Abstract
Objectives
Patients with Parkinson’s disease (PD) have deficits in lower-limb functions such as gait, which involves both cognitive and motor dysfunction. In PD theta and beta brain rhythms are associated with cognitive and motor functions, respectively. We tested the hypothesis that PD patients with lower-limb abnormalities would exhibit abnormal theta and beta rhythms in the mid-frontal cortical region during lower-limb action.
Methods
This study included thirty-nine participants; 13 PD patients with FOG (PDFOG+), 13 without FOG (PDFOG−), and 13 demographically-matched controls. We recorded scalp electroencephalograms (EEG) during a lower-limb pedaling motor task, which required intentional initiation and stopping of a motor movement.
Results
FOG scores were correlated with disease severity and cognition. PDFOG+ patients pedaled with reduced speed and decreased acceleration compared to PDFOG− patients and controls. PDFOG+ patients exhibited attenuated theta-band (4–8 Hz) power and increased beta-band (13–30 Hz) power at mid-frontal electrode Cz during pedaling. Frontal theta- and beta-band oscillations also correlated with motor and cognitive deficits.
Conclusion
Frontal theta and beta oscillations are predictors of lower-limb motor symptoms in PD and could be used to design neuromodulation for PD-related lower-limb abnormalities.
Significance
These data provide insight into mechanisms of lower-limb dysfunction in PD with FOG.
Keywords: Parkinson’s disease, lower-limb movement, freezing of gait, oscillations, frontal region
1. Introduction
Lower-body symptoms of PD include impairment in gait initiation and execution, balance, and postural instability. These symptoms can limit mobility and contribute to falls. Freezing of gait (FOG) is one of the most challenging lower-body symptoms in PD (Bloem et al., 2004; Boonstra et al., 2008). Treatments such as levodopa and deep brain stimulation (DBS) do not consistently improve lower-body symptoms in PD patients (Ambani and Van Woert, 1973; Chen, 2012; Espay et al., 2012; St George et al., 2010). Lower-limb movements combine motor and cognitive features. However, the neuronal mechanisms of lower-limb abnormalities in PD remain unknown. Understanding these mechanisms is key to decreasing morbidity and mortality from events such as falls, and to improving quality-of-life, and for new diagnostic and therapeutic approaches.
Most often, FOG occurs during gait initiation, which is the shift from standing to walking (Callisaya et al., 2016; Henriksson and Hirschfeld, 2005). Gait initiation disturbances include delayed release of anticipatory postural adjustments and slowed movement initiation in PD (Delval et al., 2014b; Rosin et al., 1997). Previous studies have observed that reduced gait initiation time and lower-body symptoms may be related to physiological alterations in both motor and cognitive systems (Killane et al., 2015; Martin et al., 2011; Walton et al., 2018). Recent work showed that gait, rather than cognition, predicts decline in cognitive domains early in PD (Morris et al., 2017), suggesting gait might be a harbinger of early cognitive decline.
Several studies have suggested a strong connection between frontal lobe impairments and executive dysfunction to FOG (Amboni et al., 2008; Giladi et al., 2007). Neuroimaging studies have suggested that medial frontal areas can account for PD-related motor-initiation deficits (Playford et al., 1992). Medial frontal regions such as supplementary motor area, leg motor cortex, and anterior cingulate cortices interact to instantiate motor and cognitive control (Singh et al., 2018; Wagner et al., 2016). Medial frontal scalp electroencephalogram (EEG) signals can identify neurophysiological signatures from these regions of both motor and cognitive control. Theta rhythms between 4–8 Hz are associated with cognitive control and are attenuated in PD (Kelley et al., 2018; Singh et al., 2018; Wagner et al., 2016). Because PD patients with FOG have greater deficits in executive function and attention compared to PD patients without FOG (Nutt et al., 2011), we hypothesized that attenuated theta activity might underlie FOG in PD. Beta rhythms between 13–30 Hz are associated with motor control and are increased with upper-limb bradykinesia and motor impairments in PD (Singh, 2018; Singh et al., 2013). This line of evidence predicts that increased frontal beta activity might also underlie impaired motor initiation in FOG.
We tested these hypotheses by investigating the neural correlates of FOG in PD by collecting cortical EEG during a pedaling task. We examined the relationship of these neural signatures with cognitive function and motor symptoms severity of PD. We found attenuated mid-frontal theta activity as well as increased frontal beta activity in PD patients with FOG. These data provide insight into the mechanisms contributing to FOG in PD.
2. Methods
2.1. Subjects and Clinical Assessments
Thirty-nine subjects (13 PD patients with FOG, “PDFOG+”; 13 PD patients without FOG, “PDFOG−”, and 13 demographically-matched healthy subjects, “controls”) participated in the study. All protocols were approved by the University of Iowa Office of the Institutional Review Board (IRB). All PD patients met UK Parkinson’s Disease Brain Bank Criteria for the diagnosis of idiopathic PD (Gibb and Lees, 1988). All subjects provided written informed consent. Because we were interested in real-world function and because there is some fall risk for unmedicated PD FOG+ patients, all PD patients were treated with levodopa medication and performed motor tasks during “ON” medication.
The patient’s visit started with questionnaires, including the Freezing of Gait Questionnaire (FGQ; scale from 0–24; higher scores are worse) (Giladi et al., 2000) to evaluate gait impairments in patients. Participants then completed multiple cognitive tasks and the pedaling motor task during EEG recording. At the end of the EEG session we administered the Montreal Cognitive Assessment (MOCA; scale from 0–30; lower scores are worse) to measure cognition (Nasreddine et al., 2005) and the motor Unified Parkinson’s Disease Rating Scale (UPDRS III; scale from 0–56; higher scores are worse) (Movement Disorder Society Task Force on Rating Scales for Parkinson’s, 2003) to measure motor symptoms of PD.
29 PD patients were recruited from University of Iowa Hospitals & Clinics (UIHC) Movement Disorders clinics. 16 patients with FOG+ were included in the study, and 3 could not perform the pedaling task. We then recruited 13 additional demographically-matched PD with FOG− from UIHC Movement Disorders clinics. Patients with a FOG score > 10 were classified as PDFOG+. All PDFOG+ also had FGQ item 3 score > 0. Patients with a FOG score < 10 were classified as PDFOG− and had FGQ item 3 score = 0. Table 1 shows the subjects’ demographic and clinical information in detail. Key variables throughout were compared via non-parametric Spearman correlation analyses and partial correlation.
Table 1.
Demographic, disease, motor, and cognitive characteristics
| Control (N = 13) | PDFOG− (N = 13) | PDFOG+ (N = 13) | p Value | |
|---|---|---|---|---|
| Gender, M/F | 8/5 | 9/4 | 8/5 | |
| Age, years | 69 (2.3) | 65.2 (2.4) | 69.5 (2.6) | >0.05a |
| Disease Duration, years | - | 4.8 (1.0) | 7.5 (1.0) | 0.06b |
| LED, mg/day | - | 682.7 (99.1) | 1158.8 (134.4) | 0.01b |
| Cognition Characteristics | ||||
| MOCA (0–30) | 26.6 (0.53) | 24.8 (0.8) | 21.8 (1.2) | <0.01a |
| Motor Characteristics | ||||
| UPDRS III (0–56) | - | 10.5 (1.7) | 19.2 (1.3) | <0.01b |
| H & Y stage, on (1–5) | - | 1.6 (0.2) | 2.4 (0.3) | 0.03b |
| Freezing of Gait (FOG) | ||||
| FOG questionnaire (0–24) | - | 3.7 (0.9) | 14.8 (0.8) | <0.01b |
Values were expressed as mean (standard error of mean).
One-way ANOVA was applied when three groups were compared followed by
two-sample t test to compare between PDFOG+ vs. control subjects.
Two-sample t test was used for comparison between PDFOG− vs PDFOG.
Abbreviations: Male, M; Female, F; Montreal Cognitive Assessment, MOCA; motor Unified Parkinson’s Disease Rating Scale, UPDRS III; Hoehn & Yahr scale, H & Y.
2.2. Lower-limb Motor Task and Analysis
We used a pedaling motor task (Fig. 1A) to study lower-limb movement control for the following reasons: (1) to minimize fall-risk in PD patients with marked gait abnormalities, (2) pedaling generates minimal movement artifact for the EEG signal, (3) similar to gait, pedaling requires bilateral coordination, and (4) pedaling kinematics analysis allows for detailed measures of lower-limb function. PDFOG+ patients can experience discontinuous changes in speed during continuous pedaling that have been considered as having freezing phenomena (Abe et al., 2003; Vercruysse et al., 2014). Patients were seated on a chair for the pedaling task and completed minimum 2 blocks of either 30 trials or 50 trials per block. PDFOG+ performed 30 trials/block due to their severe gait abnormalities. 60 trials provide adequate signal-to-noise for subsequent EEG analysis. Spectral variance decreases with the square root of the number of trials; accordingly, there was ~8-fold increase in SNR with 60 trials.
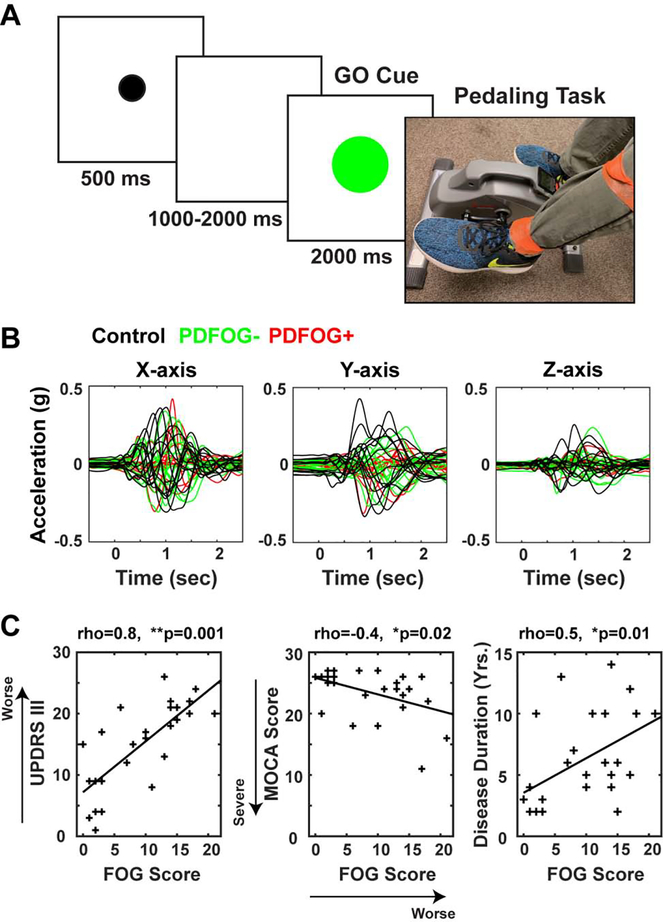
Fig. 1.
Experimental design, accelerometer signals, and relationship between FOG and motor /cognitive characteristics of PD. (A) In this task, a black “warning” cue appeared to alert the subject to pay attention. Within 1000–2000 ms, a green GO cue instructed subject to complete one rotation of the pedals. (B) Accelerometer signals were collected from tri-axial accelerometer and segmented from the GO-cue (−500 to 2500 ms) and averaged to plot the mean trace of X-, Y-, and Z-axes, in control, PDFOG−, and PDFOG+ participants. (C) FOG questionnaire scores as a measurement of lower-extremity impairment showed a significant positive correlation with UPDRS III scores and a significant negative correlation with MOCA scores (left side and middle). Correlation analysis showed a significant correlation between disease duration (DD) and FOG (right side), but not DD and MOCA scores. *p<0.05; **p<0.01. rho = correlation coefficients; FOG: Freezing of Gait; PDFOG−: PD patients without FOG; PDFOG+: PD patients with FOG. UPDRS III: motor Unified Parkinson’s Disease Rating Scale; MOCA: Montreal Cognitive Assessment.
For each trial, a warning cue (small black circle) appeared on the stimulus presentation computer for 0.5 second and then 1–2 seconds later a GO cue (green circle) appeared. The subject was instructed to complete one rotation upon seeing the GO cue. A 3-axis accelerometer was attached to the ankle of the left leg and subject initiated pedaling task from the left leg (Fig. 1A). Subject stopped the pedals in the starting position and waited for the next GO cue (3 seconds inter-trial interval).
We computed the linear speed for each pedaling trial from 3-axis accelerometer signals. We selected 0–2000 ms from the GO cue as the time-window to analyze speed and acceleration because participants took >2000 ms to complete one pedal rotation (Fig. 1B). Accelerometer signals (X-, Y-, and Z-axes) were detrended and low-pass filtered at 5 Hz. Mean speed was computed for each axis for each trial and then averaged across axes. Finally, we averaged all trials to represent the final mean speed (g*s) of the pedaling task for each subject. Additionally, to compute the time to achieve maximum acceleration, we picked the accelerometer signal (g) in which we observed highest acceleration using the Matlab “findpeaks” function. We averaged the time to reach peak acceleration across all trials to represent the mean time (peak time) for each subject. Speed and maximum acceleration time were compared via one-way analyses-of-variance (ANOVA) and post-hoc two-tailed t-tests with an alpha level of 0.05. Furthermore, we performed Spearman correlation analyses between kinematic parameters (pedaling speed and maximum acceleration time) and FOG, UPDRS III, and MOCA scores of PD patients.
2.3. EEG Recording and Analysis
EEG signals were collected during the lower-limb pedaling motor task from a customized 64-channel cap (Easycap Inc) using high-pass filter of 0.1 Hz with a sampling rate of 500 Hz (Brain Products). Online reference and ground channels were Pz and FPz, respectively. Data were epoched around the GO stimulus onset (−1000 to 3000 ms) and re-referenced to an average reference. FP1, FP2, FT10, TP9, and TP10 channels were removed, as they tend to be influenced by eyeblink / muscle artifact, leaving 59 electrodes for analysis. Of note, we used a specialized cap without FT9. Bad channels and epochs were identified using a conjunction of the FASTER algorithm and pop_rejchan from EEGlab and were subsequently interpolated and rejected respectively. Eye blinks were removed following ICA.
All analyses in the current report are from the mid-frontal Cz vertex electrode. After preprocessing, time-frequency measures were computed by complex Morlet wavelets, as explained previously (Singh et al., 2018; Singh et al., 2019). For time-frequency analysis, each epoch was cut in length (−500 to +2000 ms), frequency bands between 1–50 Hz in logarithmically-spaced bins were selected, and power was normalized by conversion to a decibel (dB) scale. The baseline for each frequency consisted of the average power from −300 to −200 ms prior to the onset of the GO stimuli. This short baseline period is common in the field since a small time sample reflects the wavelet-weighted influence of longer time and frequency periods. We restricted our analyses to electrode Cz and a-priori time-frequency Regions of Interest (tf-ROI). ROIs were preselected for the theta (3.5–7.5 Hz)- and beta (12.5–30 Hz)-bands and our time windows of interest were 0–2000 ms following the GO cue for motor execution and 0–400 ms for motor initiation. We also exported mean power values in the alpha (7.5–12.5 Hz)- and gamma (30–50 Hz)-bands in both time windows for statistical comparisons. However, according to our hypothesis we focused on theta and beta frequency bands.
To quantify time-frequency analyses, we set the size threshold for chance occurrence of the statistical cluster against 1000 permutations of group labels. We used linear regression to analyze the extent to which group differences between control, PDFOG−, and PDFOG+ account for variability in power for each tf-ROI, while controlling for MOCA. Linear regression was done in R 3.5.1, with the package “stats”. Pairwise comparisons were tested within each model using the ghlt function from the multcomp package in R. All regression results were tested with an alpha level of <0.05. Finally, we used Spearman correlation analyses to evaluate the relationships between power values during gait initiation (theta- and beta-bands; 0–400 ms) and FOG, UPDRS III, and MOCA scores of PD patients.
3. Results
3.1. Patient demographics
Table 1 shows the group comparisons for all motor, cognitive, and other clinical characteristics. No differences were found for age between PD and control groups. MOCA scores were less in PD patients compared to controls (p=0.01). PDFOG+ patients had higher FOG, higher UPDRS III, and lower cognitive (MOCA) scores than PDFOG− patients; subsequent analyses controlled for these differences.
3.2. Motor and Cognitive Assessment Scores
Previous reports have suggested that FOG can be related to motor and cognitive impairments (Kelly et al., 2012; Morris et al., 2016). Accordingly, in PD patients, we observed a positive correlation between FOG and motor function as measured by UPDRS III (Fig. 1C left side; p=0.001), and a negative correlation between FOG and cognition as measured by MOCA (Fig. 1C middle; p=0.02). We observed a positive correlation between FOG scores and disease duration (Fig. 1C right side; p=0.01), even when controlling for MOCA (partial correlation between FOG scores and disease duration: rho=0.42, p=0.03). Of note, there was no significant negative correlation between disease duration and MOCA scores (p=0.1), even when controlling for FOG (partial correlation: p=0.5). These data suggest that lower-limb movements tend to be worse with longer disease duration, while cognition is not related to duration (Morris et al., 2017).
3.3. Kinematic Results of Pedaling Task
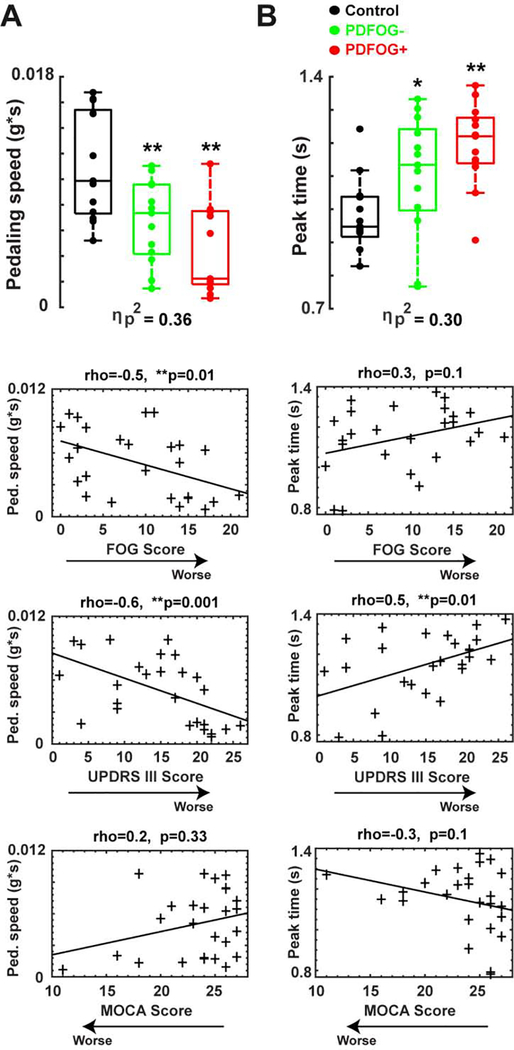
Similar to upper-limb movements (Singh et al., 2012a; Singh et al., 2012b), PD patients are impaired in lower-limb movements as measured by the speed of motor task initiation and execution. Our results indicated that PD patients executed the lower-limb pedaling motor task with significantly lower mean speed compared to control subjects (Fig. 2A; F(2,36)=9.9, p=0.001, ηp2=0.36; PDFOG+ vs. controls, p=0.001; PDFOG− vs. controls, p=0.01). Correlation results revealed relationships between pedaling speed and FOG scores (Fig. 2A; p=0.01) and UPDRS III scores (Fig. 2A; p=0.001), but not between pedaling speed and MOCA scores (Fig. 2A; p=0.33).
Fig. 2.
Pedaling kinematics. (A) Box plots show that mean pedaling linear speed was the lowest in PDFOG+, and significantly correlated with FOG scores, and UPDRS III scores, but not with MOCA scores. (B) Box plots also show that PDFOG+ patients took the most time to reach maximum acceleration (peak time) during the pedaling task, and a significant correlation was found between peak time and UPDRS III scores only, but not FOG scores and MOCA scores. *p<0.05 vs control subjects; **p<0.01 vs control subjects; **Significance correlation level <0.01. Effect size was symbolized by partial eta-squared (ηp2). rho = correlation coefficients; FOG: Freezing of Gait; UPDRS III: motor Unified Parkinson’s Disease Rating Scale; MOCA: Montreal Cognitive Assessment. PDFOG+: PD patients with FOG; PDFOG−: PD patients without FOG.
Additionally, we also computed each subjects’ time to achieve maximum acceleration and found that both groups of PD patients took longer to reach peak acceleration compared to control subjects (Fig. 2B; F(2,36)=7.8, p=0.002, ηp2=0.30; PDFOG+ vs. controls, p=0.001; PDFOG− vs. controls, p=0.04). Time to reach peak acceleration was significantly correlated with UPDRS III scores (Fig. 2B; p=0.01) but not FOG scores (Fig. 2B; p=0.1) or MOCA scores (Fig. 2B; p=0.1). These results indicate that PD patients with more severe motor symptoms exhibited greater slowing on the lower-limb motor task.
3.4. Pedaling Task-related Power Changes at Frontal Region
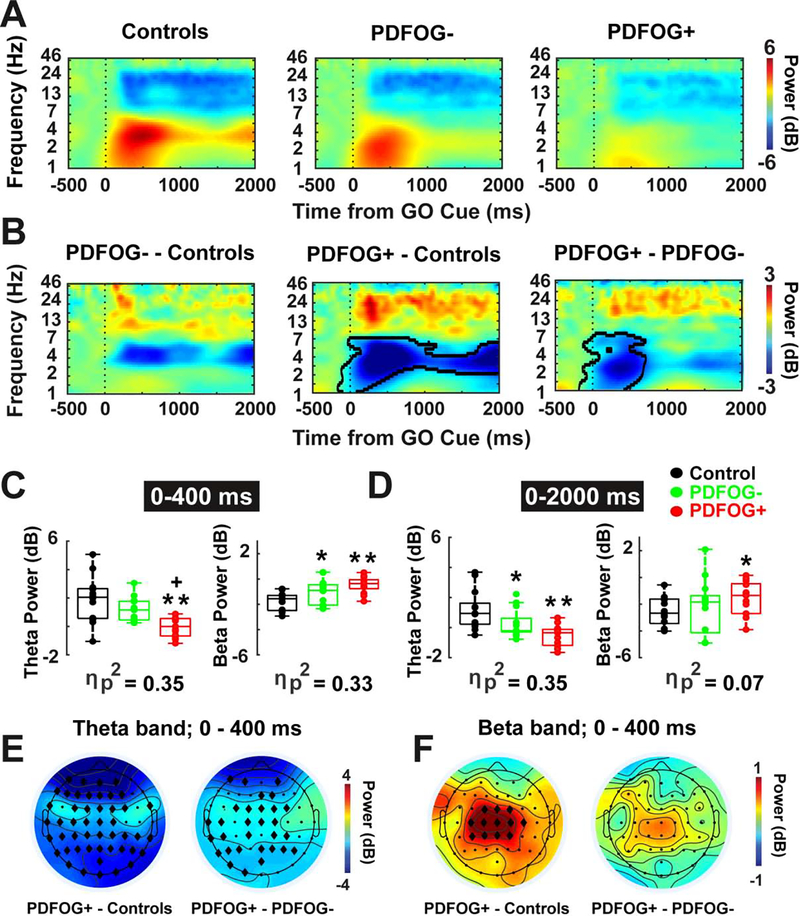
We tested the hypothesis that PDFOG+ patients had attenuated mid-frontal theta activity and increased beta activity during lower-limb movements. In support of this idea, the tf-ROI theta-band had attenuated power in PDFOG+ patients compared to both controls and PDFOG− at the time of pedaling initiation and during the execution of the pedaling task (Fig. 3A and B). Notably, as aforementioned, MOCA scores differed for PDFOG+ and PDFOG− patients. To control for these differences, we fit separate linear regression models for theta- and beta-bands power tf-ROIs, with each tf-ROI as the outcome variable and group (PDFOG+, PDFOG−, and control) and MOCA as explanatory variables. Group effects will be described with reference to the mean of control subjects, covarying for the continuous variables of MOCA.
Fig. 3.
FOG is associated with attenuated theta-band and amplified beta-band power in PD. (A-B) Time-frequency analysis showed reduced theta and amplified beta power at frontal scalp electrode (vertex or “Cz”) during pedaling task in PDFOG+ patients as compared to PDFOG− patients and control subjects. PDFOG− patients showed low theta power and high beta power compared to control subjects. (C) Box plots displayed the power values from two tf-ROIs (theta and beta power values at 0–400 ms and 0–2000 ms time windows) during pedaling task. (E-F) Topography plots (PDFOG+ patients versus controls and PDFOG+ patients versus PDFOG− patients) indicated reduced theta and increased beta activity at the frontal region in PDFOG+ patients. B: Permutation-corrected p<0.05 outlined in bold lines. *p<0.05 vs control subjects; **p<0.01 vs control subjects; +p<0.05 vs PDFOG−; Effect size was symbolized by partial eta-squared (ηp2); diamonds show significant electrodes in E and F. FOG: Freezing of Gait; PDFOG+: PD patients with FOG; PDFOG−: PD patients without FOG.
Regression analyses revealed a main effect of group on frontal theta-band power during the motor initiation epoch (0–400 ms; Fig. 3C left side; F(2,35)=9.59, p=0.0005; ηp2=0.35). Frontal theta-band power in PDFOG− was not significantly different from the control group (p=0.09). However, theta power in PDFOG+ differed significantly both from the control group (p=0.0006) and PDFOG− (p=0.02). Results also revealed a main effect of group on frontal theta power during the entire 2000 ms time window of pedaling epoch (0–2000 ms; Fig. 3D left side; F(2,35)=9.43 p=0.0005; ηp2=0.35). PDFOG+ had significantly lower theta power than controls (p=0.0006), as did PDFOG− (p = 0.02). PDFOG+ had numerically lower theta compared to PDFOG−, but it was not statistically significant (p = 0.07).
In support of our hypothesis, the tf-ROI beta-band had amplified power in PDFOG+ patients compared to controls and PDFOG− at the time of pedaling initiation and during the execution of the pedaling task (Fig. 3A and B). There was a main effect of group during the motor initiation epoch (0–400 ms; Fig. 3C right side; F(2,35)=8.81, p=0.0008; ηp2=0.33). Beta power was higher in PDFOG+ patients and PDFOG− patients compared to controls (PDFOG+ vs. controls, p=0.002 and PDFOG− vs. controls, p=0.03). No significant difference in the beta power was seen in PDFOG+ compared to PDFOG− (p=0.15). We repeated these analyses for the entire 2000 ms time window of pedaling and surprisingly, we did not observe a main effect of group in frontal beta-band power (0–2000ms; Fig. 3D right side; F(2,35)=1.39, p=0.26 ηp2=0.07). However, beta-band power was significantly higher in PDFOG+ compared to controls (p=0.04), but not in PDFOG− compared to controls (p=0.35) or PDFOG+ compared to PDFOG− (p=0.16). Scalp topography plots indicated the presence of attenuated theta-band and amplified beta-band oscillations in the frontal region during movement initiation in PDFOG+ compared to controls and PDFOG− (Fig. 3E and F).
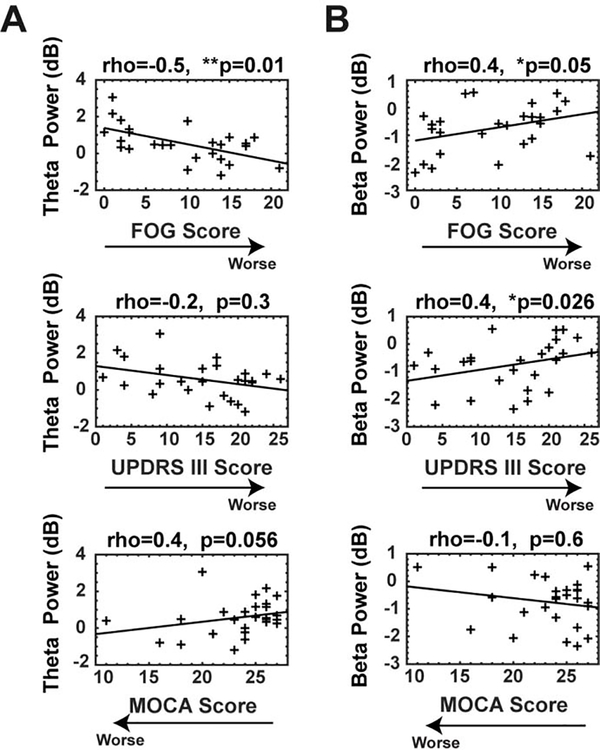
Further analysis confirmed a significant relationship between FOG scores and theta-band power values during motor initiation (0–400 ms; Fig. 4A top; p=0.01), and between FOG scores and beta-band power values during motor initiation in PD patients (0–400 ms; Fig. 4B top; p=0.05). Interestingly, MOCA scores trended to correlate with theta-band power values (0–400 ms; Fig. 4A bottom; p=0.056) and UPDRS III scores were correlated with beta-band power values in PD patients (0–400 ms; Fig. 4B middle; p=0.026). These results suggest that abnormal theta-band power may be related to cognitive control, and beta-band activity may be related to the motor control. We observed a no significant relationship between UPDRS III scores and theta-band power (0–400 ms; Fig. 4A middle; p=0.3) and between MOCA scores and beta-band power (0–400 ms; Fig. 4B bottom; p=0.6).
Fig. 4.
Correlation analysis between power values of theta and beta-band during motor initiation and motor and cognitive characteristics in PD. (A) A significant correlation was seen between theta power values and FOG scores, a trend was seen between theta power values and MOCA scores, but not between theta power and UPDRS III scores. (B) A significant correlation was observed between beta power values and FOG scores and UPDRS III scores, but not between beta power and MOCA scores. *Significance correlation level =<0.05; **Significance correlation level <0.01. rho = correlation coefficients; FOG: Freezing of Gait; UPDRS III: motor Unified Parkinson’s Disease Rating Scale; MOCA: Montreal Cognitive Assessment. All power within tf-ROIs of 0–400 ms.
We also analyzed the changes in alpha and gamma frequency bands power during pedaling task and computed the differences in power between groups. During the pedaling task at 0–400 ms, there was a main effect of group (control/PDFOG+/PDFOG−) on theta, beta and gamma power (Supplementary Material, Figure S1B). Gamma-band power was increased only for the PDFOG− group compared to control subjects for 0–400 ms (p=0.02). From 0–2000 ms, there was a main effect of group only for theta power.
4. Discussion
We explored the neural basis of FOG in PD patients. We hypothesized that attenuated theta- and enhanced beta-rhythms would underlie FOG in PD. Accordingly, we found evidence of decreased mid-frontal theta activity during motor initiation and pedaling and evidence of increased beta activity particularly during motor initiation. Linear regression suggested that this impairment was independent of differences in MOCA. These data provide new insight into the neural mechanisms of FOG in PD.
Mid-frontal theta-rhythms have been associated with cognitive control and with impairments in cognitive control in PD patients (Kelley et al., 2018; Singh et al., 2018). Our past work has suggested that frontal theta-rhythms are not modulated by levodopa (Singh et al., 2018). PDFOG+ patients had impaired cognitive control and attenuated frontal theta-rhythms relative to PDFOG− patients. PD patients may have impaired attention to gait when walking under dual-task conditions; indeed, PD patients have marked gait variability combining walking and cognitive tasks (Hausdorff et al., 2003). Neuroimaging studies suggest that FOG could be associated with dysfunction within frontoparietal regions of the cortex which subserve cognitive and executive functions (Rushworth et al., 2002; Wager et al., 2004). Further, intracortical recordings in cats demonstrated a critical role for frontal regions during gait, particularly during stepping movements (Criado et al., 1997). These studies imply frontal impairments affect motor control, and that differences in attentional and executive function networks result in impaired execution of lower-limb motor performance. Currently, targeted cognitive training has been utilized as a non-pharmacological intervention in PDFOG+ to reduce the severity of FOG and improve lower-limb movements.(Walton et al., 2018; Walton et al., 2014). It has been shown that reduced theta activity in PD patients correlates with deficits in sequence acquisition and stabilization of newly acquired movement patterns (Meissner et al., 2018) and mid-frontal theta rhythms are a mechanism of cognitive control that is impaired in PD (Cavanagh and Frank, 2014; Parker et al., 2015; Singh et al., 2018; Singh et al., 2019). Therefore, it seems that theta oscillations may contribute to sensorimotor integration needed to execute a task (Caplan et al., 2003).
Increased beta-rhythms are associated with decreased movement in PD (Singh et al., 2013; Toledo et al., 2014). Critically, levodopa attenuates beta-rhythms, and PDFOG+ patients had increased beta activity despite increased levodopa dose. These data argue that differences in beta-rhythms were not a result of differences in levodopa, although our patients were not tested during levodopa ‘OFF’ periods due to increased fall risks. During gait, frontal regions might interact with the basal ganglia nuclei via beta-band oscillations (Singh et al., 2011a; Singh et al., 2013; Toledo et al., 2014). Cortico-basal beta-band synchrony promotes tonic activity that slows down lower-limb movements implicating aberrant beta oscillations in lower-extremity abnormalities (Singh, 2018). Frontal beta rhythms may play an important role in top-down signaling to guide adjustment of preparatory and execution plans during motor tasks, and these processes may malfunction in PD (Miller and Cohen, 2001). Overall, the present data suggest an involvement of frontal theta and beta oscillations in initiation and execution of lower-extremity movements.
According to previous studies, the transition from normal walking to FOG was associated with increased theta- and beta-band power in the cortical (Shine et al., 2014) and the subcortical regions (Georgiades et al., 2019) in PDFOG+. In these studies, electrophysiological signals were either collected from cortical leads during continuous walking without any “Go Cue” or from basal ganglia region during continuous foot pedaling in virtual reality gait environment and all patients were “off” medications. Contrary to previous studies, in the current study pedaling was performed after seeing the “Go Cue” and during “on” medications. We did not investigate frontal theta and beta power during FOG episodes. Moreover, previous clinical studies have shown that FOG in PD can be associated with a paroxysmal increase in lower frequency oscillations (< 5–7 Hz), that is known clinically as ‘trembling in place’ (Gatev et al., 2006; Singh et al., 2011b). As such, the power increase in the theta frequency band during walking and freezing may be related to the mechanical oscillations transmitted into the scalp and subdural electrodes.
Similar to upper-limb movements, PD patients execute lower-limb movements with lower speed (Morris, 2000; Singh et al., 2012a; Singh et al., 2012b). Previous reports have confirmed that PDFOG+ walk with lower speed compared to PDFOG− or age-matched healthy subjects (Singh et al., 2013; Vercruysse et al., 2012b) and take more time to initiate movement (Delval et al., 2014a; Delval et al., 2014b; Rosin et al., 1997). Here, subjects initiated the lower-limb pedaling task after seeing a visual “GO” cue. Interestingly, visual and auditory cues may affect lower-limb movement, such that focusing attention on visual cues during the task might compensate for a proprioceptive processing deficit in PD (Donovan et al., 2011; Lee et al., 2012). Our results demonstrate that peak acceleration time was higher in PDFOG+, and speed to execute the task was lower, but it is also possible that patients would have shown even more impairment without the visual cue.
Overall, our data suggest an imperative role of motor and cognitive determinants in performing lower-limb movement (Amboni et al., 2013; Vercruysse et al., 2012a; Walton et al., 2018; Walton et al., 2014). Typically, lower-limb abnormalities can be diagnosed using FOG scores in advanced PD patients (Movement Disorder Society Task Force on Rating Scales for Parkinson’s, 2003). Table 1 indicates the significant differences in both motor and cognitive aspects between control subjects and PD patients, and between PDFOG+ and PDFOG−. We also found that MOCA scores were different between PDFOG+ and PDFOG− and correlated with FOG scores. MOCA is used to evaluate cognitive function, including many domains such as attention, visuospatial skills, orientation, executive functions, and memory which are often impaired in advanced PD patients (Nasreddine et al., 2005).
Potential limitations of our work include: (1) We did not study our patients “OFF” levodopa because we were interested in real-world function and “OFF” exams involve fall risks in PDFOG+ patients. Our prior work indicates that midfrontal theta abnormalities in PD do not depend on levodopa, and beta abnormalities depend on movement rather than levodopa. Since lower-limb movements are controlled by mesencephalic rhythm generator circuits controlling locomotion (Lau et al., 2015; Pozzi et al., 2019), these circuits can be cholinergic/non-dopaminergic, and are influenced by distinct top-down frontal cortical signals (Bonnet et al., 1987; Karachi et al., 2010); (2) We did not study patients during walking movements or freezing episodes. Of note, pedaling induces less movement-related artifacts, can be used in closed-loop neuromodulation experiments, and has less inherent fall risk; (3) Cortical dysfunction in PD can be complex as some PD patients may have enhanced frontal function (Cools et al., 2010). However, our analyses captured both increased and decreased patterns of frontal oscillatory activity; (4) Reduced brain activity in frontal areas is a basic abnormality in motor performance in PD (Hanakawa et al., 1999), but we did not disambiguate upper vs. lower motor movements; and (5) EEG signals recorded at the scalp are inherently limited measures of neuronal activity. We are exploring intracranial recordings, brain imaging, and experiments in animal models to further elucidate the cortical basis of theta and beta abnormalities in FOG. Basal ganglia and brain stem networks also play a key role in lower-limb movements such as gait (Singh, 2018; Singh et al., 2011a; Singh et al., 2013).
5. Conclusions
The current study suggests that synergistic interaction of motor and cognitive control systems contributes to the execution of a lower-limb motor task. Increased frontal cortical theta activity might be related to stabilization of cognitive control and decreased frontal cortical beta activity might be related to motor control as indicated by reduced susceptibility to interference to initiate and execute lower-limb movement. Our findings could link lower-limb dysfunction to a failure to recruit lower-limb motor-control processing at key moments. We also suggest that theta and beta oscillations can be used as a feature for closed-loop neuromodulation in the future to improve lower-limb function in PD and movement disorders.
Supplementary Material
Highlights.
Frontal theta and beta rhythms were associated with cognitive and motor functions.
Reduced theta and amplified beta power were observed during pedaling in Parkinson’s disease patients with freezing of gait (PDFOG+).
PDFOG+ pedaled with reduced speed compared to PDFOG− patients and controls.
Acknowledgements
The authors thank all participants in this study. AS, RCC, AIE, JFC and NN are supported by NINDS R01100849. All data and code for this study will be available on-line at narayanan.lab.uiowa.edu or PREDiCT.
Footnotes
Declaration of Competing Interest
None of the authors have any potential conflicts of interest to disclose.
Appendix A. Supplementary material
Supplementary data to this article can be found online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Asai Y, Matsuo Y, Nomura T, Sato S, Inoue S, et al. Classifying lower limb dynamics in Parkinson’s disease. Brain Res Bull 2003;61:219–26. [DOI] [PubMed] [Google Scholar]
- Ambani LM, Van Woert MH. Start hesitation--a side effect of long-term levodopa therapy. N Engl J Med 1973;288:1113–5. [DOI] [PubMed] [Google Scholar]
- Amboni M, Barone P, Hausdorff JM. Cognitive contributions to gait and falls: evidence and implications. Mov Disord 2013;28:1520–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amboni M, Cozzolino A, Longo K, Picillo M, Barone P. Freezing of gait and executive functions in patients with Parkinson’s disease. Mov Disord 2008;23:395–400. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord 2004;19:871–84. [DOI] [PubMed] [Google Scholar]
- Bonnet AM, Loria Y, Saint-Hilaire MH, Lhermitte F, Agid Y. Does long-term aggravation of Parkinson’s disease result from nondopaminergic lesions? Neurology 1987;37:1539–42. [DOI] [PubMed] [Google Scholar]
- Boonstra TA, van der Kooij H, Munneke M, Bloem BR. Gait disorders and balance disturbances in Parkinson’s disease: clinical update and pathophysiology. Curr Opin Neurol 2008;21:461–71. [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Blizzard L, Martin K, Srikanth VK. Gait initiation time is associated with the risk of multiple falls-A population-based study. Gait Posture 2016;49:19–24. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci 2003;23:4726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci 2014;18:414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R Paradoxical worsening of gait with levodopa in Parkinson disease. Neurology 2012;78:446–7. [DOI] [PubMed] [Google Scholar]
- Cools R, Miyakawa A, Sheridan M, D’Esposito M. Enhanced frontal function in Parkinson’s disease. Brain 2010;133:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JM, de la Fuente A, Heredia M, Riolobos AS, Yajeya J. Electrophysiological study of prefrontal neurones of cats during a motor task. Pflugers Arch 1997;434:91–6. [DOI] [PubMed] [Google Scholar]
- Delval A, Moreau C, Bleuse S, Tard C, Ryckewaert G, Devos D, et al. Auditory cueing of gait initiation in Parkinson’s disease patients with freezing of gait. Clin Neurophysiol 2014a;125:1675–81. [DOI] [PubMed] [Google Scholar]
- Delval A, Tard C, Defebvre L. Why we should study gait initiation in Parkinson’s disease. Neurophysiol Clin 2014b;44:69–76. [DOI] [PubMed] [Google Scholar]
- Donovan S, Lim C, Diaz N, Browner N, Rose P, Sudarsky LR, et al. Laserlight cues for gait freezing in Parkinson’s disease: an open-label study. Parkinsonism Relat Disord 2011;17:240–5. [DOI] [PubMed] [Google Scholar]
- Espay AJ, Fasano A, van Nuenen BF, Payne MM, Snijders AH, Bloem BR. “On” state freezing of gait in Parkinson disease: a paradoxical levodopa-induced complication. Neurology 2012;78:454–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatev P, Darbin O, Wichmann T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord 2006;21:1566–77. [DOI] [PubMed] [Google Scholar]
- Georgiades MJ, Shine JM, Gilat M, McMaster J, Owler B, Mahant N, et al. Hitting the brakes: pathological subthalamic nucleus activity in Parkinson’s disease gait freezing. Brain 2019;0:1–11. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. A comparison of clinical and pathological features of young- and old-onset Parkinson’s disease. Neurology 1988;38:1402–6. [DOI] [PubMed] [Google Scholar]
- Giladi N, Huber-Mahlin V, Herman T, Hausdorff JM. Freezing of gait in older adults with high level gait disorders: association with impaired executive function. J Neural Transm (Vienna) 2007;114:1349–53. [DOI] [PubMed] [Google Scholar]
- Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord 2000;6:165–70. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, et al. Mechanisms underlying gait disturbance in Parkinson’s disease: a single photon emission computed tomography study. Brain 1999;122:1271–82. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson’s disease. J Geriatr Psychiatry Neurol 2003;16:53–8. [DOI] [PubMed] [Google Scholar]
- Henriksson M, Hirschfeld H. Physically active older adults display alterations in gait initiation. Gait Posture 2005;21:289–96. [DOI] [PubMed] [Google Scholar]
- Karachi C, Grabli D, Bernard FA, Tande D, Wattiez N, Belaid H, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest 2010;120:2745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R, Flouty O, Emmons EB, Kim Y, Kingyon J, Wessel JR, et al. A human prefrontal-subthalamic circuit for cognitive control. Brain 2018;141:205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly VE, Eusterbrock AJ, Shumway-Cook A. A review of dual-task walking deficits in people with Parkinson’s disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis 2012;2012:918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killane I, Fearon C, Newman L, McDonnell C, Waechter SM, Sons K, et al. Dual Motor-Cognitive Virtual Reality Training Impacts Dual-Task Performance in Freezing of Gait. IEEE J Biomed Health Inform 2015;19:1855–61. [DOI] [PubMed] [Google Scholar]
- Lau B, Welter ML, Belaid H, Fernandez Vidal S, Bardinet E, Grabli D, et al. The integrative role of the pedunculopontine nucleus in human gait. Brain 2015;138:1284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Yoo JY, Ryu JS, Park HK, Chung SJ. The effects of visual and auditory cues on freezing of gait in patients with Parkinson disease. Am J Phys Med Rehabil 2012;91:2–11. [DOI] [PubMed] [Google Scholar]
- Martin K, Blizzard L, Garry M, Thomson R, McGinley J, Srikanth V. Gait initiation in older people--Time to first lateral movement may be the measure of choice. Gait Posture 2011;34:374–8. [DOI] [PubMed] [Google Scholar]
- Meissner SN, Krause V, Sudmeyer M, Hartmann CJ, Pollok B. The significance of brain oscillations in motor sequence learning: Insights from Parkinson’s disease. Neuroimage Clin 2018;20:448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- Morris ME. Movement disorders in people with Parkinson disease: a model for physical therapy. Phys Ther 2000;80:578–97. [PubMed] [Google Scholar]
- Morris R, Lord S, Bunce J, Burn D, Rochester L. Gait and cognition: Mapping the global and discrete relationships in ageing and neurodegenerative disease. Neurosci Biobehav Rev 2016;64:326–45. [DOI] [PubMed] [Google Scholar]
- Morris R, Lord S, Lawson RA, Coleman S, Galna B, Duncan GW, et al. Gait Rather Than Cognition Predicts Decline in Specific Cognitive Domains in Early Parkinson’s Disease. J Gerontol A Biol Sci Med Sci 2017;72:1656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s D. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003;18:738–50. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 2011;10:734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Chen KH, Kingyon JR, Cavanagh JF, Narayanan NS. Medial frontal approximately 4-Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. J Neurophysiol 2015;114:1310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Ann Neurol 1992;32:151–61. [DOI] [PubMed] [Google Scholar]
- Pozzi NG, Canessa A, Palmisano C, Brumberg J, Steigerwald F, Reich MM, et al. Freezing of gait in Parkinson’s disease reflects a sudden derangement of locomotor network dynamics. Brain 2019;142:2037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin R, Topka H, Dichgans J. Gait initiation in Parkinson’s disease. Mov Disord 1997;12:682–90. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 2002;87:2577–92. [DOI] [PubMed] [Google Scholar]
- Shine JM, Handojoseno AM, Nguyen TN, Tran Y, Naismith SL, Nguyen H, et al. Abnormal patterns of theta frequency oscillations during the temporal evolution of freezing of gait in Parkinson’s disease. Clin Neurophysiol 2014;125:569–76. [DOI] [PubMed] [Google Scholar]
- Singh A Oscillatory activity in the cortico-basal ganglia-thalamic neural circuits in Parkinson’s disease. Eur J Neurosci 2018;48:2869–78. [DOI] [PubMed] [Google Scholar]
- Singh A, Kammermeier S, Mehrkens JH, Botzel K. Movement kinematic after deep brain stimulation associated microlesions. J Neurol Neurosurg Psychiatry 2012a;83:1022–6. [DOI] [PubMed] [Google Scholar]
- Singh A, Kammermeier S, Plate A, Mehrkens JH, Ilmberger J, Botzel K. Pattern of local field potential activity in the globus pallidus internum of dystonic patients during walking on a treadmill. Exp Neurol 2011a;232:162–7. [DOI] [PubMed] [Google Scholar]
- Singh A, Levin J, Mehrkens JH, Botzel K. Alpha frequency modulation in the human basal ganglia is dependent on motor task. Eur J Neurosci 2011b;33:960–7. [DOI] [PubMed] [Google Scholar]
- Singh A, Mehrkens JH, Botzel K. Effect of micro lesions of the basal ganglia on ballistic movements in patients with deep brain stimulation. J Neurol Sci 2012b;314:175–7. [DOI] [PubMed] [Google Scholar]
- Singh A, Plate A, Kammermeier S, Mehrkens JH, Ilmberger J, Bötzel K. Freezing of gait-related oscillatory activity in the human subthalamic nucleus. Basal Ganglia 2013;3:25–32. [Google Scholar]
- Singh A, Richardson SP, Narayanan N, Cavanagh JF. Mid-frontal theta activity is diminished during cognitive control in Parkinson’s disease. Neuropsychologia 2018;117:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Trapp NT, De Corte B, Cao S, Kingyon J, Boes AD, et al. Cerebellar Theta Frequency Transcranial Pulsed Stimulation Increases Frontal Theta Oscillations in Patients with Schizophrenia. Cerebellum 2019;18:489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George RJ, Nutt JG, Burchiel KJ, Horak FB. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology 2010;75:1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Lopez-Azcarate J, Garcia-Garcia D, Guridi J, Valencia M, Artieda J, et al. High beta activity in the subthalamic nucleus and freezing of gait in Parkinson’s disease. Neurobiol Dis 2014;64:60–5. [DOI] [PubMed] [Google Scholar]
- Vercruysse S, Devos H, Munks L, Spildooren J, Vandenbossche J, Vandenberghe W, et al. Explaining freezing of gait in Parkinson’s disease: motor and cognitive determinants. Mov Disord 2012a;27:1644–51. [DOI] [PubMed] [Google Scholar]
- Vercruysse S, Gilat M, Shine JM, Heremans E, Lewis S, Nieuwboer A. Freezing beyond gait in Parkinson’s disease: a review of current neurobehavioral evidence. Neurosci Biobehav Rev 2014;43:213–27. [DOI] [PubMed] [Google Scholar]
- Vercruysse S, Spildooren J, Heremans E, Vandenbossche J, Levin O, Wenderoth N, et al. Freezing in Parkinson’s disease: a spatiotemporal motor disorder beyond gait. Mov Disord 2012b;27:254–63. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage 2004;22:1679–93. [DOI] [PubMed] [Google Scholar]
- Wagner J, Makeig S, Gola M, Neuper C, Muller-Putz G. Distinct beta Band Oscillatory Networks Subserving Motor and Cognitive Control during Gait Adaptation. J Neurosci 2016;36:2212–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton CC, Mowszowski L, Gilat M, Hall JM, O’Callaghan C, Muller AJ, et al. Cognitive training for freezing of gait in Parkinson’s disease: a randomized controlled trial. NPJ Parkinsons Dis 2018;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton CC, Shine JM, Mowszowski L, Naismith SL, Lewis SJ. Freezing of gait in Parkinson’s disease: current treatments and the potential role for cognitive training. Restor Neurol Neurosci 2014;32:411–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.