Abstract
Teprotumumab (Tepezza), an insulin-like growth factor type 1 receptor antagonist, was approved for treatment of thyroid eye disease in 2020. Teprotumumab is administered intravenously every 3 weeks for a total of eight doses. Common side effects include nausea, diarrhoea, muscle spasms, hearing impairment, dysgeusia, headaches, dry skin, infusion reactions and hyperglycaemia. We report here a 76-year-old man with Graves-related thyroid eye disease who developed a rapidly progressive cognitive decline after receiving four out of eight doses of teprotumumab (cumulative dose 4620 mg). He was admitted for workup and teprotumumab infusions were discontinued. Intravenous glucocorticoids and immunoglobulin were given which showed no improvement in clinical symptoms. He subsequently underwent plasmapheresis with resolution of his symptoms, suggesting a teprotumumab-induced encephalopathy. Further studies involving larger populations and longer durations are needed.
Keywords: neurology (drugs and medicines), endocrinology, thyroid disease, ophthalmology
Background
Teprotumumab (Tepezza), a fully human insulin-like growth factor type 1 receptor (IGF-1R)—inhibitory monoclonal antibody (mAb), was approved for treatment of thyroid eye disease (TED) in 2020. The effect of blocking IGF-1R activation and signalling pathways of teprotumumab is the first treatment shown to be beneficial in reducing proptosis and diplopia. Teprotumumab is administered intravenously every 3 weeks for a total of eight doses (10 mg/kg initial dose followed by 20 mg/kg for the next seven doses). Common side effects include nausea, diarrhoea, muscle spasms, hearing impairment, dysgeusia, headaches, dry skin, infusion reactions, alopecia, paresthesia, weight loss and hyperglycaemia. Other reported serious effects may include optic neuropathy, Hashimoto’s encephalopathy (HE), Escherichia infection, urinary retention and inflammatory bowel disease.1
Case presentation
A 76-year-old man was diagnosed with Graves' disease in 2018. He was euthyroid on methimazole 2.5 mg every other day. His TED manifestations included diplopia, proptosis and exposure keratopathy. His medical history was significant for hypertension, hyperlipidaemia and benign prostate hyperplasia. There was no family history of neurodegenerative processes. Before initiation of treatment, he lived independently, walking several miles a day and captaining his own fishing boat. Teprotumumab therapy was initiated for TED and he showed significant improvement of diplopia and proptosis after three infusions. Following the fourth infusion, his daughter noted 6 weeks of rapidly progressive cognitive decline characterised by behavioural changes, confabulation, memory deficit and delirium/delusions/mania. He became unable to carry out tasks that he had performed his entire life, especially tasks requiring motor planning; he was unaware of these deficiencies. These episodes initially presented as short, isolated incidents. However, over a 6-week period of time, they become more persistent and protracted. He was admitted to the hospital for evaluation and teprotumumab treatment was discontinued. On physical examination, vital signs were notable for a blood pressure of 143/74 mm Hg and heart rate of 95 beats/min. He was otherwise afebrile with normal oxygen saturation. He was awake and alert to person and place, but not to date, situation or current president. He was agitated with a flat affect and monophonic, hypophonic speech. He was unable to perform serial sevens or accurately draw a clock. He was not able to provide an accurate medical history. He had fluent spontaneous speech with intact reading, simple repetition, comprehension and naming without semantic or paraphasic errors; however, he had significant difficulty with complex comprehension and prolonged sentence repetition. Cranial nerves II–XII were grossly intact with normal deep tendon reflexes. Examination of heart, lungs, abdomen and extremities was otherwise unremarkable. A brain MRI and magnetic resonance angiograph (MRA) (figure 1) showed cerebral amyloid angiopathy without intracranial arterial stenosis or aneurysms, cortical atrophy or nonspecific T2 signal abnormality in the subcortical white matter. Intravenous glucocorticoids and immunoglobulin were given which showed no improvement in clinical symptoms. The patient subsequently underwent five sessions of plasmapheresis with complete resolution of his symptoms.
Figure 1.
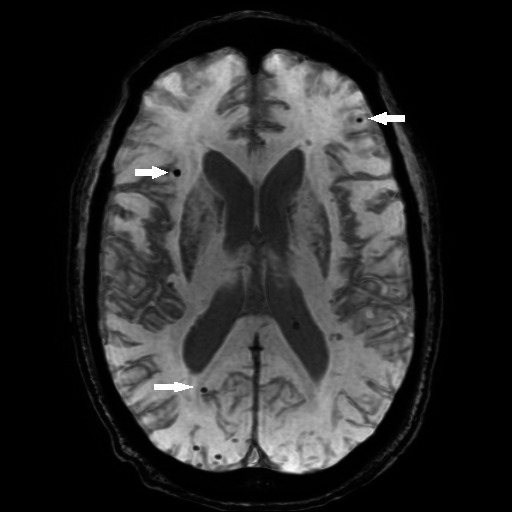
MRI/magnetic resonance angiograph showing amyloid angiopathy.
Investigations
The patient underwent an extensive testing. His cerebrospinal fluid analysis was normal except for an elevated protein of 76 mg/dL (ref 15–45). An electroencephalogram showed no epileptiform activity. A brain MRI/MRA showed cerebral amyloid angiopathy without stenosis or aneurysms in the intracranial arterial vasculature or subcortical lesions (figure 1).
Thyroid function tests were normal before, during and after discontinuation of teprotumumab. However, thyroid antibody levels noticeably changed during treatment (table 1).
Table 1.
Thyroid antibodies levels and thyroid function tests
| Tests | Before teprotumumab therapy | After teprotumumab therapy | After plasmapheresis |
| TSI (0.00–0.55 IU/L) | 1.04 | 1.06 | 0.35 |
| TgAb (0–0.9 IU/mL) | <1.0 | 18.4 | <1.0 |
| TPO (0–34 IU/mL) | <6 | 14 | <9 |
| TRAb (<1.0 U/L) | 1.6 | 1.2 | <1.10 |
| TSH (0.27–4.2 µIU/mL) | 0.311 | 1.64 | 1.01 |
| FT4 (0.93–1.7 ng/dL) | 1.52 | 1.61 | 1.41 |
| Total T3 (80–200 ng/dL) | 104.7 | 93.7 | 86.1 |
FT4, free thyroxine; T3, triiodothyronine; TgAb, thyroglobulin antibody; TPO, thyroperoxidase antibody; TRAb, thyrotropin receptor antibody; TSH, thyrotropin; TSI, thyroid stimulating immunoglobulin.
An evaluation for autoimmune encephalopathy (table 2) and human leucocyte antigen (HLA) genetic testing (box 1) were also obtained.
Table 2.
Serum autoimmune encephalopathy panel (performed by Mayo Clinic Laboratories)
| Result name | Result | Reference value |
| Glutamic acid decarboxylase 65 antibody | 0.28 nmol/L | ≤0.02 |
| Acetylcholine receptor Ganglionic Neuronal Ab | 0.00 nmol/L | ≤0.02 |
| AMPA-R Ab CBA | Negative | Negative |
| Amphiphysin Ab | Negative | <1:240 |
| AGNA-1 | Negative | <1:240 |
| ANNA-1 | Negative | <1:240 |
| ANNA-2 | Negative | <1:240 |
| ANNA-3 | Negative | <1:240 |
| CASPR2-IgG CBA | Negative | Negative |
| CRMP-5-IgG | Negative | <1:240 |
| DPPX Ab IFA | Negative | Negative |
| GABA-B-R Ab CBA | Negative | Negative |
| GFAP IFA | Negative | Negative |
| IgLON5 IFA | Negative | Negative |
| LGI1-IgG CBA | Negative | Negative |
| mGluR1 Ab IFA | Negative | Negative |
| NIF IFA | Negative | Negative |
| NMDA-R Ab CBA | Negative | Negative |
| N-type calcium channel Ab | 0.00 nmol/L | ≤0.03 |
| P/Q-type calcium channel Ab | 0.00 nmol/L | ≤0.02 |
| Purkinje cell cytoplasmic Ab type 1 | Negative | <1:240 |
| Purkinje cell cytoplasmic Ab type 2 | Negative | <1:240 |
| Purkinje cell cytoplasmic Ab type Tr | Negative | <1:240 |
GAD65 Ab is consistent with predisposition to thyrogastric disorders, including thyroiditis, pernicious anaemia and type 1 diabetes, but has low specificity for autoimmune encephalopathy. GAD65 antibody values less than 2.00 nM have a lower positive predictive value for neurological autoimmunity than values of 20.0 nM and higher.
Ab, antibody; AGNA, antiglial nuclear antibody; AMPA-R Ab CBA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antibody cell-binding assay; ANNA, anti-neuronal nuclear antibody; CASPR2-IgG CBA, contactin-associated protein 2-immunoglobulin G cell-binding assay; CRMP-5-IgG, collapsin response-mediator protein-5- Immunoglobulin G; DPPX Ab IFA, dipeptidyl peptidase-like protein 6 antibody indirect fluorescent antibody; GABA-B-R Ab CBA, gamma-aminobutyric acid-B-receptor Antibody cell-binding assay; GFAP IFA, glial fibrillary acidic protein indirect fluorescent antibody; IgLON5 IFA, immunoglobulin-like cell adhesion molecule 5 Indirect fluorescent antibody; LGI1-IgG CBA, leucine rich glioma Inactivated 1- immunoglobulin G cell-binding assay; mGluR1 Ab IFA, metabotropic glutamate receptor 1 antibody indirect fluorescent antibody; NIF IFA, neuronal intermediate filament indirect fluorescent antibody; NMDA-R Ab CBA, N-methyl-D-aspartate receptor antibody cell-binding assay.
Box 1. Human leucocyte antigen (HLA) tests, performed by LabCorp Burlington DNA, Burlington, North Carolina.
HLA haplotypes
HLA-A*01:01:01G
HLA-A*68:03:01G
HLA-B*08:01:01G
HLA-B*40:02:01G
HLA-C*07:01:01G
HLA-C*-
DRB1*03:01:01G
DRB1*11:01:01G
DRB3*01:01:02G
DRB3*02:02:01G
DQB1*02:01:01G
DQB1*03:01:01G
Other labs, including interleukin-2, interleukin-6, erythrocyte sedimentation rate, C reactive protein, heavy metals, immunoglobulin G (IgG), immunoglobulin M, SARS-CoV-2, vitamins B1, B6 and B12, were all within normal limits.
Differential diagnosis
The patient presented with rapid cognitive decline and encephalopathic symptoms of unclear aetiology. Nevertheless, the presence of high protein level in the cerebrospinal fluid, as well as rapid improvement of symptoms with plasmapheresis, indicated a possible immunologic cause supporting the diagnosis of autoimmune encephalitis syndrome.2 Although this disorder is more commonly associated with antibodies to neuronal cell surfaces/synaptic proteins and paraneoplastic syndromes, it has been reported to occur in the absence of cancer. Our patient had no known underlying malignancy. Moreover, the increase in the levels of thyroid antibodies after teprotumumab treatment suggested the possibility of autoimmune activation, raising the possibility of autoimmune encephalitis associated with teprotumumab.
In our patient, the differential diagnoses included conditions associated with disorders of delirium and progressive dementia. Most of the common causes were excluded based on clinical features, laboratory testing and neurological imaging. Our patient had Graves’ disease and normal baseline serum thyroperoxidase antibody (TPO)/thyroglobulin antibody (TgAb) antibodies; and HE3 was also considered in the differential diagnosis. HE is often characterised by a subacute onset of confusion with altered levels of consciousness, seizures and myoclonus. Although the exact aetiology of this disorder is uncertain, it is postulated that an autoimmune vasculitis, or other inflammatory process associated with immune complex deposition in the cerebral microvasculature, may be involved. These patients often have an HLA-DRB1*0301 haplotype association. Cerebrospinal fluid analysis can reveal elevated protein concentration and, in a small percentage of patients, lymphocytic pleocytosis. MRI in patients with HE is usually normal, but can demonstrate cerebral atrophy or non-specific T2 signal abnormalities in the subcortical white matter. Most patients with HE respond to glucocorticoid therapy or immunosuppressive medications. Clinical improvement with intravenous immunoglobulin (IV Ig) and plasmapheresis has been reported in the literature. Smith et al analysed the side effects of teprotumumab in 42 patients and reported one case of HE who exhibited episodic confusion with no other neurological symptoms.1
In our patient, normal TPO/TgAb levels and MRI/MRA findings excluded HE. Additionally, the poor response to glucocorticoids and immunoglobulin did not support the diagnosis.3 Finally, the complete resolution of symptoms with plasmapheresis further supports teprotumumab as a likely cause of these neurological changes.
Treatment
The patient’s symptoms initially improved with intravenous glucocorticoids, but his neurological state progressed to catatonia, mutism and persistent memory deficit. He was subsequently treated with IV Ig which was discontinued on the discovery of segmental pulmonary embolism necessitating an Inferior Vena Cava (IVC) filter placement. Since it is well known that pulmonary embolism may occur as a side effect of IV Ig administration,4 additional workup for the cause of pulmonary embolism was not performed. Following the IVC filter placement, the course of IV Ig was completed without improvement of neurologic symptoms. The treatment was then changed to plasmapheresis which was performed every other day with resolution of symptoms.
Therapeutic plasmapheresis is currently used to treat neurological disorders such as Guillain-Barre syndrome, myasthenia gravis, chronic inflammatory demyelinating polyneuropathy and autoimmune encephalitis.2 5 It is possible that plasmapheresis was effective in removing teprotumumab-induced antibodies to neuronal surfaces and synaptic proteins, resulting in neurological improvement. It would have been interesting to analyse and characterise the substances removed by apheresis; however, this was not done in our patient.
Outcome and follow-up
After five sessions of plasmapheresis, the patient’s symptoms completely resolved. He continues to function well after plasmapheresis. He is currently euthyroid without any neurological sequelae at a recent 6-month follow up. His TED response to teprotumumab was sustained.
Discussion
Recent studies have demonstrated that IGF-1R and thyroid stimulating hormone receptor (TSHR) form a signalling complex leading to cellular signalling responses such as the induction of interleukin-6, interleukin-8, interleukin-10, interleukin-16, CD-40 ligand and the regulated on activation of normal T expressed and secreted chemokine, which plays significant roles in inflammatory and autoimmune responses and may explain T-cell infiltration in TED. The activation of the IGF-1R and TSHR pathways leads to increased glycosaminoglycan and hyaluronan synthesis, activation of inflammatory responses and differentiation of CD34 and fibroblasts into myofibroblasts and adipocytes, all of which can increase orbital fat and volume and result in proptosis and optic nerve compression in TED.1 Several other studies have also supported an association between IGF-1R and TSHR signalling in the pathogenesis of TED.1 6 7 These observations support IGF-1R as a target for the treatment of TED via anti-IGF-1R antibodies such as teprotumumab. This monoclonal IgG antibody binds with high affinity to the cysteine-rich region of the alpha subunit domain of IGF-1R, displacing IGF-1 or IGF-2 and leading to internalisation and degradation of the receptor–antibody complex. Studies have also confirmed that teprotumumab has several effects on fibrocytes such as reducing cell surface expression of both IGF-1R and TSHR, attenuation of TSH-dependent interleukin-6 and interleukin-8 expression, and TSH-induced TNF-alpha production.7
Based on the strength of two successful randomised clinical trials,1 8 teprotumumab was approved by the Food and Drug Administration for use in patients with active TED. Since its approval, case reports have revealed potential effectiveness of teprotumumab in clinically stable TED and in compressive optic neuropathy.9
It has been previously shown that IGF-1/IGF-1R signalling pathway is involved in immune and inflammatory responses.10 11 The loss of IGF-1/IGF-1R signalling in the brain has been related with an increased risk of cognitive decline, Alzheimer’s disease, premature dementia, depression and anxiety.12–15 Furthermore, in animal models with overexpressed or genetically ablated IGF system, developmental anomalies and functional disturbances are observed. It is uncertain whether teprotumumab selectively affects the IGF-1R on fibrocytes of orbital tissues or if it also impacts IGF-1/IGF-1R signalling in the brain. Neurological side effects reported with teprotumumab include optic neuropathy and HE.1 However, autoimmune-mediated encephalitis syndrome has not been previously reported. This case supports the possibility of teprotumumab-induced encephalitis syndrome due to the rapid onset of symptoms after initiation of treatment in a previously neurologically-normal individual. All other causes of encephalitis were ruled out by detailed investigations. In addition, the fact that our patient responded quickly to plasmapheresis provides more supporting evidence of this aetiology. It would have been interesting to investigate the substances removed by plasmapheresis; however, this was not available. Following plasmapheresis, our patient had complete recovery of cognitive function.
Several neurological disorders may occur with the administration of mAbs, especially those targeting tumour necrosis factor (TNF) and its receptors. These disorders include optic neuritis, multiple sclerosis and different forms of peripheral demyelinating neuropathy. The prevalence of demyelinating diseases induced by biological therapies, as reported in previous studies, has been estimated 0.02%–0.20%. Peripheral neuropathies can occur early or late after initiation of mAbs therapy. Short-term follow-up indicated relatively good outcomes, sometimes after mAbs discontinuation alone, although glucocorticoids or IV Ig may be required to treat the condition. The biological therapy can occasionally be discontinued in selected cases. Prospective controlled studies have confirmed the real risks in patients receiving mAbs who develop neurological disease.16 17 This further supports the possibility of teprotumumab-induced encephalitis in our patient. Rechallenging the patient with teprotumumab could have definitively confirmed the diagnosis, although we felt this was unethical in this case.
HLA genes are well-known for susceptibility to autoimmune diseases. Our patient tested positive for HLA-DRB1*0301, which has the highest positive predictive value for Graves' disease.18 19 Moreover, DRB3*0101/*0202 heterozygosity has been reported to increase the risk of TED.18 Although certain HLA subtypes, such as HLA-DR7 or HLA-DRB4, have been reported to be associated with autoimmune encephalitis, our patient did not have these HLA subtypes.20 There are also studies investigating the interaction between IGF-1 and HLA expression. IGF-1 is shown to induce HLA-DR antigen expression in cultured human thyrocytes in vitro and negatively regulates the expression of major histocompatibility complex class I genes as well.21 Given the fact that teprotumumab acts on IGF-1 pathways, it would be a plausible hypothesis that teprotumumab treatment may increase susceptibility to autoimmune encephalitis.
Learning points.
Teprotumumab (Tepezza), a fully human insulin-like growth factor type 1 receptor—inhibitory monoclonal antibody, was recently approved for treatment of thyroid eye disease. It is the first treatment shown to be beneficial in reducing proptosis and diplopia.
Common side effects of teprotumumab include nausea, diarrhoea, muscle spasms, hearing impairment, dysgeusia, headache, dry skin, infusion reactions and hyperglycaemia.
Although the exact mechanisms for rapidly progressive cognitive decline remain unclear, clinicians should be alerted to these unexpected adverse effects that may occur during teprotumumab therapy.
This case highlights that continued evaluation of a newly approved drug may reveal new side effects which were not observed in clinical trials.
Further studies with larger populations and longer durations may be needed.
Acknowledgments
The authors have no multiplicity of interest to disclose.
Footnotes
Twitter: @tdhdthanh
Contributors: TDH: author. NTN: coauthor. EC: reviewer. MKMS: reviewer.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
The manuscript has been approved by the ethical and IRB.
References
- 1.Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med 2017;376:1748–61. 10.1056/NEJMoa1614949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nissen MS, Ryding M, Meyer M, et al. Autoimmune encephalitis: current knowledge on subtypes, disease mechanisms and treatment. CNS Neurol Disord Drug Targets 2020;19:584–98. 10.2174/1871527319666200708133103 [DOI] [PubMed] [Google Scholar]
- 3.Zvonarev V, Tregubenko P. Hashimoto encephalopathy: advanced review of clinical and scientific aspects. J Neurol Neurobiol 2020;6:1–11. [Google Scholar]
- 4.Alliot C, Rapin JP, Besson M, et al. Pulmonary embolism after intravenous immunoglobulin. J R Soc Med 2001;94:187–8. 10.1177/014107680109400412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rössling R, Prüss H. Apheresis in autoimmune encephalitis and autoimmune dementia. J Clin Med 2020;9:2683. 10.3390/jcm9092683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, Iyer S, Bauer H, et al. A stimulatory thyrotropin receptor antibody enhances hyaluronic acid synthesis in Graves' orbital fibroblasts: inhibition by an IGF-I receptor blocking antibody. J Clin Endocrinol Metab 2012;97:1681–7. 10.1210/jc.2011-2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Mester T, Raychaudhuri N, et al. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab 2014;99:E1635–40. 10.1210/jc.2014-1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med 2020;382:341–52. 10.1056/NEJMoa1910434 [DOI] [PubMed] [Google Scholar]
- 9.Slentz DH, Smith TJ, Kim DS, et al. Teprotumumab for optic neuropathy in thyroid eye disease. JAMA Ophthalmol 2021;139:244–7. 10.1001/jamaophthalmol.2020.5296 [DOI] [PubMed] [Google Scholar]
- 10.Bilbao D, Luciani L, Johannesson B, et al. Insulin-Like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease. EMBO Mol Med 2014;6:1423–35. 10.15252/emmm.201303376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suh H-S, Zhao M-L, Derico L, et al. Insulin-Like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. J Neuroinflammation 2013;10:37. 10.1186/1742-2094-10-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao X-L, Jiang S-Z, Xiong J-W, et al. The association between serum insulin-like growth factor 1 and cognitive impairments in patients with schizophrenia. Psychiatry Res 2020;285:112731. 10.1016/j.psychres.2019.112731 [DOI] [PubMed] [Google Scholar]
- 13.Moloney AM, Griffin RJ, Timmons S, et al. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging 2010;31:224–43. 10.1016/j.neurobiolaging.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 14.Yuan L-J, Zhang M, Chen S, et al. Anti-inflammatory effect of IGF-1 is mediated by IGF-1R cross talk with GPER in MPTP/MPP+-induced astrocyte activation. Mol Cell Endocrinol 2021;519:111053. 10.1016/j.mce.2020.111053 [DOI] [PubMed] [Google Scholar]
- 15.Talbot K, Wang H-Y, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012;122:1316–38. 10.1172/JCI59903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosch X, Saiz A, Ramos-Casals M, et al. Monoclonal antibody therapy-associated neurological disorders. Nat Rev Neurol 2011;7:165–72. 10.1038/nrneurol.2011.1 [DOI] [PubMed] [Google Scholar]
- 17.Gill C, Rouse S, Jacobson RD. Neurological complications of therapeutic monoclonal antibodies: trends from oncology to rheumatology. Curr Neurol Neurosci Rep 2017;17:75. 10.1007/s11910-017-0785-3 [DOI] [PubMed] [Google Scholar]
- 18.Lombardi A, Menconi F, Greenberg D, et al. Dissecting the genetic susceptibility to Graves' disease in a cohort of patients of Italian origin. Front Endocrinol 2016;7:21. 10.3389/fendo.2016.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boehm BO, Kühnl P, Manfras BJ, et al. HLA-DRB3 gene alleles in Caucasian patients with Graves' disease. Clin Investig 1992;70:956–60. 10.1007/BF00180447 [DOI] [PubMed] [Google Scholar]
- 20.Jobst BC. A genetic disposition for autoimmune encephalitis: searching for human leukocyte antigen (HLA) complex subtypes. Epilepsy Curr 2017;17:273–4. 10.5698/1535-7597.17.5.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguayo J, Wohlik N, Pérez MV, et al. [Effect of insulin-like growth factor I on HLA-DR antigen expression in cultured human thyrocytes: a component of the autoimmune process?]. Rev Med Chil 1994;122:248–52. [PubMed] [Google Scholar]


