Abstract
Autism spectrum disorder (ASD) is characterized by impaired predictive abilities; however, the neural mechanisms subsuming reward prediction errors in ASD are poorly understood. In the current study, we investigated neural responses during social and nonsocial reward prediction errors in 22 adolescents with ASD (ages 12–17) and 20 typically developing control adolescents (ages 12–18). Participants performed a reward prediction error task using both social (i.e., faces) and nonsocial (i.e., objects) rewards during a functional magnetic resonance imaging (fMRI) scan. Reward prediction errors were defined in two ways: (a) the signed prediction error, difference between the experienced and expected reward; and (b) the thresholded unsigned prediction error, the difference between expected and unexpected outcomes regardless of magnitude. During social reward prediction errors, the ASD group demonstrated the following differences relative to the TD group: (a) signed prediction error: decreased activation in the right precentral gyrus and increased activation in the right frontal pole; and (b) thresholded unsigned prediction error: increased activation in the right anterior cingulate gyrus and bilateral precentral gyrus. Groups did not differ in brain activation during nonsocial reward prediction errors. Within the ASD group, exploratory analyses revealed that reaction times and social-communication impairments were related to precentral gyrus activation during social prediction errors. These findings elucidate the neural mechanisms of social reward prediction errors in ASD and suggest that ASD is characterized by greater neural atypicalities during social, relative to nonsocial, reward prediction errors in ASD.
Keywords: Autism Spectrum Disorder, Reward Prediction Error, fMRI, Social, Social-Communication
Lay Summary:
We used brain imaging to evaluate differences in brain activation in adolescents with autism while they performed tasks that involved learning about social and nonsocial information. We found no differences in brain responses during the nonsocial condition, but differences during the social condition of the learning task. This study provides evidence that autism may involve different patterns of brain activation when learning about social information.
Introduction
The social motivation hypothesis of autism highlights that social impairments in autism spectrum disorder (ASD) may be etiologically related to functional deficits in brain reward processing systems (Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012; Clements et al., 2018). This framework suggests that core deficits in social communication and interactions in ASD may reflect decreased motivation to engage in social interactions, decreased pleasure during social interactions, and, by extension, increased motivation to interact with or seek out nonsocial rewards (Dichter, 2018). In support of this model, individuals with ASD ranging from childhood through adulthood demonstrate atypical behavioral and neural responses to a variety of social and nonsocial rewards, including monetary rewards, images of faces, images of objects, and images of preferred restricted interest stimuli (Clements et al., 2018; Dawson, Webb, & McPartland, 2005; Stavropoulos & Carver, 2018).
Whereas most research into reward processing deficits in ASD has focused on reward anticipation and receipt, reward learning in ASD has not been extensively investigated. This omission is notable given that ASD is characterized by impaired flexible responses to environmental contingencies (Sinha et al., 2014) and impaired learning more generally (e.g., Lin, Rangel, & Adolphs, 2012; Mussey, Travers, Klinger, & Klinger, 2015) that may result from atypical computation of prediction errors (Lawson, Rees, & Friston, 2014; Van de Cruys et al., 2014). The neuroimaging literature addressing reward learning among children, adolescents, and adults with ASD has found decreased frontostriatal activity during both implicit and explicit social reward learning tasks (Choi et al., 2015; Schipul & Just, 2016; Schmitz et al., 2008; Scott-Van Zeeland, Dapretto, Ghahremani, Poldrack, & Bookheimer, 2010), highlighting the potential relevance of reward learning to core ASD symptoms.
Reward prediction errors (RPEs) are a critical component of reward learning, and relatively few studies have examined RPEs in ASD. RPEs occur when there is a mismatch between an expected and a received outcome (Schultz, 1997), and RPEs serve to modify behavior to maximize the receipt of future rewards; in this way, RPEs have a powerful influence on reward learning and reward-oriented behaviors. If a reward-related outcome is as predicted, a cue-reward association is maintained, and the subsequent behavior remains unchanged. Conversely, if a reward-related outcome is more or less valuable than predicted, a positive or negative prediction error occurs, respectively, that serves to orient attention to the discrepancy to promote learning and thereby maximize future rewards (Schultz, 2015; Schultz, Dayan, & Montague, 1997).
Impaired RPEs have been suggested to be related to clinical features of ASD. Behavioral and cognitive inflexibility and insistence on sameness in ASD may result from atypical neural computation of RPEs (Lawson et al., 2014; Sinha et al., 2014; Van de Cruys et al., 2014) and reduced neural responses in the anterior cingulate gyrus have been observed in ASD during social prediction errors (Balsters et al., 2017). Our research group recently examined neural responses to RPEs using monetary rewards among adults with ASD and found relatively greater activation in the ASD group in the left insula and right frontal pole during RPEs and the left paracingulate gyrus during unexpected rewards (Mosner et al., 2019). However, that study did not assess the neural mechanisms of RPEs in the context of other types of nonsocial rewards, or in the context of social rewards, both of which represent important components of reward learning that are relevant to core features of ASD.
The goal of the present study was to assess neural mechanisms of RPEs among adolescents with ASD when rewards were delivered independently of goal-directed actions using functional magnetic resonance imaging (fMRI). The types of rewards presented included both social rewards (i.e., images of smiling faces) and object rewards (i.e., images of objects previously identified as highly interesting to individuals with ASD. hereafter referred to as “nonsocial rewards”) (Sasson, Dichter, & Bodfish, 2012), This study is an extension of our prior study examining monetary RPEs in adults with ASD (Mosner et al., 2019) in a different sample of participants. The importance of investigating different classes of rewards (i.e., social and nonsocial) was highlighted in a recent review that found that the nature of reward-related impairments in ASD is dependent in large part on the type of reward stimuli (Bottini, 2018) and by a recent meta-analysis of fMRI studies that likewise reported that patterns of atypical frontostriatal reward circuitry responses in ASD are contingent on whether rewards are social or nonsocial (Clements et al., 2018). Furthermore, since ASD is a condition that develops in childhood, adolescents rather than adults were recruited in an effort to examine neural mechanisms of ASD while participants were at a relatively young age, but with the ability to complete complex tasks.
Hypotheses were informed by the nonclinical RPE neuroimaging literature as well as our previous findings of greater frontostriatal brain activation in ASD in response to monetary RPEs in ASD (Mosner et al., 2019) and by the broader neuroimaging literature addressing reward circuitry responses to social and nonsocial rewards in ASD (Dichter, Damiano, & Allen, 2012). Although this literature is somewhat inconsistent, the preponderance of evidence suggests that ASD is characterized by greater frontostriatal impairments to social relative to nonsocial rewards in ASD (Clements et al., 2018). Therefore, we hypothesized group differences in frontostriatal regions during social RPEs given the centrality of RPEs for processing social information in ASD (Sinha et al., 2014). Because prioritization of processing nonsocial relative to social rewards in ASD has been documented using the same nonsocial stimuli as in the present study (Sasson, Elison, Turner-Brown, Dichter, & Bodfish, 2011; Sasson & Touchstone, 2014; Unruh et al., 2016), we hypothesized that we would observe no differences with respect to brain activation in response to nonsocial RPEs in ASD. We also examined relations between neural responses and ASD behavior and symptom severity.
Methods
Participants
This protocol was approved by the Institutional Review Boards at the University of North Carolina at Chapel Hill (UNC) and Duke University Medical Center. Informed consent from parents/guardians and informed assent from participants were obtained. Participants with ASD were recruited through the UNC Autism Research Registry. Participants with typical development (TD) were recruited via UNC mass emails, the UNC Child Development Registry, and letters to families through the Orange County school system.
For both groups, participants were included if they spoke English fluently and met the following criteria: (1) 12 – 18 years of age; (2) living with a parent or guardian; (3) parent or guardian able to attend research visits; (4) no siblings participating in the study; (5) no sensory deficits (i.e., not blind or deaf); (6) no significant physical impairments; (7) ability to complete the fMRI task; (8) no fMRI contraindications; (9) the ability to remain still during the scan; and (10) no history of seizures, claustrophobia, or concussions.
Additionally, participants with ASD had a diagnosis of ASD confirmed by: (1) history of a clinical ASD diagnosis; (2) a score of 15 or greater on the Social Communication Questionnaire (SCQ; Chandler et al., 2007), a parent interview which screens for the likelihood of ASD; and (3) a score consistent with “autism” or “autism spectrum” on Module 3 or 4 of the Autism Diagnostic Observation Schedule-2 (ADOS-2; Lord et al., 2012), administered by a research reliable assessor and using standard cutoffs (J.L.K.). Participants in the TD group also met the following criteria: (1) no self-reported genetic, medical, psychiatric, neurologic, or learning condition; (2) no psychotropic medication use; and (3) a score of less than 15 on the SCQ.
Sixty-five participants completed a screening visit, including 26 participants with TD (12 males, 14 females) and 39 participants with ASD (32 males, 7 females). Of these, 42 completed the fMRI scan: 20 with TD (9 males, 11 females) and 22 with ASD (19 males, 3 females).
Procedure
Participants completed two study visits. During the first visit, participants and at least one parent or guardian completed questionnaires and interviews, including: the Hollingshead Four Factor Index of Social Status; the Kaufman Brief Intelligence Test, Second Edition (KBIT-2; Kaufman & Kaufman, 2004); the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord et al., 2012) (ASD group only); and the Social Responsiveness Scale (SRS; Constantino & Todd, 2005). The ADOS-2 was video-recorded and later analyzed using the Pragmatic Rating Scale, School Age (PRS-SA; Landa, 2015), which is an observational measure of ASD characteristics in the domain of social-communication. The PRS-SA provided a detailed examination of the social use of language in a semi-naturalistic context, allowing for an exploration of the relationships between brain activation in response to RPEs and how well adolescents with ASD used social-communication to interact with the examiner. See Supplementary Materials and Measures for a fuller description of these measures. Participants also rated the social and nonsocial stimuli they would see in the scanner task on the dimensions of valence and arousal. Participants were compensated $15/hour for this 4-hour assessment. During the second visit, participants completed an fMRI practice task and mock scan session while viewing real-time feedback of their head movement on a video monitor. Participants were compensated $50 for this visit.
As illustrated in Table 1, the TD and ASD groups did not differ in race, age, ethnicity, socioeconomic status, KBIT-2 IQ Composite Scores, or KBIT-2 Nonverbal IQ. Groups were significantly different in sex ratio (i.e., less female participants in the ASD group), KBIT-2 Verbal IQ (i.e., ASD<TD), and SRS Total T Scores (i.e., ASD>TD).
Table 1.
Participant characteristics (N = 42)
| TD group | ASD group | Test statistic | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | M (SD) | n | % | M (SD) | ||||
| Sex | X2(1, N = 42) = 6.31 | 0.01** | |||||||
| Male | 9 | 45.0 | -- | 19 | 86.4 | -- | |||
| Female | 1 | 55.0 | -- | 3 | 13.6 | -- | |||
| Race | X2(1, N = 42) = 2.78 | 0.10 | |||||||
| Black or African American | 4 | 20.0 | -- | 1 | 4.5 | -- | |||
| White | 1 | 65.0 | -- | 20 | 90.9 | -- | |||
| More than one race | 3 | 15.0 | -- | 1 | 4.5 | -- | |||
| Ethnicity | X2(2, N = 42) = 4.94 | 0.08 | |||||||
| Hispanic | 0 | 0.0 | -- | 3 | 13.6 | -- | |||
| Non-Hispanic | 1 | 90.0 | -- | 19 | 86.4 | -- | |||
| Unknown or not reported | 2 | 10.0 | -- | 0 | 0.0 | -- | |||
| Age | 2 | -- | 14.75 (2.07) | 22 | -- | 14.66 (1.65) | W = 215.5 | 0.92 | |
| Socioeconomic status | 2 | -- | 51.83 (10.50) | 22 | -- | 50.89 (13.74) | W = 210 | 0.81 | |
| KBIT-2 | |||||||||
| IQ Composite | 2 | -- | 109.85 (14.87) | 22 | -- | 100.95 (17.04) | t(40) = 1.79 | 0.08 | |
| Verbal IQ | 2 | -- | 112.40 (12.41) | 22 | -- | 102.27 (15.13) | t(40) = 2.36 | 0.02* | |
| Nonverbal IQ | 2 | -- | 104.05 (14.99) | 22 | -- | 98.82 (17.82) | t(40) = 1.02 | 0.31 | |
| SRS Total T Score | 2 | -- | 44.95 (8.09) | 22 | -- | 80.55 (8.06) | W = 3, p <0.001 | <0.001*** | |
| ADOS-2 | |||||||||
| Total (SA + RRB) Score | - | -- | -- | 22 | -- | 15.64 (4.27) | -- | ||
| Calibrated Severity Score | - | -- | -- | 22 | -- | 8.45 (1.41) | -- | ||
| PRS-SA | |||||||||
| Total Score | - | -- | -- | 18 | -- | 32.94 (6.26) | -- | ||
| Discourse Management | - | -- | -- | 18 | -- | 7.00 (2.57) | -- | ||
| Presupposition | - | -- | -- | 18 | -- | 10.44 (2.36) | -- | ||
| Language | - | -- | -- | 18 | -- | 5.33 (1.68) | -- | ||
| Paralinguistics | - | -- | -- | 18 | -- | 10.17 (3.65) | -- | ||
| Degree of Impairment | - | -- | -- | 18 | -- | 2.28 (0.57) | -- | ||
Note. M = Mean; SD = Standard Deviation; Socioeconomic status measured by Hollingshead Four Factor Index; KBIT-2 = Kaufman Brief Intelligence Test, 2nd edition; ADOS-2 = Autism Diagnostic Observation Schedule, 2nd edition; SA = Social Affect; RRB= Restricted and Repetitive Behaviors; SRS = Social Responsiveness Scale; PRS-SA = Pragmatic Rating Scale-School Age. Mann Whitney Wilcoxon (W) tests are reported for variables that were not normally distributed.
= p < 0.05
= p < 0.01
= p < 0.001.
fMRI task
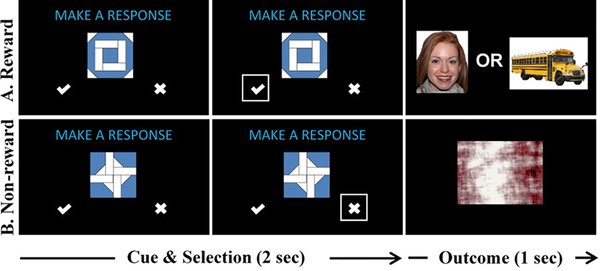
During fMRI scanning, participants completed two versions of a RPE task, each presented in separate runs: a social version, where participants viewed Happy-Direct Gaze Closed Mouth Female NimStim images (Tottenham et al., 2009) as rewards; and a nonsocial version, where nonsocial images that did not contain any faces or bodies were presented that were drawn from a set of nonsocial images used in previous ASD studies (e.g., vehicles, video game consoles) that have been identified as pleasing to individuals with ASD (Sasson et al., 2012). The task (see Figure 1) is modeled after the task described in three prior fMRI RPE studies (Addicott, Oliver, & Joseph McClernon, 2017; Mosner et al., 2019; Ramnani, Elliott, Athwal, & Passingham, 2004). Trials consisted of a cue phase and an outcome phase. During the cue phase, participants viewed one of two “quilt” patterns (i.e., Pattern A and B). During the outcome phase, participants viewed either a “reward” or a “non-reward”: rewards were images of faces (for the social runs) or objects (for the nonsocial runs), and non-rewards were scrambled images drawn from the same image sets. Participants were taught that Pattern A or Pattern B (counter-balanced across participants) predicted an unscrambled image (the rewarding image) and that the other pattern predicted a scrambled image (the non-rewarding image).
Figure 1.
Prediction Error Task. The task consisted of a cue phase, which lasted 2 seconds (first two columns above), and an outcome phase, which lasted 1 second (last column above). During the cue phase, participants were shown one of two cues (Pattern A in row 1 or Pattern B in row 2). Participants selected one of two options: (1) a “check mark” to predict that a reward would appear next (i.e., an unscrambled image); or (2) an “X” to predict that a non-reward would appear next (i.e., a scrambled image). There were two versions of the task: (1) a social version, where the reward was an unscrambled image of a smiling face; and (2) a nonsocial version, where the reward was an unscrambled image of a nonsocial object previously identified as highly pleasing to individuals with ASD (see text for details).
On each trial, the cue appeared for 2 seconds, along with the phrase “Make a Response” and two choices: a “check mark” or an “X.” Participants pressed a button as quickly as possible corresponding with their choice: the “check mark” indicated they predicted that an unscrambled image would appear next, and the “X” indicated they predicted that a scrambled image would appear next. After responding, a box outlined the selection and the outcome was shown for 1 second (see Figure 1). Participant responses did not affect outcomes. If no response was made, “Missed Response” was shown during the outcome phase and that trial was excluded from analyses. A jittered delay occurred between the cue and the outcome from 0.8 to 1.6 seconds. Prior to completing the scanning task, participants completed an 80-trial training version of the task outside of the scanner during which they were taught associations between the two patterns and the two outcomes. The practice task used different images than those used during the scan.
Participants completed four runs of the task in the scanner: two social runs and two nonsocial runs. Note that three participants with ASD did not complete all four runs for the following reasons: (1) fatigue; (2) needing to take breaks; or (3) discomfort in the scanner. Run type order was counterbalanced and each participant received one of the following orders: (a) social-nonsocial-nonsocial-social; or (b) nonsocial-social-social-nonsocial. Each run consisted of 100 trials: 50 with Pattern A and 50 with Pattern B. On 80% of the trials, the cue-outcome relationship was the same as in the training version (i.e., no prediction errors); however, in 20% of the trials, the cue-outcome relationship was the opposite of the training version (i.e., prediction errors). This resulted in four possible outcomes: expected reward, expected non-reward, unexpected reward, and unexpected non-reward. The first 11 trials did not produce prediction errors to reinforce the cue-outcome pairings that had been learned during training.
MRI Acquisition and Preprocessing
MRI acquisition and preprocessing information are presented in Supplemental Materials.
fMRI Data Analysis
Anatomical regions functionally involved in reward learning and functionally impaired in reward processing in ASD (i.e., the frontal lobes, amygdala, nucleus accumbens, insula, thalamus, caudate nucleus, anterior cingulate gyrus, and putamen) (Dichter, Felder, et al., 2012; Schmitz et al., 2008; Schultz, 2015) were defined a priori for small volume correction. These regions were generated in FSL using the Harvard-Oxford cortical and subcortical structural probabilistic atlases. Masks were thresholded at 25%, binarized, and combined into a single mask using fslmaths. Voxels were considered significant if they passed a threshold of p<.005, uncorrected, and were part of a 39-voxel cluster of contiguous significant voxels, corresponding to a family-wise corrected p<.05. This cluster size was determined by using a Monte Carlo simulation via the updated version of 3dFWHMx and using 3dClustSim programs from AFNI software package (Ward, 2000). This approach aligns with current recommendations for cluster-level correction (Cox, Chen, Glen, Reynolds, & Taylor, 2017). Localizations were based on Harvard-Oxford cortical and subcortical structural probabilistic atlases as implemented in FSLView version 5.0.10. Activations were visualized with FSLView version 5.0.10.
We then conducted a general linear model (GLM) using FEAT to examine group differences with respect to contrasts of interest. First level analyses conducted for each participant included a total of six regressors, each convolved with a double-gamma hemodynamic response function. As in Mosner et al. (2019), regressors 1 – 3 modeled the orthogonal components of the mean activation across all events from the following phases: (1) Reward cues; (2) Non-reward cues; and (3) Outcomes. We also included three regressors as parametric modulators of the outcome events that described changes relative to the mean of the outcome activation (Regressors 4 – 6). Regressor 4, the signed prediction error (Schultz, 1997), modeled positive changes for all unexpected rewards and negative changes for all unexpected reward omissions relative to the mean event activation. For a given trial ( t ) the signed prediction error (SPE) is the difference between the experienced reward on that trial ( rt ) and the expected reward for that stimulus (E[r]), see equation 1:
| 1) |
For regressor 5, we used a variant of the unsigned prediction error (e.g., Matsumoto & Hikosaka, 2009), contrasting expected and unexpected outcomes regardless of magnitude. Unexpected outcomes (large RPEs that were positive or negative) were modeled as positive deviations from the mean event activation, and expected outcomes (small RPEs that were positive or negative) were modeled as negative deviations (−1) (no trial outcomes had a prediction error of zero), see equation 2. Outcomes contain only two levels of deviation from the mean reward, allowing only a two-level contrast and not a true parametric model. We have therefore labeled this regressor as the thresholded unsigned prediction error (tUPE).
| 2) |
Regressor 6 modeled the mean of all rewarded outcomes (+1) relative to non-rewarded outcomes (−1), regardless of the magnitude of the outcome. This regressor is similar to (but not the same as) the SPE parametric regressor. Instead of modeling the magnitude of the reward surprise, it models the receipt of all rewards as producing the same response. Although it is not always included, we include it here to provide a point of comparison with previous work using the same task (Addicott et al., 2017; Mosner et al., 2019). Parametric outcome regressors were orthogonalized with respect to the mean outcome, and contrasts were defined for each outcome. Cue phase regressors were included to control for overall BOLD variance but are not the subject of the present hypotheses and thus are not presented.
For each participant, a second-level fixed-effect analysis averaged the contrasts across: (1) the two functional runs for the social task; and (2) the two functional runs for the nonsocial task. Finally, the primary method of analysis was to identify clusters that showed a main effect of Group (ASD versus TD). Group-wise activation images were calculated by a mixed effects analysis using Bayesian estimation techniques with FMRIB Local Analysis of Mixed Effects (FLAME 1+2; Smith et al., 2004; Woolrich, Ripley, Brady, & Smith, 2001). All other analyses were completed with R version 3.5.1 or SAS version 9.4. To provide context for the main results, we also include the following analyses: (a) models including gender as a covariate; (b) within group activations; (c) voxel-level corrected results; (d) group (ASD vs. TD) x task (social vs. nonsocial) interactions. Models including gender as a covariate were nearly identical to those that did not include this covariate, and so the results below reflect models without this covariate.
Results
Motion
There were no group differences in motion parameters (p’s>0.05 for x, y, z, pitch, yaw, and roll). Three participants in the ASD group and four participants in the TD group had motion in at least one run that was >2mm along at least one of the six axes. Runs with >2mm motion were excluded; however, each of these seven participants had at least one usable run remaining (i.e., <2mm motion), and these participants were included in analyses.
Missing Responses, Task Accuracy and Reaction Time
Groups did not differ on the percentage of missing responses within the social condition (W = 170.5, p = 0.30), the nonsocial condition (W = 206.5, p = 0.87), or across both conditions (W = 204, p = 0.69), In terms of task accuracy, groups did not differ in the accuracy of their responses within the nonsocial condition (W = 252.5, p = 0.16). However, there were significant group differences in task accuracy within the social condition (W = 286.5, p = 0.05) and across both social and nonsocial conditions (W = 298.5, p = 0.05). Significant differences were also found in reaction time within the social condition (t(31.08) = −4.28, p <0.001), the nonsocial condition (t(28.97) = −3.30, p = 0.003), and across both social and nonsocial conditions (t(32.75) = −4.16, p < 0.001). Overall, the TD group performed more accurately during the social condition and more slowly during both the social and nonsocial conditions, whereas the ASD group performed less accurately during the social condition and more quickly during both social and nonsocial conditions. Despite these differences, both groups performed at over 90% accuracy in both social and nonsocial conditions, suggesting that both groups understood the task. Within the ASD group, we also compared performance on the social and nonsocial tasks to explore whether participants with ASD demonstrated better responding to one type of stimulus. No differences between social and nonsocial responding were found for missing responses, task accuracy, or reaction time (p’s > 0.05) in the ASD group. Mean missing responses, task accuracy and reaction times are presented in Supplementary Figure 1.
Stimulus Ratings
Groups did not differ in ratings of Valence or Arousal of social or nonsocial stimuli, t’s(40)<1.80, p’s>.08. Mean ratings of valence and arousal are presented in Supplementary Figure 2.
Behavioral Indices of Learning During the fMRI Task
To address whether groups differed in learning behavior from choice data collected during scanning, we used multilevel logistic modeling to compare groups on the effect of accuracy on a given trial (correct or incorrect) on accuracy on the subsequent trial. There was no significant difference between groups in this behavior, γPriorAccuracy (0/1) *Group (ASD/TD) = −.94, SE = .75, t(29) = 1.57, p = 0.22. This suggests that there was no significant group difference in the association between accuracy on the prior trial and accuracy on the current trial.
fMRI Results
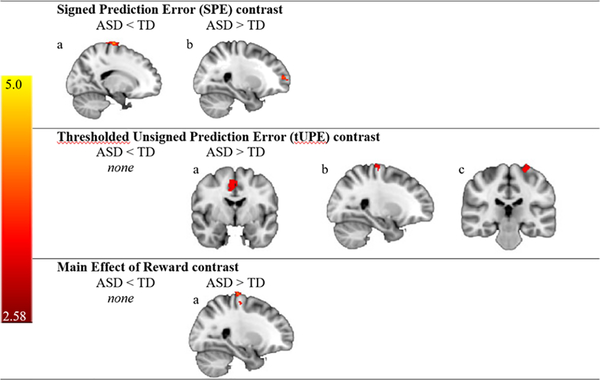
Contrasts of interest were the signed prediction error, the thresholded unsigned prediction error, and main effect of reward. For the social runs, in the signed prediction error (SPE, eq. 1) contrast, the ASD group demonstrated decreased activation relative to the TD group in one cluster in the right precentral gyrus and greater activation than the TD group in one cluster in the right frontal pole. In the thresholded unsigned prediction error (tUPE, eq. 2) contrast, there were no clusters in which the ASD group had decreased activation relative to the TD group; however, the ASD group demonstrated greater activation than the TD group in clusters in the right anterior cingulate gyrus, the right precentral gyrus, and the left precentral gyrus. For the main effect of reward, there were no clusters in which the ASD group had decreased activation relative to the TD group. However, the ASD group demonstrated greater activation than the TD group in the right precentral gyrus (see Figure 2 and Table 2). For the nonsocial runs, across all contrasts there were no clusters in which the ASD and TD groups differed in brain activation.
Figure 2.
Regions of Activation for the Social Task. Clusters that revealed group differences in voxel-wise analyses during the social version of the task are shown for the following contrasts: (1) SPE contrast: (a) ASD < TD, Right Hemisphere (RH) Precentral Gyrus, (b) ASD > TD, RH Frontal Pole; (2) tUPE contrast: (a) ASD > TD, RH Anterior Cingulate Gyrus, (b) ASD > TD, RH Precentral Gyrus, (c) ASD > TD, Left Hemisphere (LH) Precentral Gyrus; and (3) Main Effect of Reward: (a) ASD > TD, RH Precentral Gyrus. TD = typical development; ASD = autism spectrum disorder.
Table 2.
Frontostriatal functional activation clusters showing group differences in voxel-wise analyses for the social prediction error task.
| Region | Voxel Count | mm3 | Z (max) | MNI Space | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Signed Prediction Error (SPE) contrast | |||||||
| ASD < TD | |||||||
| RH Precentral Gyrus | 109 | 872 | 4.26 | 18 | -18 | 78 | |
| ASD > TD | |||||||
| RH Frontal Pole | 52 | 416 | 3.47 | 26 | 58 | 10 | |
| Thresholded Unsigned Prediction Error (tUPE) contrast | |||||||
| ASD < TD | |||||||
| ---- | ---- | ---- | ---- | ---- | ---- | ---- | |
| ASD > TD | |||||||
| RH Anterior Cingulate Gyrus | 215 | 1720 | 3.78 | 6 | -4 | 46 | |
| RH Precentral Gyrus | 80 | 640 | 3.39 | 26 | -20 | 70 | |
| LH Precentral Gyrus | 74 | 592 | 3.59 | -24 | -26 | 70 | |
| Main Effect of Reward contrast | |||||||
| ASD < TD | |||||||
| ---- | ---- | ---- | ---- | ---- | ---- | ---- | |
| ASD > TD | |||||||
| RH Precentral Gyrus | 70 | 560 | 3.46 | 26 | -22 | 76 | |
Note. Regions identified using the Harvard-Oxford Cortical and Subcortical Structural Atlases; RH = right hemisphere; LH = left hemisphere. No regions of activation were identified for the nonsocial RPE task.
See the Supporting Information section for two additional fMRI analyses: (a) within group results (Supplementary Figures 3 and 4; Supplementary Tables 1 and 2); and (b) voxel-level corrected results (Supplementary Figure 5; Supplementary Table 3). Of note, the right anterior cingulate gyrus cluster that was identified above as significant (social task, tUPE contrast, ASD>TD) remained significant in the voxel-level corrected results. No significant clusters were identified for group by task interactions.
Correlations with Task Performance
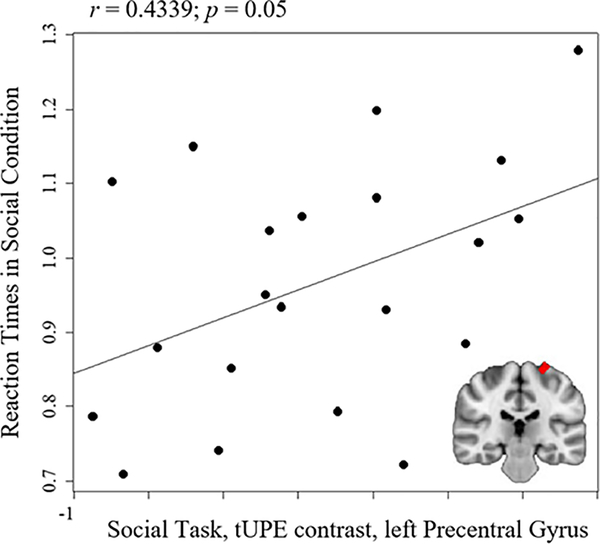
Correlations were explored between clusters that revealed group differences in voxel-wise group comparisons (i.e., clusters listed in Table 2) and task accuracy and reaction times within the ASD group. There were no significant correlations between fMRI results and accuracy (p’s > 0.05). Reaction times in the ASD group during the social condition were significantly correlated with the ASD>TD cluster in the left precentral gyrus during the social task for the tUPE contrast, (r = 0.43; p = 0.05), reflecting that quicker reaction times were associated with less activation in the left precentral gyrus (see Figure 3). This correlation was no longer significant when correcting for multiple comparisons using the Holm-Bonferroni method (p’s > 0.05).
Figure 3.
Correlation between reaction times for the social condition in the ASD group and the ASD>TD cluster in the left precentral gyrus during the social thresholded unsigned prediction error (tUPE) contrast in the ASD group.
Correlations with Clinical Measures
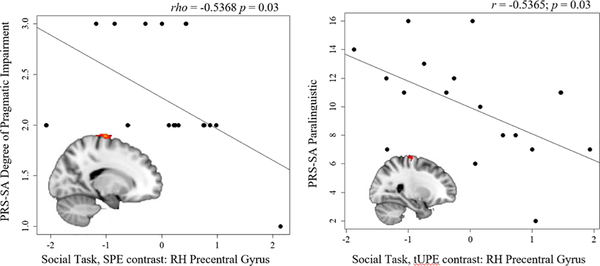
Within the ASD group, correlations were explored between functional clusters that revealed group differences and SRS total T-scores, ADOS-2 calibrated severity scores (CSS), and PRS-SA total and subdomain scores. No significant correlations were found for the SRS or the ADOS-2, p’s > 0.05. Two significant correlations were found when comparing functionally-defined clusters with the PRS-SA. First, PRS-SA Degree of Pragmatic Impairment was correlated with the TD>ASD cluster in the right precentral gyrus during the Social Task, SPE contrast (rho = −0.5368; p = 0.03; n = 17), reflecting that more severe social-communication impairments were associated with less precentral gyrus activation (see Figure 4). One participant received a score of “1” on the PRS-SA Degree of Pragmatic Impairment, and this correlation was no longer significant excluding this participant: (rho = −0.42, p = 0.1, n = 16). Second, the PRS-SA Paralinguistic subdomain was correlated with the ASD>TD cluster in the right precentral gyrus during the Social Task, tUPE contrast (r = −0.5365; p = 0.03; n = 17), reflecting that more severe impairments in paralinguistic skills (e.g., facial expressions, eye contact, gestures, intonation) were associated with less activation in the right precentral gyrus (see Figure 4). The correlations with the PRS-SA were no longer significant after correcting for multiple comparisons using the Holm-Bonferroni method (p’s > 0.05).
Figure 4.
Correlations between the Pragmatic Rating Scale—School Age (PRS-SA) and brain activation in the right hemisphere (RH) precentral gyrus during the Social Task in the ASD group. Left panel: PRS-SA Degree of Pragmatic Impairment and brain activation during the signed prediction error (SPE) contrast; Right panel: PRS-SA Paralinguistic subdomain score and brain activation during the thresholded unsigned prediction error (tUPE) contrast.
Discussion
A growing body of research indicates that ASD is characterized by deficits in reward processing in frontostriatal brain regions during adolescence and adulthood (Bottini, 2018) and most neuroimaging studies have focused on the anticipation and outcome phases of reward processing in ASD (Clements et al., 2018). Two recent studies of adults with ASD investigated neural mechanisms of RPEs, a critical component of reward-based learning, and reported altered frontostriatal responses to monetary RPEs in ASD (Balsters et al., 2017; Mosner et al., 2019). The current study extends these findings by investigating RPEs among adolescents with ASD in the context of social and nonsocial rewards. We found altered frontal activation in ASD during social RPEs, but no differences during nonsocial RPEs. Furthermore, in exploratory analyses, social RPE signaling correlated with reaction times and social-communication skills in the ASD group, whereby quicker reaction times and more severe social-communication impairments were associated with reduced social RPE activation.
Social Prediction Error Signaling
As hypothesized, social RPE signaling was decreased in frontal regions in ASD. Relative to individuals with TD, these decreases were characterized as right precentral gyrus hypoactivation in the signed prediction error (SPE) contrast and bilateral precentral gyrus hyperactivation in the thresholded unsigned prediction error (tUPE) contrast, right frontal pole hyperactivation in the SPE contrast, and anterior cingulate gyrus hyperactivation in the tUPE contrast. The anterior cingulate gyrus hyperactivation is noteworthy because: (1) this cluster was identified as significant during both uncorrected and corrected vowel-wise analyses; and (2) Balsters et al. (2017) recently reported disrupted error signal processing in this region in ASD that predicted the severity of social impairments. Together, these findingshighlight the critical role that the anterior cingulate gyrus plays in impaired prediction error signaling in ASD.
Contrary to our hypothesis, groups did not differ in activation in the dorsal or ventral striatum, regions that play critical roles in prediction error processing (Langdon, Sharpe, Schoenbaum, & Niv, 2018). However, the dorsal and ventral striatum have not previously been reported to be differentially activated in ASD in prediction error tasks (Balsters et al., 2017; Mosner et al., 2019); rather, frontal regions have consistently emerged as differentially recruited in ASD. Although multiple brain regions are involved in RPE coding (Garrison, Erdeniz, & Done, 2013), the frontal lobes appear to be principally involved in updating predictions during decision making (Vassena, Krebs, Silvetti, Fias, & Verguts, 2014; Wang et al., 2017) and vicarious reward processing (Apps & Ramnani, 2014).
We also found that the ASD group demonstrated differential precentral gyrus activation during social RPE signaling, and task-related reaction times were correlated with left precentral gyrus activation during social thresholded unsigned prediction errors in the ASD group. In other words, adolescents with ASD who responded more quickly during the social task also tended to demonstrate less activation in the left precentral gyrus when faces were expected but not presented. The precentral gyrus regulates motor execution and planning (Squire et al., 2012), and demonstrates differential activation during prediction errors in schizophrenia (Vanes, Mouchlianitis, Collier, Averbeck, & Shergill, 2018; Waltz et al., 2009). A recent EEG study found that adults with ASD responded to unpredictable visual targets with relatively greater neural motor preparation, suggesting a response pattern characterized by “over-anticipating” (Thillay et al., 2016). This is consistent with evidence for difficulty predicting the consequences of motor movements to achieve goals and the impaired use of predictive information to guide behavior in infants who later developed ASD (LeBarton & Landa, 2019). Taken together, these findings are consistent with the theory that ASD is a disorder characterized by predictive impairments (Sinha et al., 2014). With this past research in mind, it is possible that the adolescents with ASD in our study were over-anticipating when they would see unexpected social images and/or had trouble using predictions to plan motor movements, which translated to increased reaction times and associated disruptions in the precentral gyrus during social RPE signaling.
The ASD group also demonstrated changes in activation of the right precentral gyrus during social signed prediction errors (increased activation) and thresholded unsigned prediction errors (decreased activation). Although additional prediction error values would be required to map the relationship, the overall response can be approximated as an increased right precentral gyrus response to both positive and neutral stimuli and diminished right precentral gyrus response to negative stimuli. It is also noteworthy that social prediction error signaling in the right precentral gyrus was associated with social-communication impairments in the ASD group, both broadly and within the domain of paralinguistics (i.e., nonverbal communication, such as intonation, rate, facial expressions, eye contact, and gestures). This region demonstrated less activation during social prediction errors in those with more severe social-communication impairments. Interestingly, other neuroimaging studies have found a linkage between paralinguistic communication in individuals with typical development and predictive coding. For example, an EEG study found increased activity in frontal temporal brain regions associated with predictive coding during observation of gesture-accompanied-speech, suggesting that the gestures played a role in predicting upcoming speech (Biau, Torralba, Fuentemilla, de Diego Balaguer, & Soto-Faraco, 2015). When findings are viewed in light of this research, there may be a predictive coding mechanism that contributes to both social RPE processing and social-communication skills, particularly in the area of nonverbal communication; furthermore, the degree to which this mechanism is functionally disrupted may contribute to ASD symptom severity.
The ASD group also demonstrated hyperactivation in the right frontal pole during social signed prediction errors, and Mosner et al. (2019) similarly reported right frontal pole hyperactivation when processing monetary signed prediction errors among adults with ASD. The frontal pole is involved in multiple aspects of reward processing, including exploring alternate rewards (Boorman, Behrens, Woolrich, & Rushworth, 2009; Daw, O’doherty, Dayan, Seymour, & Dolan, 2006; Mansouri, Buckley, Mahboubi, & Tanaka, 2015) and effort expenditure for monetary rewards (Soutschek, Kang, Ruff, Hare, & Tobler, 2018).The right frontal pole also shows increased activation in children and adolescents with ASD during nonsocial reward anticipation in response to intranasal oxytocin (Greene et al., 2018). When considering these frontal pole functions in relation to the study task, it may be the case that, when processing unexpected social rewards, recruitment of the frontal pole reflected effort expenditure in the ASD group, given the possible challenges with prediction noted in this disorder (Sinha et al., 2014).
Overall, our social prediction error signaling findings included both neural hyperactivation and hypoactivation in the ASD group. We interpret both patterns to reflect disrupted error signal processing. Hypoactive neural responses in the anterior cingulate cortex during social prediction error signaling in ASD has been interpreted to reflect a reduction in social prediction errors in ASD and a potential mechanism underlying social deficits within the disorder (Balsters et al., 2017). Conversely, hyperactive neural signal are generally interpreted to reflect a compensatory mechanism engaged to perform a given task (e.g., Schmitz et al., 2006) or neural inefficiencies (e.g., Buchsbaum et al., 2007; Wagner et al., 2006). Manoach (2003) suggested that it may well be that variability in brain activation may best be regarded as intrinsic to heterogeneous disorders, and Dinstein and colleagues (2012) suggested that unreliable neural responses may represent a fundamental characteristic of neural processing in ASD. We thus we interpret the present findings (i.e., patterns of both hyperactivation and hypoactivation) within the framework of dysregulated and inefficient frontostriatal recruitment in ASD during social prediction error signaling
Nonsocial Prediction Error Signaling
Contrary to the social condition, adolescents with and without ASD responded similarly to nonsocial RPEs. These rewards included pictures of objects identified in previous studies as highly interesting to individuals with ASD and did not include any social content (e.g., video game consoles and vehicles; Sasson et al., 2012). ASD is characterized by restricted and repetitive patterns of behaviors and interests, and the lack group of difference during nonsocial RPEs may reflect the prioritization of processing the nonsocial stimuli used in this study that resulted in neural responses comparable to those in the TD group (Sasson et al., 2011; Sasson & Touchstone, 2014; Unruh et al., 2016). It should be noted, however, that there were no differences between the ASD and TD group: (a) when activation patterns were directly compared between social and nonsocial conditions; or (b) in how groups rated the social and nonsocial images. There were also no differences within the ASD group in their accuracy or rate of responding across the social and nonsocial tasks. Thus, although these images of objects have been identified in previous studies as more pleasing to individuals with ASD (Sasson et al., 2012), that same preference was not supported by the ratings or behavioral performance of our current sample. Future studies could help clarify the current results by using nonsocial stimuli that represent the specific restricted interests of participants.
Main Effect of Reward
Finally, we found that adolescents with ASD demonstrated hyperactivation of the right precentral gyrus when receiving social rewards but no differences between groups during the receipt of nonsocial rewards. This hyperactivation in a motor area may reflect compensatory motivation for reward perceived as diminished relative to expectations. A number of previous studies have found disrupted social reward outcome processing in ASD in several brain regions, including: hyperactivation of the bilateral insular cortex (Dichter, Richey, Rittenberg, Sabatino, & Bodfish, 2012), hypoactivation of the amygdala and anterior cingulate cortex (Kohls et al., 2013), and hypoactivation of the ventral striatum (Scott-Van Zeeland et al., 2010). These studies would seem to constrain possible interpretations of this activation. We therefore interpret this result as consistent with compensation for disrupted social reward outcome processing among individuals with ASD.
Limitations
This study has limitations that should be addressed in future studies. First, the sample size was relatively small, and the small number of females in the ASD group did not allow for an examination of sex differences in neural activation patterns. Future studies would also benefit from recruiting a larger sample across age ranges to examine developmental trajectories of RPEs in ASD. Additionally, correlations between neural activations and behavior and symptom severity did not survive corrections for multiple comparisons, and these findings should be considered exploratory until replicated in larger samples. Group by task interactions did not yield any significant clusters, and so conclusions about differences between neural responses to social and nonsocial RPE’s should be interpreted with caution. Finally, because of the demands of the task and of the neuroimaging environment, participants with ASD were adolescents with at least average IQ scores, and results may not generalize to individuals with ASD who are younger and/or with lower IQ scores.
Conclusions
The present findings indicate neural mechanisms underlying social RPEs in ASD and suggest that these neural patterns are related to the severity of social-communication impairments. Results further extend the literature addressing the neurobiological substrates of learning impairments and, by extension, insistence on sameness behaviors that are often observed in ASD (APA, 2013). These findings suggest an additional domain of social reward processing that is impaired in ASD, and in this regard, expand the social motivation hypothesis of autism (Chevallier et al., 2012). Moreover, these results are consistent with more recent theories that ASD is a disorder characterized by impaired predictive abilities (Sinha et al., 2014). This study did not find brain activation differences to nonsocial RPEs in ASD, highlighting yet another domain in which processing nonsocial rewards could potentially be prioritized in ASD and/or in which processing social rewards could be more challenging, although these speculations need to be examined in future studies (Sasson et al., 2011; Sasson & Touchstone, 2014; Unruh et al., 2016). Differential neural activation in response to social RPEs may impact learning in individuals with ASD; however, since participants in the current study learned the task prior to entering the scanner, we were unable to examine learning. Future fMRI studies will be needed to explore the degree to which aberrant neural responses to social RPEs among individuals with ASD impacts learning complex tasks.
When considered with other studies addressing the neural mechanisms of RPEs in ASD (Balsters et al., 2017; Mosner et al., 2019), the current findings have implications for ASD interventions. Many ASD therapies use rewards to motivate learning, with varying degrees of success (Sherer & Schreibman, 2005; Spreckley & Boyd, 2009; Vismara & Rogers, 2010). Unpredictable social rewards may be used during interventions to enhance social-communication skills in individuals with ASD, given our exploratory findings that social RPEs are associated with skills such as eye contact, gestures, facial expressions, and intonation. Additionally, unexpected nonsocial rewards may potentially elicit desired behaviors during an intervention, given that nonsocial RPEs were relatively unimpaired in the current sample. Since prediction errors are an important component of learning, improved understanding of social and nonsocial rewards and their impact on behavior will be critical for continued development of ASD interventions.
Supplementary Material
Acknowledgements
We extend our sincere gratitude to the families who participated in this study.
Funding Information
This research was supported by NARSAD Young Investigator Award to R. McKell Carter, MH110933 to Gabriel S. Dichter, MH113733 to Erin Walsh, and MH109667 to Tory Eisenlohr-Moul. Jessica Kinard was supported by HD40127 (Piven and Philpot). Assistance with recruitment was provided by the Clinical Translational Core of the UNC Intellectual Developmental Disabilities Research Center (HD079124). Funding sources had no direct involvement in the study design, collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication.
References
- Addicott MA, Oliver JA, & Joseph McClernon F (2017). Nicotine increases anterior insula activation to expected and unexpected outcomes among nonsmokers. Psychopharmacology (Berl), 234(7), 1145–1154. doi: 10.1007/s00213-017-4550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-V (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Apps MAJ, & Ramnani N (2014). The anterior cingulate gyrus signals the net value of others’ rewards. The Journal of neuroscience, 34(18), 6190–6200. doi: 10.1523/JNEUROSCI.2701-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters JH, Apps MA, Bolis D, Lehner R, Gallagher L, & Wenderoth N (2017). Disrupted prediction errors index social deficits in autism spectrum disorder. Brain, 140(Pt 1), 235–246. doi: 10.1093/brain/aww287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biau E, Torralba M, Fuentemilla L, de Diego Balaguer R, & Soto-Faraco S (2015). Speaker’s hand gestures modulate speech perception through phase resetting of ongoing neural oscillations. Cortex, 68, 76–85. doi: 10.1016/j.cortex.2014.11.018 [DOI] [PubMed] [Google Scholar]
- Boorman ED, Behrens TEJ, Woolrich MW, & Rushworth MFS (2009). How Green Is the Grass on the Other Side? Frontopolar Cortex and the Evidence in Favor of Alternative Courses of Action. Neuron, 62(5), 733–743. doi: 10.1016/j.neuron.2009.05.014 [DOI] [PubMed] [Google Scholar]
- Bottini S (2018). Social reward processing in individuals with autism spectrum disorder: a systematic review of the social motivation hypothesis. Research in Autism Spectrum Disorders, 45, 9–26. [Google Scholar]
- Buchsbaum MS, Buchsbaum BR, Hazlett EA, Haznedar MM, Newmark R, Tang CY, & Hof PR (2007). Relative glucose metabolic rate higher in white matter in patients with schizophrenia. Am J Psychiatry, 164(7), 1072–1081. [DOI] [PubMed] [Google Scholar]
- Chandler S, Charman T, Baird G, Simonoff E, Loucas T, Meldrum D, … Pickles A (2007). Validation of the social communication questionnaire in a population cohort of children with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry, 46(10), 1324–1332. doi: 10.1097/chi.0b013e31812f7d8d [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, & Schultz RT (2012). The social motivation theory of autism. Trends in Cognitive Sciences, 16(4), 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi US, Kim SY, Sim HJ, Lee SY, Park SY, Jeong JS, … Cheon KA (2015). Abnormal brain activity in social reward learning in children with autism spectrum disorder: an fMRI study. Yonsei Med J, 56(3), 705–711. doi: 10.3349/ymj.2015.56.3.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements CC, Zoltowski AR, Yankowitz LD, Yerys BE, Schultz RT, & Herrington JD (2018). Evaluation of the Social Motivation Hypothesis of Autism: A Systematic Review and Meta-analysis. JAMA Psychiatry, 75(8), 797–808. doi: 10.1001/jamapsychiatry.2018.1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Todd RD (2005). Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry, 57(6), 655–660. doi: 10.1016/j.biopsych.2004.12.014 [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect, 7(3), 152–171. doi: 10.1089/brain.2016.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, O’doherty JP, Dayan P, Seymour B, & Dolan RJ (2006). Cortical substrates for exploratory decisions in humans. Nature, 441(7095), 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, & McPartland J (2005). Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol, 27(3), 403–424. doi: 10.1207/s15326942dn2703_6 [DOI] [PubMed] [Google Scholar]
- Dichter GS (2018). Motivational Impairments in Autism May Be Broader Than Previously Thought. JAMA Psychiatry, 75(8), 773–774. doi: 10.1001/jamapsychiatry.2018.1078 [DOI] [PubMed] [Google Scholar]
- Dichter GS, Damiano CA, & Allen JA (2012). Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord, 4(1), 19. doi: 10.1186/1866-1955-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, & Bodfish JW (2012). Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci, 7(2), 160–172. doi: 10.1093/scan/nsq095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, & Bodfish JW (2012). Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord, 42(2), 147–160. doi: 10.1007/s10803-011-1221-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Heeger DJ, Lorenzi L, Minshew NJ, Malach R, & Behrmann M (2012). Unreliable evoked responses in autism. Neuron, 75(6), 981–991. doi: 10.1016/j.neuron.2012.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison J, Erdeniz B, & Done J (2013). Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neurosci Biobehav Rev, 37(7), 1297–1310. doi: 10.1016/j.neubiorev.2013.03.023 [DOI] [PubMed] [Google Scholar]
- Greene RK, Spanos M, Alderman C, Walsh E, Bizzell J, Mosner MG, … Dichter GS (2018). The effects of intranasal oxytocin on reward circuitry responses in children with autism spectrum disorder. J Neurodev Disord, 10(1), 12. doi: 10.1186/s11689-018-9228-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, & Kaufman NL (2004). Kaufman brief intelligence test : KBIT 2; manual. Bloomington, Minn. [u.a.: Pearson. [Google Scholar]
- Kohls G, Schulte-Ruther M, Nehrkorn B, Muller K, Fink GR, Kamp-Becker I, … Konrad K (2013). Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci, 8(5), 565–572. doi: 10.1093/scan/nss033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R (2015). Pragmatic Rating Scale-School Age. Kennedy Krieger Institute. Baltimore, MD. [Google Scholar]
- Langdon AJ, Sharpe MJ, Schoenbaum G, & Niv Y (2018). Model-based predictions for dopamine. Curr Opin Neurobiol, 49, 1–7. doi: 10.1016/j.conb.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson RP, Rees G, & Friston KJ (2014). An aberrant precision account of autism. Front Hum Neurosci, 8, 302. doi: 10.3389/fnhum.2014.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBarton ES, & Landa RJ (2019). Infant motor skill predicts later expressive language and autism spectrum disorder diagnosis. Infant Behavior and Development, 54, 37–47. [DOI] [PubMed] [Google Scholar]
- Lin A, Rangel A, & Adolphs R (2012). Impaired learning of social compared to monetary rewards in autism. Front Neurosci, 6, 143. doi: 10.3389/fnins.2012.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop S (2012). Autism Diagnostic Observation Schedule (2nd ed.). Torrance, CA: Western Psychological Services. [Google Scholar]
- Manoach DS (2003). Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res, 60(2–3), 285–298. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Buckley MJ, Mahboubi M, & Tanaka K (2015). Behavioral consequences of selective damage to frontal pole and posterior cingulate cortices. Proceedings of the National Academy of Sciences, 112(29), E3940–E3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosner MG, McLaurin RE, Kinard JL, Hakimi S, Parelman J, Shah JS, … Dichter GS (2019). Neural Mechanisms of Reward Prediction Error in Autism Spectrum Disorder. Autism Research and Treatment, 2019, 10. doi: 10.1155/2019/5469191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussey JL, Travers BG, Klinger LG, & Klinger MR (2015). Decision-making skills in ASD: performance on the Iowa Gambling Task. Autism Res, 8(1), 105–114. doi: 10.1002/aur.1429 [DOI] [PubMed] [Google Scholar]
- Ramnani N, Elliott R, Athwal BS, & Passingham RE (2004). Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage, 23(3), 777–786. doi: 10.1016/j.neuroimage.2004.07.028 [DOI] [PubMed] [Google Scholar]
- Sasson NJ, Dichter GS, & Bodfish JW (2012). Affective responses by adults with autism are reduced to social images but elevated to images related to circumscribed interests. PLoS One, 7(8), e42457. doi: 10.1371/journal.pone.0042457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Elison JT, Turner-Brown LM, Dichter GS, & Bodfish JW (2011). Brief report: Circumscribed attention in young children with autism. J Autism Dev Disord, 41(2), 242–247. doi: 10.1007/s10803-010-1038-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, & Touchstone EW (2014). Visual attention to competing social and object images by preschool children with autism spectrum disorder. J Autism Dev Disord, 44(3), 584–592. doi: 10.1007/s10803-013-1910-z [DOI] [PubMed] [Google Scholar]
- Schipul SE, & Just MA (2016). Diminished neural adaptation during implicit learning in autism. Neuroimage, 125, 332–341. doi: 10.1016/j.neuroimage.2015.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, Daly E, Smith A, Williams S, & Murphy DG (2006). Neural correlates of executive function in autistic spectrum disorders. Biological Psychiatry, 59(1), 7–16. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, van Amelsvoort T, Daly E, Smith A, & Murphy DG (2008). Neural correlates of reward in autism. Br J Psychiatry, 192(1), 19–24. doi: 10.1192/bjp.bp.107.036921 [DOI] [PubMed] [Google Scholar]
- Schultz W (1997). Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol, 7(2), 191–197. [DOI] [PubMed] [Google Scholar]
- Schultz W (2015). Neuronal Reward and Decision Signals: From Theories to Data. Physiol Rev, 95(3), 853–951. doi: 10.1152/physrev.00023.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, & Montague PR (1997). A neural substrate of prediction and reward. Science, 275(5306), 1593–1599. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, & Bookheimer SY (2010). Reward processing in autism. Autism Res, 3(2), 53–67. doi: 10.1002/aur.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer MR, & Schreibman L (2005). Individual behavioral profiles and predictors of treatment effectiveness for children with autism. J Consult Clin Psychol, 73(3), 525–538. doi: 10.1037/0022-006X.73.3.525 [DOI] [PubMed] [Google Scholar]
- Sinha P, Kjelgaard MM, Gandhi TK, Tsourides K, Cardinaux AL, Pantazis D, … Held RM (2014). Autism as a disorder of prediction. Proc Natl Acad Sci U S A, 111(42), 15220–15225. doi: 10.1073/pnas.1416797111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutschek A, Kang P, Ruff CC, Hare TA, & Tobler PN (2018). Brain stimulation over the frontopolar cortex enhances motivation to exert effort for reward. Biol Psychiatry, 84(1), 38–45. [DOI] [PubMed] [Google Scholar]
- Spreckley M, & Boyd R (2009). Efficacy of applied behavioral intervention in preschool children with autism for improving cognitive, language, and adaptive behavior: a systematic review and meta-analysis. J Pediatr, 154(3), 338–344. doi: 10.1016/j.jpeds.2008.09.012 [DOI] [PubMed] [Google Scholar]
- Squire L, Berg D, Bloom FE, Du Lac S, Ghosh A, & Spitzer NCE (2012). Fundamental neuroscience: Academic Press. [Google Scholar]
- Stavropoulos KK, & Carver LJ (2018). Oscillatory rhythm of reward: anticipation and processing of rewards in children with and without autism. Mol Autism, 9, 4. doi: 10.1186/s13229-018-0189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thillay A, Lemaire M, Roux S, Houy-Durand E, Barthelemy C, Knight RT, … Bonnet-Brilhault F (2016). Atypical Brain Mechanisms of Prediction According to Uncertainty in Autism. Front Neurosci, 10, 317. doi: 10.3389/fnins.2016.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, … Nelson C (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry research, 168(3), 242–249. doi: 10.1016/j.psychres.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh KE, Sasson NJ, Shafer RL, Whitten A, Miller SJ, Turner-Brown L, & Bodfish JW (2016). Social Orienting and Attention Is Influenced by the Presence of Competing Nonsocial Information in Adolescents with Autism. Front Neurosci, 10, 586. doi: 10.3389/fnins.2016.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Cruys S, Evers K, Van der Hallen R, Van Eylen L, Boets B, de-Wit L, & Wagemans J (2014). Precise minds in uncertain worlds: predictive coding in autism. Psychol Rev, 121(4), 649–675. doi: 10.1037/a0037665 [DOI] [PubMed] [Google Scholar]
- Vanes LD, Mouchlianitis E, Collier T, Averbeck BB, & Shergill SS (2018). Differential neural reward mechanisms in treatment-responsive and treatment-resistant schizophrenia. Psychol Med, 48(14), 2418–2427. doi: 10.1017/S0033291718000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassena E, Krebs RM, Silvetti M, Fias W, & Verguts T (2014). Dissociating contributions of ACC and vmPFC in reward prediction, outcome, and choice. Neuropsychologia, 59, 112–123. doi: 10.1016/j.neuropsychologia.2014.04.019 [DOI] [PubMed] [Google Scholar]
- Vismara LA, & Rogers SJ (2010). Behavioral treatments in autism spectrum disorder: what do we know? Annu Rev Clin Psychol, 6, 447–468. doi: 10.1146/annurev.clinpsy.121208.131151 [DOI] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, … Schlosser RG (2006). Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biol Psychiatry, 59(10), 958–965. doi: 10.1016/j.biopsych.2005.10.025 [DOI] [PubMed] [Google Scholar]
- Waltz JA, Schweitzer JB, Gold JM, Kurup PK, Ross TJ, Salmeron BJ, … Stein EA (2009). Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology, 34(6), 1567–1577. doi: 10.1038/npp.2008.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ma N, He X, Li N, Wei Z, Yang L, … Zhang X (2017). Neural substrates of updating the prediction through prediction error during decision making. Neuroimage, 157, 1–12. doi: 10.1016/j.neuroimage.2017.05.041 [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, & Smith SM (2001). Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage, 14(6), 1370–1386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.