e32835c4ea2. [DOI] [PubMed] [Google Scholar]
- 29.Wang T., Gross C., Desai A.A., et al. Endothelial cell signaling and ventilator-induced lung injury: Molecular mechanisms, genomic analyses, and therapeutic targets. Am J Physiol Lung Cell Mol Physiol. 2017;312:L452. doi: 10.1152/ajplung.00231.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X., Wang Y., Yang Y., et al. [Research status of mechanical power in ventilator-induced lung injury] Zhong Bing Ji Jiu Yi Xue. 2019;31:1549–1551. doi: 10.3760/cma.j.issn.2095-4352.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Thoracic Surgery Outcomes Research Network, Inc. Antonoff M., Backhus L. COVID-19 guidance for triage of operations for thoracic malignancies: A consensus statement from Thoracic surgery outcomes research network. J Thorac Cardiovasc Surg. 2020;160:601–605. doi: 10.1016/j.jtcvs.2020.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biniam Kidane, Jonathan Spicer, Julian O. Kim, et al. SABR-BRIDGE: Stereotactic ABlative Radiotherapy Before Resection to AvoId Delay for Early-Stage LunG Cancer or OligomEts During the COVID-19 Pandemic. Front. Oncol. 25 September 2020. 10.3389/fonc.2020.580189 [DOI] [PMC free article] [PubMed]
- 33.Whisenant J.G., Trama A., Torri V., et al. TERAVOLT: Thoracic Cancers International COVID-19 collaboration. Cancer Cell. 2020;37:742–745. doi: 10.1016/j.ccell.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]