Abstract
Background:
Emotional distress often causes cancer patients and their family caregivers (FCGs) to avoid end-of-life discussions and advance care planning (ACP), which may undermine quality of life (QoL). Most ACP interventions fail to address emotional barriers that impede timely ACP.
Aim:
We assessed feasibility, acceptability, and preliminary effects of a mindfulness-based intervention to facilitate ACP for adults with advanced-stage cancer and their FCGs.
Design:
A single-arm pilot was conducted to assess impact of a 6-week group mindfulness intervention on ACP behaviors (patients only), QoL, family communication, avoidant coping, distress, and other outcomes from baseline (T1) to post-intervention (T2) and 1 month later (T3).
Participants:
Eligible patients had advanced-stage solid malignancies, limited ACP engagement, and a FCG willing to participate. Thirteen dyads (N=26 participants) enrolled at an academic cancer center in the United States.
Results:
Of eligible patients, 59.1% enrolled. Attendance (70.8% across 6 sessions) and retention (84.6% for patients; 92.3% for FCGs) through T3 were acceptable. Over 90% of completers reported high intervention satisfaction. From T1 to T3, patient engagement more than doubled in each of three ACP behaviors assessed. Patients reported large significant decreases in distress at T2 and T3. FCGs reported large significant improvements in QoL and family communication at T2 and T3. Both patients and FCGs reported notable reductions in sleep disturbance and avoidant coping at T3.
Conclusions:
The mindfulness intervention was feasible and acceptable and supported improvements in ACP and associated outcomes for patients and FCGs. A randomized trial of mindfulness training for ACP is warranted. The study is registered at ClinicalTrials.gov with identifier NCT02367508 (https://clinicaltrials.gov/ct2/show/ NCT02367508).
Keywords: cancer, mindfulness, advance care planning, caregivers, distress, quality of life, coping, end of life
INTRODUCTION
Discussing diagnosis, prognosis, and treatment goals is essential to the advance care planning (ACP) process, whereby individuals indicate care preferences to family and healthcare providers should they become medically incapacitated.1 Preferences can be communicated verbally or using advance medical directives, wherein patients put into writing the treatments they would or would not want as their disease progresses. Timely ACP has been associated with positive outcomes for patients and family caregivers (FCGs), including earlier hospice referral,2 increased care satisfaction,3 improved preparation for death,4 and reduced complicated grief for FCGs.4,5
Despite these benefits, approximately half of the 606,000 Americans dying of cancer in 20196 will not have end-of-life (EOL) care discussions before the final month of life.7,8 Delaying or avoiding EOL discussions and ACP is associated with negative outcomes for patients and FCGs. Patients uninformed about their prognosis are 3–8 times more likely to receive non-beneficial treatments in the final week of life,8–10 which may undermine physical and emotional quality of life (QoL).11–13 Avoiding EOL discussions and ACP also prevents patients from experiencing benefits of palliative care integrated with standard cancer care earlier in the disease course, including improved QoL, mood, and survival;14 for FCGs, avoiding EOL discussions and ACP can adversely affect bereavement adjustment.15
A variety of interacting factors contribute to this avoidance.16 Advanced cancer patients vary in their willingness to engage in ACP discussions.17 Patients and FCGs typically wait for oncologists to initiate prognosis conversations,18 yet most oncologists delay discussions of prognosis, code status, advance medical directives, and hospice until the final month of life when many patients are too ill to make complex decisions.19 Patients and FCGs may also experience fear or distress surrounding ACP.20,21 To manage distress, patients and FCGs may employ an avoidant coping style by refusing to accept medical realities,22 thereby further delaying conversations about disease progression.23,24 Given potential negative consequences of avoiding EOL discussions and ACP, interventions that reduce maladaptive coping and distress surrounding these conversations are urgently needed.
One intervention showing promise in palliative care is mindfulness meditation. Mindfulness is a natural human capacity characterized by non-judgmental attention to present moment experiences without emotional reactivity.25 According to mindfulness theory, individuals who practice mindfulness develop greater self-regulation, self-awareness, and self-transcendence in service of enhanced QoL.26 In advanced cancer, mindfulness may promote greater acceptance of medical realities, thereby reducing distress, avoidant coping, and delays in ACP while improving overall QoL.
Emerging research has shown that mindfulness training reduces psychological 27–29 and physical symptoms,30–34 particularly among post-treatment cancer survivors. Mindfulness has also been shown to buffer reactivity to existential threat35 and to reduce avoidant coping.36–39 Although large-scale randomized trials of mindfulness in advanced cancer are lacking,40 several pilot studies have demonstrated reduced psychological distress in patients and FCGs,41,42 improved patient mental health,43,44 and reduced FCG burden.45 To date, no studies have assessed the effects of mindfulness on ACP behaviors among advanced-stage cancer patients and their FCGs.
Consistent with the Institute of Medicine’s recommendations for person-centered, family-oriented EOL care,1 we developed and pilot tested a mindfulness-based intervention—Mindfully Optimizing Delivery of End-of-Life Care (MODEL Care)—to support adults with advanced-stage cancer and their FCGs in approaching EOL conversations and ACP with greater ease. Our first aim was to assess feasibility and acceptability. Our second aim was to assess intervention effect sizes on ACP behaviors (patients only), QoL, family communication, avoidant coping, distress, and other outcomes.
METHODS
The study was approved by the Scientific Review Committee of the National Cancer Institute-designated Indiana University Simon Cancer Center (IUCRO-0460) and corresponding Indiana University Institutional Review Board (IRB #1312088151). Written informed consent was obtained in-person from all participants prior to enrollment.
Eligible patients: (1) were at least 18 years old, (2) were being treated for a stage IIIB, IIIC, or IV solid malignancy, (3) had an oncologist-estimated prognosis of less than 12 months,46,47 (4) had a FCG willing to participate, (5) were willing to attend 6 weekly 2-hour mindfulness training sessions, (6) had not completed a Physician Orders for Scope of Treatment (POST) form, and (7) were able to provide informed consent. Patients were excluded if they: (1) had Eastern Cooperative Oncology Group performance status >2,48 or (2) were receiving hospice care. FCGs were eligible if they were: (1) at least 18 years old, (2) invited to participate by an eligible patient, (3) willing to attend 6 weekly 2-hour mindfulness training sessions, and (4) able to provide informed consent.
Procedures
This pilot employed a single-arm design. Recruiters partnered with 4 medical oncologists and 1 oncology nurse to identify potentially eligible patients on their panel with clinic appointments in the 6-week recruitment period preceding the start of the intervention. All identified patients and FCGs were approached during clinic appointments or via telephone and were systematically screened for eligibility.
Using mail or online surveys, quantitative self-reported data were collected from dyads at baseline (T1), immediately post-intervention (T2), and 1 month post-intervention (T3). Qualitative interviews were conducted after T2, results of which are reported elsewhere.49 Participants earned a $25 gift card for each of the three completed quantitative surveys and the qualitative interview.
Intervention
Two cohorts of 6–7 dyads met as a group for 6 weekly, 2-hour experiential sessions led by a certified mindfulness facilitator with extensive training in mindfulness-based teaching methods from the Center for Mindfulness at the University of Massachusetts. The intervention was modeled after the Mindfulness-Based Stress Reduction program50 and featured formal mindfulness meditation training (i.e., body scan, sitting meditation, gentle hatha yoga, compassion meditation). Emphasis was placed on embodying interpersonal mindfulness in dialogue.51 Practices were designed to (1) cultivate adaptive, non-reactive, and non-judgmental awareness of thoughts, feelings, and bodily sensations in everyday life, and (2) be accessible and adaptable for those who were physically weak or severely ill. For example, if awareness of breath proved too difficult for a participant with dyspnea, attention was focused on other bodily sensations (e.g., feet on the floor, ice chips on the tongue). For patients unable to stand, chair adaptations for yoga were taught in tandem with standing yoga practices. In addition, simple stretching options for home practice in bed were modeled in class with the teacher lying on the floor for added visual learning. Participants were encouraged to complete formal mindfulness practices using audio recordings of practices covered in class for 20 minutes per day, 6 days per week.
ACP was introduced in Session 4. Specific ACP tools were provided, including the POST form,52 with guidance on appropriate use. To supplement discussion, participants also received a copy of the American Society of Clinical Oncology’s ACP decision-making booklet.53 Class discussion explicitly honored the variety of beliefs and values expressed in group, including informed refusal of ACP. See Table 1 for a summary of each session and the intervention’s core components.
Table 1.
MODEL Care Intervention Summary.a
| Session Theme | Mindfulness Practices | Didactics | Home Practice | |
|---|---|---|---|---|
| 1 |
Awareness: Meeting ourselves where we are in honesty and kindness |
Mindful eating (raisin exercise) Body scan (focusing on awareness of breath and body sensations) |
Course introduction and
guidelines. Defining mindfulness as being present for our lives just as they are. Mindfulness as a means for enhancing connection with those we love, identifying what is important to us, and enabling us to choose to proceed from personal values rather than emotional reactions. Introduction of interpersonal mindful dialogue skills, including listening attentively with curiosity and non-judgment, without needing to give advice or comment on others’ sharing. |
Body scan daily Eat one meal mindfully Mindfulness of one daily activity |
| 2 |
Perception and creative
responding: Struggle against “life as it is” as a source of suffering; wholeness no matter what’s here |
Body scan Introduction of gentle hatha yoga stretching Awareness of breath (AOB) sitting meditation |
Role of perception, habit-driven conditioning,
and other mental factors in the self-appraisal of
stress. Recognizing with kindness, struggle as it is reflected in the body. Use of mindfulness to enhance comfort in living with elements of life that are difficult or challenging, incorporating compassion and non-judgment. |
Alternate body scan and yoga daily Sit 10 min daily with AOB Arriving for rest: short body scan prior to sleep Keep calendar of one pleasant event each day and how it is reflected in mind and body. |
| 3 |
Relational
presence: Mindfulness in dialogue with the body as a place to learn; offer hospitality to one’s experience |
Sitting meditation Yoga practice Mindful dialogue |
Physiological and psychological bases of
stress reactivity are reviewed along with relevant mindfulness
research. Guidelines for mindful dialogue are introduced in greater depth and practiced: Pause, Relax, Open-Allow. Compassion as both attitude and behavior relating to self and others is highlighted as integral to and an outcome of practice. |
Sitting meditation, yoga, or body scan
daily Keep daily Reactivity-Responsivity Calendar as relates to communication |
| 4 |
Mindful
dialogue: Cultivating compassion & responsiveness in speech and action; communication on ACP as empowerment |
Sitting meditation Yoga practice Mindful dialogue Lovingkindness practice |
Expansion of mindful speaking and listening
guidelines allowing previously learned mindfulness practices to support
patients and their family caregivers in non-habitual, non-reactive
communication. Mindful dialogue about present moment challenges related to 1) being with change and uncertainty, and 2) discussing goals of care with healthcare providers and family members. Participants invited to open dialogue about what they value. Participants are provided information about ACP, including the POST form52 and palliative care programs in the area. |
Sitting meditation, yoga, body scan, or
lovingkindness practice Read ASCO Advanced Care Planning booklet77 and review POST form together in mindful dialogue. |
| 5 |
Mindful dialogue associated with
challenging thoughts and feelings: Meeting with practice what impedes open communication |
Sitting meditation Yoga practice Mindful dialogue Lovingkindness practice |
Using mindful dialogue guidelines, deeper
discussion of ACP as an ongoing process shared by patients, their family
members, and oncology providers grounded in the patient’s values
and preferences for goals of care. Benefits of making timely decisions about desired scope of treatment are highlighted, as well as consideration of surrogate decision-makers. ACP tools, including the POST form, are further reviewed as a means of facilitating individual choices. This dialogue honors the wide variance of beliefs and values in the room within the themes of the shared human experience of coping with the unpredictable nature of life’s changes and the preciousness of life. |
Sitting meditation, yoga, or body scan with
recorded guidance or self-guidance daily. Practicing mindful dialogue guidelines in everyday life. Consider how to support ongoing practice and mindful dialogue after the class. |
| 6 |
The rest of your
life: Making the practice your own |
Body scan Yoga Sitting meditation Lovingkindness practice |
Emphasis on the growing capacity of all
participants to adapt more easily and effectively to everyday challenges
and stressors, particularly those associated with advanced
cancer. Taking a mindful, open, conscious, and responsive—rather than reactive—approach is emphasized. Utilizing mindful communication skills, inviting each patient and family caregiver to share what has been learned in practice and any lingering questions concerning process and decisions about care preferences. Invitation for patients to continue discussing care preferences with oncology team and sign POST form at next appointment with oncologist if ready to do so. Review of core mindfulness skills and sharing of resources to support mindfulness practice after the class concludes. |
Mindfulness resources handout |
Abbreviations: ACP, advance care planning; ASCO, American Society of Clinical Oncology; POST, Physician Orders for Scope of Treatment.
All sessions were two hours and included provision of compact discs with audio recordings of guided meditations of body scan, sitting meditation, gentle hatha yoga, and lovingkindness meditation practices created by the facilitator for home practice.
Measures
Demographic and clinical characteristics were collected from participants at T1 (see Table 2). Feasibility and acceptability were measured using accrual, attendance, and retention rates through T3; participants’ responses to satisfaction and helpfulness items at T2; and participants’ responses to home practice engagement questions at T2 and T3.
Table 2.
Participant Demographics and Medical Characteristics.
| Patients (n=13) | FCGs (n=13) | |
|---|---|---|
| Age, mean (SD) | 62.91 (10.55) | 56.58 (15.62) |
| Race, N (%) | ||
| American Indian/Alaska Native | 0 (0) | 1 (7.69) |
| Asian | 0 (0) | 1 (7.69) |
| Black/African American | 1 (7.69) | 2 (15.38) |
| White/Caucasian | 12 (92.31) | 9 (69.23) |
| Sex, N (%) | ||
| Male | 7 (53.85) | 3 (23.08) |
| Female | 6 (46.15) | 10 (76.92) |
| Education, N (%) | ||
| High School/GED | 2 (15.38) | 1 (7.69) |
| Technical/Trade School | 1 (7.69) | 1 (7.69) |
| Some college | 3 (23.08) | 4 (30.77) |
| Associate’s Degree | 0 (0) | 3 (23.08) |
| Bachelor’s Degree | 3 (23.08) | 3 (23.08) |
| Master’s Degree | 3 (23.08) | 1 (7.69) |
| Other | 1 (7.69) | 0 (0) |
| Employment, N (%) | ||
| Full-time | 2 (15.38) | 5 (38.46) |
| Part-time | 1 (7.69) | 1 (7.69) |
| Self-employed | 1 (7.69) | 1 (7.69) |
| Unable to work | 3 (23.08) | 1 (7.69) |
| Homemaker | 0 (0) | 2 (15.38) |
| Retired | 5 (38.46) | 3 (23.08) |
| Other | 1 (7.69) | 0 (0) |
| Income, N (%) | ||
| Comfortable | 9 (69.23) | 8 (61.54) |
| Just enough to make ends meet | 3 (23.08) | 3 (23.08) |
| Not enough to make ends meet | 1 (7.69) | 2 (15.38) |
| Perception of Patient’s Current Condition, N (%) | ||
| Relatively healthy | 4 (30.77) | 4 (30.77) |
| Seriously ill - not terminal | 3 (23.08) | 1 (7.69) |
| Seriously ill - terminal | 6 (46.15) | 7 (53.85) |
| Skipped | 0 (0) | 1 (7.69) |
Preliminary Efficacy.
All selected outcome measures are valid, reliable, and have routinely been used in cancer care research. Patients reported ACP engagement at T1 and T3 across three behaviors: (1) having completed a POST form with a physician, (2) having discussed goals of care with their oncologist, and (3) having discussed goals of care with family.54 Patients also indicated their stage of change55 or readiness for completing each behavior by selecting responses ranging from “have not thought about it or not ready to complete” (Precontemplation), “thinking about completing in the next 6 months” (Contemplation), or “planning to complete in the next 30 days” (Preparation); if the ACP behavior was already completed, patients indicated time of completion, either “in the last 6 months” (Action) or “more than 6 months ago” (Maintenance). To assess family communication, participants completed the Openness to Discuss Cancer in the Nuclear Family (ODCNF) scale.56 Patient QoL was measured with the McGill Quality of Life Inventory total score57 while FCGs completed the Caregiver Quality of Life Index-Cancer [CQoLI-C] scale.58 Avoidant coping was measured with the cognitive avoidance subscale of the Mini-Mental Adjustment to Cancer Scale [Mini-MAC]59 and the self-distraction, denial, and behavioral disengagement subscales from the Brief COPE.60 To assess distress, participants responded to measures of depression (Patient Health Questionnaire [PHQ-8])61 and anxiety (Generalized Anxiety Disorder scale [GAD-7]).62 Sleep disturbance was assessed with a single item from the Pittsburgh Sleep Quality Index [PSQI].63 Fatigue interference was assessed with the interference subscale of the Fatigue Symptom Inventory [FSI].64,65
Data Analysis
Descriptive statistics were computed on demographic and clinical characteristics. Feasibility benchmarks included: (1) at least 50% of eligible dyads enrolling in the study, and (2) attendance rates of 70% or greater across the 6 sessions. Acceptability benchmarks included: (1) at least 70% of participants completing the study,66 and (2) at least 70% of participants reporting being mostly to completely satisfied with their intervention experience. The standardized response mean (SRM) effect size was calculated to assess magnitude of intervention effects at T2 and T3 for patients and FCGs separately. To determine SRM, mean change in T2 and T3 scores relative to T1 was calculated and divided by the standard deviation of change. For 95% confidence intervals (CIs), we computed an SRM statistic for each participant (participant’s mean change divided by the sample’s SD of change scores). Then, we used the SAS MEANS procedure with the LCLM and UCLM options to compute the lower and upper 95% confidence limits for the SRM statistic. The primary efficacy-related goal of this pilot was to estimate effect sizes; however, preliminary hypothesis tests were also performed. The two-sided paired t-test was used to determine significant (p < 0.05) responsiveness over time. Due to the small sample, marginal significance (0.05 < p < 0.10) was also reported. SRMs of 0.2, 0.5, and 0.8 indicated small, medium, and large effect sizes, respectively.67 Effect sizes of at least half a SD (≥ 0.50) are considered clinically meaningful.68−69
RESULTS
Demographic and Clinical Characteristics
Demographic and clinical characteristics of the 13 dyads are shown in Table 2. About half of the patients were male (53.9%), and the majority of FCGs were female (76.9%). FCGs were spouses (69.2%), adult children (23.1%), or family friends (7.7%) of the patient. Patients had varying cancer types and were diagnosed with advanced-stage cancer an average of 20.9 months (SD=21.4) before enrollment.
Feasibility and Acceptability
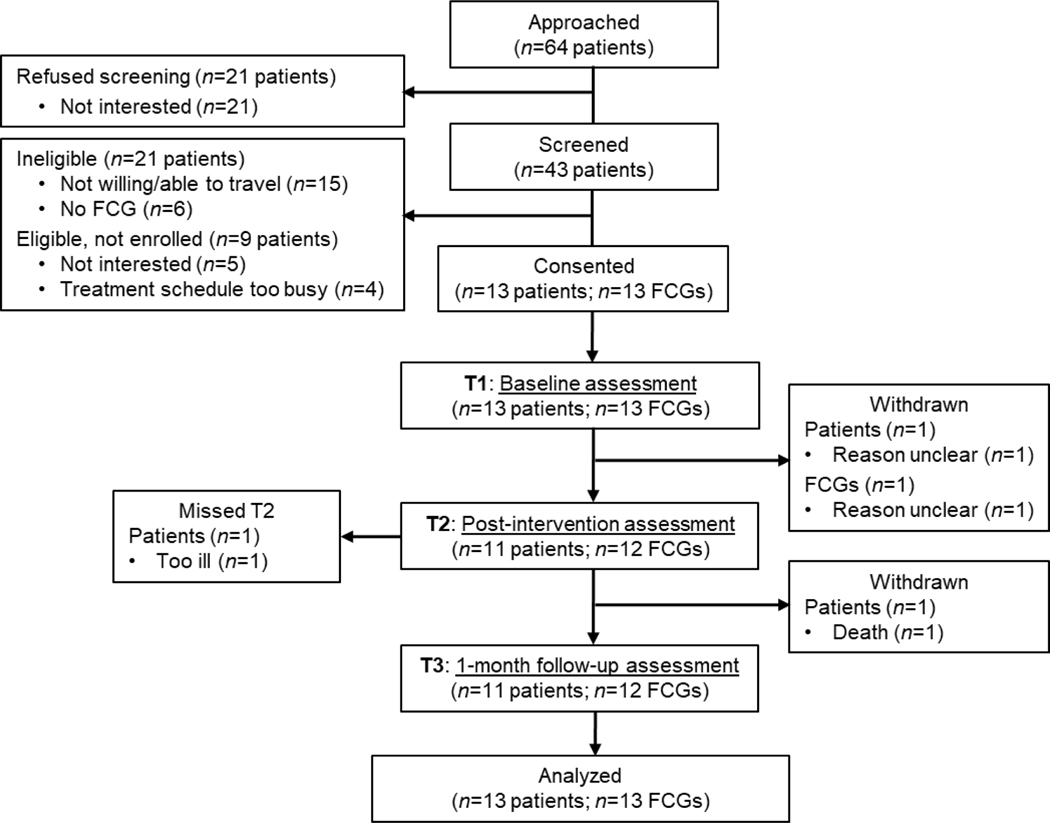
Over 6 weeks, 64 patients were approached with 43 (67.2%) assessed for eligibility (see Figure 1). Of those assessed, 22 (51.2%) were eligible and 13 (59.1%) enrolled in the study. The mean number of sessions attended was 4.3 for patients and 4.2 for FCGs. Most participants missing a session completed a brief make-up session by phone with the facilitator. One dyad withdrew after Session 1 and one patient died between T2 and T3, resulting in retention rates of 84.6% for patients and 92.3% for FCGs at T3. Notably, 91.3% of participants reported being mostly to completely satisfied with their MODEL Care experience, with the modal response being “completely satisfied.” Most participants rated the number and length of sessions as “about right” (91.3% and 87.5%, respectively). Table 3 summarizes additional acceptability items.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flow chart, including number of participants assessed at each time point.
Table 3.
Intervention Satisfaction, Helpfulness, and Home Practice.
| Survey Item | Response Scale Anchors | Patients (N=11) | FCGs (N=12) | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| SATISFACTION | |||||
| Overall, how satisfied are you with your experience in the MODEL Care course? | 1 = Completely dissatisfied 7 = Completely satisfied |
6.455 | 0.522 | 6.333 | 0.778 |
| HELPFULNESS OF MODEL CARE | |||||
| Becoming more aware of my thoughts, feelings, and bodily sensations | 1 = Not at all helpful 5 = Very helpful |
4.636 | 0.674 | 4.667 | 0.888 |
| Knowing that I am not alone in my experiences | 4.818 | 0.405 | 4.667 | 0.651 | |
| Learning how to live more in the present moment | 4.545 | 0.522 | 4.833 | 0.577 | |
| Coping with difficult thoughts, feelings, or bodily sensations | 4.455 | 0.688 | 4.250 | 1.055 | |
| Learning more about palliative care | 4.182 | 0.751 | 3.750 | 1.138 | |
| The CDs and printed materials I received helped me to practice. | 1 = Strongly disagree 5 = Strongly agree |
4.545 | 0.522 | 4.500 | 0.522 |
| The CDs and printed materials were easy to understand. | 4.727 | 0.467 | 4.917 | 0.289 | |
| The session facilitator was “tuned in” to my needs. | 4.545 | 0.688 | 4.833 | 0.577 | |
| Being involved in MODEL Care has made me feel anxious | 1.909 | 1.221 | 1.583 | 0.900 | |
| Being involved in MODEL Care has made it more difficult to deal with thoughts of cancer. | 1.364 | 0.505 | 1.417 | 0.515 | |
| How likely are you to recommend the MODEL Care course to a friend or family member in a similar situation as you? | 1 = Extremely unlikely 5 = Extremely likely |
4.636 | 0.505 | 4.750 | 0.452 |
| How likely are you to continue to use what you learned in the MODEL Care course? | 4.545 | 0.522 | 4.667 | 0.651 | |
| HOME PRACTICE HABITS | |||||
| Reported at T2 | 0–7 days | ||||
| How many days per week, on average, have you participated in FORMAL mindfulness practice during the MODEL Care course? | 3.545 | 1.635 | 3.333 | 1.435 | |
| How many days per week, on average, have you participated in INFORMAL mindfulness practice during the MODEL Care course? | 5.182 | 1.991 | 5.000 | 1.706 | |
| Reported at T3 | |||||
| How many days per week, on average, have you participated in FORMAL mindfulness practice since completing the MODEL Care course last month? | 3.000 | 2.049 | 2.917 | 1.782 | |
| How many days per week, on average, have you participated in INFORMAL mindfulness practice since completing the MODEL Care course last month? | 5.000 | 1.549 | 4.083 | 1.564 | |
Note. SD=standard deviation. All items were asked at T2, except where indicated. FORMAL mindfulness practice includes the body scan, sitting meditation, compassion meditation, and mindful movement featuring gentle hatha yoga with chair adaptations. INFORMAL mindfulness practice refers to bringing present-moment awareness to everyday activities (e.g., eating a meal, brushing teeth, washing dishes).
Intervention Effects
Table 4 shows intervention effects for patients and FCGs. At T2, patients showed a statistically significant, large reduction in depression (SRM=−0.91, p=0.01) and a marginally significant, medium reduction in cognitive avoidance (SRM=−0.64, p=0.059). Furthermore, medium effect sizes were observed for improved anxiety (SRM=−0.49), sleep disturbance (SRM=−0.54), behavioral disengagement (SRM=−0.54), and QoL (SRM=0.49). FCGs reported significant, large improvements in QoL (SRM=0.85, p=0.01) and family communication (SRM=0.88, p=0.01) and marginally significant, medium reductions in anxiety (SRM=−0.58, p=0.069), fatigue interference (SRM=−0.56, p=0.079), and cognitive avoidance (SRM=−0.53, p=0.094).
Table 4.
Patient and Family Caregiver Outcomes.
| Possible Score Range1 | T1 Mean (SD)2 | T2 Mean (SD) | T3 Mean (SD) | T1 - T2 SRM (95% CI) p-value | T1 - T3 SRM (95% CI) p-value | |
|---|---|---|---|---|---|---|
| Patient Outcomes | ||||||
| Distress | ||||||
| Depression (PHQ-8) | 0–24 | 7.91 (4.44) | 5.36 (5.14) | 4.18 (4.62) | −0.91 (−1.58, −0.24)* .0131 | −1.38 (−2.05, −0.71)* .0010 |
| Anxiety (GAD-7) | 0–21 | 4.27 (3.47) | 2.09 (2.34) | 2.18 (2.60) | −0.49 (−1.16, 0.18) .1346 | −0.83 (−1.51, −0.16)* .0199 |
| Other Symptoms | ||||||
| Sleep Disturbance (PSQI) | 0–3 | 1.18 (0.75) | 0.82 (0.75) | 0.64 (0.81) | −0.54 (−1.22, 0.13) .1039 | −0.79 (−1.47, −0.12)* .0251 |
| Fatigue Interference (FSI) | 0–10 | 4.48 (2.73) | 4.51 (3.23) | 4.40 (3.47) | 0.01 (−0.65, 0.68) .9672 | −0.21 (−0.88, 0.47) .5107 |
| Coping | ||||||
| Cognitive Avoidance (Mini-MAC) | 1–4 | 2.64 (0.53) | 2.20 (0.89) | 2.25 (0.81) | −0.64 (−1.31, 0.03) .0588 | −0.26 (−0.93, 0.41) .4062 |
| Self-Distraction (Brief COPE) | 1–4 | 2.45 (0.69) | 2.50 (1.00) | 1.86 (1.00) | 0.04 (−0.63, 0.72) .8845 | −0.91 (−1.58, −0.24)* .0131 |
| Denial (Brief COPE) | 1–4 | 1.05 (0.15) | 1.09 (0.30) | 1.09 (0.30) | 0.30 (−0.37, 0.97) .3409 | 0.30 (−0.37, 0.97) .3409 |
| Behavioral Disengagement (Brief COPE) | 1–4 | 1.27 (0.41) | 1.09 (0.30) | 1.05 (0.15) | −0.54 (−1.21, 0.13) .1039 | −0.79 (−1.46, −0.11)* .0261 |
|
Quality of Life (McGill Overall) |
0–10 | 6.73 (1.74) | 7.73 (2.49) | 8.09 (1.30) | 0.49 (−0.18, 1.16) .1367 | 0.95 (0.28, 1.62)* .0102 |
|
Family
Communication (ODCNF) |
1–4 | 2.92 (0.50) | 3.00 (0.43) | 3.09 (0.48) | 0.17 (−0.50, 0.85) .5746 | 0.33 (−0.35, 1.00) .3053 |
| Family Caregiver
Outcomes | ||||||
| Distress | ||||||
| Depression (PHQ-8) | 0–24 | 3.75 (3.82) | 2.58 (2.02) | 3.00 (3.63) | −0.37 (−1.00, 0.27) .2308 | −0.22 (−0.89, 0.45) .4825 |
| Anxiety (GAD-7) | 0–21 | 3.25 (3.33) | 1.58 (2.02) | 1.58 (2.07) | −0.58 (−1.22, 0.05) .0695 | −0.49 (−1.13, 0.14) .1169 |
| Other Symptoms | ||||||
| Sleep Disturbance (PSQI) | 0–3 | 1.25 (0.87) | 1.08 (0.79) | 0.80 (0.79) | −0.18 (−0.81, 0.46) .5505 | −0.71 (−1.42, 0.01) .0522 |
| Fatigue Interference (FSI) | 0–10 | 2.56 (2.26) | 1.44 (1.62) | 2.42 (2.55) | −0.56 (−1.19, 0.08) .0790 | 0.01 (−0.67, 0.68) .9829 |
| Coping | ||||||
| Cognitive Avoidance (Mini-MAC) | 1–4 | 2.06 (0.70) | 1.63 (0.57) | 1.31 (0.36) | −0.53 (−1.16, 0.11) .0939 | −1.05 (−1.68, −0.41)* .0039 |
| Self-Distraction (Brief COPE) | 1–4 | 2.04 (108) | 1.79 (0.84) | 1.58 (0.56) | −0.27 (−0.91, 0.36) .3653 | −0.45 (−1.09, 0.18) .1444 |
| Denial (Brief COPE) | 1–4 | 1.04 (0.14) | 1.08 (0.19) | 1.17 (0.58) | 0.29 (−0.35, 0.92) .3388 | 0.21 (−0.43, 0.84) .4910 |
| Behavioral Disengagement (Brief COPE) | 1–4 | 1.21 (0.5) | 1.04 (0.14) | 1.04 (0.14) | −0.38 (−1.01, 0.26) .2199 | −0.38 (−1.01, 0.26) .2199 |
|
Quality of
Life (CQoLI-C) |
0–4 | 2.69 (0.70) | 3.00 (0.50) | 3.06 (0.53) | 0.85 (0.21, 1.48)* .0136 | 1.02 (0.35, 1.69)* .0070 |
|
Family
Communication (ODCNF) |
1–4 | 2.83 (0.76) | 3.20 (0.68) | 3.38 (0.55) | 0.88 (0.24, 1.52)* .0110 | 0.80 (0.12, 1.47)* .0249 |
Notes. T1=baseline assessment; T2=post-intervention assessment; T3=1 month post-intervention assessment; SD=standard deviation; SRM=standardized response mean (0.2=small effect, 0.5=medium effect, 0.8=large effect);
CI=confidence interval.
Higher scores on each outcome measure indicate more of the concept being measured. As such, lower scores on all outcomes are desirable, with the exception of the quality of life measures (McGill for patients and CQoLI-C for caregivers) and the family communication measure (ODCNF), wherein higher scores are desirable.
Mean (SD) at T1 is calculated for participants who had either T2 or T3 data.
p<.05. p-value from paired t-test test.
At T3, effects were generally maintained or strengthened. Patients showed large, statistically significant improvements in depression (SRM=−1.38, p=0.001), anxiety (SRM=−0.83, p=0.02), sleep disturbance (SRM=−0.79, p=0.025), self-distraction (SRM=−0.91, p=0.013), behavioral disengagement (SRM=−0.79, p=0.026), and QoL (SRM=0.95, p=0.010). For FCGs, the large and significant effects on QoL (SRM=1.02, p=0.007) and family communication (SRM=0.80, p=0.025) were maintained and a large, significant effect on reduced cognitive avoidance (SRM=−1.05, p=0.004) emerged. In addition, a marginally significant reduction in sleep disturbance was observed with a medium-to-large effect size (SRM=−0.71, p=0.052).
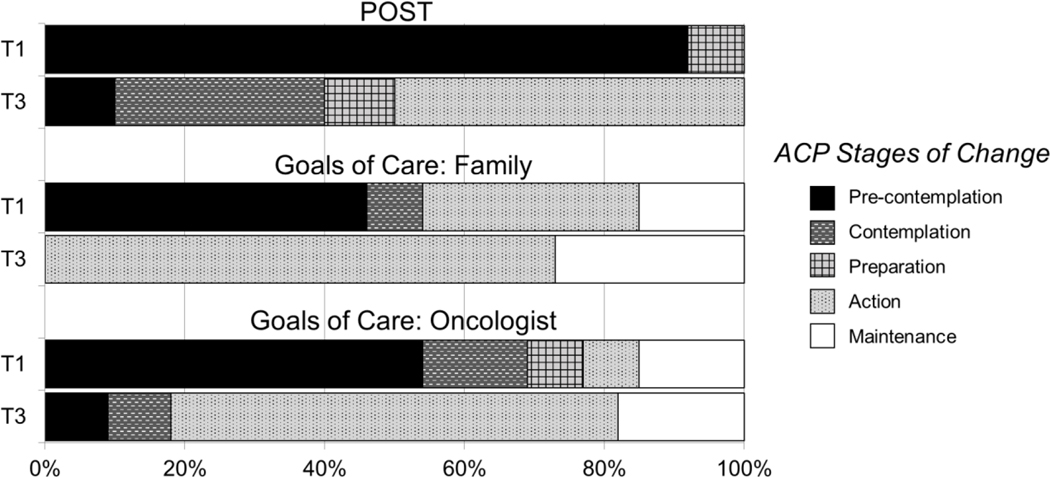
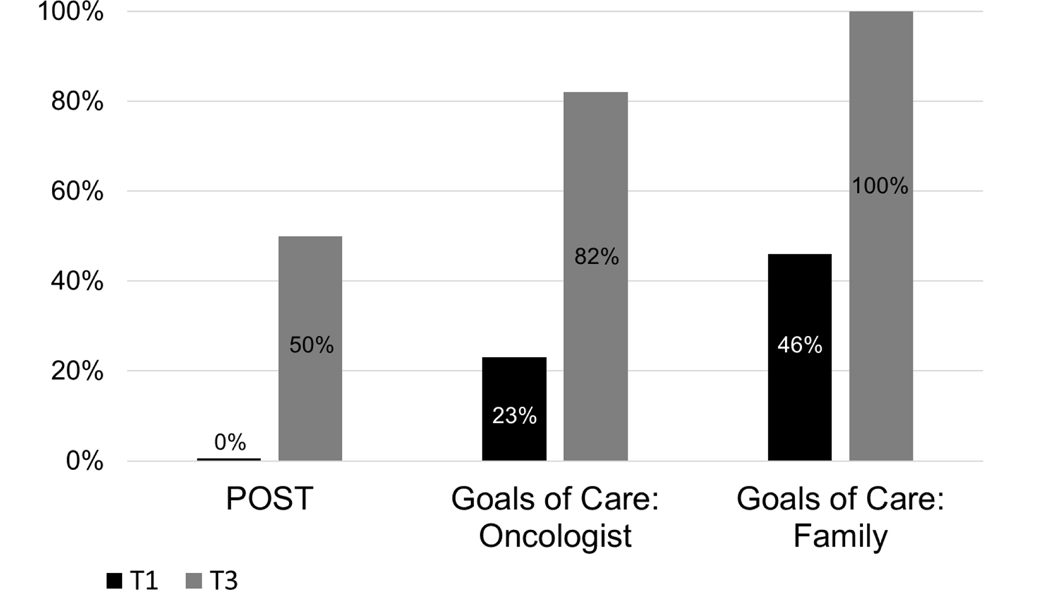
Regarding ACP stages of change from T1 to T3, 90% of patients progressed at least one stage of readiness to complete a POST form. Of those not already in Action or Maintenance stages at T1 for having a goals of care conversation with their oncologist, 100% of responding patients progressed at least 2 stages of readiness for this behavior at T3, while 75% progressed at least 1 stage on having a goals of care conversation with their family members (see Figure 2). As shown in Figure 3, patients reported marked progress across the three ACP behaviors assessed. Patients reported statistically-significant progress in having conversations about goals of care with their oncologists (p=0.03), with non-significant trends for having goals of care conversations with family members (p=.06) and completing a POST form (p=.06).
Figure 2.
Stages of change summary for patient advance care planning (ACP) behaviors from T1 to T3.
Figure 3.
Patients reporting Action or Maintenance stages of change for advance care planning behaviors from T1 to T3.
DISCUSSION
This pilot of mindfulness training in dyads of advanced cancer patients and FCGs has several important findings. First, a 6-week mindfulness training program is feasible and highly acceptable among this population. Second, preliminary effects suggest mindfulness training may significantly reduce symptoms associated with advanced-stage cancer while promoting ACP behaviors, with several results being both statistically significant and clinically meaningful.68–69 Lastly, these improvements were sustained at least 1 month post-intervention. Despite a small sample size, efficacy tests generally showed statistically significant efficacy for the outcomes that demonstrated large effect sizes and marginally significant efficacy for outcomes that demonstrated medium effect sizes. In fact, our sample of 12 individuals with both T1 and either T2 or T3 scores provides only 35% and 71% power, respectively, to detect medium (0.50) and large (0.80) effect sizes in standardized mean change scores using the two-sided paired t-test with 0.05 alpha. When alpha of 0.10 is used for assessing marginal significance, the power for 0.50 and 0.80 effect sizes is 49% and 82%, respectively, for a sample of size 12, and 46% and 79% for a sample size of 11.
Our findings suggest that participants are open to mindfulness training and are willing to complete a multi-session program. Over half of eligible patients enrolled with their FCGs, demonstrating notable openness to engage in mindfulness training late in the disease course. Sustained commitment and engagement were further exemplified by relatively high attendance, retention, and participant-reported formal and informal mindfulness practice at home, even after the intervention period. With more than 90% of completers reporting being mostly or completely satisfied with MODEL Care, mindfulness training was perceived as an acceptable intervention by this population. While many patients with cancer are uncomfortable thinking or talking about EOL,20,21,70 our recruitment, enrollment, attendance, and retention rates suggest patients and FCGs, even those with limited ACP engagement before enrollment, are eager to engage in training that may help them overcome barriers to ACP.
Mindfulness has long been shown to improve mental health outcomes,27–29 physical symptoms,30–34 and QoL71,72 in adults with cancer, and our findings are consistent with other studies.41,42 Patients and FCGs reported notable reductions in distress and avoidant coping after 6 weeks of mindfulness training and 1 month later; given that distress and avoidant coping may interfere with initiating EOL discussions, minimizing these barriers is a logical first step toward facilitating ACP behaviors among this patient population. Participants also reported improved QoL at T2 compared to T1 with increasing improvements at T3; this is a meaningful finding for a sample of patients with progressive cancer, whose QoL tends to decrease approaching EOL.
To our knowledge, this is the first study to test the effects of mindfulness training on ACP behaviors in adults with advanced cancer. Our exploratory findings suggest mindfulness training may help improve ACP engagement, evidenced not only by significantly increased completion of ACP behaviors from T1 to T3, but also through patients’ rapid progression in readiness to engage. Mindfulness may promote emotional regulation73 such that ACP behaviors may be completed with greater ease and less emotional reactivity. Mindfulness may also facilitate greater psychological flexibility (i.e., ability to connect with present-moment experiences to accept realities of one’s diagnosis and prognosis) leading to increased engagement in ACP process. By promoting present-moment awareness, nonjudgmental acceptance, and emotional regulation,74 mindfulness training may facilitate earlier EOL decision-making while patients still have the capacity to make medical decisions and articulate their preferences.
Several limitations of the current study should be noted. We used a single-arm design, which limits our ability to conclude that improved outcomes were due to MODEL Care. Other extraneous factors (e.g., passage of time) could have contributed to the observed changes. The small sample size of 13 dyads and correspondingly wide CIs on most outcomes also limit the strength of our conclusions; however, the majority of outcome effect sizes for patients and FCGs were ≥ 0.40, supporting further testing of mindfulness in this population. Participants also reported low distress levels, as evidenced by non-clinically significant PHQ-8 and GAD-7 group mean scores61,62 at every time point. Finally, most participants were English-speaking, Caucasian, and reported having a comfortable income, thereby limiting generalizability outside of these demographics. Due to our nonrandomized, single-arm design, participants knew they would receive a dyadic mindfulness-based group intervention before enrolling. It is possible that only those interested in this approach and format enrolled in the study, which may limit generalizability to those not interested in mindfulness or a dyadic, group-based intervention.
Nonetheless, results of this pilot suggest that mindfulness training could play a useful role in improving and expanding ACP uptake by preparing patients and FCGs to discuss the emotionally challenging subject of EOL with greater ease. Despite ongoing efforts to increase ACP, a recent longitudinal study found no increase in EOL discussions or use of living wills among those with cancer over a 12-year period.75 Thus, determining definitive effects of mindfulness training on ACP is essential. Future work should test the efficacy of MODEL Care in a randomized controlled trial in comparison with standard care or another established ACP intervention (e.g., Respecting Choices).76
Acknowledgements
The authors extend boundless gratitude to Gregory Kramer in developing Insight Dialogue51 and to Phyllis Hicks, Florence Meleo-Meyer, and Gregory Kramer in articulating Insight Dialogue in Interpersonal Mindfulness Practice as the cornerstone of this intervention. We thank our oncology partners at the IU Simon Cancer Center who referred patients. Finally, none of this work would be possible without the openhearted participation of the individuals who generously volunteered their time to participate in this study.
Funding
This project was funded by the Walther Cancer Foundation (0113.02). Mentoring support for Dr. Johns was provided by Dr. Victoria Champion through the National Cancer Institute of the National Institutes of Health (K05CA175048). This content is solely the responsibility of the authors and does not necessarily represent the official views of the Walther Cancer Foundation or the National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
The Authors declare that there are no conflicts of interest.
Data Accessibility
De-identified primary data collected in this study are available upon request. Please email data requests to Principal Investigator Dr. Shelley Johns at sheljohn@iu.edu.
REFERENCES
- 1.Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 2.Teno JM, Gruneir A, Schwartz Z, Nanda A, Wetle T. Association between advance directives and quality of end‐of‐life care: A national study. J Am Geriatr Soc. 2007;55(2):189–194. [DOI] [PubMed] [Google Scholar]
- 3.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz R, Boerner K, Klinger J, Rosen J. Preparedness for death and adjustment to bereavement among caregivers of recently placed nursing home residents. J Palliat Med. 2015;18(2):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrido MM, Prigerson HG. The end‐of‐life experience: Modifiable predictors of caregivers’ bereavement adjustment. Cancer. 2014;120(6):918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Cancer Society. Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society; 2019. [Google Scholar]
- 7.Peppercorn JM, Smith TJ, Helft PR, et al. American society of clinical oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol. 2011;29(6):755–760. [DOI] [PubMed] [Google Scholar]
- 8.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279(21):1709–1714. [DOI] [PubMed] [Google Scholar]
- 10.Zaros MC, Curtis JR, Silveira MJ, Elmore JG. Opportunity lost: End‐of‐life discussions in cancer patients who die in the hospital. J Hosp Med. 2013;8(6):334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith TJ, Hillner BE. Concrete options and ideas for increasing value in oncology care: the view from one trench. The Oncologist. 2010;15 Suppl 1:65–72. [DOI] [PubMed] [Google Scholar]
- 12.Earle CC, Neville BA, Landrum MB, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care. 2005;17(6):505–509. [DOI] [PubMed] [Google Scholar]
- 13.Miesfeldt S, Murray K, Lucas L, Chang CH, Goodman D, Morden NE. Association of age, gender, and race with intensity of end-of-life care for Medicare beneficiaries with cancer. J Palliat Med. 2012;15(5):548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 15.Wright AA, Keating NL, Balboni TA, Matulonis UA, Block SD, Prigerson HG. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J Clin Oncol. 2010;28(29):4457–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walling A, Lorenz KA, Dy SM, et al. Evidence-based recommendations for information and care planning in cancer care. J Clin Oncol. 2008;26(23):3896–3902. [DOI] [PubMed] [Google Scholar]
- 17.Michael N, O’Callaghan C, Clayton J, et al. Understanding how cancer patients actualise, relinquish, and reject advance care planning: implications for practice. Support Care Cancer. 2013;21(8):2195–2205. [DOI] [PubMed] [Google Scholar]
- 18.Jefford M, Tattersall MH. Informing and involving cancer patients in their own care. Lancet Oncol. 2002;3(10):629–637. [DOI] [PubMed] [Google Scholar]
- 19.Mack JW, Cronin A, Taback N, et al. End-of-life care discussions among patients with advanced cancer: a cohort study. Ann Intern Med. 2012;156(3):204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson S, Butow P, Kerridge I, Tattersall M. Advance care planning for cancer patients: a systematic review of perceptions and experiences of patients, families, and healthcare providers. Psycho-oncology. 2016;25(4):362–386. [DOI] [PubMed] [Google Scholar]
- 21.Michael N, O’Callaghan C, Clayton J, et al. Understanding how cancer patients actualise, relinquish, and reject advance care planning: implications for practice. Supportive Care in Cancer. 2013;21(8):2195–2205. [DOI] [PubMed] [Google Scholar]
- 22.Fu S, Barber FD, Naing A, et al. Advance care planning in patients with cancer referred to a phase I clinical trials program: the MD Anderson Cancer Center experience. J Clin Oncol. 2012;30(23):2891–2896. [DOI] [PubMed] [Google Scholar]
- 23.The AM, Hak T, Koeter G, van Der Wal G. Collusion in doctor-patient communication about imminent death: an ethnographic study. BMJ. 2000;321(7273):1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherlin E, Fried T, Prigerson HG, Schulman-Green D, Johnson-Hurzeler R, Bradley EH. Communication between physicians and family caregivers about care at the end of life: when do discussions occur and what is said? J Palliat Med. 2005;8(6):1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jha AP, Stanley EA, Kiyonaga A, Wong L, Gelfand L. Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion. 2010;10(1):54. [DOI] [PubMed] [Google Scholar]
- 26.Vago DR, Silbersweig DA. Self-awareness, self-regulation, and self-transcendence (S-ART): a framework for understanding the neurobiological mechanisms of mindfulness. Frontiers in Human Neuroscience. 2012;6:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piet J, Wurtzen H, Zachariae R. The effect of mindfulness-based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: a systematic review and meta-analysis. J Consult Clin Psychol. 2012;80(6):1007–1020. [DOI] [PubMed] [Google Scholar]
- 28.Zainal NZ, Booth S, Huppert FA. The efficacy of mindfulness‐based stress reduction on mental health of breast cancer patients: a meta‐analysis. Psycho‐Oncology. 2013;22(7):1457–1465. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M-F, Wen Y-S, Liu W-Y, Peng L-F, Wu X-D, Liu Q-W. Effectiveness of mindfulness-based therapy for reducing anxiety and depression in patients with cancer: a Meta-analysis. Medicine. 2015;94(45):e0897–0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psycho-oncology. 2009;18(12):1261–1272. [DOI] [PubMed] [Google Scholar]
- 31.Lengacher CA, Reich RR, Paterson CL, et al. Examination of Broad Symptom Improvement Resulting From Mindfulness-Based Stress Reduction in Breast Cancer Survivors: A Randomized Controlled Trial. J Clin Oncol. 2016;34(24):2827–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johns SA, Brown LF, Beck‐Coon K, Monahan PO, Tong Y, Kroenke K. Randomized controlled pilot study of mindfulness‐based stress reduction for persistently fatigued cancer survivors. Psycho‐Oncology. 2015;24(8):885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johns SA, Brown LF, Beck-Coon K, et al. Randomized controlled pilot trial of mindfulness-based stress reduction compared to psychoeducational support for persistently fatigued breast and colorectal cancer survivors. Supportive Care in Cancer. 2016:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niemiec CP, Brown KW, Kashdan TB, et al. Being present in the face of existential threat: The role of trait mindfulness in reducing defensive responses to mortality salience. J Pers Soc Psychol. 2010;99(2):344. [DOI] [PubMed] [Google Scholar]
- 36.Branstrom R, Kvillemo P, Moskowitz JT. A randomized study of the effects of mindfulness training on psychological well-being and symptoms of stress in patients treated for cancer at 6-month follow-up. Int J Behav Med. 2012;19(4):535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chambers SK, Foley E, Galt E, Ferguson M, Clutton S. Mindfulness groups for men with advanced prostate cancer: a pilot study to assess feasibility and effectiveness and the role of peer support. Supportive Care in Cancer. 2012;20(6):1183–1192. [DOI] [PubMed] [Google Scholar]
- 38.Labelle LE, Campbell TS, Faris P, Carlson LE. Mediators of Mindfulness‐Based Stress Reduction (MBSR): Assessing the Timing and Sequence of Change in Cancer Patients. J Clin Psychol. 2015;71(1):21–40. [DOI] [PubMed] [Google Scholar]
- 39.Henderson VP, Clemow L, Massion AO, Hurley TG, Druker S, Hébert JR. The effects of mindfulness-based stress reduction on psychosocial outcomes and quality of life in early-stage breast cancer patients: a randomized trial. Breast Cancer Res Treat. 2012;131(1):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermann FF, Burrell B, Jordan J. The acceptability and potential benefits of mindfulness-based interventions in improving psychological well-being for adults with advanced cancer: A systematic review. Complement Ther Clin Pract. 2018;30:68–78. [DOI] [PubMed] [Google Scholar]
- 41.Lengacher CA, Kip KE, Barta M, et al. A pilot study evaluating the effect of mindfulness-based stress reduction on psychological status, physical status, salivary cortisol, and interleukin-6 among advanced-stage cancer patients and their caregivers. J Holist Nurs. 2012;30(3):170–185. [DOI] [PubMed] [Google Scholar]
- 42.Milbury K, Engle R, Tsao A, et al. Pilot Testing of a Brief Couple-Based Mind-Body Intervention for Patients With Metastatic Non-Small Cell Lung Cancer and Their Partners. J Pain Symptom Manage. 2018;55(3):953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsang SC, Mok ES, Lam SC, Lee JK. The benefit of mindfulness-based stress reduction to patients with terminal cancer. J Clin Nurs. 2012;21(17–18):2690–2696. [DOI] [PubMed] [Google Scholar]
- 44.Lehto RH, Wyatt G, Sikorskii A, Tesnjak I, Kaufman VH. Home-based mindfulness therapy for lung cancer symptom management: a randomized feasibility trial. Psycho-oncology. 2015;24(9):1208–1212. [DOI] [PubMed] [Google Scholar]
- 45.van den Hurk DG, Schellekens MP, Molema J, Speckens AE, van der Drift MA. Mindfulness-based stress reduction for lung cancer patients and their partners: results of a mixed methods pilot study. Palliat Med. 2015:0269216315572720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moss AH, Lunney JR, Culp S, et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med. 2010;13(7):837–840. [DOI] [PubMed] [Google Scholar]
- 47.Robinson TM, Alexander SC, Hays M, et al. Patient–oncologist communication in advanced cancer: predictors of patient perception of prognosis. Supportive Care in Cancer. 2008;16(9):1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–656. [PubMed] [Google Scholar]
- 49.Cottingham AH, Beck-Coon K, Bernat JK, et al. Addressing personal barriers to advance care planning: Qualitative investigation of a mindfulness-based intervention for adults with cancer and their family caregivers. Palliat Support Care. 2018:1–10. [DOI] [PubMed] [Google Scholar]
- 50.Santorelli S, Kabat-Zinn J. Mindfulness-based stress reduction professional education and training resource manual: MBSR standards of practice, curriculum, and supporting materials. UMass Medical School; 2013. [Google Scholar]
- 51.Kramer G Insight dialogue: The interpersonal path to freedom. Shambhala Publications; 2007. [Google Scholar]
- 52.Indiana State Department of Health. Indiana Physician Order for Scope of Treatment (POST). State Form 55317 (R2 / 12–16). Indiana State Department of Health – IC 16–36-6; 2016. Downloaded from http://www.indianapost.org/wp-content/uploads/2016/12/Indiana-POST-Form.pdfon 1/31/19.
- 53.Rogers LQ, Fogleman A, Trammell R, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther. 2013;12(4):323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fried TR, Redding CA, Robbins ML, Paiva A, O’Leary JR, Iannone L. Stages of change for the component behaviors of advance care planning. Journal of the American Geriatrics Society. 2010;58(12):2329–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12(1):38–48. [DOI] [PubMed] [Google Scholar]
- 56.Mesters I, van den Borne H, McCormick L, Pruyn J, de Boer M, Imbos T. Openness to discuss cancer in the nuclear family: scale, development, and validation. Psychosomatic Med. 1997;59(3):269–279. [DOI] [PubMed] [Google Scholar]
- 57.Cohen SR, Mount BM, Strobel MG, Bui F. The McGill Quality of Life Questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliat Med. 1995;9(3):207–219. [DOI] [PubMed] [Google Scholar]
- 58.Weitzner MA, Jacobsen PB, Wagner H Jr., Friedland J, Cox C. The Caregiver Quality of Life Index-Cancer (CQOLC) scale: development and validation of an instrument to measure quality of life of the family caregiver of patients with cancer. Qual Life Res. 1999;8(1–2):55–63. [DOI] [PubMed] [Google Scholar]
- 59.Watson M, Law MG, Santos Md, Greer S, Baruch J, Bliss J. The Mini-MAC: further development of the mental adjustment to cancer scale. J Psychosoc Oncol. 1994;12(3):33–46. [Google Scholar]
- 60.Carver CS. You want to measure coping but your protocol’too long: Consider the brief cope. Int J Behav Med. 1997;4(1):92–100. [DOI] [PubMed] [Google Scholar]
- 61.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–173. [DOI] [PubMed] [Google Scholar]
- 62.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 63.Bysse D, Reynolds C III, Monk T. The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 64.Hann D, Jacobsen P, Azzarello L, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7(4):301–310. [DOI] [PubMed] [Google Scholar]
- 65.Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: further validation of the Fatigue Symptom Inventory. Qual Life Res. 2000;9(7):847–854. [DOI] [PubMed] [Google Scholar]
- 66.Wagner CD, Johns S, Brown LF, Hanna N, Bigatti SM. Acceptability and Feasibility of a Meaning-Based Intervention for Patients With Advanced Cancer and Their Spouses A Pilot Study. Am J Hosp Palliat Care. 2015:1049909115575709. [DOI] [PubMed] [Google Scholar]
- 67.Cohen J Statistical power analysis for behavioural sciences, Hillsdale (NJ): Lawrence Eribaum Associates. Inc:[sn]. 1988. [Google Scholar]
- 68.Norman GR, Sloan JA, Wyrwich KW. The truly remarkable universality of half a standard deviation: Confirmation through another look. Expert Rev Pharmacoecon Outcomes Res. 2004;4(5):581–585. [DOI] [PubMed] [Google Scholar]
- 69.Page P Beyond statistical significance: Clinical Interpretation of rehabilitation research literature. Int J Sports Phys Ther. 2014;9(5):726–736. [PMC free article] [PubMed] [Google Scholar]
- 70.Simon J, Porterfield P, Bouchal SR, Heyland D. ‘Not yet’and ‘Just ask’: barriers and facilitators to advance care planning—a qualitative descriptive study of the perspectives of seriously ill, older patients and their families. BMJ Support Palliat Care. 2013:bmjspcare-2013–000487. [DOI] [PubMed] [Google Scholar]
- 71.Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast-and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30(12):1335–1342. [DOI] [PubMed] [Google Scholar]
- 72.Carlson LE, Tamagawa R, Stephen J, Drysdale E, Zhong L, Speca M. Randomized-controlled trial of mindfulness-based cancer recovery versus supportive expressive group therapy among distressed breast cancer survivors (MINDSET): long-term follow-up results. Psychooncology. 2016;25(7):750–759. [DOI] [PubMed] [Google Scholar]
- 73.Tang YY, Holzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16(4):213–225. [DOI] [PubMed] [Google Scholar]
- 74.Jha AP, Stanley EA, Kiyonaga A, Wong L, Gelfand L. Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion. 2010;10(1):54–64. [DOI] [PubMed] [Google Scholar]
- 75.Narang AK, Wright AA, Nicholas LH. Trends in Advance Care Planning in Patients With Cancer: Results From a National Longitudinal Survey. JAMA Oncol. 2015;1(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacKenzie MA, Smith-Howell E, Bomba PA, Meghani SH. Respecting Choices and Related Models of Advance Care Planning: A Systematic Review of Published Evidence. Am J Hosp Palliat Care. 2018;35(6):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.American Society of Clinical Oncology. ASCO Answers: Advanced Cancer Care Planning: A Decision-Making Guide for Patients and Families Facing Serious Illness. American Society of Clinical Oncology; 2014; Alexandria, VA./References> [Google Scholar]