Keywords: IL-6, pancreatic acinar cells, proinflammatory cytokines, thiamin uptake, THTR-1, THTR-2
Abstract
Thiamin (vitamin B1) plays critical roles in normal metabolism and function of all mammalian cells. Pancreatic acinar cells (PACs) import thiamin from circulation via specific carrier-mediated uptake that involves thiamin transporter-1 and -2 (THTR-1 and -2; products of SLC19A2 and SLC19A3, respectively). Our aim in this study was to investigate the effect(s) of proinflammatory cytokines on thiamin uptake by PACs. We used human primary (h)PACs, PAC 266-6 cells, and mice in vivo as models in the investigations. First, we examined the level of expression of THTR-1 and -2 mRNA in pancreatic tissues of patients with chronic pancreatitis and observed severe reduction in their expression compared with normal control subjects. Exposing hPACs and PAC 266-6 to proinflammatory cytokines (hyper IL-6, TNF-α, and IL-1β) was found to lead to a significant inhibition in thiamin uptake. Focusing on hyper-IL-6 (which also inhibited thiamin uptake by primary mouse PACs), the inhibition in thiamin uptake was found to be associated with significant reduction in THTR-1 and -2 proteins and mRNA expression as well as in activity of the SLC19A2 and SLC19A3 promoters; it was also associated with reduction in level of expression of the transcription factor Sp1 (which is required for activity of these promoters). Finally, blocking the intracellular Stat3 signaling pathway was found to lead to a significant reversal in the inhibitory effect of hyper IL-6 on thiamin uptake by PAC 266-6. These results show that exposure of PACs to proinflammatory cytokines negatively impacts thiamin uptake via (at least in part) transcriptional mechanism(s).
NEW & NOTEWORTHY Findings of the current study demonstrate, for the first time, that exposure of pancreatic acinar cells to proinflammatory cytokines (including hyper IL-6) cause significant inhibition in vitamin B1 (thiamin; a micronutrient that is essential for normal cellular energy metabolism) and that this effect is mediated at the level of transcription of the thiamin transporter genes SLC19A2 and SLC19A3.
INTRODUCTION
Thiamin (vitamin B1) is an indispensable micronutrient for normal physiology/health of all mammalian cells due to its involvement (as a cofactor) in critical reactions including oxidative energy metabolism, ATP production, and in the maintenance of normal cellular redox state (1–3). Deficient/low intracellular levels of thiamin lead to impairment in energy metabolism, to oxidative stress, and to negative effects on the function/structure of mitochondria (2–5); these deleterious consequential effects weaken the cell and compromise its normal physiology and function. Global (systemic) thiamin deficiency leads to the development of clinical abnormalities that include neurological and cardiovascular disorders (3, 6). In contrast to the deleterious effects of cellular/systemic thiamin deficiency, optimizing the vitamin cellular/systemic homeostasis brings about positive metabolic/health benefits as seen in patients with sepsis and septic shock (7, 8).
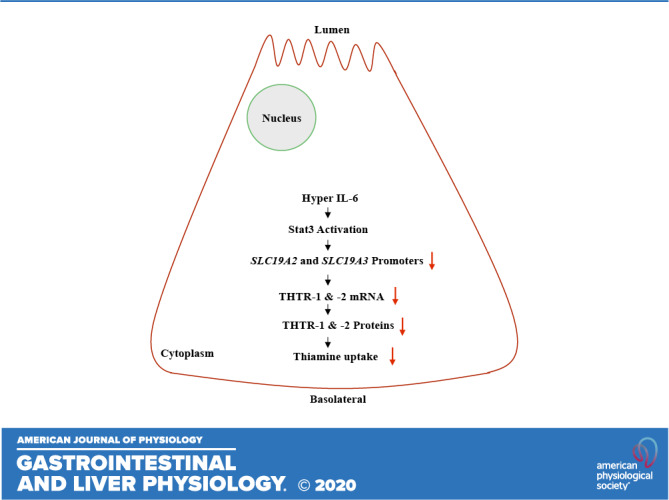
The pancreas, an organ with essential exocrine and endocrine functions, maintains a high level of thiamin and the vitamin is important for both its exocrine and endocrine functions (9–11). Cells of this organ, including its acinar cells, are among the most metabolically active cells in the body due to their high rate of protein synthesis and secretion. Like all other cells, pancreatic acinar cells (PACs) cannot synthesize thiamin endogenously and thus must obtain it from their surroundings (9–11). We have previously shown that thiamin uptake by PACs is via an efficient and specific carrier-mediated process that involves both thiamin transporter-1 and -2 (THTR-1 and -2; products of the SLC19A2 and SLC19A3 genes, respectively) (12, 13). We have also shown that exposure of these cells to certain external/environmental risk factors (e.g., alcohol, components of cigarette smoke, bacterial flagellin; 13–17) lead to a significant impairment in their ability to take in thiamin. Little is currently known about the effect of internal factors/conditions on the vitamin uptake by PACs. This include the effect of exposure of PACs to proinflammatory cytokines, levels of which are significantly induced in inflammatory conditions affecting the pancreas (as in acute and chronic pancreatitis; 18–20), as well during severe systemic inflammation (21–26). Addressing this issue is of pathophysiological relevance as these factors are known to exert profound effects on cell physiology including transport events at the cell membrane (27–32). Thus our objective in this study was to address this issue, and for that we employed three complementary models: primary human acinar cells (hPACs) obtained from organ donors, mouse-derived pancreatic acinar 266-6 cells (PAC 266-6), and mice in vivo. The results showed that exposing PACs to proinflammatory cytokines that are relevant to pancreatitis (e.g., hyper IL-6) leads to a significant inhibition in thiamin uptake. This inhibition was found to be associated with a marked reduction in level of expression of THTR-1 and -2 and appears to be mediated (at least in part) via transcriptional mechanism(s) involving the SLC19A2 and SLC19A3 genes.
MATERIALS AND METHODS
Materials
The following were obtained from commercial sources: mouse-derived pancreatic acinar 266-6 cell line from ATCC (Rockville, MD); [3H]thiamin (specific activity 12.8 Ci/mmol; radiochemical purity of 93.3%) from Moravek (Brea, CA); nitrocellulose filters (0.45 µm) from Millipore (Fisher Scientific); human and mouse hyper IL-6 from R&D Biosystems (Minneapolis, MN); Anti-THTR-1 (ab123246) from Abcam (Cambridge, MA); anti-THTR-2 (13407-1-AP) from Proteintech (Rosemont, IL); anti-Sp1 (07645) from Millipore (Billerica, MA); anti-β-actin (sc-47778) from Santa Cruz Biotechnology (Santa Cruz, CA); anti-Stat3 (no. 9139); and anti-phospho Stat3 (no.9138) from Cell Signaling Technology (Danvers, MA); and secondary antibodies anti-rabbit IRDye-800 and anti-mouse IRDye-680from LI-COR Bioscience (Lincoln, NE). Oligonucleotide primers were synthesized by Integrated DNA Technologies (San Diego, CA). All other chemicals utilized, such as unlabeled thiamin and molecular biology reagents, were of analytical grade and obtained from commercial sources.
Human Primary PACs, Treatment with Proinflammatory Cytokines, and Uptake Studies
hPACs were isolated from cadaveric pancreatic tissues of organ donors (25- to 65-yr-old males) that were provided by the Islet Cell Laboratory of the City of Hope (Duarte, CA) and the University of Louisville (Louisville, KY). Briefly, pancreases were removed from organ donors, digested to separate islets from ductal-acinar trees, and then purified as described previously (33). The isolated acinar cells were transported to University of California Irvine (protocol was approved by the Institutional Review Board: 2017-1593), and subsequently cultured in Ham’s F-12k growth medium supplemented with the following: 10% FCS, 5% BSA, 10 ng/mL epidermal growth factor, and 0.1 mg/mL soybean trypsin inhibitor, as described by us and others before (14, 33). Twenty-four hours later, the hPACs were treated (for 24 h) with the individual proinflammatory cytokines [hyper IL-6 (100 ng/mL), free IL-6 (100 ng/mL), TNF-α (20 ng/mL), or IL-1β (10 ng/mL)], suspended in Krebs-Ringer (KR) buffer and used for uptake studies. 3H-labeled (0.3 µCi/ml) and unlabeled thiamin were added to the KR buffer at the onset of incubation and uptake was terminated 10 min later (i.e., initial rate uptake; 14) with the addition of 1 mL of ice-cold KR buffer followed by rapid filtration, as described earlier (14). Levels of 3H in the washed cells were counted using liquid scintillation counter. A Bio-Rad (Hercules, CA) Dc protein assay kit was then used to determine the protein concentrations in the cell digests.
Culturing of the Mouse-Derived Pancreatic Acinar 266-6 Cells, Thiamin Uptake, and Treatment with Inhibitors
PAC 266-6 cells were cultured in complete DMEM growth medium at 37°C in a 5% CO2 environment. Confluent PAC 266-6 were treated with hyper IL-6 (100 ng/mL; 24 h), followed by examining initial rate of thiamin uptake as described previously (15). For inhibitor treatment, cells were pretreated for 1 h with Stat3 inhibitor S3I-201 (50 µM; MilliporeSigma, MA) before hyper IL-6 treatment.
Treatment of Mice with Hyper IL-6 in Vivo and Thiamin Uptake Studies
Mice were treated with hyper IL-6 (4 µg/mouse ip; 34, 35) then euthanized 24 h later followed by isolating PACs using collagenase type-IV (Sigma, St. Louis, MO) digestion method as described previously (14). Thiamin uptake studies were performed using PACs suspended in KR buffer containing [3H]thiamin. Uptake was terminated after 10 min of incubation (initial rate of uptake; 14) and 3H content of the cell lysate was determined using liquid scintillation counter. Animal use was approved by the Institutional Animal Care and Use Committee of the University of California, Irvine, CA.
Quantitative Real-Time PCR Analysis
Total RNAs were isolated from hyper IL-6-treated and untreated hPACs, PAC 266-6 and mouse PACs, as well as from pancreatic tissue of patients with chronic pancreatitis and normal control subjects and then reverse transcribed using the iScript cDNA synthesis kit from Bio-Rad. The newly synthesized cDNA samples were used for RT-qPCR with gene-specific primers (Table 1); PCR conditions were as described by us previously (14). Expression of THTR-1 and -2 mRNAs were normalized relative with β-actin and quantified using a relative relationship method (36).
Table 1.
Sequence of the primers for amplifying coding regions of thiamin transporters by real-time PCR
| Gene Name | Forward and Reverse Primers (5′–3′) |
|---|---|
| h-THTR-1 | |
| Forward | GCCAGACCGTCTCCTTGTA |
| Reverse | TAGAGAGGGCCCACCACAC |
| h-THTR-2 | |
| Forward | TTCCTGGATTTACCCCACTG |
| Reverse | GTATGTCCAAACGGGGAAGA |
| h-β-actin | |
| Forward | CATCCTGCGTCTGGACCT |
| Reverse | TAATGTCACGCACGATTTCC |
| m-THTR-1 | |
| Forward | GTTCCTCACGCCCTACCTTC |
| Reverse | GCATGAACCACGTCACAATC |
| m-THTR-2 | |
| Forward | TCATGCAAACAGCTGAGTTCT |
| Reverse | CTCCGACAGTAGCTGCTCA |
| m-β-Actin | |
| Forward | ATCCTCTTCCTCCCTGGA |
| Reverse | TTCATGGATGCCACAGGA |
THTR-1 and -2, thiamin transporter-1 and -2; h, human; m, mouse.
Transfection of PAC 266-6 with SLC19A2 and SLC19A3 Promoters and Firefly Luciferase Assay
Transient transfection of PAC 266-6 with the SLC19A2 and SLC19A3 minimal promoter-luciferase reporter constructs (previously generated and characterized in our laboratory; 37, 38) was performed using Lipofectamine 2000. Transfected cells were then exposed to hyper IL-6 (100 ng/mL; 24 h) and lysed with passive lysis buffer, followed by determination of luciferase activity using a dual-luciferase assay system (Promega).
Effect of Hyper IL-6 on Activity of the SLC19A2 and SLC19A3 Promoters in Vivo Using Transgenic Mice Expressing These Promoters
Transgenic mice carrying the SLC19A2 and SLC19A3 promoters were injected with hyper IL-6 (4 µg/mouse ip) for 24 h, followed by isolation of PACs as described previously (14, 17). Luciferase assay was then performed on PAC cells from hyper IL-6-treated and untreated mice. Luciferase activity was normalized with total protein in each sample.
Protein Isolation and Western Blot Analysis
Hyper IL-6-treated and untreated hPACs or PAC 266-6 or mouse PACs were lysed in radioimmunoprecipitation assay buffer (Sigma) in the presence of complete protease inhibitor cocktail (Roche, Branchburg, NJ). The soluble protein fraction was collected after centrifugation (14,000 rpm, 20 min) and quantified using the Dc protein assay kit. An equivalent amount of protein (30 µg) was loaded onto 4–12% bis-Tris gradient minigels (Invitrogen) and transferred onto Immobilon polyvinylidene difluoride membrane (Fisher Scientific). Afterwards, the blots were probed with anti-THTR-1 (1:1,000), anti-THTR-2 (1:1,000), anti-β-actin (1:3,000), anti-Sp1 (1:500), anti-Stat3 (1:1,000), and anti-phospho Stat3 (1:500) antibodies. Specificity of the THTR-1 (39) and that of THTR-2 (Fig. 2C and Fig. 3C) polyclonal antibodies were validated by overexpressing the human and mouse thiamin transporters in human-derived intestinal epithelial Caco-2 cells and in PAC 266-6 [Fig. 2Cii and Fig. 3Cii, respectively] The anti-Sp1 (07-645; Millipore, Billerica, MA) has been characterized and confirmed in many publications. The immunoreactive bands were then detected with the corresponding secondary antibodies anti-rabbit IRDye-800 and anti-mouse IRDye-680 (both at 1: 30,000 dilution). The densities of the specific immunoreactive bands were quantified with the use of LI-COR software (LI-COR Bioscience, Lincoln, NE).
Fig. 2.
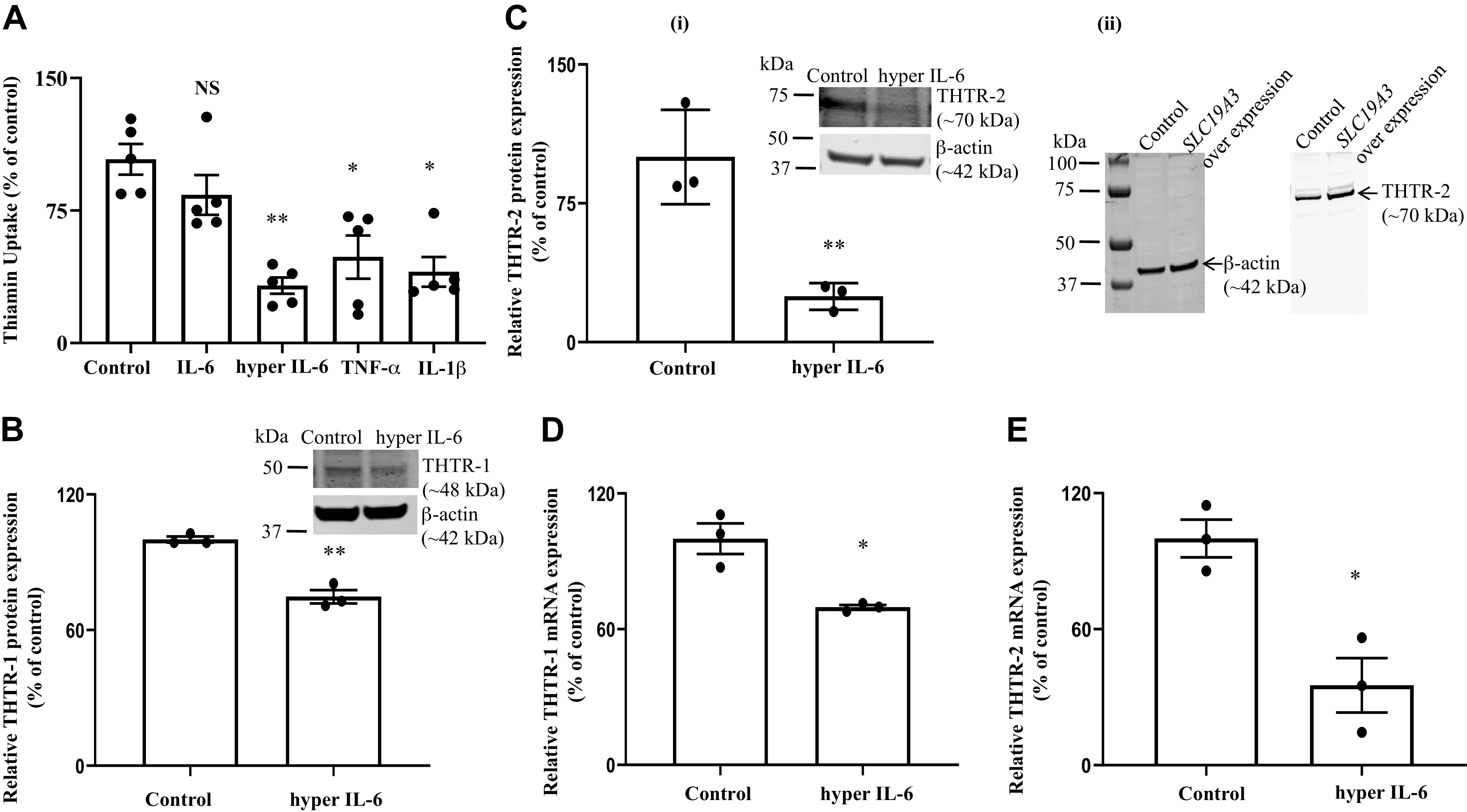
. A: effect of exposure of human pancreatic acinar cells (hPACs) to proinflammatory cytokines on thiamin uptake. hPACs were treated with proinflammatory cytokines [hyper IL-6 (100 ng/mL), free IL-6 (100 ng/mL), TNF-α (20 ng/mL), and IL-1β (10 ng/mL)] for 24 h followed by examination of carrier-mediated thiamin uptake. B–E: effect of hyper IL-6 on the level of expression of thiamin transporter-1 and -2 (THTR-1 and -2). hPACs were treated with hyper IL-6 (100 ng/mL; 24 h) followed by determination of levels of expression of THTR-1 and -2 proteins (B and C, i and ii) and mRNAs (Insets: representative Western image for THTR-1 and D and E) by Western blotting and RT-qPCR, respectively. Insets: representative Western image for THTR-1 and THTR-2 proteins. Data are means ± SE of 3–5 independent experiments. **P < 0.01; *P < 0.05; NS, not significant.
Fig. 3.
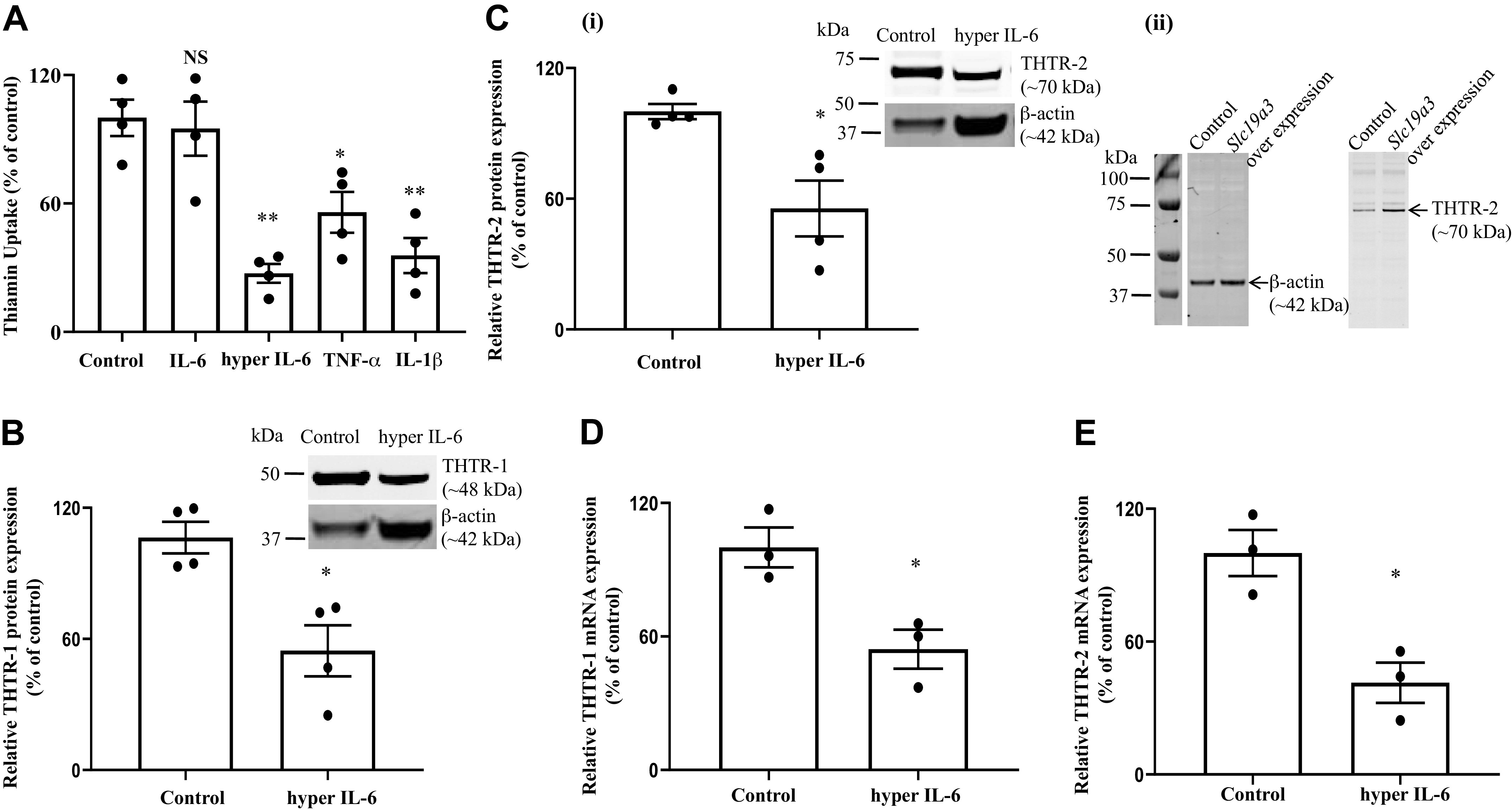
A: effect of exposure of the pancreatic acinar cell line PAC 266-6 to proinflammatory cytokines on thiamin uptake. PAC 266-6 were treated with proinflammatory cytokines [hyper IL-6 (100 ng/mL), free IL-6 (100 ng/mL), TNF-α (20 ng/mL), and IL-1β (10 ng/mL)] for 24 h, and then carrier-mediated thiamin uptake was examined. B–E: effect of hyper IL-6 treatment on level of expression of thiamin transporter-1 and -2 (THTR-1 and -2). PAC 266-6 cells were treated with hyper IL-6 and levels of expression of THTR-1 and -2 proteins (B and C, i and ii) and mRNAs (D and E) were examined. Insets: representative Western image for THTR-1 and THTR-2 proteins. Data are means ± SE of 3–4 independent experiments. **P < 0.01; *P < 0.05; NS, not significant.
Statistical Analysis
Data on carrier-mediated thiamin uptake by PACs are presented as means ± SE of four to five independent experiments and expressed as percentage relative to concurrently performed controls. Data for Western blotting, RT-qPCR and luciferase assays are expressed as means ± SE of 3–4 independent experiments. Student’s t test was used for analysis and P < 0.05 was considered as statistically significant.
RESULTS
Expression of THTR-1 and -2 in Pancreatic Tissue of Patients with Pancreatitis
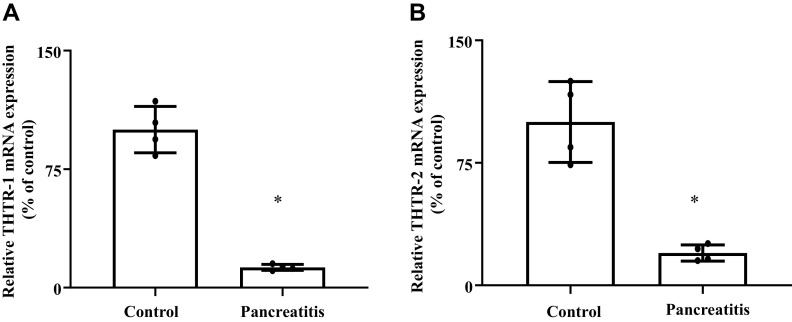
In this study, we examined the level of expression of THTR-1 and -2 mRNAs in pancreatic tissue of patients with chronic pancreatitis and compared the findings to their levels in normal (control) subjects. Diseased and normal pancreatic tissue samples were obtained from Amsbio (Cambridge, MA), and mRNA levels were determined by RT-qPCR. The results showed a significantly (P < 0.05 for both) lower level of expression of THTR-1 and -2 (Fig. 1, A and B) mRNAs in pancreatic tissues from patients with chronic pancreatitis compared with those from control subjects.
Fig. 1.
Level of expression of thiamin transporter-1 and -2 (THTR-1 and -2) mRNAs in pancreatic tissue from patients with chronic pancreatitis compared with their level in normal control subjects. Levels of expression of THTR-1 (A) and -2 (B) were determined by RT-qPCR using cDNA prepared from patients with chronic pancreatitis (n = 4) and pooled control subjects (n = 5). Data are means ± SE; *P < 0.05.
Effect of Proinflammatory Cytokines on Thiamin Uptake Physiology and Molecular Biology in Human and Mouse PACs
As mentioned earlier, PACs are exposed to elevated levels of locally and systemically generated proinflammatory cytokines (like IL-6, TNF-α, and IL-1β; 18–20) in conditions that adversely affect the physiology and health of the pancreas, as well as those that are associated with systemic inflammation (21–26). While exposure of cells to elevated levels of proinflammatory cytokines affect the physiology of membrane transport events, with the effect being differential (i.e., both stimulation and inhibition being reported; 27–32), nothing is known about their effects on thiamin uptake by PACs. This issue was addressed using human primary PACs as well as mouse PAC 266-6 cell line and mice in vivo.
Studies with hPACs.
In these studies, we examined the effect of three prominent proinflammatory cytokines whose levels are induced in chronic and acute pancreatitis, namely IL-6 (in both free and hyper IL-6 forms; the latter is a fusion protein of IL-6 with its naturally occurring soluble IL-6 receptor; 40, 41) as well as TNF-α and IL-1β on initial rate of carrier-mediated thiamin uptake by hPACs. Treatment was done for 24 h using clinically relevant concentrations of these proinflammatory cytokines [free IL-6 (100 ng/mL; 41), hyper IL-6 (100 ng/mL; 41), TNF-α (20 ng/mL; 42), and IL-1β (10 ng/mL; 43)] The results showed a significant inhibition in thiamin uptake by hPACs treated with each of the proinflammatory cytokines (P < 0.01 for hyper IL-6; P < 0.05 for both TNF-α and IL-1β), except free IL-6 (Fig. 2A). The lack of effect of free IL-6 is expected since PACs do not express significant level of the IL-6R (the latter receptor is expressed mainly in hepatocytes, neutrophils, and macrophages; 41 and unpublished observation in our laboratory). In subsequent studies, we focused on the effect of hyper IL-6 since it is a predominant cytokine in pancreatitis and is a marker for the disease (20, 44).
In another study, we examined (by means of Western blotting using specific and validated polyclonal antibodies) the effect of exposure of hPACs to hyper IL-6 on level of expression of THTR-1 and -2 proteins. The results showed a significant (P < 0.01 for both) reduction in level of both transporters in cells treated with hyper IL-6 compared with untreated controls (Fig. 2, B and Ci). We also investigated (by means of RT-qPCR) the effect of exposure of hPACs to hyper IL-6 on the level of expression of THTR-1 and -2 mRNA, with the results showing a significant (P < 0.05 for both) reduction in the level of expression of both mRNAs in treated cells compared with untreated cells (Fig. 2, D and E).
Studies with mouse-derived pancreatic acinar 266-6 cells (PAC 266-6).
To establish the suitability of the mouse-derived PAC 266-6 as model in such investigations, we examined the effect of exposing these cells to proinflammatory cytokines [free IL-6 (100 ng/mL), hyper IL-6 (100 ng/mL), TNF-α (20 ng/mL), and IL-1β (10 ng/mL)] on carrier-mediated thiamin uptake. The results showed a significant (P < 0.01 for both hyper IL-6 and IL-1β; P < 0.05 for TNF-α) inhibition in thiamin uptake by cells treated with each of the proinflammatory cytokines (but not free IL-6) compared with simultaneously processed untreated control (Fig. 3A). We also examined (by mean of Western blotting using well-validated and specific mouse antibodies) the effect of treating PAC 266-6 with hyper IL-6 on level of expression of THTR-1 and -2 at the protein levels. The results showed significant (P < 0.05 for both) inhibition in level of expression of both proteins in cells treated with hyper IL-6 compared with untreated cells (Fig. 3, B and Ci). Finally, we examined the level of mRNA expression of THTR-1 and -2 in PAC 266-6 with the results showing a significant (P < 0.05 for both) reduction in mRNA level of both transporters in hyper IL-6-treated PAC 266-6 compared with untreated control cells (Fig. 3, D and E).
Effect of in vivo exposure to hyper IL-6 on thiamin uptake by mouse PACs. In these studies, we extended our investigations to the in vivo situation and examined the effect of treating mice with hyper IL-6 (4 µg/mouse ip) on thiamin uptake physiology and molecular parameters (all assays were performed 24 h after hyper IL-6 injection). The results showed a significant (P < 0.01) inhibition on thiamin uptake by PACs of mice treated with hyper IL-6 compared with control (vehicle-treated) mice (Fig. 4A). Next, we examined the effect of in vivo exposure to hyper IL-6 on level of THTR-1 and -2 protein expression. The results showed significant inhibition in both parameters in hyper IL-6 -treated mice compared with control mice (Fig. 4, B and C). We also determined the THTR-1 and -2 mRNA expression levels in mice exposed to hyper IL-6 with the results showing a significant reduction in expression of THTR-1 (P < 0.01) and -2 (P < 0.05) in mice treated with hyper IL-6 compared with vehicle-treated mice (Fig. 4, D and E).
Fig. 4.
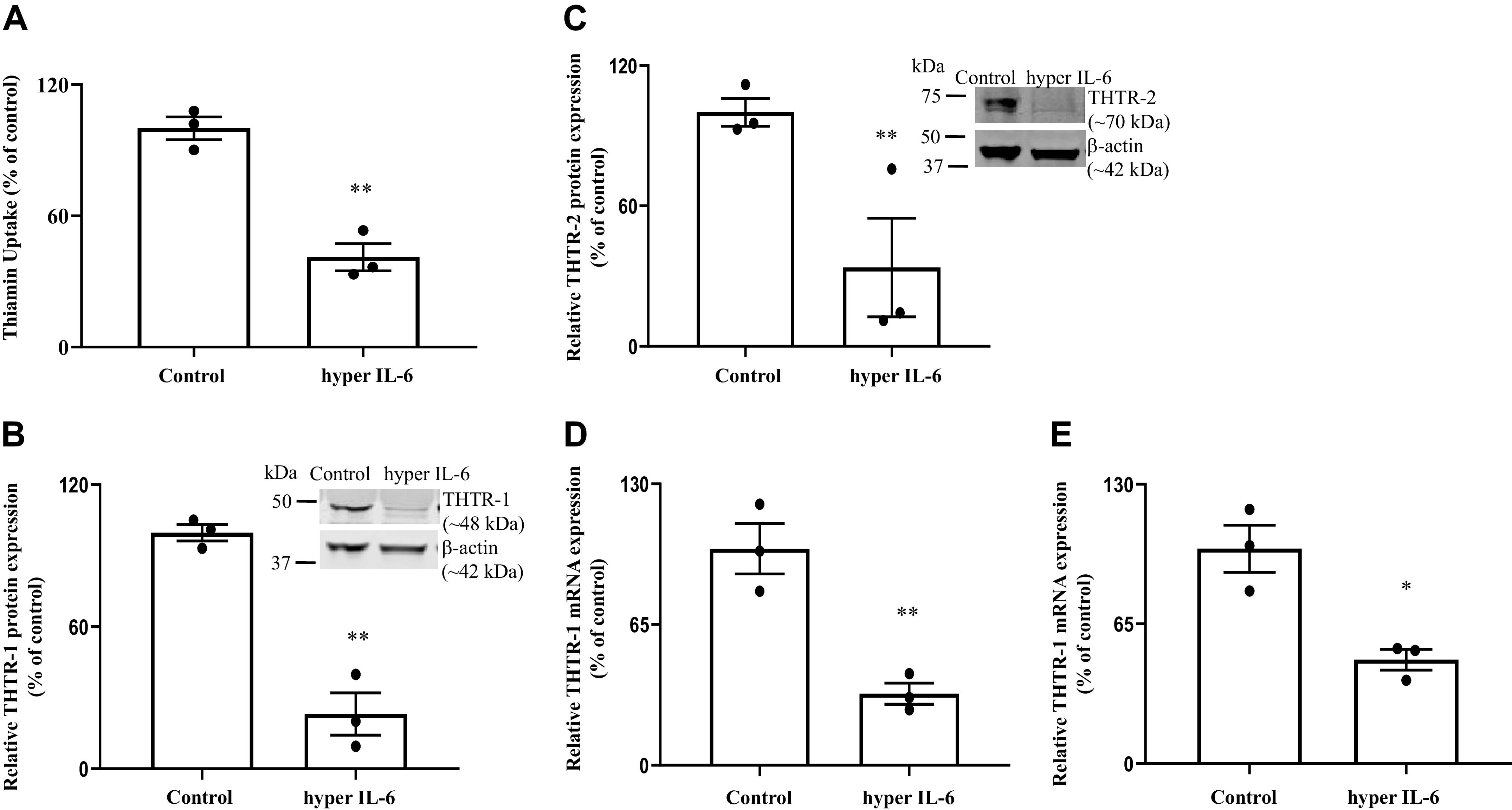
Effect of in vivo treatment of mice with hyper IL-6 on thiamin uptake by pancreatic acinar cell (PACs; A), and on level of expression of thiamin transporter-1 and -2 (THTR-1 and -2) proteins (B and C) and mRNAs (D and E). Wild-type mice were injected with hyper IL-6 (4 µg/mouse ip) followed (24 h later) by isolation of PACs, and then carrier-mediated uptake was determined. Western blotting (insets) and RT-qPCR were performed to determine the expression of THTR-1 and -2 at both protein and mRNA levels, respectively. Data are means ± SE of 3 independent experiments. **P < 0.01; *P < 0.05.
Collectively, the above-described findings show that exposure of hPACs and mouse PACs to proinflammatory cytokines leads to significant inhibition in thiamin uptake and level of expression of the uptake systems involved. The results also establish the suitability of mouse-derived PACs as suitable model in such investigations.
Transcriptional Mechanism(s) is Involved in Mediating the Inhibitory Effect of Hyper IL-6 on Thiamin Uptake by Mouse PACs
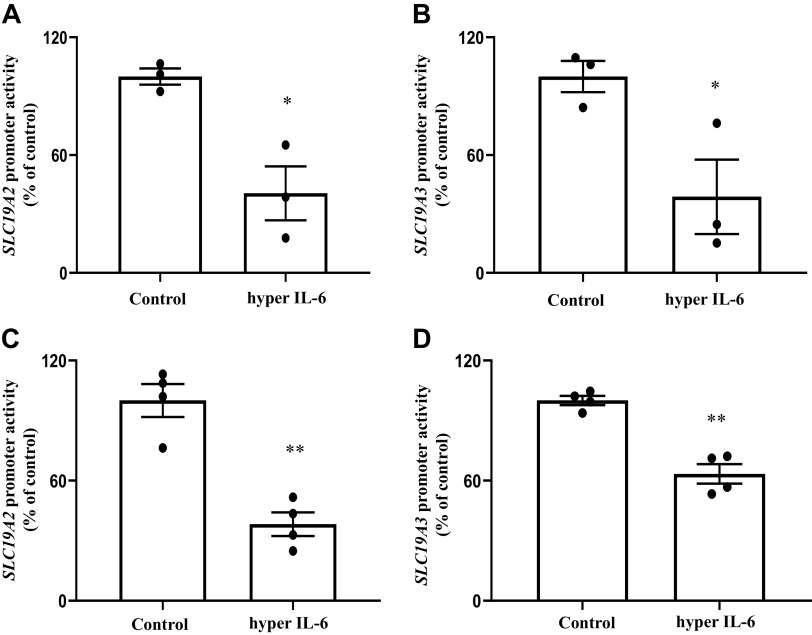
Changes in level of mRNA expression of a specific gene by external/internal factors and conditions can be accomplished via different mechanisms. One such prominent mechanism is alterations in the rate of transcription of the involved gene. To determine whether such a mechanism is involved in the effect of hyper IL-6 on expression of SLC19A2 and SLC19A3 in PACs, we exposed PAC 266-6 transfected with SLC19A2 and SLC19A3 minimal promoters fused to the luciferase reporter gene to hyper IL-6 (100 ng/mL) and then examined the rate of promoter activity. The results showed that exposure of cells to hyper IL-6 to lead to a significant (P < 0.05 for both) reduction in activity of both SLC19A2 (Fig. 5A) and SLC19A3 (Fig. 5B) promoters. To confirm these in vitro findings in in vivo situation, we expose transgenic mice carrying the human SLC19A2 and SLC19A3 promoters fused with the luciferase reporter to hyper IL-6 (4 µg/mouse ip) and examined luciferase activity in their PACs. The results showed a significant (P < 0.01 for both) reduction in SLC19A2 (Fig. 5C) and SLC19A3 (Fig. 5D) promoter activity in mice treated with hyper IL-6 compared with untreated controls.
Fig. 5.
A and B: effect of exposure of the pancreatic acinar cell line PAC 266-6 to hyper IL-6 on promoter activity. SLC19A2 and SLC19A3 minimal-promoter constructs in pGL3 basic were transfected into PAC 266-6 and exposed to hyper IL-6 (100 ng/mL) for 24 h followed by the determination of luciferase activity. C and D: effect of treating transgenic mice expressing the SLC19A2 and SLC19A3 promoters with hyper IL-6 on promoter activity in PACs. Transgenic mice expressing the SLC19A2 and SLC19A3 promoters were treated with hyper IL-6 (4 µg/mouse ip), followed by determination of luciferase activity in PACs. Data are means ± SE of 3–4 independent experiments. **P < 0.01; *P < 0.05.
Next, we examined the effect of exposure of PACs to hyper IL-6 on level of protein expression of the transcription factor Sp1. A role for this nuclear factor in regulating the activity of the SLC19A2 and SLC19A3 promoters has been demonstrated previously by our group in studies involving mutations of the predicted binding sites (cis-elements) for Sp1 in the minimal regions these promoters that are responsible for basal activities (37, 38). Thus suppression in level of expression of such a critical factor is expected to negatively impact the functionality of the SLC19A2 and SLC19A3 promoters. Our results showed a significant reduction (P < 0.05) in Sp1 level of expression in PAC 266-6 (Fig. 6A) and mouse PACs (Fig. 6B) treated with hyper IL-6 compared with untreated control cells.
Fig. 6.
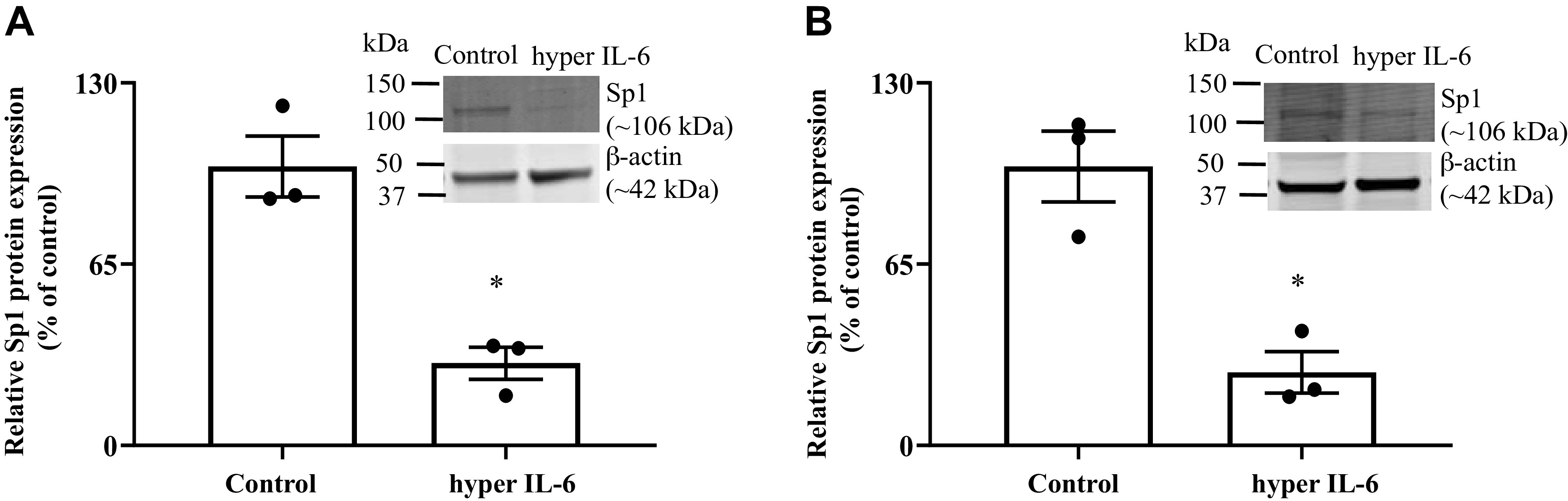
Effect of exposure of hyper IL-6 on the level of expression of Sp1 protein in pancreatic acinar cell line PAC 266-6 (A) and mouse PACs (B). PACs were exposed to hyper IL-6 (100 ng/mL) for 24 h, and the level of Sp1 protein expression was determined by Western blot (inset). Data are means ± SE of 3 independent experiments. *P < 0.05.
Intracellular Signaling Mechanism(s) Involved in Mediating the Inhibitory Effect of Hyper IL-6 on Thiamin Uptake by PAC 266-6
Previous studies have shown that Stat3 signaling pathway is involved in mediating cellular effects of hyper IL-6 (35, 41). Thus we first confirmed that in the case of PACs by showing that treating these cells with hyper IL-6 (100 ng/mL; 24 h) to lead to a significant (P < 0.05) increase in the phosphorylation of Stat3 compared with untreated cells (Fig. 7A), while such treatment with hyper IL-6 in the presence of the Stat3 specific pharmacological inhibitor S3I-201 (41) failed to do so (Fig. 7A). We then moved to examine the involvement of Stat3 signaling pathway in mediating the inhibitory effect hyper IL-6 on thiamin uptake by PACs by examining the ability of the inhibitor S3I-201 to block the inhibitory effect of hyper IL-6 on thiamin uptake. The results showed that treatment with S3I-201 to indeed lead to a significant abrogation (P < 0.05) in the inhibitory effect of hyper IL-6 on thiamin uptake by PACs (Fig. 7B).
Fig. 7.
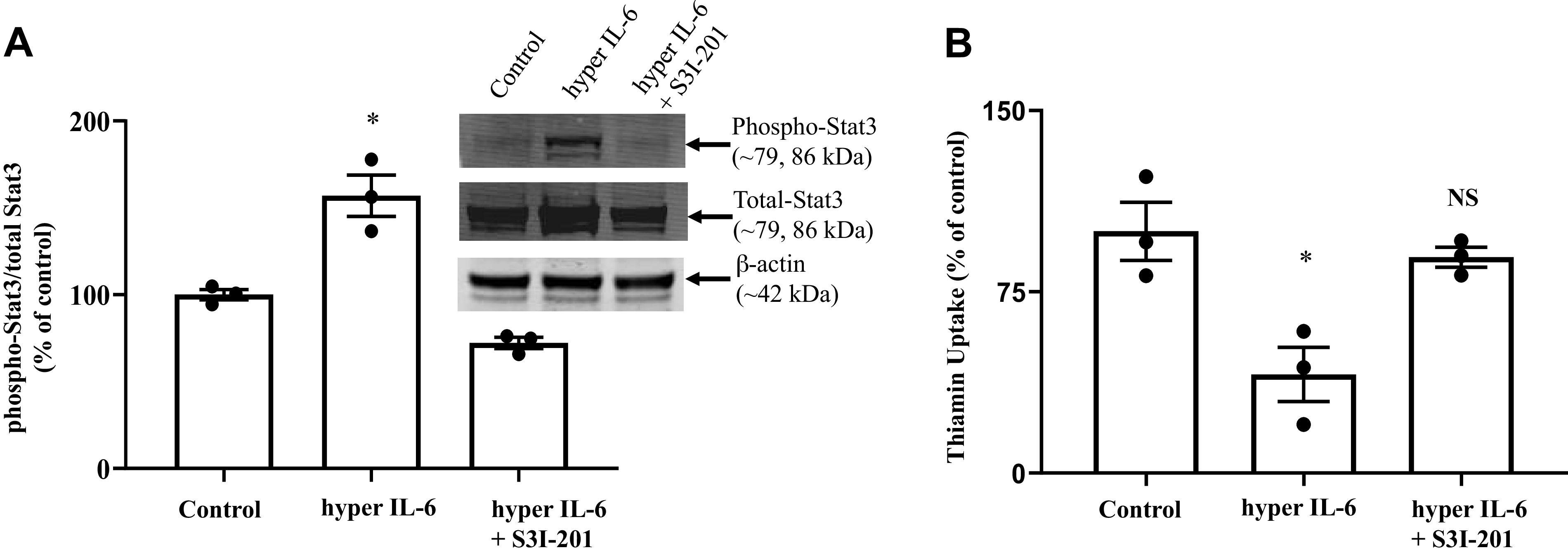
Role of Stat3 signaling pathway in mediating the effect of hyper IL-6 on thiamin uptake in the pancreatic acinar cell line PAC 266-6. A: activation of Stat3 in PAC 266-6 exposed to hyper IL-6 (100 ng/mL) and pretreated with 50 µM S3I-201 (a Stat3 inhibitor). Inset: representative Western image for phospho-Stat3 and total-Stat3. B: carrier-mediated thiamin uptake was examined in PAC 266-6 that were pretreated with S3I-201 for 1 h before hyper IL-6 treatment. Data are means ± SE of 3 independent experiments. *P < 0.05; NS, not significant.
DISCUSSION
Our aim in the present study was to examine the effect of proinflammatory cytokines on physiological and molecular parameters of thiamin uptake by PACs and to shed light on the mechanism(s) involved in any observed effect. We were prompted to do so based on recent findings showing that prolonged exposure of PACs to elevated levels of proinflammatory cytokines adversely affect their physiology and health (21–26) and our initial finding in this study showing that levels of expression of THTR-1 and -2 are significantly suppressed in pancreatic tissue of patients with chronic pancreatitis compared with that of normal control subjects. We address this issue using three complementary models namely: human primary PACs (from organ-donors), cultured PAC 266-6, and in vivo (mice).
Our studies with the human primary PACs showed that prolonged exposure of these cells to proinflammatory cytokines led to a significant inhibition in carrier-mediated thiamin uptake. Focusing on the effect of hyper IL-6 (a prominent proinflammatory cytokine in human pancreatitis; 20, 44), we found the inhibitory effect on thiamin uptake process to be associated with a significant reduction in the level of expression of the THTR-1 and -2 proteins and mRNAs. Similar findings were obtained with the mouse-derived cultured PAC 266-6 cells, as well as with mouse primary PACs exposed to hyper IL-6 in vivo.
The inhibitory effect of hyper IL-6 on the level of expression of THTR-1 and -2 appears to be mediated at the level of transcription of the SLC19A2 and SLC19A3 genes. This conclusion is based on evidence obtained from cultured PAC 266-6 transfected with promoters of these genes and exposed to hyper IL-6 in vitro and from transgenic mice carrying promoters of the SLC19A2 and SLC19A3 genes (fused to the luciferase reporter) and exposed to hyper IL-6 in vivo. In both cases, significant inhibition in the activity of the SLC19A2 and SLC19A3 promoters were observed. In other studies, we examined possible involvement of the nuclear factor Sp1 in mediating the inhibitory effects of hyper IL-6 on activity of the SLC19A2 and SLC19A3 promoters since this factor plays an important role in basal activity of these promoters (37, 38). Indeed, a significant reduction in level of expression of Sp1 was found in PACs exposed to hyper IL-6 compared with controls, suggesting possible involvement of this transcription factor in mediating the inhibitory effect of hyper IL-6 on THTR-1 and -2 expression and ultimately thiamin uptake.
Previous studies have shown that Stat3 signaling pathway is involved in mediating the effect of hyper IL-6 on physiology of a variety of cells including that of PACs (35, 41). We first confirmed that in this investigation by showing a significant increase in level of Stat3 phosphorylation in PACs treated with hyper IL-6 and that this effect is significantly abrogated in the presence of the Stat3 inhibitor, S3I-201. Our study then showed that Stat3 signaling pathway is involved in mediating the inhibitory effect of hyper IL-6 on thiamin uptake by demonstrating a marked reversal in this inhibition in the presence of S3I-201.
In summary, our findings in this study show for the first time that exposure of PACs to proinflammatory cytokines negatively affects the physiology and molecular biology of thiamin uptake by these cells, and that this effect is mediated at (at least in part) at the level of transcription of the SLC19A2 and SLC19A3 genes.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.A., P.S., T.Y., and H.M.S. conceived and designed research; K.A., P.S., T.Y. and S.A. performed experiments; K.A., P.S., and H.M.S. analyzed data; K.A., P.S., and H.M.S. interpreted results of experiments; K.A. and P.S. prepared figures; K.A., P.S., T.Y. and S.A. drafted manuscript; K.A., P.S., and H.M.S. edited and revised manuscript; K.A., P.S., T.Y., and H.M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health Grants DK-56061, AA-018071, and DK-58057 and Department of Veterans Affairs Merit Award I01BX001142.
REFERENCES
- 1.Berdanier CD. Advanced Nutrition: Micronutrients. New York: CRC, 1998. [Google Scholar]
- 2.Said HM. Thiamin. In: Encyclopedia of Dietary Supplements, edited by Coats PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD. New York: Informa Health Care USA, 2010, p. 252–257. [Google Scholar]
- 3.Tanphaichitr V. Modern Nutrition in Health and Disease, edited by Shils ME, Olsen JA, Shike M.. New York: Lea and Febiger, 1994. [Google Scholar]
- 4.Bettendorff L, Goessens G, Sluse F, Wins P, Bureau M, Laschet J, Grisar T. Thiamine deficiency in cultured neuroblastoma cells: effect on mitochondrial function and peripheral benzodiazepine receptors. J Neurochem 64: 2013–2021, 1995. doi: 10.1046/j.1471-4159.1995.64052013.x. [DOI] [PubMed] [Google Scholar]
- 5.Portari GV, Marchini JS, Vannucchi H, Jordao AA. Antioxidant effect of thiamine on acutely alcoholized rats and lack of efficacy using thiamine or glucose to reduce blood alcohol content. Basic Clin Pharmacol Toxicol 103: 482–486, 2008. doi: 10.1111/j.1742-7843.2008.00311.x. [DOI] [PubMed] [Google Scholar]
- 6.Victor MG, Adams RD, Collins A. The Wernicke-Korsakoff Syndrome and Related Neurological Disorders due to Alcoholism and Malnutrition (2nd ed.). Philadelphia, PA: F. A. Davis, 1989. [Google Scholar]
- 7.Holmberg MJ, Moskowitz A, Patel PV, Grossestreuer AV, Uber A, Stankovic N, Andersen LW, Donnino MW. Thiamine in septic shock patients with alcohol use disorders: an observational pilot study. J Crit Care 43: 61–64, 2018. doi: 10.1016/j.jcrc.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moskowitz A, Andersen LW, Cocchi MN, Karlsson M, Patel PV, Donnino MW. Thiamine as a renal protective agent in septic shock: a secondary analysis of a randomized, double-blind, placebo-controlled trial. Ann Am Thorac Soc 14: 737–741, 2017. doi: 10.1513/AnnalsATS.201608-656BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rathanaswami P, Pourany A, Sundaresan R. Effects of thiamine deficiency on the secretion of insulin and the metabolism of glucose in isolated rat pancreatic islets. Biochem Int 25: 577–583, 1991. https://europepmc.org/article/med/1805801. [PubMed] [Google Scholar]
- 10.Rathanaswami P, Sundaresan R. Effects of thiamine deficiency on the biosynthesis of insulin in rats. Biochem Int 24: 1057–1062, 1991. [PubMed] [Google Scholar]
- 11.Singh M. Effect of thiamin deficiency on pancreatic acinar cell function. Am J Clin Nutr 36: 500–504, 1982. doi: 10.1093/ajcn/36.3.500. [DOI] [PubMed] [Google Scholar]
- 12.Subramanian VS, Subramanya SB, Said HM. Relative contribution of THTR-1 and THTR-2 in thiamin uptake by pancreatic acinar cells: studies utilizing Slc19a2 and Slc19a3 knockout mouse models. Am J Physiol Gastrointest Liver Physiol 302: G572–G578, 2012. doi: 10.1152/ajpgi.00484.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanya SB, Subramanian VS, Sekar VT, Said HM. Thiamin uptake by pancreatic acinar cells: effect of chronic alcohol feeding/exposure. Am J Physiol Gastrointest Liver Physiol 301: G896–G904, 2011. doi: 10.1152/ajpgi.00308.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasan P, Anandam KY, Ramesh V, Geltz ET, Said HM. Effect of bacterial flagellin on thiamin uptake by human and mouse pancreatic acinar cells: inhibition mediated at the level of transcription of thiamin transporters 1 and 2. Am J Physiol Gastrointest Liver Physiol 316: G735–G743, 2019. doi: 10.1152/ajpgi.00048.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan P, Subramanian VS, Said HM. Effect of the cigarette smoke component, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), on physiological and molecular parameters of thiamin uptake by pancreatic acinar cells. PLoS One 8: e78853, 2013. doi: 10.1371/journal.pone.0078853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasan P, Subramanian VS, Said HM. Mechanisms involved in the inhibitory effect of chronic alcohol exposure on pancreatic acinar thiamin uptake. Am J Physiol Gastrointest Liver Physiol 306: G631–G639, 2014. doi: 10.1152/ajpgi.00420.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasan P, Thrower EC, Loganathan G, Balamurugan AN, Subramanian VS, Gorelick FS, Said HM. Chronic nicotine exposure in vivo and in vitro inhibits vitamin b1 (thiamin) uptake by pancreatic acinar cells. PLoS One 10: e0143575, 2015. doi: 10.1371/journal.pone.0143575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Beaux AC, Goldie AS, Ross JA, Carter DC, Fearon KC. Serum concentrations of inflammatory mediators related to organ failure in patients with acute pancreatitis. Br J Surg 83: 349–353, 1996. doi: 10.1002/bjs.1800830317. [DOI] [PubMed] [Google Scholar]
- 19.Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther 8: 10–25, 2017. doi: 10.4292/wjgpt.v8.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szatmary P, Gukovsky I. The role of cytokines and inflammation in the genesis of experimental pancreatitis. In: Pancreapedia: Exocrine Pancreas Knowledge Base, edited by Williams JA. Mountain View, CA: American Pancreatic Association, 2016, 42–52. 10.3998/panc.2016.29 [DOI] [Google Scholar]
- 21.Chen CC, Wang SS, Lee FY, Chang FY, Lee SD. Proinflammatory cytokines in early assessment of the prognosis of acute pancreatitis. Am J Gastroenterol 94: 213–218, 1999. doi: 10.1111/j.1572-0241.1999.00709.x. [DOI] [PubMed] [Google Scholar]
- 22.Formela LJ, Galloway SW, Kingsnorth AN. Inflammatory mediators in acute pancreatitis. Br J Surg 82: 6–13, 1995. doi: 10.1002/bjs.1800820105. [DOI] [PubMed] [Google Scholar]
- 23.Gillies N, Pendharkar SA, Asrani VM, Mathew J, Windsor JA, Petrov MS. Interleukin-6 is associated with chronic hyperglycemia and insulin resistance in patients after acute pancreatitis. Pancreatology 16: 748–755, 2016. doi: 10.1016/j.pan.2016.06.661. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki T, Hoshino M, Hayakawa T, Ohara H, Yamada T, Yamada H, Iida M, Nakazawa T, Ogasawara T, Uchida A, Hasegawa C, Miyaji M, Takeuchi T. Interleukin-6 is a useful marker for early prediction of the severity of acute pancreatitis. Pancreas 14: 1–8, 1997. doi: 10.1097/00006676-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Malmstrøm ML, Hansen MB, Andersen AM, Ersbøll AK, Nielsen OH, Jørgensen LN, Novovic S. Cytokines and organ failure in acute pancreatitis: inflammatory response in acute pancreatitis. Pancreas 41: 271–277, 2012. doi: 10.1097/MPA.0b013e3182240552. [DOI] [PubMed] [Google Scholar]
- 26.Pooran N, Indaram A, Sing P, Bank S. Cytokines (IL-6, IL-8, TNF): early and reliable predictors of severe acute pancreatitis. J Clin Gastroenterol 37: 263–266, 2003. doi: 10.1097/00004836-200309000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Abualsunun WA, Piquette-Miller M. STAT3 is involved in IL-6-mediated downregulation of hepatic transporters in mice. J Pharm Abelson Sci 21: 325s–334s, 2018. doi: 10.18433/jpps30241. [DOI] [PubMed] [Google Scholar]
- 28.Febvre-James M, Bruyère A, Le Vée M, Fardel O. The JAK1/2 inhibitor ruxolitinib reverses interleukin-6-mediated suppression of drug-detoxifying proteins in cultured human hepatocytes. Drug Metab Dispos 46: 131–140, 2018. doi: 10.1124/dmd.117.078048. [DOI] [PubMed] [Google Scholar]
- 29.Jones HN, Jansson T, Powell TL. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am J Physiol Cell Physiol 297: C1228–C1235, 2009. doi: 10.1152/ajpcell.00195.2009. [DOI] [PubMed] [Google Scholar]
- 30.Klein M, Thomas M, Hofmann U, Seehofer D, Damm G, Zanger UM. A systematic comparison of the impact of inflammatory signaling on absorption, distribution, metabolism, and excretion gene expression and activity in primary human hepatocytes and HepaRG cells. Drug Metab Dispos 43: 273–283, 2015. doi: 10.1124/dmd.114.060962. [DOI] [PubMed] [Google Scholar]
- 31.Le Vee M, Lecureur V, Stieger B, Fardel O. Regulation of drug transporter expression in human hepatocytes exposed to the proinflammatory cytokines tumor necrosis factor-alpha or interleukin-6. Drug Metab Dispos 37: 685–693, 2009. doi: 10.1124/dmd.108.023630. [DOI] [PubMed] [Google Scholar]
- 32.Simon F, Garcia J, Guyot L, Guitton J, Vilchez G, Bardel C, Chenel M, Tod M, Payen L. Impact of interleukin-6 on drug-metabolizing enzymes and transporters in intestinal cells. AAPS J 22: 16, 2020. doi: 10.1208/s12248-019-0395-x. [DOI] [PubMed] [Google Scholar]
- 33.Singh L, Bakshi DK, Vasishta RK, Arora SK, Majumdar S, Wig JD. Primary culture of pancreatic (human) acinar cells. Dig Dis Sci 53: 2569–2575, 2008. doi: 10.1007/s10620-007-0162-1. [DOI] [PubMed] [Google Scholar]
- 34.Peters M, Blinn G, Solem F, Fischer M, Meyer Zum Buschenfelde KH, Rose-John S. In vivo and in vitro activities of the gp130-stimulating designer cytokine hyper-IL-6. J Immunol 161: 3575–3581, 1998. [PubMed] [Google Scholar]
- 35.Rakemann T, Niehof M, Kubicka S, Fischer M, Manns MP, Rose-John S, Trautwein C. The designer cytokine hyper-interleukin-6 is a potent activator of STAT3-dependent gene transcription in vivo and in vitro. J Biol Chem 274: 1257–1266, 1999. doi: 10.1074/jbc.274.3.1257. [DOI] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Nabokina SM, Said HM. Characterization of the 5′-regulatory region of the human thiamin transporter SLC19A3: in vitro and in vivo studies. Am J Physiol Gastrointest Liver Physiol 287: G822–G829, 2004. doi: 10.1152/ajpgi.00234.2004. [DOI] [PubMed] [Google Scholar]
- 38.Reidling JC, Said HM. In vitro and in vivo characterization of the minimal promoter region of the human thiamin transporter SLC19A2. Am J Physiol Cell Physiol 285: C633–C641, 2003. doi: 10.1152/ajpcell.00076.2003. [DOI] [PubMed] [Google Scholar]
- 39.Ramamoorthy K, Anandam KY, Yasujima T, Srinivasan P, Said HM. Posttranscriptional regulation of thiamin transporter-1 expression by microRNA-200a-3p in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 319: G323–G332, 2020. doi: 10.1152/ajpgi.00178.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer M, Goldschmitt J, Peschel C, Brakenhoff JP, Kallen KJ, Wollmer A, Grötzinger J, Rose-John S. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat Biotechnol 15: 142–145, 1997. doi: 10.1038/nbt0297-142. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Neuhöfer P, Song L, Rabe B, Lesina M, Kurkowski MU, Treiber M, Wartmann T, Regnér S, Thorlacius H, Saur D, Weirich G, Yoshimura A, Halangk W, Mizgerd JP, Schmid RM, Rose-John S, Algül H. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J Clin Invest 123: 1019–1031, 2013. doi: 10.1172/JCI64931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anandam KY, Alwan OA, Subramanian VS, Srinivasan P, Kapadia R, Said HM. Effect of the proinflammatory cytokine TNF-α on intestinal riboflavin uptake: inhibition mediated via transcriptional mechanism(s). Am J Physiol Cell Physiol 315: C653–C663, 2018. doi: 10.1152/ajpcell.00295.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emanuelli B, Glondu M, Filloux C, Peraldi P, Van Obberghen E. The potential role of SOCS-3 in the interleukin-1beta-induced desensitization of insulin signaling in pancreatic beta-cells. Diabetes 53: S97–S103, 2004. doi: 10.2337/diabetes.53.suppl_3.S97. [DOI] [PubMed] [Google Scholar]
- 44.Pini M, Rhodes DH, Castellanos KJ, Hall AR, Cabay RJ, Chennuri R, Grady EF, Fantuzzi G. Role of IL-6 in the resolution of pancreatitis in obese mice. J Leukoc Biol 91: 957–966, 2012. doi: 10.1189/jlb.1211627. [DOI] [PMC free article] [PubMed] [Google Scholar]