Abstract
Patients with severe hypertension are at high risk for cardiovascular events. The authors hypothesized that initial treatment with a combination angiotensin receptor blocker/diuretic agent would be safe and more effective than initial treatment with a single agent for these patients. In this 6‐week, double‐blind trial, 585 patients were randomized to losartan/hydrochlorothiazide or losartan as monotherapy and titrated as needed at 2‐week intervals to reach goal blood pressure (<90 mm Hg). Almost twice as many patients achieved goal at the primary end point of 4 weeks on 50 mg losartan/12.5 mg hydrochlorothiazide vs. the losartan regimen (50–100 mg; p=0.002). Additionally, almost three times as many patients achieved goal blood pressures at 6 weeks (p<0.001). Adverse experiences on losartan/hydrochlorothiazide (43%) were significantly less than with the angiotensin receptor blocker alone (52.6%). This study confirmed the efficacy and tolerability of initial use of a fixed combination of losartan/hydrochlorothiazide vs. losartan without a thiazide.
Of the 50 million people in the United States with essential hypertension, approximately 10% have severe hypertension as defined by the World Health Organization/International Society of Hypertension (WHO/ISH) guidelines 1 , 2 , 3 , 4 , 5 , 6 (systolic blood pressure [SBP] ≥180 mm Hg or diastolic BP [DBP] ≥110 mm Hg), and are at particularly high risk for the development of stroke or coronary artery disease. Hypertension is widely acknowledged to be an important risk factor for the development of cardiovascular morbidity and mortality, and the severity of hypertension is directly correlated with the risk of adverse outcomes. 3 , 4 The first Veterans Administration Cooperative Study 5 confirmed the marked benefit of antihypertensive therapy in reducing adverse cardiovascular events in this population. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI) guidelines 1 recommend that “in some patients, it may be necessary to start treatment with more than one agent.” The recent JNC 7 guidelines take this one step further and recommend starting combination therapy for patients who require reductions of more than 20/10 mm Hg to achieve goal BP 7 ; i.e., patients with BPs of >160/100 mm Hg or 150/90 mm Hg in diabetics. According to both the WHO/ISH and JNC 7 guidelines, the goal of antihypertensive treatment is to achieve a BP <140/90 mm Hg. 7 Unfortunately, only about one third of all hypertensive patients achieve this goal 1 and, although data are limited, it is likely that the percentage of patients with severe hypertension achieving their goal BP is even lower.
Data from multiple studies 8 , 9 , 10 , 11 , 12 suggest that monotherapy results in the attainment of goal BP in only a fraction of patients with severe hypertension; a higher percent of patients with severe hypertension reach goal BP when treated with combination therapy.
The concept of using multiple medications in the initial treatment of hypertension is not a new one. Antihypertensive treatment in the 1960s and 1970s 13 , 14 , 15 generally included initial therapy with multiple drugs. The combinations were shown to be safe and effective at that time; however, as new compounds were developed, the use of monotherapy and the stepped‐care approach became the standard treatment strategy. 16
The possible limitations of the stepped‐care approach and the potential benefits of initial therapy with a combination agent have been reviewed. 17 Fewer side effects, more effective BP control, better compliance, and cost effectiveness result. Thus to compare the antihypertensive efficacy of initial combination therapy vs. monotherapy, we prospectively evaluated the efficacy and safety of initial treatment with a fixed combination agent of losartan 50 mg/hydrochlorothiazide (HCTZ) 12.5 mg and losartan 50 mg titrated as needed to 100 mg. The focus of the efficacy evaluation was the achievement of goal BP.
PATIENTS AND METHODS
This prospective, double‐blind, randomized, controlled study enrolled 585 patients from 74 sites in 16 countries. Patients were eligible for the study if they were older than the legal age of consent, had a confirmed mean sitting diastolic BP (SiDBP) >110 mm Hg and mean sitting systolic BP (SiSBP) <220 mm Hg, and were taking no more than three antihypertensive medications at the time of screening. Patients with a history of secondary hypertension of any etiology or malignant hypertension were excluded from participation. A history of myocardial infarction or angina within 6 months before the start of the study, cerebrovascular accident, transient ischemic attack, audible carotid bruits, hemodynamically significant obstructive valvular disease, or cardiomyopathy precluded a patient from participation. Additional exclusion criteria included a history of unexplained syncope within 2 years before the start of the study, atrial fibrillation, congestive heart failure, or known left ventricular ejection fraction <40%, and atrioventricular conduction disturbances. Patients on concomitant medications that could affect BP, or who regularly used nonsteroidal antiinflammatory drugs, psychotropics, or lithium were also excluded from participation. The study was reviewed and approved by institutional review committees or ethics review boards at all sites. All patients provided written informed consent.
After a 24‐hour to 3‐week baseline/washout period, during which the presence of severe hypertension was confirmed, patients were randomized in a 2:1 fashion to initial therapy with either losartan 50 mg/HCTZ 12.5 mg or losartan 50 mg. This double‐blind treatment period continued for <6 weeks with patients returning every 2 weeks for evaluation and possible titration (visits four and five). BP (average of three measurements taken at 1‐2 minute intervals) was measured at baseline, 4 hours post‐first‐dose and at Weeks 2, 4, and 6. Patients were titrated if their mean SiDBP did not reach goal (<90 mm Hg). Patients randomized to monotherapy were titrated from losartan 50 mg to losartan 100 mg to losartan 150 mg. (This latter dosage is not currently approved by the United States Food and Drug Administration for use in hypertension). Patients randomized to combination therapy were mock titrated at Week 2 and remained on losartan 50 mg/HCTZ 12.5 mg in order to maintain the blind without dose adjustment. Thereafter, patients were titrated to losartan 100 mg/HCTZ 25 mg as needed to achieve goal. For safety reasons, patients on losartan 50 mg/HCTZ 12.5 mg were titrated to losartan 100 mg/HCTZ 25 mg at the 2‐week time point if their trough SiDBP was >110 mm Hg (Figure 1).
Figure 1.
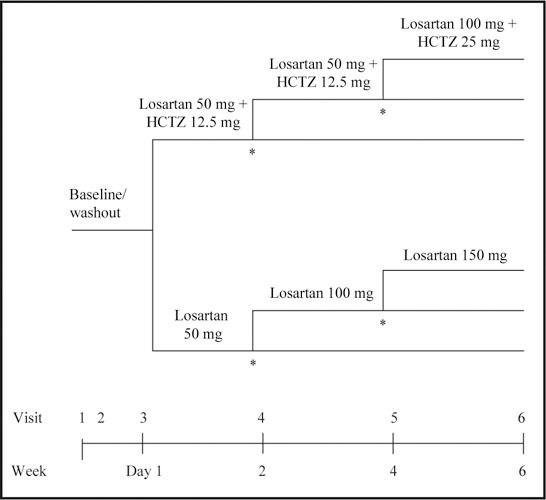
Diagram presenting overall study design including titration scheme. HCTZ=hydrochlorothiazide; SiDBP=sitting diastolic blood pressure; *titrate if SiDBP >90 mm Hg; patients with a SiDBP ≥110 mm Hg in the combination therapy arm will be titrated to losartan 100 mg + HCTZ 25 mg at Week 2
BP and heart rate were measured and adverse experiences were assessed at every visit. Complete laboratory tests and a 12‐lead electrocardiogram were performed at baseline and at Week 6. An abbreviated laboratory test (blood urea nitrogen, serum creatinine, and electrolytes) was performed at Week 4. Pregnancy tests in women of childbearing potential were performed at baseline and at Weeks 4 and 6.
RANDOMIZATION AND BLINDING
Randomization was conducted via a computer‐generated allocation schedule. Patients were randomized to the lowest allocation number available from a block of drugs (six consecutive allocation numbers) assigned to each investigator site. All bottles were labeled with the patient's allocation number, lot number, and instructions. Active tablets and the corresponding matching placebo were identical. Masked allocation schedules were provided to the site. There were no cases of unblinding during the study.
STATISTICS
Using a 2:1 randomization scheme with 340 patients treated with losartan 50 mg/HCTZ 12.5 mg and 170 patients treated with losartan 50 mg, titrated as needed, to losartan 100 mg, the planned power was at least 95% to detect an approximate 13 percentage point difference between the treatment groups at Week 4. This power computation was based on a two‐sided χ2 test performed at the 5% level of significance. Special focus on safety was based on overall clinical adverse experiences, drug‐related clinical adverse experiences, hypotension, dizziness, syncope, and worsening of renal function. Efficacy, safety, and demographic variables were compared between the two treatment groups using confidence intervals for the primary, secondary, and exploratory analyses, constructed using the Wilson's score method, p Values were computed using the likelihood‐ratio χ2 statistic. Comparison of the treatment groups with respect to mean changes in SiDBP was based on an analysis of covariance model with pretreatment SiDBP as a covariate.
Following the unblinding of the database, it was noted that some patients had violated the protocol in ways that the original rules used for data analyses did not adequately address. These violations led to the formulation of alternate data‐handling rules to confirm that the data from those patients did not bias the results and that our original conclusions were valid. Thus a sensitivity analysis was performed that modified whether patients were classified as achievers or nonachievers of goal SiDBP.
Nine patients in the combination therapy group and one patient in the monotherapy group had their achieving goal status modified for this analysis. The sensitivity analysis showed no change to the conclusions, thus the original intent‐to‐treat (ITT) analysis is presented.
RESULTS
Of the 869 patients screened for the study, 585 (67.3%) met the screening criteria and were randomized into the study. A total of 532 patients completed the study. The mean age of randomized patients was 52.7 years (22–87 years). The treatment groups were similar with respect to baseline characteristics, with the exception that a significantly higher percentage of males were randomized to monotherapy. Of the patients randomized, 321 (54.9%) were men and 264 (45.1%) were women; most patients were Caucasian. The majority of patients had hypertension for >10, years (40.9%) and were previously taking antihypertensive medications before the start of the study 457 (78.1%). Approximately 48% of patients were taking two or more antihypertensive medications before screening. Both the mean SiDBP and SiSBP at baseline were similar between the treatment groups, 171.0/113.4 mm Hg for the losartan/HCTZ combination arm and 170.5/113.3 mm Hg for the losartan monotherapy arm. A summary of patient baseline characteristics is displayed in Table I.
PRIMARY END POINT
The primary end point was the proportion of patients achieving goal mean trough SiDBP (<90 mm Hg) after 4 weeks of therapy with either losartan 50 mg/HCTZ 12.5 mg or a losartan monotherapy regimen (losartan 50 mg, titrated as needed, to losartan 100 mg). Significantly more patients achieved the goal with the combination than with the monotherapy regimen (19.6% vs. 9.9%; p=0.002; Figure 2). Note that patients randomized to combination therapy who were titrated to losartan 100 mg/HCTZ 25 mg at Week 2 for safety reasons were not considered to have achieved goal at Week 4 regardless of their BP at Week 4.
Figure 2.
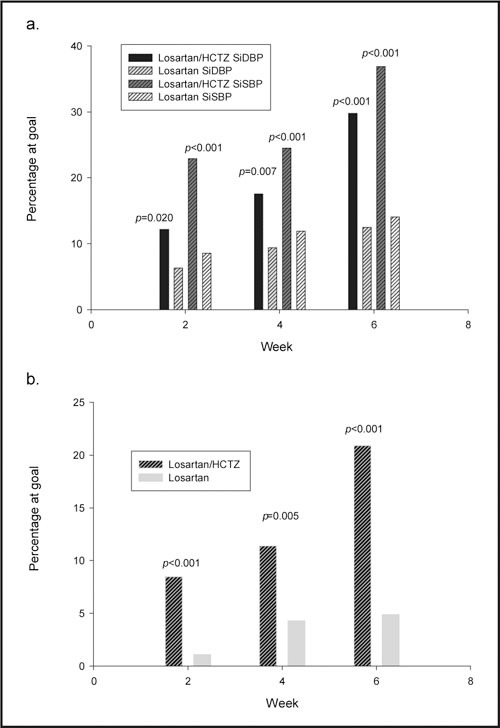
Graphs of percentage of patients achieving goal. Bar graphs showing percent of patients achieving goal blood pressure. Top graph shows SiDBP and SiSBP at goal (%) at Weeks 2, 4, and 6. Bottom graph shows percentage for both SiDBP and SiSBP at goal (%) at Weeks 2, 4, and 6. SiDBP=sitting diastolic blood pressure <90 mm Hg; SiSBP=sitting systolic blood pressure <140 mm Hg; HCTZ=hydrochlorothiazide
SECONDARY END POINTS
After 6 weeks, significantly more patients reached goal BP after one titration step of combination therapy vs. two titration steps of monotherapy (31% vs. 12.6%; p<0.001; Figure 2).
The changes from baseline in mean SiDBP and SiSBP were also examined. At Week 4, there was a significant decrease in SiDBP and SiSBP with combination therapy when vs. the monotherapy group (SiDBP: −13.6 mm Hg and −10.5 mm Hg, respectively; SiSBP: −18.0 and −12.4 mm Hg, respectively; p<0.001 for both). The decrease in SiDBP and SiSBP from baseline was greater after 6 weeks between the two groups (SiDBP: −17.8 mm Hg vs. −11.9 mm Hg, respectively; SiSBP: −25.1 and −14.1 mm Hg, respectively; p<0.001 for both). Figure 3 displays the reduction from baseline at Weeks 2, 4, and 6 (Figure 3).
Figure 3.
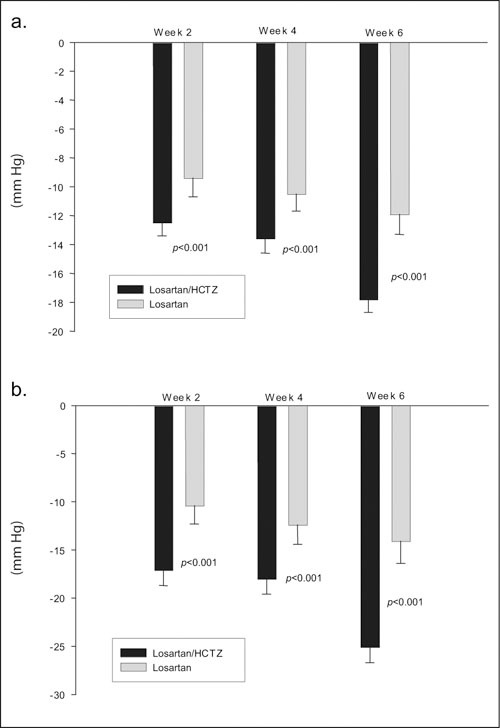
Changes in blood pressure. Bar graphs showing mean ± SE change in blood pressure (diastolic, top panel; systolic, bottom panel) at Weeks 2, 4, and 6. Losartan/HCTZ: Week 2, n=393; Week 4, n=392; Week 6, n=368; losartan: Week 2, n=192; Week 4, n=192; Week 6, n=178. HCTZ=hydrochlorothiazide; SE=standard error
POST HOC ANALYSES
The efficacy of the two treatments was assessed at Week 2. At this first‐scheduled, postrandomization visit, a significantly greater percentage of patients achieved goal SiDBP on combination than on monotherapy (12.5% vs. 6.8%; p=0.029 (Figure 2).
An analysis was also performed on the percentage of patients who achieved goal SiDBP at Week 4 and maintained goal SiDBP at week 6. Of the 77 patients on combination therapy who achieved goal SiDBP at Week 4, 55 were still at goal (11.4%) at Week 6. In contrast, of the 19 patients on monotherapy who achieved goal SiDBP at Week 4, only 9 (46.4%) patients remained at goal at Week 6.
The percentage of patients who achieved a goal trough SiSBP of <140 mm Hg was also determined (Figure 2). Patients whose SiSBP was <140 mm Hg at baseline were excluded from this analysis (n=12; five from the combination group and seven from the monotherapy group). At Week 4, twice as many patients achieved goal SiSBP on combination therapy vs. the monotherapy regimen (25.5% vs. 12.4%; p<0.001). At Week 6, more than double the patients on the combination regimen achieved goal SBP vs. the losartan monotherapy regimen (37.4% vs. 14.1%; p<0.001).
Additionally, the likelihood of achieving both target systolic and diastolic BP (<140/<90 mm Hg) was assessed on both regimens. Patients were evaluated at Weeks 2, 4, and 6. Patients receiving the combination had a significantly higher rate of achieved systolic and diastolic BP when vs. those patients taking monotherapy at all time points: Week 2 (8.7% vs. 2.6%; p=0.003), Week 4 (12.5% vs. 6.8%; p=0.029), and Week 6 (21.1% vs. 5.7%; p<0.001) (Figure 2).
An analysis for responders (mean trough SiDBP <90 mm Hg or a reduction in SiDBP from baseline of ≥10 mm Hg) was also performed. At Weeks 2, 4, and 6, the percentage of patients responding to treatment was significantly higher in the combination regimen when vs. patients taking monotherapy (Week 2: 62.3% vs. 46.4%; p<0.001; Week 4: 67.2% vs. 55.7%; p=0.007, and Week 6: 78.6% vs. 54.7%; p<0.001).
ADVERSE EVENTS
The initial use of combination therapy had a safety profile similar to monotherapy. When examining the overall safety profile between treatment groups (Table II) it revealed that there was a lower incidence in clinical adverse experiences shown in the combination therapy group as vs. the monotherapy group (43.3% vs. 52.6%). There was also a decreased incidence of renal adverse experiences (increase in serum creatinine) in the combination therapy group as vs. the monotherapy group (0.5% vs. 1.1%). Patients treated with a combination therapy also had a decreased incidence of serious clinical adverse experiences as compared with those patients on monotherapy (1.0% vs. 3.6%). None of the differences in adverse experiences attained statistical significance, none of the serious adverse experiences reported in the study were considered drug‐related or caused discontinuation. There were no deaths reported in the study.
Table II.
Summary of Adverse Experiences
| Losartan/HCTZ (n=393) | Losartan (n=192) | Estimated Difference | |||
|---|---|---|---|---|---|
| Number of Patients | n | (%) | n | (%) | (95% CI) |
| With one or more adverse experiences | 170 | (43.3) | 101 | (52.6) | −9.35 (−ndash;17.79 to −0.74) |
| Overall clinical adverse experiences at first dose | 26 | (6.6) | 17 | (8.9) | −2.24 (−7.52 to 2.12) |
| With drug‐related* adverse experiences | 62 | (15.8) | 32 | (16.7) | −0.89 (−7.65 to 5.17) |
| With serious adverse experiences | 4 | (1.0) | 7 | (3.6) | −2.63 (−6.37 to −0.19) |
| Discontinued due to adverse experiences | 7 | (1.8) | 7 | (3.6) | −1.86 (−5.66 to 0.76) |
| Discontinued due to drug‐related adverse experiences | 3 | (0.8) | 3 | (1.6) | −0.80 (−3.77 to 0.98) |
| HCTZ=hydrochlorothiazide; CI=confidence interval; *determined by the investigator to be possibly, probably, or definitely drug related | |||||
One important safety consideration when utilizing combination therapy as an initial treatment in patients with severe hypertension is the potential for first‐dose adverse experiences. DBP was assessed 4 hours after the first dose and did not differ between the 2 groups (8.7 mm Hg decrease in the combination group vs. 8.5 mm Hg decrease in the monotherapy group). The incidence of overall clinical adverse experiences occurring after the first dose was similar between patients treated with combination therapy and those treated with monotherapy (6.6% vs. 8.9%). None of the first‐dose adverse experiences from either treatment group resulted in discontinuation. There were no first‐dose adverse experiences of hypotension or syncope reported.
DISCUSSION
Nationwide, the prevalence of hypertension is increasing; however, the treatment of hypertension, although increasing, is still inadequate, with only about one third of all antihypertensive patients being controlled. 6 The current study provides the first demonstration of the safety and efficacy of initial therapy with a losartan/HCTZ combination in patients with severe hypertension. Initial combination therapy was statistically and clinically more effective in achieving goal BP than the use of monotherapy titration. The proportion of patients achieving goal mean trough SiDBP <90 mm Hg at 4 weeks was more than double on combination therapy vs. monotherapy. The percentage of patients achieving goal BP following two steps of combination titration was more than double that after three steps of monotherapy titration at 6 weeks. These results are consistent with the previously demonstrated need for multiple antihypertensive medications in this population. These results have been repeatedly observed in studies with medications from a variety of antihypertensive classes, i.e., β blockers/diuretics, angiotensin‐converting enzyme inhibitors/diuretics, etc., and underscore the lack of effectiveness of monotherapy in the treatment of severe hypertension. The proportion of patients responding to treatment (mean trough SiDBP <90 mm Hg or a decrease from baseline in mean SiDBP ≥10 mm Hg) was significantly higher in the combination group as vs. the monotherapy group.
In a study that compared irbesartan with enalapril in patients with severe hypertension, 91% of the patients on irbesartan and 93% of the patients on enalapril required full titration of monotherapy and then the addition of other agents to achieve goal BP. Of these patients, 67% and 75% of patients in the irbesartan and enalapril arms, respectively, required at least three medications. 10 In another study of severe hypertension, 11 patients were started on valsartan 160 mg or atenolol 100 mg with additional medications added if goal BP (SiDBP <95 mm Hg) was not achieved. The majority of patients on valsartan (83.6%) and atenolol (97.2%) required the addition of HCTZ at 2 weeks. Of these patients, 76.8% on valsartan/HCTZ and 60% on atenolol/HCTZ required the addition of verapamil at 4 weeks. A meta‐analysis 20 of 57 trials on the efficacy of antihypertensive agents demonstrated that the likelihood of reducing DBP by >9 mm Hg with a single agent is <5%, and thus monotherapy is likely to be inadequate for patients with a baseline DBP >100 mm Hg. Even with the upward titration of losartan/HCTZ in the current study of severe hypertensives, <30% of subjects achieved goal BPs of <140/90 mm Hg. More than two drugs are often necessary.
The percentage of patients who achieved a goal SiSBP <140 mm Hg was analyzed. More than twice as many patients at the primary end point, and almost three times as many patients at Week 6, achieved goal SiSBP on combination therapy vs. the monotherapy regimen. Patients receiving combination therapy also had a significantly higher rate of achieving goal systolic and diastolic BP vs. those patients taking monotherapy at all time points (21% vs. 6% at Week 6) (Figure 3).
Combination therapy had a safety profile similar to monotherapy. When examining the overall safety profile between treatment groups, there was a statistically nonsignificant decreased incidence in clinical adverse experiences shown in the combination therapy group vs. the monotherapy group. The rates of first‐dose adverse experiences and adverse experiences of special interest (hypotension, syncope, dizziness, and increased serum creatinine) were low and did not differ between the two treatment groups.
One of the most important potential long‐term benefits of using a low‐dose combination medication as initial therapy is the effect on patient compliance. Drug intolerance is one of the more common reasons for the discontinuation of antihypertensive therapy. 22 Discontinuation rates of up to 76% in the first year of treatment on newly diagnosed hypertensive patients have been noted by Conlin et al. 21 There are multiple reasons for this phenomenon, but a high rate of discontinuation may lead to a lack of BP control. The stepcare approach suggests starting at a dose that has generally been shown to be ineffective for patients with severe hypertension and then titrating to a maximally tolerated or recommended dose before adding a second agent. 10 , 11 , 12 Adverse experiences are dose dependent, thus the patients with severe hypertension who are at the greatest risk for inadequate treatment are at additional risk of adverse events from high doses of medications. 21 Data 19 , 23 demonstrate that early BP control with few medication changes leads to substantially improved compliance compared with regimens requiring multiple medication changes. Thus a treatment strategy that utilizes initial treatment with an effective, well‐tolerated agent might lead to improved compliance and outcomes.
Data from The Institute for Effectiveness Research (TIER) 24 in 6007 patients receiving a fixed dose of an angiotensin‐II antagonist/diuretic show a high rate of persistence (remaining on therapy) at 12 months (72.2%). 1 , 25 In addition, a recent publication reported that combination therapy was more cost effective than monotherapy.
CONCLUSION
This study is the first to confirm the superior efficacy and tolerability of the initial use of combination therapy as vs. monotherapy in patients with severe hypertension. In a difficult‐to‐treat population, the combination achieved systolic and diastolic BP control in approximately 20% of patients by 6 weeks. A treatment strategy utilizing initial treatment with combination therapy might lead to better compliance with long‐term antihypertensive therapy and an eventual reduction in cardiovascular events. Increased efficacy provided by initial combination therapy might provide important clinical benefits in this patient population.
Acknowledgment: The authors would like to thank Gilbert W. Gleim, PhD,Merck Research Laboratories, West Point, PA for his editorial assistance in the preparation of this manuscript.
References
- 1. Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure . The sixth report of the Joint National Committee on prevention, detection, and treatment of high blood pressure (JNC VI). Arch Intern Med. 1997;157:2413–2446. [DOI] [PubMed] [Google Scholar]
- 2. 1999 World Health Organization—International Society of Hypertension Guidelines for the Management of Hypertension . Guidelines Subcommittee. J Hypertens. 1999;17:151–183. [PubMed] [Google Scholar]
- 3. Neaton JD, Kuller L, Stamler J, et al. Impact of systolic and diastolic blood pressure on cardiovascular mortality. In: Laragh JHand Brenner BM, eds. Hypertension: Pathophysiology, Diagnosis and Management, 2nd ed, New York , NY : Raven Press, 1995:127–144. [Google Scholar]
- 4. Anderson KM, Wilson PW, Odell PM, et al. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–362. [DOI] [PubMed] [Google Scholar]
- 5. Veterans Administration Cooperative Study Group on Antihypertensive Agents . Effects of treatment of morbidity in hypertension. Results in patients with diastolic blood pressure averaging 115 through 129 mm Hg. JAMA. 1970;213:1143–1152. [PubMed] [Google Scholar]
- 6. Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. [DOI] [PubMed] [Google Scholar]
- 7. Chobanian AV, Bakris GL, Black HR, et al., for The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. [DOI] [PubMed] [Google Scholar]
- 8. Dunlay MC, Fitzpatrick V, Chrysant S, et al. Losartan potassium as initial therapy in patients with severe hypertension. J Hum Hypertens. 1995;9(11):861–867. [PubMed] [Google Scholar]
- 9. Oparil S, Ripley E, on behalf of Candesartan Study Investigators. Candesartan cilexetil enhances blood pressure reduction severe (state 3, JNC‐VI) hypertensive patients inadequately controlled with HCTZ. Am J Hypertens. 1998;11(4 part 1):121A. [Google Scholar]
- 10. Larochelle P, Flack JM, Marbury TC, et al., for the Irbesartan Multicenter Investigators. Effects and tolerability of irbesartan versus enalapril in patients with severe hypertension. Am J Cardiol. 1997;80:1613–1615. [DOI] [PubMed] [Google Scholar]
- 11. Cifkova R, Peleska J, Hradec J, et al. Valsartan and atenolol in patients with severe essential hypertension. J Hum Hypertens. 1998;12(8):563–567. [DOI] [PubMed] [Google Scholar]
- 12. Hansson L, Zanchetti A, Carruthers SG, et al., for the HOT Study Group. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 13. Hutchison JC. Long‐term treatment of hypertension with a preparation combining rauwolfia and bendroflumethiazide. Med Times August. 1967;95(8):860–868. [PubMed] [Google Scholar]
- 14. Shaw CW. The treatment of hypertension with a combination of veratrum and epithiazide. Br J Clin Prac Jan. 1966;20(1):28–30. [PubMed] [Google Scholar]
- 15. Freis ED. Hypertension: challenge in preventive medicine. Prev Med. 1973;2:7–9. [DOI] [PubMed] [Google Scholar]
- 16. Moser M. Evolution of the treatment of hypertension from the 1940s to JNC V. Am J Hypertens Mar. 1997;10(3):2S–8S. [DOI] [PubMed] [Google Scholar]
- 17. Neutel MJ, Smith DHG, Weber MA. Low‐dose combination therapy: an important first‐line treatment in the management of hypertension. AJH. 2001;14:286–292. [DOI] [PubMed] [Google Scholar]
- 18. Waeber B, Brunner HR. Rationale for the use of very low‐dose combinations as first‐line treatment of hypertension. J Hypertens. 2001;19(suppl 4):S3–S8. [PubMed] [Google Scholar]
- 19. Ambrosioni E. Healthcare benefits of very‐low‐dose combination treatment used in the management of hypertension. J Hypertens. 2001;19(suppl 4):S29–S36. [PubMed] [Google Scholar]
- 20. Elliott WJ. Average number of drugs and mean change in blood pressure in clinical trials: implications for antihypertensive monotherapy. Am J Hypertens. 2002;4(part 2):29A. [Google Scholar]
- 21. Conlin PR, Gerth WC, Fox J. Four‐year persistence patterns among patients initiating therapy with the angiotensin II receptor antagonist losartan versus other antihypertensive drug classes. Clin Ther. 2001;23(12):1999–2010. [DOI] [PubMed] [Google Scholar]
- 22. Sica DA. Fixed‐dose combination antihypertensive drugs. Do they have a role in rational therapy? Drugs. 1994;48:16. [DOI] [PubMed] [Google Scholar]
- 23. Caro JJ, Speckman JL, Salas M, et al. Effect of initial drug choice on persistence with antihypertensive therapy: the importance of actual practice data. Can Med Assoc J. 1999;150:41–46. [PMC free article] [PubMed] [Google Scholar]
- 24. Hilleman D. Pharmacoeconomics of combination antihypertensive therapy. Blood Press Monit. 2001;5(suppl 1): S31–S36. [Google Scholar]
- 25. Data on file . The Institute for Effectiveness Research LLC, a subsidiary of Merck‐Medco Managed Care LLC, a Merck Company, Bridgewater , NJ . [Google Scholar]


