Abstract
This study was aimed to compare the physicochemical properties and bacterial community structure of tray-packaged fresh lamb meat under different storage temperatures, such as 4°C (chilling), −1.5°C (supercooling), −4°C (superchilling) and −9°C (sub-freezing). The total viable counts (TVC), total volatile base nitrogen (TVB-N), bacterial diversity and metabolic pathways were investigated. The results indicated that the shelf life of superchilling and sub-freezing storage was over 70 d, which was significantly longer than that of chilling and supercooling storage. TVC and TVB-N values showed an increasing trend and were correlated well (R2>0.92). And the TVB-N values of lamb meat were exceeded the tolerable limit (15 mg/100 g) only found under chilling and supercooling storage during storage period. At the genus level, Pseudomonas was the core spoilage bacteria then followed Brochothrix for chilling and supercooling storage. Pseudomonas, Ralstonia, Psychrobacter and Acinetobacter were the dominant spoilage bacteria for superchilling and sub-freezing storage. Furthermore, the bacterial community diversity of lamb meat stored at chilling and supercooling storage decreased with the storage time prolonged, which was opposite to the outcome of meat stored under superchilling and sub-freezing storage. For chilling and supercooling storage, the abundance of main metabolisms (carbohydrate metabolism and amino acid metabolism, etc.) of bacteria increased with the storage time prolonged, which was opposite to superchilling storage. This may be related to the bacteria community diversity and the formation of dominant spoilage bacteria. In conclusion, this work provides data for the preservation of fresh lamb meat which will benefit the meat industry.
Keywords: fresh lamb meat, supercooling, superchilling, sub-freezing, quality
Introduction
Fresh meat, recognized as one of the most perishable food, is very conducive to the growth of microorganisms, for its high water content (aw>0.85), optimal pH for microbial growth (5.5−6.5), as well as abundant nutrients that provide energy (Buncic et al., 2014; Doulgeraki et al., 2012; Nychas et al., 2008). It has been confirmed that the spoilage of fresh meat is caused mainly by microorganisms especially bacteria (Pellissery et al., 2020). Several studies have demonstrated that bacterial communities play an important role in spoilage of meat, and the spoilage status is closely related to the species and amount of bacteria under specific storage conditions (Kaur et al., 2021; Li et al., 2019; Mansur et al., 2019). The composition and change of spoilage microflora of fresh meat are primarily determined by many factors including the slaughter and processing environment, chilling method, packaging, and storage temperature (Erkmen and Bozoglu, 2016; Nychas et al., 2008; Pellissery et al., 2020).
In the process of fresh meat, chilling is applied to preserve the carcass from microbiological contamination (Reid et al., 2017). Carcass must be chilled immediately after slaughter and dressing to ensure quality and safety (Buncic et al., 2014; Nychas et al., 2008). Apart from the conventional chilling and fast chilling, spray chilling, multistep chilling and very fast chilling are also applied in modern meat production enterprises (Zhang et al., 2019). Very fast chilling can be recognized as a special chilling technology which demands the temperature of muscle should be chilled to −1°C within 5 h after slaughter (Joseph, 1996). Research has reported that very fast chilling could provide tender meat by inhibiting the occurrence of “cold shortening” (Zhang et al., 2019). Due to the faster decrease of carcass surface temperature, very fast chilling can reduce evaporative losses and improve the economic effectiveness in meat industry (McGeehin et al., 2002). The chilling rate of carcass is recognized as an important hazard control point for abattoirs, and the faster freezing rate showed stronger inhibition of microbial proliferation (Zhang et al., 2019). Compared to the conventional chilling treatment, very fast chilling treatment can significantly reduce the number of microorganisms on the surface of lamb carcasses (Omer et al., 2015). However, there are few studies about the effects of very fast chilling on the composition and change of bacteria in fresh meat during storage.
Storage temperature is considered the main factor that affects the shelf life of fresh meat (Nychas et al., 2008). Storage of foods at temperatures above freezing and below 15°C is known as refrigerated or chilling storage (Karel et al., 1975). Chilling and frozen storage are the most two popular storage techniques on the market in the past few decades (Lu et al., 2019). The application of frozen storage could extend the shelf life of fresh meat significantly compared to chilling storage. Freezing and thawing can result in the reduction of water holding capacity, the increase of drip loss and the denaturation of protein, which affect the quality of fresh meat (Leygonie et al., 2012). The temperature range of supercooling is between 0°C and below its usual freezing point without the phase change solidification (formation of ice crystals) occurring (Stonehouse and Evans, 2015). Moreover, supercooling storage could inhibit the activity of enzymes and the growth of microorganisms in fresh meat, thereby the shelf life of fresh meat could be prolonged by 1.4−4 times at least compared to ordinary chilling storage (Magnussen et al., 2008; Pomponio et al., 2018; Zhou et al., 2010). In recent years, superchilling and sub-freezing are explored to be a new storage technology (Lu et al., 2020; Soyer et al., 2010; Wang et al., 2018). The temperature range of superchilling is lowered to 1°C–2°C below the initial freezing point of the product, with the conversion of some water into ice, which makes it less available for deteriorative processes (Aune, 2003; Duun and Rustad, 2007). The temperature range of sub-freezing is between superchilling and freezing temperature. Based on the results of low field nuclear magnetic resonance, −6°C, −9°C, and −12°C were designated as sub-freezing temperatures (Qian et al., 2018). It has been reported that the growth of toxin-producing microorganisms and bacteria on the meat surfaces could be inhibited by the low storage temperatures (Magnussen et al., 2008). To date, there have been many studies focused on the quality of fresh meat, but the change of microbial composition under different storage temperatures has rarely been reported.
Thus, the purpose of the work was to evaluate the effect of different storage temperatures (4°C, −1.5°C, −4°C, and −9°C) on the shelf life, bacterial communities and dominant spoilage bacteria of very fast chilled fresh lamb meat. Spoilage indicators, including total viable counts (TVC), total volatile base nitrogen (TVB-N) and thiobarbituric acid reactive substance (TBARS) were used to evaluate shelf life. The dynamic change of spoilage-related bacterial communities and dominant spoilage bacteria were also determined. Additionally, the potential mechanism of spoilage bacteria was investigated by functional analyses. The results may provide a novel approach to extend the shelf life of fresh lamb meat.
Materials and Methods
Sample collection and storage
A total of 24 seven-month old male lamb carcasses (small-tail Han sheep) with the same feeding system were collected at a commercial abattoir (Hebei Jinhong Halal Meat, Baoding, China). After slaughtered, the lamb carcasses were immediately chilled in a chilling room at −35°C air temperature with air velocity of 3 m/s. Room temperature was recorded with a temperature probe (LK-U, Changzhou blue light electronic, Changzhou, China) placed in the surrounding of carcass area, and the core temperature of carcass was measured by inserting a temperature probe into the 12th to 13th Musculi longissimus thoracis (M. longissimus) about 2 cm. When the temperature of the center of M. longissimus decreased to −1°C, carcasses were transferred to the conventional chilling room (0°C−4°C) for 24 h. The muscle samples of M. longissimus from both sides of each carcass were cut off and the visible fat and connective tissues were removed immediately after chilling. The M. longissimus from one lamb carcass were packed into one sterile sampling bag (Shockmixer-1, Guangdong Huankai Microbial Sci. & Tech., Guangzhou, China). All the sterile sampling bags were transported to the laboratory on ice. In the laboratory, every piece of M. longissimus was divided equally into 5 pieces and totally 10 pieces were obtained from one lamb carcass. Every piece of lamb meat was packed into a polypropylene tray (14.2 cm×10 cm×2 cm) and covered with a film with an O2 permeability of 18,500 cm3 m−2 day−1 at 25°C and 1 atm. The packages from one lamb carcass were randomly stored at different temperatures and marked as chilling, supercooling, superchilling and sub-freezing, respectively. Every treatment had 60 samples and 6 pieces of lamb meat from 6 lamb carcasses in each treatment were collected randomly at sampling timepoints. The collected samples were placed on ice for the determination of TVC, TVB-N and TBARS. 10 g of lamb meat were segmented from each samples and stored at −80°C for bacterial analysis.
Bacterial enumeration
The TVC was measured in accordance to a reference method of Wang et al. (2020a) with minor adjustment. 10 g of lamb meat were weighed accurately by using sterile scissors and added into a sampling bag (Shockmixer-1, Guangdong Huankai Microbial Sci. & Tech., Guangzhou, China) containing 90 mL of sterile saline. The collected samples were extracted in a sterile equalizer mixer (JN-400i, Ningbo Jiangnan Instrument Factory, Ningbo, China) for 1 min. The extraction was serially diluted with sterile saline containing 0.9% NaCl and 0.1 mL of diluent was plated onto agar plates (CM101, Beijing Luqiao Technology, Beijing, China) for counting. The plates were incubated at 36±1°C for 48 h. The results were expressed in Log CFU/g.
Physicochemical parameters
TVB-N
The TVB-N concentration was measured in accordance to Chinese standard GB 5009.228 (National Health and Family Planning Commission of China, 2016). The fresh lamb meat was minced by a meat mincer (JYL-C022E, Joyoung, Jinan, China). Minced lamb meat (10 g) was transferred into the distillation tube. Then 75 mL distilled water was added and homogenized for 30 min. At last, 1 g magnesium oxide was added into the distillation tube containing the processed sample. The content of TVB-N was determined by the Automatic Kjeldahl nitrogen analyzer (K9840, Hanon Instruments, Jinan, China). The results were expressed as mg of TVB-N per 100 g of lamb meat.
TBARS
The TBARS concentration was measured in accordance to a reference method described by Sinnhuber et al. (1977) with minor modifications. Minced lamb meat (2 g) was weighed into the 50 mL centrifugation tube containing 3 mL of 1% thiobarbituric acid solution, 17 mL of 2.5% trichloroacetic acid-HCl solution, and 1 mL of butylated hydroxytoluene. The mixture was heated in boiling water for 30 min and then cooled to room temperature. Then the mixture was mixed with isometric chloroform, followed by centrifugation at 845 g for 10 min. The absorbance of the supernatant was determined at 532 nm. The TBARS value was expressed as mg of malonaldehyde/kg of the minced lamb meat.
DNA extraction, PCR amplification and sequencing
The total DNA was extracted from lamb samples using the FastDNA® Spin Kit for Soil (MP Biomedicals, Solon, Ohio, USA), following the manufacturer’s instructions. 1% agarose gel was used to check the DNA extract, and NanoDrop 2000 UV-vis spectrophotometer was used to determine the DNA concentration and purity. To investigate the bacterial community diversity, the hypervariable region V3–V4 of the bacterial 16S rRNA gene were amplified with primer pairs 338F (5'-ACTCCTACGGGAGGCAGCAG-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3') by an ABI GeneAmp® 9700 PCR thermocycler (ABI, CA, USA). The PCR mixtures were carried out in 20 μL reactions including 0.8 μL (5 μM) forward and reverse primers each, 4 μL 5×TransStart FastPfu buffer, 2 μL 2.5 mM dNTPs, 0.4 μL TransStart FastPfu DNA Polymerase, 10 ng template DNA, and complemented ddH2O to 20 μL. The amplification of the 16S rRNA genes consisted of initial denaturation at 95°C for 3 min, followed by 30 cycles of denaturing at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 45 s, single extension at 72°C for 10 min, and end at 10°C. The amplified product was visualized on 2% agarose gel and further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Axygen, CA, USA) according to the manufacturer’s instructions. The target segment was sequenced on Illumina MiSeq PE300 platform (Shanghai Majorbio Bio-pharm Technology, Shanghai, China). The raw sequence data was submitted to the NCBI as BioProject PRJNA700819.
Processing of sequencing data
The raw 16S rRNA gene sequencing reads were demultiplexed, quality-filtered by FASTP version 0.20.0 (Chen et al., 2018) and merged by FLASH version 1.2.7 (Magoc and Salzberg, 2011). In brief, the operational taxonomic units (OTUs) with 97% similarity cutoff were clustered using UPARSE version 7.1 (Edgar, 2013; Stackebrandt and Goebel, 1994), and chimeric sequences were identified and removed to guarantee the tags effectively. The taxonomy of each OTU representative sequence was analyzed by RDP Classifier version 2.2 (Wang et al., 2007) against the 16S rRNA database (silva 138/16s_bacteria) using the confidence threshold of 0.7.
Statistical analysis
All of the tests were carried out in sextuplicate. One-way ANOVA implemented in SPSS 20.0 software (IBM, Chicago, IL, USA) was used to analyse the differences in mean values for TVC, TVB-N, and TBARS. Duncan's multiple range test was performed to verify significant differences (p<0.05) among samples. For sequencing data, Mothur program (version: 1.30.2) and QIIME pipeline were implemented to analysis the alpha and beta diversity. The bacterial community structural component was analyzed based on the statistical software package R (version 3.1.2). PICRUSt2 and Genomes (KEGG) pathways were applied to predict the functional genes of bacterial communities.
Results and Discussion
Bacterial enumeration
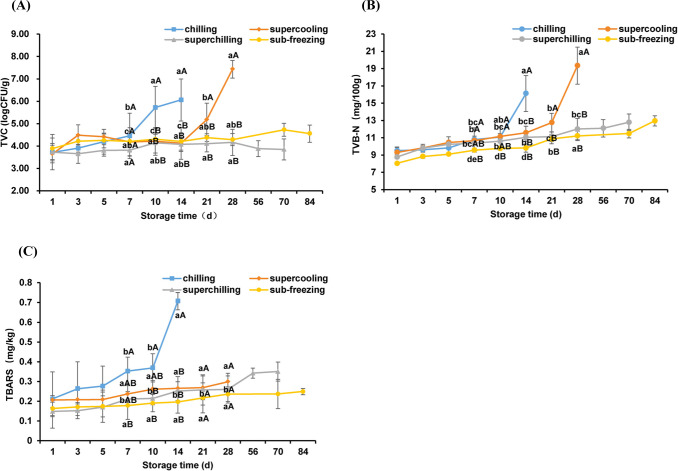
Microorganism is one of the main factors affecting the shelf life of fresh meat (Hong et al., 2012). Changes in TVC of all four treatments were shown in Fig. 1A. The initial count for TVC was about 3.8 Log CFU/g for four treatments and increased with the prolonging of storage time. The TVC values of chilling and supercooling increased remarkably at 10 d and 21 d respectively compared with 1 d (p<0.05). The shelf life of chilling and supercooling treatment were 14 d and 28 d, respectively. At the end of storage, the TVC values of chilling and supercooling were up to 7−8 Log CFU/g, which exceeded the upper tolerable limit for fresh meat (Bolton et al., 2009), whereas the TVC values for superchilling and sub-freezing were still below 5 Log CFU/g. These results indicated that the low temperatures could inhibit the activity of microorganisms, consistent with previous results (Lu et al., 2019; Pellissery et al., 2020).
Fig. 1. Changes in total viable counts (TVC), total volatile base nitrogen (TVB-N) and thiobarbituric acid reactive substance (TBARS) of fresh lamb samples during storage at different temperatures.
(A) TVC changes, (B) TVB-N changes, (C) TBARS changes. The error bars represent the standard deviation between replicates of the same experiment. Different capital letters within same line show significant differences according to ANOVA one way analysis followed by Duncan’s multiple range test (p<0.05). Different small letters within different line at the same storage time show significant differences according to ANOVA one way analysis followed by Duncan’s multiple range test (p<0.05).
TVB-N and TBARS
TVB-N is an important index to reflect the freshness of meat (Wang et al., 2020b). As shown in Fig. 1B, the TVB-N values showed an increasing trend for all treatments during storage. The TVB-N of chilling exhibited the highest rate of growth from 11.07 mg/100 g to 16.12 mg/100 g on the 14 d. The significant change of TVB-N occurred after 21 d storage for supercooling (p<0.05). Meanwhile, the TVB-N of superchilling and sub-freezing were still lower than 15 mg/100 g even at 84 d. According to the current Chinese hygienic standard (GB 2707–2016), the upper limit of TVB-N value in fresh meat is 15 mg/100 g (Lu et al., 2019). It has been reported that spoilage bacteria metabolites could cause the protein to be degraded, which lead to the production of TVB-N, and the low temperatures can inhibit bacterial reproduction (Chung et al., 2019; Mansur et al., 2019; Muela et al., 2010). These results indicated that the increase of TVB-N was influenced by the storage temperature. In the commercial refrigeration condition, the shelf of fresh lamb meat is less than 2 weeks, hardly over-passes 21 days (Berruga et al., 2005; Karel et al., 1975). Based on the results of TVC and TVB-N, the shelf life of fresh lamb meat could extend more than 70 days at superchilling or sub-freezing storage.
The TBARS value is mainly used as a marker of lipid oxidation of meat by evaluating the formation of the secondary products (Dai et al., 2014). As shown in Fig. 1C, the TBARS values for all treatments increased linearly throughout the storage period. The TBARS value of chilling exhibited a higher rate of increase compared to other treatments at d 14 (p<0.05), which is same as TVC and TVB-N for chilling. It is reported that when the TBARS value exceeds 0.5 mg/kg, the trained sensory panels could detect some unpleasant flavors (Hansen et al., 2004). On the last day of storage, the TBARS values of supercooling, superchilling and sub-freezing were still lower than the limit of 0.5 mg/kg. The accumulation of TBARS may contribute to the hydroperoxide production by lipid oxidation and the hydroperoxide is likely to be degraded rapidly, and these reactions is correlated with the occurrence of rancidity and the activity of spoilage bacteria (Ganhão et al., 2011; Li et al., 2019). The present results indicated that the lower temperature (−1.5°C, −4°C, and −9°C) could restrain lipid oxidation to some degree.
For all treatments, there is a good correlation between TVC and TVB-N (R2>0.92), which is in agreement with previously reported results (Hernández-Macedo et al., 2012; Li et al., 2019). These results indicated that TVC and TVB-N could be used as spoilage indicators of fresh lamb meat. Except for superchilling and sub-freezing samples, TVC and TBARS were correlated well (R2>0.92) (Table 1). The TBARS values are affected by the activity of microorganisms and the oxidation of meat itself (Resconi et al., 2018; Tuboly et al., 2003). Compared with 4°C and –1.5°C storage, the lower temperature (−4°C and −9°C) inhibited the activity of microorganisms and the oxidation process of meat itself. Therefore, TVC and TBARS were poorly correlated for superchilling and sub-freezing treatment.
Table 1. Correlation between total viable counts (TVC), total volatile base nitrogen (TVB-N) and thiobarbituric acid reactive substance (TBARS) in each sample.
| Treatment | Chilling | Supercooling | Superchilling | Sub-freezing | |
|---|---|---|---|---|---|
| TVB-N | Correlation coefficient | 0.992 | 0.994 | 0.934 | 0.927 |
| Significance | 0.081 | 0.006 | 0.066 | 0.073 | |
| TBARS | Correlation coefficient | 1.000 | 0.929 | 0.855 | 0.780 |
| Significance | 0.019 | 0.071 | 0.145 | 0.220 |
Bacterial diversity
Alpha diversity and beta diversity metrics
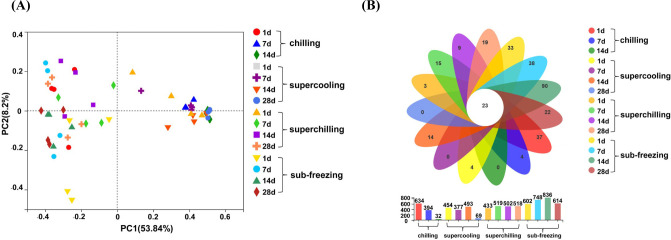
According to the results of TVC, the samples representative time points were selected including d 1, d 7 and d 14 for chilling, d 1, d 7, d 14, and d 28 for supercooling, superchilling and sub-freezing in the present study. Data of 90 samples in total at 15 representative time points were analyzed. A total of 4,038,356 sequences were retained, after removing short and low-quality reads, singletons, triplicates, and chimeras. The total abundance, 4027 OTUs were clustered (identity 97%), ranging from 29,783 to 59,934 (Table S1). Good's coverage (more than 99%) indicated all the sequencing data could cover the most of bacteria in lamb meat samples (Table S2). The richness and diversity of bacterial communities were evaluated by Chao1, Sobs and Shannon indexes (Yang et al., 2018). For d 14 of chilling, d 28 of supercooling, superchilling and sub-freezing, the values of Chao1, Sobs and Shannon in chilling and supercooling were lower than the initial values, which was contrary to the results of superchilling and sub-freezing (Table S3). The change of Chao1, Sobs and Shannon indicated that the richness and diversity dropped rapidly in chilling and supercooling and some bacteria became the dominant spoilage bacteria in samples with the extension of storage time. It has reported that various kinds of initial bacteria may contaminate the meat, whereas only a small number of species related to meat spoilage become the major spoilage bacteria at the end of storage (Benson et al., 2014; Chaillou et al., 2015). These results indicated that the change of bacterial community structure and the formation of dominant spoilage bacteria were influenced by storage temperature, consistent with previous results (Kaur et al., 2021). Furthermore, superchilling and sub-freezing storage may extend the shelf of lamb meat by inhibiting the growth of dominant spoilage bacteria and reduce the further risk of microbial at a certain extent.
To compare the differences in bacterial community composition among samples, PCoA was an effective method (Fig. 2A). The samples of chilling stored on d 7 were separated compared to d 1, suggesting that the effect of storage time for the bacterial community composition was significant. Meanwhile, the samples of chilling stored on d 7 and d 14 were clustered together, suggesting that the bacterial community composition trended to be stable. The bacterial community composition of supercooling and superchilling became stable after d 7, which had the same trend as chilling. There was an interesting observation that the change of bacterial community composition was not significant for sub-freezing during storage period, probably due to that the low temperature (−9°C) inhibited the growth of the main spoilage bacteria, thereby resulting in a relatively stable bacterial structure. In addition, the Venn diagram revealed that 23 taxa were common to all samples including Pseudomonas, Ralstonia, Acinetobacter, Brochothrix, Psychrobacter, Lactococcus, Vagococcus and Macrococcus, etc. (Fig. 2B), which explained the relative content of these bacteria is highly related to the structure of the bacterial community.
Fig. 2. The change of bacterial communities and shared bacteria in fresh lamb samples.
(A) Principal coordinate analysis (PCoA) of bacterial communities among different fresh lamb samples during storage, (B) Venn diagrams for numbers of shared and unique genera among different fresh lamb samples during storage.
Diversity changes of lamb associated bacterial community at phylum and genus levels
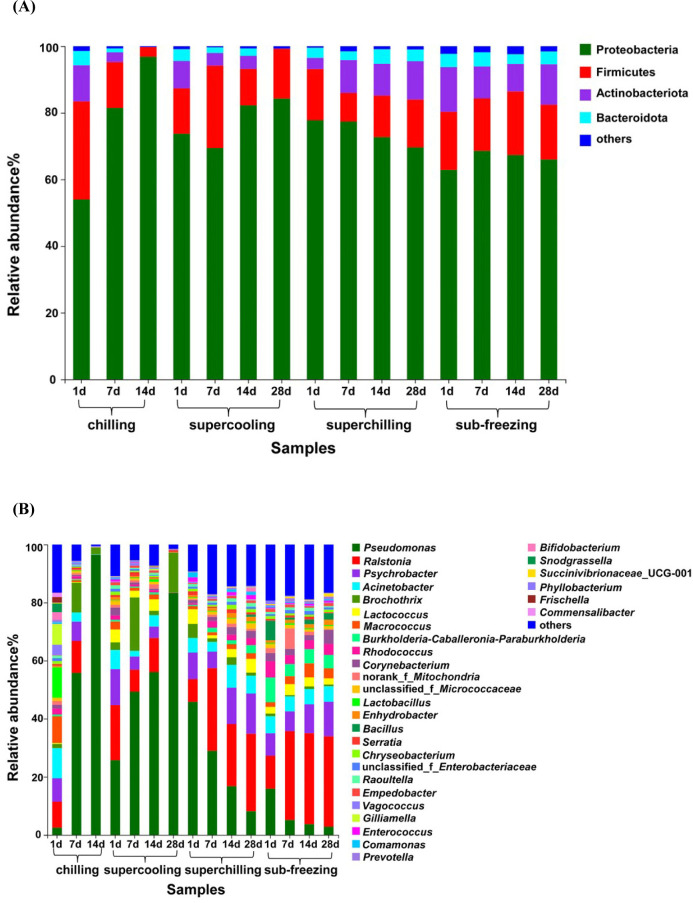
At the phylum level, a total of four bacteria were identified including Proteobacteria, Firmicutes, Actinobacteriota and Bacteroidota. Proteobacteria and Firmicutes (together represented >80% of all OTUs) were the major bacteria during storage (Fig. 3A). As the storage time increased, the proportion of Actinobacteriota and Bacteroidota decreased from 11%−14% to <3% for chilling and supercooling. Conversely, the proportion of Actinobacteriota and Bacteroidota increased from 6% to 14% for superchilling. The proportion of Actinobacteriota and Bacteroidota had no significantly change during storage for sub-freezing.
Fig. 3. The relative abundance (%) of major bacteria at different levels in fresh lamb samples.
Relative abundance (%) of major bacteria among different treatments at phylum (A) and genus (B) level in fresh lamb samples.
Bacterial community dynamics were also evaluated at the genus level (Fig. 3B). At the beginning of storage, the major bacteria including Acinetobacter, Macrococcus, Lactobacillus accounted for 29% and Pseudomonas accounted for only 3% for chilling. At the end of storage (d 14), Pseudomonas accounted for more than 96% which lead to a significant decrease in the bacterial diversity compared to d 1. Similar observations have been reported that Pseudomonas could account for 96% of the spoilage flora in air packing (Bailey et al., 1979; Enfors et al., 1979; Kumosinski, 1988). Pseudomonas and Brochothrix were the dominant genus during storage and they had a significant increase compared to d 1 for supercooling. At the early storage period, Psychrobacter and Pseudomonas were recognized as the main spoilage bacteria for superchilling and sub-freezing, and the proportion gradually decreased with the storage time prolonged. At the end of storage (d 28), Ralstonia became the dominate spoilage bacteria for superchilling and sub-freezing. In another study, Ralstonia was also isolated in dairy and meat processing environments (Liu et al., 2016). Ralstonia could enhance the incorporation of E. coli O157:H7 into dual-species biofilms under various temperature and nutrient availability conditions (Liu et al., 2015). There are rare reports about Ralstonia in meat, it could provide potential transference routes for foodborne pathogens (Lynch et al., 2009). The present result indicated that we need pay attention to the Ralstonia at superchilling and sub-freezing temperature. At the end of storage for chilling and supercooling, the relative content of Ralstonia was significantly lower than that of Pseudomonas (p<0.05). The result demonstrated that Ralstonia was less competitive than Pseudomonas during storage at chilling and supercooling temperature. Meanwhile, the bacterial diversity of superchilling and sub-freezing were also higher than that of supercooling on d 28, explaining that most of bacteria were inhibited and the dominated spoilage bacteria did not occur at –4°C and –9°C, which was in line with the results of TVC and TVB-N. These results indicated that the dynamic changes in bacterial community were greatly affected by the storage temperature.
In aerobic packing, Pseudomonas and Brochothrix are identified as the main spoilage bacteria for raw meat (Doulgeraki et al., 2012). More accurately, Pseudomonas is particularly involved in the spoilage of beef and pork at chilling temperatures (5°C) (Ercolini et al., 2006; Liu et al., 2006). Compared with Brochothrix, Pseudomonas became the dominate spoilage bacteria owing to its faster growth during storage period. In a study by Tsigarida et al (Tsigarida et al., 2003), they applied the gel cassette system to replace broth to simulate the spoilage environment and drew the similar conclusion. Glucose, as the first source of energy, which is metabolized more rapidly by Pseudomonads rather than Brochothrix (Erkmen and Bozoglu, 2016). Pseudomonads could oxidize glucose and glucose-6-phosphate to form D-gluconate, pyruvate and 6-phosphogluconate (Gill, 1976). Once the glucose reserves are exhausted, lactic acid is the next energy source, and then followed by amino acids (Casaburi et al., 2015). At this period, protein also can be used as the source of energy (Gill, 1976). The change in available nutrients is beneficial to the growth of microorganisms that can decompose protein (Nychas et al., 2008). In aerobically packaged meat, that the Pseudomonas could become the main spoilage bacteria was due to the ability to decompose protein (Doulgeraki and Nychas, 2013). In addition to Pseudomonads, some bacteria also have the spoilage potential such as Acinetobacter, Moraxella, and Flavobacterium (Ercolini et al., 2006). These bacterial species could compete with other organisms for oxygen, thereby promoting the growth of Pseudomonads (Gill, 1976). Among different spoilage organisms, Pseudomonas favors the breakdown of proteins and amino acids, and could produce off-odor in meat (Casaburi et al., 2015; Smit et al., 2009). Pseudomonas, as the lipolytic bacteria, could promote the lipolysis (rancidity) to produce aldehydes, ketones, and short-chain fatty acids, which results in the formation of rancid flavors (Erkmen and Bozoglu, 2016). In addition, Pseudomonas, Acinetobacter and Moraxella have been reported that they are the main spoilage bacterial in aerobical packing meat at different storage temperature (–1°C−25°C) (Pellissery et al., 2020). Pseudomonas, as the common spoilage bacteria, are also isolated from spoiled meat at low temperatures (4°C) in aerobic storage, consistent well with this study (Ercolini et al., 2007; Ercolini et al., 2010). In this study, the growth of Pseudomonas was obvious to be inhibited owing to the fact that storage temperature was far lower than the optimal growth temperature (25°C−35°C) of Pseudomonas such as −4°C and −9°C. Therefore, the bacterial community diversity increased compared to the initial storage (d 1) for superchilling and sub-freezing. These results indicated that lamb meat stored below −4°C can effectively inhibit the reproduction of Pseudomonas and then prolong the shelf life of fresh meat. However, there are still many unknown bacteria worthy of further research.
Functional properties of the bacterial community
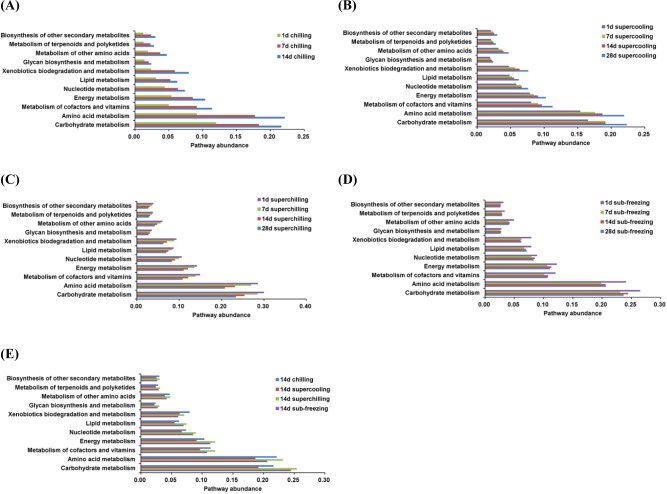
The spoilage potential of microorganism depends on their ability to produce metabolites related to spoilage (Li et al., 2019). As shown in Fig. 4 (A, B, C, and D), for samples of chilling and supercooling, the abundance of genes related to carbohydrate metabolism and amino acid metabolism began to increase after 1 d, but these two metabolic pathways were just the opposite in the samples of superchilling. For sub-freezing, the amino acid metabolism showed a trend of falling at the beginning and subsequent rising, but the carbohydrate metabolism was no regular change. For chilling, the abundance of amino acid metabolism gradually exceeded the abundance of carbohydrate metabolism with storage time increased. This may result from the fact that Pseudomonas as the dominant spoilage bacteria and it exhibited the ability to promote lipolysis and break down the protein. The abundance of two metabolic pathways decreased for superchilling and sub-freezing and this may be due to the less proportion of Pseudomonas. From this point, Pseudomonas, as the main spoilage bacteria, was also in line with the trend of two main metabolic pathways. The results also presented that the longer the storage time was, the stronger abundance of metabolism was inhibited at low storage temperature. Moreover, the abundance of metabolic pathways was also compared on d 14 for all treatments. As shown in Fig. 4E, the top two metabolic pathways are carbohydrate metabolism and amino acid metabolism, which confirms the results of the previous studies (Li et al., 2019; Reis et al., 2016).
Fig. 4. Abundance of functional properties related to microbial metabolism in fresh lamb samples.
(A), (B), (C), and (D) Changes of metabolic pathways in chilling, supercooling, superchilling and sub-freezing samples during storage, (E) Compare of functional properties that related to microbial metabolism in the four fresh lamb samples at day 14.
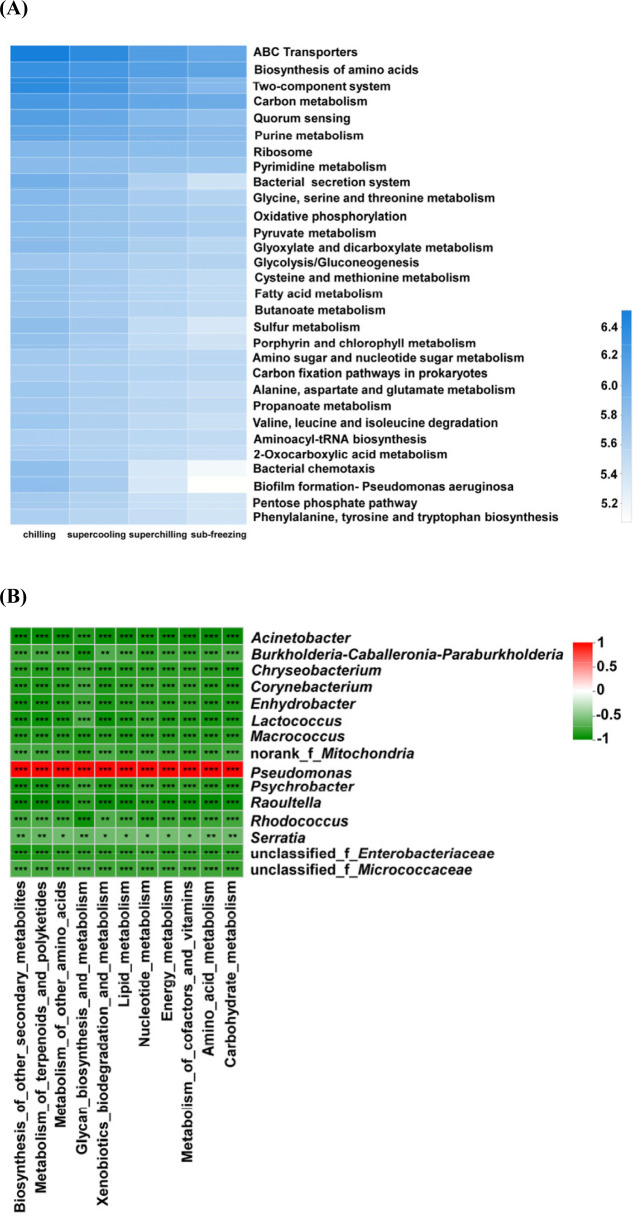
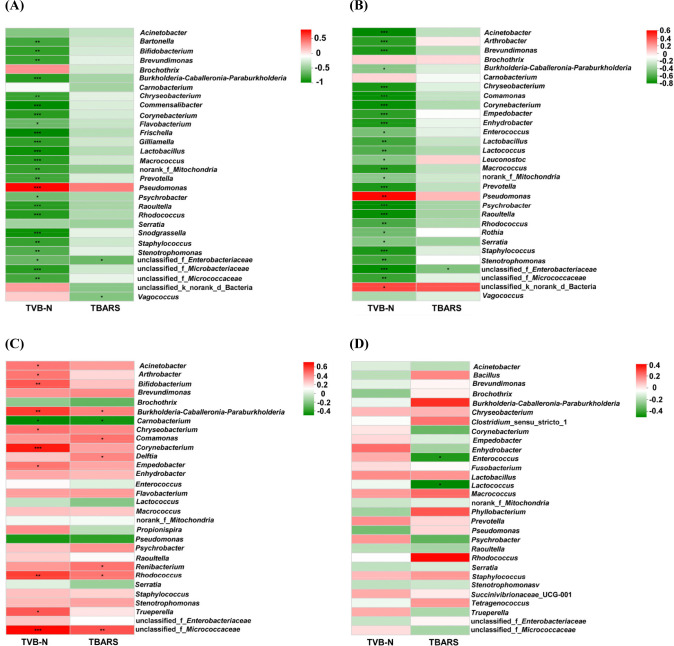
On the level of the thirdly metabolic pathway, the microbial metabolism was also inferred (Fig. 5A and B). The abundance of main metabolic pathways including ABC transporters, Biosynthesis of amino acids, Two-component system and Carbon metabolism for chilling were higher than other treatments. For chilling, the dominant microorganisms were Pseudomonas on d 14. The cold adaptability of Pseudomonas is related to various mechanisms of tolerance to cold-induced stress and the high level of unsaturated fat in the cell membrane (Moreno and Rojo, 2014). Pseudomonas could accelerate the oxidation of free fatty acids, the promotion of proteolytic degradation, and the formation of some volatile compounds including biogenic amines and sulfide formation, etc. (Dainty et al., 1985; Kakouri and Nychas, 1994; Lambert et al., 1991). Meanwhile, the metabolic pathways for chilling related to fatty acid and amino metabolisms such as arginine and proline metabolism, pyruvate and glycerophospholipid metabolism were higher than other treatments. At the same time, the TVC, TVB-N and TBARS values in chilling were higher than these in supercooling, superchilling and sub-freezing significantly (p<0.05) on d 14. Previous study showed that the content of TVB-N in aerobic packing beef is higher, which is likely to be caused by Pseudomonas taetrolens and Pseudomonas fragi (Ercolini et al., 2011). The present results proved that Pseudomonas is significantly positively correlated with TVB-N for chilling and supercooling (p<0.05) (Fig. 6). This finding confirmed that Pseudomonas could have a great impact on the spoilage process of meat. It could infer that Pseudomonas promotes the breakdown of protein and fat by improving related metabolic activities, which leads to the increase of TVB-N and TBARS values.
Fig. 5. Heatmap of metabolic pathways related to bacteria in fresh lamb samples.
Heatmap of bacterial metabolic pathways in fresh lamb stored on the day 14 at different temperatures. Metabolic inference based on the 16S rRNA sequence. The heatmap only showed the relative abundance of metabolic pathways were in top 30, (B) Heatmap of the correlation between the differential abundant genus and metabolic pathway on the day 14 at different temperatures. Red represents a positive correlation, green represents a negative correlation, and the darker the color, the greater the correlation.
Fig. 6. Correlation between bacterial, total volatile base nitrogen (TVB-N) and thiobarbituric acid reactive substance (TBARS) in fresh lamb samples.
Red represents a positive correlation, green represents a negative correlation, and the darker the color, the greater the correlation. * represents a significant difference. * 0.01<p≤0.05, ** 0.001<p≤0.01, *** p≤0.001. (A) chilling, (B) supercooling, (C) superchilling, (D) sub-freezing.
Conclusion
Storage temperature could significantly affect the shelf life and bacterial communities of the very fast chilled tray-packaged lamb meat. Compared to store at chilling or supercooling, the shelf life of very fast chilled lamb meat could be extended to over 70 d when stored at superchilling or sub-freezing. At the genus level, the dominant microorganisms were Pseudomonas followed by Brochothrix for chilling and supercooling storage, and the dominant microorganisms were Pseudomonas, Ralstonia, Psychrobacter, Acinetobacter and Brochothrix for superchilling and sub-freezing storage. Pseudomonas was the core spoilage microorganism that might improve the related metabolic activity (carbohydrate metabolism and amino acid metabolism) to promote the decomposition of protein and lipid to produce metabolites. Accordingly, it can monitor the abundance of Pseudomonas to predict the freshness of fresh tray-packaged lamb meat. Further studies on the response mechanism of spoilage microorganisms to the environmental factors are deserved to explore.
Acknowledgements
This research was financially supported by the Fundamental Research Funds for the China Agriculture Research System (CARS-38), Agricultural Science and Technology Innovation Program (CAAS-ASTIP-2020-IFST-03), and S & T Program of Hebei (No. 20327115D).
Conflicts of Interest
The authors declare no potential conflicts of interest.
Author Contributions
Conceptualization: Zhang D, Hou C. Data curation: Liang C, Zhang D, Zhang Z, Hou C. Formal analysis: Zheng X. Methodology: Wen X, Yan T. Software: Liang C. Validation: Liang C. Investigation: Liang C, Wen X, Yan T. Writing - original draft: Liang C. Writing - review & editing: Liang C, Zhang D, Zheng X, Wen X, Yan T, Zhang Z, Hou C.
Ethics Approval
This article does not require IRB/IACUC approval because there are no human and animal participants.
Supplementary Materials
Supplementary Tables
References
- Aune EJ. Superchilling of foodstuff, a review. 21st Congress ICR; Washington, DC, USA: 2003. [Google Scholar]
- Bailey JS, Reagan JO, Carpenter JA, Schuler GA, Thomson JE. Types of bacteria and shelf-life of evacuated carbon dioxide-injected and ice-packed broilers. J Food Prot. 1979;42:218–221. doi: 10.4315/0362-028X-42.3.218. [DOI] [PubMed] [Google Scholar]
- Benson AK, David JRD, Gilbreth SE, Smith G, Nietfeldt J, Legge R, Kim J, Sinha R, Duncan CE, Ma J, Singh I, Schaffner DW. Microbial successions are associated with changes in chemical profiles of a model refrigerated fresh pork sausage during an 80-day shelf life study. Appl Environ Microbiol. 2014;80:5178–5194. doi: 10.1128/AEM.00774-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berruga MI, Vergara H, Gallego L. Influence of packaging conditions on microbial and lipid oxidation in lamb meat. Small Rumin Res. 2005;57:257–264. doi: 10.1016/j.smallrumres.2004.08.004. [DOI] [Google Scholar]
- Bolton E, Little C, Aird H, Greenwood M, Mclauchlin J, Meldrum R, Surman-Lee S, Tebbutt G, Grant K. [Accessed at July 23, 2018];Guidelines for assessing the microbiological safety of ready-to-eat foods placed on the market. 2009 http://webarchive.nationalarchives.gov.uk/20110930033343/http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1259151921557 Available from.
- Buncic S, Nychas GJ, Lee MRF, Koutsoumanis K, Hébraud M, Desvaux M, Chorianopoulos N, Bolton D, Blagojevic B, Antic D. Microbial pathogen control in the beef chain: Recent research advances. Meat Sci. 2014;97:288–297. doi: 10.1016/j.meatsci.2013.04.040. [DOI] [PubMed] [Google Scholar]
- Casaburi A, Piombino P, Nychas GJ, Villani F, Ercolini D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015;45:83–102. doi: 10.1016/j.fm.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Chaillou S, Chaulot-Talmon A, Caekebeke H, Cardinal M, Christieans S, Denis C, Hélène Desmonts M, Dousset X, Feurer C, Hamon E, Joffraud JJ, La Carbona S, Leroi F, Leroy S, Lorre S, Macé S, Pilet MF, Prévost H, Rivollier M, Roux D, Talon R, Zagorec M, Champomier-Vergès MC. Origin and ecological selection of core and food-specific bacterial communities associated with meat and seafood spoilage. ISME J. 2015;9:1105–1118. doi: 10.1038/ismej.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhou Y, Chen Y, Gu J. FASTP: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KH, Park MS, Kim HY, Bahk GJ. Growth prediction and time-temperature criteria model of Vibrio parahaemolyticus on traditional Korean raw crab marinated in soy sauce (ganjang-gejang) at different storage temperatures. Food Control. 2019;98:187–193. doi: 10.1016/j.foodcont.2018.11.021. [DOI] [Google Scholar]
- Dai Y, Lu Y, Wu W, Lu XM, Han ZP, Liu Y, Li XM, Dai RT. Changes in oxidation, color and texture deteriorations during refrigerated storage of ohmically and water bath-cooked pork meat. Innov Food Sci Emerg. 2014;26:341–346. doi: 10.1016/j.ifset.2014.06.009. [DOI] [Google Scholar]
- Dainty RH, Edwards RA, Hibbard CM. Time course of volatile compound formation during refrigerated storage of naturally contaminated beef in air. J Appl Bacteriol. 1985;59:303–309. doi: 10.1111/j.1365-2672.1985.tb03324.x. [DOI] [PubMed] [Google Scholar]
- Doulgeraki AI, Ercolini D, Villani F, Nychas GJE. Spoilage microbiota associated to the storage of raw meat in different conditions. Int J Food Microbiol. 2012;157:130–141. doi: 10.1016/j.ijfoodmicro.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Doulgeraki AI, Nychas GJE. Monitoring the succession of the biota grown on a selective medium for pseudomonads during storage of minced beef with molecular-based methods. Food Microbiol. 2013;34:62–69. doi: 10.1016/j.fm.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Duun AS, Rustad T. Quality changes during superchilled storage of cod (Gadus morhua) fillets. Food Chem. 2007;105:1067–1075. doi: 10.1016/j.foodchem.2007.05.020. [DOI] [Google Scholar]
- Edgar RC. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Enfors SO, Molin G, Ternström A. Effect of packaging under carbon dioxide, nitrogen or air on the microbial flora of pork stored at 4°C. J Appl Bacteriol. 1979;47:197–208. doi: 10.1111/j.1365-2672.1979.tb01746.x. [DOI] [PubMed] [Google Scholar]
- Ercolini D, Casaburi A, Nasi A, Ferrocino I, Di Monaco R, Ferranti P, Mauriello G, Villani F. Different molecular types of Pseudomonas fragi have the same overall behaviour as meat spoilers. Int J Food Microbiol. 2010;142:120–131. doi: 10.1016/j.ijfoodmicro.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Ercolini D, Ferrocino I, Nasi A, Ndagijimana M, Vernocchi P, La Storia A, Laghi L, Mauriello G, Guerzoni ME, Villani F. Monitoring of microbial metabolites and bacterial diversity in beef stored under different packaging conditions. Appl Environ Microbiol. 2011;77:7372–7381. doi: 10.1128/AEM.05521-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercolini D, Russo F, Blaiotta G, Pepe O, Mauriello G, Villani F. Simultaneous detection of Pseudomonas fragi, P. lundensis, and P. putida from meat by use of a multiplex PCR assay targeting the carA gene. Appl Environ Microbiol. 2007;73:2354–2359. doi: 10.1128/AEM.02603-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercolini D, Russo F, Torrieri E, Masi P, Villani F. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl Environ Microbiol. 2006;72:4663–4671. doi: 10.1128/AEM.00468-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkmen O, Bozoglu TF. Spoilage of meat and meat products. John Wiley & Sons; New Jersey, NJ, USA: 2016. pp. 279–295. [DOI] [Google Scholar]
- Ganhão R, Estévez M, Morcuende D. Suitability of the TBA method for assessing lipid oxidation in a meat system with added phenolic-rich materials. Food Chem. 2011;126:772–778. doi: 10.1016/j.foodchem.2010.11.064. [DOI] [Google Scholar]
- Gill CO. Substrate limitation of bacterial growth at meat surfaces. J Appl Bacteriol. 1976;41:401–410. doi: 10.1111/j.1365-2672.1976.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Hansen E, Juncher D, Henckel P, Karlsson A, Bertelsen G, Skibsted LH. Oxidative stability of chilled pork chops following long term freeze storage. Meat Sci. 2004;68:479–484. doi: 10.1016/j.meatsci.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Hernández-Macedo ML, Contreras-Castillo CJ, Tsai SM, Da Cruz SH, Sarantopoulos CIGL, Padula M, Dias CTS. Gases and volatile compounds associated with micro-organisms in blown pack spoilage of Brazilian vacuum-packed beef. Lett Appl Microbiol. 2012;55:467–475. doi: 10.1111/lam.12004. [DOI] [PubMed] [Google Scholar]
- Hong H, Luo Y, Zhu S, Shen H. Application of the general stability index method to predict quality deterioration in bighead carp (Aristichthys nobilis) heads during storage at different temperatures. J Food Eng. 2012;113:554–558. doi: 10.1016/j.jfoodeng.2012.07.012. [DOI] [Google Scholar]
- Joseph RL. Very fast chilling of beef and tenderness: A report from an EU concerted action. Meat Sci. 1996;43:217–227. doi: 10.1016/0309-1740(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Kakouri A, Nychas GJE. Storage of poultry meat under modified atmospheres or vacuum packs: Possible role of microbial metabolites as indicator of spoilage. J Appl Bacteriol. 1994;76:163–172. doi: 10.1111/j.1365-2672.1994.tb01612.x. [DOI] [PubMed] [Google Scholar]
- Karel M, Fennema OR, Lund DB. Physical principles of food preservation. Marcel Dekker; New York, NY, USA: 1975. pp. 133–172. [Google Scholar]
- Kaur M, Williams M, Bissett A, Ross T, Bowman JP. Effect of abattoir, livestock species and storage temperature on bacterial community dynamics and sensory properties of vacuum packaged red meat. Food Microbiol. 2021;94:103648. doi: 10.1016/j.fm.2020.103648. [DOI] [PubMed] [Google Scholar]
- Kumosinski TF. Use of thermodynamic linkage to study the salt-induced solubility change of soybean isolate. J Agric Food Chem. 1988;36:669–672. doi: 10.1021/jf00081a065. [DOI] [Google Scholar]
- Lambert AD, Smith JP, Dodds KL. Shelf life extension and microbiological safety of fresh meat: A review. Food Microbiol. 1991;8:267–297. doi: 10.1016/S0740-0020(05)80002-4. [DOI] [Google Scholar]
- Leygonie C, Britz TJ, Hoffman LC. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012;91:93–98. doi: 10.1016/j.meatsci.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Li N, Zhang YX, Wu Q, Gu Q, Chen MT, Zhang Y, Sun X, Zhang J. High-throughput sequencing analysis of bacterial community composition and quality characteristics in refrigerated pork during storage. Food Microbiol. 2019;83:86–94. doi: 10.1016/j.fm.2019.04.013. [DOI] [PubMed] [Google Scholar]
- Liu F, Guo YZ, Li YF. Interactions of microorganisms during natural spoilage of pork at 5°C. J Food Eng. 2006;72:24–29. doi: 10.1016/j.jfoodeng.2004.11.015. [DOI] [Google Scholar]
- Liu NT, Bauchan GR, Francoeur CB, Shelton DR, Lo YM, Nou X. Ralstonia insidiosa serves as bridges in biofilm formation by foodborne pathogens Listeria monocytogenes, Salmonella enterica, and enterohemorrhagic Escherichia coli. Food Control. 2016;65:14–20. doi: 10.1016/j.foodcont.2016.01.004. [DOI] [Google Scholar]
- Liu NT, Nou X, Bauchan GR, Murphy C, Lefcourt AM, Shelton DR, Lo YM. Effects of environmental parameters on the dual-species biofilms formed by Escherichia coli O157:H7 and Ralstonia insidiosa, a strong biofilm producer isolated from a fresh-cut produce processing plant. J Food Prot. 2015;78:121–127. doi: 10.4315/0362-028X.JFP-14-302. [DOI] [PubMed] [Google Scholar]
- Lu X, Zhang Y, Xu B, Zhu L, Luo X. Protein degradation and structure changes of beef muscle during superchilled storage. Meat Sci. 2020;168:108180. doi: 10.1016/j.meatsci.2020.108180. [DOI] [PubMed] [Google Scholar]
- Lu X, Zhang Y, Zhu L, Luo X, Hopkins DL. Effect of superchilled storage on shelf life and quality characteristics of M. longissimus lumborum from Chinese yellow cattle. Meat Sci. 2019;149:79–84. doi: 10.1016/j.meatsci.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Lynch MF, Tauxe RV, Hedberg CW. The growing burden of foodborne outbreaks due to contaminated fresh produce: Risks and opportunities. Epidemiol Infect. 2009;137:307–315. doi: 10.1017/S0950268808001969. [DOI] [PubMed] [Google Scholar]
- Magnussen OM, Haugland A, Torstveit Hemmingsen AK, Johansen S, Nordtvedt TS. Advances in superchilling of food: Process characteristics and product quality. Trends Food Sci Technol. 2008;19:418–424. doi: 10.1016/j.tifs.2008.04.005. [DOI] [Google Scholar]
- Magoc T, Salzberg SL. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur AR, Song EJ, Cho YS, Nam YD, Choi YS, Kim DO, Seo DH, Nam TG. Comparative evaluation of spoilage-related bacterial diversity and metabolite profiles in chilled beef stored under air and vacuum packaging. Food Microbiol. 2019;77:166–172. doi: 10.1016/j.fm.2018.09.006. [DOI] [PubMed] [Google Scholar]
- McGeehin B, Sheridan JJ, Butler F. Optimising a rapid chilling system for lamb carcasses. J Food Eng. 2002;52:75–81. doi: 10.1016/S0260-8774(01)00088-7. [DOI] [Google Scholar]
- Moreno R, Rojo F. Features of pseudomonads growing at low temperatures: Another facet of their versatility. Environ Microbiol Rep. 2014;6:417–426. doi: 10.1111/1758-2229.12150. [DOI] [PubMed] [Google Scholar]
- Muela E, Sañudo C, Campo MM, Medel I, Beltrán JA. Effect of freezing method and frozen storage duration on instrumental quality of lamb throughout display. Meat Sci. 2010;84:662–669. doi: 10.1016/j.meatsci.2009.10.028. [DOI] [PubMed] [Google Scholar]
- National Health and Family Planning Commission of China . Chinese Standard GB 5009.228–2016. Determination of total volatile basic nitrogen in foods. China Standards Press of China; Beijing, China: 2016. [Google Scholar]
- Nychas GJE, Skandamis PN, Tassou CC, Koutsoumanis KP. Meat spoilage during distribution. Meat Sci. 2008;78:77–89. doi: 10.1016/j.meatsci.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Omer MK, Hauge SJ, Østensvik Ø, Moen B, Alvseike O, Røtterud OJ, Prieto M, Dommersnes S, Nesteng OH, Nesbakken T. Effects of hygienic treatments during slaughtering on microbial dynamics and contamination of sheep meat. Int J Food Microbiol. 2015;194:7–14. doi: 10.1016/j.ijfoodmicro.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Pellissery AJ, Vinayamohan PG, Amalaradjou MAR, Venkitanarayanan K. Spoilage bacteria and meat quality. In: Biswas AK, Mandal PK, editors. In Meat quality analysis. Academic Press; London, UK: 2020. pp. 307–334. (ed) [DOI] [Google Scholar]
- Pomponio L, Bukh C, Ruiz-Carrascal J. Proteolysis in pork loins during superchilling and regular chilling storage. Meat Sci. 2018;141:57–65. doi: 10.1016/j.meatsci.2018.03.022. [DOI] [PubMed] [Google Scholar]
- Qian S, Li X, Wang H, Sun Z, Zhang C, Guan W, Blecker C. Effect of sub-freezing storage (−6, −9 and −12°C) on quality and shelf life of beef. Int J Food Sci Technol. 2018;53:2129–2140. doi: 10.1111/ijfs.13800. [DOI] [Google Scholar]
- Reid R, Fanning S, Whyte P, Kerry J, Lindqvist R, Yu Z, Bolton D. The microbiology of beef carcasses and primals during chilling and commercial storage. Food Microbiol. 2017;61:50–57. doi: 10.1016/j.fm.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Reis MM, Reis MG, Mills J, Ross C, Brightwell G. Characterization of volatile metabolites associated with confinement odour during the shelf-life of vacuum packed lamb meat under different storage conditions. Meat Sci. 2016;113:80–91. doi: 10.1016/j.meatsci.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Resconi VC, Bueno M, Escudero A, Magalhaes D, Ferreira V, Campo MM. Ageing and retail display time in raw beef odour according to the degree of lipid oxidation. Food Chem. 2018;242:288–300. doi: 10.1016/j.foodchem.2017.09.036. [DOI] [PubMed] [Google Scholar]
- Sinnhuber RO, Yu TC. The 2-thiobarbituric acid reaction, an objective measure of the oxidative deterioration occurring in fats and oils. J Jpn Oil Chem Soc. 1977;26:259–267. doi: 10.5650/jos1956.26.259. [DOI] [Google Scholar]
- Smit BA, Engels WJM, Smit G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl Microbiol Biotechnol. 2009;81:987–999. doi: 10.1007/s00253-008-1758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyer A, Özalp B, Dalmiş Ü, Bilgin V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010;120:1025–1030. doi: 10.1016/j.foodchem.2009.11.042. [DOI] [Google Scholar]
- Stackebrandt E, Goebel BM. Taxonomic note: A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–849. doi: 10.1099/00207713-44-4-846. [DOI] [Google Scholar]
- Stonehouse GG, Evans JA. The use of supercooling for fresh foods: A review. J Food Eng. 2015;148:74–79. doi: 10.1016/j.jfoodeng.2014.08.007. [DOI] [Google Scholar]
- Tsigarida E, Boziaris IS, Nychas GJE. Bacterial synergism or antagonism in a gel cassette system. Appl Environ Microbiol. 2003;69:7204–7209. doi: 10.1128/AEM.69.12.7204-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuboly E, Lebovics VK, Gaál Ö, Mészáros L, Farkas J. Microbiological and lipid oxidation studies on mechanically deboned turkey meat treated by high hydrostatic pressure. J Food Eng. 2003;56:241–244. doi: 10.1016/S0260-8774(02)00260-1. [DOI] [Google Scholar]
- Wang H, Qin X, Li X, Wang X, Gao H, Zhang C. Changes in the microbial communities of air- and water-chilled yellow-feathered broilers during storage at 2°C. Food Microbiol. 2020a;87:103390. doi: 10.1016/j.fm.2019.103390. [DOI] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, He Z, Gan X, Li H. Interrelationship among ferrous myoglobin, lipid and protein oxidations in rabbit meat during refrigerated and superchilled storage. Meat Sci. 2018;146:131–139. doi: 10.1016/j.meatsci.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Wang Z, Shi Y, Zhou K, Zhou H, Li X, Li C, Wang Z, Xu B. Effects of different thermal temperatures on the shelf life and microbial diversity of Dezhou-braised chicken. Food Res Int. 2020b;136:109471. doi: 10.1016/j.foodres.2020.109471. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhu L, Zhang Y, Liang R, Luo X. Microbial community dynamics analysis by high-throughput sequencing in chilled beef longissimus steaks packaged under modified atmospheres. Meat Sci. 2018;141:94–102. doi: 10.1016/j.meatsci.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mao Y, Li K, Luo X, Hopkins DL. Effect of carcass chilling on the palatability traits and safety of fresh red meat. Compr Rev Food Sci Food Saf. 2019;18:1676–1704. doi: 10.1111/1541-4337.12497. [DOI] [PubMed] [Google Scholar]
- Zhou GH, Xu XL, Liu Y. Preservation technologies for fresh meat: A review. Meat Sci. 2010;86:119–128. doi: 10.1016/j.meatsci.2010.04.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables