Abstract
While elevated low‐density lipoprotein cholesterol is the primary target of hypercholesterolemia treatment, high triglycerides and low high‐density lipoprotein cholesterol are also important targets for therapy. Correcting these lipid abnormalities should be an integral part of therapy in hypertensive individuals. Medications such as the fibrates are effective and well tolerated for reducing triglycerides and increasing high‐density lipoprotein cholesterol, and their use has resulted in a reduction in cardiovascular events. Fibrates are also recommended as adjunct therapy for patients receiving statins whose low‐density lipoprotein cholesterol or non‐high‐density lipoprotein cholesterol is not reduced to goal levels. The combination of a statin and a fibrate may, however, raise the risk of myopathy and rhabdomyolysis. Gemfibrozil, one of the fibrates, but not fenofibrate, interferes with statin glucuronidation, which may increase the risk of myopathydue to elevations in statin serum levels. This may at least partially explain the lower incidence of myopathy with fenofibrate compared with gemfibrozil when combined with statins. Combination therapy with a fibrate and a statin is a potentially useful therapy for patients with atherogenic lipid profiles, for which fenofibrate appears to be a more appropriate choice due to less myopathic potential.
Elevated low‐density lipoprotein cholesterol (LDL‐C) is a major risk factor for coronary heart disease (CHD). 1 Epidemiologic data support a strong and continuously graded relationship between serum cholesterol levels and the risk of premature death from CHD among individuals both with and without preexisting cardiovascular disease. 1 , 2 Therapy aimed at lowering LDL‐C significantly reduces the risk of CHD events. 1 Other common dyslipidemias, however, including elevated triglyceride and low high‐density lipoprotein cholesterol (HDL‐C) levels, are also significant independent risk factors for CHD and should be targeted for treatment in high‐risk patients. 1 , 3 , 4 , 5 The ratio of total cholesterol to HDL‐C may represent an important marker for cardiovascular risk, with a ratio below 3.5 being associated with lower risk for CHD. Each 1% increase in HDL‐C is correlated with a 2%–3% reduction in CHD risk. 5 Hypertriglyceridemia (≥150 mg/dL) and low HDL‐C (<40 mg/dL in men; <50 mg/dL in women) are also commonly associated as components of the metabolic syndrome with formation of highly atherogenic triglyceride‐rich remnants and small dense LDL particles. 1 Because a large number of hypertensive patients also have dyslipidemia and may have the metabolic syndrome with a low HDL‐C level and elevated triglycerides, it is appropriate to review data on the treatment of these entities in The Journal of Clinical Hypertension.
CLINICAL BENEFITS OF FIBRATE THERAPY
Fibric acid derivatives (fibrates) are among the most effective and well tolerated therapies for reducing triglyceride levels, increasing HDL‐C levels, and consequently, for treating atherogenic dyslipidemia. 1 , 6 These agents act primarily by activating peroxisome proliferator‐activated receptor α, thus changing the transcription of genes that regulate lipid metabolism. 6 Fibrates reduce triglycerides by 20%–50% and increase HDL‐C by 10%–35%; greater increases occur in patients with severe hypertriglyceridemia. 1 The fibrates clofibrate and gemfibrozil generally reduce LDL‐C levels by up to 10% in patients with primary hypercholesterolemia, although LDL‐C may increase in patients with marked hypertriglyceridemia. Fenofibrate appears to be more effective than clofibrate or gemfibrozil in controlling serum LDL‐C. This agent reduces LDL‐C by 15%–20% in patients with primary hypercholesterolemia. 1 , 7 , 8 Fibrates can also reduce the proportion of atherogenicdense LDL particles. 9
In large‐scale outcome studies, including the Helsinki Heart Study 10 and the Veterans Affairs High‐Density Lipoprotein Cholesterol Intervention Trial (VA‐HIT), 11 , 12 the use of gemfibrozil resulted in significant reductions in the primary and secondary prevention of CHD events and stroke. In the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study 13 involving 9795 patients with diabetes, therapy with micronized fenofibrate over an average of 5 years reduced the primary end point of coronary events (CHD death or nonfatal myocardial infarction) by 11%. This change, however, did not achieve statistical significance (p=0.16). When the secondary end point of total cardiovascular events was analyzed, a significant 11% reduction in total cardiovascular events was demonstrated (p=0.035) along with a 24% reduction in nonfatal myocardial infarction (p=0.01) and a 21% reduction in coronary revascularizations (p=0.003). One of the possible contributors to the lack of statistical significance for the primary end point was that a greater proportion of patients in the placebo group compared with the fenofibrate group had been placed on statin therapy during the trial (17% vs. 8%; p<0.0001).
Fibrates are generally recommended for patients with very high triglyceride levels (≥500 mg/dL) to prevent pancreatitis. 1 , 3 Guidelines also suggest that after achieving LDL‐C goals, fibrates should be considered for patients at high risk for CHD with low HDL‐C levels and/or high triglycerides (atherogenic dyslipidemia). This type of lipid profile is often noted in patients with type 2 diabetes or the metabolic syndrome (commonly seen in individuals with hypertension). Fibrates have also been recommended as an adjunct to 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitor (statin) therapy for patients unable to achieve LDL‐C and non‐HDL‐C goals. 1 , 3 Although large‐scale outcome studies have not been reported with combination therapy with a fibrate plus a statin, combination therapy does result in significantly higher HDL‐C and lower triglyceride, total cholesterol, and LDL‐C levels, as well as improved total cholesterol/HDL‐C ratios, compared with statin monotherapy. 14 , 15
There are, however, some concerns that the addition of a fibrate to a statin will increase the risk of myopathy, i.e., muscle aches or weakness with creatinine kinase (CK) levels >10 × the upper limit of normal (ULN), and rhabdomyolysis. 16 , 17 The risk of myopathy may differ among the available fibrates. 3 This article will review the available data on the safety and tolerability of fibrates as monotherapy and in combination with statins.
SAFETY AND TOLERABILITY OF FIBRATE MONOTHERAPY
The most common adverse events (AEs) resulting from fibrate use are gastrointestinal symptoms such as dyspepsia. 1 , 4 , 8 In addition, biliary cholesterol concentrations can increase with all fibrates, leading to an increased risk of gallstones. 6 Gemfibrozil may be associated more frequently with gastrointestinal symptoms than other fibrates, with complaints of dyspepsia reported by up to 40% of patients in randomized trials. 6 , 11 The rate of AEs with fenofibrate in clinical trials of up to 12 months' duration involving approximately 2700 patients was 6.3% (gastrointestinal symptoms were reported by 3.8% of patients). 18 Fibrates are contraindicated in patients with gallbladder disease, hepatic dysfunction, severe renal dysfunction, unexplained elevations of liver function tests, and primary biliary cirrhosis. 1 , 4 The most serious safety risk associated with fibrates is that of myopathy. This complication is rare, but failure to discontinue drug therapy when it occurs can result in rhabdomyolysis, leading to possible kidney failure and death. 19 In general, fibrates are well tolerated.
In the Helsinki Heart Study, in which 4081 asymptomatic middleaged men with primary dyslipidemia were randomized to treatment with gemfibrozil or placebo, there were no cases of myopathy reported during either the initial 5‐year study 10 or an additional 3.5‐year follow‐up. 20 VA‐HIT 11 compared the effects of gemfibrozil with placebo for 5 years in 2531 men with CHD and low HDL‐C. No major AEs, including myopathy, were reported. The incidence of elevated CK or aspartate aminotransferase levels was similar between the two groups. In the Bezafibrate Infarction Prevention (BIP) study, 21 3090 patients with ischemic CHD and high triglyceride levels, low HDL‐C, or both were randomized to bezafibrate or placebo for over 6 years. The overall rate of AEs was the same in both treatment groups (69%), with seven patients (0.45%) on placebo reporting muscle pain, compared with five patients (0.32%) in the bezafibrate group. CK levels >2 × ULN (390 U/L for men; 260 U/L for women) were reported in four patients (0.26%) receiving bezafibrate and one patient (0.06%) in the placebo group. The Diabetes Atherosclerosis Intervention Study (DAIS), 22 which included 418 patients with type 2 diabetes who were randomized to micronized fenofibrate or placebo for at least 3 years, reported only one patient (in the placebo group) with a muscle‐related serious AE. The total incidence of serious AEs was low and similar between the two treatment groups. Fenofibrate was also well tolerated in the FIELD study, 13 in which a good safety profile was demonstrated irrespective of concomitant lipid‐lowering therapy. Both gastrointestinal (19% and 20% with placebo and fenofibrate, respectively) and musculoskeletal symptoms (15% in both groups) were reported at similar rates in the two treatment groups, and a similar number of patients in each group discontinued the study drug (10% and 11%, respectively). CK elevations >10 × ULN occurred in three patients in the placebo group (n=4900) compared with four in the fenofibrate group (n=4895), indicating a low rate of myopathy. Rhabdomyolysis was equally rare, occurring in one patient on placebo and three patients on fenofibrate. Individual cases of rhabdomyolysis have been reported in clinical practice with fibrate monotherapy in patients with renal failure and hypothyroidism and in older patients; rhabdomyolysis is less common among individuals with normal creatinine levels at baseline. 23 , 24 , 25
The rates of myopathy with lipid‐lowering drugs were examined in population‐based cohorts in the United Kingdom during the period 1991–1997. 26 These cohorts included 17,219 patients who had received at least one prescription for a lipid‐lowering drug, 28,974 who had been diagnosed with hyperlipidemia but had not been prescribed a lipid‐lowering drug, and 50,000 individuals without a diagnosis of hyperlipidemia. The rate of myopathy with all lipid‐lowering drugs was 2.3/10,000 person years of therapy, which exceeded the rates of 0 and 0.2/10,000 person years in the non—lipidlowering population and the general population, respectively. Myopathy was reported with both fibrate and statin monotherapy, with a 5.5‐fold higher incidence associated with fibrates compared with statins, despite the low absolute risk with both classes of agents.
Managed care data on 252,460 patients reported a total of 24 hospitalized cases of rhabdomyolysis. 27 The incident rate per 10,000 patient years of therapy was 3.70 for gemfibrozil alone and 2.82 for gemfibrozil and fenofibrate when all data were combined. This represented a 5.5‐fold increase over the incidence rate of 0.44 for the statins; thus, the number needed to treat to cause one case of rhabdomyolysis in 1 year with fibrate monotherapy would be 3546 patients. The risk was increased for patients older than 65 years and with diabetes.
Overall, fibrate monotherapy is safe and well tolerated, with a low absolute risk of myopathy. Importantly, complaints of myalgia are fairly common with statins (myalgia is not associated with fibrate monotherapy), but these symptoms are generally not associated with frank myopathy. 28 One study designed to track all muscle complaints found that among 815 hyperlipidemic patients receiving lipid‐lowering therapy, 165 (20%) complained of mild myalgia or weakness related to treatment. 29 In a study among 508 patients with familial hypercholesterolemia treated with simvastatin for 2 years, 45 patients (8.9%) reported myalgia; only nine patients (1.8%) discontinued therapy due to musculoskeletal complaints. 30 Lastly, in the Heart Protection Study (N=20,536), 31 the simvastatin and placebo groups had similar rates of reported muscle pain or weakness (32.9% vs. 33.2%, respectively) and of withdrawal from study treatment due to muscle symptoms (0.5% for both). The rate of frank myopathy with simvastatin (0.1%) was low, however, and was not significantly higher than that with placebo (0.04%).
RISK OF MYOPATHY WITH STATIN‐FIBRATE THERAPY
The potential risk of myopathy and rhabdomyolysis with statin‐fibrate combination therapy was first reported in 1990 with the combination of gemfibrozil and lovastatin. 32 This led to changes in prescribing information for all statins, with warnings about the combined use of gemfibrozil with a statin. Later reports showed increased rates of rhabdomyolysis when gemfibrozil was combined with cerivastatin, initially leading to labeling changes indicating a contraindication for the combined use of these specific agents. 17 , 33 Subsequent analyses by US Food and Drug Administration (FDA) researchers clarified that cerivastatin monotherapy was more likely to cause rhabdomyolysis than other statins 19 , 33 and that higher statin doses had higher rates of myopathy. Based on these risks, cerivastatin was removed from the market in 2001. 34 For all statins, a threshold dose exists where myopathy risks outweigh LDL‐C‐lowering benefits (Figure I). 35
Figure 1.
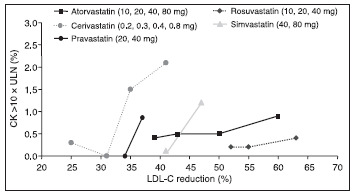
The incidence ofmyopathy, defined as a creatinine kinase (CK) level >10 × the upper limit of normal (ULN), increases with increasing dose for all statins, most markedly with cerivastatin. Data are derived for each agent from prescribing information, summary basis for approval, US Food and Drug Administration, and published trial results. LDL‐C=low‐density lipoprotein cholesterol. Reproduced with permission from Am J Cardiol. 2003;92:23K–29K. 35
One review found that the rate of myopathy was similar for all lipid‐lowering drugs used as monotherapy, but rose from a range of 0.1%–0.5% with monotherapy to 0.5%–2.5% with combination therapy. 36 A review of 36 published clinical trials and 29 case reports of statin‐fibrate therapy, including a total of 1674 patients, found that the incidence of myopathy or rhabdomyolysis was 0.12% based on two cases of myopathy, both of which occurred with the use of gemfibrozil and a statin. 17 , 37 Risk factors for myopathy associated with statin‐fibrate therapy include increased age, female gender, renal or liver disease, diabetes, hypothyroidism, debilitation, surgery, trauma, excessive alcohol intake, and heavy exercise. 17
In 2000, a joint American College of Cardiology/American Heart Association/National Heart, Lung, and Blood Institute Advisory Committee on the use and safety of statins reviewed eight controlled clinical trials of statin‐fibrate therapy involving almost 600 patients. 38 This review found that 1% of patients experienced a CK level >3 × ULN without muscle symptoms and 1 % withdrew from therapy because of muscle discomfort; none of the findings were considered serious by the investigators, and no cases of rhabdomyolysis or myoglobinuria had been reported. The statin safety advisory committee concluded that the combination of a moderately dosed statin with a fibrate “appears to have a relatively low incidence of myopathy, especially when used in persons without multiple‐system disease or multiple medications.” 38
DIFFERENCES IN RISK OF MYOPATHY AMONG FIBRATES
There appear to be important differences in myopathy risk among fibrates when combined with statins. A review of data from the FDA's Adverse Event Reporting System 39 found that fenofibrate‐statin therapy was associated with significantly fewer reports of rhabdomyolysis than gemfibrozil‐statin therapy. The rhabdomyolysis rates per million gemfibrozil prescriptions dispensed in combination with cerivastatin and other statins were approximately 33 times and 15 times greater, respectively, than the corresponding rates with fenofibrate therapy (Table I).
Table I.
Reported Cases of Rhabdomyolysis With Combination Fibrate‐Statin Therapy
| Medication | No. of Cases Reported* | No. of Prescriptions Dispensed**,† | No. of Cases Reported per Million Prescriptions |
|---|---|---|---|
| Fenofibrate | |||
| With cerivastatin | 14 | 100,000 | 140 |
| With other statins | 2 | 3,419,000 | 0.58 |
| Total | 16 | 3,519,000 | 4.58 |
| Gemfibrozil | |||
| With cerivastatin | 533 | 116,000 | 4600 |
| With other statins | 57 | 6,641,000 | 8.6 |
| Total | 590 | 6,757,000 | 87 |
| *Adverse Event Reporting System, US Food and Drug Administration, Rockville, MD; **National Prescription Audit Plus Report, IMS Health, Fairfield, CT; †Concomitancy Report, Verispan LLC, Yardley, PA. Data derived from Am J Cardiol. 2005;95:120–122. 39 | |||
Fibrates are believed to increase the risk of myopathy when combined with a statin by altering statin catabolism. 28 Statins, including simvastatin, atorvastatin, and cerivastatin, have been found in animal and human studies to undergo extensive glucuronidation. Gemfibrozil also undergoes glucuronidation, which interferes with statin catabolism and thus causes an increase in plasma statin concentrations. 40 Not all fibrates have this effect on statin metabolism. A study in human hepatocytes found that while gemfibrozil inhibited the lactonization and glucuronidation of simvastatin, fenofibrate did not appreciably affect any of the metabolic pathways of simvastatin. 41 Although fenofibrate also undergoes glucuronidation, the pattern of isoform selectivity for fenofibrate is different than that for gemfibrozil and statins, with less potential for interference with statin metabolism. Concomitant administration of gemfibrozil doubles the peak plasma concentration of simvastatin (Figure 2). 42 In contrast, fenofibrate has no significant effects on the plasma concentrations of simvastatin (Figure 3). 43 In the recently completed FIELD trial, 13 no cases of rhabdomyolysis were seen in fenofibrate‐treated patients in whom concomitant statin therapy was initiated during the trial.
Figure 2.
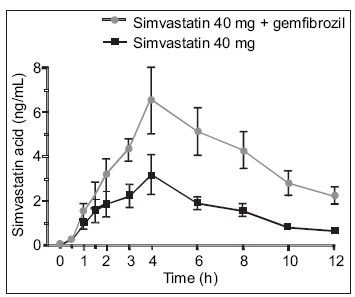
In a study of 10 healthy volunteers given gemfibrozil 600 mg b.i.d. or placebo for 3 days and a single 40‐mg dose of simvastatin on Day 3, plasma concentrations of simvastatin were significantly increased by gemfibrozil pretreatment, compared with placebo. Reproduced with permission from Clin Pharmacol Ther. 2000;68:122–129. 42
Figure 3.
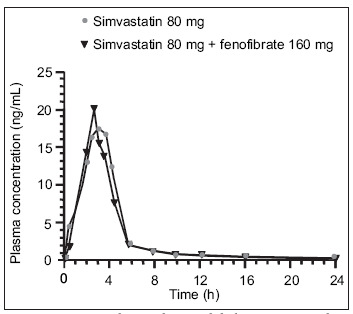
In a randomized, open‐label crossover study, 12 subjects received an 80‐mg dose of simvastatin for 7 days in the first treatment period. In the second treatment period, patients received a 160‐mg dose of micronized fenofibrate for 7 days, followed by a 160‐mg dose of micronized fenofibrate with an 80‐mg dose of simvastatin for an additional 7 days. No clinically significant pharmacokinetic drug interaction between fenofibrate and simvastatin was observed. Reproduced with permission from J Clin Pharmacol. 2004;44:1054–1062. 43
A recent crossover study in 20 healthy volunteers demonstrated that neither atorvastatin nor simvastatin had a significant effect on the pharmacokinetics of Insoluble Drug Delivery MicroParticle (IDD‐P [SkyePharma PLC, London, England, United Kingdom]) fenofibrate. 44 And in a study in 23 healthy adult volunteers, concomitant administration of fenofibrate and pravastatin did not affect the pharmacokinetics of either agent. 45 , Table II summarizes the lack of pharmacokinetic interactions between statins and fenofibrate compared with gemfibrozil.
Table II.
Statin‐Fibrate Combination Therapy: Pharmacokinetic Interactions
| Statin | Gemfibrozil | Fenofibrate |
|---|---|---|
| Atorvastatin | ? in Cmax* | No effect** |
| Simvastatin | 2‐fold ? in Cmax*** | No effect† |
| Pravastatin | 2‐fold ? in Cmax†† | No effect††† |
| Rosuvastatin | 2‐fold ? in Cmax ‡ | No effect‡, ‡‡ |
| Fluvastatin | No effect†† | No effect |
| Lovastatin | 2.8‐fold ? in Cmax ‡‡‡ | Not available |
| Cerivastatin | 2–3‐fold ? in Cmax § | No effect†† |
| ?=increase; Cmax=peak plasma concentration; *Arch Intern Med. 2003;163:553–564 37 ; **TriCor [package insert]. Abbott Park, IL: Abbott Laboratories; 2004; ***Clin Pharmacol Ther. 2000;68:122–129 42 ; †J Clin Pharmacol. 2004;44:1054–1062 43 ; ††Am J Cardiol. 2002;90:50K–60K 50 ; †††J Clin Pharmacol. 2000;40:316–323 45 ; ‡CRESTOR [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2005; ‡‡ Clin Ther. 2003;25:459–471 49 ; ‡‡‡ Clin Pharmacol Ther. 2001;69:340–345 48 ;§ Clin Pharmacol Ther. 2002;72:685–691 47 | ||
The safety and tolerability of fibrates with other lipid‐lowering drugs has not been well studied. No drug interaction was observed between extended‐release niacin and IDD‐P fenofibrate. 44 A study of concomitant therapy with fenofibrate and the cholesterol absorption inhibitor ezetimibe, compared with component monotherapy, in 625 patients with mixed dyslipidemia found that this combination was well tolerated. 46 The rate of AEs was similar in all treatment groups, and no serious AEs, elevations of CK >10 × ULN, or cases of myopathy or rhabdomyolysis were reported. The incidence of myalgia was low (ranging from 1.1% to 3.1%) and similar across all treatment groups.
CONCLUSIONS
Fibrates lower elevated triglycerides, raise HDL‐C, reduce LDL‐C, and reduce the density of LDL particles. In clinical studies, fibrates have been generally well tolerated. The most serious risk associated with fibrates is that of myopathy, which is fairly low with fibrate monotherapy but is increased with fibrate‐statin combination therapy. Significant risk factors for myopathy with fibrate‐statin therapy include age older than 70 years, female gender, renal disease, liver disease, diabetes, hypothyroidism, debilitation, surgery, trauma, excessive alcohol intake, and heavy exercise.
The risk of myopathy differs among fibrates; gemfibrozil has been associated with the highest rates of reported rhabdomyolysis when used concomitantly with statins, whereas fenofibrate has been associated with the lowest rates. Findings from experimental studies suggest that gemfibrozil raises serum levels of statins and thus increases the risk of myopathy by inhibiting the glucuronidation of statins. Fenofibrate, on the other hand, does not appear to block statin glucuronidation. This may help explain the lower incidence of rhabdomyolysis associated with fenofibrate‐statin combination therapy. Combination of a fibrate and a statin is a clinically useful treatment regimen that results in beneficial changes in atherogenic lipid fractions. In patients who may benefit from combination statin‐fibrate therapy, fenofibrate appears to be a more appropriate choice than gemfibrozil due to lower myopathic potential.
References
- 1. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 2. Pekkanen J, Linn S, Heiss G, et al. Ten‐year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322: 1700–1707. [DOI] [PubMed] [Google Scholar]
- 3. Grundy SM, Cleeman JI, Bairey‐Merz CN, et al, For the Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110: 227–239. [DOI] [PubMed] [Google Scholar]
- 4. Ashen MD, Blumenthal RS. Low HDL cholesterol levels. N Engl J Med. 2005;353: 1252–1260. [DOI] [PubMed] [Google Scholar]
- 5. Gotto AM Jr. High‐density lipoprotein cholesterol and triglycerides as therapeutic targets for preventing and treating coronary artery disease. Am Heart J. 2002;144(suppl 6):S33–S42. [DOI] [PubMed] [Google Scholar]
- 6. Knopp RH. Drug treatment of lipid disorders. N Engl J Med. 1999;341: 498–511. [DOI] [PubMed] [Google Scholar]
- 7. Pauciullo P, Marotta G, Rubba P, et al. Serum lipoproteins, apolipoproteins and very low density lipoprotein subfractions during 6‐month fibrate treatment in primary hypertriglyceridaemia. J Intern Med. 1990;228: 425–430. [DOI] [PubMed] [Google Scholar]
- 8. Leaf DA, Connor WE, Illingworth DR, et al. The hypolipidemic effects of gemfibrozil in type V hyperlipidemia: a double‐blind, crossover study. JAMA. 1989;262: 3154–3160. [PubMed] [Google Scholar]
- 9. Winkler K, Weltzien P, Friedrich I, et al. Qualitative effect of fenofibrate and quantitative effect of atorvastatin on LDL profile in combined hyperlipidemia with dense LDL. Exp Clin Endocrinol Diabetes. 2004;112: 241–247. [DOI] [PubMed] [Google Scholar]
- 10. Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary‐prevention trial with gemfibrozil in middle‐aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317: 1237–1245. [DOI] [PubMed] [Google Scholar]
- 11. Rubins HB, Tobins SJ, Collins D, et al, For the Veterans Affairs High‐Density Lipoprotein Cholesterol Intervention Trial Study Group. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high‐density lipoprotein cholesterol. N Engl J Med. 1999;341: 410–418. [DOI] [PubMed] [Google Scholar]
- 12. Bloomfield Rubins H, Davenport J, Babikian V, et al, For the VA‐HIT Study Group. Reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: the Veterans Affairs HDL Intervention Trial. (VA‐HIT). Circulation. 2001;103: 2828–2833. [DOI] [PubMed] [Google Scholar]
- 13. The Field Study Investigators . Effects of long‐term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366: 1849–1861. [DOI] [PubMed] [Google Scholar]
- 14. Vega GL, Ma PT, Cater NB, et al. Effects of adding fenofibrate. (200 mg/day) to simvastatin (10 mg/day) in patients with combined hyperlipidemia and metabolic syndrome. Am J Cardiol. 2003;91: 956–960. [DOI] [PubMed] [Google Scholar]
- 15. Ellen RL, McPherson R. Long‐term efficacy and safety of fenofibrate and a statin in the treatment of combined hyperlipidemia. Am J Cardiol. 1998;81: 60B–65B. [DOI] [PubMed] [Google Scholar]
- 16. Farnier M. Combination therapy with an HMG‐CoA reductase inhibitor and a fibric acid derivative: a critical review of potential benefits and drawbacks. Am J Cardiovasc Drugs. 2003;3: 169–178. [DOI] [PubMed] [Google Scholar]
- 17. Shek A, Ferril MJ. Statin—Fibrate combination therapy. Ann Pharmacother. 2001;35: 908–917. [DOI] [PubMed] [Google Scholar]
- 18. Roberts WC. Safety of fenofibrate?US and worldwide experience. Cardiology. 1989;76: 169–179. [DOI] [PubMed] [Google Scholar]
- 19. Chang JT, Staffa JA, Parks M, et al. Rhabdomyolysis with HMG‐CoA reductase inhibitors and gemfibrozil combination therapy. Pharmacoepidemiol Drug Saf. 2004;13: 417–426. [DOI] [PubMed] [Google Scholar]
- 20. Huttunen JK, Heinonen OP, Manninen V, et al. The Helsinki Heart Study: an 8.5‐year safety and mortality follow‐up. J Intern Med. 1994;235: 31–39. [DOI] [PubMed] [Google Scholar]
- 21. The BIP Study Group . Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000;102: 21–27. [DOI] [PubMed] [Google Scholar]
- 22. Diabetes Atherosclerosis Intervention Study Investigators . Effect of fenofibrate on progression of coronary‐artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001;357: 905–910. [PubMed] [Google Scholar]
- 23. Layne RD, Shbai AS, Stark LJ. Rhabdomyolysis and renal failure associated with gemfibrozil monotherapy. Ann Pharmacother. 2004;38: 232–234. [DOI] [PubMed] [Google Scholar]
- 24. Kanterewicz E, Sanmarti R, Riba J, et al. Bezafibrate induced rhabdomyolysis. Ann Rheum Dis. 1992;51: 536–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clou?tre Y, Leblanc M, Ouimet D, et al. Fenofibrate‐induced rhabdomyolysis in two dialysis patients with hypothyroidism. Nephrol Dial Transplant. 1999;14: 1047–1048. [DOI] [PubMed] [Google Scholar]
- 26. Gaist D, Rodriguez LA, Huerta C, et al. Lipid‐lowering drugs and risk of myopathy: a population‐based follow‐up study. Epidemiology. 2001;12: 565–569. [DOI] [PubMed] [Google Scholar]
- 27. Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipidlowering drugs. JAMA. 2004;292: 2585–2590. [DOI] [PubMed] [Google Scholar]
- 28. Thompson PD, Clarkson P, Karas RH. Statin‐associated myopathy. JAMA. 2003;289: 1681–1690. [DOI] [PubMed] [Google Scholar]
- 29. Franc S, Dejager S, Bruckert E, et al. A comprehensive description of muscle symptoms associated with lipid‐lowering drugs. Cardiovasc Drugs Ther. 2003;17: 459–465. [DOI] [PubMed] [Google Scholar]
- 30. de Sauvage Nolting PR, Buirma RJ, Hutten BA, et al, and the Dutch ExPRESS Investigators Grou .. Two‐year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia. (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia. [ExPRESS FH]). Am J Cardiol. 2002;90: 181–184. [DOI] [PubMed] [Google Scholar]
- 31. Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo controlled trial. Lancet. 2002;360: 7–22. 12114036 [Google Scholar]
- 32. Pierce LR, Wysowski DK, Gross TP. Myopathy and rhabdomyolysis associated with lovastatin‐gemfibrozil combination therapy. JAMA. 1990;264: 71–75. [PubMed] [Google Scholar]
- 33. Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med. 2002;346: 539–540. [DOI] [PubMed] [Google Scholar]
- 34. US Food and Drug Administration. Bayer voluntarily withdraws Baycol. Available at: http:www.fda.govbbstopicsANSWERS2001ANS01095.html. Accessed October 6, 2005.
- 35. Brewer HB Jr. Benefit‐risk assessment of rosuvastatin 10 to. milligrams. Am J Cardiol. 2003;92: 23K–29K. [DOI] [PubMed] [Google Scholar]
- 36. Hodel C. Myopathy and rhabdomyolysis with lipid‐lowering drugs. Toxicol Lett. 2002;128: 159–168. [DOI] [PubMed] [Google Scholar]
- 37. Ballantyne CM, Corsini A, Davidson MH, et al. Risk for myopathy with statin therapy in high‐risk patients. Arch Intern Med. 2003;163: 553–564. [DOI] [PubMed] [Google Scholar]
- 38. Pasternak RC, Smith SC Jr, Bairey‐Merz CN, et al. ACC/ AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40: 567–572. [DOI] [PubMed] [Google Scholar]
- 39. Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95: 120–122. [DOI] [PubMed] [Google Scholar]
- 40. Prueksaritanont T, Zhao JJ, Ma B, et al. Mechanistic studies on metabolic interactions between gemfibrozil and statins. J Pharmacol Exp Ther. 2002;301: 1042–1051. [DOI] [PubMed] [Google Scholar]
- 41. Prueksaritanont T, Tang C, Qiu Y, et al. Effects of fibrates on metabolism of statins in human hepatocytes. Drug Metab Dispos. 2002;30: 1280–1287. [DOI] [PubMed] [Google Scholar]
- 42. Backman JT, Kyrklund C, Kivisto KT, et al. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68: 122–129. [DOI] [PubMed] [Google Scholar]
- 43. Bergman AJ, Murphy G, Burke J, et al. Simvastatin does not have a clinically significant pharmacokinetic interaction with fenofibrate in humans. J Clin Pharmacol. 2004;44: 1054–1062. [DOI] [PubMed] [Google Scholar]
- 44. Penn R, Williams RX III, Guha‐Ray DK, et al. Pharmacokinetics of IDD‐P fenofibrate in combination with atorvastatin, simvastatin, and extended‐release niacin. Clin Ther.In press. [DOI] [PubMed] [Google Scholar]
- 45. Pan WJ, Gustavson LE, Achari R, et al. Lack of a clinically significant pharmacokinetic interaction between fenofibrate and pravastatin in healthy volunteers. J Clin Pharmacol. 2000;40: 316–323. [DOI] [PubMed] [Google Scholar]
- 46. Farnier M, Freeman MW, Macdonell G, et al, For the Ezetimibe Study Group. Efficacy and safety of the coadministration of ezetimibe with fenofibrate in patients with mixed hyperlipidaemia. Eur Heart J. 2005;26: 897–905. [DOI] [PubMed] [Google Scholar]
- 47. Backman JT, Kyrklund C, Neuvonen M, et al. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin Pharmacol Ther. 2002;72: 685–691. [DOI] [PubMed] [Google Scholar]
- 48. Kyrklund C, Backman JT, Kivisto KT, et al. Plasma concentrations of active lovastatin acid are markedly increased by gemfibrozil but not by bezafibrate. Clin Pharmacol Ther. 2001;69: 340–345. [DOI] [PubMed] [Google Scholar]
- 49. Martin PD, Dane AL, Schneck DW, et al. An open‐label, randomized, three‐way crossover trial of the effects of coadministration of rosuvastatin and fenofibrate on the pharmacokinetic properties of rosuvastatin and fenofibric acid in healthy male volunteers. Clin Ther. 2003;25: 459–471. [DOI] [PubMed] [Google Scholar]
- 50. Davidson MH. Combination therapy for dyslipidemia: safety and regulatory considerations. Am J Cardiol. 2002;90: 50K–60K. [DOI] [PubMed] [Google Scholar]


