Abstract
Purpose:
Clinical research in India has been besieged by controversies. While studies have addressed other stakeholders, few have addressed the patient. The present study was conducted to assess the extent of awareness and understanding about the nature and conduct of CR among people of Mumbai.
Methods:
Institutional Ethics Committee approval was taken (EC/OA-12/15) and written informed consent was obtained. Adults who were residents of Mumbai were enrolled. A prevalidated and published 48-item questionnaire based on six themes, namely awareness and participation, voluntariness and autonomy, compensation, confidentiality, safety, and involvement in CR were administered. Perception based on themes and association of variables such as age, gender, socioeconomic class, and education on this perception was assessed. Descriptive statistics along with Chi-square test/Chi-square test for trend and crude odds ratio (cOR) were assessed.
Results:
Of the 453 participants approached, 400 (age 32 [18–96]) consented. Only 210/400 (52.5%) were aware of CR and 194/400 (48.5%) said they needed permission for participation. Only 226/400 (56.5%) were aware of their rights and 111/400 (27.75%) felt that clinical trial participants received compensation. The socioeconomic class influenced awareness of CR (P < 0.00001; r2= 0.495) as did the age (P < 0.0001; r2= 0.82). Men were less likely to need permission to participate relative to women (cOR [95% confidence interval (CI)] 2.47 [1.6, 3.6] [P < 0.00001]). Those who had heard of CR were twice more willing to participate (cOR [95% CI] 1.72 (1.2, 2.6); P = 0.008).
Conclusions:
There is a greater need to improve awareness, especially about safety, compensation, and confidentiality in CR.
Keywords: Clinical research, compensation, confidentiality, patient safety, public awareness
INTRODUCTION
India has emerged as one of the key destinations for the conduct of clinical research (CR) over the last decade.[1] A slew of regulatory changes was introduced in the country in recent times to foster growth of CR and protect patient rights. These include, among others, the mandatory registration of ethics committees, specification of conditions required for the conduct of clinical trials, and defining the quantum of compensation for trial-related injuries.[2]
Studies which assessed public awareness and attitude toward CR in India found that people lack adequate understanding about compensation for adverse outcomes and safety of the participants enrolled in trials.[3,4,5,6] Similarly, a meta-analysis of seven studies including three from India found that an overwhelming 64% denied participation in research owing to reasons such as mistrust of the trial organization, concerns about safety and efficacy, and breach of confidentiality.[7] Studies have shown that creating awareness, changes the attitude toward clinical trials, enrolment, and the benefits of participation.[8] Furthermore, a well-informed public is always in a better position to safeguard their rights.
Understanding the existing perceptions and knowledge about CR among people is crucial for designing better awareness programs. Mumbai, a cosmopolitan city in India, is home to people from diverse cultural backgrounds, ethnicities, and religions. In addition, a large number of clinical trial sites are located in Mumbai.[9] Despite this, there is no data on the public attitudes and perceptions toward research in Mumbai.
The present study was conducted with the primary objective of evaluating public knowledge and perceptions about CR in the city. A secondary objective was to study the association of variables such as age, gender, and socioeconomic class with these perceptions.
METHODS
Ethics
The Institutional Ethics Committee of Seth GS Medical College and KEM Hospital approved this study and written informed consent was obtained from the participants. The Trial was registered in the Clinical Trial Registry of India (CTRI/2017/07/009066).
Study design
This was a cross-sectional study.
Study site and duration
The study was conducted in Mumbai between June 2015 and October 2016.
Study instrument
A 48-item prevalidated and published questionnaire developed by Tal Burt et al.,[5] was used after obtaining permission from the author. The questionnaire was translated into two regional languages, namely Marathi and Hindi by a study team member and the translation authentication was performed by the respective subject experts. Reliability assessment was done for the translated version prior to administration.
Sample size and sampling
The sample size of 400 participants for the study was calculated using Yamane equation.[10] The study team members screened the potential participants from across the 24 administrative wards of Mumbai by visiting public places and residential areas in each ward. Those above 18 years of age and who provided written, informed consent and confirmed their willingness to answer the study questionnaire were eligible and enrolled in the study.
Study procedure
The questionnaire was self-administered, and each participant was given adequate time to answer. Demographic details for the language, monthly income, gender, education, occupation, and age were collected for each participant. Socioeconomic class of the study participants was assessed using the modified Kuppuswamy scale 2015.[10]
Outcome measures
Responses given to the six themes and expressed in proportions and association of that response with variables such as age, gender, socioeconomic class (with education being part of socioeconomic class).
Statistical analysis
Data were analyzed using both descriptive and inferential statistics. The age was expressed as median [range] and gender, language, socioeconomic class, and education were expressed as proportions. The association between the response to the main question (in all six themes) with age, gender, and socioeconomic class was analyzed using the Chi-square for trend, and the strength of this association was expressed as crude odds ratio along with 95% confidence intervals (CIs). All analyses were performed at 5% significance using GraphPad Software version 5.0 manufactured by Graphpad Software Inc. 2365, Northside Dr. Suite 560 San Diego, CA 92108, USA.
RESULTS
A total of 453 participants were screened and counseled out of which 400 participants agreed to participate. A total of 53 participants declined to participate.
Age: Only 353/400 (88.25%) participants had mentioned their age and majority (168/353, 47.59%) were between the age group of 18–30 years. Gender: More than 50% of the participants were male. Socioeconomic class as per the Kuppuswamy scale: Three hundred and eighty-three out of four hundred (95.75%) participants had mentioned information pertaining to their socioeconomic class and more than >60% belonged to the upper-middle class. Language: More than 50% were Marathi speakers. Education as per Kuppuswamy scale: Three hundred and ninety-seven out of 400 (99.25%) had mentioned their education and 191/400 (48.1%) were either graduates, postgraduates or had a professional degree [Table 1].
Table 1.
Demographics of the study participants as per socioeconomic class, education, and language
| Variables | n (%) |
|---|---|
| Age (n=353) | |
| 18-30 | 168 (47.59) |
| 31-50 | 147 (41.64) |
| Above 50 | 38 (10.76) |
| Gender (n=400) | |
| Males | 233 (58.25) |
| Females | 167 (41.75) |
| Socioeconomic class (n=383) | |
| Upper middle class | 262 (68.40) |
| Upper class | 47 (13.31) |
| Upper lower class | 56 (15.86) |
| Lower middle class | 15 (4.2) |
| Lower class | 3 (0.84) |
| Language (n=398) | |
| Marathi | 225 (56.53) |
| Hindi | 101 (25.37) |
| Gujarati | 28 (7.03) |
| Sindhi | 10 (2.51) |
| English | 6 (1.5) |
| Other | 28 (7.14) |
| Education (n=397) | |
| Professional | 28 (41.05) |
| Graduate or postgraduate | 163 (7.05) |
| Intermediate | 73 (18.38) |
| High school certificate | 76 (19.14) |
| Middle school certificate | 38 (9.57) |
| Primary school education | 19 (4.7) |
Overall response with respect to the six themes [Table 2]
Table 2.
Response based on themes
| Themes and related questions | Response | Percentage |
|---|---|---|
| Awareness and participation | ||
| Have you heard about clinical research? | 210/400 | 52.5 |
| From whom did you hear about clinical research? | Doctor - 46/210 | 21.9 |
| Media - 37/210 | 17.6 | |
| Internet - 17/210 | 8.09 | |
| Relatives - 18/210 | 8.5 | |
| Friends - 17/210 | 8.09 | |
| Colleagues - 10/210 | 4.76 | |
| Other sources like company training, school, etc., - 24/210 | 11.42 | |
| Multiple sources - 41/210 | 52 | |
| Are you willing to participate in clinical trials? | Yes - 238/400 (59.5%) | 59.5 |
| If yes, what type of study would you like to participate? | Noninterventional - 101/238 | 42.43 |
| Low-risk observational studies like single blood draw - 28/238 | 11.76 | |
| Multiple visit interventional study - 30/238 | 12.60 | |
| Multiple types of studies - 75/238 | 31.51 | |
| If No, can you state a reason | Concern about safety - 57/157 | 36.30 |
| Lack of time - 30/157 | 19.10 | |
| Lack of trust - 13/157 | 8.29 | |
| Voluntariness and autonomy | ||
| Participation in research is entirely voluntary | True - 359/400 | 89.8 |
| Would you have to take permission from someone else to participate in research? | Yes - 196/400 | 49 |
| If yes who would it be? | Family members - 139/196 | 70.91 |
| Confidentiality | ||
| Confidentiality is a matter of importance to research participants | Yes - 324/400 | 81 |
| Compensation | ||
| Participants in clinical research get adequate compensation for any adverse outcomes | Not aware - 180/400 | 45 |
| No - 103/400 | 25.75 | |
| Yes - 112/400 | 28 | |
| Not answered - 05/400 | 1.25 | |
| Safety | ||
| Human participants in clinical research are treated like experimental animals (“human Guinea Pigs”) | 64/400 | 16 |
| Researchers make sure research is safe for participants. | 223/400 | 55.8 |
| Importance of clinical research | ||
| Clinical research benefits society | 342/400 | 85.5 |
A little over half (210/400, 52.5%) had heard about research and were willing to participate (238/400, 59.5%). An overwhelming (359/400, 89.8%) felt that participation in clinical trials is voluntary, yet (196/400, 48.5%) said they would need permission from a family member or their family physician to participate in research. Majority (342/400, over 80%) had a positive perception about research; however, awareness about compensation for adverse outcomes during study conduct was low (45% – not aware) [Table 2].
Associations within the themes
A few associations were found 1) Awareness with willingness - those who were aware of CR were approximately twice more willing to participate relative to those who were not aware of CR [cOR(95% C.I 2); 1.725(1.15-2.57)] 2) Autonomy and willingness - Those who had autonomy were twice as likely to participate relative to those who did not [cOR(95% C.I); 2.372(1.58-3.57)] 3) Gender and autonomy- Women were twice more likely to need permission to participate relative to men [cOR(95% C.I); 2.5 (1.61-3.64)] [Table 3].
Table 3.
Associations within the themes
| Associations | Awareness | Number of participants |
cOR (95% CI) | |
|---|---|---|---|---|
| Willing | Not willing | |||
| Awareness with willingness to participate | Yes | 138 | 72 | 1.725 (1.15-2.57) |
| No | 100 | 90 | ||
| Associations | Autonomy | Willing | Not willing | cOR (95% CI) |
| Autonomy to participate with willingness to participate | No | 142 | 63 | 2.372 (1.58-3.57) |
| Yes | 95 | 100 | ||
| Associations | Participants | No autonomy to participate | Autonomy to participate | cOR (95% CI) |
| Gender and autonomy to participate | Women (n=167) | 103 | 64 | 2.5 (1.61-3.64) |
| Men (n=233) | 92 | 141 | ||
cOR: Crude odds ratio, CI: Confidence interval
Association of variables on the six themes
Socioeconomic class
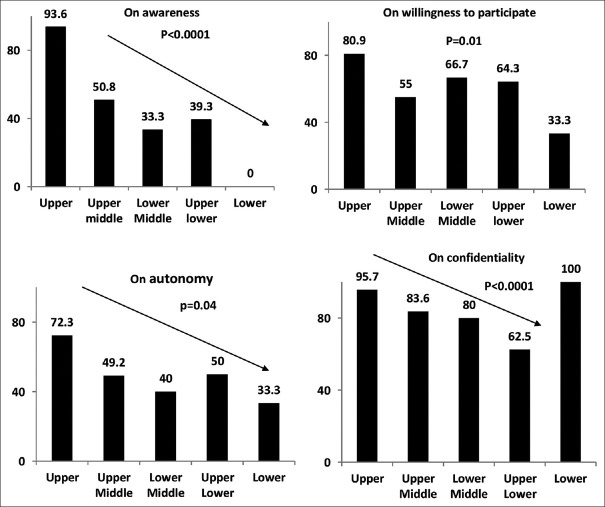
The socioeconomic class was associated with awareness (lower awareness in [lower socioeconomic classes relative to other classes (P < 0.00001)], willingness to participate [decrease in willingness to participate among lower class; P = 0.012], voluntariness [A large number required permission to participate in lower classes; P = 0.041], and understanding of confidentiality [higher in upper classes) among the study participants (P < 0.05)] [Figure 1].
Figure 1.
Association of socioeconomic class with the themes
Age
The age was seen to be associated with awareness [reduced awareness with increasing age; P = 0.00006], willingness [reluctance to participate with rising age; P = 0.0004] and confidentiality (older individuals less concerned about confidentiality, P = 0.009).
Education
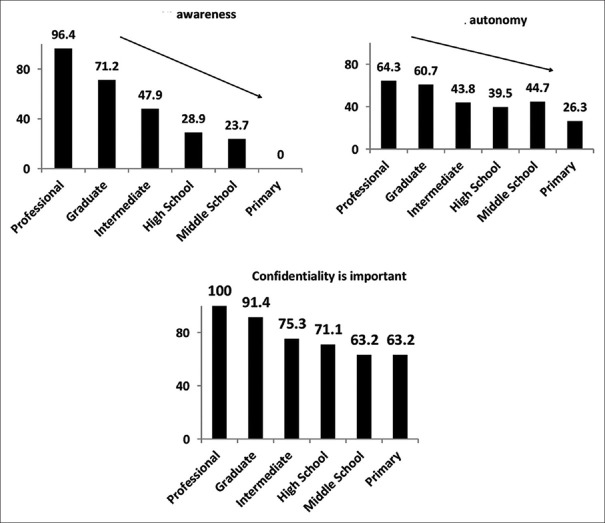
Education was also associated with a participant's willingness to participate (willingness being higher with greater education P < 0.0001), need for permission (less educated needed permission; P = 0.001), belief about confidentiality (more highly educated felt that confidentiality is important; P < 0.0001), belief about safety (individuals with higher education had better perception about safety; P = 0.006) [Figure 2].
Figure 2.
Association of education on the themes related to clinical research
DISCUSSION
We conducted a cross-sectional study among lay people in various administrative wards of the city of Mumbai and found that a majority (>50%) individuals were aware of CR and as many as 60% were willing to participate in the research. The extent of awareness seen by us (52%) was much higher than in the study by Burt et al. in Delhi and Joshi et al. in Pune where awareness was only 26% and 25%, respectively, among the population sampled[4,5] This variation was seen despite the fact that all three surveys were done in urban cities of India with similar literacy levels and a large number of young people (mean age [±standard deviation]: 32 [±12] in our study vs. 39.6 [±16.6] in Burt et al. vs. 39 [±14] in Joshi et al.). The extent of awareness reported in the studies conducted in Delhi and Pune as well as our study in Mumbai are expressed in proportions without CIs, and the true difference, therefore, cannot be assessed.
Physicians were stated as being the main source of knowledge about CR by most participants (21.9%) in our study. In the Pune study, similarly, 72% of participants knew about CR through physicians.[4] The greater willingness to participate in research among our participants was associated with low risk and noninterventional studies. This was similar to a study by Decosta et al.[11] conducted in rural North India, which found that participants preferred CR involving interview-based and low-risk studies that had a single blood sample collection.[12] Another study by Thaker et al. which assessed the reasons for consent refusal in CR had identified “concerns about the risk” as one among the several factors influencing the decision to participate.[13]
Individuals who were aware of CR were more willing to participate in CR as per our study. This finding was corroborated by the findings from the “Haris interactive” survey conducted in a high-income country[14] in 2001 where it was seen that the majority (75%) of interviewed participants would have enrolled in a trial had they been made aware of it.[8]
An overwhelming number (89.8%) of individuals believed that participation in CR is voluntary yet; very few had the freedom to participate without consulting a family member or a physician. The “need to seek permission” before participating in a clinical trial reflects the social fabric of the country where decision-making process of an individual is invariably a “joint” decision of the individual with his/her family or even community.[15] Studies from various developing settings indicate that the decision of the females to participate in CR was especially guided by their spouse or a family member.[16] The low recruitment of women in CR with a high dropout is a reflection of this limited autonomy in women.[17]
The willingness to participate was better among individuals from upper socioeconomic class, as well as younger and individuals with a higher education. One of the reasons suggested by Unger et al., for less willingness among lower-income groups is the concern about excess expenditure which might be incurred during participation.[18] Our observation, however, differs from a study conducted by Chu et al., in the urban and rural areas of South Korea who found no correlation between age, gender, socioeconomic class, and education on the willingness to participate.[19] Social systems have the ability to impact an individual's attitude, knowledge, and decision-making regarding participating in CR;[20] and therefore, the setting in which CR is conducted may influence perceptions.
We found that many of the participants (68%) believed that the confidentiality of research participants is adequately protected by researchers. The belief in physicians (as researchers) among Indian patients is further corroborated by a study conducted by Doshi et al. in India that assessed the reasons that motivate participants to consent for nontherapeutic trials and found that 88% considered participation mainly on the physician's request.[21]
We observed that a majority (55%) of the participants perceived that research was safe for participants, and this observation is similar to a study conducted in Mexico by González-Saldivar et al. in which they found 68.95% of the individuals who had not participated in trials felt “protected in case of a serious adverse event related to the experimental drugs.”[22] In our study, we found that 45% of the participants were not very well aware of compensation for adverse research-related events, and 25.7% believed that compensation is not given in CR. Despite regulations for compensation in case of serious adverse events,[23,24,25,26] the lack of awareness remains a concern.
An overwhelming number of participants in our study emphasized the importance of CR with 93% stating that “Clinical Research was important in the development of new treatment.” This observation is similar to the findings from a qualitative study conducted in Ghana, where participants believed that trial studies are needed to determine efficacy and to “come out with new knowledge on whether the drugs were suitable for human beings to use.”[27]
Our study is limited by the fact that it is cross-sectional and therefore inferences about causality cannot be truly drawn. The distribution of the participants with respect to socioeconomic class may not represent the actual census data for the city of Mumbai as classification was based on the Kuppuswamy scale[12] and census data on socioeconomic class was unavailable at the time of conduct. There is selection bias in the study due to the operational challenges such as denial of permission by some residential societies, and thus, those who agreed when approached were ultimately enrolled. A pre-validated questionnaire was used, and only reliability analysis was done for the translated versions.
CONCLUSION
In summary, our study showed that potential participants in Mumbai were aware of CR and their rights as research participants. However, they were less aware of key aspect like compensation for trial-related injuries and safety of trial participants in CR. The study provides baseline awareness about CR in the city, and awareness programs about CR will help promote patient engagement in trials beyond mere participation.
Financial support and sponsorship
This study was funded by the Research Society of Seth GS Medical College and KEM Hospital.
Conflicts of interest
Authors have no competing interest to state.
Acknowledgments
We are grateful to Dr. Tal Burt for allowing us to use the pre-validated version of his questionnaire for our study.
REFERENCES
- 1.Poongothai S, Unnikrishnan R, Balasubramanian J, Nair MD, Mohan V. Why are clinical trials necessary in India? Perspect Clin Res. 2014;5:55–9. doi: 10.4103/2229-3485.128018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gogtay NJ, Ravi R, Thatte UM. Regulatory requirements for clinical trials in India: What academicians need to know. Indian J Anaesth. 2017;61:192–9. doi: 10.4103/ija.IJA_143_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sridharan K, Mehta M, Sivaramakrishnan G. Awareness and attitude of general public about clinical trials from a developing country. Am J Exp Clin Res. 2016;3:146–8. [Google Scholar]
- 4.Joshi VD, Oka GA, Kulkarni AA, Bivalkar VV. Public awareness and perception of clinical trials: Quantitative study in Pune. Perspect Clin Res. 2013;4:169–74. doi: 10.4103/2229-3485.115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burt T, Dhillon S, Sharma P, Khan D, Mv D, Alam S, et al. PARTAKE survey of public knowledge and perceptions of clinical research in India. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0068666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapadia K. Understanding patients perspective on clinical research in Indian population. J Clin Res Bioeth. 2015;6:1–6. [Google Scholar]
- 7.Shah JY, Phadtare A, Rajgor D, Vaghasia M, Pradhan S, Zelko H, et al. What leads Indians to participate in clinical trials? A meta-analysis of qualitative studies. PLoS One. 2010;5:e10730. doi: 10.1371/journal.pone.0010730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris Interactive. Misconceptions and lack of awareness greatly reduce recruitment for cancer clinical trials. [Last accessed on 2019 May 19];Health Care News. 2001 1:1–3. Available from: http://www.harrisinteractive.com/news/newsletters/healthnews/HI_HealthCareNews2001Vol1_iss3.pdf . [Google Scholar]
- 9.Clinical Trial Registry-India. [Last accessed on 2018 Mar 20]. Available from: http://ctri.nic.in/Clinicaltrials/advancesearchmain.php .
- 10.Israel GD. Determining Sample Size. University of Florida. 1992. [Last accessed on 2019 May 19]. pp. 1–5. Available from: https://www.gjimt.ac.in/wp-content/uploads/2017/10/2_Glenn-D.-Israel_Determining-Sample-Size.pdf .
- 11.DeCosta A, D'Souza N, Krishnan S, Chhabra MS, Shihaam I, Goswami K. Community based trials and informed consent in rural north India. J Med Ethics. 2004;30:318–23. doi: 10.1136/jme.2002.001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oberoi SS. Updating income ranges for Kuppuswamy's socio-economic status scale for the year 2014. Indian J Public Health. 2015;59:156–7. doi: 10.4103/0019-557X.157540. [DOI] [PubMed] [Google Scholar]
- 13.Thaker SJ, Figer BH, Gogtay NJ, Thatte UM. An audit of consent refusals in clinical research at a tertiary care center in India. J Postgrad Med. 2015;61:257–63. doi: 10.4103/0022-3859.166515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Country Classification. Data Sources, Country Classifications and Aggregation Methodologies. [Last accessed on 2018 Aug 17]. Available from: http://www.un.org/en/development/desa/policy/wesp/wesp_current/2014wesp_country_classification.pdf .
- 15.Lobato L, Bethony JM, Pereira FB, Grahek SL, Diemert D, Gazzinelli MF, et al. Impact of gender on the decision to participate in a clinical trial: A cross-sectional study. BMC Public Health. 2014;14:1156. doi: 10.1186/1471-2458-14-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall PA, Adebamowo CA, Adeyemo AA, Ogundiran TO, Strenski T, Zhou J, et al. Voluntary participation and comprehension of informed consent in a genetic epidemiological study of breast cancer in nigeria. BMC Med Ethics. 2014;15:38. doi: 10.1186/1472-6939-15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloom T. A question of evidence. BMJ. 2016:355. doi: 10.1136/bmj.i6316. [DOI] [PubMed] [Google Scholar]
- 18.Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: A prospective survey study. JAMA Oncol. 2016;2:137–9. doi: 10.1001/jamaoncol.2015.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu SH, Kim EJ, Jeong SH, Park GL. Factors associated with willingness to participate in clinical trials: A nationwide survey study. BMC Public Health. 2015;15:10. doi: 10.1186/s12889-014-1339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.How Cultural Competence and Cultural Factors Affect Minority Recruitment to Clinical Trials. [Last accessed on 2012 June 12]. Available from: https://knect365.com/clinical-trials-innovation/article/bc8a5c9c-83e1-4411-a057-7b1366ad0548/cultural-socioeconomic-trial-execution.19 .
- 21.Doshi MS, Kulkarni SP, Ghia CJ, Gogtay NJ, Thatte UM. Evaluation of factors that motivate participants to consent for non-therapeutic trials in India. J Med Ethics. 2013;39:391–6. doi: 10.1136/medethics-2012-100755. [DOI] [PubMed] [Google Scholar]
- 22.González-Saldivar G, Rodríguez-Gutiérrez R, Viramontes-Madrid JL, Salcido-Montenegro A, Carlos-Reyna KE, Treviño-Alvarez AM, et al. Participants’ perception of pharmaceutical clinical research: A cross-sectional controlled study. Patient Prefer Adherence. 2016;10:727–34. doi: 10.2147/PPA.S96021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mondal S, Abrol D. [Last accessed on 2016 Aug 01];Clinical Trials Industry in India: A Systematic Review. Working Paper 179, Institute for Studies in Industrial Development New Delhi. 2015 [Google Scholar]
- 24.Suvarnapathaki K. Indian regulatory update 2013. Perspectives in Clinical Research. 2013;4:237–8. doi: 10.4103/2229-3485.120174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gogtay NJ, Ravi R, Thatte UM. Regulatory requirements for clinical trials in India: What academicians need to know Indian J Anaesth. 2017;61:192–9. doi: 10.4103/ija.IJA_143_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Compensation Formula. [Last accessed on 2018 Aug17]. Available at http://www.cdsco.nic.in/writereaddata/formula2013SAE.pdf .
- 27.Chatio S, Baiden F, Achana FS, Oduro A, Akazili J. Zhou X, editor. Knowledge and Perceptions about Clinical Trials and the Use of Biomedical Samples: Findings from a Qualitative Study in Rural Northern Ghana. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0152854. [DOI] [PMC free article] [PubMed] [Google Scholar]