Abstract
People with HIV (PWH) are at an increased risk of developing nonalcoholic fatty liver disease (NAFLD). Interleukin (IL)-18 is regulated by inflammasomes in response to pathogens and danger signals and has been implicated in both the pathogenesis of NAFLD and HIV disease progression. We hypothesized that increased IL-18 may be associated with NAFLD and liver injury in PWH. This was an observational study of 125 PWH and 59 individuals without HIV in the Boston area. Participants with known hepatitis B, hepatitis C, and excessive alcohol use were excluded. IL-18 was measured in serum by enzyme-linked immunosorbent assay. Liver lipid content was assessed by liver-to-spleen computed tomography (CT) attenuation ratio. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and IL-18 levels were higher in PWH than in controls. In PWH, log10 IL-18 was associated with log10AST (r = 0.34, p = .0001), log10ALT (r = 0.33, p = .0002), log10HIV RNA (r = 0.29, p = .002), and inversely associated with liver-to-spleen ratio (r = −0.24, p = .02). In addition, log10 IL-18 was associated with log10 triglycerides (r = 0.26, p = .003), log10 MCP-1 (monocyte chemoattractant protein-1; r = 0.33, p = .0004), log10caspase-1 (r = 0.35, p < .0001), log10LPS (r = 0.28, p = .004), and inversely associated with high-density lipoprotein (r = −0.28, p = .002), and CD4+/CD8+ T cell ratio (r = −0.24, p = .007). In controls without HIV, log10 IL-18 was also associated with log10ALT (r = 0.44, p = .0005). After adjusting for potential confounders, the relationships between IL-18 and AST (p = .004) and ALT (p = .003) remained significant, and the relationship between IL-18 and liver-to-spleen ratio (p = .02). Increased inflammasome activation and subsequent monocyte recruitment in PWH may contribute to the development and progression of NAFLD. Clinical Trials Registration. NCT00455793.
Keywords: NAFLD, NASH, inflammasome, interleukin-18
Introduction
With the success of antiretroviral therapy and improved life expectancy from decreased AIDS-related morbidities, metabolic complications such as nonalcoholic fatty liver disease (NAFLD) are increasing in people with HIV (PWH). NAFLD prevalence estimates range from 30% to 40% in PWH,1 and histologic studies suggest increased severity of NAFLD in PWH compared with non-HIV patients.2 Traditional risk factors such as insulin resistance and dyslipidemia are more common in PWH and are implicated in the development of NAFLD. In addition, chronic low-grade inflammation, which is persistent in PWH despite viral suppression, and intestinal microbial translocation and dysbiosis present in PWH are important factors that have also been associated with the pathogenesis of NAFLD.3
More recently, the role of inflammasomes has been elucidated in the progression of NAFLD by modulating the inflammatory response.4 The activation of inflammasome components is evident in nonalcoholic steatohepatitis (NASH), and it is also required for the development of fibrosis.5,6 In particular, NLRP3 inflammasomes are cytosolic multiprotein complexes located in liver immune cells and hepatocytes that sense pathogens or host cell damage to activate caspase-1, which in turn produces effector cytokines interleukin-1β (IL-1β) and interleukin-18 (IL-18). These cytokines create a proinflammatory and profibrotic milieu by increasing the expression of secondary proinflammatory cytokines and chemokines including tumor necrosis factor alpha and monocyte chemoattractant protein-1/chemokine ligand 2 (MCP-1/CCL2), recruiting inflammatory cells, and activating hepatic stellate cells.4 MCP-1-mediated migration of monocytes is an important contributor to inflammation and disease progression in NAFLD.7,8 In NAFLD, repeated exposures to endotoxins, saturated fatty acids, cholesterol esters, and reactive oxygen species are thought to perpetuate inflammation through inflammasome activation and lead to disease progression.4
Consistent with this mechanism, IL-18 has previously been implicated in NASH in individuals without HIV. Hepatic expression of inflammasome components including pro-IL-18 is significantly increased in individuals with NASH compared with those with simple steatosis.6 The levels of IL-18 in circulation are also significantly increased in PWH and have been implicated in the pathogenesis of HIV disease progression and in a nonhuman primate monkey model of HIV.9–11 To our knowledge, the relationship between fatty liver disease and proinflammatory cytokine IL-18 in PWH has not yet been reported. We hypothesized that increased levels of IL-18, as a marker of increased inflammasome activation, may be associated with noninvasive measures of NAFLD and liver injury in PWH.
Methods
This study reports on new analyses from an observational study of men and women with HIV infection and simultaneously recruited matched controls without HIV.12,13 Participants were recruited from the Boston area from community centers and infectious disease clinics. PWH and individuals without HIV were recruited from the same communities, and family members, partners, and friends of PWH were also encouraged to enroll in an attempt to ensure the two groups would be similar with respect to demographic characteristics and cardiovascular risk factors. Other than HIV disease, inclusion and exclusion factors were identical for both groups. Participants recruited were 18–60 years of age and had no known cardiac disease or symptoms suggestive of any current or prior cardiac disease (including angina, arrhythmias, valvular disease, pericarditis, and congestive heart failure). Participants with renal disease, creatinine levels >1.0 mg/dL or creatinine clearance <60 mL/min were excluded to minimize the risk of contrast nephropathy. In addition to the eligibility criteria outlined in the original study, for this study, participants with known history of hepatitis B, hepatitis C, and excessive alcohol intake were also excluded. Excessive alcohol intake was defined as >21 standard drinks per week in men and >14 drinks per week in women.14 All participants provided informed consent to participate. This study was approved by the institutional review board of Massachusetts General Hospital.
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), glucose, hemoglobin A1c, lipids, HIV-1 RNA level (reverse transcription polymerase chain reaction [RT-PCR]; Roche Amplicor Monitor; lower limit of detection, 50 copies/mL), CD4+ T cells, and CD8+ T cells were measured by MGH laboratory using standard techniques. Using serum collected after an overnight fast, IL-18 was measured by enzyme-linked immunosorbent assay (ELISA; R&D, Minneapolis, MN) and caspase-1 was measured according to manufacturers' instructions (Cell Technology, Hayward, CA). MCP-1 was measured by ELISA (R&D) and LPS (Associates of Cape Cod, East Falmouth, MA) was measured according to the manufacturers' instructions. Measurements of serum markers were performed in duplicate with appropriate controls. Sample variation was acceptable with a percent coefficient of variation (%CV) of 25% or less. Samples exceeding the %CV cutoff were repeated.
To characterize hepatic steatosis, measurements of liver and spleen attenuation were measured on noncontrast computed tomography (CT) images by a reader blinded to HIV status as well as clinical and biomarker results. Three circular regions with an area of at least 2 cm2 on three axial CT slices were measured in the liver with the spleen as an internal control.15 The liver-to-spleen ratio was calculated from the mean liver density measurements divided by the mean spleen density measurements, and the ratio <1 was defined as NAFLD as previously described.16,17 A lower liver-to-spleen ratio indicates higher hepatic lipid content.18 A single-slice abdominal CT was used to derive measurements of visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) areas.
Liver enzymes, triglycerides, HIV RNA, and inflammatory markers were log10-transformed owing to non-normal distributions. Pearson correlation coefficients were assessed for investigating relationships between continuous variables. Multivariable linear regression was performed to adjust for potential confounders that may affect liver enzymes or liver fat content. Comparisons between the two groups were analyzed using Student's t-test for normally distributed variables and by Wilcoxon rank-sum test for non-normally distributed variables. Statistical significance was defined as p < .05. Statistical analyses were performed using SAS JMP Pro (Cary, NC).
Results
Characteristics of participants
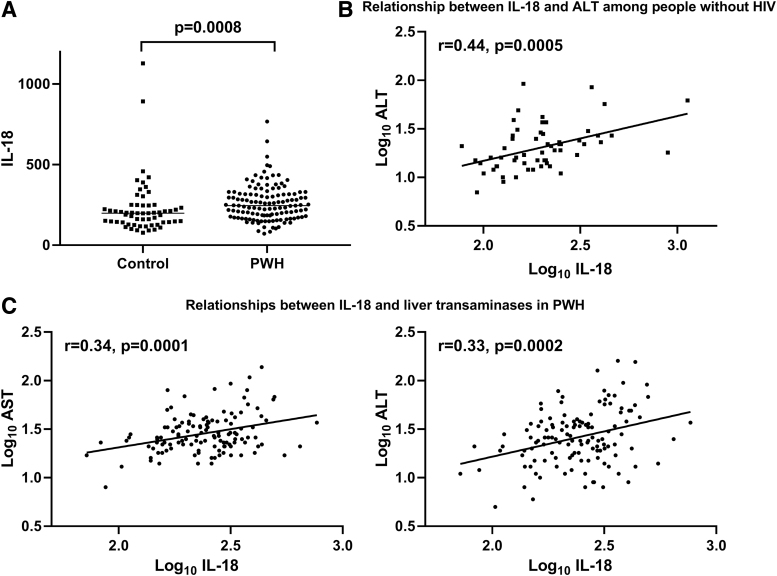
A total of 125 PWH and 59 matched controls without HIV were included in the current analysis. The PWH and participants without HIV were similar in age (46.5 ± 8.2 vs. 45.4 ± 7.1 years; p = .32), sex (69.6% vs. 66.1% male; p = .63), and body mass index (BMI; 27.9 ± 5.5 vs. 27.3 ± 4.9 kg/m2; p = .49). Demographic, metabolic, and immunologic characteristics of participants are summarized in the Supplementary Table S1. AST [26 (21–37) vs. 23 (17–29) U/L; p = .009] and ALT [25 (17–38) vs. 19 (14–27) U/L; p = .005] were higher in PWH than in controls. IL-18 [245.8 (179.5–317.0) vs. 198.6 (141.7–246.6) pg/mL; p = .0008] (Fig. 1A) and MCP-1 [262.0 (179.0–358.0) vs. 223.5 (170.3–275.0) pg/mL; p = .02] were also higher in PWH than in controls.
FIG. 1.
(A) IL-18 is higher in PWH than in people without HIV. Line represents median. (B) Log10 IL-18 levels are associated with Log10 ALT in individuals without HIV. (C) Log10 IL-18 levels are associated with log10 AST and log10 ALT in PWH. ALT, alanine aminotransferase; AST, aspartate aminotransferase; IL, interleukin; PWH, people with HIV.
IL-18 is elevated in PWH with NAFLD defined by CT liver-to-spleen ratio
Among PWH, log10 IL-18 was higher in the NAFLD group than in the non-NAFLD group defined by CT liver-to-spleen ratio (2.51 ± 0.15 vs. 2.36 ± 0.18; p = .01).
Markers of fatty liver disease are associated with IL-18 and MCP-1
Among PWH, log10 IL-18 had a positive relationship with log10 AST (r = 0.34, p = .0001) and log10 ALT (r = 0.33, p = .0002) (Table 1 and Fig. 1). The relationship between log10 IL-18 and log10 ALT was also significant in individuals without HIV (r = 0.44, p = .0005). Similarly, log10 MCP-1 had a positive correlation with log10 AST (r = 0.27, p = .004) and log10 ALT (r = 0.26, p = .006) among PWH. Log10 IL-18 showed a significant correlation with liver-to-spleen ratio (r = −0.24, p = .02) in PWH.
Table 1.
Relationships of Liver, Metabolic, and Inflammatory Indices with Interleukin-18 and Monocyte Chemoattractant Protein-1 in People with HIV
| Log10 (IL-18) |
Log10 (MCP-1) |
|||
|---|---|---|---|---|
| r | p | r | p | |
| Liver | ||||
| Log10 (AST) | 0.34 | .0001 | 0.27 | .004 |
| Log10 (ALT) | 0.33 | .0002 | 0.26 | .006 |
| Liver/spleen ratio | −0.24 | .02 | −0.20 | .06 |
| Glucose | ||||
| Fasting glucose | 0.08 | .40 | 0.09 | .33 |
| Log10 (hemoglobin A1c) | −0.09 | .32 | −0.001 | .99 |
| Lipids | ||||
| Total cholesterol | −0.02 | .83 | −0.06 | .52 |
| LDL | −0.01 | .87 | −0.05 | .63 |
| HDL | −0.28 | .002 | −0.25 | .009 |
| Log10 (triglycerides) | 0.26 | .003 | 0.25 | .007 |
| Fat distribution | ||||
| BMI | −0.04 | .64 | 0.06 | .53 |
| Log10 (VAT) | 0.12 | .19 | 0.22 | .02 |
| Log10 (SAT) | −0.15 | .09 | −0.004 | .97 |
| Inflammatory markers | ||||
| Log10 (MCP-1) | 0.33 | .0004 | — | — |
| Log10 (IL-18) | — | — | 0.33 | .0004 |
| Log10 (LPS) | 0.28 | .004 | 0.02 | .86 |
| Log10 (caspase-1) | 0.35 | <.0001 | 0.15 | .13 |
| HIV disease parameters | ||||
| Log10 (viral load) | 0.29 | .002 | −0.03 | .74 |
| Log10 (CD4+ T cell count) | −0.17 | .06 | 0.12 | .21 |
| Log10 (CD8+ T cell count) | 0.13 | .16 | 0.24 | .01 |
| Log10 (CD4+/CD8+ ratio) | −0.24 | .007 | −0.05 | .61 |
| Adjusted relationships with log10 (IL-18) | β | p | ||
| Log10 (AST)a | 0.31 | .004 | ||
| Log10 (ALT)a | 0.43 | .003 | ||
| Liver-to-spleen ratiob | 0.63 | .02 | ||
Bold values denote statistical significance at p-value < 0.05.
Multivariate analysis adjusting for age, gender, BMI, log10 triglycerides, statin use, log10 HIV RNA, and log10 CD4 count.
Multivariate analysis adjusting for age, gender, BMI, log10 triglycerides, fasting glucose, and log10 VAT area.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HDL, high-density lipoprotein; IL, interleukin; LDL, low-density lipoprotein; MCP-1, monocyte chemoattractant protein-1; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
IL-18 and MCP-1 are associated with triglycerides and high-density lipoprotein
Among PWH, log10 IL-18 had a positive correlation with log10 triglycerides (r = 0.26, p = .003) and inverse correlation with high-density lipoprotein (HDL; r = −0.28, p = .002). The inverse correlation between log10 IL-18 and HDL was also significant in HIV-negative individuals (r = −0.27, p = .04). Log10 MCP-1 had a positive correlation with log10 triglycerides (r = 0.25, p = .007) and inverse correlation with HDL (r = −0.25, p = .009).
IL-18 is associated with markers of inflammation, microbial translocation, and HIV disease parameters
In PWH, log10 IL-18 was associated with log10 MCP-1 (r = 0.33, p = .0004), log10 caspase-1 (r = 0.35, p < .0001), and log10 LPS (r = 0.28, p = .004). There was a significant positive correlation with log10 IL-18 and log10 HIV viral load (r = 0.29, p = .002) and an inverse relationship between log10 IL-18 and log10 CD4+/CD8+ ratio (r = −0.24, p = .007) in PWH.
Multivariable analysis
The relationship between log10 IL-18 with log10 AST (β = 0.31, p = .004) remained significant after adjusting for age, gender, BMI, log10 triglycerides, statin use, log10 HIV RNA, and log10 CD4 count. The relationship between log10 IL-18 with log10 ALT (β = 0.43, p = .003) remained significant after adjusting for age, gender, BMI, log10 triglycerides, statin use, log10 HIV RNA, and log10 CD4 count.
In a model that also included MCP-1, the relationship between log10 IL-18 with log10 AST (β = 0.36, p = .007) remained significant after adjusting for age, gender, BMI, log10 triglycerides, statin use, log10 HIV RNA, log10 CD4 count, and log10 MCP-1. The relationship between log10 IL-18 with log10 ALT (β = 0.52, p = .002) remained significant after adjusting for age, gender, BMI, log10 triglycerides, statin use, log10 HIV RNA, log10 CD4 count, and log10 MCP-1.
Adjusting for relevant metabolic parameters, the relationship between log10 IL-18 with liver-to-spleen ratio (β = −0.63, p = .02) remained significant after adjusting for age, gender, BMI, log10 triglycerides, fasting glucose, and log10 VAT area.
To assess if HIV status affected the relationship of IL-18 with ALT, AST, or liver-to-spleen ratio, we performed an analysis among all participants including HIV status, log10 IL-18 as covariates as well as the interaction term (HIV × log10 IL-18) for the outcomes of log10 ALT, log10 AST, and liver-to-spleen ratio, and we did not see significant interaction of HIV status on the relationships of log10 IL-18 with ALT (p = .76 for interaction), AST (p = .18 for interaction), or liver-to-spleen ratio (p = .32 for interaction).
Sensitivity analysis
In a sensitivity analysis excluding individuals with detectable HIV RNA, we observed similar findings including higher IL-18 in PWH than in controls [232.0 (171.3–300.2) vs. 198.6 (141.7–246.6) pg/mL; p = .008] and higher MCP-1 in PWH than in controls [251.5 (176.8–364.8) vs. 223.5 (170.3–275.0) pg/mL; p = .04]. We also observed similar relationships between log10 IL-18 with log10 ALT (r = 0.39, p < .0001), log10 AST (r = 0.40, p < .0001), and liver-to-spleen ratio (r = −0.24, p = .04) among PWH with undetectable HIV RNA.
Discussion
In this observational study of PWH who do not have known hepatitis B, hepatitis C, or excessive alcohol use, we observed that circulating IL-18 was higher in individuals with evidence of NAFLD by CT liver-to-spleen ratio <1, and that IL-18 was positively related to ALT and AST levels and inversely associated with CT liver-to-spleen ratio, even after controlling for relevant covariates. Furthermore, IL-18 was positively associated with MCP-1, a marker of an important inflammatory mechanism in the development of steatohepatitis that is activated downstream of inflammasome activation. These findings suggest a potential role of the inflammasome/IL-18 pathway and downstream MCP-1 pathway in NAFLD progression through hepatic inflammation in PWH. The relationship of IL-18 with liver transaminases and hepatic steatosis assessed by CT scan remained significant even after controlling for traditional risk factors for NAFLD. To our knowledge, this is the first report to support the potential role of inflammasome activation in NAFLD in PWH.
IL-18 and the inflammasome have been implicated in the pathogenesis of chronic liver diseases including NAFLD in the general population. Knockout mouse models of NLRP3 inflammasome or IL-18 are spared from the development of significant steatohepatitis and fibrosis, whereas knockin models of inflammasome or IL-18 lead to significant liver damage.6,19,20 In a previous study by Wree et al., inflammasome components including pro-IL-18 expression in the liver were significantly increased in individuals with NASH compared with those with hepatic steatosis in non-HIV-infected population.6 Serum levels of IL-18 trended to be higher in a population with NAFLD than in controls in one study although it did not reach statistical significance,21 and increases in IL-18 have been associated with metabolic risk factors closely related to NAFLD such as obesity, insulin resistance, and hypertriglyceridemia.22–24 Our findings are consistent with this body of work in the non-HIV population demonstrating that IL-18 may contribute to the progression of NAFLD. As expected, serum IL-18 was positively associated with serum caspase-1 levels, which is upstream in the inflammasome pathway. Indeed, in this study, we observed strong relationships between IL-18 and ALT, indicative of hepatic inflammation in NASH, among individuals without HIV and in PWH, suggesting that the inflammasome pathway is associated with liver injury among individuals with or without HIV. Among PWH who have even higher IL-18 levels suggestive of heightened inflammasome activation, the greater degree of inflammasome activation may further contribute to the severity of NAFLD progression seen in PWH.
Similarly, the MCP-1/CCR2 pathway has been implicated in the pathogenesis of NAFLD,7,25 and the blockade of CCR2 and CCR5 is being studied as a therapeutic target for NAFLD.26 In HIV, monocyte activation in NAFLD progression and fibrosis development has been demonstrated by Maurice et al. with increased levels of sCD163 and sCD14 in PWH with NAFLD compared with PWH without liver disease.27 Our data showing an association between MCP-1 and noninvasive markers of NAFLD add to the evidence that monocyte recruitment and infiltration are important steps in the pathogenesis of NAFLD in PWH. Furthermore, association of IL-18 with both LPS and MCP-1 in our study may underscore the ability of inflammasome to sense danger signals such as endotoxins from increased microbial translocation and its known role in activating MCP-1-mediated monocyte recruitment to drive NAFLD progression.
This study is hypothesis generating and has limitations. It was not specifically designed to recruit participants with diagnosed NAFLD, and the assessments for NAFLD did not include liver biopsy, which is the gold standard to assess the degree of both steatosis and fibrosis, or magnetic resonance imaging-derived proton density fat fraction to assess hepatic steatosis radiographically. Second, our study measured circulating IL-18 and MCP-1 levels and did not assess tissue-specific expression in the liver and liver-specific inflammasome components. Finally, owing to the cross-sectional nature of the study, the causal directionality of the associations is uncertain. Nevertheless, the initial observations brought forth by this study may provide novel insights into the key inflammatory components of NAFLD in PWH.
Future studies using liver biopsies are needed to confirm our findings and also to elucidate precise mechanisms involving the inflammasome pathways in NAFLD development and progression in PWH. In addition, these findings may aid in the development of effective therapies. Among current investigational therapeutic studies targeting the MCP-1/CCR2 pathway, cenicriviroc, a dual antagonist of CCR2 and CCR5, has been shown to prevent progression of liver fibrosis in participants with NASH and is currently undergoing a phase 3 trial for the treatment of NASH with fibrosis in the general population.26 Investigational therapies modulating the inflammasome pathway to prevent NAFLD progression are in preclinical stages. In one study with NASH murine models, a selective NLRP3 inflammasome inhibitor MCC950 reduced markers of inflammasome activation, MCP-1, ALT/AST, and the severity of liver inflammation and fibrosis.28 If demonstrated to be effective, these investigational pharmacotherapies targeting specific inflammatory pathways may also be relevant for study in the treatment of NASH in PWH, who have a heightened risk of developing liver disease and metabolic complications linked to ongoing inflammation.
Conclusion
In conclusion, the proinflammatory cytokine, IL-18, which is a component of inflammasomes, has been known to be higher in PWH, and in this study, we identified a new relationship between IL-18 and liver enzymes and hepatic steatosis. These findings from this hypothesis-generating study suggest that increased inflammasome activation may be a potential mechanism in the pathogenesis of hepatic inflammation and NAFLD in PWH warranting further future investigation.
Supplementary Material
Authors' Contributions
J.H.S., J.B.S., and J.L. contributed to design and concept of the study analysis. K.V.F., S.E.L., J.A.R., M.T.L., T.H.B., and J.L. conducted data collection. J.H.S., J.B.S., and J.L. conducted data analysis. J.H.S. and J.B.S. wrote the first draft of the article. J.H.S., J.B.S., T.L.S., K.E.C., M.T.L., T.H.B., and J.L. critically reviewed and finalized the article. All authors contributed to subsequent drafts, reviewed, and approved the final version of the article.
Author Disclosure Statement
T.L.S. received investigator-initiated grant from Novo Nordisk for an unrelated project. T.H.B. received equity in Excision BioTherapeutics unrelated to this project. J.L. was a consultant for Viiv Healthcare and Gilead Sciences, all unrelated to this project. All other authors have no reported conflicts of interest.
Funding Information
This work was supported by the National Institutes of Health (Grants No. K23HL092792 and RO1HL123351 to J.L., R01HL141132 to T.H.B., 5T32DK007028-45 to J.H.S, T32MH079785 to J.A.R., and M01-RR-01066 and ULI RR025758 for support of the MGH Clinical Research Center) and Bristol Myers Squibb. Funding sources had no role in the design of the study, data analysis, or the writing of the article.
Supplementary Material
References
- 1. Seth A, Sherman KE: Fatty liver disease in persons with HIV infection. Top Antivir Med 2019;27:75–82 [PMC free article] [PubMed] [Google Scholar]
- 2. Vodkin I, Valasek MA, Bettencourt R, Cachay E, Loomba R: Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: A case-control study. Aliment Pharmacol Ther 2015;41:368–378 [DOI] [PubMed] [Google Scholar]
- 3. Buzzetti E, Pinzani M, Tsochatzis EA: The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016;65:1038–1048 [DOI] [PubMed] [Google Scholar]
- 4. Szabo G, Petrasek J: Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol 2015;12:387–400 [DOI] [PubMed] [Google Scholar]
- 5. Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G: Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 2011;54:133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wree A, McGeough MD, Peña CA, et al. : NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med (Berl) 2014;92:1069–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haukeland JW, Damås JK, Konopski Z, et al. : Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol 2006;44:1167–1174 [DOI] [PubMed] [Google Scholar]
- 8. Kazankov K, Jørgensen SMD, Thomsen KL, et al. : The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol 2019;16:145–159 [DOI] [PubMed] [Google Scholar]
- 9. Shapiro L, Puren AJ, Barton HA, et al. : Interleukin 18 stimulates HIV type 1 in monocytic cells. Proc Natl Acad Sci U S A 1998;95:12550–12555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmad R, Sindhu STA, Toma E, Morisset R, Ahmad A: Elevated levels of circulating interleukin-18 in human immunodeficiency virus-infected individuals: Role of peripheral blood mononuclear cells and implications for AIDS pathogenesis. J Virol 2002;76:12448–12456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kearns AC, Robinson JA, Shekarabi M, Liu F, Qin X, Burdo TH: Caspase-1-associated immune activation in an accelerated SIV-infected rhesus macaque model. J Neurovirol 2018;24:420–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lo J, Abbara S, Shturman L, et al. : Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS 2010;24:243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fitch KV, Srinivasa S, Abbara S, et al. : Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis 2013;208:1737–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chalasani N, Younossi Z, Lavine JE, et al. : The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005–2023 [DOI] [PubMed] [Google Scholar]
- 15. Puchner SB, Lu MT, Mayrhofer T, et al. : High-risk coronary plaque at coronary CT angiography is associated with nonalcoholic fatty liver disease, independent of coronary plaque and stenosis burden: Results from the ROMICAT II trial. Radiology 2015;274:693–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lo J, Lu MT, Kim EA, et al. : Statin effects to reduce hepatosteatosis as measured by computed tomography in patients with human immunodeficiency virus. Open Forum Infect Dis 2016;3:ofw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeb I, Li D, Nasir K, Katz R, Larijani VN, Budoff MJ: Computed tomography scans in the evaluation of fatty liver disease in a population based study: The multi-ethnic study of atherosclerosis. Acad Radiol 2012;19:811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park YS, Park SH, Lee SS, et al. : Biopsy-proven nonsteatotic liver in adults: Estimation of reference range for difference in attenuation between the liver and the spleen at nonenhanced CT. Radiology 2011;258:760–766 [DOI] [PubMed] [Google Scholar]
- 19. Wree A, Eguchi A, McGeough MD, et al. : NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation and fibrosis. Hepatology 2014;59:898–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finotto S: Severe hepatic injury in interleukin 18 (IL-18) transgenic mice: A key role for IL-18 in regulating hepatocyte apoptosis in vivo. Gut 2004;53:392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vecchiet J, Falasca K, Cacciatore P, et al. : Association between plasma interleukin-18 levels and liver injury in chronic hepatitis C virus infection and non-alcoholic fatty liver disease. Ann Clin Lab Sci 2005;35:415–422 [PubMed] [Google Scholar]
- 22. Esposito K, Pontillo A, Ciotola M, et al. : Weight loss reduces interleukin-18 levels in obese women. J Clin Endocrinol Metab 2002;87:3864–3866 [DOI] [PubMed] [Google Scholar]
- 23. Hung J, McQuillan BM, Chapman CML, Thompson PL, Beilby JP: Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol 2005;25:1268–1273 [DOI] [PubMed] [Google Scholar]
- 24. Falasca K, Manigrasso MR, Racciatti D, et al.: Associations between hypertriglyceridemia and serum ghrelin, adiponectin, and IL-18 levels in HIV-infected patients. Ann Clin Lab Sci 2006:36:59–66 [PubMed] [Google Scholar]
- 25. Greco D, Kotronen A, Westerbacka J, et al. : Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol 2008;294:G1281–G1287 [DOI] [PubMed] [Google Scholar]
- 26. Friedman SL, Ratziu V, Harrison SA, et al. : A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018;67:1754–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maurice JB, Garvey L, Tsochatzis EA, et al. : Monocyte-macrophage activation is associated with nonalcoholic fatty liver disease and liver fibrosis in HIV monoinfection independently of the gut microbiome and bacterial translocation. AIDS 2019;33:805–814 [DOI] [PubMed] [Google Scholar]
- 28. Mridha AR, Wree A, Robertson AAB, et al. : NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 2017;66:1037–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.