Abstract
Functional magnetic resonance imaging (fMRI) has been widely used to examine the relationships between brain function and phenotypic features in neurodevelopmental disorders. Techniques such as resting-state functional connectivity (FC) have enabled the identification of the primary networks of the brain. One fMRI network, in particular, the default mode network (DMN), has been implicated in social-cognitive deficits in autism spectrum disorders (ASD) and attentional deficits in attention deficit hyperactivity disorder (ADHD). Given the significant clinical and genetic overlap between ASD and ADHD, surprisingly, no reviews have compared the clinical, developmental, and genetic correlates of DMN in ASD and ADHD and here we address this knowledge gap. We find that, compared with matched controls, ASD studies show a mixed pattern of both stronger and weaker FC in the DMN and ADHD studies mostly show stronger FC. Factors such as age, intelligence quotient, medication status, and heredity affect DMN FC in both ASD and ADHD. We also note that most DMN studies make ASD versus ADHD group comparisons and fail to consider ASD+ADHD comorbidity. We conclude, by identifying areas for improvement and by discussing the importance of using transdiagnostic approaches such as the Research Domain Criteria (RDoC) to fully account for the phenotypic and genotypic heterogeneity and overlap of ASD and ADHD.
Impact statement
In this work, we review the default mode network in autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD), as well as comorbid ASD+ADHD literature. Such a review has not been constructed in the field of cognitive neuroscience at this time, and it would greatly aid other behavioral and cognitive neuroscientists in identifying gaps in the field. In addition, the need to consider disorders to be on a continuum, as suggested by the Research Domain Criteria (RDoC), is important while identifying abnormal patterns in resting-state functional connectivity. This timely review will impact the field in a meaningful way, such that more research on the overlaps between ASD and ADHD is conducted along a spectrum.
Keywords: ADHD, ASD, attention deficit hyperactivity disorder, autism spectrum disorders, default mode network, resting fMRI
Introduction
Functional magnetic resonance imaging (fMRI) has shed light on the functional activity of the human brain. “Functional connectivity” (FC) is defined as correlations of spontaneous functional activity between different brain regions. These functionally correlated or connected regions collectively form functional networks (van den Heuvel et al., 2010; see review by Lee et al. 2013). Two fMRI techniques have been widely used to build a functional atlas of the human brain: task fMRI (Ehrsson et al., 2000) and resting fMRI (rfMRI) (Biswal et al., 1995; van den Heuvel and Hulshoff Pol, 2010). In task fMRI, subjects are asked to perform simple tasks, such as auditory, motor, working memory, or visual tasks, whereas fMRI data are acquired to identify brain regions associated with such tasks (Downar et al., 2001; Moshfeghi et al., 2016). In contrast, rfMRI does not involve task performance, instead, subjects are asked to lie still, relax, and focus on a fixation cross presented on a screen (van den Heuvel et al., 2010).
Understanding rfMRI FC provides valuable information regarding the inherent functional organization of the human brain and may provide a deeper understanding of brain–behavior relationships. The rfMRI FC approach has several advantages and disadvantages. It is a useful technique in that comparisons can be made across the developmental lifespan (Fox et al., 2012). FC also displays improved signal-to-noise ratio, provides the ability to image a variety of human clinical populations (e.g., depression, schizophrenia, autism-diagnosed individuals), and circumvents task-related confounds (Fox and Greicius, 2010). In addition, rfMRI FC is more efficient than task-related fMRI in obtaining better signal-to-noise ratio, given that during a standard fMRI task, many trials are required, and a large amount of time is spent on averaging data to obtain signal/activation maps (Fox and Greicius, 2010). Indeed, Fox and colleagues note that 20% of signal-to-noise ratio is seen in task activation studies, whereas rfMRI shows 3:1 improved signal-to-noise ratio, demonstrating superior signal quality over task fMRI. This provides significant advantages when trying to perform clinical neuroimaging in human subjects, as neurological abnormalities can potentially be detected in a shorter time. Disadvantages of FC include unclear performance due to ambiguity of mental state, the purely correlational nature (rather than causal), and difficulty in obtaining truly “resting” state data especially from children (Fox et al., 2012). Despite these limitations, the baseline networks of rfMRI have been reliably identified and rfMRI FC is widely used in behavioral and cognitive neuroscience.
Among rfMRI networks, one network of interest is the default mode network (DMN) (Raichle et al., 2001). The DMN primarily comprises the posterior cingulate cortex (PCC), the anterior cingulate cortex (ACC), medial prefrontal cortex (mPFC), medial temporal lobes (MTL), angular gyrus (AG), and the precuneus (Greicius et al., 2003; Supekar et al., 2010). The DMN has been shown to exhibit increased activity during rfMRI, with PCC and ACC areas displaying greater activity at rest than during task-based activities (Greicius et al., 2003).
Numerous rfMRI FC studies have been conducted with various clinical populations. To date, studies examining neurodevelopmental disorders have explored the influences of varying methodological approaches and developmental trends using FC. Uddin and colleagues (2010) conducted a review of atypical brain development in a variety of disorders, including autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD). However, to the best of our knowledge, a review of the ASD and ADHD rfMRI literature has not been conducted for the past 10 years, during which time the number of rfMRI studies has grown significantly. Having a systematic review would help to understand these trends and may further help in identifying robust neural signatures of these disorders. Therefore, the goal of this review is to highlight existing ASD and ADHD research efforts examining DMN FC while presenting the rationale for conducting ASD and ADHD research in the context of the Research Domain Criteria (RDoC) (Garvey et al., 2016). The RDoC is a framework created to understand clinical research integrating knowledge from genomics and neuroscience findings. The research in these domains may then ultimately be utilized to parse out clearer diagnostic criteria for psychiatric disorders. Garvey and associates (2016) outline clearly in the RDoC proposal guide that “RDoC classification assumes that the dysfunction in neural circuits can be identified with the tools of clinical neuroscience, including electrophysiology, functional neuroimaging, and new methods for quantifying connections in vivo.” To this end, we hope to use the RDoC framework to inform this review, and identify how ASD and ADHD lie on a clinical “spectrum.”
Existing Reviews of the DMN in ASD and ADHD Studies
A recent review by Padmanabhan and colleagues (2017) reviewed studies related to the DMN with a primary focus on ASD studies on both functional and structural findings. In addition, this review focused on DMN abnormalities with regards to social-cognitive dysfunction, task-related DMN abnormalities, prominent neurobiological abnormalities (i.e., excitation–inhibition imbalances), intrinsic FC differences in DMN implicated nodes, atypical cellular organization, and structural abnormalities associated with the DMN (Padmanabhan et al., 2017). Along with Padmanabhan and colleagues, a review focusing on the necessity of finding biomarkers in ASD and ADHD was published by Uddin and colleagues (2017). Although these topics are of interest in DMN research, the Padmanabhan DMN review did not emphasize methodological approaches and the implications of clinical abnormalities in the social-cognitive domain across the ASD spectrum. Rather, results were reported as to which DMN area reflected social-cognitive deficits. Further, in Uddin et al. (2017), the primary focus was not on the DMN and did not address ASD+ADHD comorbidity, or how the DMN was affected in comorbid ASD+ADHD. The primary focus of the Uddin and associates was related to biomarker approaches in ASD and ADHD. Stigler and colleagues (2011) included more information regarding the FC of areas such as the mPFC, PCC, and AG, however with very little information regarding clinical assessments used in the studies. Despite a wealth of information on ASD, ADHD, and comorbid ASD+ADHD, previous studies have not focused on specifically reviewing findings of the DMN in ASD and ADHD. Therefore, the primary goal of this review article is to focus on studies that captured the DMN as one of the main networks of interest. Given the shared connectivity patterns between ASD and ADHD, this review article seeks to identify commonalities in connectivity patterns, as well as identify differences between the two groups in developmental, clinical, and genetic factors.
Review Search Mechanism
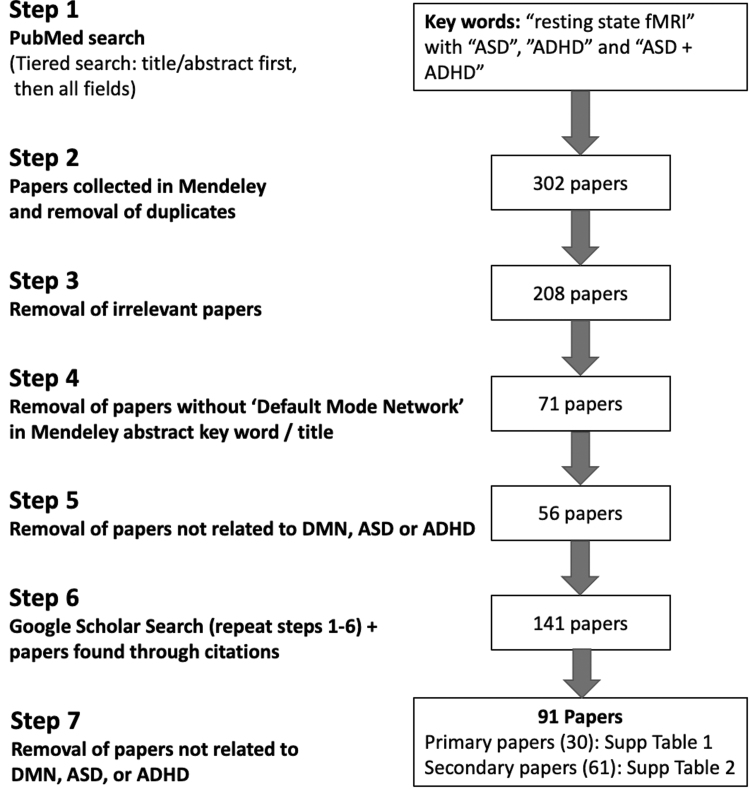
We conducted our search on PubMed, with specific keywords in both abbreviated and expanded forms. The goal was to narrow our search to articles published between 2010 and 2020 (i.e., since the Uddin et al., 2010 review) on ASD and ADHD that focused on FC in the DMN. Our search included “resting state, fMRI” within the “Title/Abstract” with “ASD,” “ADHD,” and “ASD+ADHD.” We next repeated the same keyword search as listed earlier, but the search was not limited just to “Title/Abstract” but “All Fields.” This two-tier search mechanism was applied as precedence was given to the “Title/Abstract” first and “All Fields” was checked to make sure all relevant manuscripts were captured. The searches cited earlier were repeated with “autism” instead of “ASD” and “Attention Deficit Hyperactivity Disorder” instead of “ADHD.”
Search results were collected by using Mendeley, and duplicate manuscripts were removed by using the “check for duplicates” function in Mendeley. This yielded 302 articles. Based on information provided in the abstract of the manuscripts, we narrowed the 302 manuscripts to 208 manuscripts after removing irrelevant articles. Next, we narrowed the 208 manuscripts to 71 DMN manuscripts after excluding manuscripts that did not include “Default Mode Network” in the Mendeley displayed abstract keyword list, or article title. Articles that were not related to the DMN in ASD or ADHD were removed, and this reduced the 71 DMN articles to 56. In addition to these 56 DMN articles, any other relevant DMN FC articles not captured by PubMed were included as they were cited in one of the 56 manuscripts in our list. Further, to account for any additional manuscripts related to this topic, a Google Scholar search was performed by applying the same search scheme described earlier. The total number of articles after this process was 141:60 DMN only articles (55 from Mendeley, 3 from Yerys et al., 2015; 1 from Google Scholar missed by Mendeley, and 1 newly published in press), and 81 additional studies that relate to the DMN and FC.
We then categorized the 141 articles as “primary” and “secondary”; primary articles are those that directly addressed DMN in ASD or ADHD and secondary are articles that are relevant to this topic. Based on this categorization, we were able to further remove articles that were not relevant to this review, and this reduced the 141 articles to 91.
Our final review is based on these 91 articles, 30 of which were primary articles (Supplementary Table S1) and 61 were secondary (Supplementary Table S2). Several articles were added into the review after additional suggestions from reviewers. See Figure 1 for a complete overview of our selection process.
FIG. 1.
Flow chart of article search mechanism.
Developmental Trends of the DMN in ASD and ADHD
One of the primary ongoing discussions in the neurodevelopmental literature involves developmental trajectories and delays in ASD and ADHD. Understanding FC of the DMN in ASD and ADHD will enable researchers to find commonalities, as well as differences in connectivity patterns between these disorders. These patterns, in turn, could have significant implications for the presence or absence of key clinical symptoms in ASD and ADHD.
Mixed rfMRI developmental trends of both underconnectivity and overconnectivity are found in the ASD literature (Lynch et al., 2013; Yao et al., 2016), leading to differing DMN FC observations. Lynch et al. (2013) posited that there may be a “switch” during the period of development in ASD children where connectivity patterns switch from hyperconnectivity to hypoconnectivity. This was discussed in depth by Uddin et al. (2013a), identifying a particular trend in children with ASD related to salience network hyperconnectivity. The study by Uddin et al. (2013a) found that using a classifier approach, ASD versus typically developing (TD) connectivity was distinguished from the TD group with 83% accuracy, 67% sensitivity, and 100% specificity. This study conclusively identified that this unique feature in ASD children may help in identifying specific biomarkers for ASD children. A further review by Uddin et al. (2013b) addresses this mixed trend of hyper and hypoconnectivity, with reduced connectivity predominantly found in adults with ASD, and overconnectivity predominantly found in children with ASD. Trends of increased FC in the anterior module of the DMN have been observed in ASD (Jann et al., 2015). Local overconnectivity in the ASD group was found in occipital and posterior temporal regions, and underconnectivity was found in middle/posterior cingulate, and medial prefrontal regions (Maximo et al., 2013). Studies have also identified underconnectivity in ASD, specifically reduced functional connectivity densities (FCDs) in ASD versus TD in both inter- and intra-hemispheric FCDs in the PCC, lingual/parahippocampal gyrus, and post central gyrus (Lee et al., 2016).
A review of functional and structural magnetic resonance imaging (MRI) studies summarized these trends of both over and underconnectivity in DMN regions in ASD versus TD comparisons. Specifically, structural abnormalities included abnormal findings in cortical gray and white matter volume in individuals with ASD. In addition, increased head circumference, increased total brain volume, and brain overgrowth were found in early developmental stages in ASD individuals (Stigler et al., 2011). Further, individuals with autism spectrum conditions (ASCs) compared with the TD group were found to have reduced connectivity of the DMN, including areas such as the mPFC. Using independent components analysis, von dem Hagen and colleagues noted reduced seed based connectivity between the insula and amygdala. In addition, the salience network incorporating insula, MTL network and amygdala demonstrated reduced inter-network connectivity in individuals with ASCs (von dem Hagen et al., 2013). Further studies demonstrated decreased connectivity between posterior and frontal regions in the DMN (i.e., precuneus/posterior cingulate gyrus) (Yao et al., 2016) and increased evidence of an excitation/inhibition balance found in ASD individuals associated with poor segregation of the DMN (Yerys et al., 2015). Decreased long-range FC, as opposed to local increased FC, was observed in ASD versus TD, particularly decreased FC between the precuneus and mPFC/ACC in ASD patients (Assaf et al., 2010). DMN FC in the bilateral inferior parietal lobule and PCC were decreased in an ASD group than Healthy Control children in a study by Funakoshi and colleagues (2016).
In contrast to the mixed FC trends in ASD, ADHD studies displayed a more global trend of widespread and increased FC. Young adolescents and children have shown increases in FC in the prefrontal regions as well as widespread increased FC across the whole brain, particularly increased FC in the right inferior frontal gyrus and bilateral mPFC in ADHD children versus TD (Bos et al., 2017). Specific maturational delays and lags were found in DMN connectivity between DMN regions and task positive networks (TPNs), such as the fronto-parietal network (FPN) and ventral attentional network (VAN). These delays strongly suggest the theory of a “developmental delay hypothesis” in ADHD tied to the delayed maturation of DMN networks, which may contribute to ADHD-related behavioral deficits in patients (Sripada et al., 2014). Further evidence of hyperconnectivity was found between two attentional networks (dorsal and ventral attention networks), as well as within the DMN and ventral attention network (Sidlauskaite et al., 2016), leading to potential links between clinical relationships in attention and DMN FC. Although it is unclear as to whether ADHD DMN evolves across the lifespan, evidence has shown atypical brain FC in DMN regions implicated in ADHD patients. Abnormal brain FC was displayed in ADHD adults between the dorsal ACC and PCC, providing evidence that ADHD adults mirrored younger, typically developing individuals during brain development (Sato et al., 2012). These findings provide further credence to the developmental delay hypothesis in that ADHD symptoms are tied to poor maturation of the DMN. However, it is unclear as to whether this delay can be attributed to other cognitive and intellectual disability that can be comorbid in ADHD.
Summary
Mixed trends of overconnectivity and underconnectivity in ASD point to the overall presence of varied clinical symptoms (Assaf et al., 2010; Chen et al., 2016; Chien et al., 2015; Jann et al., 2015; Lynch et al., 2013; Plitt et al., 2015; Stigler et al., 2011; von dem Hagen et al., 2013; Yao et al., 2016; Yerys et al., 2015) and restricted and repetitive behavior (Lee et al., 2016). In contrast, ADHD FC studies indicate specific attentional deficits such as mind wandering and attentional fluctuations, which are directly linked to DMN FC aberrations between the cerebellar DMN (cerDMN) and regions in the visual and dorsal attentional networks in an ADHD versus TD comparison (Kucyi et al., 2015). In addition to these behavioral deficits, delayed segregation of prefrontal brain areas have been identified in individuals with ADHD (Bos et al., 2017). Although ASD DMN networks present a mixed pattern of both over and underconnectivity, ADHD DMN patterns primarily exhibit overconnectivity in DMN regions. Further, maturational lags in ADHD DMN connectivity demonstrate that they may play an important role in behavioral symptoms of ADHD.
Associations of DMN FC with Clinical Factors
The ASD studies have shown relationships between clinical deficits and DMN FC. Atypically increased intrinsic FC has been shown to be positively correlated to social deficits, as measured with the Autism Diagnostic Inventory—Revised (ADI-R) and Social Responsive Scores (SRS) (Chien et al., 2015). Other ASD studies have shown relationships between ASD symptoms measured with the SRS and the DMN (Elton et al., 2016; Plitt et al., 2015), as well as numerous studies delineating relationships between social cognition processing/social impairments and the DMN FC (Glerean et al., 2016; Jung et al., 2015; Lynch et al., 2013; von dem Hagen et al., 2013; Weng et al., 2010).
In contrast to social deficits in ASD, clinical deficits related to the ADHD DMN areas include issues with attention and impulsivity. Sudre et al. (2017) noted that association with ADHD symptoms correlated strongly with the right superior longitudinal fasciculus tract. Decreased correlations between hyperactive/impulsive symptoms and FC between the right superior temporal gyrus and the precuneus/cuneus to the DMN have been noted (Elton et al., 2014), as well as decreased negative correlations between the cerDMN and the dorsal attention network (DAN) with increased inattentive symptoms (Kucyi et al., 2015).
Correlations between behavioral measures and these DMN networks identified negative correlations between TPNs and inattention, confirming that a greater lag of DMN maturation and interconnections with the TPNs corresponded to increased symptoms of inattention as found on the Conners Parent Rating Scale—Long Version (CPRS-LV) (Keith Conners et al., 1998; Sripada et al., 2014).
Genetic Studies and the DMN
The majority of existing DMN-related genetic research has been performed in ADHD populations by utilizing a categorical, rather than a dimensional RDoC approach. In ASD literature, to the best of our knowledge, no research has focused on genetic studies related to the DMN. An interesting genomic study performed by Esteller-Cucala et al. (2020) has identified that ADHD-associated alleles are traced back to an older time frame for when alleles evolved, and the frequency of variants associated with ADHD has decreased since the Paleolithic times.
In ADHD individuals, shared heritability was demonstrated for 58 out of 66 possible family pairs (87.9%), suggesting that nuclear and extended families demonstrated shared heritability in ADHD for DMN regions (Sudre et al., 2017). Sudre and colleagues found correlations between cognitive control and DANs at trend level. With regards to clinical symptomatology, associations between heritable FC of the DMN and hyperactivity/impulsivity, as well as inattentive symptoms were found in a study of 52 nuclear families and 24 multigenerational extended families (Sudre et al., 2017), highlighting an important link between genetic and clinical symptoms in ADHD. Decreased DMN FC was associated with increased symptoms of hyperactivity/impulsivity and inattention. van der Meer et al. (2017) found more conclusive evidence between genetic and behavioral links in ADHD, citing a positive correlation between stress exposure and FC of the supramarginal gyrus within the DMN for S-allele carriers compared with L-allele homozygotes (van der Meer et al., 2017).
Finally, an overall link between increased FC in the DMN with the interaction between 5-HTTLPR (a serotonin transporter) and stress exposure (environment) was noted (van der Meer et al., 2017), concluding a significant gene × environment interaction involved in affecting the DMN in ADHD. van der Meer and colleagues also noted specifically a clinical–gene interplay, noting that the interaction between 5-HTTLPR genotype and stress exposure was associated with differences in FC in the executive control network and the DMN, which significantly affected the processing of emotional stimuli in ADHD individuals. In the executive control network, S-allele carriers in ADHD individuals were found to have an increased negative association with stress exposure and FC than those with L-alleles. L-alleles, in contrast, displayed a more positive relationship between stress exposure and FC of the posterior hub of the DMN (van der Meer et al., 2017). In conclusion, van der Meer and colleagues also noted that higher DMN FC during rest was directly related to greater trait rumination (i.e., focusing on negative, self-referential thinking) (Berman et al., 2011; cited in van der Meer et al., 2017) whereas decreased cognitive control was related to rumination and worrying (Beckwé et al., 2014; cited in van der Meer et al., 2017).
A review outlined by Dennis and Thompson (2013) underlies the relationship between genetic abnormality-based disorders, such as Fragile X syndrome and comorbid ASD (Dennis and Thompson, 2013). Fragile X syndrome is a single-gene mutation that is associated with elevated risk of ASD. The review noted that Fragile X accounted for 5% of ASD cases (Budimirovic and Kaufmann, 2011). Existing studies have shown that the DMN is significantly affected by genetic factors after looking at family pedigrees and heritability factors (Glahn et al., 2010). Indeed, Glahn and colleagues highlighted that a greater need for research investigating the link between various psychiatric illnesses and the shared changes in the DMN network among these illnesses is warranted. Given the sparse amount of studies on this topic, further inquiry is needed for using an RDoC framework to see whether genetic factors common in ASD and ADHD (and other neurodevelopmental disorders) are linked to similar patterns of FC in these disorders.
ASD+ADHD Comorbid Group Studies: A Brief Discussion
Existing and growing evidence suggest that ADHD and ASD genotypes overlap significantly (Bathelt et al., 2020; Chantiluke et al., 2014; Christakou et al., 2012; Mulligan et al., 2009; Simonoff et al., 2008). Recent studies have indicated that ASD and ADHD present shared endophenotypes, which need to be explored further in DMN research (Kernbach et al., 2018). A study investigating 35,073 study participants including children and mothers with ASD and ADHD diagnoses identified a 2.0% prevalence rate for ADHD (ages 6–12 years in children), and 0.8% for ASD; 0.2% of the full sample (19% ASD, 9.6% ADHD) had comorbid ASD+ADHD (Musser et al., 2014). Rommelse et al. (2010) discussed that ASD-ADHD occur at a high co-frequency such that 20–50% of children with ADHD meet criteria for ASD, and 30–80% of ASD children meet criteria for ADHD. With this evidence of shared prevalence rates, DMN FC deficits between both disorders are shared as well and further investigated later.
This was recently studied by Wang et al. (2019), who demonstrated strong evidence that dysfunction of the DMN is a central feature of co-occurrence in ASD and ADHD, and strongly supports the theory of a clinically combined phenotype. Wang and colleagues investigated DMN connectivity, specifically intrinsic functional connectivity (iFC) in four cohorts: 162 ASD, 79 co-diagnosed ASD+ADHD, 83 ADHD, and 177 TD participants. On examining the DMN FC both within (intra-iFC) and between (inter-iFC), the ASD+ADHD group demonstrated increased social impairment, and decreased intra-iFC in the bilateral PCC, and increased inter-iFC between the DMN-somatomotor networks in comparison to the ASD-only group. More interestingly, the strength of intra-iFC in the DMN was found to be associated with increased autistic trait severity across the ASD group, especially the ASD+ADHD group (Wang et al., 2019). This recent study clearly demonstrates the growing need to utilize an RDoC approach, and combine comorbid ASD+ADHD presentations with existing ASD and ADHD groups to understand shared deficits (both behavioral and imaging related) between the groups.
Despite growing evidence of shared clinical and genetic overlaps between the two disorders, ASD+ADHD comorbidity has been discussed sparingly in the literature. To the best of our knowledge, only a few studies have focused on comorbid ASD+ADHD, with DMN as the main network of interest. More comparative studies between comorbid ASD+ADHD, ASD, and ADHD groups such as Wang and colleagues would present a clearer picture of DMN connectivity profiles, and how they are similar to or different from each other. Comorbid ASD+ADHD presentations have emerged as an important clinical group, as opposed to studies that presented ASD and ADHD as separate groups (Johnston et al., 2013; Leitner, 2014; Pondé et al., 2010). Further, shared heritability has been an important topic discussed in the current literature (Craig et al., 2016; Musser et al., 2014; Rommelse et al., 2010), but it has not been examined with respect to DMN connectivity. This is a gap in the literature that needs to be addressed.
Surveying the current literature, previous studies have focused on ASD and ADHD as separate disorders (Ray et al., 2014; Strang et al., 2014; Taurines et al., 2012) with definitions before the DSM-5 listing autism and ADHD as distinct clinical disorders as opposed to existing on one clinical spectrum (Regier et al., 2013). Here, an RDoC-based approach would serve as an excellent method for understanding similarities between ASD and ADHD. ASD and ADHD have been compared against other disorders, such as Tourette's syndrome (TS) (Kern et al., 2015), but with little discussion to DMN FC. Instead, much of the discussion was focused exclusively on neurobiological factors such as neuroinflammation, excitoxicity, and other factors that led to similar neural profiles (i.e., long-range underconnectivity, and short-range overconnectivity patterns). Interestingly, Kern and colleagues did mention that ASD, ADHD, and TS belong under a larger umbrella of neurodevelopmental disorders due to these shared neural profiles. This strengthens the argument for an RDoC-like dimensional approach, as opposed to a categorical one (Kern et al., 2015).
Finally, existing studies looking at ASD, ADHD, and ASD+ADHD groups investigated conditions such as response time variability in the three groups (Tye et al., 2016), and recent studies on sluggish cognitive tempo (SCT); however, with little mention or emphasis on the DMN FC of the three groups (Camprodon-Rosanas et al., 2019; Duncan et al., 2019). Duncan et al. (2019) noted that SCT is highly comorbid with ASD, with social cognitive deficits in comorbid SCT-ASD individuals related to clinical symptoms such as increased ASD symptomatology and internalizing symptoms. Although such clinical correlates are extremely important in understanding ASD symptomatology, these are disparate and unrelated to the DMN. The conclusion is that far more studies must be performed while comparing the DMN between ASD, ADHD, and ASD+ADHD (such as Wang et al., 2019), and they must utilize an RDoC approach investigating genetic, clinical, and neuroimaging methods to fully understand how the two disorders overlap.
Additional Considerations
Head motion
One of the most important parameters to consider in FC research is the presence of head motion. Given that subjects during resting-state scans are required to lie as still as possible, the presence of head motion creates undesirable artifacts in FC data (Power et al., 2012, 2013). These artifacts can directly affect DMN FC, causing them to be incomplete or disrupted (Power et al., 2012). Thus, Power et al. (2012) proposed a technique referred to as data “scrubbing,” in which high motion data points are eliminated entirely from FC analyses (Power et al., 2012). This process has been shown to significantly improve seed correlations of the DMN. Given these concerns regarding head motion, it comes as no surprise that ASD and ADHD studies also present such issues. In particular, ASD and ADHD studies are vulnerable to the effects of head motion, given that ASD and ADHD subjects often exhibit difficulties lying still in the scanner, and are prone to greater movement compared with healthy controls (Chen et al., 2016). However, Yerys et al. (2015) noted significant DMN interactions with salience, processing, and coordinating motor response networks in ASD subjects that remained even after controlling for head motion.
In addition, Maximo et al. (2013) considered global signal regression (GSR) to remove motion artifacts and its impacts on FC results. After including GSR in one of the analysis, Maximo et al. (2013) noted that removal of the GSR resulted in emphasizing overconnectivity effects in the ASD group in the left lateral temporal cortex (Maximo et al., 2013), thereby creating no changes in the group comparison (Saad et al., 2012). Maximo et al. (2013) used low motion subsamples and noted local overconnectivity in the ASD group in occipital and posterior temporal regions, as well as underconnectivity in the medial prefrontal regions and middle/posterior cingulate regions. This suggested that even with the use of low motion subsamples, cingulate and medial frontal underconnectivity highlighted atypical patterns of DMN FC. The use of low motion subsamples may have improved the correlations of DMN regions. This study utilized only high functioning individuals and did not represent the lower spectrum of ASD individuals.
In ADHD studies, controlling for mean relative head displacement did not change the significance of results; that is, the same regions exhibiting greater FC in ADHD compared with healthy controls appeared significant both when controlling and when not controlling for motion (Kucyi et al., 2015). However, harmful effects of head motion correction have been noted by Sun et al. (2012), where the removal of global mean signal may have accounted for anti-correlations in their DMN results (Sun et al., 2012).
Intelligence quotient
Multiple studies have indicated that high-functioning subjects are predominantly used in ASD research (Assaf et al., 2010; Ebisch et al., 2011; Tyszka et al., 2014; Vissers et al., 2012), as lower functioning individuals are difficult to scan in the MRI machine (Mazzone and Curatolo, 2010). Given the general predominance of high intelligence quotient (IQ) subjects, existing ASD studies may be biased in study design given that lower IQ individuals (e.g., individuals with more severe ASD symptomatology) are typically excluded due to IQ cutoffs. Therefore, studies generally draw on an exclusively high IQ sample of ASD participants, which may not capture the full variability and clinical heterogeneity of ASD. This issue is also present in ADHD studies. Kucyi et al. (2015) noted that when studying the cerDMN in ADHD subjects, one limitation of the study was that high IQ subjects were used. IQ and cognitive ability significantly affect inclusion/exclusion criteria in both ASD and ADHD studies. In a study tracking subjects with 1 year of brain imaging data, ASD subjects without intellectual disabilities were found to show no outcome improvement when IQ was included in a brain-based regression model (Plitt et al., 2015). Further, the study noted that FC involving many regions including the DMN was highly predictive of future autistic traits, and the change of these traits and behavior over time. However, these findings were exclusively based on male subjects.
Sample size
In addition to the factors, sample size is also an important consideration in interpreting results. The ASD studies present difficulties in interpreting clinical scores with regards to small sample sizes as well as difficulties with DMN feature classification through machine-learning techniques (Plitt et al., 2015). Having a larger sample size would eliminate these issues in ASD and ADHD research, since a larger subject pool would enable greater feature classification (with machine learning), advanced understanding of DMN regional FC especially in ADHD (van der Meer et al., 2017), stronger interpretation of clinical scores due to a wide range of subjects, and reduced random effects. Other issues with small sample sizes in ADHD research include the inability to distinguish subject variability between ADHD subtypes (i.e., inattentive, hyperactive, combined subtypes) (Kucyi et al., 2015). Larger sample sizes would also allow to better capture variance associated with a disorder, by allowing for application of a “divide and conquer” approach (i.e., dividing low and normal verbal IQ individuals into sub-groups because of the low number of individuals with verbal IQ <85) to yield improved results (Katuwal et al., 2016). Further, Katuwal et al. (2016) demonstrated that dividing ASD populations into more homogeneous subgroups improved ASD classification accuracy by using structural brain features (Katuwal et al., 2016). As such, translating these efforts into DMN FC studies would prove particularly useful in identifying relationships between DMN abnormalities and sample size-related changes.
Discussion
Summary of connectivity trends
In this review, we highlight the need for a more comprehensive patient profile in ASD and ADHD research. Specifically, this review identifies the need for a more nuanced approach and to view neuropsychiatric disorders as existing along quantitative dimensions spanning various domains of dysfunction. More importantly, the review recognizes the considerable overlap in symptom expression, leading to high rates of comorbidity between ASD and ADHD. This is in contrast to the traditional, purely taxonomic approach regarding ASD and ADHD as two separate and distinct disorders as currently found in the literature.
Findings of mixed trends in ASD studies with over and underconnectivity in DMN regions present concerns for measuring trends across a clinical spectrum (Assaf et al., 2010; DiMartino et al., 2011; Glerean et al., 2016; Maximo et al., 2013; Stigler et al., 2011; Venkat Raghavan et al., 2017). Although underconnectivity and overconnectivity appear to be linked to a wide range of social, communicative, deficits and delays in children and adults with ASD, ADHD studies link DMN dysfunction to their associated attentional deficits, punctuating the role of the DMN in the core symptoms associated with these two disorders. One reason for the mixed trends in ASD could be that ASD symptomatology is far more nuanced than expected; for example, differing delays in social cognitive networks could explain and lead to specific underdevelopment in specific areas, that is, decreased connectivity in regions associated with social cognition, but typical development of other behaviors and connectivity patterns. Wang et al. (2019) found this trend, mentioning that decreased iFC strength in the bilateral PCC of the DMN was noted in ASD patients, which could underlie the severity of ASD traits. Wang et al. also found that having comorbid ASD+ADHD may further worsen the hypoconnectivity demonstrated within the social cognition-related DMN regions in ASD patients, leading to increased dysfunctions in social cognition. Comorbid individuals may have one disorder “acting up” more than the other, that is, ASD symptoms playing more of a role in patterns associated with ASD DMN connectivity patterns in ASD+ADHD patients.
The ADHD articles present a different picture, with primarily increased connectivity. The increased connectivity in the DMN-DAN and increased connectivity between the salience—VAN suggest that specific behavioral deficits are rooted in these key networks, as opposed to a mixed pattern of connectivity (Sidlauskaite et al., 2016). Interestingly, on taking a closer look, these same attentional networks also present a maturational lag as noted by Sripada et al. (2014), particularly in the DMN and interconnections with the FPN and VAN. These findings denote a clear distinction between ASD and ADHD in that ASD symptoms are primarily characterized by more heterogeneous symptoms that are readily reflected in both under and overconnectivity; The ADHD symptoms, on the other hand, are more centralized and focused to specific attentional network dysfunctions related to the DMN, and specific developmental delays that attribute to these dysfunctions. In summary, linking ASD behavioral traits with specific connectivity patterns is a far more complex task and increasingly difficult to identify as opposed to ADHD traits, which have a distinct, consistent set of networks that consistently malfunction. Here, understanding and comparing comorbid ASD+ADHD connectivity data with ASD or ADHD groups would greatly help in teasing apart unique connectivity patterns between the two disorders.
Future Directions: RDoC Methodology in DMN Studies
A review by Dougherty and colleagues (2016) of structural brain imaging findings comparing ASD and ADHD children indicated the utility of an RDoC approach (Insel et al., 2010). Dougherty and colleagues (2016), however, reviewed both shared and some unique brain structure abnormalities in ASD and ADHD. Although distinct social deficits in ASD (Chien et al., 2015; Elton et al., 2016; Jung et al., 2015; Lynch et al., 2013; Venkataraman et al., 2015; von dem Hagen et al., 2013; Weng et al., 2010) present symptomatology differences compared with attentional deficits found in ADHD (Elton et al., 2014; Kucyi et al., 2015; Lee et al., 2017; Sripada et al., 2014), it is important to note that ASD and ADHD are also present as comorbid disorders (Chantiluke et al., 2014; Leitner, 2014). Current diagnostic practices fail to account for overlapping symptom dimensions, creating inaccurate dichotomies that obscure clinical realities (Mayes et al., 2012). Given these existing difficulties, using an RDoC approach would potentially help alleviate many of the diagnostic ambiguities, such as incorrect classification of comorbid ASD+ADHD individuals into either ASD or ADHD cohorts. Open-source databases such as ABIDE and ADHD-200 should be used (see Wang et al., 2019) to further understand the DMN due to the large sample sizes present in these databases. In addition to these approaches, more studies examining the effects of medication status on DMN FC across the ASD spectrum should be considered, as well as in ADHD subtypes. As noted in the study by Kernbach et al. (2018), ASD and ADHD present shared neural signatures in the DMN, DAN, and salience network regions. Understanding these similarities would help with RDoC classification, and bridge ASD and ADHD as overlapping clinical disorders with shared network patterns. Additional comorbidity in diseases such as benign childhood epilepsy with centrotemporal spikes with ADHD (Xiao et al., 2015) as well as social cognition related studies (Silani et al., 2013) are important and interesting avenues for future DMN studies.
Given the aforementioned gaps regarding comorbid ASD+ADHD literature, there are many avenues as to which future DMN studies can advance RDoC goals. Further, this approach can lead to the identification of neural correlates of brain dysfunction along the continuum and may provide more insights than the current research practice of searching for brain markers that are based on group differences (Dougherty et al., 2016). Finally, studies examining comorbid ASD+ADHD individuals should be compared against ASD and ADHD groups using longitudinal studies. This would add to the RDoC hypothesis of measuring developmental brain changes across the lifespan. The developmental brain delay approach posits that a specific cluster of genetic deficits (i.e., mutations or genetic abnormalities) found in early childhood may be responsible for clinical deficits in disorders such as autism. For example, a deletion in the gene such as a 16p 11.2 or 22q11.2 deletion greatly increases the probability of developing a neurodevelopmental disorder (Moreno-De-Luca et al., 2013). Given the existing challenges of comorbidity that confounds clinical diagnoses along with shared heritability in ASD and ADHD (Rommelse et al., 2010), it is important to consider diagnosing ADHD and ASD by using a cross-diagnostic, RDoC approach to account for clinical and genetic factors that are shared between the two disorders. Given that there are no ASD+ADHD comorbid DMN articles to compare the studies cited in this review, it is difficult to generalize DMN trends across ASD+ADHD comorbid disorders. Further limitations in ASD studies include the predominance of high-functioning ASD individuals as opposed to the entire clinical spectrum, and little research comparing DMN FC in ADHD subtypes (Fair et al., 2012). This topic has been recently explored by investigating ADHD subtypes versus ASD populations (Harikumar, 2018), and future studies should examine comparisons between ADHD subtypes, comorbid ASD+ADHD, and typically developing groups.
Finally, given the RDoC emphasis on the association between developmental trajectories and clinical symptoms in ASD and ADHD, further longitudinal investigations in ASD and ADHD will be necessary for the developmental course of the links between DMN and clinical presentation. Variables such as age, gender, and cognitive developmental status of subjects also affect the FC results presented in these cross-sectional studies, and they create an ongoing challenge for research in ASD and ADHD.
Supplementary Material
Author Disclosure Statement
The authors present no conflicts of interest at this time.
Funding Information
No funding was received.
Supplementary Material
References
- Assaf M, Jagannathan K, Calhoun VD, et al. . 2010. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage 53:247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathelt J, Caan MWA, Geurts HM. 2020. More similarities than differences between ADHD and ASD in functional brain connectivity. pp. 1–17. DOI: 10.31234/osf.io/4tfmn [DOI] [Google Scholar]
- Beckwé M, Deroost N, Koster EHW, et al. . 2014. Worrying and rumination are both associated with reduced cognitive control. Psychol Res 78:651–660 [DOI] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, et al. . 2011. Depression, rumination and the default network. Soc Cogn Affect Neurosci 6:548–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, et al. . 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Bos DJ, Oranje B, Achterberg M, et al. . 2017. Structural and functional connectivity in children and adolescents with and without attention deficit/hyperactivity disorder. J Child Psychol Psychiatry 58:810–818 [DOI] [PubMed] [Google Scholar]
- Budimirovic DB, Kaufmann WE. 2011. What can we learn about autism from studying fragile X syndrome? Dev Neurosci 33:379–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camprodon-Rosanas E, Pujol J, Martínez-Vilavella G, et al. . 2019. Brain structure and function in school-aged children with sluggish cognitive tempo symptoms. J Am Acad Child Adolesc Psychiatry 58:256–266 [DOI] [PubMed] [Google Scholar]
- Chantiluke K, Christakou A, Murphy CM, et al. . 2014. Disorder-specific functional abnormalities during temporal discounting in youth with Attention Deficit Hyperactivity Disorder (ADHD), Autism and comorbid ADHD and Autism. Psychiatry Res 223:113–120 [DOI] [PubMed] [Google Scholar]
- Chen H, Duan X, Liu F, et al. . 2016. Multivariate classification of autism spectrum disorder using frequency-specific resting-state functional connectivity-A multi-center study. Prog Neuropsychopharmacol Biol Psychiatry 64:1–9 [DOI] [PubMed] [Google Scholar]
- Chien H-Y, Lin H-Y, Lai M-C, et al. . 2015. Hyperconnectivity of the right posterior temporo-parietal junction predicts social difficulties in boys with autism spectrum disorder. Autism Res 8:427–441 [DOI] [PubMed] [Google Scholar]
- Christakou A, Murphy CM, Chantiluke K, et al. . 2012. Disorder-specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with Autism. Mol Psychiatry 18:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig F, Margari F, Legrottaglie AR, et al. . 2016. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat 12:1191–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM. 2013. Mapping connectivity in the developing brain. Int J Dev Neurosci 31:525–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, et al. . 2011. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry 69:847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty CC, Evans DW, Myers SM, et al. . 2016. A comparison of structural brain imaging findings in autism spectrum disorder and attention-deficit hyperactivity disorder. Neuropsychol Rev 26:25–43 [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, et al. . 2001. The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. Neuroimage 14:1256–1267 [DOI] [PubMed] [Google Scholar]
- Duncan A, Tamm L, Birnschein AM, et al. . 2019. Clinical correlates of sluggish cognitive tempo in adolescents with autism spectrum disorder. Autism 23:1354–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJH, Gallese V, Willems RM, et al. . 2011. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp 32:1013–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, et al. . 2000. Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol 83:528–536 [DOI] [PubMed] [Google Scholar]
- Elton A, Alcauter S, Gao W. 2014. Network connectivity abnormality profile supports a categorical-dimensional hybrid model of ADHD. Hum Brain Mapp 35:4531–4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Di Martino A, Hazlett HC, et al. . 2016. Neural connectivity evidence for a categorical-dimensional hybrid model of autism spectrum disorder. Biol Psychiatry 80:120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller-Cucala P, Maceda I, Børglum AD, et al. . 2020. Genomic analysis of the natural history of attention-deficit/hyperactivity disorder using Neanderthal and ancient Homo sapiens samples. Sci Rep 10:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, et al. . 2012. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Halko M, Eldaief M, et al. . 2012. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation. Neuroimage 62:2232–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M. 2010. Clinical applications of resting state functional connectivity. Front Syst Neurosci 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi Y, Harada M, Otsuka H, et al. . 2016. Default mode network abnormalities in children with autism spectrum disorder detected by resting-state functional magnetic resonance imaging. J Med Invest 63:204–208 [DOI] [PubMed] [Google Scholar]
- Garvey M, Avenevoli S, Anderson K. 2016. The National Institute of Mental Health Research domain criteria and clinical research in child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry 55:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Winkler AM, Kochunov P, et al. . 2010. Genetic control over the resting brain. Proc Natl Acad Sci U S A 107:1223–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glerean E, Pan RK, Salmi J, et al. . 2016. Reorganization of functionally connected brain subnetworks in high-functioning autism. Hum Brain Mapp 37:1066–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, et al. . 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar A. 2018. The default mode network in autism spectrum disorder and attention deficit hyperactivity disorder: a comparative study. Doctoral dissertation. San Diego, CA: San Diego State University [Google Scholar]
- Insel T, Cuthbert B, Garvey M, et al. . 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders [DOI] [PubMed]
- Jann K, Hernandez LM, Beck-Pancer D, et al. . 2015. Altered resting perfusion and functional connectivity of default mode network in youth with autism spectrum disorder. Brain Behav 5:e00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K, Dittner A, Bramham J, et al. . 2013. Attention deficit hyperactivity disorder symptoms in adults with autism spectrum disorders. Autism Res 6:225–236 [DOI] [PubMed] [Google Scholar]
- Jung M, Mody M, Saito DN, et al. . 2015. Sex differences in the default mode network with regard to autism spectrum traits: a resting state fMRI study. PLoS One 10:e0143126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katuwal GJ, Baum SA, Cahill ND, et al. . 2016. Divide and conquer: sub-grouping of ASD improves ASD detection based on brain morphometry. PLoS One 11:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith Conners C, Sitarenios G, Parker JDA, et al. . 1998. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 26:257–268 [DOI] [PubMed] [Google Scholar]
- Kern JK, Geier DA, King PG, et al. . 2015. Shared brain connectivity issues, symptoms, and comorbidities in autism spectrum disorder, attention deficit/hyperactivity disorder, and Tourette syndrome. Brain Connect 5:321–335 [DOI] [PubMed] [Google Scholar]
- Kernbach J, Satterthwaite T, Bassett D, et al. . 2018. Shared endo-phenotypes of default mode dysfunction in attention deficit/hyperactivity disorder and autism spectrum disorder. Transl Psychiatry 8:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Hove MJ, Biederman J, et al. . 2015. Disrupted functional connectivity of cerebellar default network areas in attention-deficit/hyperactivity disorder. Hum Brain Mapp 36:3373–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Lee JE, Lee JE, et al. . 2017. Altered functional connectivity in default mode network in Internet gaming disorder: influence of childhood ADHD. Prog Neuropsychopharmacol Biol Psychiatry 75:135–141 [DOI] [PubMed] [Google Scholar]
- Lee JM, Kyeong S, Kim E, et al. . 2016. Abnormalities of inter- and intra-hemispheric functional connectivity in autism spectrum disorders: a study using the autism brain imaging data exchange database. Front Neurosci 10:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Smyser CD, Shimony JS. 2013. Resting state fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol 34:1866–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner Y. 2014. The co-occurrence of autism and attention deficit hyperactivity disorder in children—what do we know? Front Hum Neurosci 8:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CJ, Uddin LQ, Supekar K, et al. . 2013. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biol Psychiatry 74:212–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximo JO, Keown CL, Nair A, et al. . 2013. Approaches to local connectivity in autism using resting state functional connectivity MRI. Front Hum Neurosci 7:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL, Mayes RD, et al. . 2012. Autism and ADHD: overlapping and discriminating symptoms. Res Autism Spectr Disord 6:277–285 [Google Scholar]
- Mazzone L, Curatolo P. 2010. Conceptual and methodological challenges for neuroimaging studies of autistic spectrum disorders. Behav Brain Funct 6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-De-Luca A, Myers SM, Challman TD, et al. . 2013. Developmental brain dysfunction: revival and expansion of old concepts based on new genetic evidence. Lancet Neurol 12:406–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshfeghi Y, Triantafillou P, Pollick FE. Understanding Information Need: an fMRI Study. In Proceedings of the 39th International ACM SIGIR conference on Research and Development in Information Retrieval, Pisa, Italy, 2016, pp. 335–344 [Google Scholar]
- Mulligan A, Anney RJL, O'Regan M, et al. . 2009. Autism symptoms in attention-deficit/hyperactivity disorder: a familial trait which correlates with conduct, oppositional defiant, language and motor disorders (Journal of Autism and Developmental Disorders DOI: 10.1007/s10803-008-0621-3). J Autism Dev Disord 39:210–211 [DOI] [PubMed] [Google Scholar]
- Musser ED, Hawkey E, Kachan-Liu SS, et al. . 2014. Shared familial transmission of autism spectrum and attention-deficit/hyperactivity disorders. J Child Psychol Psychiatry 55:819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Lynch CJ, Schaer M, et al. . 2017. The default mode network in autism. Biol Psychiatry Cogn Neurosci Neuroimaging 2:476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitt M, Barnes KA, Wallace GL, et al. . 2015. Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proc Natl Acad Sci U S A 112:E6699–E6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondé MP, Novaes CM, Losapio MF. 2010. Frequency of symptoms of attention deficit and hyperactivity disorder in autistic children. Arq Neuropsiquiatr 68:103–106 [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, et al. . 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, et al. . 2013. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage 76:439–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, et al. . 2001. A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Miller M, Karalunas S, et al. . 2014. Structural and functional connectivity of the human brain in autism spectrum disorders and attention-deficit/hyperactivity disorder: a rich club-organization study. Hum Brain Mapp 35:6032–6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Kuhl EA, Kupfer DJ. 2013. The DSM-5: classification and criteria changes. World Psychiatry 12:92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse NNJ, Franke B, Geurts HM, et al. . 2010. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry 19:281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, et al. . 2012. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato JR, Hoexter MQ, Fujita A, et al. . 2012. Evaluation of pattern recognition and feature extraction methods in ADHD prediction. Front Syst Neurosci 6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidlauskaite J, Sonuga-Barke E, Roeyers H, et al. . 2016. Altered intrinsic organisation of brain networks implicated in attentional processes in adult attention-deficit/hyperactivity disorder: a resting-state study of attention, default mode and salience network connectivity. Eur Arch Psychiatry Clin Neurosci 266:349–357 [DOI] [PubMed] [Google Scholar]
- Silani G, Lamm C, Ruff CC, et al. . 2013. Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. J Neurosci 33:15466–15476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, et al. . 2008. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 47:921–929 [DOI] [PubMed] [Google Scholar]
- Sripada CS, Kessler D, Angstadt M. 2014. Lag in maturation of the brain's intrinsic functional architecture in attention-deficit/hyperactivity disorder. Proc Natl Acad Sci U S A 111:14259–14264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigler KA, McDonald BC, Anand A, et al. . 2011. Structural and functional magnetic resonance imaging of autism spectrum disorders. Brain Res 1380:146–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang JF, Kenworthy L, Dominska A, et al. . 2014. Increased gender variance in autism spectrum disorders and attention deficit hyperactivity disorder. Arch Sex Behav 43:1525–1533 [DOI] [PubMed] [Google Scholar]
- Sudre G, Choudhuri S, Szekely E, et al. . 2017. Estimating the heritability of structural and functional brain connectivity in families affected by attention-deficit/hyperactivity disorder. JAMA Psychiatry 74:76–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Cao Q, Long X, et al. . 2012. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naïve boys with attention deficit hyperactivity disorder. Psychiatry Res 201:120–127 [DOI] [PubMed] [Google Scholar]
- Supekar K, Uddin L, Prater K, et al. . 2010. Development of functional and structural connectivity within the default mode network in young children. Neuroimage 52:290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurines R, Schwenck C, Westerwald E, et al. . 2012. ADHD and autism: differential diagnosis or overlapping traits? A selective review. Atten Defic Hyperact Disord 4:115–139 [DOI] [PubMed] [Google Scholar]
- Tye C, Johnson KA, Kelly SP, et al. . 2016. Response time variability under slow and fast-incentive conditions in children with ASD, ADHD and ASD+ADHD. J Child Psychol Psychiatry 57:1414–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyszka JM, Kennedy DP, Paul LK, et al. . 2014. Largely typical patterns of resting-state functional connectivity in high-functioning adults with autism. Cereb Cortex 24:1894–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Dajani DR, Voorhies W, et al. . 2017. Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Transl Psychiatry 7:e1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, et al. . 2013a. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry 70:869–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. 2010. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. 2013b. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci 7:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. 2010. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 20:519–534 [DOI] [PubMed] [Google Scholar]
- van der Meer D, Hartman CA, Pruim RHR, et al. . 2017. The interaction between 5-HTTLPR and stress exposure influences connectivity of the executive control and default mode brain networks. Brain Imaging Behav 11:1486–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkat Raghavan L, Bharadwaj S, Wourms V, et al. . 2017. Brain resting state functional connectivity is preserved under sevoflurane anesthesia in patients with pervasive developmental disorders—a pilot study. Brain Connect 7:250–257 [DOI] [PubMed] [Google Scholar]
- Venkataraman A, Duncan JS, Yang DY-J, et al. . 2015. An unbiased Bayesian approach to functional connectomics implicates social-communication networks in autism. Neuroimage Clin 8:356–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts HM. 2012. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev 36:604–625 [DOI] [PubMed] [Google Scholar]
- von dem Hagen EAH, Stoyanova RS, Baron-Cohen S, et al. . 2013. Reduced functional connectivity within and between “social” resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci 8:694–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Xu M, Ji Y, et al. . 2019. Altered social cognition and connectivity of default mode networks in the co-occurrence of autistic spectrum disorder and attention deficit hyperactivity disorder. Aust N Z J Psychiatry 53:760–771 [DOI] [PubMed] [Google Scholar]
- Weng S-J, Wiggins JL, Peltier SJ, et al. . 2010. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res 1313:202–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Li L, An D, et al. . 2015. Altered attention networks in benign childhood epilepsy with centrotemporal spikes (BECTS): a resting-state fMRI study. Epilepsy Behav 45:234–241 [DOI] [PubMed] [Google Scholar]
- Yao Z, Hu B, Xie Y, et al. . 2016. Resting-state time-varying analysis reveals aberrant variations of functional connectivity in autism. Front Hum Neurosci 10:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Gordon EM, Abrams DN, et al. . 2015. Default mode network segregation and social deficits in autism spectrum disorder: evidence from non-medicated children DMN in children with ASD. Neuroimage Clin 9:223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.