Abstract
Introduction
The aim of this study was to evaluate the efficacy of a serious exergame in improving the neuropsychiatric symptoms of patients with neurocognitive disorders.
Methods
X‐Torp is a serious exergame combining motor and cognitive activities. Ninety‐one subjects (mean age = 81.7 years, mean Mini‐Mental State Examination = 18.3) were recruited in 16 centers. Centers were randomized into intervention and control centers. Subjects underwent assessment for cognitive and behavioral symptoms at baseline (BL), the end of the intervention (W12), and 12 weeks after the end of the intervention (W24).
Results
The comparison of neuropsychiatric symptoms between BL and W12 and W24 showed that subjects of the intervention group improved in apathy between BL and W12. Mixed analysis (time BL, W12, W24 x group) indicated a significant increase in apathy and neuropsychiatric symptoms in the control subjects.
Discussion
The use of X‐Torp improved neuropsychiatric symptoms, particularly apathy. Future studies should more consistently use behavioral and neuropsychiatric symptoms as outcome measures.
Keywords: Alzheimer's disease, apathy, cognitive disorders, exergame, neuropsychiatry, technology
1. BACKGROUND
Being active and exercising help people with Alzheimer's disease (AD) feel better. A literature review 1 indicated that physical exercise improves functionality and performance of daily living activities; neuropsychiatric symptoms; cardiovascular and cardiorespiratory fitness; and cognitive components such as sustained attention, visual memory, and frontal cognitive function in patients with mild to severe AD, even in those individuals with genetically driven autosomal dominant AD. Physical activities are also recommended for cerebral vascular disease.
Physical activities can be practiced individually or in a group. Many programs have thus been developed for people with cognitive impairment. 2 , 3 Today, information and communication technologies (ICTs) are progressively expanding in the health field. Recently, increasing attention has been devoted to the field of neurocognitive disorders (NCD), in which ICTs are used to both support and improve the assessment of behavior and cognition, 4 , 5 as well for therapeutic purposes. 6 ICTs may also be helpful in the fields of motor activities 7 and gait and balance. 8 Specifically, this might be done using exergames, defined as the combination of physical exercise with an interactive video game in which the game commands can be provided by body movements captured by sensors. A systematic review 9 addressed the effects of exergame training on cognitive functions and cognitive states in healthy older adults. Overall, exergaming was shown to yield very inconsistent benefits only on specific cognitive functions (mainly executive functions) and appeared to be approximately as beneficial as other forms of physical exercise. In people with dementia, a systematic literature review 10 indicated that exergaming may well be a feasible intervention. However, only a few controlled studies with very small samples have investigated its effectiveness, showing some positive effects on physical, cognitive, and emotional functioning. Most of the studies thus far have focused on cognitive symptoms, whereas neuropsychiatric symptoms have been only marginally considered. This is unfortunate, given the impact of this symptomatology on the daily lives of patients and caregivers.
In 2015, the exergame X‐Torp was developed specifically for subjects with cognitive disorders. The results of the first pilot study 11 suggested that X‐Torp is a usable exergaming solution to stimulate positive emotions in patients with mild or moderate cognitive impairment.
The aim of the present cluster randomized trial was to evaluate the efficacy of X‐Torp for improving neuropsychiatric symptoms in patients with cognitive disorders in comparison with the usual care.
RESEARCH CONTEXT
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations. While there is convincing evidence on the efficacy of physical exercise on cognition and physical health in people with neurocognitive disorders—via classical stimulation or serious games—the role of exergames (combining physical and cognitive stimulation) on neuropsychiatric disorder (NCD) symptoms is largely unexplored.
Interpretation: Our findings support the hypothesis that exergames can help prevent the aggravation of behavioral symptoms, such as apathy, in older adults with NCD.
Future directions: The article suggests the importance of including assessment of neuropsychiatric symptoms in clinical trials evaluating the efficacy of interventions using serious games in people with NCD. While studies may fail to find improvements in cognition, maintaining low apathy over time may be a good target for non‐pharmacological interventions.
2. METHODS
2.1. X‐Torp application
X‐Torp is an exergame from the Az@GAME project and winner of the Call for Projects in e‐Health, ranking No. 1 for Investments for the Future: “Health and autonomy in the living space.” X‐Torp stimulates cognitive capacities, promotes physical activity, and helps maintain social ties (see: http://www.innovation‐alzheimer.fr/azgame‐en/).
The game is based on the principles of endurance and stimulation to encourage players to practice regular physical activity. The scenario mode combines action game dynamics (naval battle mode) with exploration of open environments. To stimulate cognitive abilities (cognitive training mode), the game has a scenario with many objectives to achieve, involving playful mini‐games and orientation exercises (see: http://www.innovation‐alzheimer.fr/azgame‐eco‐2/).The players control a submarine in real time with their stationary movements, involving mainly the lower limbs (e.g., walking and running in place to move the submarine forward, using the arms to turn or shoot). Hence, several actions involving the lower and upper limbs can be combined. When the players make a movement to give a command, the submarine performs the action as long as they keep doing the movement. When they stop, the submarine stops the action. During the exploration of the environment, players can tackle islands where they receive missions in the form of 2D mini‐games and puzzles inspired by the classic neuropsychological tests used in clinical practice. During the mini‐games, they use only their hands, and a virtual hand follows their movements. The players select an icon by positioning the virtual hand over it and holding the hand position for about one second. Hence, they are considered physically active when they navigate or battle on the sea and physically inactive when they play mini‐games in missions on the islands.
The game is distributed via the e‐health solution Curapy (https://www.curapy.com/jeux/x‐torp/). The therapists create individual accounts for each patient. The account opens a free service in each center, giving access to games and patient follow‐up. Therapists can also access the e‐health program to modify/adjust the game difficulty based on the results. The equipment to run X‐Torp was provided to each center before the beginning of the study. A team engineer (AD) went to each center to install the equipment and explain its use. An online manual was also available (https://youtu.be/WFyX3tSQOr0). The caregivers of each center could also call the engineer in the event of a technical problem.
X‐Torp was controlled by a desktop PC (Dell Precision M4600, Intel Core i7 2°ø2.2 GHz processor, 3Gbytes of RAM, AMD Fire Pro M5950 graphic card) and displayed on a high‐resolution wide screen (68 cm°ø121 cm). Subjects interacted with the exergame using a RGB‐D (Red Green Blue+Depth) KinectTM (V.2, Microsoft, USA) and customized software (Software Development Kit, Microsoft, USA).
2.2. Population and study design
To have a representative sample of the different types of care centers in France, subjects were recruited in memory centers, daycare centers, and nursing homes. All provided informed written consent before starting the study. The study was performed in compliance with the Declaration of Helsinki and was approved by the ethics committee of CCP Sud Méditérranée, number 2017‐A00132‐51. Individuals were included if they were older than 60 years, had a Mini‐Mental State Examination (MMSE) score between 12 and 24/30, and met the diagnostic criteria for mild or major NCD based on the Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5, 2013). Subjects were not included if they had major hearing or visual impairments, had a history of premorbid intellectual disability, or had already used X‐Torp.
Randomization between the intervention group using X‐Torp and the control group (standard care given in each center) was done by center (cluster randomized trial) rather than by subject to control for possible cross‐contamination of treatment or diffusion effects. There were eight daycare centers (four control centers, four intervention centers); six nursing homes (three control centers, three intervention centers); and six memory centers (two control centers, four intervention centers). Among them, three centers (one memory center, one daycare center, one nursing home) did not include any subjects. The selection and screening of the patients corresponding to the inclusion criteria were performed by physicians and psychologists at each center. Inclusion was effective only after the study psychologist had determined that each recruited subject met the study criteria. Patients underwent a cognitive and behavioral assessment battery including the MMSE, 12 the Neuropsychiatric Inventory (NPI), 13 and the Apathy Inventory, clinician's version (AI), 14 for quantitative apathy assessment. Assessments were performed by the study neuropsychologist at baseline (BL), Week 12 (end of the training period, W12), and Week 24 (12 weeks after the end of the training sessions, W24). The MMSE was performed only at BL and W24. During the trial, the medical staff of the center had no access to the game results.
Each patient in the intervention group participated in two X‐Torp sessions per week, over a period of 12 weeks, for a total of 24 sessions. A session consisted of 15 minutes of gameplay. The two weekly sessions were interspersed with 2 days without playing the serious game to ensure regular frequency of use throughout the intervention period. The intervention group centers were free to organize individual or group sessions (up to five participants), depending on their organizational constraints and the characteristics of the patients. Three daycare centers and one memory center organized group sessions. In the case of group sessions, the centers had to make sure that each patient had an effective 15‐minute session. To better familiarize the patients with X‐Torp and encourage their engagement, the first 6 weeks (first 12 sessions) consisted of 15 minutes using X‐Torp in the naval battle mode, including exploration of the environment while performing physical exercise. The next 6 weeks (last 12 sessions) consisted of 10 minutes in naval battle mode plus 5 minutes in cognitive training mode using the cognition‐stimulating mini‐games. Each subject started with the easiest level for each game. Game difficulty could remain fixed or be gradually increased according to the therapist's judgment to keep the game challenging while avoiding feelings of incompetence.
2.3. Statistical analysis
Data are presented as mean (standard deviation [SD]) for quantitative variables and as frequency and percentage for qualitative variables (sex, education level, diagnosis).
At BL, comparisons between the groups (control vs. intervention group/type of center) were performed using the Student's t‐test or Wilcoxon‐Mann‐Whitney test for quantitative variables and χ2 for qualitative variables.
To analyze the progression of the cognitive and behavioral scores through the three time points (BL, W12, W24), we used linear mixed models, controlling for the effects of patient age at study onset, sex, years of education, group, time, diagnosis, and type of center. The mixed model was tested using successively the MMSE score (score/30), the NPI score (score/134), and the AI score (score/2) as the dependent variable. As the study was a cluster randomization trial, the type of center was considered a random factor. Variables with a P‐value < .10 in univariate analysis were included in the multivariate model. The interaction between group x time was also tested. Variables with a P‐value < .05 were considered significant. The 95% confidence intervals are also reported.
Next, we performed more detailed analyses focused on the subjects in the intervention group to explore the effects of the type of center on game performance and adherence. For the subjects in the intervention group, we had data available for each game session, including the total duration (in minutes), the number of steps, and the number of cognitive games played. In addition, a ratio between the cognitive game count/step count × 10,000 was also calculated. Game data were compared among the types of center using a Kruskal‐Wallis test. To test the link between progression of the NPI total score from BL to W12 and the game data, Spearman correlations were used.
The analyses were performed using R software 3.3.0 and the SAS Enterprise Guide 7.1.
3. RESULTS
3.1. Demographic and clinical characteristics
The study included 125 subjects. Among them, 34 performed the cognitive and behavioral assessment only at BL and were not included in the analysis. Thus, the final sample consisted of 91 subjects. Thirty‐five subjects (39%) were included in daycare centers, 30 (33%) in memory centers, and 26 (29%) in nursing homes. The demographic and clinical characteristics of the subjects are presented in Table 1. No significant differences in terms of demographic and clinical characteristics were found between the control group and the intervention group, except for sex (more male subjects in the intervention group). Table 2 presents the characteristics of the subjects according to the type of center. Patients from memory centers were younger and had higher MMSE scores, whereas patients from nursing homes were older and had lower MMSE scores.
TABLE 1.
Demographic and clinical characteristics of the subjects at baseline (BL)
| Overall‐n = 91 | Control group‐n = 54 | Intervention group‐n = 37 | |||||
|---|---|---|---|---|---|---|---|
| Mean | [SD] | Mean | [SD] | Mean | [SD] | P* | |
| Age | 81.7 | [7.9] | 81.4 | [8.9] | 82.1 | [6.3] | .884 |
| Education years | 8.4 | [3.8] | 8.4 | [3.6] | 8.6 | [4.2] | .795 |
| MMSE | 18.3 | [3.6] | 18.1 | [3.4] | 18.7 | [3.8] | .498 |
| NPI | 15.9 | [11.3] | 16.4 | [11.4] | 14.8 | [11.2] | .604 |
| AI | 3.8 | [3.3] | 4.0 | [3.2] | 3.6 | [3.6] | .583 |
| N | (%) | N | (%) | N | (%) | P** | |
|---|---|---|---|---|---|---|---|
| Group | |||||||
| Control | 54 | (59.3) | |||||
| Intervention | 37 | (40.7) | |||||
| Sex | .004 | ||||||
| Female | 60 | (65.9) | 42 | (77.8) | 18 | (48.6) | |
| Male | 31 | (34.1) | 12 | (22.2) | 19 | (51.4) | |
| Diagnosis: severity level | 1.000 | ||||||
| Mild NCD (MMSE ≥21) | 25 | (27.8) | 15 | (27.8) | 10 | (27.8) | |
| Major NCD (MMSE <21) | 65 | (72.2) | 39 | (72.2) | 26 | (72.2) | |
| Missing data | 1 | 0 | 1 |
Abbreviations: AI, Apathy Inventory; MMSE, Mini‐Mental State Examination; NCD, neurocognitive disorder; NPI, Neuropsychiatric Inventory; SD, standard deviation.
TABLE 2.
Comparisons at baseline between types of center
| Day care center–n = 35 | Memory center–n = 30 | Nursing home–n = 26 | |||||
|---|---|---|---|---|---|---|---|
| Mean | [SD] | Mean | [SD] | Mean | [SD] | P | |
| Age | 82.5 | [8.0] | 78.5 | [6.6] | 84.3 | [8.2] | .003 |
| Education years | 7.9 | [4.3] | 9.3 | [3.7] | 8.2 | [3.0] | .445 |
| MMSE | 18.0 | [3.7] | 19.6 | [3.5] | 17.2 | [3.0] | .039 |
| NPI | 15.4 | [8.4] | 17.6 | [12.7] | 14.5 | [12.2] | .545 |
| AI | 3.0 | [3.3] | 4.8 | [3.5] | 3.6 | [3.0] | .204 |
| N | (%) | n | (%) | N | (%) | P | |
|---|---|---|---|---|---|---|---|
| Group | .029 | ||||||
| Control | 17 | (48.6) | 16 | (53.3) | 21 | (80.8) | |
| Intervention | 18 | (51.4) | 14 | (46.7) | 5 | (19.2) | |
| Sex | .418 | ||||||
| Female | 25 | (71.4) | 17 | (56.7) | 18 | (69.2) | |
| Male | 10 | (28.6) | 13 | (43.3) | 8 | (30.8) | |
| Diagnostic | .175 | ||||||
| Mild NCD (MMSE ≥21) | 10 | (28.6) | 11 | (37.9) | 4 | (15.4) | |
| Major NCD (MMSE <21) | 25 | (71.4) | 18 | (62.1) | 22 | (84.6) | |
| Missing data | 0 | 1 | 0 |
Abbreviations: AI, Apathy Inventory; MMSE, Mini‐Mental State Examination; NCD, neurocognitive disorder; NPI, Neuropsychiatric Inventory; SD, standard deviation.
3.2. Changes in the cognitive and behavioral scores in the intervention and control groups
The comparison of MMSE scores between BL and W24 showed no significant differences in the control group (BL mean: 18.1, SD 3.4 vs. W24 mean: 18, SD 4.2) or the intervention group (BL mean: 18.7, SD 3.8 vs. W24 mean: 19, SD 4.6), suggesting that the global level of cognitive functioning was stable in the two groups. Figure 1 shows the score change between BL, W12, and W24 for the NPI and AI scales.
FIGURE 1.
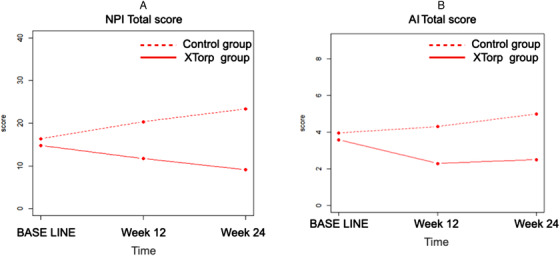
Evolution of Neuropsychiatric Inventory (NPI; A) and Apathy Inventory (AI; B) total scores between baseline, week 12 and week 24
Multivariate analyses are shown in Table 3. The MMSE scores were collected only at BL and W24. Subjects from memory centers had higher scores than nursing home subjects (AdjCoeff = 2.6, confidence interval [CI] 95% = [0.4; 4.9], P = .024). No significant effect of time, group, or group × time interaction was found.
TABLE 3.
Multivariate analyses for NPI score and AI score
| MMSE | NPI total score | AI total score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Adj Coeff | [CI 95%] | P | Adj Coeff | [CI 95%] | P | Adj Coeff | [CI 95%] | P | |
| Sex | |||||||||
| Female | −1.59 | [−3.20; 0.05] | .057 | – | – | – | −2.47 | [−3.96; −0.97] | .014 |
| Male | Ref | – | – | – | ref | ||||
| Type of center | |||||||||
| Day care center | 1.27 | [−0.94; 3.48] | .256 | – | – | – | – | – | – |
| Memory center | 2.63 | [0.35; 4.92] | .024 | – | – | – | – | – | – |
| Nursing home | Ref | – | – | – | – | – | – | ||
| Time | |||||||||
| BL | Ref | Ref | ref | ||||||
| W12 | – | – | – | 3.39 | [−0.19; 6.97] | .063 | 0.36 | [−0.46; 1.18] | .388 |
| W24 | −0.10 | [−0.96; 1.76] | .748 | 6.79 | [2.92; 10.66] | .001 | 0.95 | [0.07; 1.84] | .035 |
| Group | |||||||||
| Control | Ref | Ref | ref | ||||||
| Intervention | −0.19 | [−2.15; 1.76] | .847 | −1.81 | [−8.80; 5.18] | .610 | −1.27 | [−3.01; 0.48] | .153 |
| Interaction time × Group | .577 | .008 | .032 | ||||||
Abbrevitions: AI, Apathy Inventory; BL, baseline; CI, confidence interval; MMSE, Mini‐Mental State Examination; NCD, neurocognitive disorder; NPI, Neuropsychiatric Inventory; W12, week 12; W24, week 2.
For the NPI total score, there was a significant effect of time (with a significant change observed between BL and W24, P = .001) and a significant group × time interaction (P = .008). Specifically, subjects from the control group showed no modification in the NPI score between BL and W12 (P = .063) but showed an increase from BL to W24 (AdjCoeff = 6.8, CI 95% = [2.9; 10.7], P = .001). In the intervention group, there was no change at W12 and W24 compared to BL (respectively: P = .309 and P = .150; see Figure 1A).
For the AI score, there was a significant effect of time (with a significant change observed between BL and W24, P = .035) and a significant group × time interaction (P = .032). Specifically, in the control group, there was no difference between BL and W12 (P = .388), but there was an increase at W24 compared to BL (AdjCoeff = 1.0, CI 95% = [0.1; 1.8], P = .035). In the intervention group, there was a decrease at W12 compared to BL (AdjCoeff = –1.3, CI 95% = [–2.6; 0.0], P = .044) but no difference between BL and W24 (P = .162; see Figure 1B). Moreover, women had lower AI scores compared to men (AdjCoeff = –2.5, CI 95% = [–4.0; –1.0], P = .01).
3.3. X‐Torp game data
Among the 37 patients from the intervention group, one person had missing game information data due to a download dysfunction. Patients played for an average of 19 sessions (SD = 6.4) for a mean total game duration of 318.1 minutes (SD = 118.8). They played on average 31.6 cognitive games (SD = 21.7; see Table 4).
TABLE 4.
Study of game performance for the intervention group
| Overall—N = 36 | Daycare center n = 17 | Memory center n = 14 | Nursing Home n = 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | [SD] | Mean | [SD] | Mean | [SD] | Mean | [SD] | P* | |
| Sessions total time | 318.1 | [118.8] | 303.7 | [99.6] | 358.5 | [136.0] | 253.6 | [109.0] | .045 |
| Steps count | 16687.7 | [6920.4] | 16258.6 | [6296.2] | 18905.9 | [7399.6] | 11935.4 | [6025.3] | .072 |
| Cognitive game count | 31.6 | [21.7] | 28.4 | [17.3] | 41.6 | [25.4] | 14.4 | [8.8] | .052 |
| Ratio cognitive game count/steps count × 10,000 | 17.7 | [9.9] | 17.1 | [10.1] | 20.9 | [9.8] | 10.5 | [6.3] | .096 |
Notes: Session total time: in minutes.
Cognitive Game count: mean number of mini games played.
The total game time of the sessions was significantly different among daycare centers, memory centers, and nursing homes (respectively: mean = 303.7, SD = 99.6; mean = 358.5, SD = 136.0; mean = 253.6, SD = 109.0; P = .045). There was a difference in the number of cognitive games played (daycare center: mean = 28.4, SD = 17.3; memory center: mean = 41.6, SD = 25.4; nursing home: mean = 14.4, SD = 8.8; P = .052).
The higher the number of cognitive games played, the greater the decrease in the NPI total score from BL to W12 (Spearman rho = –0.52; P = .028) and the higher the number of steps the subjects took (Spearman rho = 0.61, P < .001). Using the cognitive game count/step count × 10,000 ratio, the higher the ratio, the greater the decrease in the NPI total score from BL to W12 (Spearman rho = –0.75; P < .001), suggesting that the combination of physical and cognitive activities had a significant effect in decreasing neuropsychiatric symptoms. No significant correlation with the AI was found.
4. DISCUSSION
The objective of this study was to evaluate the efficacy of X‐Torp—a serious exergame to train physical and cognitive activity—on neuropsychiatric symptoms and global cognitive functioning in patients suffering from cognitive disorders, in comparison to the usual management in various clinical settings. Results indicated that, while the neuropsychiatric symptoms in the control group worsened over time, the neuropsychiatric symptoms and most particularly apathy decreased in the X‐Torp group during the training period (W12) and remained stable after the end of the training (W24). Furthermore, a significant correlation between the time spent using X‐Torp and the improvement in the NPI total score was found for the X‐Torp group. It is interesting to note that the difference observed for the NPI score between the X‐Torp group and the control group at the end of the intervention (W12 = 9.6; BL score = 15.9, difference at BL = 1.6, non‐significant), was bigger than that observed between pharmacological treatments used in the standard care for this population—such as cholinesterase inhibitor donepezil (3.5) and the standard care (NPI BL score ranging between 15 and 23). 15
Several studies have indicated the positive effect of physical exercise on neuropsychiatric symptoms as assessed by the NPI 16 or depression scales. 17 , 18 , 19 The use of exergames may offer an entertaining and relatively safe way of exercising by providing an enriched environment combining cognitive and physical stimulation in a positive emotional context. Most of the published studies on exergaming have focused on cognitive outcomes in healthy older adults 9 or those with mild cognitive disorders and dementia. 10 , 20 Only a few studies have investigated emotional functioning. 21 Recently, Padala et al. 22 showed that exergames may overcome some of the barriers to exercise and result in good exercise adherence by acting on apathy. The diagnostic criteria define apathy as a quantitative reduction in goal‐directed activity in comparison with the patient's previous level of functioning. 23 Apathy and depression show some overlap in terms of prevalence and brain circuits, but they can be differentiated. Thus, apathy should be measured as an independent outcome variable. In this study, the active X‐Torp subjects were significantly less apathetic after 12 weeks compared to the control subjects. This is in line with the findings of the preliminary study performed using X‐Torp, suggesting an increase in motivation. 11 The effect on apathy was also observed in cognitive training using serious games. 6 This motivational impact must be considered in relation to the results obtained in the context of non‐pharmacological interventions. Undertaking a structured occupational therapeutic intervention improves apathy in patients living with dementia and is much more beneficial than the patients’ free use of the same amount of time in a non‐structured environment. 24 “Tailor‐made” activities have also been recommended, depending on the individual's interests, needs, abilities, and capacities 25 , 26 and in line with their perceived self and identity. 27 In this way, ICT tools may play an important role in non‐pharmacological treatment for apathy, as they facilitate the development of customized activities for each patient. 28
Even though the results here are promising, the study has several limitations. First, the number of subjects was relatively small, and their profiles were quite heterogeneous (outpatients consulting memory centers, daycare centers, and nursing homes). We wanted to test the feasibility and efficacy of using X‐Torp in different settings and with different patient profiles to assess the limits of its usability. Further studies should be performed on a bigger sample to confirm these promising results.
Second, as the study was performed in different contexts and with patients having different cognitive and motor profiles, the subjects did not benefit from the same amount of physical and cognitive stimulation. In total, the protocol indicated that each patient who used X‐Torp had to benefit from 6 hours of stimulation. Results indicated that, overall, the total session time was closer to 5 hours and even lower for nursing home patients. Interestingly, the number of cognitive games and the amount of physical activity performed correlated with improvements in the NPI score, suggesting that there is a quantitative relationship between amount of time spent playing and reduction in neuropsychiatric symptoms.
Third, as the study was performed in a clinical environment, it was difficult to have a full record of the pharmacological treatment changes between the beginning and end of the study. Yet it would interesting to determine whether a non‐pharmacological treatment such as an exergame is able to help reduce the use of medications targeting neuropsychiatric symptoms and whether the effects observed in the study were due to changes in pharmacological treatments, even if unlikely, over the study period.
5. CONCLUSIONS
In conclusion, this study showed that an exergame like X‐Torp is a promising solution to help slow down the aggravation of neuropsychiatric symptoms in people with NCD. While slowing down cognitive decline in these patients is important, neuropsychiatric symptoms often have a bigger impact on the patients’ and caregivers’ quality of life than the cognitive symptoms. In NCD, apathy has been shown to be associated with a decrease in quality of life and impaired activities of daily living, 29 , 30 , 31 as well as an increase in caregiver distress or burden. 32 Apathy has also been associated with faster cognitive and functional decline in such neurodegenerative disorders as, for instance, Parkinson's disease, 33 Huntington's disease 34 and AD. 35 For these reasons, finding non‐pharmacological solutions to reduce apathy, or at least slow down its aggravation, may be relevant, even if no big effects on cognitive and physical health are found. Future studies using serious games or exergames should consider systematically adding neuropsychiatric symptoms as outcome measures to assess the efficacy of different forms of non‐pharmacological interventions on a larger spectrum of symptoms affecting quality of life.
Although promising, using exergames for older adults with NCD has some limitations. In recent papers, we performed strengths, weaknesses, opportunities, threats (SWOT) analyses and provided recommendations for the use of serious games and exergames in these populations. 7 , 36 The difficulties reported in previous studies included the higher fatigability of people with cognitive disorders in exergames compared to healthy older adults, 11 and, for several subjects, low motivation to play serious games when not accompanied by a family or professional caregiver. 37 In addition, the SWOT analyses pointed out that using ICT may be challenging for people who are not familiar with them, for several reasons. These include difficulties in acquiring, installing, maintaining, and using the game interfaces and the need to have a therapist or caregiver present. Similarly, subjects may not be interested in using videogames and thus refuse to use them. As for every intervention, exergames should meet individual preferences and thus may not be adapted to all older people. Finally, it is possible to observe side effects such as fatigue, headache due to screen exposure, or risk of falls, which again point to the importance of adapting the game to each patient's physical and cognitive profile and to monitor the training.
CONFLICTS OF INTEREST
Pierre Foulon is employed by GENIOUS Healthcare, which developed the game.
ACKNOWLEDGMENTS
This work was supported by a grant from the French Ministry of Health—PRME 2016. Additional support came from the Association IA and the JL Noisiez Foundation. Thanks to all patients who participated in this study. Thanks also to the participating care centers—nursing homes: Ancilla, les Amaryllis, les Broussailes, Valrose, and Pauliani; daycare centers: Antibes, Cantazur, Fondation GL Noisiez, France Alzheimer 06, L'olivier, Simone Riff, and Villa Helios; and memory centers: Bastia, Pitié Salpetrière, Sainte Marie, and Institut Claude Pompidou. French Ministry of Health—PRME 2016.
Robert P, Albrengues C, Fabre R, et al. Efficacy of serious exergames in improving neuropsychiatric symptoms in neurocognitive disorders: Results of the X‐TORP cluster randomized trial. Alzheimer's Dement. 2021;7:e12149. 10.1002/trc2.12149
REFERENCES
- 1. Salma SS, Sandreschi PF, Da Silva FC, et al. What are the benefits of exercise for Alzheimer's Disease? A systematic review of the past 10 years. J Aging Phys Act. 2015;23:659‐668. [DOI] [PubMed] [Google Scholar]
- 2. Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer‐type dementia. Ann Intern Med. 2018;168(1):30‐38. [DOI] [PubMed] [Google Scholar]
- 3. McGough EL, Lin S‐Y, Belza B, et al. A scoping review of physical performance outcome measures used in exercise interventions for older adults with Alzheimer disease and related dementias. J Geriatr Phys Ther. 2019;42(1):28‐47. [DOI] [PubMed] [Google Scholar]
- 4. Robert PH, Konig A, Andrieu S, et al. Recommendations for ICT use in Alzheimer's disease assessment: monaco CTAD expert meeting. J Nutr Health Aging. 2013;17(8):653‐660. [DOI] [PubMed] [Google Scholar]
- 5. Konig A, Aalten P, Verhey F, et al. A review of current information and communication technologies: can they be used to assess apathy?. Int J Geriatr Psychiatry. 2013;29(4):345‐358. [DOI] [PubMed] [Google Scholar]
- 6. Robert P, Manera V, Derreumaux A, et al. Efficacy of a web app for cognitive training (MeMo) regarding cognitive and behavioral performance in people with neurocognitive disorders: randomized controlled trial. J Med Internet Res. 2020;22(3):e17167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manera V, Ben‐Sadoun G, Aalbers T, et al. Recommendations for the use of serious games in neurodegenerative disorders: 2016 delphi panel. Front Psychol. 2017;8:1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Padala KP, Padala PR, Lensing SY, et al. Efficacy of wii‐fit on static and dynamic balance in community dwelling older veterans: a randomized controlled pilot trial. J Aging Res. 2017;2017:4653635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stojan R, Voelcker Rehage C. A Systematic review on the cognitive benefits and neurophysiological correlates of exergaming in healthy older adults. J Clin Med. 2019;8(5):734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Santen J, Droes RM, Holstege M, et al. Effects of exergaming in people with dementia: results of a systematic literature review. J Alzheimers Dis. 2018;63(2):741‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ben‐Sadoun G, Sacco G, Manera V, et al. Physical and cognitive stimulation using an exergame in subjects with normal aging, mild and moderate cognitive impairment. J Alzheimer's Dis. 2016;53:1299‐1314. [DOI] [PubMed] [Google Scholar]
- 12. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 13. Cummings JL, Mega MS, Gray K, Rosemberg Thompson S, Gornbein T. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;41:1374‐1382. [DOI] [PubMed] [Google Scholar]
- 14. Robert PH, Clairet S, Benoit M, et al. The apathy inventory: assessment of apathy and awareness in Alzheimer's disease, Parkinson's disease and mild cognitive impairment. Int J Geriatr Psychiatry. 2002;17(12):1099‐1105. [DOI] [PubMed] [Google Scholar]
- 15. Lockhart IA, Orme ME, Mitchell SA. The efficacy of licensed‐indication use of donepezil and memantine monotherapies for treating behavioural and psychological symptoms of dementia in patients with Alzheimer's disease: systematic review and meta‐analysis. Dement Geriatr Cogn Disord Extra. 2011;1(1):212‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nascimento CMC, Teixeira CVL, Gobbi LTB, Gobbi S, Stella F. A controlled clinical trial on the effects of exercise on neuropsychiatric disorders and instrumental activities in women with Alzheimer's disease. Braz J Phys Ther. 2012;16:197‐204. [DOI] [PubMed] [Google Scholar]
- 17. Teri L, Gibbons LE, McCurry SM, et al. Exercise plus behavioral management in patients with Alzheimer disease a randomized controlled trial. JAMA. 2003;290(15):2015‐2022. [DOI] [PubMed] [Google Scholar]
- 18. Rolland Y, Pillard F, Klapouszczak A, et al. Exercise program for nursing home residents with Alzheimer's disease: a 1‐Year randomized, controlled trial. J Am Geriatr Soc. 2007;55(2):158‐165. [DOI] [PubMed] [Google Scholar]
- 19. Williams CL, Tappen RM. Exercise training for depressed older adults with Alzheimer's disease. Aging Ment Health. 2008;12(1):72‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao Y, Feng H, Wu X, et al. Effectiveness of exergaming in improving cognitive and physical function in people with mild cognitive impairment or dementia: systematic review. JMIR Serious Games. 2020;8(2):e16841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bamidis PD, Fissler P, Papageorgiou SG, et al. Gains in cognition through combined cognitive and physical training: the role of training dosage and severity of neurocognitive disorder. Front Aging Neurosci. 2015;7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Padala KP, Malloy TR, Lensing SY, Bopp MM, Sullivan DH, Padala PR. P4‐665: exercice adherence in early Alzheimer's dementia: what exercice adherence in early Alzheimer's dementia: what roles do executive function and apathy play?. Alzheimer's Dement. 2019;15(7S_Part_30):P1586‐P. [Google Scholar]
- 23. Robert P, Lanctot KL, Aguera‐Ortiz L, et al. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur Psychiatry. 2018;54:71‐76. [DOI] [PubMed] [Google Scholar]
- 24. Ferrero‐Arias J, Goñi‐Imízcoz M, González‐Bernal J, Lara‐Ortega F, da Silva‐González A, Díez‐Lopez M. The efficacy of nonpharmacological treatment for dementia‐related apathy. Alzheimer Dis Assoc Disord Dement. 2011;25(3):213‐219. [DOI] [PubMed] [Google Scholar]
- 25. Theleritis C, Siarkos K, Politis AA, Katirtzoglou E, Politis A. A systematic review of non‐pharmacological treatments for apathy in dementia. Int J Geriatr Psychiatry. 2018;33(2):e177‐e92. [DOI] [PubMed] [Google Scholar]
- 26. Starkstein S, Hayhow B. Apathy in dementia: time to StandUp. Am J Geriatr Psychiatry. 2019;27(4):406‐407. [DOI] [PubMed] [Google Scholar]
- 27. Cohen‐Mansfield J, Dakheel‐Ali M, Thein K, Marx MS. The impact of stimulus attributes on engagement of nursing home residents with dementia. Arch Gerontol Geriatr. 2009;49(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manera V, Abrahams S, Aguera‐Ortiz L, et al. Recommendations for the nonpharmacological treatment of apathy in brain disorders. Am J Geriatr Psychiatry. 2019;28(4):410‐420. [DOI] [PubMed] [Google Scholar]
- 29. Nijsten JMH, Leontjevas R, Smalbrugge M, Koopmans RTCM, Gerritsen DL. Apathy and health‐related quality of life in nursing home residents. Qual Life Res. 2019;28(3):751‐759. [DOI] [PubMed] [Google Scholar]
- 30. D'lorio A, Vitale C, Piscopo F, et al. mpact of anxiety, apathy and reduced functional autonomy on perceived quality of life in Parkinson's disease. Parkinsonism Relat Disord. 2017;43:114‐117. [DOI] [PubMed] [Google Scholar]
- 31. Fritz NE, Boileau NR, Stout JC, et al. Relationships among apathy, health‐related quality of life, and function in Huntington's disease. J Neuropsychiatry Clin Neurosci. 2018;30(3):194‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lorenzo‐López L, de Labra C, Maseda A, et al. Caregiver's distress related to the patient's neuropsychiatric symptoms as a function of the care‐setting. Geriatr Nurs. 2017;38(2):110‐118. [DOI] [PubMed] [Google Scholar]
- 33. Zhou Z, Müller M, Kanel P, et al. Apathy rating scores and β‐amyloidopathy in patients with Parkinson disease at risk for cognitive decline. Neurology. 2020;94(4):e376‐e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andrews SC, Langbehn DR, Craufurd D, et al. Apathy predicts rate of cognitive decline over 24 months in premanifest Huntington's disease. Psychol Med. 2020:1‐7. [DOI] [PubMed] [Google Scholar]
- 35. Ruthirakuhan M, Herrmann N, Vieira D, Gallagher D, Lanctôt KL. The roles of apathy and depression in predicting Alzheimer disease: a longitudinal analysis in older adults with mild cognitive impairment. Am J Geriatr Psychiatry. 2019;27(8):873‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robert PH, König A, Amieva H, et al. Recommendations for the use of serious games in people with Alzheimer's disease, related disorders and frailty. Front Aging Neurosci. 2014;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manera V, Petit P‐D, Derreumaux A, et al. Kitchen and cooking,’ a serious game for mild cognitive impairment and Alzheimer's disease: a pilot study. Front Aging Neurosci. 2015;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]


