INTRODUCTION
Head-and-neck squamous cell carcinoma (HNSCC) affects approximately 830,000 people each year worldwide.[1] Despite using combinations of surgery, radiotherapy, and chemotherapy, the 5-year overall survival of HNSCC is only 40%–50%.[2] The programmed death 1 (PD-l)/programmed death ligand 1 (PD-L1) axis has emerged as a key mechanism of immune escape by HNSCC.[3] This immune checkpoint system can accordingly be targeted with specific drugs blocking the tumor's immunosuppressive signaling, thus boosting the antitumor immune response.[4]
Several methods have been proposed for PD-L1 status assessment by immunohistochemistry (IHC) including the use of a combined positive score (CPS).[5] This integrated scoring system considers the expression of this immune checkpoint biomarker on the cell membrane of both tumor and tumor-associated inflammatory cells.[6] Recent trials investigating the efficacy of first-line immune-checkpoint inhibition with the anti-PD-1 drug pembrolizumab in recurrent and/or metastatic HNSCC (KEYNOTE-048 and KEYNOTE-012) showed that PD-L1 expression is associated with an increased objective response rate, with better response when CPS ≥20.[7,8] Around 50%–60% of HNSCC tumor cells express PD-L1 when assessed with tumor proportion score, [9] but this percentage increases to 85% when considering both tumor and surrounding immune cells (as measured with CPS).[8] The two different modalities of assessment partly explain the different prevalence of PD-L1 expression. The latter has also been reported in other studies involving the head-and-neck region.[10,11] In response to the approval by several drug agencies of checkpoint inhibitor therapy in biomarker-selected populations of HNSCC patients with recurrent/metastatic disease, it is reasonable to anticipate that pathologists will be increasingly requested to assess PD-L1 CPS on HNSCC specimens to guide the selection of afflicted patients for immunotherapy. Currently, most centers may not routinely employ CPS in reporting PD-L1 expression in other tumor histotypes (e.g., nonsmall-cell lung carcinoma). Therefore, pathologists need targeted training programs to reliably learn how to assess this score and improve standardization among different medical centers.
The COVID-19 pandemic has prompted the full adoption of available, but perhaps underused, digital solutions for e-learning purposes.[12] Pathologists have an advantage in this evolution to digital imaging given their acquired experience over the past two decades with virtual or whole slide images used for applications such as telepathology. If leveraged properly, digital pathology can become the backbone of a training network where digital slides can be shared to improve training and accelerate knowledge transfer.[13] Online webinars for distance learning (as the name implies, seminars and learning events that take place on web platforms) can be easily hosted employing several user-friendly platforms,[14] and when coupled with virtual microscopy using whole slide imaging (WSI) can be shared globally with a large pathology audience. Further, this allows digital pathology to no longer just be confined to utilization at academic institutions but can help meet the need to train many general pathologists.
The aim of this paper is to present the results of an e-learning event targeting pathologists regarding PD-L1 CPS assessment in HNSCC, with a critique on the efficacy of using this technology as a mechanism of contemporary knowledge delivery.
MEETING ORGANIZATION
The e-learning event “PD-L1 Key learning in HNSCC” was organized by Global Studio SRL over several days (December 2019 to May 2020) and ended with a final webinar on June 12, 2020. There were 3 learning days with overall 28 sessions comprising both traditional lectures and interactive slide viewing sessions for overall 6 h of learning activity, with involvement of a mean of six lecturers of the faculty for each event. The final meeting was supported by Zoom videoconferencing software (Zoom Video Communications, Inc., San Jose, CA, USA). After subscription, all participants received a permanent link with meeting ID and password for all online material. The faculty included Albino Eccher (University and Hospital Trust of Verona, Verona), Gabriella Fontanini (University of Pisa, Pisa), Nicola Fusco (European Institute of Oncology, Milan), Paolo Graziano (Foundation IRCCS “Casa Sollievo della Sofferenza,” San Giovanni Rotondo, Foggia), Elena Guerini Rocco (European Institute of Oncology, Milan), Maurizio Martini (Catholic University-Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Rome), Patrizia Morbini (University of Pavia and IRCCS Policlinico S. Matteo, Pavia and Chief of Head and Neck Pathology Study Group for Italian Society for Anatomic Pathology and Cytology [SIAPEC]), Giancarlo Troncone (University of Naples Federico II, Naples), and Elena Vigliar (University of Naples Federico II, Naples). Attendees were 86 pathologists from all over Italy. All of the events were structured with lectures and interactive practical sessions involving case discussions.
PROGRAMMED DEATH LIGAND 1 COMBINED POSITIVE SCORE ASSESSMENT AND SLIDE DIGITIZATION
Attendees were asked to assess CPS on digital slides of twenty representative anonymized HNSCC cases before the course. The slides were made available on the web platform 1 month ahead of the course. The HNSCC cases came from two different institutions and comprised both surgical specimens and small biopsies, as well as lymph nodes with metastases. This allowed for wide variability in terms of fixation and possible artifacts. Specifically, PD-L1 IHC was scored as summarized in Table 1.[16]
Table 1.
Combined positive score assessment
| Definition | |
CPS = 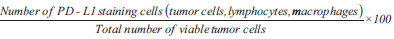
| |
| Key points | |
| At least 100 viable tumor cells present in the stained slide to be considered adequate | |
| Scores range from 0 to 100 with the maximum score reported=100 | |
| Staining must be evaluated at 20×magnification | |
| Tumor cell evaluation considered to be partial or complete linear membrane staining of any intensity | |
| Lymphocytes and macrophages evaluation considered to be any staining at any intensity | |
| Denominator | |
| Include | Exclude |
| All viable invasive tumor cells | Necrotic or nonviable tumor cells |
| All types of immune cells | |
| In situ component | |
| Stromal cells and other benign cells | |
| Any stained debris or artifacts | |
| Numerator | |
| Include | Exclude |
| All viable invasive PD-L1 positive tumor cells | Mononuclear inflammatory cells associated with ulcers, carcinoma in situ or benign structures |
| Lymphocytes and macrophages (that lie in the same 20×field of invasive tumor) | Plasma cells and granulocytes |
| In situ component | |
| Stromal cells and other benign cells | |
| Any necrotic cells or debris | |
Modified from reference.[16] CPS: Combined Positive Score, PD-L1: Programmed death-ligand 1
For this analysis, any convincing partial or complete linear membrane staining (≥1+) of viable tumor cells that is perceived as distinct from cytoplasmic staining was considered to represent PD-L1 staining and included in scoring. Only lymphocytes and macrophages (i.e., mononuclear inflammatory cells) within tumor nests and/or adjacent supporting stroma within a ×20 microscopic field were included in scoring. The cases (H and E and PD-L1 staining) were digitized with a Hamamatsu NanoZoomer scanner at ×40, uploaded on a shared web platform provided by Nikon, and viewed with NDP.view2 software.[15] Digital slide size ranged from 328 megabytes to 4 gigabytes. For this analysis, tumor sections were stained using the anti-PD-L1 antibody clone 22C3 (PharmDx, Dako Agilent, Santa Clara, CA, USA) on a Dako Autostainer Link 48 platform.[16] All attendees received basic clinical information on each case and were asked to render their CPS score before the course using a standardized form in a spreadsheet. For each case, participants were required to assess adequacy, positivity for the CPS cutoffs ≥1 and ≥20, and a CPS value range (e.g., 45–50). The forms were made available to the faculty members to guide the discussion of the most controversial cases and critical points. The reference CPS for each case was established by the faculty consensus.
MEETING OVERVIEW
The meeting started with a presentation by an oncologist (Paolo Bossi, University and Hospital Trust of Brescia) on the new therapeutic perspectives with checkpoint inhibitors for patients with HNSCC and the main results of clinical trials. Then, Gabriella Fontanini and Patrizia Morbini explained the scientific background and the rationale of new guidelines for PD-L1 IHC assessment using the CPS in HNSCC specimens. Lectures ended with Maurizio Martini illustrating the practical rules to follow with CPS evaluation, how to count tumor and inflammatory cells and important pitfalls. These key points regarding CPS assessment are summarized in Table 1. The lectures took about 30 min each with the use of both WSI and still images, while the majority of e-learning was dedicated to interactive sessions of about 4–5 h each with digital slides. Participants were divided into “online classrooms” of ten attendees for discussions with faculty members. A survey for concordance of both faculty members and learning participants was performed.
RESULTS OF THE MEETING
The twenty cases were representative of different HNSCC cases from the head-and-neck region and showed a CPS score ranging from 0 to 100. Representative images of cases highlighting membrane staining in tumor cells and cytoplasmic staining in inflammatory cells at ×20 magnification are presented in Figure 1. The concordance rate of attendees for single a case with CPS ≥1 and ≥20 ranged between 39.13% and 95.65%. The more challenging cases had a CPS around 1 or 20, which are in fact the two thresholds considered in the trials, with eligibility for first-line therapy at CPS ≥1 and clinical interest for outcome for CPS ≥20.[4,16] Intraclass correlation coefficient (ICC) for agreement on assigning CPS was calculated among all participants and among faculty members. There was an ICC of 0.646 (confidence interval: 0.381–0.791) for attendees, while faculty showed an ICC of 0.948 (confidence interval: 0.904–0.976). For some of the more challenging cases with CPS around 1 or 20, the participants had an ICC of 0.176 (0.015–0.996) and 0.410 (0.102–0.999), respectively. All of the attendees reported considerable satisfaction with this e-learning course, and in particular, pathologists were comfortable using digital slides. Evaluation of the course by the attendees was assessed with a general questionnaire with Likert scale-like question and space for personal comments. Attendees assessed the cases with their own personal workstations, so there was no standardization of viewing modality in terms of displays used or bandwidth.
Figure 1.
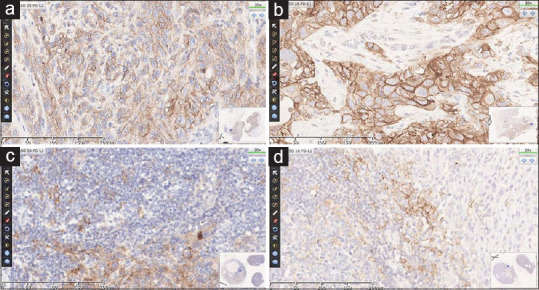
Programmed death-ligand 1 immunostaining of head and neck squamous cell carcinoma. Representative staining of tumor cells (a and b) and inflammatory cells (c and d) is shown at ×20. The combined positive score of the cases were 100, 85, 80 and 27 respectively
DISCUSSION
Currently and increasingly, pathologists are being requested to assess PD-L1 CPS in HNSCC given the widespread availability of checkpoint inhibitor therapy. Reliable assessment of this vital biomarker requires not only expertise in head-and-neck pathology but also specific practice and tailored training for PD-L1 biomarker assessment to achieve standardized and reproducible scoring among pathologists. For example, awareness of expected staining patterns and intensity is not always straightforward and careful screening of the stained tumor section is needed to establish which areas of the tumor or groups of cells needs to be included or excluded from the numerator and denominator of the CPS formula. The COVID-19 pandemic has emphasized the need for alternatives to in-person meetings. Indeed, digital pathology allows participants to interactively view and discuss cases in real time. Moreover, many more pathologists are able to join in, perhaps even exceeding the number typically permitted using traditional large multiheaded microscopes that have limited capacity or employing WSI in conference rooms of limited size or lack of adequate technical infrastructure. Finally, but not least importantly, it is also an advantage for participants to gain expertise with their own equipment or evaluate whether they have the equipment needed.
Digital pathology plays an important role in accelerating the progression of healthcare by supporting collaboration of highly specialized teams through several possibilities to exchange medical information, not only in webinar meetings.[13] Its application has led to the simplification and widespread access to remote interdisciplinary expertise, as well as to improving medical education.[17] The results of this project demonstrate the effectiveness of digital pathology solutions in providing high-quality training to general pathologists on real-life practical cases, overcoming the potential barriers of geography and the availability of adequate materials. Attendees used their own workstations, and despite the heterogeneity in terms of the types of computer monitors (e.g., size, resolution, use of eye-saver mode and optimization features for long-time screen work, other modalities such as tablets) and used and internet bandwidth for each location (e.g., optic fiber still not available all over the country, possible some minor lagging issues in visualization and navigation of slides), no major issues were reported by the participants. Even though standardization of viewer settings is often requested for validation studies,[18,19] online training events such as the study described herein point to the value of adopting digital imaging for such training events. Such events offer scientific value by facilitating the dissemination of information in a standard and safe (such as in the pandemic situation) manner, including the cost-effectiveness of saving time and resources by not travelling. The digital solution worked well even in the presence of intrinsic differences of the histological samples. The HNSCC cases comprised tissue from both surgical specimens and small biopsies, as well as lymph nodes metastases, allowing for a great variability in terms of fixation and presence of artifacts. On the other hand, immunohistochemical staining was standardized on a single antibody and platform as required in pembrolizumab registration study.[16] Concerning preanalytical issues, WSI has shown to be reliable for assessment of almost any type of slide, from frozen section to cytological samples[20,21,22] when proper evaluation is critical and may require the consultation of an expert.[23]
The CPS assessment is often performed on small biopsy material, as candidate patients present with advanced cancer are often unfit for large surgery as are those with recurrence after adjuvant therapy. After the evaluation of H and E slides for viable tumor cells and an associated inflammatory background, the assessment of CPS requires careful microscopic estimation of the number of cells at ×20 magnification. The difficulties that pathologists may encounter when rendering a CPS (e.g., ambiguity of cell staining) are the same on a digital and glass slide. Hence, guided training by experts is important to achieve reliable results which is of benefit to patients. Finally, the success of this project was also attributed to the collaboration of experts from different institutions and their effort towards the harmonization of workflow and CPS scoring in HNSCC pathology. It is anticipated that webinars and digital pathology will be increasingly used for training in the future and to support collaborations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors and organization are thankful to all the meeting attendees.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2021/12/1/1/306285
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 3.Horton JD, Knochelmann HM, Day TA, Paulos CM, Neskey DM. Immune evasion by head and neck cancer: Foundations for combination therapy. Trends Cancer. 2019;5:208–32. doi: 10.1016/j.trecan.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. 2019;99:104460. doi: 10.1016/j.oraloncology.2019.104460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagni F, Guerini-Rocco E, Schultheis AM, Grazia G, Rijavec E, Ghidini M, et al. Targeting immune-related biological processes in solid tumors: We do need biomarkers. Int J Mol Sci. 2019;20:5452. doi: 10.3390/ijms20215452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulangara K, Zhang N, Corigliano E, Guerrero L, Waldroup S, Jaiswal D, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143:330–7. doi: 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 7.Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–65. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 8.Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 9.Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, et al. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNγ That induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76:1031–43. doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girolami I, Pantanowitz L, Mete O, Brunelli M, Marietta S, Colato C, et al. Programmed death-ligand 1 (PD-L1) Is a potential biomarker of disease-free survival in papillary thyroid carcinoma: A systematic review and meta-analysis of PD-l1 immunoexpression in follicular epithelial derived thyroid carcinoma. Endocr Pathol. 2020;31:291–300. doi: 10.1007/s12022-020-09630-5. [DOI] [PubMed] [Google Scholar]
- 11.Girolami I, Pantanowitz L, Munari E, Martini M, Nocini R, Bisi N, et al. Prevalence of PD-L1 expression in head and neck squamous precancerous lesions: A systematic review and meta-analysis. Head Neck. 42:3018–30. doi: 10.1002/hed.26339. [DOI] [PubMed] [Google Scholar]
- 12.Pagni F, Malapelle U, Doglioni C, Fontanini G, Fraggetta F, Graziano P, et al. Digital pathology and PD-L1 testing in non small cell lung cancer: A workshop record. Cancers (Basel) 2020;12:1800. doi: 10.3390/cancers12071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aghdam MRF, Vodovnik A, Hameed RA. Role of telemedicine in multidisciplinary team meetings. J Pathol Inform. 2019;10:35. doi: 10.4103/jpi.jpi_20_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bottanelli F, Cadot B, Campelo F, Curran S, Davidson PM, Dey G, et al. Science during lockdown-From virtual seminars to sustainable online communities. J Cell Sci. 2020;133:jcs249607. doi: 10.1242/jcs.249607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamamatsu NDP.View2 Software. [Last accessed on 2020 Sep 10]. Available from: https://www.hamamatsu.com/resources/pdf/sys/SBIS0066E_NDPVIEW2.pdf .
- 16.Dako PD-L1 IHC 22C3 pharmDx Interpretation Manual – Head and Neck Squamous Cell Carcinoma (HNSCC) [Last accessed on 2020 Sep 10]. Available from: https://www.agilent.com/cs/library/usermanuals/public/29314_22c3_pharmDx_hnscc_interpretation_manual_us.pdf .
- 17.Liang ZC, Ooi SBS, Wang W. Pandemics and their impact on medical training: Lessons from Singapore. Acad Med. 2020;95:1359–61. doi: 10.1097/ACM.0000000000003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pantanowitz L, Sinard JH, Henricks WH, Fatheree LA, Carter AB, Contis L, et al. Validating whole slide imaging for diagnostic purposes in pathology: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137:1710–22. doi: 10.5858/arpa.2013-0093-CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunelli M, Beccari S, Colombari R, Gobbo S, Giobelli L, Pellegrini A, et al. iPathology cockpit diagnostic station: Validation according to College of American Pathologists Pathology and Laboratory Quality Center recommendation at the Hospital Trust and University of Verona. Diagn Pathol. 2014;9:S12. doi: 10.1186/1746-1596-9-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietz RL, Hartman DJ, Pantanowitz L. Systematic review of the use of telepathology during intraoperative consultation. Am J Clin Pathol. 2020;153:198–209. doi: 10.1093/ajcp/aqz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cima L, Brunelli M, Parwani A, Girolami I, Ciangherotti A, Riva G, et al. Validation of remote digital frozen sections for cancer and transplant intraoperative services. J Pathol Inform. 2018;9:34. doi: 10.4103/jpi.jpi_52_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girolami I, Pantanowitz L, Marietta S, Brunelli M, Mescoli C, Parisi A, et al. Diagnostic concordance between whole slide imaging and conventional light microscopy in cytopathology: A systematic review. Cancer Cytopathol. 2020;128:17–28. doi: 10.1002/cncy.22195. [DOI] [PubMed] [Google Scholar]
- 23.Eccher A, Neil D, Ciangherotti A, Cima L, Boschiero L, Martignoni G, et al. Digital reporting of whole-slide images is safe and suitable for assessing organ quality in preimplantation renal biopsies. Hum Pathol. 2016;47:115–20. doi: 10.1016/j.humpath.2015.09.012. [DOI] [PubMed] [Google Scholar]


