Abstract
Kaposi's sarcoma (KS) is an AIDS-defining malignancy that can improve or worsen with antiretroviral therapy (ART). We aimed at identifying clinical, HIV-related, and sociodemographic factors associated with either progression or nonprogression (regression or stable disease) of ART-treated HIV-associated KS in patients with limited cutaneous disease. We conducted a prospective cohort study of ART-treated HIV-associated KS cases. Clinical, HIV-related, and sociodemographic variables were collected at baseline, and patients were followed up to determine treatment outcomes. Cox regression, linear mixed effects model, and Spearman's rank correlation were used for analysis. Half (50%) of the study participants had KS regression or stable disease, whereas the other half (50%) had disease progression during the treatment and follow-up period. Among the data analyzed, presence of KS nodules at baseline (hazard ratio = 5.47; 95% confidence interval = 1.32–22.65; p = .02) was an independent predictor of poor treatment outcome. Progressors and nonprogressors were indistinguishable in the changes they experienced in the HIV plasma viral load and CD4 counts as a result of ART. Even when cutaneous presentation is limited, the presence of nodular morphotype KS lesions should be considered an indicator for combined ART plus chemotherapy. Temporal trends in CD4 counts and HIV viral loads did not correlate with treatment outcome in ART-treated HIV-associated KS.
Keywords: Kaposi's sarcoma, HIV, antiretroviral therapy, outcomes, predictors
Introduction
Kaposi's sarcoma (KS) is the most prevalent malignancy among HIV-infected individuals.1 KS is a vascular malignancy that causes high morbidity and mortality.2 Besides most commonly occurring in HIV-infected individuals [epidemic KS (EpKS)], KS is also a common malignancy in organ transplant recipients (iatrogenic KS). It can also be found in HIV-seronegative individuals in sub-Saharan Africa (SSA) (endemic KS), and is also reported in elderly men in some areas near the Mediterranean (classic KS).3
According to World Health Organization (WHO) global cancer estimates from 2018, KS is a leading cancer in both incidence and mortality in Zambia and other SSA countries.4 Antiretroviral therapy (ART) has had an effect on reducing the burden of EpKS globally, and especially in developed countries.5 However, many developing countries still experience a high burden of disease despite the rollout and wide availability of ART.6,7
Treatment of EpKS in SSA is usually ART alone for nondisseminated cutaneous disease, or ART plus cytotoxic cancer chemotherapy for advanced disease (disseminated cutaneous with or without visceral involvement).8 The commonly used first-line chemotherapy regimen for KS treatment in Zambia is Adriamycin/Doxorubicin, Bleomycin, and Vincristine (ABV). When available, liposomal doxorubicin is the preferred first-line option. Paclitaxel is sometimes used as first line but usually reserved for those who do not respond to first-line treatment because of its high cost.
Outcomes for ART-treated EpKS are variable, with individuals having either KS remission, stable disease, or KS progression.9,10 ART-only treatment of KS in the developed world seems to be more effective than in Africa. It is unclear why ART has had a less significant impact on KS in Africa. It could be sociodemographic/economic related to lack of recognition of the disease until it is painfully symptomatic, or it could be other factors. Unfortunately, most of the KS cases seen in most settings, including Zambia, present late with poor treatment outcomes.11 Thus, the clinical factors associated with KS disease progression or remission in ART-treated limited cutaneous EpKS patients are still poorly understood, with most previous studies mainly focused on outcomes of chemotherapy-treated KS patients with disseminated disease.12,13
This study aimed at characterizing a cohort of cutaneous EpKS individuals with limited disease to identify clinical, HIV-related, and sociodemographic factors that predict outcomes of an ART-only treatment regimen. Determining these factors could guide clinicians on which patients are likely to respond favorably to ART only, which might require short-interval follow-up, and which subjects require combined ART and cytotoxic cancer chemotherapy, as is recommended for disseminated/advanced EpKS.
Materials and Methods
Study design
This was a prospective cohort study conducted at the Dermatology and Venereology Division of the Adult Hospital of the University Teaching Hospitals (UTH), Lusaka, Zambia. It was conducted in the period between November 2017 and February 2020.
The primary objective of this study was to recruit EpKS patients with limited cutaneous disease, and determine their clinical, sociodemographic, and HIV disease parameters associated with progression or regression upon ART treatment.
The study protocol was approved by the University of Zambia Biomedical Research Ethics Committee, the Zambia National Health Research Authority, and the University of Nebraska IRB. Informed consent was obtained from all the study participants.
Study participants
Recruitment was done after obtaining informed consent. Participants also had to be ART naive or on ART for <2 weeks. We recruited both males and females >18 years of age who met the inclusion criteria. These were individuals with cutaneous EpKS, AIDS Clinical Trials Group (ACTG) stage T0 and S0. Mild pedal edema was an exception to the ACTG T0 staging. Limited disease was considered as lesions involving <10% of the body surface area. If mucous membranes were involved, only flat lesions were considered for inclusion.
Key variables collected at baseline included age, gender, smoking status, alcohol status, time since the first KS lesion was noticed, time since HIV diagnosis, presence of mucous membrane lesions, number of KS lesions, type of KS lesions, CD4 counts, and HIV viral loads.
Diagnosis of clinically suspicious KS lesions was made by hematoxylin and eosin staining followed by Kaposi's Sarcoma-Associated Herpesvirus (KSHV) Latency-Associated Nuclear Antigen (LANA) immunohistochemistry on biopsied KS lesions. Only LANA-positive cases were approached for recruitment.
Longitudinal follow-up
The study participants were followed up monthly for a variable period of time depending on treatment response, with one patient followed up to 560 days. Those who were lost to follow-up were censored, whereas an event was considered as worsening/progression of KS lesions. At each follow-up visit, HIV viral loads and CD4 counts were performed to determine the levels of viral suppression and immune reconstitution. In addition, a thorough physical examination was performed to assess whether the participants were experiencing regression of KS lesions, stable disease, or changes in number and/or size of KS lesions. Follow-up was ended and chemotherapy commenced for participants that had an event (progression of KS) during the follow-up period. Progression of KS lesions was considered as appearance of new lesions, any increase in size of existing lesions, increase in lymphedema, and/or involvement of visceral organs.
Statistical analysis
Baseline characteristics were analyzed using descriptive statistics. Continuous variables (e.g., CD4 counts and HIV viral loads) at baseline are presented as medians and interquartile range, categorical or dichotomous variables are presented as proportions. CD4 count was also dichotomized into > or ≤200 cells/μL.
We used Kaplan–Meier curves to describe the progression-free survival, whereas the log-rank test was used to compare groups. Univariate and multivariate Cox regression were used to determine the association between exposures of interest and time to disease progression. Variables with p values <.1 and those expected to predict disease progression based on how they associate with KS development or severity were included in the multivariate cox regression model. The other parameter used for building and selecting the best multivariate model was the likelihood ratio chi-square test, and the model with the largest likelihood value was picked. There were some patients with missing data on some variables. In this case, a complete case analysis was done since only one patient had missing information for each of those variables.
Spearman's rank correlation was used to determine whether or not there was a correlation between baseline CD4 counts and HIV viral loads in progressors and nonprogressors, and in the combined cohort. A linear mixed-effects model for longitudinal data was used to compare changes in mean CD4 counts and HIV viral loads between progressors and nonprogressors at different time points during the follow-up period. The model was selected because our data were highly unbalanced, with patients presenting at variable time periods. The model accounted for a linear correlation among the repeated measures over time and allowed for heterogeneity of the variance over time. The model included an effect of treatment outcome, time, and outcome/time interaction. The model had an unstructured covariance of random effects since we wanted a correlated random intercept and slope. All statistical tests were two-sided. p Values <.05 were considered statistically significant. Stata version 15 (StataCorp LLC, College Station, TX) was used to perform all statistical analyses.
Results
A total of 28 eligible participants were successfully enrolled and followed up during the study period. Fourteen (50%) of these had disease progression, whereas 14 (50%) had KS regression or stable disease. Table 1 shows the baseline characteristics of the study participants.
Table 1.
Baseline Characteristics of Study Participants (by Outcome)
| KS regressors or stable disease | KS progressors | |
|---|---|---|
| Age in years [IQR] | 33 [30–39] | 33 [31–43] |
| Males | 9 (64%) | 6 (43%) |
| Smoking | 8 (57%) | 4 (29%) |
| Alcohol | 10 (71%) | 12 (86%) |
| Months since first lesion [IQR] | 3 [3–9] | 3 [2–6] |
| Days since HIV diagnosis [IQR] | 14 [2–30] | 7 [3–30] |
| Days on ART [IQR] | 2 [0–14] | 6 [2–14] |
| Lesions in mucous membranes | 6 (43%) | 5 (38%) |
| More than 5 KS lesions present | 9 (69%) | 10 (71%) |
| CD4 count as cells/μL [IQR] | 88 [53–234] | 128 [109–223] |
| HIV viral load as copies/mL [IQR] | 30,539 [1,125–489,428] | 20,033 [1,693–180,726] |
ART, antiretroviral therapy; IQR, interquartile range; KS, Kaposi's sarcoma.
No variable was significantly associated with disease progression on univariate Cox regression (Table 2).
Table 2.
Crude Hazard Ratios for Predictors of Antiretroviral Therapy-Treated Kaposi's Sarcoma Disease Progression
| HR | 95% CI | p | |
|---|---|---|---|
| Age | 0.96 | 0.90–1.03 | .26 |
| Male | 1.03 | 0.35–3.01 | .95 |
| Smoking | 0.65 | 0.20–2.11 | .48 |
| Alcohol | 2.36 | 0.52–10.75 | .27 |
| Education | |||
| Secondary | 3.03 | 0.77–12 | .27 |
| Tertiary | 2.0 | 0.40 – 9.84 | .41 |
| Multiple KS lesions (>3 lesions) | 1.43 | 0.44–4.61 | .55 |
| Lymphedema | 1.16 | 0.36–3.77 | .80 |
| KS lesion type (nodule) | 3.16 | 0.96–10.42 | .06 |
| Mucous membrane lesions | 1.56 | 0.47–5.12 | .47 |
| More than 4 affected areas | 1.61 | 0.55–4.70 | .39 |
| HIV viral load | 0.99 | 0.99–1 | .16 |
| CD4 count >200 cells/μL | 1.96 | 0.63–6.04 | .24 |
CI, confidence interval; HR, hazard ratio.
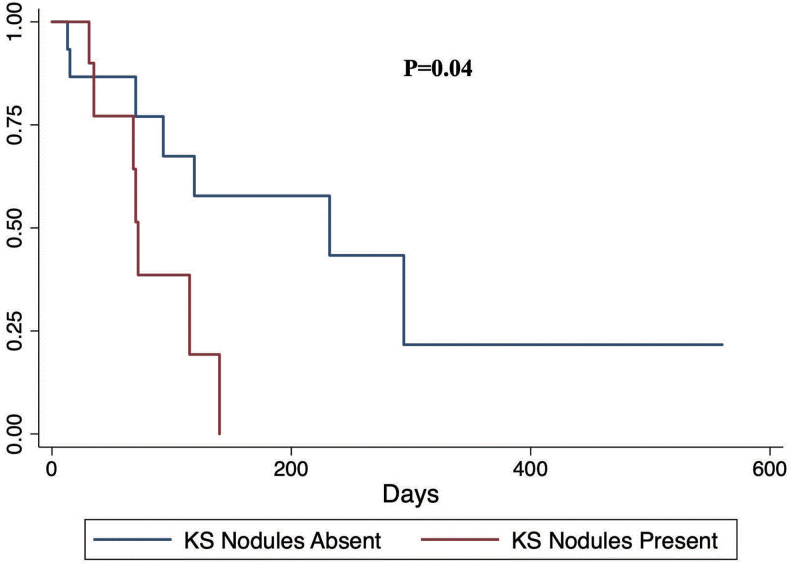
We performed multivariate Cox regression to determine predictors of treatment outcomes after controlling for possible confounders. We observed that presence of KS nodules was associated with increased risk of KS progression after controlling for confounders (Table 3) and (Fig. 1). We tested the proportional hazards assumption for our multivariable model and found that there was no departure from the proportional hazards assumption for the global test or for any of the predictors.
Table 3.
Adjusted Hazard Ratios for Predictors of Antiretroviral Therapy-Treated Kaposi's Sarcoma Disease Progression
| HR | 95% CI | p | |
|---|---|---|---|
| Lymphedema | 2.92 | 0.63–13.50 | .17 |
| Smoking | 0.26 | 0.06–1.24 | .09 |
| KS lesion type (nodule) | 5.47 | 1.32–22.65 | .02 |
Bold value represents significant p value (<0.05).
FIG. 1.
Kaplan–Meier progression-free survival comparing individuals with KS nodules versus those without KS nodules. The p value is a log-rank test p value. KS, Kaposi's sarcoma. Color images are available online.
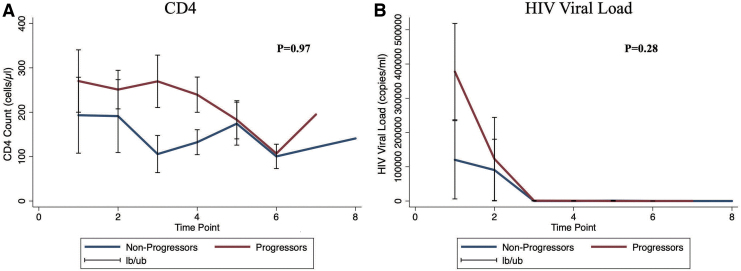
We used the Spearman's rank correlation to determine whether or not there was a correlation between baseline CD4 count and HIV viral load in progressors and nonprogressors. We found that there was a negative but statistically insignificant correlation between CD4 count and HIV viral load in progressors (⍴ = −0.13; p = .67), in nonprogressors (⍴ = −0.30; p = .43), and in the combined cohort (⍴ = −0.19; p = .39). We also compared the pattern of change in CD4 counts and HIV viral loads between progressors and nonprogressors during the follow-up period. Nineteen (19) patients had repeated measurements of CD4 counts and viral loads in the database. We found that there was no statistically significant difference in CD4 count (interaction coefficient = −0.56, p = .97) and HIV viral load (interaction coefficient = −60,958, p = .28) trends over time in progressors compared with those who had KS regression or stable disease (Fig. 2).
FIG. 2.
Longitudinal changes in CD4 counts (A) and HIV viral loads (B), and comparisons between progressors and nonprogressors. The lb/ub indicates 95% confidence intervals. On the x-axis are the time points that indicate when the patients presented, irrespective of the actual month/duration. Color images are available online.
Discussion
This study was designed to identify easily discernible clinical parameters that predict outcomes of ART-treated limited cutaneous EpKS. Understanding these factors could enhance the individualization of management of early-diagnosed EpKS and, therefore, improve treatment outcomes.
Presence of nodules with or without patches and plaques was found to be significantly associated with increased risk of EpKS disease progression after adjusting for other predictors of progression. Patches and plaques often represent earlier stages of disease, as KS lesions begin as flat dyspigmentations or patches that develop into raised plaques and then evolve to become raised nodules.14 Therefore, presence of cutaneous nodules may be a poor prognostic indicator of ART-treated EpKS because they likely represent an underlying advanced disease status and hence more dysregulated immune system.
Mild lymphedema of the lower limbs was associated with a higher risk of KS progression. However, this risk was not statistically significant. Lymphedema has previously been reported to be an indicator of poor outcomes for ART-treated EpKS patients.10 In our study, we only included individuals with mild lymphedema (pitting edema that subsides with limb elevation) and excluded those with moderate to severe lymphedema. Our statistically insignificant finding could be due to the small sample size or due to lower stage of lymphedema. Nevertheless, ART-treated EpKS patients with any stage of lymphedema should be monitored closely for disease progression.
After adjusting for other predictors, smoking was associated with reduced risk of KS progression. However, this association was not statistically significant. Some previous studies have observed and reported that smoking reduces the risk of EpKS and classical KS development.15,16 In addition, cigarette smoke concentrates have been found to specifically inhibit KSHV infection of cells in vitro by as high as 50%.17 Based on our findings and previous studies, it is worth exploring the potential benefits of nicotine patches for treatment of localized cutaneous KS lesions.
There was a negative correlation between baseline HIV viral loads and CD4 counts in progressors, nonprogressors, and our combined study cohort. Higher viral loads were associated with lower CD4 counts, although this finding was not statistically significant. We also observed that there were no significant differences in changes in HIV viral loads and CD4 counts in progressors compared with nonprogressors over time. High HIV viral loads are associated with an increased risk of KS development,18 whereas low CD4 counts are also associated with an increased risk of KS development.19,20 However, several studies have reported KS development in HIV-infected individuals who are on ART, with normal CD4 counts and low or suppressed HIV viral loads.21–23 Furthermore, some studies have found no correlation between CD4 count, CD4%, and HIV viral load with clinical manifestations of HIV infection.24 Our study findings on no baseline correlation between CD4 count and HIV viral load may be due to the small sample size or due to the high variability of the HIV viral load. Nevertheless, our data suggest that temporal longitudinal trends in CD4 counts and HIV viral loads during ART treatment of EpKS are not correlated with treatment outcomes.
Study Limitations
A limitation for our study is the small number of cutaneous KS patients with limited disease recruited into our study. This is mainly due to the fact that the majority KS patients presenting to our clinic and hospital have advanced disease. Most KS patients in our country and region do not have the custom of seeking medical attention early, unless they become debilitated and unable to work. A larger cohort needs to be recruited, perhaps from the community rather than from the hospital clinic, and followed up to confirm these initial observations. Another limitation was that we left out lymph node involvement as a possible predictor of outcomes. Furthermore, another major limitation was the loss to follow-up of a significant proportion of individuals. These individuals were mainly those who were responding well to treatment.
Conclusion
At initiation of ART as treatment for cutaneous EpKS patients, presence of nodular KS may be a poor prognostic indicator. Changes in HIV viral load and CD4 counts over time do not correlate with treatment outcome.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by the Fogarty International Center, National Cancer Institute, and National Institutes of Health under award numbers K43TW011095 to ON, and D43TW010354 and U54CA221204 to CW. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
References
- 1. Semango GP, Charles RM, Swai CI, et al. : Prevalence and associated risk factors for Kaposi's sarcoma among HIV-positive patients in a referral hospital in Northern Tanzania: A retrospective hospital-based study. BMC Cancer 2018;18:1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin-Carbonero L, Palacios R, Valencia E, et al. : Long-term prognosis of HIV-infected patients with Kaposi sarcoma treated with pegylated liposomal doxorubicin. Clin Infect Dis 2008;47:410–417 [DOI] [PubMed] [Google Scholar]
- 3. Li S, Bai L, Dong J, Sun R, Lan K: Kaposi's sarcoma-associated herpesvirus: Epidemiology and molecular biology. Adv Exp Med Biol 2017;1018:91–127 [DOI] [PubMed] [Google Scholar]
- 4. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424 [DOI] [PubMed] [Google Scholar]
- 5. Chang E, Mapakshi SR, Mbang P, et al. : Impact of protease inhibitors on HIV-associated Kaposi sarcoma incidence: A systematic review. J Acquir Immune Defic Syndr 2018;79:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ngalamika O, Minhas V, Wood C: Kaposi's sarcoma at the University Teaching Hospital, Lusaka, Zambia in the antiretroviral therapy era. Int J Cancer 2015;136:1241–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rohner E, Valeri F, Maskew M, et al. : Incidence rate of Kaposi sarcoma in HIV-infected patients on antiretroviral therapy in Southern Africa: A prospective multicohort study. J Acquir Immune Defic Syndr 2014;67:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freeman EE, Busakhala N, Regan S, et al. : Real-world use of chemotherapy for Kaposi's sarcoma in a large community-based HIV primary care system in Kenya. BMC Cancer 2020;20:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nyirenda M, Ngongondo M, Kang M, et al. : Early progression and immune reconstitution inflammatory syndrome during treatment of mild-to-moderate Kaposi sarcoma in sub-Saharan Africa and South America: Incidence, long-term outcomes and effects of early chemotherapy. J Acquir Immune Defic Syndr 2020;84:422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Volkow P, Cesarman-Maus G, Garciadiego-Fossas P, Rojas-Marin E, Cornejo-Juarez P: Clinical characteristics, predictors of immune reconstitution inflammatory syndrome and long-term prognosis in patients with Kaposi sarcoma. AIDS Res Ther 2017;14:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kasturia SE, Gunthel C, Zeng C, Nguyen ML: Severe Kaposi sarcoma in an Urban Public Hospital. AIDS Res Hum Retroviruses 2017;33:583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bekolo CE, Soumah MM, Tiemtore OW, et al. : Assessing the outcomes of HIV-infected persons receiving treatment for Kaposi sarcoma in Conakry-Guinea. BMC Cancer 2017;17:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mtonga W, Mujajati A, Munkombwe D, et al. : Therapeutic outcomes in AIDS-associated Kaposi's sarcoma patients on antiretroviral therapy treated with chemotherapy at two tertiary hospitals in Lusaka, Zambia. Curr HIV Res 2018;16:231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antman K, Chang Y: Kaposi's sarcoma. N Engl J Med 2000;342:1027–1038 [DOI] [PubMed] [Google Scholar]
- 15. Luu HN, Amirian ES, Scheurer ME: The interaction between smoking status and highly active antiretroviral therapy (HAART) use on the risk of Kaposi's sarcoma (KS) in a cohort of HIV-infected men. Br J Cancer 2013;108:1173–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson LA, Lauria C, Romano N, et al. : Risk factors for classical Kaposi sarcoma in a population-based case-control study in Sicily. Cancer Epidemiol Biomarkers Prev 2008;17:3435–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ford PW, Hamden KE, Whitman AG, et al. : Cigarette smoke concentrate inhibits Kaposi's sarcoma-associated herpesvirus infection. Virus Res 2005;114:172–176 [DOI] [PubMed] [Google Scholar]
- 18. Dubrow R, Qin L, Lin H, et al. : Association of CD4+ T-cell count, HIV-1 RNA viral load, and antiretroviral therapy with Kaposi sarcoma risk among HIV-infected persons in the United States and Canada. J Acquir Immune Defic Syndr 2017;75:382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lupia R, Wabuyia PB, Otiato P, Fang CT, Tsai FJ: Risk factors for Kaposi's sarcoma in human immunodeficiency virus patients after initiation of antiretroviral therapy: A nested case-control study in Kenya. J Microbiol Immunol Infect 2017;50:781–788 [DOI] [PubMed] [Google Scholar]
- 20. Min J, Katzenstein DA: Detection of Kaposi's sarcoma-associated herpesvirus in peripheral blood cells in human immunodeficiency virus infection: Association with Kaposi's sarcoma, CD4 cell count, and HIV RNA levels. AIDS Res Hum Retroviruses 1999;15:51–55 [DOI] [PubMed] [Google Scholar]
- 21. von Braun A, Braun DL, Kamarachev J, Gunthard HF: New onset of kaposi sarcoma in a human immunodeficiency virus-1-infected homosexual man, despite early antiretroviral treatment, sustained viral suppression, and immune restoration. Open Forum Infect Dis 2014;1:ofu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Power DG, Mulholland PJ, O'Byrne KJ: AIDS-related Kaposi's sarcoma in a patient with a normal CD4 count. Clin Oncol (R Coll Radiol) 2008;20:97. [DOI] [PubMed] [Google Scholar]
- 23. Stebbing J, Powles T, Bower M: AIDS-associated Kaposi's sarcoma associated with a low viral load and a high CD4 cell count. AIDS 2008;22:551–552 [DOI] [PubMed] [Google Scholar]
- 24. Shah I: Correlation of CD4 count, CD4% and HIV viral load with clinical manifestations of HIV in infected Indian children. Ann Trop Paediatr 2006;26:115–119 [DOI] [PubMed] [Google Scholar]