Abstract
Immunoengineering is a new discipline that creates and applies engineering tools and principles to investigate and modulate the immune system. It spans from the molecular scale to the scale of populations and is critically important in both health and disease. This perspective discusses the rapid development of immunoengineering as a field, including advances to research and education. On the research side, immunoengineering is poised to revolutionize technologies for tissue engineering, drug delivery, and medical devices, among others. Immunoengineering is shown to unlock new tools for biomedical discovery and innovation and has the potential to safely and effectively treat myriad diseases, from cancer to infectious diseases to type 1 diabetes and autoimmune diseases in novel ways. On the educational side, it is described how immunoengineering centers and educational focus areas are being created at leading universities. Further, data is presented to show how grant agencies are making major investments into the field and high-impact research and translational biotechnologies are being developed.
Keywords: immunoengineering, biomaterials, biomedical engineering, immunology, education
INTRODUCTION
Since the founding of the Society For Biomaterials (SFB) 45 years ago, there have been tremendous achievements in biomaterials research, from the basic to the translational. Many of these discoveries and innovations have focused on the long-standing interests of biomaterials scientists and engineers in areas such as tissue engineering, drug delivery, and implantable devices. The newest SFB Special Interest Group (SIG), “Immune Engineering,” founded five years ago, is in many ways a natural outgrowth of these interests and goals. For example, immune engineering, or has it has also come to be commonly referred to as “immunoengineering,” involves approaches to improve the interactions of tissue engineered constructs and transplanted tissue with the host, to enhance long-circulating drug delivery nanoparticulates, and to prevent fibrosis from occurring with implanted biomedical devices. Thus, immunoengineering is at the forefront of many active areas within SFB and biomedical research more broadly.
Immunoengineering is a new discipline that creates and applies engineering tools and principles to investigate and modulate the immune system. Immunoengineering research spans from the molecular scale to the scale of populations and is critically important in both health and disease. It is attracting significant interest from academia, industry, and funding bodies as immunoengineering has the potential to revolutionize multiple fields of medicine including oncology, infectious diseases, autoimmunity, and transplantation, among others.
Although in some ways a natural outgrowth, in other ways the shift in biomaterials research represented by immunoengineering can also be seen as revolutionary. This is because historically, biomaterials research was often most interested in designing biomaterials that were as close as possible to being biologically inert, exhibiting no interactions with the body. For applications ranging from implants to circulating particles, the goal was to minimize biological interactions and a favorite biomaterial to minimize these interactions was often poly(ethylene glycol) (PEG). While for certain applications this approach is still quite common and useful, there is now also an increased awareness that an ideal biomaterial-host interaction, from tissue engineering scaffold to drug delivery device, is not necessarily prevention of interaction with the body, but instead, a bioactive or healing interaction with the body. For such an exquisite interaction to take place requires careful understanding and engineering of the immune system; it requires immunoengineering.
IMMUNOENGINEERING RESEARCH IMPACT
Research thrusts in immunoengineering have grown from the progress and vision of biomaterials scientists and engineers, especially from those with interests in biomimetic materials. Biomimetic materials are materials designed to emulate biological functions, usually by mimicking the physical, chemical, and biological properties found in living systems.1 Designing materials to emulate and interact with the immune system is especially intriguing due to the rapid pace that new immunological discoveries are being made in the basic sciences and the increasing opportunities to engineer and innovate synthetic systems that can re-create similar biology. As there are many areas of health and disease that are touched by immunology, immunoengineering can function as an enabling technology to drive fundamental discoveries to increase understanding, quantify and model the ever-increasing large biomedical data sets being generated, and treat seemingly disparate diseases through new paradigms.
Areas of medicine where immunoengineering research can make a translational impact include generating immune tolerance in cases of autoimmune diseases such as rheumatoid arthritis, type 1 diabetes, ulcerative colitis, Crohn’s disease, multiple sclerosis, lupus, and others. These diseases are caused by the immune system attacking the host, and immunoengineering can design biomaterials that signal the immune system to stop these attacks on “self” and to allow healing to take place.2 In a similar manner, immunoengineered materials could potentially obviate the need for immunosuppressants for the recipients of transplants. Key components for such a technology to be safe and effective are two layers of specificity: antigen-specificity so that the tolerance is specific to just the cells and tissues that are to be protected and immunomodulatory specificity so that, for example, regulatory T cells are stimulated to protect without stimulation of cytotoxic T cells. A careful balance is required so that autoimmunity is not made worse and yet the patient’s immune system can still respond in a robust way to pathogens or infectious agents.
Immunoengineering is also important in the field of sustained drug delivery, where the goal is to deliver a desired biomolecule to particular cells of the body over time in a durable fashion. While historically, PEG-coated particles have provided for extended circulation in the blood, for many applications, such as tumor-targeting, the current delivery approaches are insufficient (typically <1% of dose delivered to the target).3 Rather than conjugation of a synthetic polymer to the particle surface, biomimetic, immunoengineering approaches that disguise the surface as self, either by coating immune cell membranes on particle surfaces4 or conjugating biomimetic peptides to particle surfaces5 enables synthetic particles to become camouflaged from the immune system. Such stealthy nanosystems could function for extended periods as drug delivery devices, detoxification agents, or sensors, among other applications.
Immunoengineering for Vaccines and Therapeutics
To combat infectious diseases, it is desirable to engineer new vaccines that have enhanced efficacy, safety, and scalability. New types of immunoengineered vaccines are being constructed out of diverse materials such as liposomes carrying DNA to protect against malaria6 and biodegradable polymeric nanoparticles that acts as a nicotine vaccine.7 Engineering the glycosylation of immunomodulatory molecules, a type of glycoengineering, can also be used to further enhance the immunogenicity of antigens,8 and improve the design of vaccines and immunotherapies.9 Another important aspect of vaccines is their administration and biomaterials can be used to construct an array of microneedles that can painlessly deliver vaccines in a safe and easy-to-use manner.10,11 The use of biomaterials-based immunoengineering strategies are also opening up new avenues for vaccine translation, such as the development of mRNA vaccines for infectious diseases including for SARS-CoV-2.12
A particularly exciting aspect of immunoengineered vaccines are developments towards cancer vaccines. The premise is that instead of developing a therapeutic that must target and destroy every cancer cell in a patient’s body, the therapeutic need only reach a few target immune cells, where the therapeutic can train the immune system to activate, proliferate, and mount a systemic anti-cancer cellular immune response throughout the body. For example, mRNA encoding tumor antigen can be intracellularly delivered in nanoparticles to dendritic cells that can induce cytotoxic T cells to kill triple negative breast cancer in a mouse model, and act in combination with CTLA-4 immune checkpoint inhibition.13 In an alternative approach, the tumor microenvironment can also be reprogrammed in situ utilizing biodegradable nanoimmunomaterials to generate an antigen-specific systemic cellular immune response without needing a priori knowledge of the cancer-specific antigens.14 Cancer vaccines can also be designed based on stimulation of the innate arm of the immune system and immunoengineered nanoparticles can be utilized to dramatically boost potency. Poly(beta-amino ester) nanoparticles were found to encapsulate cyclic dinucleotides (STING agonists) and efficiently deliver them to the cytosol of immune cells, generating anti-tumor efficacy in combination with anti-PD-1 blocking antibody in a melanoma mouse model.15 In a different, antigen-specific approach, David Mooney and coworkers demonstrated that cationic polymer polyethylenimine coated mesoporous silica microrods can enhance the activity of adsorbed antigen and lead to systemic anti-tumor immunity in mouse models of melanoma.16 Such approaches have the potential to treat metastatic cancer in entirely new ways, including with improved efficacy and safety compared to conventional treatments of chemotherapy, surgery, and radiation.
More broadly for cancer therapy, immunoengineering is creating new knowledge and translational therapeutics across a wide range of length scales. On the molecular to macromolecular length scale, new computational protein engineering approaches are generating biomimetic materials that mimic immune cytokines such as IL-2, but with higher affinity and designed selectivity, improving anti-tumor activity in multiple mouse models.17 On the scale of 10–100 nm, nanoparticles are being precisely designed so that their physical core characteristics (including size, shape, and elasticity) and their chemical surface characteristics (polymer coating, targeting ligands, penetration enhancers) are well-controlled and facilitate immune cell interactions and function.18 On the micron-scale, biodegradable biomimetic particles are being constructed that mimic antigen-presenting cells by displaying from their surfaces both an antigen-specific Signal 1 to engage T cell receptors and a co-stimulatory Signal 2 to activate the T cells. In addition to the biochemical functionalization of their surfaces, their physical properties, including the size19 and shape20 of these artificial antigen presenting cells (aAPCs), was found to modulate their activation of cytotoxic T cells in vitro and in vivo, with flatter surfaces from elongated ellipsoidal particles dramatically outperforming spherical particles.
Cellular engineering of immune cells ex vivo for adoptive T cell immunotherapy is showing strong promise in the clinic, especially chimeric antigen receptor (CAR) T cells for the treatment of blood cancers. To improve the performance further, immunoengineering approaches are utilizing molecular engineering such as creating immunomaterials to boost CAR T cell activity in vivo,21 adding new functionality by genetically encoding secretable proteins like cytokines,22 or by incorporation of genetically encoded cellular imaging modalities23 to enable tracking of location and phenotype. To enable imaging, cell labeling can be genetically encoded in engineered CAR T cells using herpes simplex virus type 1 thymidine kinase for PET imaging and firefly luciferase for optical imaging and markers for T cell function can be encoded through the use of engineered promoters.23 Intriguingly, engineering the specialized metabolism of T cells can also further improve efficacy and durability.24
Immunoengineering for Regeneration and Discovery
Just as immunoengineering approaches open up new pathways for treatments that harness the immune system to attack an invader, they also hold the keys to engineer new pathways for healing. The emerging field of regenerative immunology leverages immunoengineering approaches to probe immune cell interactions and design improved biomaterials. For example, it was demonstrated that while extracellular matrix-derived biological scaffolds can promote tissue repair through an increase in T helper 2 cells and IL-4 production (type 2 immune microenvironment), a synthetic material, polycaprolactone, leads to an increase in T helper 17 cells, IL-17 production, and activity of CD9hi+IL-36γ+ macrophages that create fibrosis (type 17 immune microenvironment).25
The role of macrophages is critical to promote healing responses and spatially and temporally engineered drug delivery systems can facilitate this control through precise macrophage phenotype switching.26 In an alternative biomaterials-based research approach, localized donor-specific immune tolerance in allotransplantation can be achieved via CCL22 releasing microparticles to recruit regulatory T cells.27
Understanding and engineering the chemical and physical properties of synthetic biomaterials can also enable one to direct their immunomodulatory behavior. In particular, the foreign body response to implanted devices can be inhibited through the design of geometry, with spheres 1.5 mm and larger in diameter being most effective,28 and through surface conjugation, such as coating with select molecules containing triazole.29 Immunoengineered encapsulation materials led to long-term health of transplanted pancreatic islet cells and functional glucose control was achieved in a diabetes model.28 In an alternative approach, researchers have created Fas ligand-immunoengineered biomaterials to cause apoptosis of T effector cells and induce localized immunomodulation to achieve allogenic islet graft survival in diabetic mice.30 Diverse biomaterials-based immunoengineering approaches, from immunomodulatory scaffolds to tolerogenic vaccines and aAPCs, are opening up new avenues of treatment for type 1 diabetes.31
For varied immunoengineering approaches, large data sets can be generated where biomedical data science techniques and systems biology analysis have the potential to unlock new understanding. For example, whole-genome sequencing of patient tumor and normal tissue can lead to computational prediction of neoantigen targets, single cell RNA sequencing (scRNA-seq) combined with high-dimensional data visualization software can enable discovery of transcriptomes and identification of the key immune cells in a microenvironment, and multiplexed imaging can be analyzed by deep learning to capture information of individual immune cells within a 3D spatial context.32
Finally, immunoengineering technology is allowing researchers to probe the immune system and create representative ex vivo models to further discovery. Biomaterials are being used to map lymphatic drainage and lymph node targeting in vivo, and physical features of a material such as size, shape, and charge are critical to transport.33 Immunoengineered organoids are also being developed ex vivo to mimic the B-cell zone of lymphoid tissue and aspects of germinal centers.34 Through these approaches, the immune system is increasingly being analyzed quantitatively so that design principles can be established to motivate the next round of innovative technologies, tools, and therapeutics.
IMMUNOENGINEERING AS A NEW DISCIPLINE
As the previous research description demonstrates, over the past decade, immunoengineering has been laying the foundation to establish itself as a new discipline. Early use of the term, “immunoengineering,” can be traced back to a 2012 Science Translational Medicine article by Melody Swartz, Sachiko Hirosue, and Jeffrey Hubbell entitled “Engineering approaches to immunotherapy” that declared “the nascent field of immunoengineering aims to provide new approaches to our understanding, application, and therapeutic manipulation of immunology.”35
Immunoengineering in the Literature
Prior to this article being published in 2012, the terms “immunoengineering” and “immune engineering” were rarely found in the scientific literature, and since this key publication, there has been steady growth in the usage of these terms (Figure 1). To analyze the growth of the “immunoengineering” term in the biomedical literature, a publication search was conducted using PubMed. The results of the PubMed searches for the term “immunoengineering” and the closely related term “immune engineering” are shown in Figure 1. Interestingly, while the term “immunoengineering” is still young, it is already increasingly being used in the scientific literature with strong year-over-year growth in its usage since 2012. While many more scientific manuscripts are published each year on the subject of immunoengineering without using this keyword (i.e. using “immunotherapy,” “immunomodulatory,” etc.), Figure 1A clearly demonstrates that this is a rapidly growing field. Interestingly, the majority of these “immunoengineering” articles are biomaterials-related, further demonstrating the closeness of these two fields (Figure 1A). This is not too surprising, as the early pioneers in immunoengineering have approached the field with their own biomaterials toolboxes. A review of the literature also reveals that the keyword “immune engineering” is used much less frequently than the term “immunoengineering” and that it is not necessarily as associated with biomaterials research as the “immunoengineering” articles tend to be (Figure 1B). In terms of growth rate, combined publications using either term (“immunoengineering” or “immune engineering”) are growing at a rapid rate of an approximately 53% increase each year, from 2 publications in 2012 to 111 total publications spanning 2012–2019. In contrast, the term “immune” as used in PubMed publications is growing too, but is representative of a more mature field, and it has an 8-fold slower growth rate of approximately 6.6% growth each year (30,921 publications in 2012 and 313,024 cumulative publications 2012–2019).
Figure 1.

A PubMed search reveals the growth of the terms (a) “Immunoengineering” and (b) “Immune Engineering” in the scientific literature.
As the field of Immunoengineering has developed, a natural home has been in the Society For Biomaterials, where Immune Engineering has been an official SIG since 2014, under the founding leadership of Ankur Singh, Susan Thomas, Lance Kam, and Benjamin Keselowsky. Upon its founding, “the purpose of the Immune Engineering SIG is to bring together emerging ideas and provide a venue for professional interaction to a large number of academic and industrial research groups and scientists.” Over the years, the SFB SIG has organized many immunoengineering scientific sessions that have allowed the discipline to reach a critical mass. In a similar vein, the Controlled Release Society recently launched the “Immuno Delivery” Focus Group to focus on “new approaches in drug delivery to selectively target immune cells in peripheral tissues, diseased tissues, and at the primary sites of immunological reactions.” Immunoengineering focus areas and tracks are likely to grow within these societies as well as gain increased recognition within other related national and international societies as well.
Immunoengineering in Education
Just as there has been growth in the research dimension of immunoengineering, there has also been growth in the educational dimension of immunoengineering. Several major universities in the United States have now added tracks or focus areas associated with immunoengineering. At the University of Chicago, the Institute for Molecular Engineering was established in 2011 (now the Pritzker School of Molecular Engineering) with a theme in “Immuno-Engineering.” Georgia Tech established its Center for Immunoengineering, a member of the Georgia Immunoengineering Consortium, in 2013 and the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory offers an “Immunoengineering” research focus area. Johns Hopkins University’s Department of Biomedical Engineering offers a graduate and undergraduate focus area in “Immunoengineering,” and requires undergraduate and Master’s students to choose one such focus area as part of the major. Duke University’s Biomedical Engineering Department also has a research program and Master’s concentration in “Immune Engineering.” Immunoengineering programs at these institutions, and others across the country, are growing as these universities have been increasingly hiring new faculty with “immunoengineering” focused faculty candidate job advertisements. Industry is interested in the skill sets that come with such a multidisciplinary training experience, and an immunoengineering focus area can prepare one for a career in biotechnology, medical devices, and the pharmaceutical industry, among others. For an interested undergraduate, such a course of study frequently meets the pre-med requirements as well. Thus, this growth at educational institutions allows undergraduate and graduate students to benefit from new courses and labs that prepare the students for exciting careers in academia, industry, and medicine.
Immunoengineering Funding
Externally funded research and education initiatives from investigators at universities in the United States have also increased in parallel with this growth. For example, at the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Division of Discovery Science & Technology (Bioengineering) has a Program Area in “Technologies for Immunoengineering” that is led by Program Director David Rampulla. Furthermore, intramurally, the NIBIB has a new “Section on Immunoengineering” led by Investigator Kaitlyn Sadtler. These recent developments demonstrate a long-term structural interest by the NIH in the field of immunoengineering. Moreover, funded “immunoengineering” projects have been on a steady increase at the NIH over several years (Figure 2) and cut across many different NIH institutes (including NCI, NINDS, NIAID, NIGMS, NIAMS, NIA, NIDCR, and NIBIB). The growth is remarkable, as an “immunoengineering” keyword search in NIH Grant Reporter shows that the fiscal year funding has jumped from $0 in 2014 to $30M in funding in 2019, just five short years. This dramatic rise in funding comes due in part due to traditional R01 grants, but also especially due to new multiple investigator grants that bring together leaders from disparate backgrounds to work together in this highly multidisciplinary area. For example, recent large immunoengineering-related NIH awards include Immunoengineering T32 Training Grants (to Georgia Tech and Cornell University), an Immuno-engineering to Improve Immunotherapy U54 Center Grant (to University of Pennsylvania), a P30 Cancer Center Grant (to MIT), and an Immunoengineering P41 Biomedical Technology Research Resource Grant (to Johns Hopkins University). This new funding can critically catalyze the growth of this research field, leading to translational impact.
Figure 2.
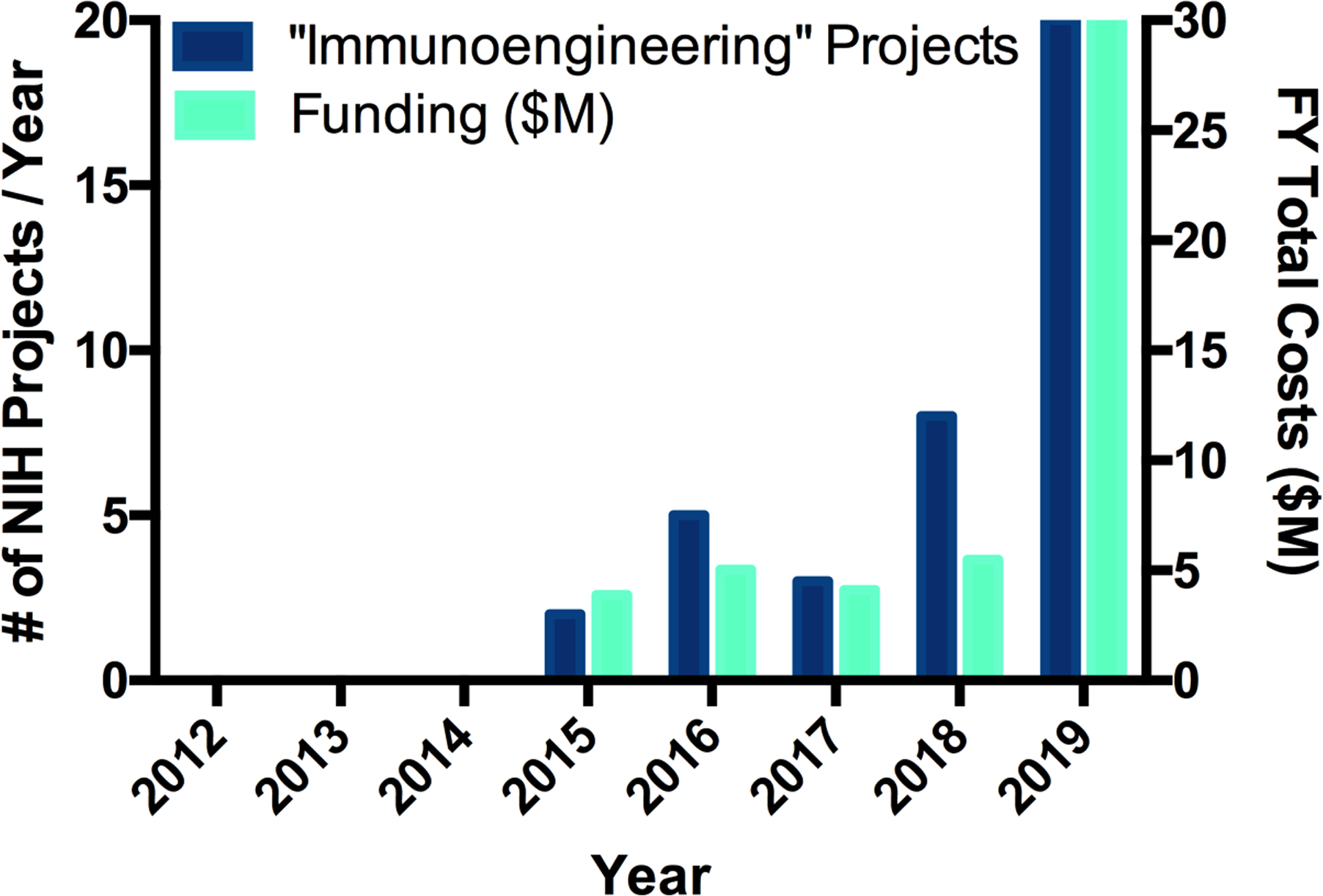
An NIH RePORTER search demonstrates the rapid growth in the number of NIH-funded “Immunoengineering” projects and their level of funding
CHALLENGES AND OPPORTUNITIES
While there has been rapid progress in the field of immunoengineering, there are also numerous challenges in the field. One persistent challenge is the limitation of current animal models. Models that may work well for one aspect of a therapeutic response (such as effectiveness against a patient-supplied human tumor growing in a mouse) may not be very relevant to an actual patient with the same tumor due to multiple factors such as immunocompromised animals, differences in physiological transport, and differences in tumor microenvironment. Future improvements to meet this challenge could take many forms from in vivo animal experiments with engineered humanized immune systems,36 to ex vivo systems of tissue engineered human-like organs on a chip,37,38 to sophisticated in silico multi-scale and multi-physics models that can simulate critical aspects of an individual patient.39 In each of these cases, it is a new engineering technology that can help lead the way in immunoengineering research. One of the greatest overarching challenges to immunoengineering research is its complexity, due to the many systems and players governing immune responses. One example is the evolving understanding of the role and function of macrophages in health and disease, encompassing a wide spectrum from stimulating inflammation and neutralizing pathogens to wound healing and tissue repair to immune regulation and anti-inflammatory activity.40,41 New research approaches are also adding further complexity, such as by engineering chimeric antigen receptors on the surfaces of macrophages to enable enhanced cellular functions for cancer immunotherapy.42 Yet, with challenges to immunology complexity also comes opportunities for immunoengineering approaches to elucidate mechanisms and facilitate understanding. In particular, new information can be gleaned through the use of engineered technologies to better characterize individual immune cells via scRNA-seq and multiplexed imaging combined with powerful computational tools. Thus, immunoengineering, as a field, is able to act as a self-catalyst, accelerating its own development. Convergence43 is also critical to the success of immunoengineering, and drawing on fields outside of its largely biomaterials-based beginnings, to areas such as biomedical data science, computational medicine, and public health, should further quicken its progress and broaden its impact.
CONCLUSIONS
It is clear that there has been rapid growth of the immunoengineering field over the last eight years. During this time period, there has been a convergence of disciplines including biomedical engineering, immunology, material science, medicine, chemistry, biology, chemical engineering, computer science, and others to create a new discipline. Immunoengineering centers and educational focus areas are being created at leading universities, grant agencies are making major investments into the field, and high-impact research and translational biotechnologies are being developed. Immunoengineering holds the promise of being able to vanquish diseases that have been plagues for too long, from cancer to infectious diseases, while also enabling brand new approaches for treating type 1 diabetes, multiple sclerosis, and so many other autoimmune diseases. By unlocking new tools for discovery, we can better understand the function of the immune system and improve our medical devices and transplantation protocols. While this future is very exciting, it also requires continued perseverance against complex challenges. Through multidisciplinary teams, from biomaterials engineers to biomedical data scientists to clinicians, traditional boundaries and limitations can be overcome and immunoengineering can reach its full potential.
ACKNOWLEDGEMENT
JJG thanks the National Institute of Biomedical Imaging and Bioengineering (P41EB028239) and the National Cancer Institute (R01CA228133) of the NIH, the Juvenile Diabetes Research Foundation (1-PNF-2019-782-S-B and 1-INO-2020-923-A-N), and the Bloomberg~Kimmel Institute for Cancer Immunotherapy at Johns Hopkins for support.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
JJG reports board membership and equity/options with the biotechnology companies AsclepiX Therapeutics, Dome Therapeutics, and VasoRx. Any potential conflicts of interest are managed by the Johns Hopkins University Committee on Outside Interest.
REFERENCES
- 1.Green JJ, Elisseeff JH. Mimicking biological functionality with polymers for biomedical applications. Nature 2016;540(7633):386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gammon JM, Jewell CM. Engineering Immune Tolerance with Biomaterials. Adv Healthc Mater 2019;8(4):e1801419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials 2016;1(5). [Google Scholar]
- 4.Wei X, Zhang G, Ran D, Krishnan N, Fang RH, Gao W, Spector SA, Zhang L. T-Cell-Mimicking Nanoparticles Can Neutralize HIV Infectivity. Adv Mater 2018;30(45):e1802233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 2013;339(6122):971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fotoran WL, Santangelo R, de Miranda BNM, Irvine DJ, Wunderlich G. DNA-Loaded Cationic Liposomes Efficiently Function as a Vaccine against Malarial Proteins. Mol Ther Methods Clin Dev 2017;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu Y, Smith D, Frazier E, Hoerle R, Ehrich M, Zhang C. The next-generation nicotine vaccine: a novel and potent hybrid nanoparticle-based nicotine vaccine. Biomaterials 2016;106:228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson DS, Hirosue S, Raczy MM, Bonilla-Ramirez L, Jeanbart L, Wang R, Kwissa M, Franetich JF, Broggi MAS, Diaceri G and others. Antigens reversibly conjugated to a polymeric glyco-adjuvant induce protective humoral and cellular immunity. Nat Mater 2019;18(2):175–185. [DOI] [PubMed] [Google Scholar]
- 9.Buettner MJ, Shah SR, Saeui CT, Ariss R, Yarema KJ. Improving Immunotherapy Through Glycodesign. Front Immunol 2018;9:2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMuth PC, Li AV, Abbink P, Liu J, Li H, Stanley KA, Smith KM, Lavine CL, Seaman MS, Kramer JA and others. Vaccine delivery with microneedle skin patches in nonhuman primates. Nat Biotechnol 2013;31(12):1082–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev 2012;64(14):1547–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis SARS CoV-2 Infection. U.S. National Library of Medicine; 2020. [Google Scholar]
- 13.Liu L, Wang Y, Miao L, Liu Q, Musetti S, Li J, Huang L. Combination Immunotherapy of MUC1 mRNA Nano-vaccine and CTLA-4 Blockade Effectively Inhibits Growth of Triple Negative Breast Cancer. Mol Ther 2018;26(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzeng SY, Patel KK, Wilson DR, Meyer RA, Rhodes KR, Green JJ. In situ genetic engineering of tumors for long-lasting and systemic immunotherapy. Proc Natl Acad Sci U S A 2020;117(8):4043–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson DR, Sen R, Sunshine JC, Pardoll DM, Green JJ, Kim YJ. Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomedicine 2018;14(2):237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li AW, Sobral MC, Badrinath S, Choi Y, Graveline A, Stafford AG, Weaver JC, Dellacherie MO, Shih TY, Ali OA and others. A facile approach to enhance antigen response for personalized cancer vaccination. Nat Mater 2018;17(6):528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva DA, Yu S, Ulge UY, Spangler JB, Jude KM, Labao-Almeida C, Ali LR, Quijano-Rubio A, Ruterbusch M, Leung I and others. De novo design of potent and selective mimics of IL-2 and IL-15. Nature 2019;565(7738):186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toy R, Roy K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioengineering & Translational Medicine 2016;1(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer RA, Sunshine JC, Perica K, Kosmides AK, Aje K, Schneck JP, Green JJ. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small 2015;11(13):1519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sunshine JC, Perica K, Schneck JP, Green JJ. Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells. Biomaterials 2014;35(1):269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma L, Dichwalkar T, Chang JYH, Cossette B, Garafola D, Zhang AQ, Fichter M, Wang C, Liang S, Silva M and others. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 2019;365(6449):162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther 2015;15(8):1145–54. [DOI] [PubMed] [Google Scholar]
- 23.Minn I, Rowe SP, Pomper MG. Enhancing CAR T-cell therapy through cellular imaging and radiotherapy. Lancet Oncol 2019;20(8):e443–e451. [DOI] [PubMed] [Google Scholar]
- 24.Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nature Reviews Immunology 2014;14(7):435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sommerfeld SD, Cherry C, Schwab RM, Chung L, Maestas DR Jr., Laffont P, Stein JE, Tam A, Ganguly S, Housseau F and others. Interleukin-36gamma-producing macrophages drive IL-17-mediated fibrosis. Sci Immunol 2019;4(40). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien EM, Risser GE, Spiller KL. Sequential drug delivery to modulate macrophage behavior and enhance implant integration. Adv Drug Deliv Rev 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher JD, Zhang W, Balmert SC, Aral AM, Acharya AP, Kulahci Y, Li J, Turnquist HR, Thomson AW, Solari MG and others. In situ recruitment of regulatory T cells promotes donor-specific tolerance in vascularized composite allotransplantation. Sci Adv 2020;6(11):eaax8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS and others. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater 2015;14(6):643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K and others. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol 2016;34(3):345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Headen DM, Woodward KB, Coronel MM, Shrestha P, Weaver JD, Zhao H, Tan M, Hunckler MD, Bowen WS, Johnson CT and others. Local immunomodulation Fas ligand-engineered biomaterials achieves allogeneic islet graft acceptance. Nat Mater 2018;17(8):732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stabler CL, Li Y, Stewart JM, Keselowsky BC. Engineering immunomodulatory biomaterials for type 1 diabetes. Nature Reviews Materials 2019;4(6):429–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finotello F, Rieder D, Hackl H, Trajanoski Z. Next-generation computational tools for interrogating cancer immunity. Nat Rev Genet 2019;20(12):724–746. [DOI] [PubMed] [Google Scholar]
- 33.Schudel A, Francis DM, Thomas SN. Material design for lymph node drug delivery. Nature Reviews Materials 2019;4(6):415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purwada A, Singh A. Immuno-engineered organoids for regulating the kinetics of B-cell development and antibody production. Nat Protoc 2017;12(1):168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swartz MA, Hirosue S, Hubbell JA. Engineering approaches to immunotherapy. Sci Transl Med 2012;4(148):148rv9. [DOI] [PubMed] [Google Scholar]
- 36.Allen TM, Brehm MA, Bridges S, Ferguson S, Kumar P, Mirochnitchenko O, Palucka K, Pelanda R, Sanders-Beer B, Shultz LD and others. Humanized immune system mouse models: progress, challenges and opportunities. Nat Immunol 2019;20(7):770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh A. Biomaterials innovation for next generation ex vivo immune tissue engineering. Biomaterials 2017;130:104–110. [DOI] [PubMed] [Google Scholar]
- 38.Kamm RD, Bashir R, Arora N, Dar RD, Gillette MU, Griffith LG, Kemp ML, Kinlaw K, Levin M, Martin AC and others. Perspective: The promise of multi-cellular engineered living systems. APL Bioeng 2018;2(4):040901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niederer SA, Lumens J, Trayanova NA. Computational models in cardiology. Nat Rev Cardiol 2019;16(2):100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol 2015;33:643–75. [DOI] [PubMed] [Google Scholar]
- 41.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8(12):958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty NE and others. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nature Biotechnology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Council NR. Convergence: Facilitating Transdisciplinary Integration of Life Sciences, Physical Sciences, Engineering, and Beyond. Washington, DC: The National Academies Press; 2014. [PubMed] [Google Scholar]


