Abstract
We present a rare case of mesenteric venous infarction in a 36-year-old man due to coronavirus disease-19 (COVID-19). Although COVID-19 usually presents with respiratory disease, multi-system manifestations are increasingly reported. Coagulopathy manifestations are also reported on imaging, including in vascular thrombosis, embolus, and organ infarction. Because the clinical variables poorly predict or suspect coagulopathy and its complications, it is important to be aware of imaging manifestations of coagulopathy complications of COVID-19.
Keywords: Mesenteric venous infarction, COVID-19, CT imaging, Coagulopathy
Introduction
Since the emergence of novel coronavirus, SARS-CoV-2, in December 2019, approximately 143 million people have been infected worldwide with approximately 3.05 million deaths to date [1,2]. A portion of those who recover from the acute infection is left with lingering symptoms as well as significant health effects and morbidity. The most common symptoms persisting beyond active coronavirus disease (COVID-19) infection include fatigue, dyspnea, cough, chest pain, joint pain, and cognitive deficits. Medical complications are thought to arise from acute infection, hyperinflammatory state, inadequate immune response, and persistent viral activity. According to the Centers for Disease Control (CDC), these include dysfunction of organs and/or organ systems, such as respiratory failure, cardiovascular complications, thromboembolic disease, neurologic complications, and inflammatory disorders. Abdominal manifestations occur in approximately 3% to 40.7% of patients infected with SARS-CoV-2, including anorexia, nausea, vomiting, diarrhea, abdominal pain, belching, abdominal distension, and GI hemorrhage [3]. Intestinal manifestations of COVID-19 discovered on abdominopelvic diagnostic imaging include intestinal inflammation, bowel wall thickening, fluid-filled colon, pneumatosis intestinalis, pneumoperitoneum, bowel ischemia, and intussusception. We present a rare case of gastrointestinal symptoms and features consistent with mesenteric venous ischemia in the setting of SARS-CoV-2 infection.
Case presentation
A 36-year-old previously healthy male with recent COVID-19 infection presented to the emergency department with acute onset abdominal pain. The patient otherwise had a noncontributory medical history and family history, specifically with an absence of any known coagulation disorders. The patient continued to test positive for SARS-CoV-2 via polymerase chain reaction (PCR) at the time of presentation.
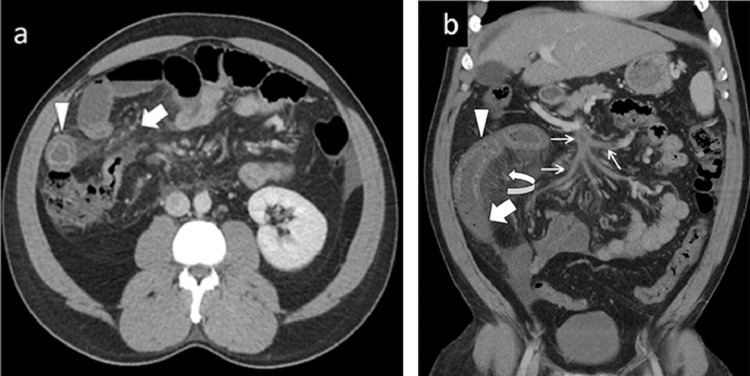
Contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis revealed mesenteric ischemia with thrombosis of the portal venous confluence, also involving the splenic vein, superior mesenteric vein, and main portal vein (Fig. 1). Due to the imaging findings suggestive of acute mesenteric venous ischemia and infarction, the patient was urgently brought to the operating room.
Fig. 1.
(A) Axial contrast-enhanced CT of the abdomen demonstrated circumferential bowel wall thickening with a “target sign” (arrowhead). Diffuse mesenteric fat stranding (bold arrow) due to edema, and mild ascites also seen. (B) Coronal CT abdomen and pelvis showing circumferential bowel wall thickening with differential enhancement of the bowel loops. Proximal bowel segment shows increased enhancement (arrowhead), whereas distal segment shows reduced enhancement (bold arrow) suggesting infarction. Diffuse thrombosis within the superior mesenteric vein and its branches (thin arrows) and congestion of mesenteric venous arcade (curved arrow).
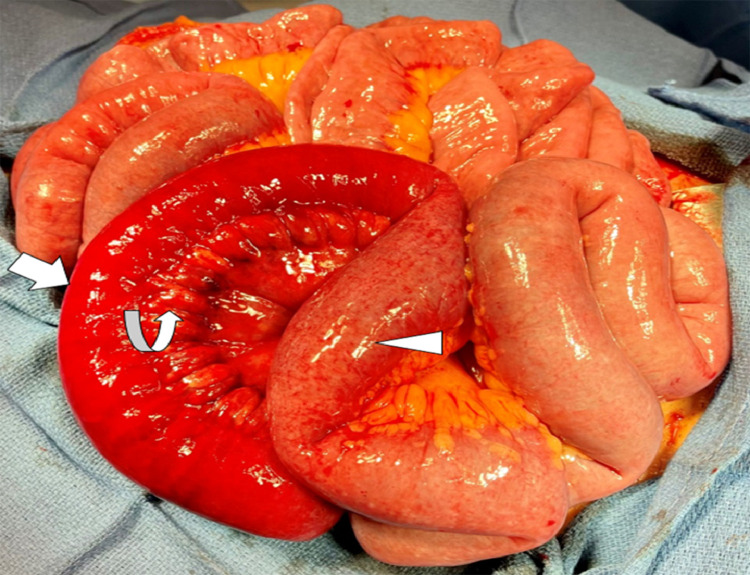
Intraoperative gross examination demonstrated a bowel loop with a distal segment of dusky, ischemic bowel and a proximal segment of hyperemic bowel secondary to mesenteric venous engorgement, consistent with CT findings of ileal loop ischemia (Fig. 2). Resection of the mid-to-distal ileum followed by small bowel anastomosis was performed due to these findings.
Fig. 2.
Intraoperative image shows dusky, ischemic, and non-viable bowel loop (bold arrow) suggesting infarction. Curved arrow points to the congested and engorged mesenteric veins. Proximal bowel loop with slight discoloration (arrow head) is in earlier stage of ischemia which showed hyper enhancement on CT.
The patient underwent intraoperative liver biopsy due to the proximal extent of the thrombus involving the portal venous confluence, which demonstrated microvesicular steatosis with a fibrosis score of 3 out of 4. The liver biopsy was negative for periodic acid-Schiff (PAS) stain and reticulin stain. There was a normal hepatocellular plate. The patient denied alcohol use. The following laboratory tests were performed due to the biopsy findings: alpha-1-antitrypsin, 206; ceruloplasmin, 29; DRVVT screen, 59; protein C antigen, 73; PTT lupus anticoagulant, 42.
The patient subsequently underwent several laboratory tests to aid in diagnosis. Testing for factor V Leiden mutation, prothrombin gene mutation, protein C deficiency, protein S deficiency, mitochondrial antibody screen, and anti-phospholipid antibody syndrome was performed and all resulted within normal limits. Given this evaluation did not reveal a pre-existing coagulation disorder, it was determined that the thrombosis was due to the hypercoagulable state of the patient's coronavirus disease. The patient is being followed by the surgery clinic and has been successfully recovering after the hospital discharge.
Discussion
COVID-19 is a novel infectious disease in the coronavirus family, most frequently causing substantial respiratory illness. Classic symptoms of the SARS-CoV-2 virus include fever, cough, and dyspnea. An increasing number of studies have demonstrated extrapulmonary manifestations of COVID-19 affecting the hematologic, cardiovascular, renal, gastrointestinal and hepatobiliary, endocrine, neurologic, ophthalmologic, and dermatologic systems.
A recent meta-analysis by Ye et al. reports that gastrointestinal manifestations occur in approximately 3% to 40.7% of patients, with the most common symptoms being diarrhea, anorexia, nausea, vomiting, abdominal pain, belching, abdominal distension, and gastrointestinal hemorrhage. Diarrhea was the most frequently reported gastrointestinal symptom, occurring in 2% to 38.1% of infected patients. Anorexia was present in 9.3% to 66.7% of infected patients. Patients experiencing nausea and vomiting were reported at 1% to 19.5%. Abdominal pain was reported in 1.2% to 19.1% of patients [3].
According to Goldberg-Stein et al. abdominal pain was the most common indication for patients who underwent abdominopelvic CT and it was the most common symptom associated with positive findings on CT exam. It was reported that 54% of cases of abdominal pain in any location and 40% of cases of unspecified abdominal pain were associated with positive findings on abdominopelvic CT. Approximately 57% of abdominopelvic CT exams performed on patients with COVID-19 had positive findings, most frequently abnormalities of the gastrointestinal tract (31%). Of gastrointestinal tract abnormalities, mural thickening was reported most often [4].
Imaging features frequently described on abdominal imaging of COVID-positive patients with GI symptoms are bowel wall thickening, fluid-filled dilated colon, pneumatosis, pneumoperitoneum, intussusception, vascular thrombosis, mesenteric ischemia, and ascites [5]. Evidence of these features highlights the need to consider and evaluate for other manifestations of COVID-19 such as lung parenchymal findings.
Although not completely understood, the mechanism of gastrointestinal manifestations is likely multifactorial and several mechanisms have been demonstrated. SARS-CoV-2 enters host cells via binding angiotensin-converting-enzyme-2 (ACE2) receptor and serine protease TMPRSS2, which are expressed primarily in the respiratory system, but also in the small bowel, esophagus, and colon, thus potentially the route of direct tissue damage [3]. There has been identification of viral nucleocapsid protein in gastric, duodenal, and rectal epithelial cells and glandular enterocytes as well as viral RNA in stool. Histopathologic examination has visualized endothelial inflammation in intestinal submucosal vessels along with mesenteric ischemia, suggesting vascular injury. Inflammatory tissue damage has also been proposed due to identification of plasma cells, lymphocytes, and interstitial edema within the stomach, duodenum, and rectum. Severe inflammation is thought to play a role in the hypercoagulable state seen with coronavirus disease [6].
Intestinal manifestations of COVID-19 include intestinal inflammation, bowel obstruction, ileus, bowel ischemia, and intussusception. Khader et al. present a case of colitis of the cecum and ascending colon in a previously healthy female infected with SARS-CoV-2 [7]. Similarly, Sattar et al. present two cases of colitis, one patient with involvement of the ascending, transverse, descending, and sigmoid colon, and the other patient with involvement of the proximal transverse colon. They also present a unique case of a colonic ileus with air in the bowel wall [8]. Intestinal inflammation is a common finding in patients infected with SARS-CoV-2 who report nonspecific abdominal pain.
Gastrointestinal symptoms are often the presenting symptoms in early multisystem inflammatory syndrome in children (MIS-C). A retrospective study performed by Sahn et al. identified inflammatory bowel changes in 85.7% of patients with MIS-C who underwent abdominopelvic CT or ultrasound. These changes most frequently reflected terminal ileitis. Mesenteric fat stranding and right lower quadrant mesenteric lymphadenopathy were present in 71% of patients involved in the study. One patient underwent ileocolic resection due to bowel obstruction. Histopathologic examination of the resected specimen showed significant signs of inflammation. Marked transmural lymphocytic inflammation and acute enteritis were visualized. An ileocolic mass discovered on gross examination demonstrated coalescent lymph nodes with necrotizing lymphadenitis. Additionally, there was inflammation of the mesentery, arteritis with lymphocyte and fibrin deposition, and venous microthrombi. These findings suggest direct inflammatory damage to the intestine [9].
We present a patient with mesenteric venous ischemia and infarction due to SARS-CoV-2, a rare gastrointestinal manifestation that has not been well described in the literature. O'Shea et al. evaluated the imaging manifestations of COVID-19-associated coagulopathy. Their study found that 26% of patients with COVID-19 who underwent imaging had imaging manifestations of coagulopathy. The most common findings were pulmonary embolism (PE) and arterial and venous thromboembolism; however, bowel ischemia and infarct were present in 11% of patients with coronavirus disease-associated coagulopathy. Three of these patients had multi-site involvement with PE, hepatic infarction, or cerebellar parenchymal infarction. Gastrointestinal thromboses involving the mesenteric vasculature were present in these cases [10]. Revzin et al. also present a case of bowel ischemia due to a large thrombus in the superior mesenteric artery [11]. Coagulation abnormalities due to SARS-CoV-2 are associated with worse outcomes [12]. While coagulopathy is a common finding in patients with coronavirus disease, mesenteric venous ischemia is an uncommon etiology for abdominal pain compared to other sources, such as intestinal inflammation.
In addition to superior mesenteric vein thrombosis, our patient was also found to have new-onset microvesicular steatosis with a fibrosis score of 3 out of 4 . A meta-analysis performed by Mao et al. determined that patients with severe COVID-19 were more likely to experience abdominal pain and have abnormal liver function. They calculated a pooled prevalence of gastrointestinal symptoms in 15% of infected patients and liver injury in 19% of infected patients. Commonly identified signs of liver injury were elevated ALT, elevated AST, elevated total bilirubin, and decreased albumin. They report that 10% of patients with COVID-19 may present with gastrointestinal symptoms only and thus may receive a delayed diagnosis [13]. Given that patients with gastrointestinal involvement have a higher tendency to develop severe disease, there should be a high level of suspicion for COVID-19 in patients presenting with digestive symptoms.
Conclusions
Mesenteric vein thrombosis is an uncommon etiology for bowel ischemia, and our patient had a rare case of mesenteric venous ischemia and infarction due to infection with SARS-CoV-2. Clinical variables poorly predict imaging manifestations of coagulopathy. Multisystem involvement is common both in regards to gastrointestinal inflammation and coagulopathy and thus thorough evaluation may be necessary.
Footnotes
Acknowledgment: We acknowledge our patient, for providing a great learning opportunity.
Competing Interest: The authors declare that they have no conflict of interest. Compliance with ethical standards.
Financial disclosure: Nothing to disclose
Patient Consent: The authors declare that informed consent was obtained from the patient in regards to using information for this case report.
Authors’ Contribution: EC: manuscript writing, OS: surgeon, PN: images contribution, RA: manuscript edit, JS: images interpretation.
References
- 1.COVID-19. (2021). Retrieved from centers for disease control and prevention: Available at: https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- 2.WHO Coronavirus (COVID-19) Dashboard. (2021). Retrieved from World Health Organization: Available at: https://covid19.who.int.
- 3.Ye L, Yang Z, Liu J, Liao L, Wang F. Digestive system manifestations and clinical significance of coronavirus disease 2019: a systematic literature review. J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15323. doi: [DOI] [PubMed] [Google Scholar]
- 4.Goldberg-Stein S., Fink A., Paroder V., Kobi M., Yee J., Chernyak V. Abdominopelvic CT findings in patients with novel coronavirus disease 2019 (COVID-19) Abdominal Radiology. 2020;45(9):2613–2623. doi: 10.1007/s00261-020-02669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lui K, Wilson MP, Low G. Abdominal imaging findings in patients with SARS-CoV-2 infection: a scoping review. Abdom Radiol (NY) 2021;46(3):1249–1255. doi: 10.1007/s00261-020-02739-5. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S. Extrapulmonary manifestations of COVID-19. Nature medicine. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 7.Khader M., Al Bishawi A., Kambal A., Abdelmajid A. SARS-CoV-2 infection presenting as colitis with chest and abdomen CT findings. Radiology Case Reports. 2020;15(11):2427–2432. doi: 10.1016/j.radcr.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sattar Y., Connerney M., Rauf H., Saini M., Ullah W., Mamtani S. Three cases of COVID-19 disease with colonic manifestations. The American Journal of Gastroenterology. 2020 doi: 10.14309/ajg.0000000000000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahn B., Eze O.P., Edelman M.C., Chougar C.E., Thomas R.M., Schleien C.L. Features of intestinal disease associated with COVID-related multisystem inflammatory syndrome in children. Journal of pediatric gastroenterology and nutrition. 2021;72(3):384–387. doi: 10.1097/MPG.0000000000002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'shea A., Parakh A., Hedgire S., Lee S.I. Multisystem assessment of the imaging manifestations of coagulopathy in hospitalized patients with coronavirus disease (COVID-19) American Journal of Roentgenology. 2021;216(4):1088–1098. doi: 10.2214/AJR.20.24132. [DOI] [PubMed] [Google Scholar]
- 11.Revzin M.V., Raza S., Warshawsky R., D'agostino C., Srivastava N.C., Bader A.S. Multisystem imaging manifestations of covid-19, part 1: viral pathogenesis and pulmonary and vascular system complications. Radiographics. 2020;40(6):1574–1599. doi: 10.1148/rg.2020200149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M. Hematological findings and complications of COVID-19. American journal of hematology. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao R., Qiu Y., He J.S., Tan J.Y., Li X.H., Liang J. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. The lancet Gastroenterology & hepatology. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]