Abstract
Personalized cognitive counseling (PCC) is an evidence-based intervention designed to modify HIV-related risk behavior. We assessed the impact of PCC on sexual behavior, drinking expectancy, and incidence of sexually transmitted infections (STIs) in a six-month randomized controlled trial among 153 HIV-uninfected men who have sex with men (MSM) and transgender women (TW) in Peru. Study retention was ≥90%, with three HIV infections (3 Control) and 19 cases of GC/CT (10 Control, 9 PCC) at six months. There was a decline in condomless receptive anal intercourse in the Control (0.74, 95% CI: 0.60–0.91; p<0.01) and PCC arms (0.72, 0.55–0.94; p=0.02) at six-month follow-up. There was a decrease in drinking expectancy at six months among participants endorsing alcohol use in the PCC arm (0.89, 0.83–0.96; p<0.01), versus no change in the Control arm (0.98, 0.92–1.04; p=0.54). PCC was efficacious in reducing drinking expectancy and HIV risk among MSM and TW in Peru.
RESUMEN:
La consejería cognitiva personalizada (CCP) es una intervención basada en evidencia diseñada para poder modificar el comportamiento asociado con el riesgo de contraer VIH. Evaluamos el impacto de CCP en el compartimiento sexual, el drinking expectancy, y la incidencia de infecciones de transmisión sexual (ITS) a través de un estudio controlado aleatorio que duró seis meses e incluyó 153 hombres sin VIH que tienen relaciones sexuales con hombres (HSH) y mujeres transgéneros (MT) en Perú. La retención en el estudio fue ≥90%, con tres infecciones de VIH (3 Control) y 19 casos de GC/CT (10 Control, 9 CCP) a los seis meses. Hubo una disminución de las relaciones sexuales receptivas sin preservativos dentro del grupo Control (0.74, 95% CI: 0.60–0.91; p<0.01) y el grupo CCP (0.72, 0.55–0.94; p=0.02) a los seis meses. También hubo una disminución en el drinking expectancy a los seis meses dentro de los participantes quienes tomaban alcohol dentro del grupo CCP (0.89, 0.83–0.96; p<0.01), versus ningún cambio dentro del grupo Control (0.98, 0.92–1.04; p=0.54). La CCP fue eficaz en disminuir el drinking expectancy y el riesgo de contraer VIH dentro de HSH y MT en Perú.
Keywords: Personalized cognitive counseling (PCC), Men who have sex with men (MSM), Transgender women (TW), Drinking expectancy, HIV prevention
INTRODUCTION:
Personalized cognitive counseling (PCC) is an evidence-based intervention designed to modify HIV-related risk behavior among men who have sex with men (MSM) (1). This participant-focused approach seeks to retrospectively understand and prospectively manage cognitive processes underlying high-risk sexual behavior (2–5). Developed to address the prevention needs of men who repeatedly tested for HIV after recurrent episodes of condomless anal intercourse (CAI) with HIV-infected or unknown serostatus partners, PCC was conceptualized by Dilley et al. based on the hypothesis that the decisions of these men to engage in high-risk acts were governed by “self-justifications,” e.g., thoughts, attitudes, and beliefs in the “heat of the moment” that undercut the transmission risk involved, and thereby “allowed” the desired, but known risky, behavior to occur (6). In an attempt to help these men reduce their future risk of HIV infection, PCC was designed to make clients aware of their self-justifications by asking them to recall details of their decision-making during a recent CAI episode in “the cold light of day,” objectively re-assessing the risks involved in the encounter. During the intervention, a counselor encourages the client to consider ways he might manage similar sexual encounters differently in the future (7). While PCC has been successfully employed in the United States (U.S.) (2, 6, 8–13), it has not previously been implemented with MSM or TW in Latin America.
Because substance use is disproportionately prevalent among MSM and TW worldwide (14–21), and substantially increases the risk of HIV transmission when it occurs prior to or during sex (22–24), recent studies in the U.S. have raised the possibility of adapting PCC to address sexual risk behavior fueled by alcohol and/or substance use (9). Peru offers an optimal environment to address this question as alcohol use is common (25) and associated with high-risk sexual behaviors among MSM and TW in the country (26, 27). Alcohol use disorders (AUDs) are also highly prevalent (28–25) in the region, and have been associated with HIV risk-taking behaviors and delays in HIV diagnosis (29, 30). Additionally, Peru’s HIV epidemic is concentrated among MSM and TW, with estimated prevalences of 16.4% and 18.5%, respectively (31). Due to its emphasis on examining self-justifications for risky behavior, including substance use (e.g., “I was so high, it [high-risk sex] just happened”) PCC may effectively modify “drinking expectancy,” or an expectation of improved mood, social connectedness, and/or problematic behavior with alcohol use (32, 33).
To better understand how PCC may reduce high-risk sexual behavior among MSM and TW, we piloted a randomized controlled trial (RCT) to assess the logistics, feasibility, and effect of a PCC intervention on sexual risk behavior, drinking expectancy, and prevalence of HIV and other sexually transmitted infections (STIs) among MSM and TW reporting condomless receptive anal intercourse (cRAI) in Lima, Peru. Research was conducted as part of a study evaluating a combination HIV prevention approach based on rectal STI testing, counseling, and treatment completed between June, 2017 and May, 2018. We hypothesized that individual counseling tailored to recent high-risk behavior (PCC) would contribute to short-term reductions in: 1) High-risk sexual practices, including cRAI; 2) Drinking expectancy, as measured by the Drinking Expectancy Questionnaire for MSM (DEQ-MSM); and 3) Biological outcomes, including incidence of rectal GC/CT and HIV infection.
MATERIALS AND METHODS
Screening procedures
Potential participants were recruited between June and December, 2017 from community venues by peer recruiters of the Asociación Civil Via Libre, a non-governmental organization promoting HIV and STI research, testing, and treatment in Lima, Peru. Enrollment in the screening protocol was limited to individuals who: 1) Were at least 18 years of age; 2) Were assigned male sex at birth; 3) Had not previously tested positive for HIV; and 4) Reported cRAI with ≥1 HIV-infected or unknown serostatus male partner in the previous six months. Potential subjects were invited to participate in an HIV prevention study based on an STI screening platform, including testing for rectal Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT).
Participants completed a computer-assisted self-interview (CASI) of demographic characteristics, sexual risk behavior, and substance use. Questions addressed participant sexual orientation (heterosexual, bisexual, homosexual), sexual role (activo [insertive], pasivo [receptive], moderno [versatile], and other), and number of recent sexual partners and types of sexual activities. Sexual acts with the three most recent contacts were also addressed, including intercourse type (anal, vaginal, oral), condom use and sexual position (insertive, receptive, both) for each act, and event-specific alcohol and drug use by both the participant and their partner.
Study physicians performed a medical history and physical exam assessing for STI signs/symptoms, and collected blood and rectal swabs for STI screening. All swab specimens were tested for GC and CT using the Gen-Probe Aptima II assay (Hologic, San Diego, CA, USA) at the Universidad Peruana Cayetano Heredia Sexual Health Laboratory in San Martin de Porres, Peru. Participants with clinically symptomatic urethritis or proctitis, or asymptomatic, laboratory-diagnosed GC/CT infection, were treated per CDC Guidelines (34).
All participants underwent a 4th Generation Rapid HIV-1/2 assay (Alere Determine, Alere). Positive results were confirmed by immunofluorescence assay. Baseline syphilis testing was performed using RPR screening (RPRnosticon, Biomérieux, Marcy l’Etoile, France) and TPPA confirmation (Serodia TPPA, Fujirebio, Malvern, PA, USA). Confirmed syphilis cases were treated according to stage of infection, per CDC guidelines (34). Participants with newly diagnosed HIV infection were referred to local HIV treatment centers (including Via Libre). Participants were compensated 15 Nuevos soles (US $5.00) for transportation and provided with condoms and sachets of lubricant.
All screened participants received standard HIV risk reduction counseling based on CDC Project RESPECT-2 guidelines (35), and incorporating cognitive and action-oriented strategies, similar to motivational interviewing (36). Specifically, study counselors discussed recent sexual risk behavior and perceived risks for HIV infection with participants prior to HIV testing. Post-test discussions addressed condom use, partner notification, and follow-up testing to reduce risk of future STI/HIV acquisition.
Randomization and enrollment
HIV-uninfected MSM and TW who reported cRAI with ≥1 HIV-infected or unknown serostatus male partner(s) in the last six months and were diagnosed with asymptomatic rectal GC/CT infection or symptomatic proctitis were invited to enroll in the PCC trial. Most participants were asymptomatic and enrolled at the 2-week follow-up screening visit after receiving results of nucleic acid testing and appropriate antibiotic therapy. All participants provided signed informed consent to participate in a study “to see if treating rectal STIs and providing counseling on how to reduce sexual risk behavior can help reduce people’s risk of acquiring HIV infection.”
After providing signed consent, participants (n=100) were randomized using a previously designed random permuted block scheme. Participants with rectal GC/CT infection were assigned to PCC (Intervention) or Standard Counseling (Control). Allocation assignments were concealed in opaque, sealed, sequentially numbered envelopes opened in numerical order by the study coordinator at randomization. No deviation from allocation order or wasting of envelopes was reported. An additional 53 participants, identified by matching GC/CT-uninfected controls with GC/CT-infected cases according to age and number of recent receptive anal intercourse partners, were enrolled in a concurrent analysis of cytokine levels in rectal mucosa. As these individuals underwent the standardized counseling procedures at all visits, they were included as subjects in the Control arm of this analysis.
Intervention and control follow-up procedures
At three- and six-month visits, participants underwent a physical examination assessing for symptomatic infection, received repeat HIV and rectal GC/CT testing, and completed a CASI survey addressing the same behaviors as at baseline. Control participants were offered standard HIV counseling procedures at each visit, while intervention participants received standard counseling and PCC at the three- and six-month visits.
All study counselors proficient in standard counseling procedures were trained to deliver PCC by a Spanish-speaking expert (FN) with extensive experience training staff in PCC methods and practices. Training sessions included lectures and role-play exercises, and were provided at the beginning of the study and again right before initiating PCC procedures at the three-month follow-up visits.
PCC participants first completed a self-justification evaluation instrument adapted for the local context, and met with a study counselor to review their responses. As an example, one of the self-justifications was, “I drank more than I thought and I cannot control my actions when I am drunk.” Counselors asked participants to recount a recent sexual encounter involving CAI, paying attention to environmental cues, interpersonal interactions, and emotions and cognitive processes at each step of the encounter. Participants and their counselor then reviewed the self-justifications employed during the interaction together, using objective, “offline” analysis to explore the “online” cognitive processes they used to justify sexual behavior during the episode. Counselors highlighted moments where critical behavioral decisions occurred, identifying self-justifications employed to explain risk behavior in those moments, and developing strategies to manage future encounters where risk behavior might occur. A key component of the counseling was linking the prior episode of CAI with the recent rectal GC/CT diagnosis as a strategy to highlight flaws in previously used self-justifications. The participants also rehearsed risk reduction strategies with the counselor by role-playing interactions where their high-risk sexual behaviors typically occur.
Outcome measures
The validated DEQ-MSM scale was administered at three- and six-month visits. This ten-item survey, developed in Australia in 2011 (33), uses a five-point Likert scale to measure the perceived impact of alcohol consumption on mood and decision-making processes among MSM, with higher scores reflecting an expectation of improved mood, social connectedness, and/or problematic behavior with alcohol use (32). Because expectations about the effects of a substance influence post-use behavior, drinking expectancies are hypothesized as a key mediating variable between alcohol use and sexual risk-taking (33). A 2015 validation study among MSM and TW in Peru found that the Spanish version had excellent reliability (32).
The primary behavioral outcome was prevalence of self-reported cRAI with an HIV-infected or unknown serostatus partner at six months. Secondary outcomes included number of episodes of CAI in the last thirty days, and three sexual risk behaviors limited to the last three partners: number of CAI events, number of sexual encounters involving alcohol, and number of CAI events with HIV-infected or unknown serostatus partners involving alcohol. To obtain information on both the overall scope of risk behavior and event-specific correlates of risk behavior, we asked participants about the total number of CAI events in the last thirty days, as well as risk behaviors with each of their last three partners. We also measured laboratory-confirmed diagnoses of new rectal GC, CT, and HIV infections at each visit.
Statistical analysis
Descriptive characteristics by study arm were calculated with median and interquartile range (IQR) or mean and standard deviation (SD) where appropriate, for continuous variables and proportions for categorical variables. Differences between baseline characteristics of participants in the two arms were assessed using two-sided t-test for parametric continuous variables, Wilcoxon rank-sum for non-parametric continuous variables, and Chi square test for categorical variables.
The treatment effect was assessed by intention-to-treat analyses conducted according to participants’ random allocation (PCC = 50; Control = 103), without regard to study procedures, and based on all observed study data. Complete case analysis was performed for variables with missing data; ≤5% of data was missing for any variable. We used generalized estimating equation (GEE) Poisson models to evaluate group-specific linear trends in outcomes across two study visits, with robust standard errors to account for within-subject correlation. The treatment effect was captured in the Poisson model by the time-by-treatment interaction.
We performed a sensitivity analysis to assess the robustness of observed associations when restricted only to GC/CT-positive participants (i.e., excluding the 53 GC/CT-negative matched controls). As the primary findings were similar, the original analysis was used to maintain statistical power. All analyses were conducted using Stata 12.0 (StataCorp, College Town, TX).
Human subjects protections
All study procedures were reviewed and approved by the Institutional Review Boards of the University of California, Los Angeles and the Asociación Civil Via Libre prior to initiation of any activities. All participants underwent informed consent procedures, providing written informed consent prior to participation. The clinical trial was registered with www.ClinicalTrials.gov (Protocol Number NCT03010020).
RESULTS:
We screened a total of 613 individuals and enrolled 153 HIV-uninfected MSM (n=118) and TW (n=31). Symptomatic proctitis or laboratory-diagnosed GC/CT infection was present in 100 participants randomized to receive either PCC (Intervention) or standard post-test counseling (Control). All 53 GC/CT-uninfected participants received standard post-test counseling (Control).
Sample characteristics
Baseline demographic characteristics and sexual risk behaviors of participants in the Intervention (n = 50) and Control (n = 103) arms were similar (Table I). The median age in both groups was 24 years, and most participants reported having completed some college or technical school. The most frequently reported sexual role was pasivo (receptive; 55.6%), and more than 70% of MSM and TW reported CAI with ≥1 of their last three sexual partners. More than half of all participants (53.6%) met AUDIT-10 criteria for an AUD, and 42.5% reported alcohol use prior to sex with ≥1 of their last three partners.
Table I.
Baseline characteristics and self-reported sexual risk behaviors with the last three partners among MSM and TW in Lima, Peru, 2017, stratified by treatment arm; N=153
| Characteristic | Control (n=103) | PCC (n=50) |
|---|---|---|
| Age (n=153) | 24 (21, 30) | 24.5 (21, 29) |
| Education (n=151) | ||
| Secondary or less | 42 (43.7) | 15 (45.8) |
| University/technical | 58 (56.3) | 26 (54.2) |
| Sexual orientation (n=149) | ||
| Hetero/bisexual | 12 (11.8) | 6 (12.8) |
| Homosexual | 71 (69.6) | 29 (61.7) |
| Transgender | 19 (18.6) | 12 (25.5) |
| Sexual role (n=151) | ||
| Pasivo | 54 (52.4) | 30 (62.5) |
| Moderno | 49 (47.6) | 18 (37.5) |
| Alcohol use disorders (AUD; n=153) | ||
| No AUD or social drinker | 47 (45.6) | 24 (48.0) |
| Hazardous use | 41 (39.8) | 15 (30.0) |
| Harmful use | 15 (14.6) | 11 (22.0) |
| Drinking Expectancy Questionnaire (DEQ-MSM) Scores (3-Month; n=145) | ||
| Total | 2.5 (0.8) | 2.7 (0.8) |
| Condomless anal intercourse (CAI) with ≥1 of the last three partners (n=153) | ||
| No | 30 (29.1) | 11 (22.0) |
| Yes | 73 (70.9) | 39 (78.0) |
| Alcohol use prior to sex with ≥1 of the last three partners (n=153) | ||
| No | 61 (59.2) | 27 (54.0) |
| Yes | 42 (40.8) | 23 (46.0) |
| Alcohol use prior to CAI with ≥1 of the last three partners (unknown status or HIV-positive partners only; n=153) | ||
| No | 80 (77.7) | 33 (66.0) |
| Yes | 23 (22.3) | 17 (34.0) |
Age, Median (IQR); DEQ-MSM, Mean (SD); Categorical variables, N (%); Bold text = p<0.05
Study Retention
Study completion rates were similar between the two arms: 99/103 (96%) participants in the Control arm returned for the three-month visit, compared with 48/50 (96%; p=0.97) in the Intervention arm. For the six-month visit, 96/103 (93%) participants in the Control arm were evaluated, compared with 45/50 (90%; p=0.49) participants in the Intervention arm.
Sexual risk behavior outcomes
There was a significant reduction in prevalence of ≥1 episode of cRAI (primary outcome) in the last month in the Control (0.74, 95% CI: 0.60–0.91; p<0.01) and Intervention arms (0.72, 0.55–0.94; p=0.02) at six months, though there was no significant difference in the reduction in cRAI prevalence between the two groups (p=0.83). There were no statistically significant differences between groups in self-reported number of CAI events in the last month or sexual risk behaviors with the last three partners (Table II).
Table II.
Effects of Personalized Cognitive Counseling (PCC) on self-reported substance use and sexual risk behaviors among MSM and TW in Lima, Peru, 2017; N=153
| Outcomes | Mean | Rates of change in mean value of outcomes over time | PCC (n=50) vs. Control (n=103) | |||||
|---|---|---|---|---|---|---|---|---|
| 3-Mo | 6-Mo | Rate | 95% CI | P value | RR | 95% CI | P value | |
| Number of condomless anal intercourse (CAI) events in the last month | ||||||||
| PCC | 2.26 | 1.41 | 0.69 | 0.51, 0.93 | 0.01 | 0.89 | 0.55, 1.43 | 0.63 |
| Control | 2.34 | 1.49 | 0.74 | 0.58, 0.93 | 0.01 | |||
| Number of condomless anal intercourse (CAI) events with three most recent partners | ||||||||
| PCC | 0.96 | 0.89 | 0.94 | 0.65, 1.35 | 0.73 | 0.99 | 0.62, 1.58 | 0.95 |
| Control | 0.94 | 0.89 | 0.95 | 0.72, 1.25 | 0.72 | |||
| Alcohol use disorder identification test (AUDIT) score | ||||||||
| PCC | 6.96 | 4.68 | 0.70 | 0.57, 0.87 | <0.01 | 0.76 | 0.60, 0.97 | 0.03 |
| Control | 7.08 | 6.41 | 0.94 | 0.82, 1.07 | 0.35 | |||
| Analyses below limited to participants who endorsed alcohol use, n=139 (Control, n=91; PCC, n=48) | ||||||||
| Number of sexual encounters with three most recent partners involving alcohol | ||||||||
| PCC | 0.42 | 0.44 | 1.07 | 0.70, 1.57 | 0.81 | 1.18 | 0.67, 2.07 | 0.57 |
| Control | 0.62 | 0.55 | 0.90 | 0.62, 1.30 | 0.58 | |||
| Number of CAI events with unknown serostatus or HIV-positive partners involving alcohol use (three most recent partners) | ||||||||
| PCC | 0.10 | 0.15 | 1.52 | 0.67, 3.41 | 0.31 | 1.98 | 0.70, 5.58 | 0.20 |
| Control | 0.26 | 0.19 | 0.74 | 0.39, 1.44 | 0.38 | |||
| AUDIT score | ||||||||
| PCC | 7.07 | 4.83 | 0.72 | 0.59, 0.88 | 0.01 | 0.79 | 0.62, 1.00 | 0.05 |
| Control | 8.06 | 7.12 | 0.91 | 0.80, 1.05 | 0.20 | |||
| Drinking expectancy questionnaire (DEQ-MSM) score | ||||||||
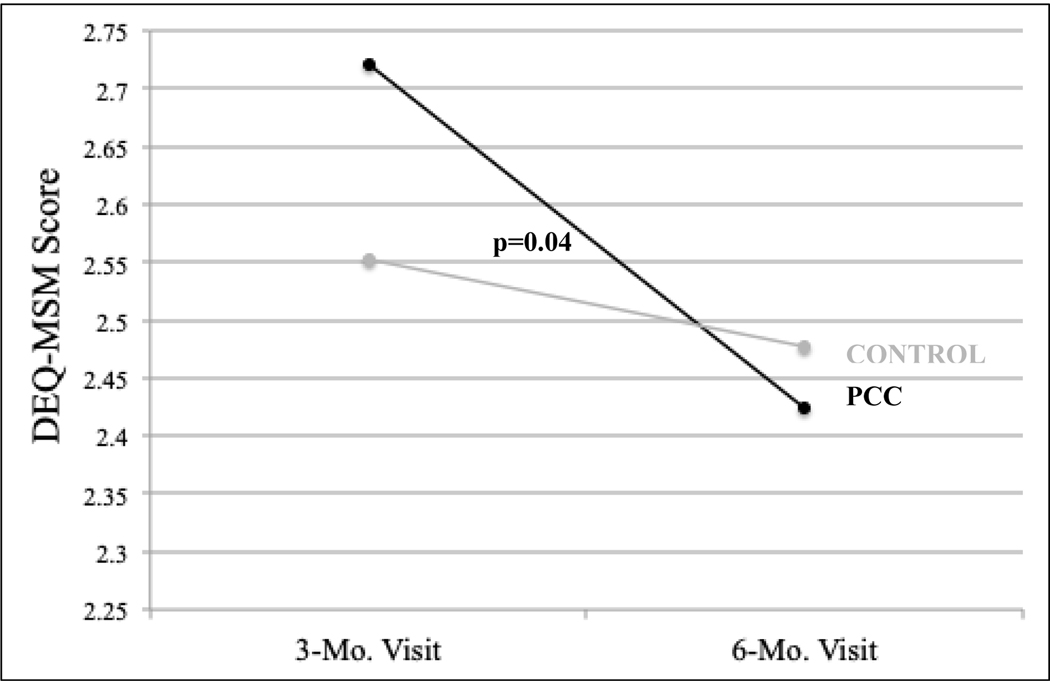
| PCC | 2.72 | 2.42 | 0.89 | 0.83, 0.96 | <0.01 | 0.91 | 0.82, 0.99 | 0.04 |
| Control | 2.54 | 2.48 | 0.98 | 0.92, 1.04 | 0.54 | |||
Alcohol use disorder identification test (AUDIT)
At the six-month evaluation, there was a significant decrease in mean AUDIT score among MSM and TW in the Intervention arm (Change in mean AUDIT score over the three-month period: 0.70, 0.57–0.87; p<0.01) compared to no change in the control arm (0.94, 0.82–1.07; p=0.35).
Drinking expectancy questionnaire (DEQ-MSM)
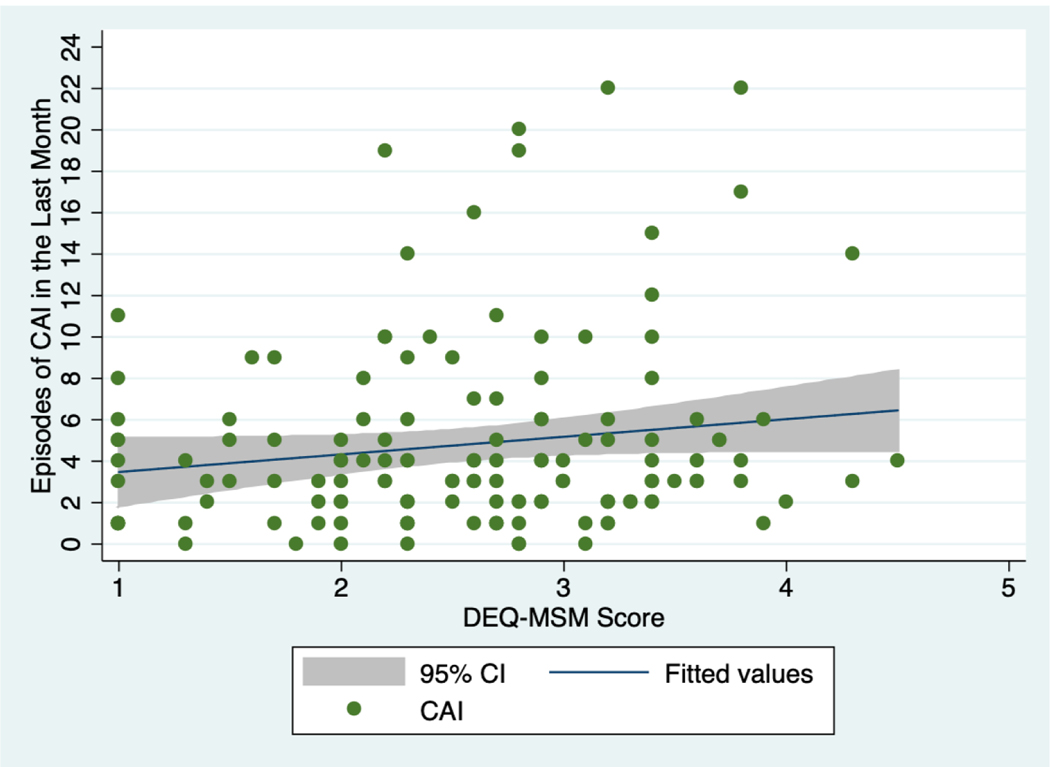
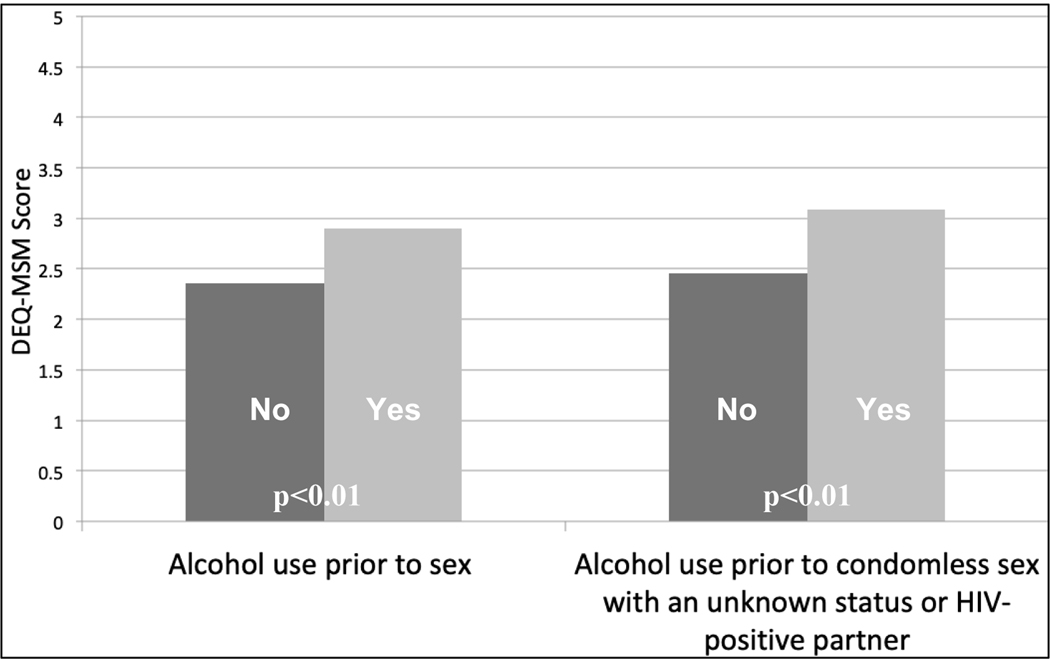
At the three-month evaluation, there was a trend towards association between DEQ-MSM score and baseline number of self-reported episodes of CAI in the previous month (Figure 1). Mean DEQ-MSM scores were also higher among participants who reported substance use prior to sex in both arms (2.90 vs. 2.36, p<0.01) and among those who reported alcohol use prior to CAI with an unknown serostatus or known HIV-positive partner (3.09 vs. 2.46, p<0.01; Figure 2). At the six-month evaluation, there was a significant decrease in mean DEQ-MSM score among MSM and TW in the Intervention arm who endorsed alcohol use (Change in mean DEQ-MSM score over the three-month period: 0.89, 0.83–0.96; p<0.01), compared to no change in the Control arm (0.98, 0.92–1.04; p=0.54; Table II; Figure 3).
Figure 1.
Association between Drinking Expectancy Questionnaire (DEQ-MSM) scores and baseline number of self-reported episodes of condomless anal intercourse (CAI) in the last month for MSM and TW in Lima, Peru, 2017; N=140
Figure 2.
Mean Drinking Expectancy Questionnaire (DEQ-MSM) scores at the three-month follow-up visit for MSM and TW in Lima, Peru, 2017, stratified by self-report of alcohol use prior to sex and alcohol use prior to condomless anal intercourse (CAI) with ≥1 of the last three partners (unknown status or HIV-positive partners only); N=145
Figure 3.
Drinking Expectancy Questionnaire (DEQ-MSM) scores among MSM and TW who endorsed alcohol use in Lima, Peru, 2017, stratified by treatment arm; N=139
Biological outcomes
We identified ten new HIV infections (6 Control, 4 Intervention) and 29 rectal GC/CT cases (18 Control, 11 Intervention) across both arms at the three-month follow-up visit. At six months, we diagnosed three new cases of HIV infection (3 Control, 0 Intervention) and 19 rectal GC/CT cases (10 Control, 9 Intervention). There were no significant differences in the prevalence of new HIV and/or rectal GC/CT infections between arms at either follow-up visit.
DISCUSSION:
To our knowledge, our study is the first RCT to assess the effect of PCC on sexual risk behavior and drinking expectancy among MSM and TW in Latin America. While there were no significant differences in the pre-specified primary outcomes of cRAI and persistent or recurrent rectal GC/CT infection at the 6-month follow-up visit, there were no new HIV infections identified among PCC participants at the six-month visit compared to three new HIV diagnoses in the Control arm. In our sample, higher DEQ-MSM scores were associated with greater reported frequency of CAI and alcohol use prior to sex. Notably, at the 6-month evaluation there was a significant decrease in mean DEQ-MSM score among MSM and TW in the PCC arm compared to no change in the Control arm. As alcohol use among MSM and TW in Peru is common and frequently associated with high-risk sexual behaviors (27, 26), these findings provide support for further research into how to adapt and use PCC as a strategy to address sexual risk behavior occurring in the context of alcohol use in this population.
In both the PCC and Control arms in our study, there was a significant decline in self-reported prevalence of cRAI in the last month at both follow-up evaluations. This reduction could be due to social desirability bias in both groups leading to over-reported condom use at follow-up visits. While we did not find significant differences in biological outcomes between the two arms, there were three new HIV infections in the Control group compared to none in the PCC group at six months. This difference highlights the fact that there are likely factors other than self-reported CAI influencing HIV transmission among MSM and TW in Lima, such as network prevalence of bacterial STIs, patterns of HIV serosorting, and undisclosed sexual risk behavior. While the current risk reduction counseling provided in Peru has not proven effective for modifying behavior among repeat testers (37, 38), PCC may offer an effective alternative for reducing HIV-related behavior by using interactive storytelling and role-playing exercises that consider overlapping contexts of sexual risk, and are not limited to conversations about condom use (39, 40). Although the small size of our pilot study prevents any definitive conclusions, findings support further study of the efficacy of PCC as part of combination HIV/STI prevention interventions for MSM and TW in Lima.
To be successful, combination STI and HIV prevention approaches among MSM and TW in Peru should consider how substance use influences sexual interactions and consider incorporating harm reduction techniques. To this point, our findings highlight an association between higher drinking expectancy scores and frequency of sexual risk behavior, particularly in the context of alcohol use before sex. Notably, high drinking expectancy scores are a potentially modifiable component of AUDs, and more than fifty percent of participants in our study met AUDIT-10 criteria for hazardous drinking. These results are consistent with previous studies showing associations between drinking expectancies and frequency and quantity of drinking (33), and the intent to both drink and engage in high-risk sexual behaviors (30, 41). Future research is needed to explore the role drinking expectancy may play in mediating sexual risk-taking and alcohol use, particularly among MSM and TW.
To our knowledge, ours is the first study to demonstrate an association of PCC and reduced drinking expectancy among MSM and TW. Although the exact mechanism of this effect is unclear, by facilitating meaningful explorations of self-justifications for risky behavior, including those involving the disinhibitory effects of alcohol, PCC may motivate individuals to critically reflect on their attitudes toward, and consumption of, alcohol as a strategy to reduce their HIV risk. While recent literature has proposed a role for PCC in addressing substance use among MSM (6, 9), exactly how to adapt this intervention will depend on the primary substance used, the degree of dependence, and the local sociocultural context of substance use (10). Accordingly, our study makes a substantial contribution to the existing literature regarding PCC by providing support for, and direction to, efforts to adapt counseling to address sexual risk behavior in the context of alcohol use in Peru. Future studies enrolling a larger sample size for a longer follow-up period may have greater power to detect significant effects of PCC on CAI, alcohol use prior to CAI, and HIV/STI incidence.
Our findings should be considered in the context of several limitations. While the pre-specified primary outcome of our trial was reduction in prevalence of self-reported CAI with HIV-positive or unknown serostatus partners at six months, findings from our formative qualitative research, as well as interim content analysis of PCC sessions conducted during the trial, both suggested the central importance of alcohol use in guiding high-risk sexual behavior in Peru’s local context. As a result, we modified our analysis plan to incorporate these secondary outcomes, and will focus on the intersections of alcohol use, sexual risk behavior, and HIV/STI incidence in future trials of PCC in Peru. In addition, all behavioral outcomes were assessed via self-report. While participants completed a computer-assisted self-interview, some individuals may have underreported sexual risk behavior due to social desirability. Because PCC discussions often focused on alcohol use, social desirability bias may have also led participants in the Intervention arm to report reduced alcohol use in subsequent follow-up surveys (42).
The fact that half of the Control participants were GC/CT-negative at baseline may have affected frequency of risk behavior in this arm, though participants were matched based on number of RAI partners to ensure similar risk profiles. Moreover, as described in the Methods section, results of a sensitivity analysis limited to GC/CT-positive MSM and TW were comparable to analyses including the GC/CT-negative controls. Similarly, our results may not be generalizable to all MSM and TW in Lima because we collected a convenience sample of behaviorally high-risk volunteers in a rectal STI screening and HIV prevention trial. In addition to the enrollment criteria, the recruitment site is an HIV research center, and our sample is likely to be at higher risk for HIV infection than the general population of MSM and TW in Peru. Despite these limitations, our findings provide important information on the development and effect of a PCC intervention on sexual risk behavior and substance use among MSM and TW in Peru at high risk for HIV and STI acquisition.
CONCLUSIONS:
While our pilot assessment of PCC did not find an effect of the intervention on CAI, our study supports the feasibility and potential efficacy of PCC in reducing HIV risk behavior in the context of alcohol use among MSM and TW in Lima, Peru. Results show DEQ-MSM score is associated with alcohol-associated CAI in this population, and the adapted PCC intervention was efficacious in reducing drinking expectancy among MSM and TW who endorsed alcohol use. While overall reductions in sexual risk behavior were similar between arms, no new HIV infections were observed among participants who received PCC at the prior visit. As the HIV epidemic in Peru continues to dramatically and disproportionately affect MSM and TW, and alcohol use remains a predominant mediator in HIV risk-taking behavior (29), there is a critical need to develop and evaluate interventions that address overlapping facets of risk behavior. Additional research with a larger sample size is needed to assess the impact of PCC on post-STI behavior change, substance use, and HIV/STI incidence among MSM and TW in Latin America.
ACKNOWLEDGEMENTS:
We would like to thank the study participants and staff who devoted their time and efforts to make this project possible.
FUNDING: Funding for this work was provided by the US National Institute of Health grants NIH R25 MH087222 and NIH R34 MH 105272 to JLC.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS:
Ethical Approval for Research Involving Human Participants: All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Boards of the University of California, Los Angeles and the Asociación Civil Via Libre and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (and registered with www.ClinicalTrials.gov; Protocol Number NCT03010020).
Informed Consent: Informed consent was obtained from all individual participants included in the study
Disclosure of Potential Conflicts of Interest: The authors declare that they have no conflicts of interest to disclose
REFERENCES:
- 1.CDC. Compendium of Evidence-Based Interventions and Best Practices for HIV Prevention: Personalized Cognitive Counseling. Centers for Disease Control and Prevention; 2018. [Google Scholar]
- 2.Dilley JW, Woods WJ, Sabatino J, Lihatsh T, Adler B, Casey S, et al. Changing sexual behavior among gay male repeat testers for HIV: a randomized, controlled trial of a single-session intervention. J Acquir Immune Defic Syndr. 2002;30(2):177–86. [DOI] [PubMed] [Google Scholar]
- 3.Gold RS. AIDS education for gay men: towards a more cognitive approach. AIDS Care. 2000;12(3):267–72. [DOI] [PubMed] [Google Scholar]
- 4.Nelson KM, Simoni JM, Pearson CR, Walters KL. ‘I’ve had unsafe sex so many times why bother being safe now?’: the role of cognitions in sexual risk among American Indian/Alaska Native men who have sex with men. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2011;42(3):370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah D, Thornton S, Burgess AP. Sexual Risk Cognitions Questionnaire: a reliability and validity study. AIDS Care. 1997;9(4):471–80. [DOI] [PubMed] [Google Scholar]
- 6.Coffin PO, Santos GM, Colfax G, Das M, Matheson T, DeMicco E, et al. Adapted personalized cognitive counseling for episodic substance-using men who have sex with men: a randomized controlled trial. AIDS Behav. 2014;18(7):1390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPhee B, Skinta MD, Paul J, Dilley JW. Single-session personalized cognitive counseling to change HIV risk behavior among HIV-negative men who have sex with men: a two-part case study. Cognitive and Behavioral Practice. 2012;19:328–37. [Google Scholar]
- 8.Dilley JW, Woods WJ, Loeb L, Nelson K, Sheon N, Mullan J, et al. Brief cognitive counseling with HIV testing to reduce sexual risk among men who have sex with men: results from a randomized controlled trial using paraprofessional counselors. J Acquir Immune Defic Syndr. 2007;44(5):569–77. [DOI] [PubMed] [Google Scholar]
- 9.Santos GM, Coffin PO, Vittinghoff E, DeMicco E, Das M, Matheson T, et al. Substance use and drinking outcomes in Personalized Cognitive Counseling randomized trial for episodic substance-using men who have sex with men. Drug Alcohol Depend. 2014;138:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight KR, Das M, DeMicco E, Raiford JL, Matheson T, Shook A, et al. A roadmap for adapting an evidence-based HIV prevention intervention: personal cognitive counseling (PCC) for episodic substance-using men who have sex with men. Prevention science : the official journal of the Society for Prevention Research. 2014;15(3):364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbst JH, Raiford JL, Carry MG, Wilkes AL, Ellington RD, Whittier DK. Adaptation and National Dissemination of a Brief, Evidence-Based, HIV Prevention Intervention for High-Risk Men Who Have Sex with Men. MMWR supplements. 2016;65(1):42–50. [DOI] [PubMed] [Google Scholar]
- 12.Wiersema JJ, Santella AJ, Canady P, Jordan AO. Self-Justifications for Unsafe Sex Among Incarcerated Young Men Who Have Sex with Men and Are Living with HIV: Results from a New York City Jail-Based Pilot Intervention. Journal of community health. 2019. [DOI] [PubMed] [Google Scholar]
- 13.Dilley JW, Schwarcz S, Murphy J, Joseph C, Vittinghoff E, Scheer S. Efficacy of personalized cognitive counseling in men of color who have sex with men: secondary data analysis from a controlled intervention trial. AIDS Behav. 2011;15(5):970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin TW, Morgenstern J. Drug-use patterns among men who have sex with men presenting for alcohol treatment: differences in ethnic and sexual identity. Journal of urban health : bulletin of the New York Academy of Medicine. 2005;82(1 Suppl 1):i127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stall R, Wiley J. A comparison of alcohol and drug use patterns of homosexual and heterosexual men: the San Francisco Men’s Health Study. Drug Alcohol Depend. 1988;22(1–2):63–73. [DOI] [PubMed] [Google Scholar]
- 16.Balan ICF Timothy; Pando Maria A.; Marone Ruben O.; Barreda Victoria; Dolezal Curtis; Carballo-Dieguez Alex; Avila Maria M. High Substance Use and HIV Risk Behavior Among Young Argentine Men Who Have Sex with Men. AIDS and behavior. 2018;22(4):1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomkins A, Ahmad S, Cannon L, Higgins SP, Kliner M, Kolyva A, et al. Prevalence of recreational drug use reported by men who have sex with men attending sexual health clinics in Manchester, UK. International journal of STD & AIDS. 2018;29(4):350–6. [DOI] [PubMed] [Google Scholar]
- 18.Sabin LL, Beard J, Agyarko-Poku T, DeSilva M, Ashigbie P, Segal T, et al. “Too Much Sex and Alcohol”: Beliefs, Attitudes, and Behaviors of Male Adolescents and Young Men Who have Sex with Men in Ghana. Open AIDS J. 2018;12:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pham QD, Nguyen TV, Nguyen PD, Le SH, Tran AT, Nguyen LT, et al. Men who have sex with men in southern Vietnam report high levels of substance use and sexual risk behaviours but underutilise HIV testing services: a cross-sectional study. Sex Transm Infect. 2015;91(3):178–82. [DOI] [PubMed] [Google Scholar]
- 20.Hunter LJ, Dargan PI, Benzie A, White JA, Wood DM. Recreational drug use in men who have sex with men (MSM) attending UK sexual health services is significantly higher than in non-MSM. Postgraduate medical journal. 2014;90(1061):133–8. [DOI] [PubMed] [Google Scholar]
- 21.Secor AM, Wahome E, Micheni M, Rao D, Simoni JM, Sanders EJ, et al. Depression, substance abuse and stigma among men who have sex with men in coastal Kenya. AIDS (London, England). 2015;29 Suppl 3:S251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drumright LN, Little SJ, Strathdee SA, Slymen DJ, Araneta MR, Malcarne VL, et al. Unprotected anal intercourse and substance use among men who have sex with men with recent HIV infection. J Acquir Immune Defic Syndr. 2006;43(3):344–50. [DOI] [PubMed] [Google Scholar]
- 23.Colfax G, Coates TJ, Husnik MJ, Huang Y, Buchbinder S, Koblin B, et al. Longitudinal patterns of methamphetamine, popper (amyl nitrite), and cocaine use and high-risk sexual behavior among a cohort of san francisco men who have sex with men. Journal of urban health : bulletin of the New York Academy of Medicine. 2005;82(1 Suppl 1):i62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colfax G, Vittinghoff E, Husnik MJ, McKirnan D, Buchbinder S, Koblin B, et al. Substance use and sexual risk: a participant- and episode-level analysis among a cohort of men who have sex with men. American journal of epidemiology. 2004;159(10):1002–12. [DOI] [PubMed] [Google Scholar]
- 25.Deiss RG, Clark JL, Konda KA, Leon SR, Klausner JD, Caceres CF, et al. Problem drinking is associated with increased prevalence of sexual risk behaviors among men who have sex with men (MSM) in Lima, Peru. Drug Alcohol Depend. 2013;132(1–2):134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrera MC, Konda KA, Leon SR, Deiss R, Brown B, Calvo GM, et al. Impact of alcohol use on sexual behavior among men who have sex with men and transgender women in Lima, Peru. Drug Alcohol Depend. 2016;161:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vagenas P, Lama JR, Ludford KT, Gonzales P, Sanchez J, Altice FL. A systematic review of alcohol use and sexual risk-taking in Latin America. Revista panamericana de salud publica = Pan American journal of public health. 2013;34(4):267–74. [PMC free article] [PubMed] [Google Scholar]
- 28.Herrera MC, Konda KA, Leon SR, Brown B, Calvo GM, Salvatierra HJ, et al. Do Subjective Alcohol Screening Tools Correlate with Biomarkers Among High-Risk Transgender Women and Men Who Have Sex with Men in Lima, Peru? AIDS Behav. 2017;21(Suppl 2):253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludford KT, Vagenas P, Lama JR, Peinado J, Gonzales P, Leiva R, et al. Screening for drug and alcohol use disorders and their association with HIV-related sexual risk behaviors among men who have sex with men in Peru. PloS one. 2013;8(8):e69966-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vagenas P, Ludford K, Gonzales P. Being Unaware of Being HIV-Infected is Associated with Alcohol Use Disorders and High-Risk Sexual Behaviors Among Men Who have Sex with Men in Peru. AIDS Behav. 2014;18:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UNAIDS. UNAIDS Data 2017: Peru Joint United Nations Programme on HIV/AIDS 2017. [PubMed]
- 32.Vagenas P, Wickersham JA, Calabrese SK, Lama JR, Benites CM, Pun M, et al. Validation of the ‘drinking expectancy questionnaire for men who have sex with men’ in Peru. Drug and alcohol review. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullens AB, Young RM, Dunne MP, Norton G. The Drinking Expectancy Questionnaire for Men who have Sex with Men (DEQ-MSM): a measure of substance-related beliefs. Drug and alcohol review. 2011;30(4):372–80. [DOI] [PubMed] [Google Scholar]
- 34.Workowski KA, Bolan GA. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm Rep. 2015;64(3):1–138. Update: https://www.cdc.gov/std/tg2015/default.htm. [PMC free article] [PubMed] [Google Scholar]
- 35.Metcalf CA, Douglas JM Jr., Malotte CK, Cross H, Dillon BA, Paul SM, et al. Relative efficacy of prevention counseling with rapid and standard HIV testing: a randomized, controlled trial (RESPECT-2). Sex Transm Dis. 2005;32(2):130–8. [DOI] [PubMed] [Google Scholar]
- 36.Berg RC, Ross MW, Tikkanen R. The effectiveness of MI4MSM: how useful is motivational interviewing as an HIV risk prevention program for men who have sex with men? A systematic review. AIDS education and prevention : official publication of the International Society for AIDS Education. 2011;23(6):533–49. [DOI] [PubMed] [Google Scholar]
- 37.Leaity S, Sherr L, Wells H, Evans A, Miller R, Johnson M, et al. Repeat HIV testing: high-risk behaviour or risk reduction strategy? AIDS (London, England). 2000;14(5):547–52. [DOI] [PubMed] [Google Scholar]
- 38.Deiss RG, Segura ER, Clark JL, Konda KA, Leon SR, Caceres CF, et al. Testing for HIV and sexually transmitted infections among men who have sex with men (MSM) in Lima, Peru: opportunities for treatment and risk modification. APHA; San Francisco: 2012. [Google Scholar]
- 39.Eaton LA, Huedo-Medina TB, Kalichman SC, Pellowski JA, Sagherian MJ, Warren M, et al. Meta-analysis of single-session behavioral interventions to prevent sexually transmitted infections: implications for bundling prevention packages. Am J Public Health. 2012;102(11):e34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin JS, Whitlock E, O’Connor E, Bauer V. Behavioral counseling to prevent sexually transmitted infections: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(7):497–508, w96–9. [DOI] [PubMed] [Google Scholar]
- 41.Wells BE, Starks TJ, Parsons JT, Golub S. Conflict and expectancies interact to predict sexual behavior under the influence among gay and bisexual men. Journal of health psychology. 2014;19(7):821–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eaton LA, Kalichman SC, Kalichman MO, Driffin DD, Baldwin R, Zohren L, et al. Randomised controlled trial of a sexual risk reduction intervention for STI prevention among men who have sex with men in the USA. Sex Transm Infect. 2018;94(1):40–5. [DOI] [PubMed] [Google Scholar]