Abstract
This study investigated whether the predictive ability of the Finnish Diabetes Risk Score (FINDRISC) can be improved among people with HIV by adding a marker of insulin resistance. In this longitudinal analysis of the Multicenter AIDS Cohort Study and the Women's Interagency HIV Study, HIV-positive and HIV-negative participants without prevalent diabetes were included. FINDRISC score and the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) were calculated at baseline. Cox proportional hazards models were used to examine associations between baseline risk scores and time to incident diabetes (first self-report of diabetes medication use). Model discrimination (Uno's c-statistic) and calibration (observed vs. cumulative probability of diabetes) were assessed for FINDRISC, HOMA-IR, and combined FINDRISC and HOMA-IR. Overall, 2,527 men (1,299 HIV-positive and 1,228 HIV-negative, median age = 44) and 2,446 women (1,841 HIV-positive and 605 HIV-negative, median age = 41) were included. Over 47,040 person-years of follow-up, diabetes incidence rates per 1,000 person-years were 9.5 in HIV-positive men, 7.1 in HIV-negative men, 14.5 in HIV-positive women, and 15.1 in HIV-negative women. FINDRISC discrimination (HIV-positive men c = 0.64 [0.55, 0.74], HIV-negative men c = 0.74 [0.68, 0.79], HIV-positive women c = 0.68 [0.64, 0.71], and HIV-negative women c = 0.73 [0.66, 0.79]) was significantly better than that of HOMA-IR. FINDRISC was better calibrated than HOMA-IR in each of the four groups. Adding HOMA-IR did not improve FINDRISC discrimination/calibration. Diabetes risk prediction with FINDRISC was suboptimal in men and women with HIV, and its performance was not improved with addition of HOMA-IR. The optimal method for identifying people living with HIV at-risk for diabetes is yet to be identified.
Keywords: insulin resistance, dysglycemia, risk prediction, HIV
Introduction
Type 2 diabetes is a significant comorbidity among people living with HIV (PWH). The national diabetes prevalence in PWH has surpassed that of the general U.S. population, reaching 12% in 2010.1 In addition, some cohort studies have shown higher diabetes incidence in PWH compared with those without HIV.2,3 HIV-related risk factors, such as increased systemic inflammation and exposure to early era antiretroviral therapies, increase diabetes risk.4–9 There is evidence that traditional risk factors, such as increasing age and obesity, are more influential in increasing diabetes risk among PWH.10 HIV clinical guidelines therefore recommend detecting individuals at risk for diabetes and addressing risk factors11–13; however, the optimal method for identifying at-risk PWH is yet to be identified.
Diabetes risk scores can be useful tools to identify PWH at risk for diabetes. For instance, the Finnish Diabetes Risk Score (FINDRISC)14 is a widely validated short questionnaire found to be effective for diabetes risk screening in the U.S. general population.15,16 In the HIV population, FINDRISC has been identified as a promising screening tool17 but its performance in the U.S. HIV population has been found to be suboptimal.18 Because PWH experience a high burden of insulin resistance owing to antiretroviral medication use and chronic systemic inflammation,9,19–21 adding a measure of insulin resistance may improve FINDRISC performance in the HIV population. Specifically, the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), which measures the relationship between plasma glucose and insulin concentrations,22 has been shown to be a strong predictor of diabetes development in the general population,23 and has potential for improving FINDRISC performance in PWH.
We assessed the comparative performance of FINDRISC and HOMA-IR separately and whether combining them improves prediction of incident diabetes among men and women living with and without HIV.
Methods
Study design and population
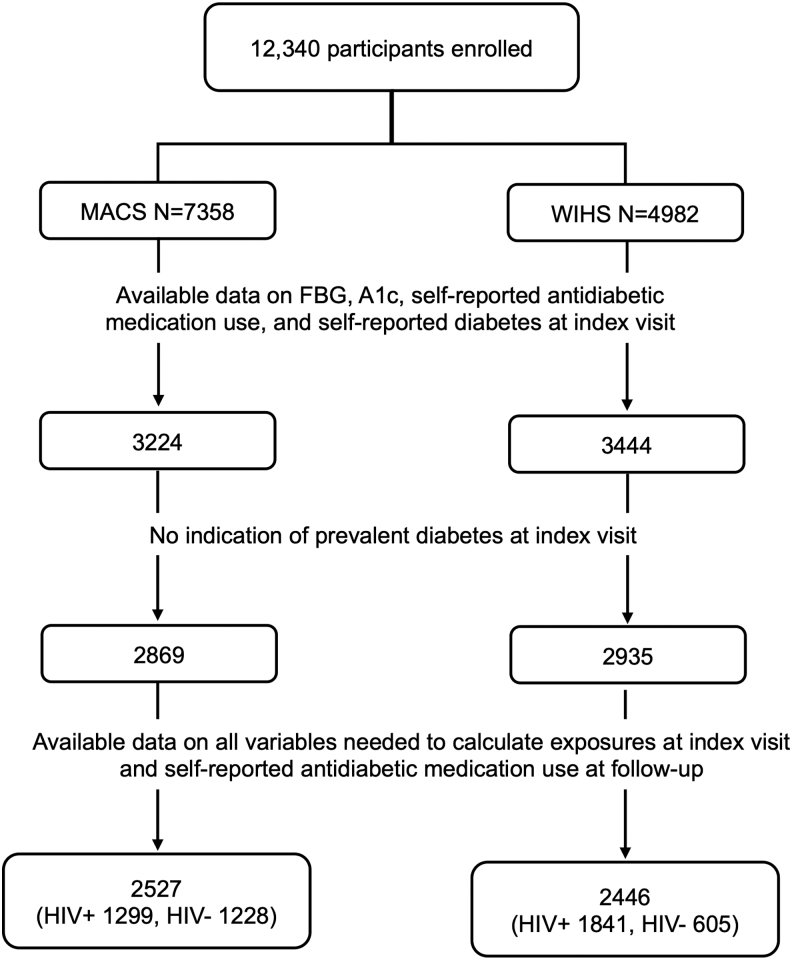
Our analyses included HIV-positive and HIV-negative participants from the Multicenter AIDS Cohort Study (MACS)24 and the Women's Interagency HIV Study (WIHS).25 Since their inception in 1984 and 1993, respectively, MACS has enrolled 7,358 men (3,270 HIV-positive and 4,088 HIV-negative) and WIHS has enrolled 4,982 women (3,677 HIV-positive and 1,305 HIV-negative). Participants attended a study visit every 6 months, where they underwent a comprehensive physical examination, blood sampling, and complete interviewer-administered questionnaires, which collected information on demographics, disease characteristics, and specific antiretroviral therapy use. The MACS and WIHS study protocols and consent forms were approved by the Institutional Review Board at each study site, and all participants provided written informed consent.
For the current analysis, we first identified 3,224 MACS and 3,444 WIHS participants that had an index visit, defined as the first visit in which data on fasting blood glucose, hemoglobin A1c, self-reported anti-diabetes medication use, and self-reported diabetes were concurrently available. Fasting blood glucose and hemoglobin A1c were collected since April 1999 in MACS and beginning in October 2000 in WIHS. In both studies, data on self-reported anti-diabetes medication use and self-reported diabetes were collected since study inception. From MACS and WIHS participants with an index visit, we excluded 355 (11%) MACS participants and 509 (15%) WIHS participants with prevalent diabetes (defined as fasting blood glucose ≥7.0 mmol/L or hemoglobin A1c ≥6.5%, or self-reported anti-diabetes medication use, or self-reported diabetes). From the remaining 2,869 MACS and 2,935 WIHS participants, we included participants with complete data for analyses.
Primary exposures, outcome, and participant characteristics
The exposures were the index visit values of FINDRISC and HOMA-IR for each participant. FINDRISC is a questionnaire that yields a score obtained by summing risk points based on participant age (0, 2, or 3 points), body mass index (0, 1, or 3 points), waist circumference (0, 3, or 4 points), history of elevated fasting blood glucose (0 or 5 points), and self-reported history of hypertension medication use (0 or 2 points). For our analysis, we used measured fasting blood glucose but FINDRISC uses a self-report for history of elevated blood glucose. We used the FINDRISC concise model that excludes physical activity and fruit and vegetable consumption14; this model has been validated in a biracial U.S. population.26 HOMA-IR was computed as [fasting insulin (μIU/mL) × fasting glucose (mmol/L)]/22.5,22 where values ≥2.0 indicate insulin resistance. All biochemical assays in MACS and WIHS were centralized and carried out by trained laboratory staff using standardized methods.24,25
The outcome of interest was incident diabetes, defined as the first time after the index visit at which the participant self-reported diabetes medication use. This diabetes outcome definition was used because it is the outcome for which FINDRISC was validated.14
Participant demographic characteristics (race, age, body mass index, and self-reported health insurance status) were summarized at the index visit. The proportion of participants with prediabetes (defined as fasting blood glucose 5.55–6.99 mmol/L or hemoglobin A1c 5.7%–6.4%) was also summarized at the index visit. Among HIV-positive participants, we calculated the proportion reporting stavudine use, ritonavir use, or any protease inhibitor use as of the index visit. The proportion of HIV-positive participants with undetectable HIV RNA, defined as HIV-1 RNA less than the lower limit of detection, was also obtained.
Statistical analysis
The ability of FINDRISC, HOMA-IR, and FINDRISC in combination with HOMA-IR for discriminating participants who developed diabetes from those who did not was assessed using Uno's c-statistic.27 Values falling between 0.50 and 0.59 indicated poor discrimination, 0.60–0.69 suboptimal discrimination, 0.70–0.79 acceptable discrimination, and ≥0.80 good discrimination.
Calibration was assessed by comparing the predicted cumulative probability of developing diabetes determined from a Cox regression model against the observed probability of developing incident diabetes for each exposure by sex/HIV group. Specifically, the ith participant had time (ti) to incident diabetes, an indicator for whether or not they experienced the event (δi = 0 if no event, δi = 1 if event), and an index visit value for each predictor (Xi), which is FINDRISC or HOMA-IR value.
From each Cox model, we estimated the probability that the ith participant developed diabetes by 10 years, Fi (10) by evaluating the expression 1 – S0(10)e(β·Xi), where S0(10) is the value of the underlying survival function at 10 years, e is the inverse of the natural logarithm function, β is the log(relative hazard), and Xi is the value of either FINDRISC or HOMA-IR of the ith participant. When both FINDRISC and HOMA-IR were included in the same model, Xi is the constellation of index visit values of both FINDRISC and HOMA-IR for the ith participant.
The estimated probabilities Fi (10) for all participants were divided into tertiles, and the median value within each tertile was determined. The estimated median was then compared with the observed F(10) value from 1 – Kaplan–Meier curve at 10 years calculated from the (ti, δi) in a given tertile. An exposure was deemed well calibrated if the estimated median within a tertile was close to 1 – Kaplan–Meier curve at time t = 10 years and its corresponding 95% confidence interval. Ten years corresponded to the 38th percentile of follow-up time in MACS and 63rd percentile of follow-up time in WIHS. Individuals not reporting diabetes medication use were censored at the date of their last study visit. Analyses were conducted in SAS version 9.4.
Results
We included 1,299 HIV-positive and 1,228 HIV-negative men and 1,841 HIV-positive and 605 HIV-negative women in our analyses. Median (interquartile range) index visit dates were December 2002 (June 2000, August 2005) for HIV-positive men, August 2002 (October 2001, July 2003) for HIV-negative men, November 2002 (July 2001, January 2012) for HIV-positive women, and January 2003 (February 2002, December 2011) for HIV-negative women (Fig. 1).
FIG. 1.
Participant selection flow chart.
At the index visit, median ages were 44 years in men and 41 years for women and more women were of African American race than men (65% vs. 26%). Baseline prediabetes (fasting blood glucose, 5.55–6.99 mmol/L or hemoglobin A1c, 5.7%–6.4%) was more prevalent among women than men (31% vs. 25%). Women also had a higher median body mass index than men (27.8 kg/m2 vs. 25.1 kg/m2). Among HIV-positive participants, 52% men and 49% women had history of protease inhibitor use at the index visit, with 23% of all HIV-positive participants reporting a history of ritonavir use. An estimated 49% men and 45% women had viral suppression (HIV-1 RNA less than the lower limit of detection) at the index visit (Table 1).
Table 1.
Participant Characteristics by Sex and HIV Status at the Index Visit
| |
MACS |
WIHS |
||
|---|---|---|---|---|
| Characteristics | HIV positive (n = 1,299) | HIV negative (n = 1,228) | HIV positive (n = 1,841) | HIV negative (n = 605) |
| African American race (%) | 31 | 20 | 64 | 68 |
| Age (years) | 42 (35–48) | 46 (39–53) | 42 (35–47) | 39 (30–46) |
| Body mass index (kg/m2) | 24.7 (22.6–27.3) | 25.5 (23.1–28.3) | 27.6 (23.8–33.1) | 28.7 (23.8–34.5) |
| Prediabetesa (%) | 26 | 24 | 30 | 34 |
| Insulin (mmol/L) | 78.5 (58.3–112.5) | 70.1 (53.5–98.6) | 69.5 (41.7–111.1) | 55.6 (34.7–104.2) |
| Fasting blood glucose (mmol/L) | 5.0 (4.6–5.4) | 4.9 (4.6–5.3) | 4.7 (4.4–5.1) | 4.6 (4.3–5.0) |
| History of stavudine use (%) | 40 | NA | 35 | NA |
| History of protease inhibitor use (%) | 52 | NA | 49 | NA |
| History of ritonavir use (%) | 23 | NA | 23 | NA |
| CD4 cell count (cells/mm3) | 511 (343–721) | 883 (704–1,082) | 461 (289–656) | 970 (770–1,212) |
| Undetectable HIV RNAb (%) | 49 | NA | 45 | NA |
Values presented are percentages or median (IQR). The index visit is the first visit at which fasting blood glucose, hemoglobin A1c, self-reported antidiabetes medication use, and self-reported diabetes data were concurrently available.
Defined as fasting blood glucose 5.55–6.94 mmol/L or hemoglobin A1c 5.7%–6.4%.
Defined as HIV-1 RNA below limit of quantification.
IQR, interquartile range; MACS, Multicenter AIDS Cohort Study; WIHS, Women's Interagency HIV Study.
Index visit values for FINDRISC and HOMA-IR exposures by sex and HIV status are given in Table 2. HIV-positive and HIV-negative women had higher FINDRISC scores (median score 6) than HIV-positive (median score 3) and HIV-negative men (median score 4). Higher scores among women were driven by a greater prevalence of obesity, higher waist circumference, and hypertension. Overall, men presented with higher HOMA-IR values (indicating greater insulin resistance) than women. HOMA-IR values were also higher in HIV-positive than HIV-negative participants (Table 2). Insulin resistance (HOMA-IR ≥ 2.0) was more prevalent among men: 67% HIV-positive and 60% HIV-negative men, compared with 53% HIV-positive and 42% HIV-negative women.
Table 2.
Values for Primary Exposures at the Index Visit by HIV Status in 2,527 Multicenter AIDS Cohort Study (MACS) and 2,446 Women's Interagency HIV Study (WIHS) Participants
| |
MACS |
WIHS |
|||
|---|---|---|---|---|---|
| FINDRISC components | Risk points | HIV positive (n = 1,299) | HIV negative (n = 1,228) | HIV positive (n = 1,841) | HIV negative (n = 605) |
| Age, n (%) | |||||
| <45 years | 0 | 823 (63) | 557 (45) | 1,186 (64) | 432 (71) |
| 45 to <55 years | 2 | 392 (30) | 437 (36) | 549 (30) | 138 (23) |
| 55 to 65 years | 3 | 84 (6) | 234 (19) | 106 (6) | 35 (6) |
| Body mass index, n (%) | |||||
| <25 kg/m2 | 0 | 686 (53) | 531 (43) | 607 (33) | 190 (31) |
| 25 to <30 kg/m2 | 1 | 460 (35) | 488 (40) | 552 (30) | 145 (24) |
| ≥30 kg/me | 3 | 153 (12) | 209 (17) | 682 (37) | 270 (45) |
| Waist circumference, n (%) | |||||
| Men <37.0 inches, women <31.5 inches | 0 | 888 (68) | 681 (55) | 424 (23) | 166 (27) |
| Men 37.0 to <40.2, women 31.5 to <34.6 | 3 | 246 (19) | 266 (22) | 387 (21) | 106 (18) |
| Men ≥40.2 inches, women ≥34.6 inches | 4 | 165 (13) | 281 (23) | 1,030 (56) | 333 (55) |
| Fasting blood glucose, n (%) | |||||
| <5.55 mmol/L | 0 | 1,057 (81) | 1,029 (84) | 1,650 (90) | 555 (92) |
| 5.55 to <6.99 mmol/L | 5 | 242 (19) | 199 (16) | 191 (10) | 50 (8) |
| History of HTN medication, n (%) | |||||
| No | 0 | 1,166 (90) | 1,089 (89) | 1,469 (80) | 521 (86) |
| Yes | 2 | 133 (10) | 139 (11) | 372 (20) | 84 (14) |
| FINDRISC score, median (IQR)a | 3 (0–6) | 4 (1–7) | 6 (4–8) | 6 (3–7) | |
| HOMA-IR, median (IQR) | 2.5 (1.8–3.7) | 2.2 (1.6–3.3) | 2.1 (1.3–3.6) | 1.7 (1.1–3.2) | |
Index visit defined as the first visit at which fasting blood glucose, hemoglobin A1c, self-reported antidiabetic medication use, and self-reported diabetes data were concurrently available.
Score ranges from 0 to 17. Higher scores reflect higher risk for developing drug-treated diabetes.
HTN, hypertension; FINDRISC, Finnish Diabetes Risk Score; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance.
Regarding diabetes development, 237 of 2,527 men and 268 of 2,446 women self-reported diabetes medication use after index visit. Of these, 185 (78%) men and 180 (67%) women also had either fasting blood glucose ≥126 mg/dL or hemoglobin A1c ≥6.5%. However, 355 (14%) men and 179 (7%) women had fasting blood glucose ≥126 or hemoglobin A1c ≥6.5% and did not self-report diabetes medication use; hence, they were not counted as diabetes cases in our analysis. Incidence rates for self-reported diabetes medication use over 47,040 person-years of follow-up were 9.5 cases per 1,000 person-years for HIV-positive men, and 7.1 cases per 1,000 person-years for HIV-negative men. Among women, incidence rates for self-reported diabetes medication use were 14.5 cases per 1,000 person-years for HIV-positive, and 15.1 cases per 1,000 persons-years for HIV-negative women.
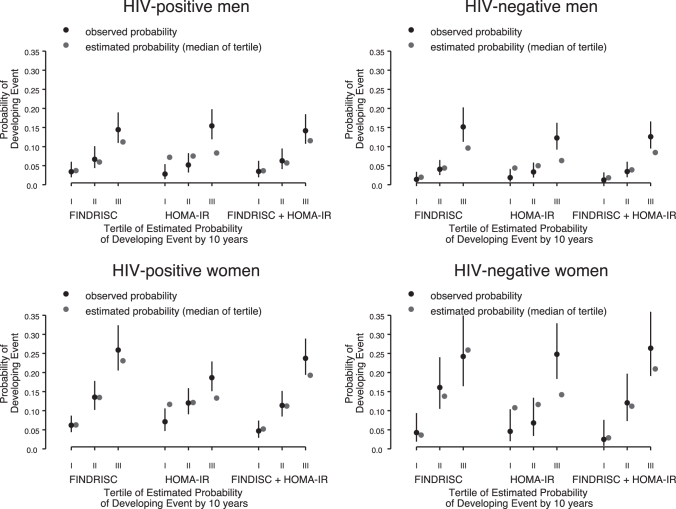
FINDRISC and HOMA-IR had similar discrimination in HIV-positive and HIV-negative men (Table 3). FINDRISC discrimination was better than that of HOMA-IR in both HIV-positive (c = 0.68 vs. 0.62, p = .006) and HIV-negative women (c = 0.73 vs. 0.64, p = .012). FINDRISC was better calibrated than HOMA-IR in all four groups, although the model underestimated diabetes risk in HIV-negative men in the highest risk tertile (Fig. 2). Adding HOMA-IR to FINDRISC did not improve the discrimination or calibration of FINDRISC.
Table 3.
Concordance Statistics (c) to Quantify the Ability of FINDRISC, HOMA-IR, and FINDRISC+HOMA-IR to Discriminate Risk of Incident Diabetes by Sex and HIV Status
| Sex/HIV group | n (%) with event | FINDRISC, c (95% CI) | HOMA-IR, c (95% CI) | FINDRISC+HOMA-IR, c (95% CI) |
|---|---|---|---|---|
| HIV-positive men (n = 1,299) | 130 (10) | 0.64 (0.55–0.74) | 0.67 (0.57–0.76) | 0.66 (0.55–0.76) |
| p = .599 (vs. FINDRISC) | p = .068 (vs. FINDRISC) | |||
| HIV-negative men (n = 1,228) | 107 (9) | 0.74 (0.68–0.79) | 0.72 (0.66–0.78) | 0.75 (0.70–0.80) |
| p = .602 (vs. FINDRISC) | p = .069 (vs. FINDRISC) | |||
| HIV-positive women (n = 1,841) | 197 (11) | 0.68 (0.64–0.71) | 0.62 (0.57–0.66) | 0.68 (0.65–0.72) |
| p = .006 (vs. FINDRISC) | p = .422 (vs. FINDRISC) | |||
| HIV-negative women (n = 605) | 71 (12) | 0.73 (0.66–0.79) | 0.64 (0.56–0.71) | 0.73 (0.67–0.79) |
| p = .012 (vs. FINDRISC) | p = .613 (vs. FINDRISC) |
CI, confidence interval; FINDRISC, Finnish Diabetes Risk Score; HOMA-IR, homeostatic model assessment of insulin resistance.
FIG. 2.
Observed (1 − Kaplan–Meier) versus estimated median cumulative probability in tertiles of reporting anti-diabetes medication use by 10 years by sex/HIV group using FINDRISC, HOMA-IR, and FINDRISC+HOMA-IR. FINDRISC, Finnish Diabetes Risk Score; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance.
Discussion
To inform diabetes risk screening practices in HIV care, we assessed whether the ability of FINDRISC to predict diabetes development in PWH can be improved by adding a measure of insulin resistance. We found FINDRISC and HOMA-IR had similar discrimination among men, whereas FINDRISC was superior among women. Calibration with FINDRISC was superior to that of HOMA-IR. However, FINDRISC discrimination was suboptimal in HIV-positive men and women, suggesting HIV-specific diabetes risk factors are not being captured in this tool. Of importance, despite a high prevalence of insulin resistance, adding HOMA-IR to FINDRISC did not improve FINDRISC performance. Screening tools that effectively capture diabetes risk in the HIV population are yet to be developed. Until these are available, FINDRISC could be used for diabetes risk screening in the HIV population, with caution around its suboptimal discrimination.
FINDRISC performance did not differ by HIV status but was suboptimal (c < 0.7) in HIV-positive men and women in this study. In HIV-positive women, FINDRISC discrimination mirrored our previous findings in WIHS,18 whereas in HIV-positive men, discrimination was lower than what was found in a London HIV population of men and women.17 FINDRISC performance did not differ between men and women; however, the risk score did capture a risk difference by sex. Specifically, women had a higher risk score (6 vs. 3), which reflected the greater prevalence of obesity and hypertension in women, and later translated into higher diabetes incidence.
FINDRISC was well calibrated for all groups except for HIV-negative men, where the model underestimated diabetes development in the highest risk group. As previously suggested,26 different score cutoffs for men and women, or recalibration of the algorithm components may be needed to improve its ability to predict diabetes development across sex groups.
Whereas addition of hemoglobin A1c has been found to improve FINDRISC performance in the U.S.,16 we found addition of HOMA-IR did not improve model performance in any of the groups studied. Furthermore, the performance of HOMA-IR alone was suboptimal in this study, and poorer than what has been reported in the general population.28 Because diabetes develops through a progressive failure of pancreatic β-cell function accompanied by increased insulin resistance,29 HOMA-IR can reflect different stages or paths in the natural history of diabetes. Indeed HOMA-IR is a measure of insulin resistance, which may be a better indicator of diabetes development in its early stages,30 and a limited predictor of fully developed and treated diabetes, as explored in this study.
In addition, HOMA-IR is tightly linked to body mass index, and it is possible that it may be a better predictor in those with obesity,31 something that was not examined in this study. Similarly, antiretroviral therapy is associated with insulin resistance,32 and its prediction ability may be affected by HIV medication class, which was not explored in this study. Overall, the usefulness of HOMA-IR as a stand-alone or add-on risk screening tool remains unclear in the setting of HIV infection.
Diabetes risk scores are simple tools that can be integrated in routine HIV care to identify PWH at-risk for diabetes. Although an HIV-specific diabetes risk score does not exist, available tools can be used in this effort. For instance, the D:A:D equation predicts 6-month diabetes risk in PWH4 and could be used by HIV providers with access to the online calculator. Furthermore, risk scores such as the American Diabetes Association Risk Score, the Q-Diabetes Risk Score, and FINDRISC have also been tested in PWH, with FINDRISC achieving superior performance.17,18 Ideally, HIV-specific risk score should be developed and current efforts in MACS and WIHS are focused on in this endeavor. Until an HIV-specific risk score is finalized, FINDRISC could be used in routine HIV care to allocate diagnostic testing and prevention resources more cost-effectively.
Our findings should be interpreted in light of some limitations. Because physical activity and fruit and vegetable consumption data were not complete in MACS and WIHS, we used the concise FINDRISC model; that said, these lifestyle measures remain important considerations in addressing diabetes risk. Our diabetes definition, although aligned with the FINDRISC outcome definition, excludes blood glucose and hemoglobin A1c measures and does not include undiagnosed and untreated diabetes cases or those not self-reporting diabetes medication use.
Conclusion
Type 2 diabetes is a prevalent comorbidity among PWH that will continue to increase as this population ages. Identification of at-risk PWH and early intervention should thus become routine practice in HIV care. Although tools like FINDRISC can be used in this endeavor, more work is needed to better understand and screen for diabetes risk in PWH. This will require investigating the natural history of this condition in PWH and developing HIV-specific risk assessment models. Expanding HIV care to identify and address cardiometabolic diseases such as diabetes is a necessary step to promote the quality of life and healthy aging of the HIV population.
Acknowledgments
Data in this article were collected by the Multicenter AIDS Cohort Study (MACS), and by the Women's Interagency HIV Study, now the MACS/WIHS Combined Cohort Study (MWCCS). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D'Souza, Stephen Gange, and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194.
Authors' Contributions
K.I.G. and T.T.B. designed the study, provided guidance for statistical analyses, provided interpretation of study findings, and drafted the article. M.F.S. conducted the statistical analyses, contributed to interpretation of findings, critically revised the article and approved submission. P.T., K.N.A., M.K.A., and I.O. contributed to study design, provided guidance for statistical analyses and interpretation of findings, critically revised the article and approved submission.
Disclaimer
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was funded by the by the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK111024). K.I.G. was supported by the National Heart, Lung, And Blood Institute (K01HL149479). T.T.B. and P.C.T. were supported in part by the National Institute of Allergy and Infectious Diseases (K24 AI120834 and K24 AI108516, respectively). K.N.A. was supported by the National Institute of Allergy and Infectious Diseases (U01 AI069918) and NIA (R01 AG053100). The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Human Genome Research Institute (NHGRI), National Institute on Aging (NIA), National Institute of Dental & Craniofacial Research (NIDCR), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Neurological Disorders and Stroke (NINDS), National Institute of Mental Health (NIMH), National Institute on Drug Abuse (NIDA), National Institute of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
References
- 1. Hernandez-Romieu AC, Garg S, Rosenberg ES, Thompson-Paul AM, Skarbinski J: Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care 2017;5:e000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown TT, Cole SR, Li X, et al. : Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the Multicenter AIDS Cohort Study. Arch Intern Med 2005;165:1179–1184 [DOI] [PubMed] [Google Scholar]
- 3. Tien PC, Schneider MF, Cox C, et al. : Association of HIV infection with incident diabetes mellitus: Impact of using hemoglobin A1C as a criterion for diabetes. J Acquir Immune Defic Syndr 2012;61:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petoumenos K, Worm SW, Fontas E, et al. : Predicting the short-term risk of diabetes in HIV-positive patients: The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. J Int AIDS Soc 2012;15:17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salehian B, Bilas J, Bazargan M, Abbasian M: Prevalence and incidence of diabetes in HIV-infected minority patients on protease inhibitors. J Natl Med Assoc 2005;97:1088–1092 [PMC free article] [PubMed] [Google Scholar]
- 6. Sanjay K, Bharti K, Navneet A, Unnikrishnan AG: Understanding diabetes in patients with HIV/AIDS Diabetol Metab Syndr 2011;3:2 [DOI] [PMC free article] [PubMed]
- 7. Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M, Justice AC: Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology 2004;40:115–119 [DOI] [PubMed] [Google Scholar]
- 8. De Wit S, Sabin CA, Weber R, et al. : Incidence and risk factors for new-onset diabetes in HIV-infected patients: The data collection on adverse events of anti-hiv drugs (D:A:D) Study. Diabetes Care 2008;31:1224–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ledergerber B, Furrer H, Rickenbach M, et al. : Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis 2007;45:111–119 [DOI] [PubMed] [Google Scholar]
- 10. Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. : HIV infection and the risk of diabetes mellitus. AIDS 2009;23:1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aberg JA, Gallant JE, Ghanem KG, et al. : Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014;58:1–10 [DOI] [PubMed] [Google Scholar]
- 12. Department of Health and Human Services: Side Effects of HIV Medicines—HIV and Diabetes. 2019. Available at https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-and-diabetes. Accessed January14, 2021
- 13. Ryom L, Cotter A, De Miguel R, et al.: EACS Governing Board. 2019. update of the European AIDS Clinical Society Guidelines for treatment of people living with HIV version 10.0. HIV Med. 2020. [Epub ahead of print]; doi: 10.1111/hiv.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lindström J, Tuomilehto J: The Diabetes Risk Score: A practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725–731 [DOI] [PubMed] [Google Scholar]
- 15. Zhang L, Zhang Z, Zhang Y, Hu G, Chen L: Evaluation of Finnish Diabetes Risk Score in screening undiagnosed diabetes and prediabetes among U.S. adults by gender and race: NHANES 1999–2010. PLoS ONE 2014;9:e97865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Hu G, Zhang L, Mayo R, Chen L: A Novel Testing Model for opportunistic screening of pre-diabetes and diabetes among U.S. adults. PLoS ONE 2015;10:e0120382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mok J, Goff L, Peters B, Duncan A: Comparison of risk tools to estimate type 2 diabetes risk in an urban HIV cohort. HIV Drug Therapy Conference; October 2016, Glasgow, United Kingdom, 2016 [Google Scholar]
- 18. Galaviz KI, Schneider MF, Tien CP, et al. : Predicting diabetes risk among HIV-positive and HIV-negative women. AIDS 2018;28:2767–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feeney ER, Mallon PW: Insulin resistance in treated HIV infection. Best Pract Res Clin Endocrinol Metab 2011;25:443–458 [DOI] [PubMed] [Google Scholar]
- 20. Hulgan T: Factors associated with insulin resistance in adults with hiv receiving contemporary antiretroviral therapy: a brief update. Current HIV/AIDS Rep 2018;15:223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tien PC, Schneider MFE, Cole SR, et al. : Antiretroviral therapy exposure and insulin resistance in the Women's Interagency HIV study. J Acquir Immune Defic Syndr 2008;49:369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 23. Song Y, Manson JE, Tinker L, et al. : Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: The Women's Health Initiative Observational Study. Diabetes Care 2007;30:1747–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Detels R, Jacobson L, Margolick J, et al. : The multicenter AIDS Cohort Study, 1983 to …. Public Health 2012;126:196–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adimora AA, Ramirez C, Benning L, et al. : Cohort Profile: The Women's Interagency HIV Study (WIHS). Int J Epidemiol 2018;47:393–394i [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kulkarni M, Foraker RE, McNeill AM, et al. : Evaluation of the modified FINDRISC to identify individuals at high risk for diabetes among middle-aged white and black ARIC study participants. Diabetes Obes Metab 2017;19:1260–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uno H, Cai T, Pencina MJ, D'Agostino RB, Wei LJ: On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 2011;30:1105–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rutter MK, Wilson PWF, Sullivan LM, Fox CS, D'Agostino RB, Meigs JB: Use of alternative thresholds defining insulin resistance to predict incident type 2 diabetes mellitus and cardiovascular disease. Circulation 2008;117:1003–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Skyler JS, Bakris GL, Bonifacio E, et al. : Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017;66:241–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wallace TM, Levy JC, Matthews DR: Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 31. Owei I, Umekwe N, Provo C, Wan J, Dagogo-Jack S: Insulin-sensitive and insulin-resistant obese and non-obese phenotypes: Role in prediction of incident pre-diabetes in a longitudinal biracial cohort. BMJ Open Diabetes Res Care 2017;5:e000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dagogo-Jack S: HIV Therapy and diabetes risk. Diabetes Care 2008;31:1267. [DOI] [PubMed] [Google Scholar]