Abstract
Phasic dopamine activity is evoked by reliable predictors of food reward and plays a role in cue-triggered, goal-directed behavior. While this important signal is modulated by physiological state (e.g. hunger, satiety), the mechanisms by which physiological state is integrated by dopamine neurons is only beginning to be elucidated. Activation of central receptors for glucagon-like peptide-1 (GLP-1R) via long-acting agonists (e.g., Exendin-4) suppresses food intake and food-directed motivated behavior, in part, through action in regions with dopamine cell bodies, terminals, and/or neural populations that directly target the mesolimbic dopamine system. However, the effects of GLP-1R activation on cue-evoked, phasic dopamine signaling remain unknown. Here, in vivo fiber photometry was used to capture real-time signaling dynamics selectively from dopamine neurons in the ventral tegmental area of male and female transgenic (tyrosine hydroxylase-Cre; TH:Cre+) rats trained to associate an audio cue with the brief availability of a sucrose solution. Cue presentation evoked a brief spike in dopamine activity. Administration of Exendin-4 (Ex4; 0, 0.05, 0.1μg) to the lateral ventricle both dose-dependently suppressed sucrose-directed behaviors and the magnitude of cue-evoked dopamine activity. Moreover, the amplitude of cue evoked dopamine activity was significantly correlated with subsequent sucrose-directed behaviors. While female rats exhibited overall reduced dopamine responses to the sucrose-paired cue relative to males, there was no significant interaction with Ex4. Together, these findings support a role for central GLP-1Rs in modulating a form of dopamine signaling that influences approach behavior and provide a potential mechanism whereby GLP-1 suppresses food-directed behaviors.
Keywords: ventral tegmental area, dopamine, satiety, reward, motivation, fiber photometry
1. Introduction
Cues that are predictive of food hold powerful command over food-directed behavior, triggering craving and overeating in humans [1], incentive motivation [2], and increased appetitive behavior and overeating in rodents [3]. While the neurocircuitry underlying cue-driven feeding is complex and distributed, there is substantial evidence that the mesolimbic dopamine system plays a critical role. Reliable predictors of food reward evoke brief (e.g. 1–2s, phasic) spikes in dopamine cell body activity [4–6] and dopamine release in the nucleus accumbens [7–9]. Likewise, modulation of mesolimbic dopamine influences food cue-evoked behavior [10, 11]. While considerable debate remains ([12], for example), recent work demonstrates that phasic responses of dopamine neurons and phasic dopamine release in the nucleus accumbens (NAc) are reinforcing [13, 14] and strongly promote goal-directed behaviors triggered by reward predictive cues [4, 15–18].
It is fundamentally advantageous for both humans and animals to utilize learned associations between external environmental cues and reinforcement in the service of obtaining substances required for homeostatic balance (e.g., food and water). It is also advantageous for these goal-directed behaviors to be regulated by changes in homeostatic states (e.g. hunger and satiety; see [19–22] for review). Particularly in the context of feeding behaviors, deviations from homeostasis and the signals that relate them have potent modulatory effects on both the expression of goal-directed behaviors and on dopamine signaling in response to both primary food reward and cues associated with food reward [8, 23–25].
Glucagon-like peptide-1 (GLP-1) is a neuropeptide and hormone derived from the nucleus of the solitary tract (NTS) and distal intestines, respectively. Peripheral and central activation of GLP-1 receptors (GLP-1R) strongly reduces food intake and body weight (For review see [26]), and GLP-1 analogs have been developed and approved for the treatment of Type II diabetes and obesity. GLP-1Rs act throughout the brain [27, 28], including neural substrates within the mesolimbic pathway, to not only reduce food intake and body weight, but also to suppress goal-directed behaviors for food reinforcement [29–35]. Thus, among the many gut- and brain-derived signals that relay hunger/satiety states, central GLP-1R signaling and its influence on the mesolimbic system provides one mechanism through which satiety factors might suppress goal-directed behaviors. While ongoing investigations have identified site-specific GLP-1R-mediated actions on goal-directed behaviors [29–40] as well as putative roles of GLP-1Rs in modulating phasic dopamine signaling [41–43], it remains unknown whether central GLP-1Rs modulate food cue-evoked phasic dopamine signaling. This is a critical gap in the literature considering the importance of phasic dopamine signaling for cue-evoked approach behavior and reinforcement.
Here, we utilize in vivo fiber photometry in transgenic rats to measure calcium (Ca2+) transients from VTA dopamine neurons in real-time. Food restricted male and female rats were trained to associate a 1s audio cue with brief access to a sucrose solution. Following training, rats received a central infusion of the GLP-1 receptor agonist Exendin-4 (Ex4) just prior to test sessions. Rats were trained under food restriction to increase motivation to respond to sucrose and to mitigate central effects of endogenous GLP-1. Ca2+ transients occurred spontaneously but were also time-locked to key features of the behavioral paradigm. Regression analyses determined the interactions between measures of goal-directed behavior, sex, and event-related VTA phasic dopamine neuron activity. We found selective modulation of cue-evoked dopamine responses by central GLP-1R activation which was correlated with subsequent goal-directed behaviors.
2. Materials and methods
2.1. Subjects
Male (n = 10) and female (n = 12; randomly cycling) Long Evans rats expressing Cre recombinase under the control of the tyrosine hydroxylase promoter [TH:Cre+; [44]; Rat Research Resource Center, RRRC#: 659] were individually housed after weaning within a temperature and humidity controlled room and on a 12:12hr light:dark schedule (lights on 0700hr). Rats were maintained on ad libitum food and water unless otherwise noted, Four rats (n = 2 males and n = 2 randomly cycling females) were used to determine the penetrance and specificity of virally delivered constructs. Eight (n = 3 males and n = 5 randomly cycling females) were used to determine the relationship between Ca2+ transients and dopamine neuron excitability. Ten (n = 5 males and n = 5 randomly cycling females) were used to determine the effects of Ex4 on sucrose-directed behavior and dopamine transient activity. An additional 8 rats were initially and identically used but ultimately excluded due to criteria detailed in section 2.7. Rats used in behavioral paradigms weighed >250g and were moderately food restricted with 18g of food per day throughout the duration of their training and experiments. This modest amount of food restriction permitted gradual weight gain throughout training and testing. Animal care and use was in accordance with the National Institutes for Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago.
2.2. Behavior
All training and experimental sessions took place during the light phase in standard operant chambers (ENV-009A-CT, Med Associates Inc.). Rats were trained to expect availability of a retractable sipper containing a 0.3M sucrose solution 1s after the onset of a tone (cue; 4.5kHz, 1s duration). Licks at the sipper were timestamped using a contact lickometer and controller (ENV-252M; ENV-250, Med Associates Inc.). A trial consisted of the 1s cue and 20s sipper availability followed by a randomly selected, variable inter-trial interval (32–48s). Daily sessions consisted of 30 trials for 10 consecutive days, after which, surgery was performed.
2.3. Surgery
Male and female rats were anesthetized with ketamine hydrochloride (100mg/kg, i.p.) and xylazine hydrochloride (10mg/kg, i.p.) for stereotaxic surgery. First, a Cre-dependent virus containing the construct for a genetically encoded Ca2+ indicator (AAV1.Syn.Flex.GCaMP6f.WPRE.SV40, University of Pennsylvania Vector Core) was unilaterally administered to the ventral tegmental area (VTA; 1μL of 0.5e13 GC/ml: AP −5.4, ML −0.7, DV −8.15, mm relative to bregma) using a rate of 0.1μL/min and a 5min post infusion period to allow for diffusion before the injector was removed. Then, an optic fiber (flat 400μm core, 0.48NA, Doric Lenses Inc.) was implanted in the VTA just above the injection site (AP −5.4, ML −0.7, DV −8.00, mm relative to bregma). Finally, an infusion cannula (26Ga Cannula, PlasticsOne) was implanted above the lateral ventricle (LV; AP −0.9, ML −1.8, DV: −2.6, mm relative to bregma). All animals received post-operative analgesia (0.1 mL of 5 mg/ml meloxicam, s.c.) and were housed in their home cage for two weeks to allow sufficient time for recovery and construct expression. During this time, rats had ad libitum access to food. After two weeks, food restriction, for rats trained in the behavioral paradigm, resumed.
2.4. Fiber Photometry
LEDs (465, 405nm, Doric Lenses) were used to excite GCaMP6f in order to measure Ca2+ activity. As Ca2+ binds GCaMP6f, the conformation of the GFP moiety changes to increase fluorescent efficiency and the fluorescence due to 465nm light increases, but the fluorescence due to 405nm (the isosbestic point) light remains unchanged [45]. Intensity of the 465nm and 405nm light were sinusoidally modulated at 211Hz and 531Hz, respectively, for all recording sessions [46], then were coupled to a filter cube (FMC4 contains excitation filters at 405nm, 460–490nm and emission filters at 500–550nm, Doric Lenses) and converged into an optical fiber patch cord, which was mated to the fiber optic implant. GCaMP6f fluorescence was collected by the same fiber and focused onto a photoreceiver (Visible Femtowatt Photoreceiver Model 2151, Newport). A lock-in amplifier and data acquisition system (RZ5P; Tucker Davis Technologies), was used to control the LEDs and independently demodulate the fluorescence brightness due to 465nm (Ca2+ dependent) and 405nm (Ca2+ independent) excitation (recorded as mV arriving from the photoreceiver). Behavioral events (e.g. cue, licks) were timestamped and sent as digital inputs to the same data acquisition system and recorded in software (Synapse Suite, Tucker Davis Technologies). To calculate fluorescence due specifically to fluctuations in Ca2+, corrected for bleaching and movement artifacts, a subtraction of the 465nm signal by the 405nm signal in the frequency domain was made and then inverted to recover a ratiometric time domain Ca2+ signal (ΔF/F). The subtracted signal was smoothed using a custom fifth order bandpass butterworth filter (Figure 2D; cutoff frequencies: 0.05Hz, 2.25Hz).
Figure 2.
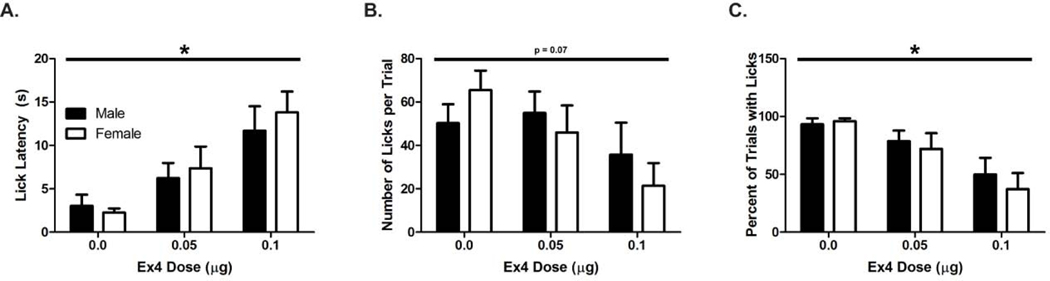
Ex4 dose-dependently suppresses indices of sucrose-directed behavior (n=5 male and n=5 female rats). (A) Latency to begin licking increases with Ex4. (B) Number of licks per trial trend towards a decrease with increasing doses of Ex4. (C) Percent of trials with licks decreases with increasing doses of Ex4. All effects were independent of sex. Data are mean ± SEM, *: p < 0.01 and p = 0.07 represent the main effect of Ex4 dose.
2.5. Signal normalization
For comparability of task-related responses across recording sessions, the smoothed fourier subtracted Ca2+ specific signal of each session was normalized by the mean transient Ca2+ amplitude of that session. A transient was defined as a point that exceeds 3 standard deviations of the overall signal above the previous point. The normalized signal was then aligned to cue onset in order to quantify the activity of dopamine neurons. All data processing was performed using custom MATLAB scripts (which are available upon request to corresponding author).
2.6. Pharmacology
To determine the relationship between Ca2+ transients and dopamine neuron activity, a cohort of 8 (n = 3 males and n = 5 randomly cycling females) rats were prepared for photometry. Rats were injected, on 4 different days, 10min prior to a recording sessions (no behavioral paradigm; 20min of recording). Injections were of either the D2 receptor antagonist raclopride (Cat. No. R121; 2mg/kg i.p) or the D2 receptor agonist quinpirole (Cat. No. Q102; 20μg/kg s.c.; D2 receptor agonist) or their vehicle (physiological saline). Drugs were acquired from Sigma-Aldrich, Inc. Injection order was counterbalanced across rats and each drug session had an accompanying saline control session. During test sessions, the frequency of spontaneous transients was measured. To determine the effects of GLP-1R activation on behavior and dopamine transient activity, Ex4 was acquired from Bachem (Cat. No. 4044219), was dissolved (0, 0.05, 0.1μg) in artificial cerebrospinal fluid (Cat. No. 3525, Tocris) and administered into the lateral ventricle in a 1μL volume (30Ga injector protruding 2mm past the guide, Plastics One Inc.) 45min prior to a behavioral test session using a counterbalanced, within-subjects design. The 0.1μg dose was chosen based on previous literature demonstrating robust suppression of food intake and food motivated behaviors [47]. Two days of no testing separated test sessions for all experiments.
2.7. Immunohistochemistry and verification of recording sites
Following completion of experiments, rats were deeply anesthetized with sodium pentobarbital (100mg/kg) and transcardially perfused with 0.01M PBS followed by 10% buffered formalin solution (HT501320, Sigma Aldrich, Inc). Brains were removed and stored in formalin for 24hr and then transferred to 20% sucrose in 0.01M PBS. All brains were sectioned at 40μm on a freezing stage microtome (SM2010R, Leica Biosystems). VTA sections were collected and processed to label for GFP (as an indicator of GCaMP6f expression) and tyrosine hydroxylase (TH) via immunohistochemistry. Antibody incubations were done at 4°C (washes and other steps at room temperature). Tissues were permeabilized in 0.3% Triton-X 100 for 30min and were blocked in 2% normal donkey serum for 10min. Sections were incubated in rabbit anti-TH (AB152, Sigma Aldrich) and chicken anti-GFP (AB13907, Abcam) antibodies overnight (~18hr). Primary antibodies were diluted in the following solution: KPBS containing 2% normal donkey serum, followed by KPBS washes (8 changes, 10 min each). Secondary antibody (Cy3 conjugated donkey anti-rabbit and AF488 conjugated donkey anti-chicken; Jackson Immunoresearch) incubations were performed overnight. Sections were then mounted onto glass slides, air dried, and coverslipped with 50% glycerol in KPBS mountant. Only data from subjects with GCaMP6f expression and VTA fiber placement were included in statistical analyses. Rats (n = 8) were excluded because of missed placement of the optic fiber in regions other than the VTA (n = 2) or because of poor tissue quality and the inability to confirm construct expression and fiber placement (n = 6). Quantification of the specificity of Cre-dependent GCaMP6f expression were performed in a separate cohort of TH:Cre+ rats (n = 2 male and n = 2 female rats).
2.8. Statistical analyses
The specificity of the GCaMP6f expression was quantified using descriptive statistics in the form of percent colocalization with TH. Influence of D2 receptor pharmacology on dopamine neural activity (frequency of Ca2+ transients) were analyzed using paired t-tests against each drug’s vehicle-paired session. Behavior was analyzed using two-way repeated measure analysis of variance (ANOVA) with Ex4 dose as a within-subjects variable and sex as a between-subjects variable. The magnitude of all transients and cue-evoked transients (mean of signal during 0s to 1s after cue onset) were analyzed via multiple linear regression with Ex4 dose as a within-subjects variable and sex as a between-subjects variable. The time course of the cue-aligned dopamine dynamics was analyzed by first averaging the signal into 1s bins and then using two-way repeated measures analysis of variance (ANOVA) for each sex, separately, with time (−5 to +10s relative to cue onset) and Ex4 dose as within-subjects variables. Sidak-corrected pairwise comparisons were used to compare 1s bins for each dose of Ex4 relative to vehicle. We determined the first and last quartile for latency to first lick and compared dopamine responses from these two quartiles when data were aligned to cue onset or first lick using unpaired t-tests. To isolate the relationship between cue evoked transients and behavior, multiple linear regressions of the behavioral measures (lick latency and number of licks per trial) were performed with the magnitude of cue-evoked transient as a within-subjects variable and sex as a between-subjects variable, while accounting for the variance due to Ex4 dose. All statistical analyses were computed using the coding environment R (https://www.r-project.org/) with an α level for significance at 0.05.
3. Results
3.1. Selective expression of calcium-dependent fluorescent construct captures dynamic fluctuations in dopamine signaling
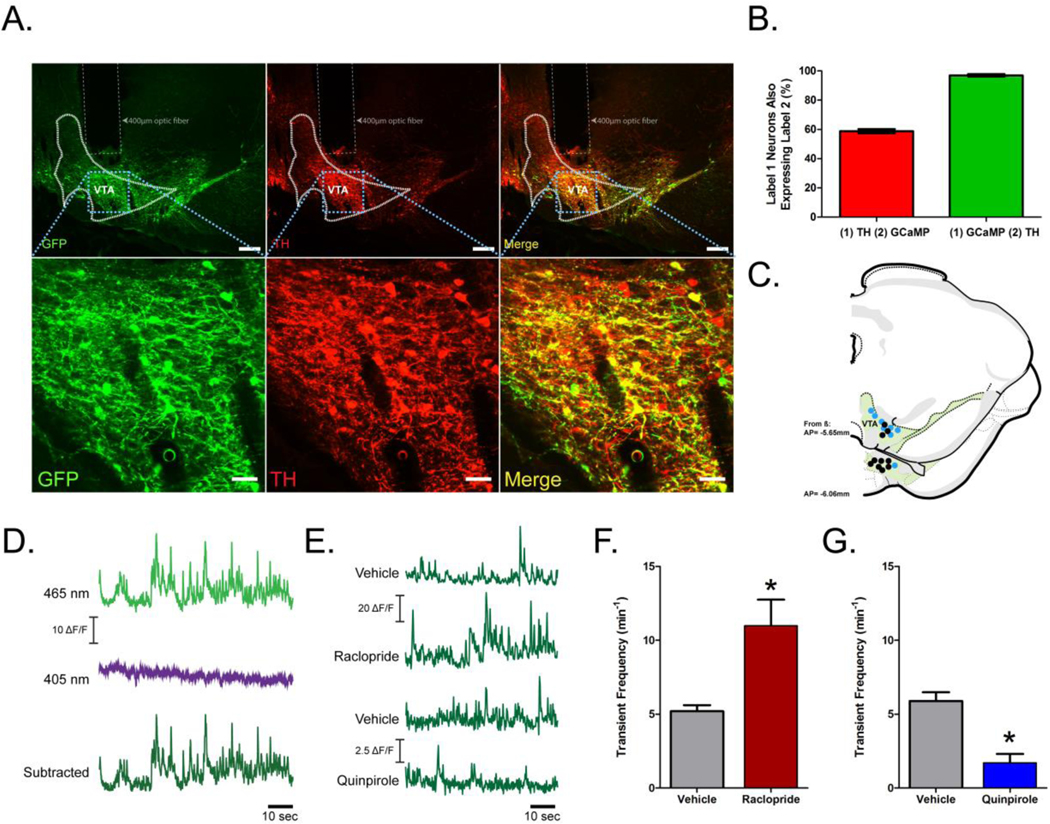
In order to selectively measure activity in VTA dopamine neurons, we delivered an adeno-associated virus containing a Cre-dependent GCaMP6f construct followed by implant of a fiber optic into the VTA of TH:Cre+ rats. To quantify the specificity of GCaMP6f expression, sections containing the VTA from a cohort of rats (n = 2 males and 2 randomly cycling females) were labeled with GFP and TH antibodies (Figure 1A). TH-positive [85.24 ± 17.7 mean ± SEM TH cells/section] and GFP-positive [52.1 ± 11.9 mean ± SEM GFP cells/section] cells were separately counted on each section and a merged image (Figure 1A, enlarged inset) to identify and quantify co-labeled cell bodies (Figure 1B). The viral construct had good penetrance [58.80 ± 1.40% of cells that labeled for TH were co-labeled for GCaMP6f] and selectivity [97.0 ± 0.90% of cells that labeled for GCaMP6f were co-labeled for TH; Figure 1B] similar to other reports [48]. Placement of fiber optic tips from all rats included in analyses were verified in the VTA and shown in Figure 1C.
Figure 1.
Selective expression of calcium-dependent fluorescent construct in dopamine neurons captures dynamic fluctuations in dopamine signaling. (A) Representative images (top panels; scale bar = 200μm) with a high magnification of the VTA (bottom panels; scale bar = 100 μm) with GFP label in green, TH label in red, and a merge. (B) Quantification of neurons expressing TH that also labeled for GFP [(1) TH (2) GCaMP] and neurons labeling for GFP that also expressed TH [(1) GCaMP (2) TH] (n=4 rats). (C) Location of optic fiber tips from all rats used in photometry recordings. Solid blue and black circles superimposed on coronal sections modified from the Swanson brain atlas [83] represent optic fiber placements for rats used in D2 receptor and Ex4 pharmacology experiments, respectively. (D) Representative traces from the VTA of real-time Ca2+ dependent signal (465nm), Ca2+ independent signal (405nm), and fourier subtracted, dopamine activity signal. (E) Representative traces of dopamine activity after vehicle, raclopride or quinpirole. Quantification of transient frequency after vehicle versus raclopride (F) and vehicle versus quinpirole (G); n=8 rats. Data in panels B, F & G are mean ± SEM, *: p < 0.01.
Fiber photometry was used to record activity from VTA dopamine neurons. To remove contributions to the fluorescent signal from photobleaching and movement artifacts in all fiber photometry experiments, the Ca2+ independent signal (in response to 405nm light; Figure 1D purple trace, for example) was subtracted from the Ca2+ dependent signal (in response to 465nm light; Figure 1D, light green, for example) in the frequency domain. The subtracted signal was returned to the time domain (Figure 1D, dark green, for example). To further validate fluctuations in the subtracted fluorescent signal as resulting from changes in dopamine activity, rats (n = 3 males and n = 5 randomly cycling females) were injected with dopamine D2 receptor drugs (representative traces shown in Figure 1E). D2 autoreceptor antagonism by raclopride increases burst firing in dopamine neurons [49] and phasic dopamine release in the nucleus accumbens [50]. D2 receptor agonism by quinpirole suppresses dopamine neuron firing rate and inhibits phasic dopamine release in the nucleus accumbens [51]. Thus, increasing or decreasing D2 autoreceptor activity, decreases or increases the firing rate of dopamine neurons [52], respectively. Administration of raclopride significantly increased transient frequency [5.2 ± 0.4 min−1 versus 11.0 ± 1.8 min−1 for vehicle versus raclopride, respectively; t(7) = 3.59, p < 0.01; Figure 1F] whereas administration of quinpirole significantly attenuated transient it [5.9 ± 0.6 min−1 versus 1.7 ± 0.6 min−1 for vehicle versus quinpirole, respectively; t(7) = 10.63, p < 0.01; Figure 1G]. Thus, fluorescent signals measured in the VTA were sensitive to drugs acting on the D2 receptor.
3.2. GLP-1R activation suppresses sucrose directed behavior
We administered Ex4 via the lateral ventricle in male (n = 5) and female (n = 5 randomly cycling) rats and recorded several behavioral measures (latency to first lick, number of licks per trial, percent of trials with at least one lick) in response to cue-predicted sucrose availability (Figure 2). As previous work indicated sex differences with respect to GLP-1R signaling and food reward [53], we used sex as a between-subjects variable in our analyses. For latency to first lick (Figure 2A), we found a main effect of Ex4 dose [F(2,16) = 12.63; p < 0.001], but no main effect of sex [F(1,8) = 0.25; p = 0.63] nor an interaction [F(2,16) = 0.26; p = 0.77]. There was no difference in lick latency between vehicle and 0.05μg Ex4 [p = 0.11]. However, the 0.1μg dose of Ex4 caused a significant increase in latency relative to vehicle [2.7 ± 1.4 versus 12.8 ± 1.4 s for vehicle versus 0.1 μg, respectively; t(16) = 5.0, p = 0.003]. For number of licks per trial (Figure 2B), there was a trend for an effect of Ex4 dose [F(2,16) = 3.11; p = 0.07], no main effect of sex [F(1,8) = 0.15; p = 0.71] and no interaction [F(2, 16) = 0.83; p = 0.45]. On some trials, rats failed to engage in any licking behavior following cued spout availability. We therefore examined the relationship between Ex4 dose and sex on the percentage of trials in which at least one lick was emitted (Figure 2C) and found a main effect of Ex4 dose [F(2,16) = 13.22; p < 0.001], no main effect of sex [F(1,8) = 0.32; p = 0.59] and no interaction [F(2,16) = 0.3; p = 0.75]. There was no difference in the percent of trials with licks emitted between vehicle and 0.05μg Ex4 [p = 0.14]. However the 0.1 μg dose significantly decreased the percent of trials with licks relative to vehicle than in the 0.1 μg Ex4 dose [94.7 ± 7.6 versus 43.7 ± 7.6 percent for vehicle and 0.1μg dose, respectively; t(16) = 5.09, p = 0.002]. Thus, Ex4 suppressed latency to begin licking and the percent of trials with a lick similarly in both male and female rats.
3.3. Central GLP-1R activation selectively suppresses cue evoked dopamine activity in the VTA
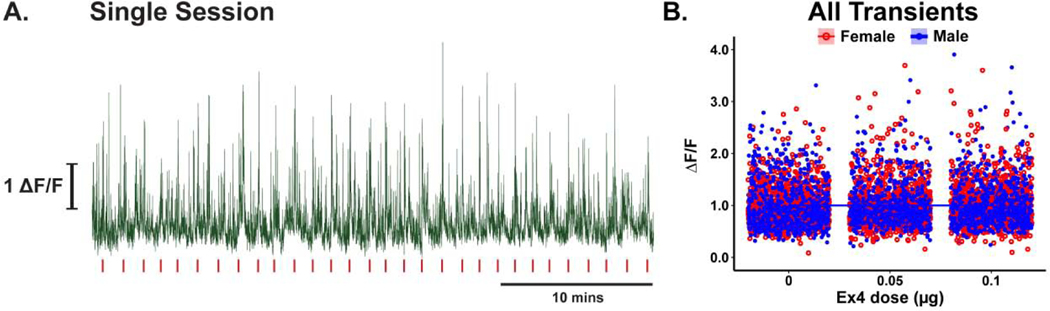
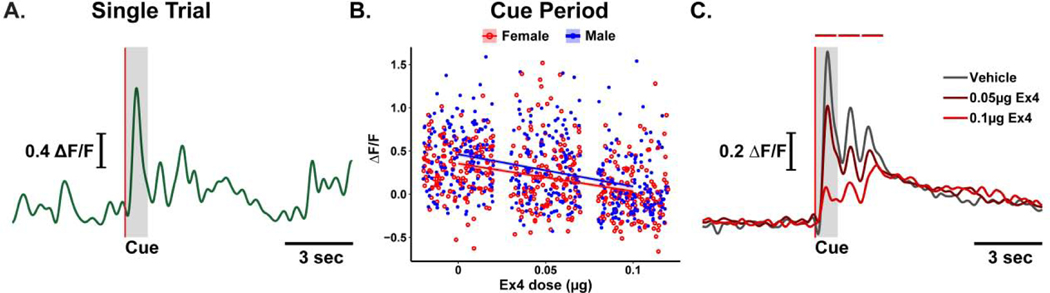
Dopamine transients occur throughout the recording session (Figure 3A, for example). To determine if Ex4 modulated dopamine transients, regardless of when they occur, we measured the magnitude of all transients recorded across behavioral sessions. Linear regression [r2 < 0.01, F(3,4992) = 0.04, p = 0.99, Figure 3B] indicated no relationship with increasing dose of Ex4 [p = 0. 77], no main effect of sex [p = 0.94], and no interaction between Ex4 dose and sex [p = 0.75]. Dopamine transients are specifically evoked by the sucrose-predictive cue (Figure 4A, for example). To determine if Ex4 specifically modulated cue-evoked phasic dopamine activity, we isolated transients that occurred during the 1s cue period predicting sucrose availability. Here, regression [r2 = 0.15, F(3,896) = 52.19, p < 0.001; Figure 4B] of cue evoked transients identified a significant decrease in transient magnitude by dose [β = −0.16, p < 0.001] and that cue-evoked transients, in general, were larger in males [β = 0.08, p < 0.001]. There was no significant interaction between Ex4 dose and sex [p = 0.36].
Figure 3.
Spontaneous VTA dopamine transients are not modulated by central Ex4. (A) Trace of dopamine activity across the entire behavioral session from a representative rat (vehicle session; red vertical ticks represent the time of cue administration). (B) Multiple linear regression of the magnitude of all dopamine transients (symbols) reveals no effect of Ex4 for either males (n=5 rats; blue symbols) or females (n=5 rats; red symbols).
Figure 4.
Central Ex4 suppresses cue evoked dopamine activity. (A) Dopamine activity aligned to cue onset (vertical red line) in a representative trial. (B) Multiple linear regression of each cue response (symbols) reveals a dose-dependent suppression of cue-evoked transient magnitude for both males (n=5; blue symbols) and females (n=5; red symbols). (C) Ex4 dose-dependently suppresses averaged dopamine activity aligned to cue onset (vertical red line) collapsed across sex (n=10 rats). Horizontal red bars above the trace represent times when dopamine activity following 0.1 μg Ex4 were significantly different from vehicle, p < 0.01. Lines represent group means.
Given the difference between cue-evoked transient magnitude between males and females was modest and there was no interaction with Ex4 dose, we combined data from male and female rats and plotted dopamine dynamics for the 5s before and 10s after cue onset (Figure 4C). For analysis, we averaged data across 1s bins. A two-way repeated measures ANOVA showed that there was a significant interaction between time and dose of Ex4 [F(28,252) = 4.49, p < 0.001]. Post-hoc analysis indicated that dopamine activity after the 0.1μg dose was significantly suppressed relative to vehicle during the first [0.39 ± 0.03 versus 0.04 ± 0.03 ΔF/F for vehicle and 0.1μg dose, respectively; t(9) = 7.74, p < 0.001], second [0.36 ± 0.03 versus 0.09 ± 0.03 ΔF/F for vehicle and 0.1μg dose, respectively; t(9) = 6.14, p < 0.001] and third bins [0.33 ± 0.03 versus 0.17 ± 0.03 ΔF/F for vehicle and 0.1μg dose, respectively; t(9) = 3.58, p = 0.013] after cue onset. There were no significant differences in dopamine activity between vehicle and 0.05μg dose.
3.4. Magnitude of cue-evoked dopamine is correlated with indices of behavior
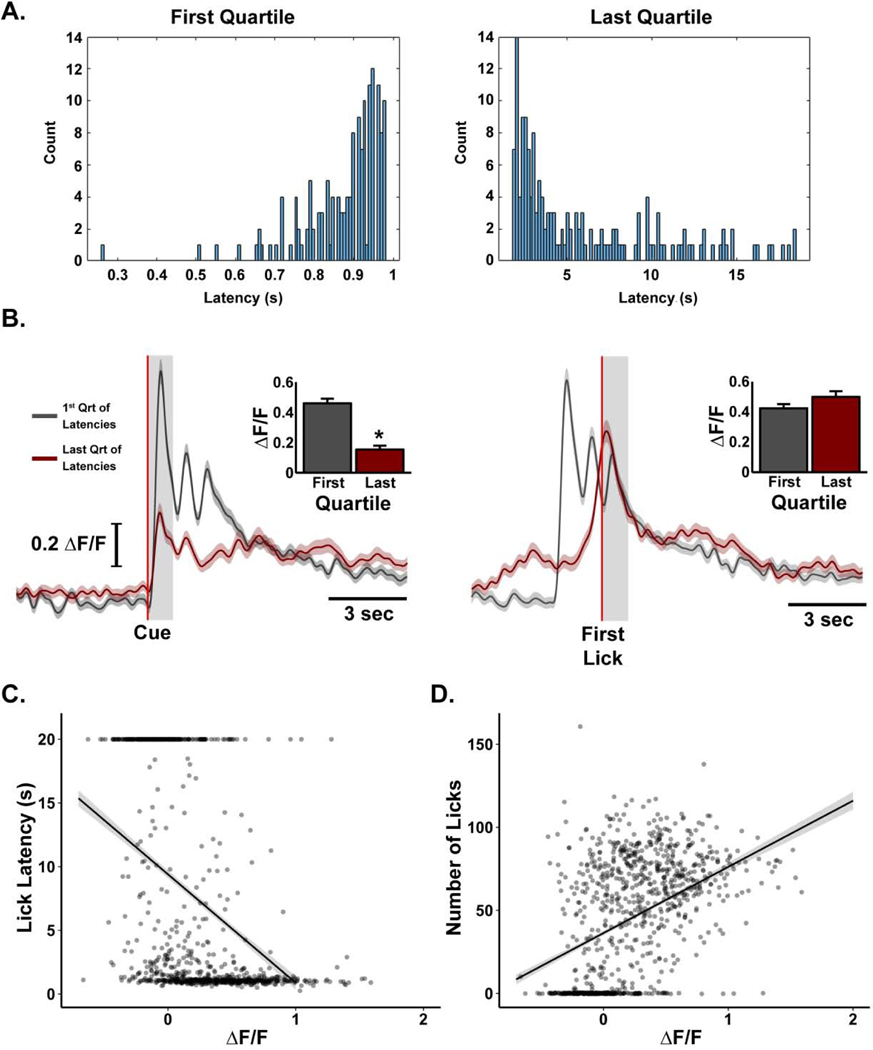
To determine if the magnitude of cue-evoked dopamine activity was related to the onset of licking, we compared cue-evoked dopamine activity on short (defined by the first quartile of all latencies; Figure 5A, left) versus long (defined by the last quartile of all latencies, Figure 5A, right) latency trials. For these subsets of trials, the dopamine activity was aligned to either cue (Figure 5B, left) or first lick (Figure 5B, right) onset. Dopamine activity during the 1s cue was significantly larger for short [0.5 ± 0.03 ΔF/F] versus long [0.2 ± 0.03 ΔF/F] latency trials [t(310) = 7.81, p < 0.001; Figure 5B left inset]. In contrast, dopamine activity in the 1s after first lick did not differ [0.4 ± 0.03 versus 0.5 ± 0.04 ΔF/F for short versus long latency trials, respectively; t(310) = −1.62, p = 0.11; Figure 4B right inset]. These results suggest that large increases in dopamine activity are correlated with rapid approach behavior and that suppression of cue-evoked responses may weaken subsequent goal-directed action. To further explore this possibility, we examined the relationship between the magnitude of cue-evoked dopamine transients and lick latency across all trials (Figure 5C) and found a significant negative correlation [r2 = 0.37, F(2,897) = 262.9, p < 0.001; β = −8.52, p < 0.001]. We found a positive relationship between the magnitude of cue-evoked dopamine activity and number of licks emitted per trial [r2 = 0.26, F(2,897) = 158, p < 0.001; β = 39.86, p < 0.001; Figure 5D]. Together these results suggest that the magnitude of cue evoked dopamine activity biases approach and licking behavior.
Figure 5.
Magnitude of cue evoked dopamine activity is correlated with indices of sucrose-directed behavior. (A) Distributions of short (n = 152 trials) versus long (n = 160 trials) first-lick latencies. (B) Cue-evoked (left) but not first-lick-evoked (right) average dopamine activity is significantly greater for short (grey) versus long (red) latency trials. Vertical red lines indicate the onset of the behavioral event. Insets are quantifications of the averaged dopamine activity in the first second after the behavioral event as denoted by the grey shaded areas on the traces. Data are mean ± SEM, *: p < 0.01. (C) Multiple linear regression shows a negative relationship between lick latency and cue-evoked dopamine activity and a (D) positive relationship between number of licks per trial and cue-evoked dopamine activity for every trial across all behavioral sessions (n=900 trials).
4. Discussion
Phasic activity of VTA dopamine neurons and NAc dopamine release are critical neurophysiological substrates underlying goal-directed behaviors, particularly behaviors that become invigorated based on cue-reward relationships. Importantly, physiological states like hunger and satiety have robust effects on phasic dopamine signaling (reviewed in [22]). In the current manuscript we focused on central GLP-1R signaling, a potent inhibitor of food intake and food-motivated behaviors (For review see [26]), to determine its modulatory impact on cue-driven phasic dopamine signaling. We captured phasic dopamine signaling in awake and behaving male and female rats by utilizing in vivo fiber photometry while they performed a Pavlovian conditioning task and revealed a selective, dose-dependent suppression of cue-evoked phasic dopamine signaling that correlated with reductions in approach and licking behavior.
The VTA is a heterogeneous structure with respect to both cell types (e.g. dopamine, GABA) and projection targets (e.g. prefrontal cortex; dorsomedial shell, lateral shell, core of the nucleus accumbens) [54–57]. With respect to cell types, we are confident that we are recording only from dopamine neurons given our use of transgenic TH-Cre rats [44, 48] and Cre-dependent expression of GCaMP (Figure 1B). With respect to projection targets, our optical fiber placements were within the paranigral/parabrachial pigmented region in the posterior half of rostrocaudal extent of the VTA ([58]; see Figure 1A and C for placements). Dopamine cell bodies in this VTA territory project to the dorsomedial nucleus accumbens shell subregion [58] – where phasic dopamine plays a role in encoding reward value [59]. However, the present work cannot resolve the responses of individual dopamine neurons based on their target. Future studies will combine photometry using fluorescent dopamine sensors (e.g. dLight1,2; [60]) and projection-site specific recording. It will be especially important to compare the effects of GLP1-R on modulation of phasic dopamine in different striatal regions. Indeed, while dopamine signaling in the nucleus accumbens relates to reward, substantia nigra [61] and dorsal striatal [62] dopamine signaling may be more closely linked with post-ingestive feedback and satiety.
We examined the effects of Ex4 on dopamine transients and, when analyzing all transients regardless of when they occur, found no effect. Assuming that cell body transient activity is well correlated with dopamine concentration fluctuations in terminal regions, these data are consistent with microdialysis studies where Ex4 alone had no impact on dopamine levels in the nucleus accumbens but instead suppressed drug-stimulated dopamine levels [63–65]. As shown previously [6, 8] and recapitulated here, phasic dopamine activity was evoked by a reliable predictor of food reward. When analysis was restricted to transient activity during the cue, Ex4 caused a dose-dependent suppression. The specificity of central Ex4 effects on cue-evoked activity suggests that GLP-1R activation modulates VTA dopamine neuron excitability in response to cue-related inputs. Indeed, GLP-1 producing neurons of the hindbrain nucleus tractus solitarius (NTS) directly project to the VTA [35] and GLP-1R is expressed in the VTA [27, 28] – suggesting the potential for direct effects of central Ex4 on VTA receptors. Indeed, GLP-1R activation in the VTA decreases chow intake [47], high-fat diet intake [35], and sugar pellet rewards earned in a progressive ratio operant test [47]. Recent work has suggested that the effects of VTA GLP-1R activation on behavior may depend on an anterior-posterior gradient. For example, effects of Ex4 on alcohol-induced locomotion were restricted to the posterior VTA [66, 67]. Moreover, Ex4 administration in the posterior VTA suppresses cocaine-seeking behaviors. [68, 69]. However, a systematic investigation of the impact of anterior-posterior VTA GLP-1R activation on cocaine-seeking has not been performed. While our optic fiber placements were in the posterior VTA, systematically varying the anterior-posterior placement may further elucidate a gradient for GLP-1R modulation of dopamine cell bodies.
The impact of GLP-1R on cue-evoked phasic dopamine signaling may also be mediated by action at cell bodies with afferent projections to the VTA [30]. A promising candidate is the lateral dorsal tegmental nucleus (LDTg) - which directly projects to the VTA [70, 71] and modulates VTA dopamine neuron activity [70, 72–74]. Most importantly, peripherally administered fluorescent Ex4 accumulates in the LDTg [29], is a site of robust GLP-1R expression [27] and Ex4 modulation of feeding behaviors [29]. Future site-specific GLP-1R manipulation will be needed to map circuit level mechanisms for Ex4 modulation of phasic dopamine signaling during goal-directed behaviors.
Cue-evoked VTA dopamine activity was strongly correlated with sucrose-directed behavior. The magnitude of dopamine activity was significantly higher in response to the cue when rats rapidly approached the spout relative to long latency trials. Importantly, even on long latency trials, there was a sharp increase in dopamine activity when rats finally approached the spout. This is consistent with prior work from our lab [8] and others [75, 76] where phasic dopamine release in the nucleus accumbens was correlated with the initiation of approach behavior. Here we extend this work to clearly implicate dopamine cell body activity in cue-evoked approach behavior as well (see also [77, 78]). Indeed, using regression analyses, we found further support for critical association between VTA phasic dopamine signaling and food-directed behavior (correlations with lick latency and number of licks).
It is important to note that the rats in the present experiments were food restricted. While this is a common practice in behavioral neuroscience to facilitate responding for food reinforcement, it has critical implications for both phasic dopamine activity and putative GLP-1R signaling. Hunger enhances phasic firing of VTA dopamine neurons and phasic dopamine responses to cues that predict food reward [23, 24, 79]. Moreover, GLP-1R blockade and chronic pre-pro-glucagon (GLP-1 precursor) knockdown is associated with hyperphagia [80], suggesting endogenous roles of GLP-1 in suppressing hunger. Thus, one interpretation of the findings that is consistent with previous literature [23, 24] is that in the hungry state, phasic dopamine activity exhibits heightened sensitivity to cues that predict food. Our data suggest that this sensitivity can be then attenuated by central GLP-1R signaling – similar to effects of leptin that have been reported [81]. Food deprivation also silences endogenous GLP-1 neural activity [82, 83]. Thus, a physiological role of GLP-1R activity in modulating cue-evoked phasic dopamine signaling remains a critical area for exploration. Besides loss-of-function experiments, other approaches that modulate GLP-1R signaling (i.e. modulations of meal size, gastric distension, etc. [82, 84, 85]) will provide valuable insight.
Central Ex4 administration has been linked with nausea, malaise, and interoceptive stress [86, 87], and highlights an important caveat in the data described here. Malaise and negative affect have been shown to suppress phasic dopamine signaling, an effect that can be mediated by central GLP-1R signaling [42]. Moreover, central GLP-1R signaling at select brain regions (e.g. the medial NTS [86, 88]) produces indices of nausea and malaise in rodents. Ventricular doses of Ex4 similar to those used in the current study are sufficient to induce reductions in food intake and body weight, but also increase measures of malaise [86]. Thus, it remains possible that Ex4 effects on phasic dopamine signaling could be secondary to malaise or negative affect. Likewise, systemic [89] or central [90] Ex4 reduce indices of general locomotor behavior. Site specific delivery of Ex4 at doses that are sufficient to reduce motivation but subthreshold for malaise or motor impairment [35] will be a critical area for future investigation. Still, the power of using a cue in the present study – and measuring the dopamine response to it – is that the dopamine response is evoked by a salient sensory stimulus that occurs regardless of the animal’s behavior. We additionally found that the magnitude of the dopamine response predicts subsequent behavior even after accounting for the variance in the dopamine response due to dose of Ex4. Thus, current findings provide crucial insight into the mechanisms through which interoceptive stress might be relayed to mesolimbic pathways and ultimately affect goal-directed behaviors.
Recent data has identified sex as a modifier of GLP-1R regulation of feeding behaviors [37, 38, 53, 91, 92]. For example, lateral ventricular infusion of Ex4 more potently suppressed responding for food reward under a progressive ratio but not consumption of food, in females [53]. Thus, we included sex as a biological variable in our analyses. We found that Ex4 delivered to the lateral ventricle suppressed cue-triggered approach (latency to first lick) as well as consumption of sucrose (number of licks per trial) to similar degrees in female and male rats. On the surface, this result is surprising given previous reports. However, sex differences in behavioral responses to Ex4 are dependent on site of action. For example, GLP-1R signaling in the lateral hypothalamic area (LHA) plays endogenous roles in feeding behaviors, and acute blockade of LHA GLP-1Rs only has an effect in males and not in females during lever pressing tasks [37]. Similarly, GLP-1R activation in the supramammillary nucleus has more potent effects on food motivated behaviors in males compared to females [38]. In contrast, GLP-1R activation in the VTA had more potent effects on motivated behaviors in females compared to males [38]. Estrous cycle stage also plays an important role in modulating goal-directed behaviors. Intra-LHA administration of Ex4 has more potent effects on food-motivated behaviors in the estrus phase, whereas there is no effect in the metestrus/diestrus stage [37], highlighting the importance of sex hormones and their interactions with food-motivated behaviors. Indeed, central estrogen blockade attenuates the anorexigenic effects of Ex4 in both female and male rats [53]. Importantly, the studies here were performed in males and randomly cycling females with Ex4 delivery to the lateral ventricle. Thus, future studies examining the interactions between estrus phase, sex hormones, phasic dopamine signaling, and selective sites of central GLP-1R signaling are warranted.
There are ample reports of sex differences with respect to dopamine signaling generally ([93], for review) and phasic dopamine signaling in particular [94–97]. We and others have previously reported enhanced electrically-evoked dopamine release in females relative to males [94, 98]. Yet, in the context of cue-evoked VTA dopamine activity, here we observed a lower magnitude dopamine response in females. Resolving these conflicting findings can be challenging given that comparisons are being made between recordings from cell bodies in the VTA with release in terminal regions, like the nucleus accumbens and dorsal striatum. While cell body and release dynamics relative to food-predictive cues are very similar, mechanisms at dopamine terminals capable of modulating the magnitude of dopamine release independent of cell body effects have been identified [75, 99, 100]. Concluding whether females have greater or smaller dopamine responses relative to males is also dependent on comparing results from studies where dopamine signaling is electrically-evoked, when release probability is high, versus dopamine signaling that is cue-evoked– which is presumably being driven by a subset of inputs to dopamine cell bodies [101]. Thus, it is important to highlight that sex as a biological variable in the context of the magnitude of cue-driven phasic dopamine signaling remains understudied.
5. Conclusions
Physiological states, such as hunger and satiety, have potent effects on goal-directed behaviors. The clinically relevant long-acting GLP-1 analog Ex4 suppressed the phasic response of VTA dopamine neurons to sucrose-predictive cues. The suppressed response, in turn, was correlated with decreased sucrose-directed behaviors. Central GLP-1R activation thus holds potential for tuning a form of dopamine signaling that biases approach in response to environmental cues that are associated with food reinforcement.
Highlights.
Central glucagon-like peptide-1 (GLP-1) receptor agonism by Exendin-4 suppresses sucrose-directed appetitive behaviors
Phasic dopamine activity evoked by a cue predictive of sucrose is suppressed by central GLP-1 receptor activation.
The degree of approach toward sucrose reward is correlated with cue-evoked phasic dopamine activity.
Acknowledgements
We thank Dr. Alexander P. Demos and Dr. Jamie D. Roitman for their help with MATLAB coding and statistical analysis. This work was supported by NIH grants R01 DK105155 (M.R.H, S.E.K, M.F.R.) and R01 DA025634 (M.F.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Boswell RG, Kober H. Food cue reactivity and craving predict eating and weight gain: a meta-analytic review. Obes Rev. 2016,17:159–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Berridge KC Evolving Concepts of Emotion and Motivation. Front Psychol. 2018,9:1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Petrovich GD Forebrain networks and the control of feeding by environmental learned cues. Physiol Behav. 2013,121:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Coddington LT, Dudman JT The timing of action determines reward prediction signals in identified midbrain dopamine neurons. Nat Neurosci. 2018,21:1563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lak A, Stauffer WR, Schultz W. Dopamine prediction error responses integrate subjective valuefrom different reward dimensions. Proc Natl Acad Sci U S A. 2014,111:2343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schultz W, Dayan P, Montague PR A neural substrate of prediction and reward. Science. 1997,275:1593–9. [DOI] [PubMed] [Google Scholar]
- [7].Day JJ, Roitman MF, Wightman RM, Carelli RM Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007,10:1020–8. [DOI] [PubMed] [Google Scholar]
- [8].Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004,24:1265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, et al. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008,321:1690–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wyvell CL, Berridge KC Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000,20:8122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Halbout B, Marshall AT, Azimi A, Liljeholm M, Mahler SV, Wassum KM, et al. Mesolimbic dopamine projections mediate cue-motivated reward seeking but not reward retrieval in rats. Elife. 2019,8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Berke JD What does dopamine mean? Nat Neurosci. 2018,21:787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009,324:1080–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013,16:966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Medic N, Ziauddeen H, Vestergaard MD, Henning E, Schultz W, Farooqi IS, et al. Dopamine modulates the neural representation of subjective value of food in hungry subjects. J Neurosci. 2014,34:16856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, et al. Mesolimbic dopamine signals the value of work. Nat Neurosci. 2016,19:117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fischbach-Weiss S, Reese RM, Janak PH Inhibiting Mesolimbic Dopamine Neurons Reduces the Initiation and Maintenance of Instrumental Responding. Neuroscience. 2018,372:306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].du Hoffmann J, Nicola SM Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J Neurosci. 2014,34:14349–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ferrario CR, Labouebe G, Liu S, Nieh EH, Routh VH, Xu S, et al. Homeostasis Meets Motivation in the Battle to Control Food Intake. J Neurosci. 2016,36:11469–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rossi MA, Stuber GD Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab. 2018,27:42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu CM, Kanoski SE Homeostatic and non-homeostatic controls of feeding behavior: Distinct vs. common neural systems. Physiol Behav. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hsu TM, McCutcheon JE, Roitman MF Parallels and Overlap: The Integration of Homeostatic Signals by Mesolimbic Dopamine Neurons. Front Psychiatry. 2018,9:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cone JJ, Roitman JD, Roitman MF Ghrelin regulates phasic dopamine and nucleus accumbens signaling evoked by food-predictive stimuli. J Neurochem. 2015,133:844–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cone JJ, McCutcheon JE, Roitman MF Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 2014,34:4905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wilson C, Nomikos GG, Collu M, Fibiger HC Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995,15:5169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kanoski SE, Hayes MR, Skibicka KP GLP-1 and weight loss: unraveling the diverse neural circuitry. Am J Physiol Regul Integr Comp Physiol. 2016,310:R885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999,403:261–80. [DOI] [PubMed] [Google Scholar]
- [28].Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab. 2015,4:718–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Reiner DJ, Leon RM, McGrath LE, Koch-Laskowski K, Hahn JD, Kanoski SE, et al. Glucagon-Like Peptide-1 Receptor Signaling in the Lateral Dorsal Tegmental Nucleus Regulates Energy Balance. Neuropsychopharmacology. 2018,43:627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, et al. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab. 2013,305:E1367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mietlicki-Baase EG, Ortinski PI, Reiner DJ, Sinon CG, McCutcheon JE, Pierce RC, et al. Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J Neurosci. 2014,34:6985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Richard JE, Anderberg RH, Goteson A, Gribble FM, Reimann F, Skibicka KP Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PloS one. 2015,10:e0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dossat AM, Lilly N, Kay K, Williams DL Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011,31:14453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dossat AM, Diaz R, Gallo L, Panagos A, Kay K, Williams DL Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am J Physiol Endocrinol Metab. 2013,304:E1314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Alhadeff AL, Rupprecht LE, Hayes MR GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012,153:647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology. 2015,40:327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lopez-Ferreras L, Richard JE, Noble EE, Eerola K, Anderberg RH, Olandersson K, et al. Lateral hypothalamic GLP-1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Mol Psychiatry. 2018,23:1157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lopez-Ferreras L, Eerola K, Mishra D, Shevchouk OT, Richard JE, Nilsson FH, et al. GLP-1 modulates the supramammillary nucleus-lateral hypothalamic neurocircuit to control ingestive and motivated behavior in a sex divergent manner. Mol Metab. 2019,20:178–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD, et al. Endogenous Glucagon-like Peptide-1 Receptor Signaling in the Nucleus Tractus Solitarius is Required for Food Intake Control. Neuropsychopharmacology. 2017,42:1471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hsu TM, Noble EE, Liu CM, Cortella AM, Konanur VR, Suarez AN, et al. A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Mol Psychiatry. 2018,23:1555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang XF, Liu JJ, Xia J, Liu J, Mirabella V, Pang ZP Endogenous Glucagon-like Peptide-1 Suppresses High-Fat Food Intake by Reducing Synaptic Drive onto Mesolimbic Dopamine Neurons. Cell Rep. 2015,12:726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fortin SM, Chartoff EH, Roitman MF The Aversive Agent Lithium Chloride Suppresses Phasic Dopamine Release Through Central GLP-1 Receptors. Neuropsychopharmacology. 2016,41:906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fortin SM, Roitman MF Central GLP-1 receptor activation modulates cocaine-evoked phasic dopamine signaling in the nucleus accumbens core. Physiol Behav. 2017,176:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011,72:721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lutcke H, Murayama M, Hahn T, Margolis DJ, Astori S, Zum Alten Borgloh SM, et al. Optical recording of neuronal activity with a genetically-encoded calcium indicator in anesthetized and freely moving mice. Front Neural Circuits. 2010,4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, Zalocusky KA, et al. Intact-Brain Analyses Reveal Distinct Information Carried by SNc Dopamine Subcircuits. Cell. 2015,162:635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012,32:4812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Decot HK, Namboodiri VM, Gao W, McHenry JA, Jennings JH, Lee SH, et al. Coordination of Brain-Wide Activity Dynamics by Dopaminergic Neurons. Neuropsychopharmacology. 2017,42:615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Andersson JL, Nomikos GG, Marcus M, Hertel P, Mathe JM, Svensson TH Ritanserin potentiates the stimulatory effects of raclopride on neuronal activity and dopamine release selectivity in the mesolimbic dopaminergic system. Naunyn Schmiedebergs Arch Pharmacol. 1995,352:374–85. [DOI] [PubMed] [Google Scholar]
- [50].Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008,28:8821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Anzalone A, Lizardi-Ortiz JE, Ramos M, De Mei C, Hopf FW, Iaccarino C, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012,32:9023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gentet LJ, Williams SR Dopamine gates action potential backpropagation in midbrain dopaminergic neurons. J Neurosci. 2007,27:1892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Richard JE, Anderberg RH, Lopez-Ferreras L, Olandersson K, Skibicka KP Sex and estrogens alter the action of glucagon-like peptide-1 on reward. Biol Sex Differ. 2016,7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].de Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim CK, et al. A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron. 2019,101:133–51 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Morales M, Margolis EB Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017,18:73–85. [DOI] [PubMed] [Google Scholar]
- [56].Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012,491:212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lammel S, Steinberg EE, Foldy C, Wall NR, Beier K, Luo L, et al. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron. 2015,85:429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007,56:27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sackett DA, Saddoris MP, Carelli RM Nucleus Accumbens Shell Dopamine Preferentially Tracks Information Related to Outcome Value of Reward. eNeuro. 2017,4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong WH, et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science. 2018,360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, et al. A Neural Circuit for Gut-Induced Reward. Cell. 2018,175:665–78 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tellez LA, Han W, Zhang X, Ferreira TL, Perez IO, Shammah-Lagnado SJ, et al. Separate circuitries encode the hedonic and nutritional values of sugar. Nat Neurosci. 2016,19:465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology. 2013,38:1259–70. [DOI] [PubMed] [Google Scholar]
- [64].Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS One. 2013,8:e77284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One. 2013,8:e69010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Jerlhag E. Alcohol-mediated behaviours and the gut-brain axis; with focus on glucagon-like peptide-1. Brain Res. 2019:146562. [DOI] [PubMed] [Google Scholar]
- [67].Vallof D, Kalafateli AL, Jerlhag E. Brain region specific glucagon-like peptide-1 receptors regulate alcohol-induced behaviors in rodents. Psychoneuroendocrinology. 2019,103:284–95. [DOI] [PubMed] [Google Scholar]
- [68].Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, et al. Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology. 2018,43:2000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, et al. Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology. 2016,41:1917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Cornwall J, Cooper JD, Phillipson OT Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990,25:271–84. [DOI] [PubMed] [Google Scholar]
- [71].Lodge DJ, Grace AA The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006,103:5167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Steidl S, Veverka K. Optogenetic excitation of LDTg axons in the VTA reinforces operant responding in rats. Brain Res. 2015,1614:86–93. [DOI] [PubMed] [Google Scholar]
- [73].Steidl S, Wang H, Ordonez M, Zhang S, Morales M. Optogenetic excitation in the ventral tegmental area of glutamatergic or cholinergic inputs from the laterodorsal tegmental area drives reward. Eur J Neurosci. 2017,45:559–71. [DOI] [PubMed] [Google Scholar]
- [74].Steidl S, O’Sullivan S, Pilat D, Bubula N, Brown J, Vezina P. Operant responding for optogenetic excitation of LDTg inputs to the VTA requires D1 and D2 dopamine receptor activation in the NAcc. Behav Brain Res. 2017,333:161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mohebi A, Pettibone JR, Hamid AA, Wong JT, Vinson LT, Patriarchi T, et al. Dissociabled opamine dynamics for learning and motivation. Nature. 2019,570:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wassum KM, Ostlund SB, Maidment NT Phasic mesolimbic dopamine signaling precedes and predicts performance of a self-initiated action sequence task. Biol Psychiatry. 2012,71:846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Engelhard B, Finkelstein J, Cox J, Fleming W, Jang HJ, Ornelas S, et al. Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons. Nature. 2019,570:509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].da Silva JA, Tecuapetla F, Paixao V, Costa RM Dopamine neuron activity before action initiation gates and invigorates future movements. Nature. 2018,554:244–8. [DOI] [PubMed] [Google Scholar]
- [79].Branch SY, Goertz RB, Sharpe AL, Pierce J, Roy S, Ko D, et al. Food restriction increases glutamate receptor-mediated burst firing of dopamine neurons. J Neurosci. 2013,33:13861–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011,31:3904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].van der Plasse G, van Zessen R, Luijendijk MC, Erkan H, Stuber GD, Ramakers GM, et al. Modulation of cue-induced firing of ventral tegmental area dopamine neurons by leptin and ghrelin. Int J Obes (Lond). 2015,39:1742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Maniscalco JW, Zheng H, Gordon PJ, Rinaman L. Negative Energy Balance Blocks Neural and Behavioral Responses to Acute Stress by “Silencing” Central Glucagon-Like Peptide 1 Signaling in Rats. J Neurosci. 2015,35:10701–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Maniscalco JW, Rinaman L. Overnight food deprivation markedly attenuates hindbrain noradrenergic, glucagon-like peptide-1, and hypothalamic neural responses to exogenous cholecystokinin in male rats. Physiol Behav. 2013,121:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kreisler AD, Rinaman L. Hindbrain glucagon-like peptide-1 neurons track intake volume and contribute to injection stress-induced hypophagia in meal-entrained rats. Am J Physiol Regul Integr Comp Physiol. 2016,310:R906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hayes MR, Bradley L, Grill HJ Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009,150:2654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology. 2012,62:1916–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kinzig KP, D’Alessio DA, Seeley RJ The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002,22:10470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].De Jonghe BC, Holland RA, Olivos DR, Rupprecht LE, Kanoski SE, Hayes MR Hindbrain GLP-1 receptor mediation of cisplatin-induced anorexia and nausea. Physiol Behav. 2016,153:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Erreger K, Davis AR, Poe AM, Greig NH, Stanwood GD, Galli A. Exendin-4 decreases amphetamine-induced locomotor activity. Physiol Behav. 2012,106:574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Anderberg RH, Richard JE, Hansson C, Nissbrandt H, Bergquist F, Skibicka KP GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology. 2016,65:54–66. [DOI] [PubMed] [Google Scholar]
- [91].Vogel H, Wolf S, Rabasa C, Rodriguez-Pacheco F, Babaei CS, Stober F, et al. GLP-1 and estrogen conjugate acts in the supramammillary nucleus to reduce food-reward and body weight. Neuropharmacology. 2016,110:396–406. [DOI] [PubMed] [Google Scholar]
- [92].Maske CB, Jackson CM, Terrill SJ, Eckel LA, Williams DL Estradiol modulates the anorexic response to central glucagon-like peptide 1. Horm Behav. 2017,93:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Becker JB, Chartoff E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology. 2019,44:166–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Conway SM, Puttick D, Russell S, Potter D, Roitman MF, Chartoff EH Females are less sensitive than males to the motivational- and dopamine-suppressing effects of kappa opioid receptor activation. Neuropharmacology. 2019,146:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yoest KE, Cummings JA, Becker JB Oestradiol influences on dopamine release from the nucleus accumbens shell: sex differences and the role of selective oestradiol receptor subtypes. Br J Pharmacol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Shams WM, Sanio C, Quinlan MG, Brake WG 17beta-Estradiol infusions into the dorsal striatum rapidly increase dorsal striatal dopamine release in vivo. Neuroscience. 2016,330:162–70. [DOI] [PubMed] [Google Scholar]
- [97].Cummings JA, Jagannathan L, Jackson LR, Becker JB Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend. 2014,135:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Walker QD, Rooney MB, Wightman RM, Kuhn CM Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000,95:1061–70. [DOI] [PubMed] [Google Scholar]
- [99].Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012,75:58–64. [DOI] [PubMed] [Google Scholar]
- [100].Mateo Y, Johnson KA, Covey DP, Atwood BK, Wang HL, Zhang S, et al. Endocannabinoid Actions on Cortical Terminals Orchestrate Local Modulation of Dopamine Release in the Nucleus Accumbens. Neuron. 2017,96:1112–26 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tian J, Huang R, Cohen JY, Osakada F, Kobak D, Machens CK, et al. Distributed and Mixed Information in Monosynaptic Inputs to Dopamine Neurons. Neuron. 2016,91:1374–89. [DOI] [PMC free article] [PubMed] [Google Scholar]