Abstract
The emerging concept of the vasculome suggests that microvessels contribute to function and dysfunction in every organ. In the brain, aging and comorbidities such as hypertension and diabetes significantly influence a wide variety of neurodegenerative and cerebrovascular disorders, but the underlying mechanisms are complex and remain to be fully elucidated. Here, we hypothesize that aging, hypertension and diabetes perturb gene networks in the vasculome. Microvascular endothelial cells were isolated from mouse brain and heart, and their transcriptomes were profiled with microarrays. For aging, we compared 5 mo vs 15 mo old C57BL6 male mice. For hypertension, we compared 4 mo old normotensive BPN vs hypertensive BPH male mice. For diabetes, we compared 3 mo old diabetic db/db mice with their matching C57BLKS controls. Four overall patterns arose from these comparative analyses. First, organ differences between brain and heart were larger than effects of age and co-morbidities per se. Second, across all conditions, more genes were altered in the brain vasculome compared with the heart. Third, age, hypertension and diabetes perturbed the brain and heart vasculomes in mostly distinct ways, with little overlap. Fourth, nevertheless, a few common pathways were detected in the brain, expressed mostly as a suppression of immune response. These initial drafts of the brain and heart vasculomes in the context of aging and vascular comorbidities should provide a framework for designing future investigations into potential targets and mechanisms in CNS disease.
Keywords: Stroke, Brain injury, Dementia, Neurovascular unit, Genomics, Comorbidities
1. Introduction
All CNS diseases comprising stroke, brain injury and neurodegeneration kill vulnerable neurons and cause dysfunction in surviving neurons. However, it is now recognized that saving neurons alone may not be enough. The pathophysiology of all CNS diseases is now being increasingly re-interpreted within the context of a disrupted neurovascular unit (Lok et al. 2007). Brain function requires crosstalk between all cell types in the CNS. After injury and disease, the progression of pathology is similarly based on the dynamic interplay of injurious and compensatory cell-cell signaling between neurons, glia and vascular cells (Xing and Lo 2017). Hence, the balance between neuronal death and neuronal repair is regulated by non-cell autonomous gliovascular mechanisms.
Within this conceptual framework, the cerebrovasculature may play an essential role. Indeed, the emerging concept of the vasculome suggests that microvessels are not merely “empty pipes” for blood delivery (Guo et al., 2012a, b). Instead, the dense microvascular structures in each organ may even be thought of as endocrine and paracrine systems embedded within every organ. Previous studies have shown that the vasculome may contribute to circulating blood biomarkers (Ning et al. 2010), provide a signaling niche for neurogenesis and neuroplasticity (Marie et al. 2018), act as a regulator of microglia and neuroinflammation (Xing et al. 2018), and perhaps even provide a source of trophic factors to protect neurons against injury and disease (Guo et al. 2008; Guo et al., 2012a, b; Guo et al. 2016). If this is true, then one should expect that modifying factors known to affect blood vessels may also influence the ability of the vasculome to support CNS homeostasis. Aging and vascular comorbidities such as hypertension and diabetes significantly influence a wide variety of CNS disorders. Within the context of the neurovascular unit, the cerebral endothelium may contribute to a wide range of underlying mechanisms in CNS injury and disease (Bharadwaj et al. 2017; Faraco and Iadecola, 2013; Erdo et al., 2017; Rojas-Gutierrez et al. 2017; Shi et al. 2016;). Hence, it is likely that neural effects of aging and various comorbidities may be mediated by specific responses in the vasculome (Guo et al., 2012a, b; Guo et al. 2016).
In this proof-of-principle study, we hypothesize that aging, hypertension and diabetes will perturb the brain vasculome in specific ways, and these patterned responses may ultimately play key roles in influencing the progression of CNS disease. As a start, we sought to map and compare the brain and heart vasculomes in young versus old, normotensive versus hypertensive, and control versus diabetic mice.
2. Methods and materials
2.1. Preparation of endothelial cells
All procedures followed animal protocols approved by Massachusetts General Hospital Institutional Animal Care and Use Committee, consistent with the NIH Guide for the Care and Use of Laboratory Animals. For age comparisons, we used 5 and 15 mo old male C57BL/6 mice (NIA, Charles River). For assessing hypertension, we used 4 mo old male BPH and BPN mice (Jackson Labs). For assessing the effects of diabetes, we used the type II diabetes mouse model BKS·Cg-Dock7m +/+ Lepr db/J (also known as db/db mice) and its wild-type control C57BLKS/J mice (Jackson Labs, all male) at 3 mo of age. The endothelial cells were extracted from the brain cortex and heart of mice, as in our previous report (Guo et al., 2012a, b). Briefly, mice were anesthetized by isofluorane and perfused with 40 ml HBSS (Invitrogen). The cerebral cortex and heart were dissected and after removing surface large blood vessels, tissue was combined from 5 mice, minced and digested in 2 mg/ml Collagenase/Dispase (Roche) at 37°C for 30–40 min with vigorous shaking. The digested tissue were mechanically dissociated by titrating through 14 Gauge needles, then filtered through a 70 μM cell strainer (Becton Dickinson Labware, Bedford, MA), and centrifuged at 500 × g for 5 min at 4 °C. The cell pellets were then resuspended in cold HBSS (without Ca2+/Mg2+) and incubated with anti-PECAM 1 antibody-coated Dynabeads (Invitrogen), with gentle rotation for 30 min at 4 °C. The antibody-bead-bound endothelial cells were recovered and washed with magnetic separator in HBSS, then for the RNA preparation with RNeasy Micro Plus kit (Qiagen).
2.2. Transcriptome profiling with microarray and data analysis
Three RNA samples for each organ, under each condition, were individually hybridized to Affymetrix GeneChip Mouse 430 2.0 arrays; provided by Translational Genomics Core Services of Partners Healthcare Personalized Medicine. All samples were used only when RNA integrity number (RIN) scores were verified to be larger than 7.0, by RNA Pico Kit on Agilent Bioanalyzer 2001. Microarray hybridization and scanning was performed after amplification with the NuGEN Ovation WTA Pico kit and fragmentation and labeling with Encore Biotin Module. Raw expression data for each chip was collected and normalized using RMA algorithm. All chips passed these manual quality checks. A total of 45,037 probes were detected through microarray analysis. After removing the probes with maximum intensity across all the samples < 50, a total of 16,571 genes were identified. All statistical analyses were performed with the statistics software R (Version 2.6.2; available from http://www.r-project.org) and R packages developed by BioConductor project (available from http://www.bioconductor.org). Genes with p < .05 and fold change > 1.2 were considered as differentially expressed genes (DEGs). GSEA (Gene Set Enrichment Analysis) was used to identify the effected biological pathways. For a specific pathway (canonical pathway) from different dataset, the normalized enrichment score (NES) and the significance p value were calculated. A canonical pathway is considered as significantly affected, with p value < .05. Except for the database of GO, some disease database were also used for screening, including DisGeNET, GWAS (Genome-wide association study) and OMIM (Online Mendelian Inheritance in Man).
2.3. Quantitative real-time polymerase chain reaction (qRT-PCR)
Representative up- and downregulated genes were selected for brain and heart under all 3 conditions, and expression was checked with RT-PCR. Relative expressions of selected genes were confirmed by qRT-PCR, with pre-designed Taqman primers from Applied Biosystems. First strand cDNA was synthesized with QuantiTect reverse transcription system (Qiagen). The level of gapdh was used as housekeeping gene for normalization, the fold change of specific gene was measured with 2−ΔΔCt method. Overall, there was good correlation between microarray and RT-PCR validations: R2 = 0.81.
3. Results
Brain and heart endothelial cells were isolated from all mice and subjected to microarray analysis. A total of 16,571 genes were identified after removing probes that with maximum intensity < 50 across all samples. Probes with the highest intensity assigned to each gene were used for analysis.
In order to confirm the purity and quality of our preparations, expression levels were assessed for representative endothelial genes versus parenchymal genes. In brain samples, the expression of neuron, astrocyte, microglia and oligodendrocyte genes were all much lower than those of pan-endothelial markers such as Vwf, Icam1, Pecam1 and Vcam1 (Fig. 1A). In heart samples, cardiomyocyte and fibroblast genes were lower than all pan-endothelial markers (Fig. 1A). Organ specificity was also observed. Brain endothelial-specific markers (Ocln, Slc2a1, Tnfrsf19) were enriched in the brain samples, and heart endothelial-specific markers (Fabp4, Cd36, Meox2) were enriched in the heart samples (Fig. 1A).
Fig. 1.
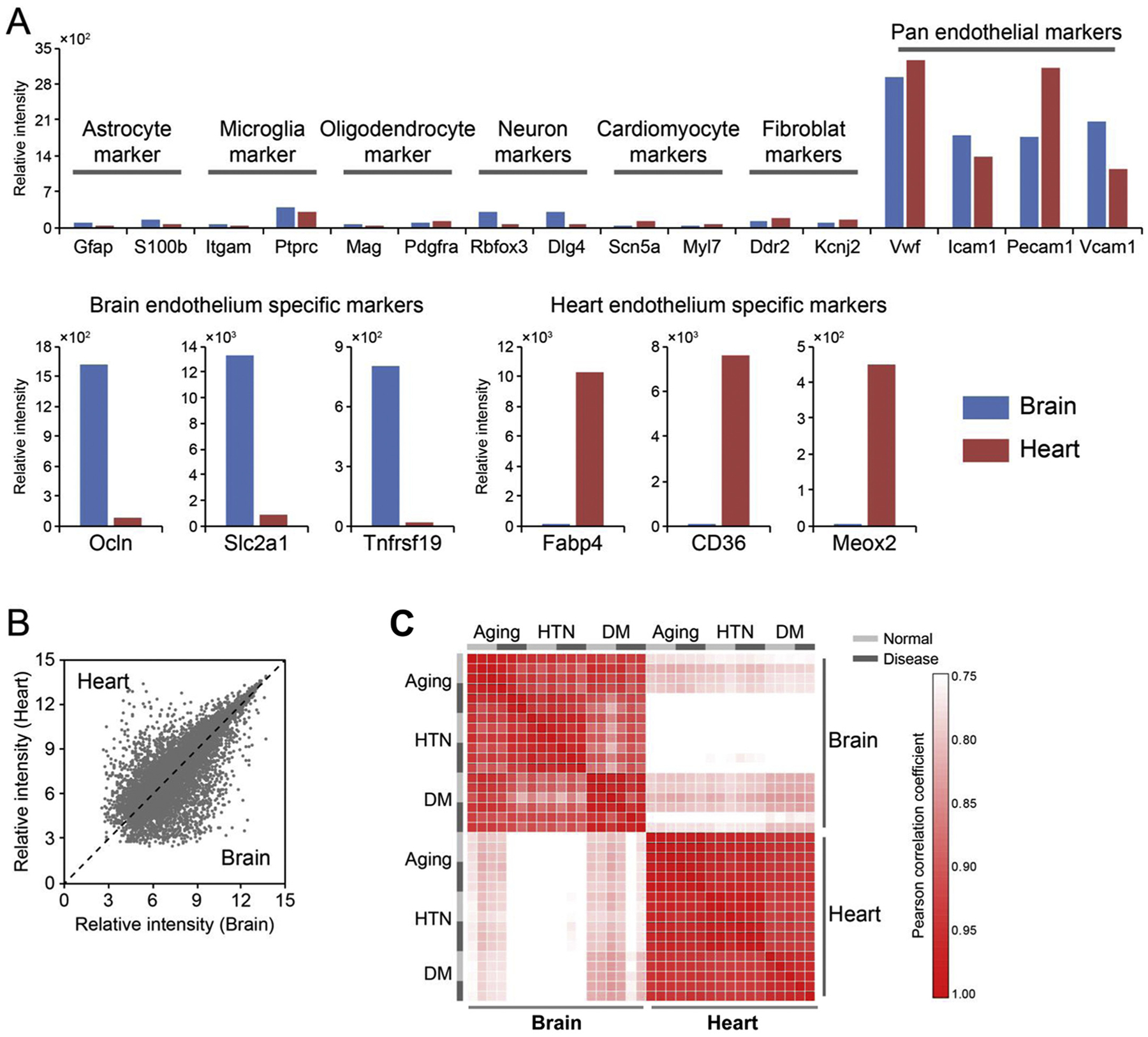
Endothelial-specific gene expression in the vasculome. (A) Markers of pan-endothelial genes were enriched compared with parenchymal genes in both brain and heart vasculomes in normal status (young, normal blood pressure and normal blood glucose), suggesting lack of contamination by neurons and glia (in brain) and myocardial cells (in heart). (B) Low correlation of gene expression between brain and heart. (C) Heat map demonstrates that endothelial gene expression is more strongly dependent on tissue location instead of being affected by age or comorbidities.
Based on these endothelial preparations, we then compared the transcriptomes in brain and heart of young male (5 mo old) versus old male (15 mo old) C57BL6 mice, 4 mo old hypertensive BPH male mice versus normotensive BPN male mice, and 3 mo old diabetic db/db male mice versus their C57BLKS/J male controls. In agreement with our previous studies, brain and heart vasculomes showed unique signatures (Fig. 1B). Interestingly, organ differences between brain and heart were larger than any changes induced by age, hypertension or diabetes (Fig. 1C).
Age and vascular co-morbidities appeared to induce mostly unique responses in the brain and heart vasculomes. Changes in gene expression did not correlate well between any of the 3 conditions with R values below 0.1, except when comparing the aged versus diabetic brain vasculome, where a modest degree of similarity was noted (R value equals 0.44, Fig. 2A). Using a cut-off of 1.2-fold change and p < .05, upregulated and downregulated genes were calculated for the different groups (Table 1). These numbers demonstrated that the brain vasculome may be more sensitive than the heart vasculome, with a higher number of altered genes across all conditions (Table 1). The heart vasculome appeared to be affected more by hypertension and diabetes compared to age, whereas in the brain, aging induced larger changes compared to hypertension and diabetes (Table 1). These comparisons also underscored the observations that there was almost no overlap in vasculome response after aging, hypertension and diabetes (Fig. 2B). Principal component analysis confirmed that the brain was affected more than the heart (Fig. 2C).
Fig. 2.
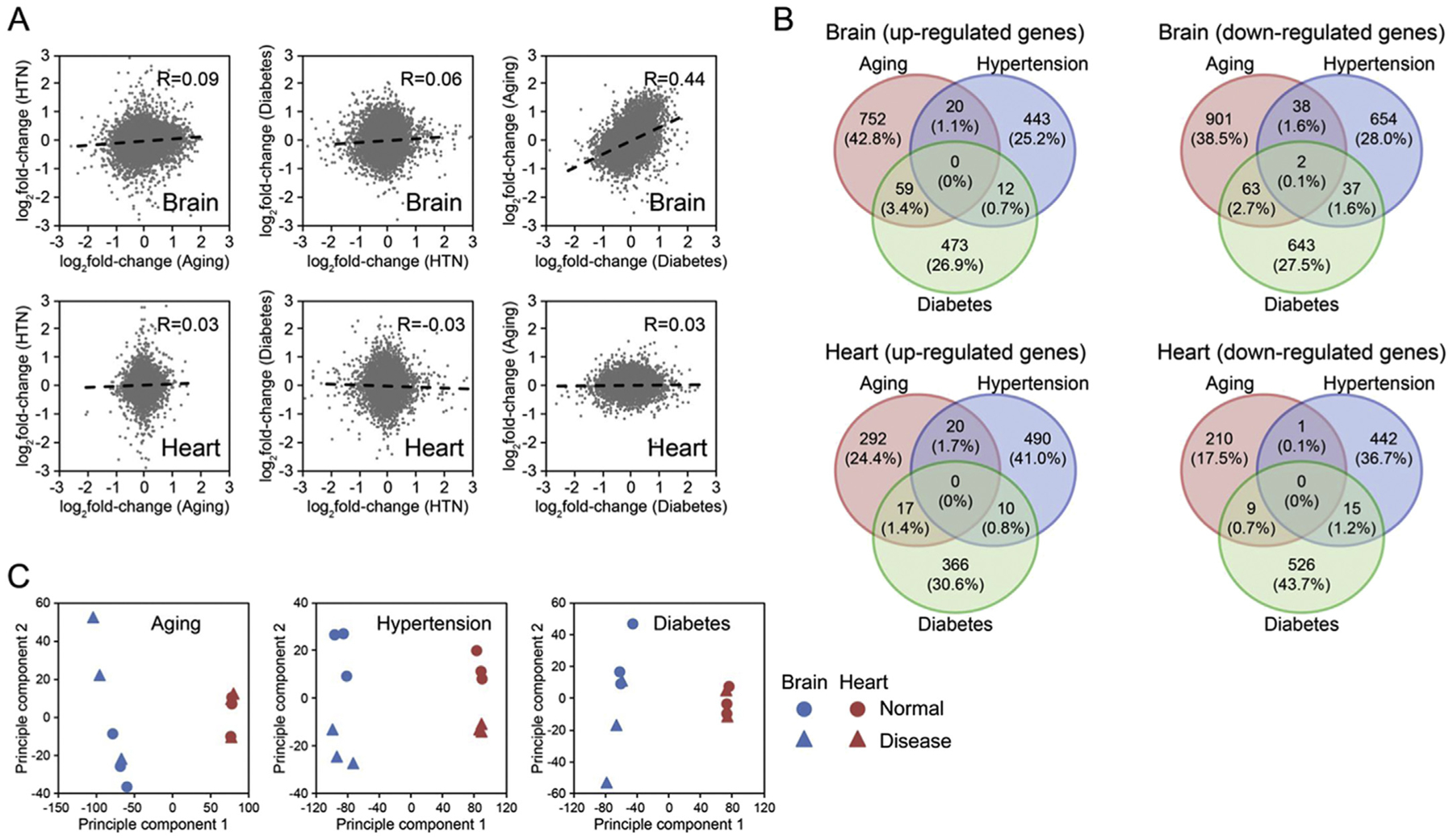
Comparison of gene perturbations in the aged, hypertensive and diabetic vasculomes from brain and heart. (A) Low correlations of gene alterations across all 3 conditions. Note, however, that a relatively higher correlation R = 0.44 is observed between aged and diabetic brain vasculomes. (B) Venn diagrams show mostly unique responses in upregulated and downregulated genes across all 3 conditions. (C) Principal component analysis confirms that effects of tissue location are larger than effects of age or comorbidities.
Table 1.
Number of differentially expressed genes.
| Brain | Heart | |||||
|---|---|---|---|---|---|---|
| DEG | Aging | Hypertension | Diabetes | Aging | Hypertension | Diabetes |
| Up | 831 | 475 | 544 | 329 | 520 | 393 |
| Down | 1004 | 731 | 745 | 220 | 458 | 550 |
| Total | 1835 | 1206 | 1289 | 549 | 978 | 943 |
Based on these transcriptomic changes, we next used GSEA pathway analysis to ask whether corresponding patterns in biological function may be present for the aged, hypertensive and diabetic vasculomes. In the brain, aging seemed to upregulate pathways that may be related to compensatory regulation of neuronal processes, hypertension activated pathways related to mitochondrial responses and apoptosis, and diabetes activated pathways related to insulin response and downregulated pathways of carbohydrate metabolism (Fig. 3A). In spite of the overall pattern of distinct brain vasculome responses, one common feature was noted in that immune-related processes seemed to be suppressed across all conditions. In the heart vasculome, distinct responses were also noted with little overlap between the 3 conditions (Fig. 3B). For example, aging upregulated lipid metabolism and may also speed up cellular respiration, reactive oxygen species metabolism and coagulation. Hypertension appeared to perturb lipid metabolism in both directions – some pathways were upregulated and others downregulated. Diabetes appeared to induce apoptosis and cell cycle processes and downregulate immune-related responses.
Fig. 3.
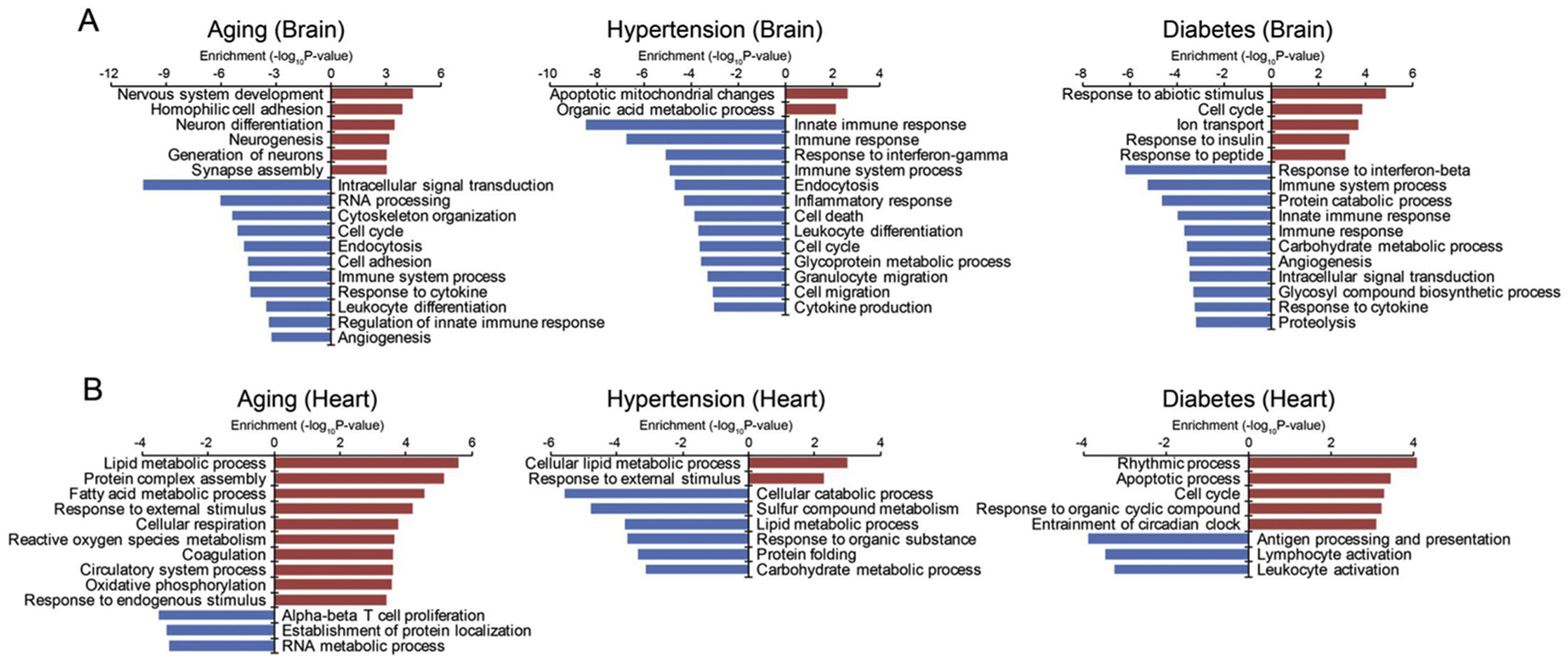
Comparative pathway analysis in the aged, hypertensive and diabetic vasculomes from brain and heart. (A) Up- and downregulated pathways in brain vasculome show mostly unique response in the 3 conditions, although some common immune-related pathways were detected. (B) Up- and downregulated pathways in heart vasculome also show little overlap between the 3 conditions.
To further assess the common pattern of immune suppression in the brain vasculome, network responses were mapped for subsets of linked genes (Fig. 4A). According to this analysis, altered immune pathways mainly involved cytokine/chemokine signaling, toll-like receptors, Fc gamma receptors, interferon and complement cascades. In spite of these common responses in networks, however, individual gene responses did not show significant overlap between aging, hypertension and diabetes. Suppression of these immune-related pathways appeared to be mediated by different specific genes with very little intersection across all 3 conditions. Only a handful of genes were uniformly affected by aging, hypertension and diabetes, including Was (Wiskott-Aldrich syndrome), Tab2 (TFG-beta activated kinase 1/MAP3K7 binding protein 2), Irf3 (interferon regulatory factor 3)and Clcf1 (cardiotrophin like cytokine factor) (Fig. 4A). Quantitative Z-scores of pathway activation confirmed the distinct response of each pathway (Fig. 4B). Cytokine/chemokine response and toll-like receptor signaling were significantly downregulated in both aging and hypertension. Interferon response was mainly affected by diabetes, whereas hypertension inhibited the expression of complement factors.
Fig. 4.
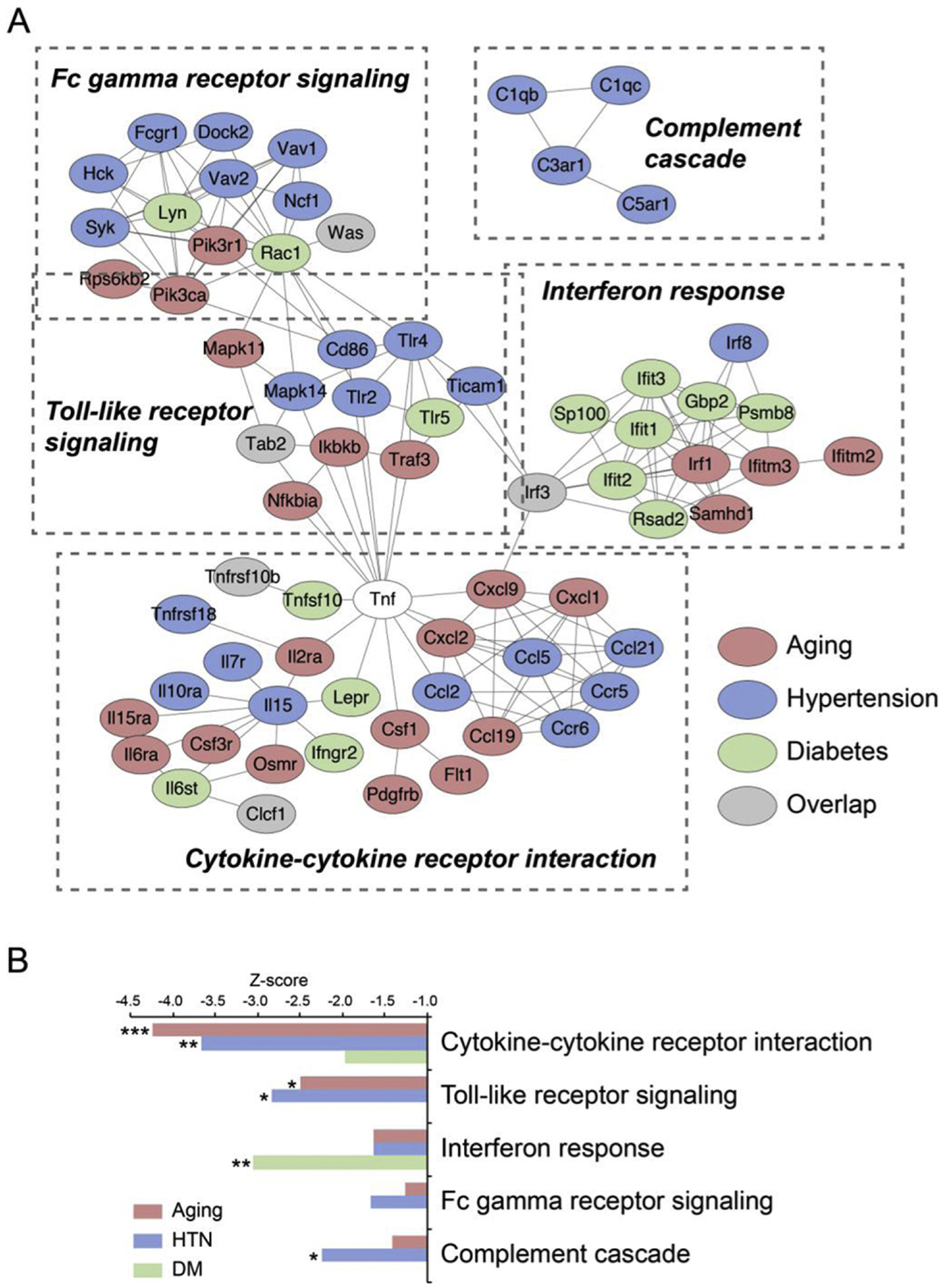
Subsets of immune network alterations in brain vasculome. (A) Altered immune pathways comprise cytokine-cytokine receptor interactions, Toll-like signaling, complement cascades, and Fc gamma receptor signaling. Note that in spite of the overall suppression of immune function, the responses to aging, hypertension and diabetes were mostly unique and showed very little common genes (gray indicates genes affected in all 3 conditions). (B) Quantitative z-scores of specific pathway downregulation.
Finally, we asked whether these responses in the brain and heart vasculomes were connected to human disease genes that were obtained from the following databases: DisGeNET, GWAS and OMIM (Table 2). An initial comparison suggested that our brain and heart vasculomes may be linked to genes that are implicated in CNS and cardiovascular disease (Table 2). Aging, hypertension and diabetes seemed to contribute to brain disorders in different ways, whereas in the heart, age and vascular comorbidities may contribute to disease equally (Fig. 5).
Table 2.
List of diseases enriched with differentially expressed genes of mouse brain and heart vasculomes.
| Concept ID | Disease | Aging | Hypertension | Diabetes | All | ||||
|---|---|---|---|---|---|---|---|---|---|
| DEG | p | DEG | p | DEG | p | DEG | p | ||
| Brain-related diseases | |||||||||
| C0006105 | Brain abscess | 1 | 0.309 | 2 | 0.029 | 4 | 0.001 | 7 | 0.000 |
| C3888102 | Frontotemporal dementia with motor neuron disease | 2 | 0.012 | 1 | 0.044 | 1 | 0.055 | 4 | 0.001 |
| C0234379 | Resting Tremor | 4 | 0.001 | 2 | 0.022 | 2 | 0.031 | 6 | 0.001 |
| C3160718 | Parkinson disease | 16 | 0.006 | 11 | 0.01 | 8 | 0.196 | 31 | 0.001 |
| C3279222 | Cerebellar hypoplasia and atrophy | 10 | 0.000 | 1 | 0.656 | 5 | 0.035 | 14 | 0.003 |
| C4024946 | Focal white matter lesions | 2 | 0.005 | 0 | 0.255 | 2 | 0.002 | 3 | 0.003 |
| C0752156 | Dural arteriovenous fistula | 1 | 0.064 | 0 | 0.255 | 2 | 0.002 | 3 | 0.003 |
| C0085200 | Lewy bodies | 2 | 0.005 | 2 | 0.001 | 1 | 0.035 | 3 | 0.003 |
| C0151699 | Intracranial hemorrhages | 6 | 0.088 | 5 | 0.035 | 7 | 0.006 | 15 | 0.004 |
| C3714756 | Intellectual disability | 152 | 0.000 | 64 | 0.675 | 71 | 0.769 | 264 | 0.005 |
| C0007766 | Intracranial aneurysm | 9 | 0.356 | 8 | 0.094 | 13 | 0.003 | 28 | 0.005 |
| C0751003 | Brain aneurysm | 2 | 0.070 | 0 | 0.484 | 3 | 0.004 | 5 | 0.008 |
| C0086543 | Cataract | 42 | 0.102 | 30 | 0.043 | 26 | 0.416 | 94 | 0.009 |
| C0751837 | Gait ataxia | 12 | 0.014 | 8 | 0.025 | 4 | 0.539 | 22 | 0.010 |
| C1290398 | Cerebral arterial aneurysm | 6 | 0.455 | 5 | 0.215 | 12 | 0.000 | 21 | 0.010 |
| C0025362 | Mental retardation | 115 | 0.000 | 50 | 0.616 | 54 | 0.772 | 200 | 0.030 |
| Heart-related diseases | |||||||||
| C0027051 | Myocardial Infarction | 31 | 0.001 | 48 | 0.003 | 47 | 0.003 | 114 | 0.000 |
| C0007222 | Cardiovascular Diseases | 31 | 0.001 | 45 | 0.009 | 49 | 0.001 | 112 | 0.000 |
| C0010054 | Coronary arteriosclerosis | 25 | 0.029 | 49 | 0.001 | 48 | 0.001 | 109 | 0.000 |
| C0011849 | Diabetes mellitus | 45 | 0.020 | 83 | 0.001 | 80 | 0.002 | 190 | 0.000 |
| C0003850 | Arteriosclerosis | 45 | 0.000 | 64 | 0.003 | 52 | 0.124 | 146 | 0.000 |
| C0020538 | Hypertensive disease | 43 | 0.001 | 70 | 0.001 | 54 | 0.130 | 151 | 0.000 |
| C1301700 | Cardiovascular morbidity | 2 | 0.019 | 3 | 0.018 | 3 | 0.017 | 8 | 0.000 |
| C0018801 | Heart failure | 27 | 0.011 | 52 | 0.000 | 33 | 0.312 | 103 | 0.000 |
Fig. 5.
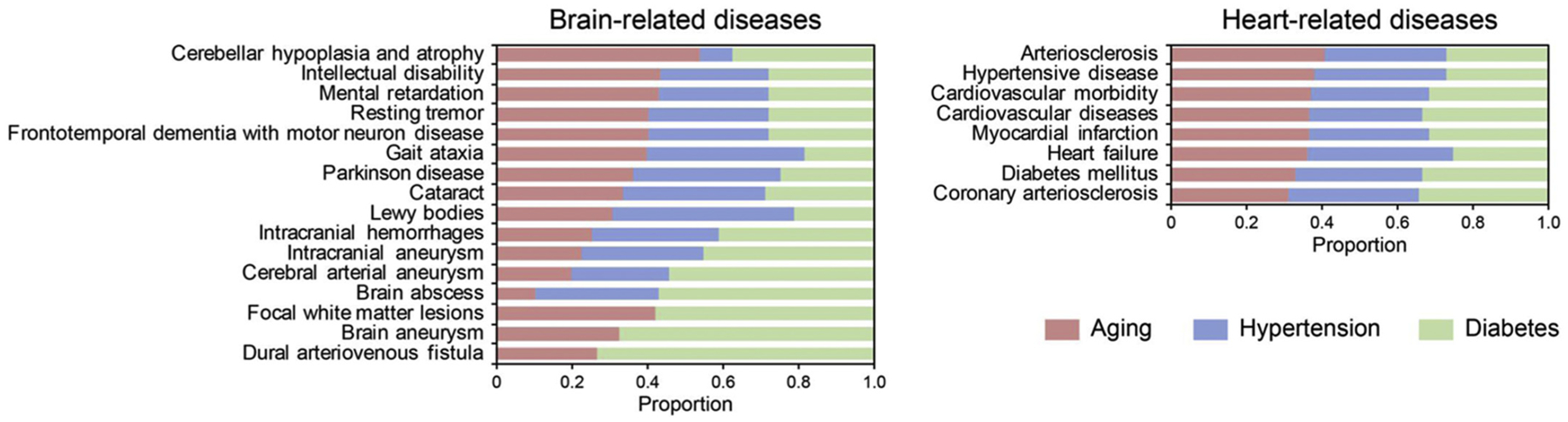
Connection between brain (left panel) and heart (right panel) vasculomes and human disease genes. In the heart, the effects appear equally distributed. In the brain, age, hypertension and diabetes may connect to CNS disease in different ways.
4. Discussion
In this study, we used microarray analyses to map and compare gene expression profiles in endothelial cells isolated from brains and hearts of various mouse models of aging, hypertension and diabetes. Our initial findings suggest that (i) vasculome differences between brain and heart were larger than any effects of age, hypertension, and diabetes; (ii) more genes were altered in the brain compared to the heart across all 3 conditions; (iii) age, hypertension and diabetes perturbed the brain and heart vasculomes in mostly distinct ways; but (iv) a small set of common responses were detected in the aging, hypertensive and diabetic brain vasculome as a suppression of immune response.
The present findings continue to build on the emerging concept of the vasculome as framework for understanding how the brain (and other organ systems) may respond to injury and disease. The major implications here appear three-fold. First, in spite of how age and vascular comorbidities all appear to be common risk factors for brain and heart disease, there was very little overlap in our vasculome responses. Indeed, tissue-dependent differences in gene expression profiles at baseline were larger than any of the effects caused by hypertension and diabetes per se. Hence, it may be difficult to always seek common targets when pursuing therapeutics in brain and heart. Second, although it is common to associate CNS and cardiac pathophysiology with inflammation, it was somewhat surprising to note the relative suppression rather than amplification of immune responses in the vasculome in the context of aging, high blood pressure and diabetes. Indeed, even with the observation of overall downregulation in immune responses within the brain vasculome, this network phenomenon appeared to be induced via different genes. Once again, this may mean that it is not easy to always seek common targets at a gene-specific level to ameliorate similar perturbations at a network level for different diseases. Nevertheless, recent studies now suggest that more fully mapping the interactome may help us identify neighborhoods where comorbidities and diseases should overlap (Menche et al. 2015). How these approaches may be used to delve deeper into our initial draft of the diseased vasculome warrants further study. Understanding how to rescue the aged or diseased vasculature (Das et al. 2018) may eventually lead us to novel therapeutic approaches for a wide spectrum of CNS and cardiovascular disorders.
Recently, findings from both cell biology and in vivo physiology have suggested that crosstalk exists between brain and heart. Cerebrovascular and cardiovascular disease share many common characteristics (Courties et al. 2014; Gonzales-Portillo et al., 2016). Injury to the heart causes injury to the brain and vice versa (Ishikawa et al. 2013; Ning et al., 2013). Therefore, we were especially interested in asking whether any coordinated responses were detected in the brain and heart vasculomes during aging, hypertension and diabetes. Surprisingly, we were unable to detect common patterns. In the heart, age and vascular comorbidities affected pathways broadly related to cell cycle, rhythmic processes and metabolism. In the brain, the common response across all 3 conditions appeared to be mostly related to a complex suppression of immune responses. However, it remains possible that in spite of the dissimilar baselines, connected responses may still be evoked post-stroke or cardiac arrest, and these gene responses warrant further investigation.
Taken together, this study provides proof-of-principle that the brain and heart vasculomes can be mapped for conditions and comorbidities that are suspected of affecting mechanisms of disease. However, there are several caveats to keep in mind. First, we pooled our endothelial cells from whole cortex in brain and from large parts of myocardium in heart, so we do not have information on potential differences in arterioles versus venules versus capillaries. Furthermore, the quantitative distribution of the vascular tree may be different in heart versus brain. Therefore, it is possible that organ-specific gene signatures may reflect both parenchymal signaling as well as differential proportions of endothelial cells from different sized vessels. In the present study, larger surface blood vessels were removed in our tissue processing procedure, so at least 90% of endothelial cells should be isolated from microvessels < 100 μm in diameter in both organs. Nevertheless, it should be important for future studies to examine whether the vasculome may respond differently to disease at different levels of the microvascular architecture (Vanlandewijck et al., 2018). Second, we only measured mRNA profiles. The vasculome should also be mapped for miRNA, protein, metabolomics etc. Third, the endothelium is regulated by interactions with adjacent cells such as pericytes and astrocytes in the brain (Pekny et al. 2015; Sweeney and Ayyadurai, 2016). The associated vasculome of these perivascular cells warrant further investigation. In fact, aging and co-morbidities can affect all cell types in the neurovascular unit (Cai et al., 2017). For example, hypertension can activate perivascular macrophages (Faraco et al., 2016), diabetes can perturb glymphatic function and overall cerebral metabolism (Andersen et al., 2017; Jiang et al., 2017), and smooth muscle vascular regulation may be significantly modified in the aging brain (Lubomirov et al., 2017). It will be important to ask how responses in the whole neurovascular unit may interact with the vasculome and influence function and dysfunction in the CNS. Fourth, we only measured the vasculome at a single timepoint. It remains possible that endothelial pathways evolve at different rates in BPH versus db/db mice. So the lack of common gene signatures could be due to different temporal progression of metabolically compromised conditions in diabetes versus hypertension. Further studies may be useful to more carefully assess temporal profiles of the vasculome under various aging and comorbid conditions. Finally, it is now known that significant differences may exist between rodent and human CNS cells in response to injury (Du et al. 2017). In this initial analysis, we were able to indirectly connect our mouse vasculomes with human disease databases, suggesting that the effects of aging and vascular co-morbidities may influence the pathophysiology of CNS and cardiac disorders. However, it remains possible that some of these links may not always translate well. It may be useful for future studies to examine gene expression profiles in diseased human brains and hearts for validation.
In summary, this preliminary study provides the first comparative map of the brain and heart vasculomes in mouse models of aging, hypertension and diabetes. Insofar as age and vascular comorbidities influence the pathogenesis of CNS disease, this vasculome database may provide an initial framework for future hypothesis generation and investigation.
Acknowledgments
Supported in part by grants from the Rappaport Foundation and NIH.
References
- Andersen JV, Christensen SK, Nissen JD, Waagepetersen HS, 2017. Improved cerebral energetics and ketone body metabolism in db/db mice. J Cereb Blood Flow Metab. 37, 1137–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj P, Wijesekara N, Liyanapathirana M, Newsholme P, Ittner L, Fraser P, Verdile G, 2017. The link between type 2 diabetes and neurodegeneration: roles for amyloid-β, amylin, and tau proteins. J. Alzheimers Dis 59, 421–432. [DOI] [PubMed] [Google Scholar]
- Cai W, Zhang K, Li P, Zhu L, Xu J, Yang B, Hu X, Lu Z, Chen J, 2017. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: An aging effect. Ageing Res Rev. 34, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courties G, Moskowitz MA, Nahrendorf M, 2014. The innate immune system after ischemic injury: lessons to be learned from the heart and brain. JAMA Neurol. 71, 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Treviño-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA, 2018. Impairment of an endothelial NAD(+)-H(2)S signaling network is a reversible cause of vascular aging. Cell 173, 74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Deng W, Wang Z, Ning M, Zhang W, Zhou Y, Lo EH, Xing C, 2017. Differential subnetwork of chemokines/cytokines in human, mouse, and rat brain cells after oxygen-glucose deprivation. J. Cereb. Blood Flow Metab 37, 1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdő F, Denes L, de Lange E, 2017. Age-associated physiological and pathological changes at the blood-brain barrier: a review. J. Cereb. Blood Flow Metab 37, 4–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Iadecola C, 2013. Hypertension: a harbinger of stroke and dementia. Hypertension 62, 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, Racchumi G, Murphy M, Rooijen N, Anrather J, Iadecola C, 2016. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 126, 4674–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales-Portillo C, Ishikawa H, Shinozuka K, Tajiri N, Kaneko Y, Borlongan CV, 2016. Stroke and cardiac cell death: two peas in a pod. Clin. Neurol. Neurosurg 142, 145–147. [DOI] [PubMed] [Google Scholar]
- Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, Stins MF, Wang X, Dedhar S, Lo EH, 2008. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc. Natl. Acad. Sci. U. S. A 105, 7582–7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Som AT, Waeber C, Lo EH, 2012a. Vascular neuroprotection via TrkB- and Akt-dependent cell survival signaling. J. Neurochem 123 (Suppl 2), 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Zhou Y, Xing C, Lok J, Som AT, Ning M, Ji X, Lo EH, 2012b. The vasculome of the mouse brain. PLoS One 7 (e52665). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Lok J, Zhao S, Leung W, Som AT, Hayakawa K, Wang Q, Xing C, Wang X, Ji X, Zhou Y, Lo EH, 2016. Effects of controlled cortical impact on the mouse brain vasculome. J. Neurotrauma 33, 1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Tajiri N, Vasconcellos J, Kaneko Y, Mimura O, Dezawa M, Borlongan CV, 2013. Ischemic stroke brain sends indirect cell death signals to the heart. Stroke 44, 3175–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, Sadry N, Nedergaard M, Chopp M, Zhang Z, 2017. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 37, 1326–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, Lo EH, 2007. Cell-cell signaling in the neurovascular unit. Neurochem. Res 32, 2032–2045. [DOI] [PubMed] [Google Scholar]
- Lubomirov LT, Papadopoulos S, Putz S, Welter J, Klockener T, Weckmuller K, Ardestani MA, Filipova D, Metzler D, Metzner H, Staszewski J, Zittrich S, Gagov H, Schroeter MM, Pfitzer G, 2017. Aging-related alterations in eNOS and nNOS responsiveness and smooth muscle reactivity of murine basilar arteries are modulated by apocynin and phosphorylation of myosin phosphatase targeting sub-unit-1. J Cereb Blood Flow Metab. 37, 1014–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C, Pedard M, Quirié A, Tessier A, Garnier P, Totoson P, Demougeot C, 2018. Brain-derived neurotrophic factor secreted by the cerebral endothelium: A new actor of brain function? J Cereb Blood Flow Metab. 38, 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menche J, Sharma A, Kitsak M, Ghiassian SD, Vidal M, Loscalzo J, Barabási AL, 2015. Disease networks. Uncovering disease-disease relationships through the incomplete interactome. Science 347, 1257601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning M, Sarracino DA, Kho AT, Guo S, Lee SR, Krastins B, Buonanno FS, Vizcaíno JA, Orchard S, McMullin D, Wang X, Lo EH, 2010. Proteomic temporal profile of human brain endothelium after oxidative stress. Stroke 42, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning M, Lo EH, Ning PC, Xu SY, McMullin D, Demirjian Z, Inglessis I, Dec GW, Palacios I, Buonanno FS, 2013. The brain’s heart - therapeutic opportunities for patent foramen ovale (PFO) and neurovascular disease. Pharmacol. Ther 139, 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A, 2015. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 131, 323–345. [DOI] [PubMed] [Google Scholar]
- Rojas-Gutierrez E, Muñoz-Arenas G, Treviño S, Espinosa B, Chavez R, Rojas K, Flores G, Díaz A, Guevara J, 2017. Alzheimer’s disease and metabolic syndrome: a link from oxidative stress and inflammation to neurodegeneration. Synapse. [DOI] [PubMed] [Google Scholar]
- Shi Y, Thrippleton MJ, Makin SD, Marshall I, Geerlings MI, de Craen AJ, van Buchem MA, Wardlaw JM, 2016. Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J. Cereb. Blood Flow Metab 36, 1653–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Ayyadurai S, Zlokovic BV, 2016. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 19, 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, Sun Y, Raschperger E, Räsänen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C, 2018. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475–480. [DOI] [PubMed] [Google Scholar]
- Xing C, Lo EH, 2017. Help-me signaling: non-cell autonomous mechanisms of neuroprotection and neurorecovery. Prog. Neurobiol 152, 181–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C, Li W, Deng W, Ning M, Lo EH, 2018. A Potential Gliovascular Mechanism for Microglial Activation: Differential Phenotypic Switching of Microglia by Endothelium Versus Astrocytes. J Neuroinflamm. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]


