It has been described that a subset of children with ASD show an improvement in their behavioral symptoms during the course of a fever, a sign of systemic inflammation1,2. Here we elucidate the molecular and neural mechanisms that underlie the beneficial effects of inflammation on social behavior deficits. We compared an environmental model of neurodevelopmental disorders in which mice were exposed to maternal immune activation (MIA) during embryogenesis3,4 with mice genetically deficient for Contactin-associated protein-like 2 (Cntnap2)5, Fragile X mental retardation-1 (Fmr1)6, and Sh3 and multiple ankyrin repeat domains 3 (Shank3)7. We establish that social behavior deficits in MIA-exposed offspring can be temporarily rescued by the inflammatory response elicited by lipopolysaccharide (LPS) administration. This behavioral rescue was accompanied by a reduction in neural activity in the primary somatosensory cortex dysgranular zone (S1DZ), whose hyperactivity was previously implicated in the manifestation of MIA behavioral phenotypes8. In contrast, we did not observe an LPS-induced rescue of social deficits in monogenic models. We demonstrate that the differences in responsivity to the LPS treatment between the MIA and the monogenic models emerge from differences in the levels of cytokine production. LPS treatment in monogenic mutant mice did not induce comparable amounts of interleukin-17a (IL-17a) as in MIA offspring, and bypassing this difference by directly delivering IL-17a into S1DZ was sufficient to promote sociability in monogenic mutant mice as well as in MIA offspring. Conversely, abrogating IL-17 receptor subunit a (IL-17Ra) expression in the S1DZ neurons eliminated the ability of LPS to reverse the sociability phenotypes in MIA offspring. Our data support a novel neuroimmune mechanism underlying neurodevelopmental disorders, whereby production of IL-17a during inflammation can ameliorate the expression of social behavior deficits by directly affecting neural activity in the central nervous system.
The beneficial effects of infection and ensuing inflammation on neurological disorders have been noted9. For example, a subset of children with autism spectrum disorder (ASD) exhibit temporary but significant improvement of their behavioral symptoms during episodes of fever, a sign of systemic inflammation1,2. However, a mechanistic understanding of how fever-associated immune responses translate into behavioral relief, both at the molecular and neural level, is still lacking. ASD etiology includes both environmental and genetic risk factors10. Animal models of ASD in which mice harbor mutations in ASD risk genes including Cntnap25, Fmr16, and Shank37 show behavioral abnormalities including social interaction deficits11,12. Similar behavioral abnormalities are observed in mouse models for environmental ASD risk factors like exposure to maternal inflammation13. We sought to explore the mechanisms that allow for the fever-associated rescue of sociability deficits using both genetic and environmental mouse models for neurodevelopmental disorders.
LPS rescues sociability in MIA offspring
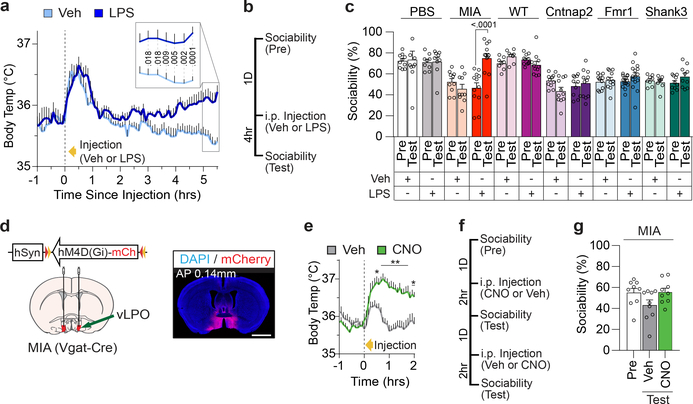
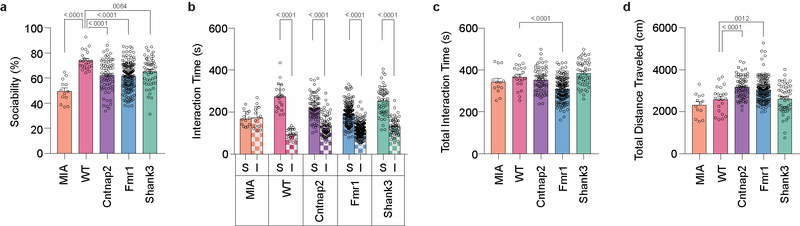
A febrile response can be exogenously induced by injecting animals with a low dose of LPS14. Indeed, intraperitoneal (i.p.) LPS administration (50μg/kg) in control animals - offspring born to mothers injected with PBS - led to a significant increase in body temperature (~0.5–1.0 °C) ~4–5 hours after the treatment (Fig. 1a). To investigate whether LPS injection can ameliorate sociability deficits, we compared sociability in adult males one day before LPS injection (pretest, Pre) and 4 hours after injection (Test), when the increase in body temperature initiates (Fig. 1b). As previously reported, MIA offspring born to mothers injected with Poly(I:C) (polyinosinic:polycytidylic acid) at embryonic day 12.5 (E12.5) exhibited impaired social approach behavior during the pretest12–15. The Cntnap2, Fmr1 and Shank3 monogenic models also displayed sociability deficits albeit with a marked variability during the pretest (Extended Data Fig. 1). We therefore compared MIA offspring to monogenic mutant animals that exhibited a sociability index lower than ~62%, the mean value for the three monogenic lines (Extended Data Fig. 1a).
Figure 1. Immune stimulation rescues sociability deficits in MIA offspring.
a, Body temperature profile following Veh or LPS injection in PBS-offspring (Veh n=11, LPS n=11; from 5 independent experiments). Initial spike in body temperature is due to handling stress. Veh,Vehicle; LPS, lipopolysaccharide. b,c, Mice were tested for sociability (% time investigating social object / total time investigating both social and inanimate objects) one day prior to LPS injection (Pre). Mice were then tested for sociability four hours after either Veh or LPS injection (Test) (PBS-Veh n=10, PBS-LPS n=9, MIA-Veh n=10, MIA-LPS n=12, WT-Veh n=8, WT-LPS n=11, Cntnap2-Veh n=11, Cntnap2-LPS n=11, Fmr1-Veh n=11, Fmr1-LPS n=15, Shank3-Veh n=8, Shank3-LPS n=10; from 3 independent experiments). d, Virus encoding inhibitory DREADD (AAV2-hSyn-DIO-hM4D(Gi)-mCherry) was targeted to the vLPO of Vgat-Cre MIA mice. Scale bar represents 2mm. vLPO, ventral part of the lateral preoptic nucleus. e, Body temperature profile following Veh or CNO injection. f,g, Mice were tested for sociability one day prior to injection (Pre). The following two days, mice received counterbalanced injections of either Veh or CNO. Sociability was assessed two hours post-injection. For experiments d-g, n=9 for all groups; from 2 independent experiments. *P<0.05, **P<0.01 calculated by two-way repeated measures ANOVA with Bonferroni’s (a,e) and Sidak’s (c) post-hoc tests, and one-way repeated measures ANOVA with Tukey’s post-hoc test (g). Graphs indicate mean ± s.e.m.
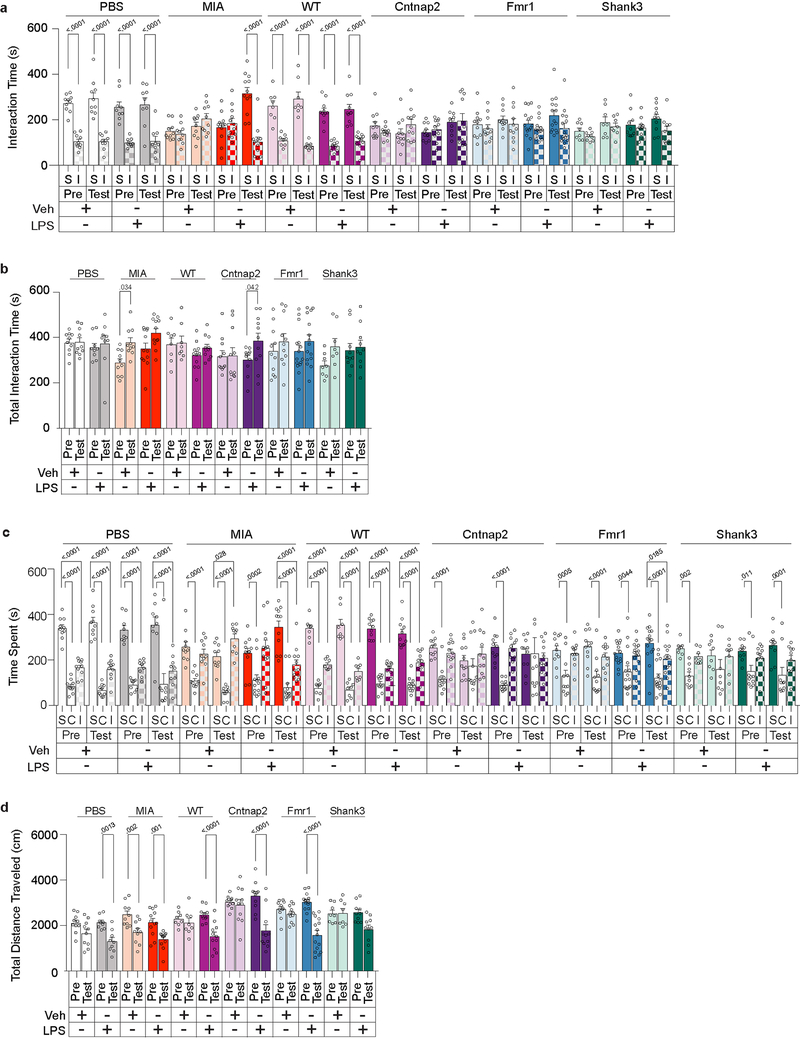
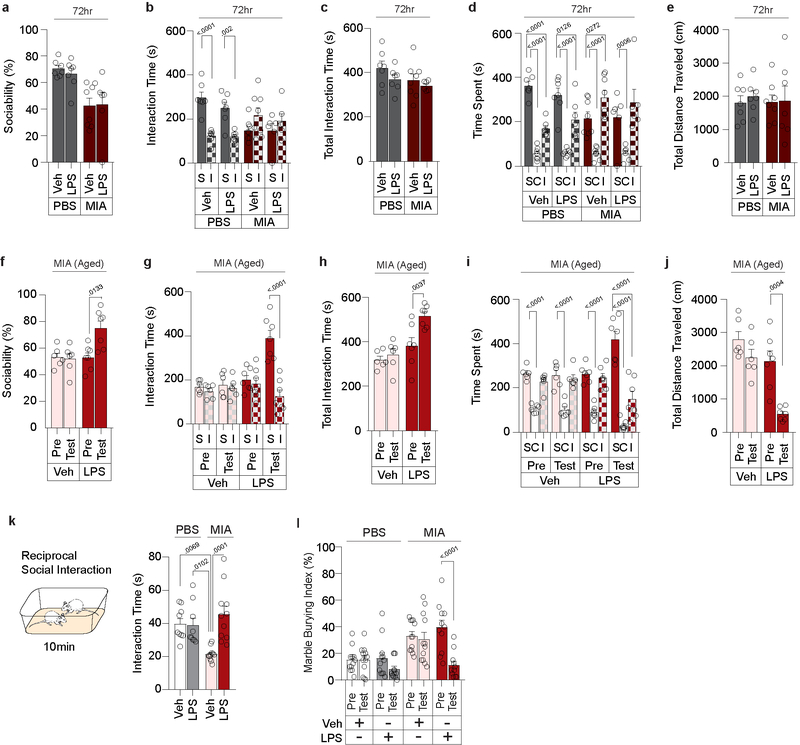
LPS injection in MIA offspring robustly rescued their characteristic deficits in sociability; these animals displayed an interest in social objects indistinguishable from controls (Fig. 1c and Extended Data Fig. 2). LPS injection did not affect baseline sociability in control offspring. LPS-induced sociability rescue was absent 72 hours following the treatment, paralleling the transient nature of fever-associated improvement observed in ASD children1 (Extended Data Fig. 3a–e). Furthermore, LPS-induced rescue was observed in both young (2–5 months) and aged (9–12 month) mice (Fig. 1c and Extended Data Fig. 3f–j), suggesting that this is a generalizable phenomenon, not confined to early adulthood. LPS-induced rescue of MIA sociability deficits was also evident when assayed using a reciprocal social interaction test (Extended Data Fig. 3k). Lastly, LPS treatment in MIA offspring also rescued an enhanced marble burying behavior (Extended Data Fig. 3l), showing that inflammation can relieve several MIA-associated behavioral phenotypes. Intriguingly, unlike in MIA offspring, LPS treatment failed to restore sociability in mutant mice deficient for Cntnap2, Fmr1, and Shank3 (Fig. 1c and Extended Data Fig. 2), indicating that rescue by LPS-driven inflammation may be applicable only to a subset of animal models for neurodevelopmental disorders.
Acute fever does not restore sociability
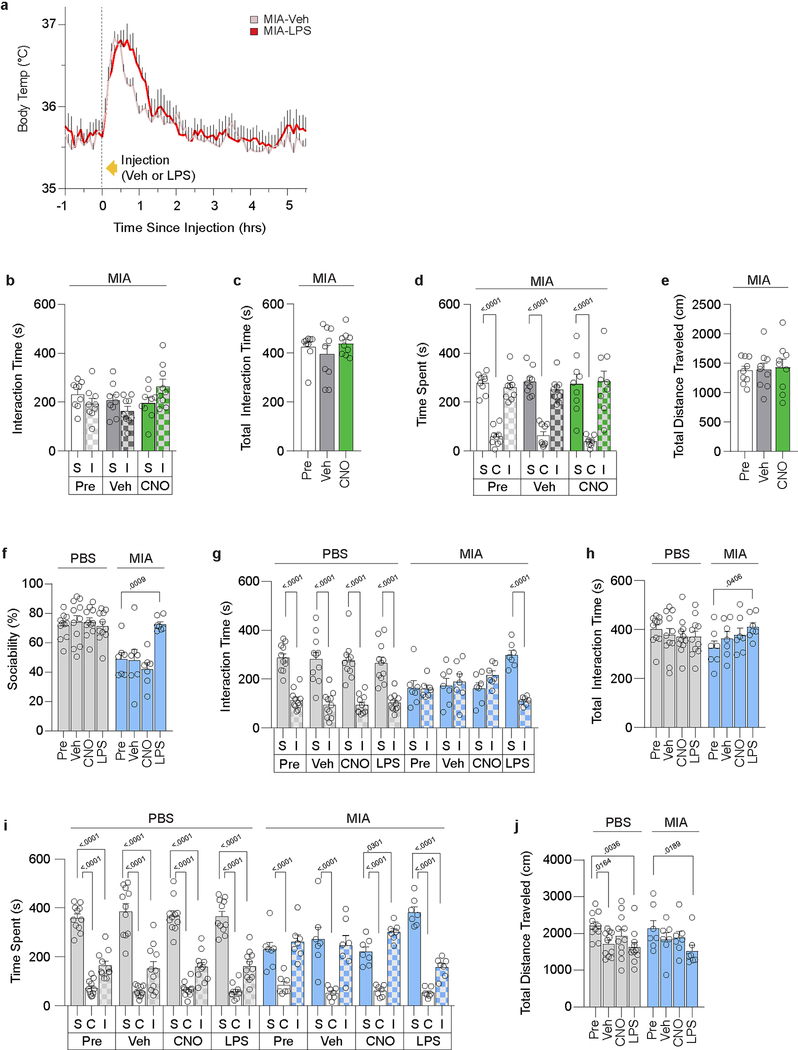
We next probed whether fever has a role in the observed behavioral rescue. Unlike in control offspring, LPS treatment did not induce changes in body temperature in MIA animals (Extended Data Fig. 4a), suggesting that the febrile response might not be the main factor contributing to the rescue. To directly test whether fever is dispensable, we sought to increase the animals’ body temperature without inducing systemic inflammation by targeting inhibitory DREADDs (designer receptors exclusively activated by designer drugs)17 to GABAergic neurons in the ventral part of the lateral preoptic nucleus (vLPO) of Vgat-Cre mice18 (Fig. 1d). As previously reported19, inhibition of these neurons led to an increase in body temperature of ~1°C (Fig. 1e). Induction of febrile response alone, however, failed to promote social preference in MIA offspring (Fig. 1f, g and Extended Data Fig. 4b–e), confirming that fever per se is not the main driver of the LPS-induced rescue. Of note, MIA Vgat-Cre mice exhibited comparable sociability deficits, were unaffected by CNO treatment and showed an increase in sociability upon LPS treatment (Extended Data Fig. 4f–j).
LPS reduces c-Fos induction in the S1DZ
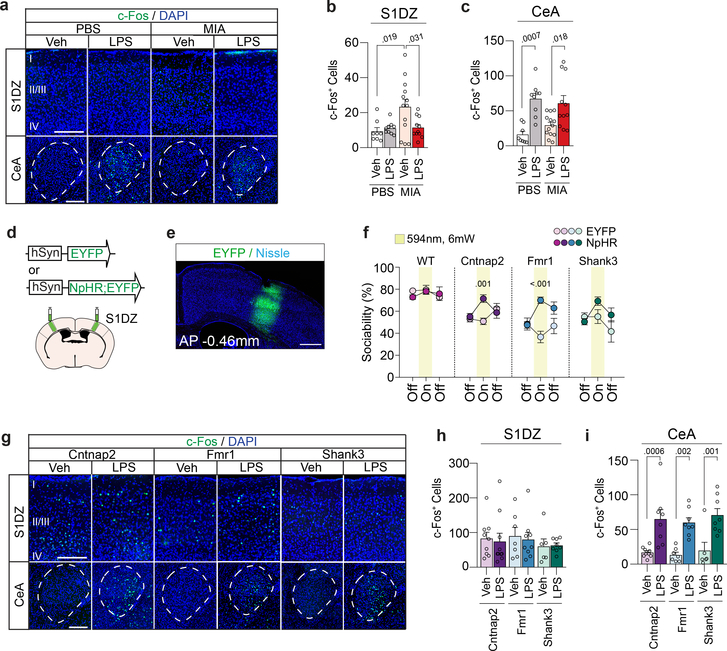
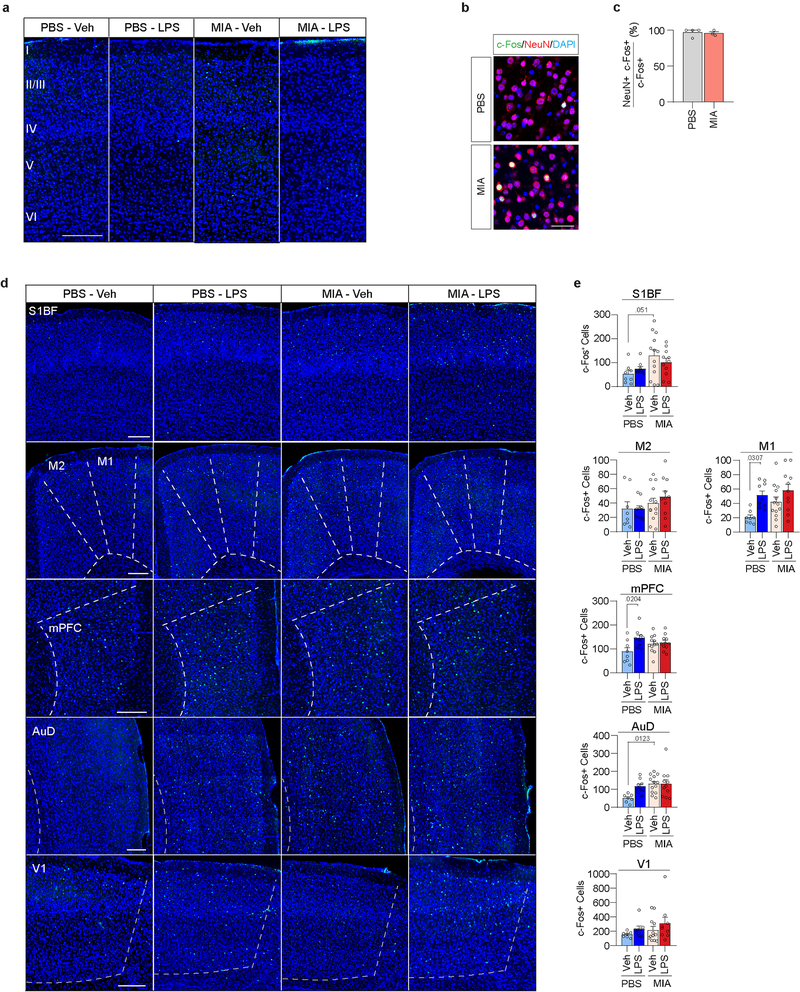
We previously established that adult MIA offspring display cortical abnormalities preferentially localized to S1DZ, a subregion of the S1 cytoarchitecturally defined by the absence of a discernable fourth layer (Extended Data Fig. 5). The cortical phenotype is characterized by an overall increase in neural activity, that, when reduced, can acutely rescue MIA-induced deficits in social behaviors8. We therefore investigated whether LPS-induced behavioral rescue in MIA offspring is accompanied by changes in S1DZ neural activity. MIA offspring exhibited an increase in the number of S1DZ cells expressing c-Fos, a marker for neuronal activation, relative to control offspring. However, in LPS-treated MIA offspring, the number of c-Fos+ S1DZ neurons was reduced to the level of control offspring (Fig. 2a, b and Extended Data Fig. 6a–c). LPS injections did not elicit a generalized, brain-wide effect on c-Fos expression in MIA offspring; the number of c-Fos+ neurons either remained unchanged, as in several cortical regions examined, or increased, such as in the central amygdala (CeA), a region known to be activated by LPS20 (Fig. 2a,c and Extended Data Fig. 6d,e). Therefore, LPS-induced behavioral rescue in MIA offspring was accompanied by a reduction in S1DZ neural activity.
Figure 2. Immune stimulation reduces hyperactivation in the S1DZ of MIA offspring.
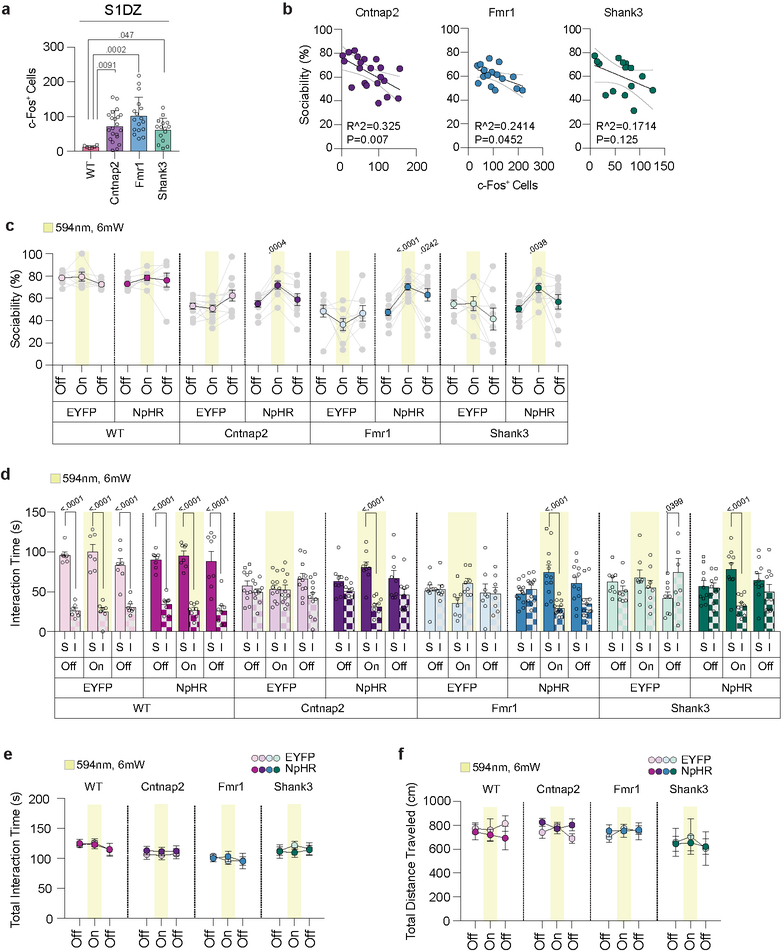
a, Representative images illustrating c-Fos (green) expression in the S1DZ and CeA following Veh or LPS injection. Scale bar represents 200μm. Numerals indicate cortical layers. S1DZ, Primary somatosensory cortex, dysgranular zone; CeA, Central amygdala. b,c, Quantification of c-Fos expressing cells in the S1DZ (b) and CeA (c) following Veh or LPS injection in the S1DZ (b) and CeA (c). For experiments a-c, PBS-Veh n=8, PBS-LPS n=9, MIA-Veh n=13, MIA-LPS n=11; from 3 independent experiments. d,e, AAV encoding either EYFP or EYFP fused to eNpHR was bilaterally injected into the S1DZ of monogenic mutant animals. Scale bar represents 500μm. f, Performance on sociability was assessed in the presence and absence of optical inhibition. For experiments e,f, WT-EYFP n=7, WT-eNpHR n=8, Cntnap2-EYFP n=11, Cntnap2-eNpHR n=9, Fmr1-EYFP n=8, Fmr1-eNpHR n=12, Shank3-EYFP n=8, Shank3-eNpHR n=10; from 6 independent experiments. g, Representative images illustrating c-Fos expression in the S1DZ and CeA following Veh or LPS injection in monogenic mutant mice. Scale bar represents 200μm. h,i, Quantification of c-Fos expressing cells following Veh or LPS injection in the S1DZ (h) and CeA (i). For experiments g-i, Cntnap2-Veh n=9, Cntnap2-LPS n=10, Fmr1-Veh n=7, Fmr1-LPS n=9, Shank3-Veh n=6, Shank3-LPS n=8; from 3 independent experiments. Statistics calculated by two-way ANOVA with Tukey’s post-hoc test (b,c) and Sidak’s post-hoc test (h,i), and two-way repeated measures ANOVA with Sidak’s post-hoc test (f). Graphs indicate mean ± s.e.m.
Dysregulation of neural activity and deficits in interneuron function in S1 have been previously associated with various genetic mouse models for neurodevelopmental disorders21–23. We, therefore, sought to determine whether increased neural activity can also be observed in the S1DZ of monogenic mutant mice. The number of c-Fos+ S1DZ neurons was increased compared to that of WT animals, and the magnitude of this increase correlated with the severity of the sociability deficits, notably in Cntnap2 and Fmr1 mutant animals (Extended Data Fig. 7a,b). These data suggest that increased neural activity in S1DZ may contribute to the expression of sociability deficits also in monogenic mutant mice, similarly to what we have described for MIA offspring8. Consistent with this idea, optogenetically reducing neural activity in the S1DZ could rescue sociability deficits in Cntnap2 and Fmr1 mutant animals. Shank3 mutant mice also showed an increase in sociability upon photoinhibition, but it was not significantly different from that of control animals expressing EYFP (Fig. 2d–f and Extended Data Fig. 7c–f). Therefore, reducing neural activity in the S1DZ was sufficient to restore sociability in Cntnap2 and Fmr1 mutant mice as well as in MIA offspring8. Unlike in MIA offspring, however, LPS treatment failed to reduce the number of c-Fos+ S1DZ neurons in monogenic mutant mice (Fig. 2g–i).
IL-17a restores sociability deficits
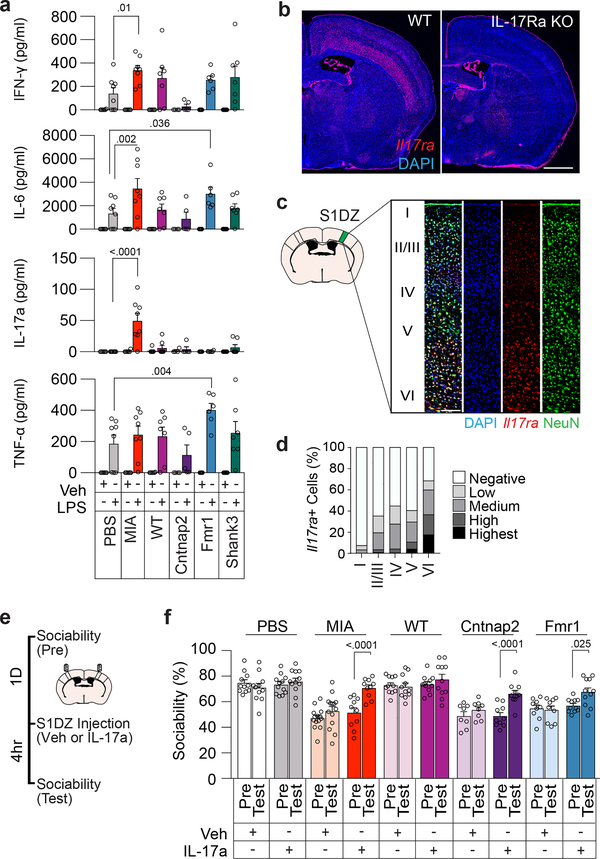
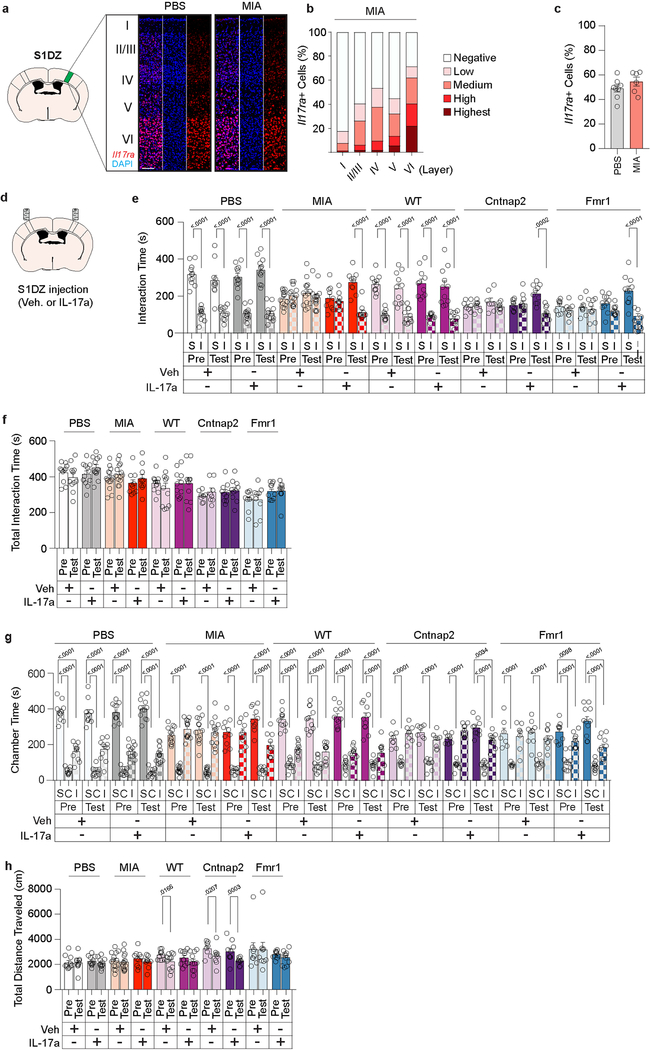
LPS injection is known to increase production of inflammatory cytokines24. We observed that administration of LPS results in a robust increase in plasma levels of IFN-γ, IL-6, and TNF-α (Fig. 3a). Intriguingly, IL-17a, whose orthologue in C. elegans25 has previously been implicated in promoting social behaviors, was prominently upregulated in MIA offspring, but not in monogenic mutant mice or in control animals (Fig. 3a). Furthermore, we noted that the receptor subunit A for IL-17a (IL-17Ra) is expressed in cortical neurons, including in the S1DZ (Fig. 3b–d and Extended Data Fig.8a–c). These data suggested that the increased levels of IL-17a upon LPS treatment in MIA, but not in monogenic mutant mice, may directly impact the S1DZ to restore sociability. Consistent with this idea, direct administration of recombinant IL-17a into the S1DZ was sufficient to increase sociability in not only MIA offspring, but also in Cntnap2 and Fmr1 mutant mice (Fig. 3e, f and Extended Data Fig. 8d–h).
Figure 3. IL-17a rescues sociability deficits in both MIA offspring and monogenic mutant mice.
a, IFN-γ, IL-6, IL-17a, and TNF-α levels in plasma following Veh or LPS injection (PBS-Veh n=6, PBS-LPS n=8, MIA-Veh n=6, MIA-LPS n=8, WT-Veh n=6, WT-LPS n=7, Cntnap2-Veh n=4, Cntnap2-LPS n=5, Fmr1-Veh n=5, Fmr1-LPS n=6, Shank3-Veh n=7, Shank3-LPS n=7; from 3 independent experiments). b, Il17ra expression in WT and IL-17Ra KO animals at AP −0.58mm from 1 independent experiment. Scale bar represents 1mm. c, Co-labeling of Il17ra and NeuN in the S1DZ from 2 independent experiments. Scale bar represents 100μm. d, Quantification of Il17ra expression within the S1DZ according to cortical layer in PBS offspring (n=8; from 2 independent experiments). e,f, Mice were tested for sociability one day prior to injection (Pre) and following bilateral administration of Veh or IL-17a into the S1DZ (Test). (PBS-Veh n=11, PBS-IL-17a n=12, MIA-Veh n=14, MIA-IL-17a n=10, WT-Veh n=11, WT-IL-17a n=11, Cntnap2-Veh n=8, Cntnap2-IL-17a n=10, Fmr1-Veh n=9, Fmr1-IL-17a n=11; from 6 independent experiments). Statistics calculated by two-way ANOVA with Dunnett’s post-hoc test (a) and two-way repeated measures ANOVA with Sidak’s post-hoc test (f). Graphs indicate mean ± s.e.m.
LPS-induced rescue requires IL-17a
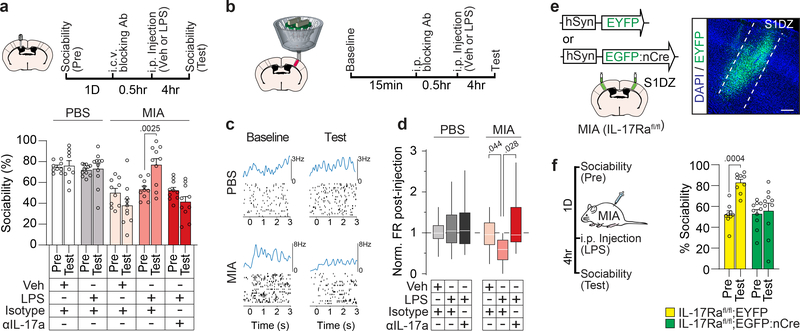
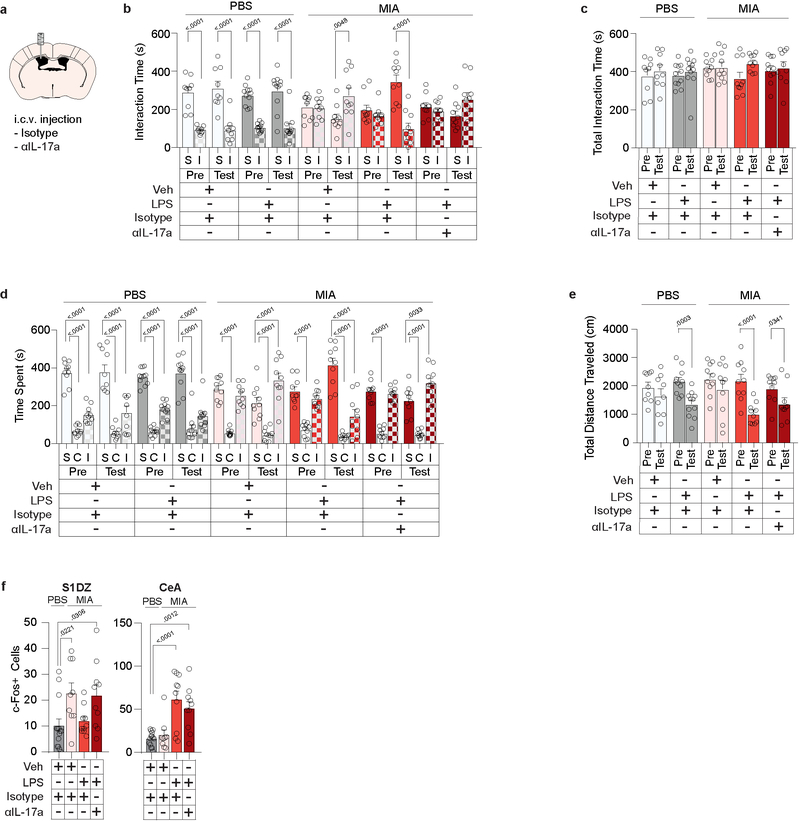
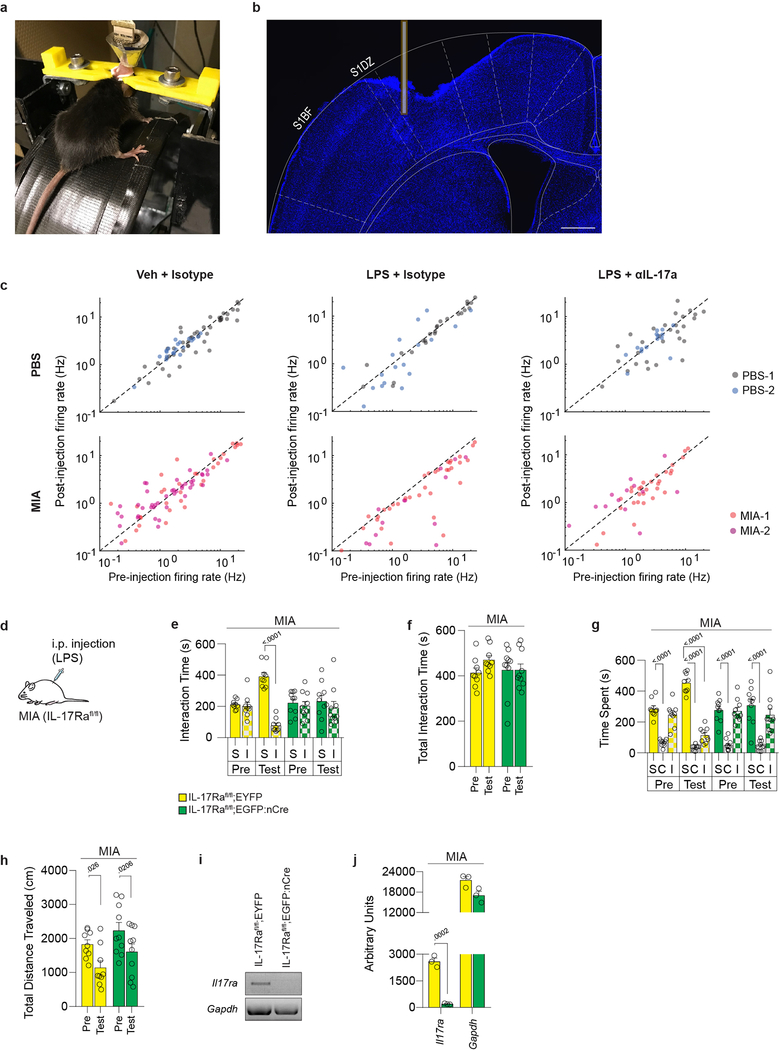
To further determine whether IL-17a mediates LPS-driven behavioral rescue, we inhibited IL-17a activity in the brain via intracerebroventricular (i.c.v.) injection of blocking antibodies. Antibodies against IL-17a prevented both the LPS-induced rescue of sociability (Fig. 4a and Extended Data Fig. 9a–e) and the reduction of c-Fos expression in the S1DZ of MIA offspring (Extended Data Fig. 9f). To directly assay the effects of LPS on neural activity we used multi-electrode arrays to measure the firing rate of S1DZ neurons in awake animals (Extended Data Fig. 10a, b). Upon LPS treatment, we observed a decrease in overall firing rate that was prevented by blocking IL-17a in MIA offspring. Neither LPS nor IL-17a blocking antibody treatment changed the firing rate in the S1DZ of control offspring (Fig. 4b–d and Extended Data Fig. 10c). Furthermore, LPS injection failed to restore sociability to MIA offspring deficient for IL-17Ra in the S1DZ neurons (Fig. 4e, f and Extended Data Fig. 10d–j). Our data collectively demonstrate that IL-17a mediates the restoring effects of inflammation on social behaviors by directly acting on IL-17Ra+ S1DZ neurons.
Figure 4. IL-17a is necessary for LPS-induced rescue of sociability deficits and reduction of S1DZ neural activity in MIA offspring.
a, Mice were tested for sociability one day prior to injection (Pre). The following day blocking antibody against IL-17a (αIL-17a) or control isotype antibody was administered i.c.v. 30 minutes prior to Veh or LPS injection. Sociability was assessed four hours following Veh or LPS injection (Test) (PBS-Veh-Isotype n=9, PBS-LPS-Isotype n=11, MIA-Veh-Isotype n=10, MIA-LPS-Isotype n=10, MIA-LPS-αIL-17a n=10; from 7 independent experiments). b-d, Firing rate of S1DZ neurons before and four hours after Veh or LPS injection in PBS and MIA offspring pretreated with isotype control antibody or blocking antibody against IL-17a. c, Example raster plot with firing rate profile before (Baseline) and after (Test) LPS treatment from an PBS and MIA mouse pretreated with isotype control antibody. At time 0 mice began walking on the wheel rotating at 7.5cm/s. Data collected between 1–2 seconds were included in analysis. d, Normalized firing rate change following treatment represented as box-whisker plots indicating median, interquartile range, and data limits as defined by Tukey (PBS-Veh-Isotype n=65 cells, PBS-LPS-Isotype n=42 cells, PBS-LPS-αIL-17a n=40 cells, MIA-Veh-Isotype n=75 cells, MIA-LPS-Isotype n=48 cells, MIA-LPS-αIL-17a n=43 cells; from 2 MIA offspring and 2 PBS offspring in 12 independent experiments). e, Lentivirus encoding either EYFP or EGFP fused to nuclear Cre (nCre) was bilaterally injected into the S1DZ of IL-17Rafl/fl MIA offspring. Scale bar represents 200μm. f,g, Mice were tested for sociability one day prior to injection (Pre). The following day, mice were tested for sociability four hours after LPS injection (Test). For experiments e,f, IL-17Rafl/fl;EYFP n=9, IL-17Rafl/fl;EGFP:nCre n=10; from 5 independent experiments. Statistics calculated by two-way repeated measures ANOVA with Bonferroni’s post-hoc test (a,f) and two-way ANOVA with Tukey’s post-hoc test (d). Graphs indicate mean ± s.e.m.
Discussion
Our and other group’s previous data suggested that increased IL-17a production in the pregnant mothers may present as a risk factor for neurodevelopmental disorders in offspring26,27. Based on our current findings, we propose that in adult MIA offspring the same type of cytokine is beneficial and ameliorates sociability phenotypes during episodes of inflammation. LPS treatment led to an increase in IL-17a levels in the blood selectively in MIA offspring, but not in other monogenic mutant mice, suggesting that inflammatory responses may result in beneficial effects only for those individuals who have their immune systems primed by prenatal exposure to immune activation or by other environmental factors. A better understanding of the role of a primed immune system among patients with neurodevelopmental disorders may help identify those whose behavioral symptoms are likely to improve upon exposure to fever-associated inflammation. Furthermore, elucidating the mechanisms by which IL-17a can induce two opposing behavioral outcomes depending on when its upregulation occurs, during embryonic fetal brain development or in the adult brain, may provide opportunities to devise novel therapeutic as well as preventive treatments for ASD-associated behavioral symptoms.
Methods
Animals
All experiments were performed according to Guide for the Care and Use of Laboratory Animals and were approved by the National Institutes of Health and the Committee on Animal Care at Massachusetts Institute of Technology. C57BL/6 were purchased from Taconic Biosciences for generating PBS and MIA offspring. Vgat-Cre (028862) were purchased from Jackson Laboratories and inbred. For monogenic experiments, C57BL/6, Cntnap2 (017482), Fmr1 (003025), and Shank3 (017688) mice were purchased from Jackson Laboratories and inbred after colonizing with SFB from donor mice. IL-17Rafl/fl and IL-17Ra KO were previously described28,29. All mice were males aged 2–5 months, unless otherwise specified.
Maternal immune activation
Mice were mated overnight with females carrying SFB in their guts26. On E12.5, pregnant female mice were weighed and injected with a single dose (20 mg/kg i.p.) of poly(I:C) (P9582, Sigma Aldrich) or PBS vehicle. Each dam was returned to its cage and left undisturbed until the birth of its litter. All pups remained with the mother until weaning on postnatal day 21–28 (P21–P28), at which time mice were group housed at a maximum of 5 per cage with same-sex littermates. Mating between Vgat-Cre(c/c) males and WT females were used to make MIA Vgat-Cre mice.
Stereotaxic surgery
Surgeries were carried out using aseptic technique. Mice were anesthetized using a mixture of ketamine (100mg/kg, i.p.) and xylazine (10mg/kg, i.p.). Mice were given pre-operative slow-release buprenorphine (1.0mg/kg s.c.). For manipulating body temperature with vLPO inhibition, Vgat-Cre mice received bilateral stereotaxic injections of virus (200nl/side) at rates of <0.1ml/min (AAV2-hSyn-DIO-hM4Di-mCherry (Addgene)). Virus was bilaterally targeted to the vLPO (AP+0.15, ML±0.50, DV-4.9, lambda was raised 600μm above bregma). For experiments involving optical manipulation in S1DZ, WT, Cntnap2, Fmr1, and Shank3 mice received bilateral stereotaxic injections of virus (400ul/side) at a rate of <0.1ml/min (AAV2-hSyn-EYFP, AAV2-hSyn-eNpHR3.0-EYFP). Viruses were bilaterally targeted to the S1DZ (AP-0.50, ML±2.6, DV-0.80, lambda was level with bregma). In order to deliver light into the S1DZ, a 300μm optic fiber was superficially implanted in the S1DZ. For experiments involving genetic knockout of IL-17Ra in S1DZ, IL-17Rafl/fl mice received bilateral stereotaxic injections of virus (800nl/side) at a rate of <0.1ml/min (pLenti-hSyn-EYFP, pLenti-hSyn-EGFP:nCre). Viruses were bilaterally targeted to the S1DZ. All lentiviruses were made in house. For administration of cytokines into S1DZ, cannula (PlasticsOne) were implanted superficially within the S1DZ. For central administration of blocking antibodies, cannula were implanted above the right lateral ventricle (AP-0.30, ML+1.0, DV-1.35, lambda was raised 600μm above bregma). Cannula were fitted with dummy cannula (PlasticsOne) to maintain cannula patency following surgery.
Tracking of body temperature
Body temperature was measured using the Anipill remote temperature monitoring system (007894–001, DSI). Adult male mice were implanted with an Anipill capsule in the abdominal cavity. Experiments were carried out >3 weeks following surgery. Mice were singly housed the day before the experiment. Ambient temperature was maintained at 23.5°C, consistent with the ambient temperature of the vivarium. Body temperature was sampled in 5-minute increments. LPS or saline (Veh) injections occurred between 11:00–13:00 for experiments assaying the effects of LPS on body temperature in PBS and MIA offspring.
Immunohistochemistry
Animals were transcardially perfused with cold PFA (4% in PBS). Brains were kept in PFA overnight at 4°C prior to vibratome sectioning (Leica VT1000s). Brains were cut at 50μm thickness for c-Fos quantification. Brains were cut at 100μm thickness for all other experiments. Prior to antibody labeling, sections were incubated in blocking solution (0.4% Triton X-100 and 2% goat serum in PBS) for 30min. Sections were then incubated in blocking solution containing primary antibodies overnight at room temperature. Primary antibodies used were chicken anti-GFP (1:1000, Ab5450, Abcam), rabbit anti-c-Fos (1:500, ABE457, Millipore), rabbit anti-DsRed (1:1000, 632496, Clontech), and mouse anti-NeuN (1:1000, MAB377, Millipore). Sections were washed in wash buffer (0.4% Triton X-100 in PBS) three times before secondary antibody labeling. Sections were incubated in blocking solution containing secondary antibodies and DAPI (1:5000, D1306, Thermofisher) for three hours at room temperature. Images of stained slices were acquired using a confocal microscope (LSM710, Carl Zeiss) with a 10x, 20x, or 40x objective lens.
In situ hybridization
Animals were transcardially perfused with cold PBS. Brains were extracted and embedded in optimal cutting temperature (OCT) compound on dry ice. Sections were cut at 20μm thickness on a cryostat. In situ hybridizations were performed using RNAscope 2.5 HD Assay-Red kit (322350, Advanced Cell Diagnostics) using a probe targeting the Il17ra transcript (Mm-Il17ra-O1, 566131, Advanced Cell Diagnostics). The probe was designed to target region 444–882 of the Il17ra transcript (NM_008359.2). Modifications to the kit protocol to improve adherence of tissue to the slide include an extension of fixation time to 30min and the addition of a humidified bake step at 40°C immediately prior to probe hybridization. Sections were counterstained with DAPI. Images were acquired using a confocal microscope (LSM710, Carl Zeiss) with a 10x or 20x objective lens. Il17ra and DAPI expression was quantified using QuPath30. Cells were divided into the following categories based on level of Il17ra expression: low = 1–3 puncta, medium = >3–9 puncta, high = >9–15 puncta, highest = >15 puncta.
In situ hybridization followed by immunohistochemistry
For experiments assaying the overlap of Il17ra and NeuN expression, immunohistochemistry for NeuN was performed following a modified in situ hybridization protocol. The RNAscope 2.5 HD Assay-Red kit in situ protocol was modified in the following ways: Sections were baked at 60°C, followed by a 10min fixation step with 4% PFA at room temperature. Sections were then stored in 70% ethanol at 4°C overnight. Sections were permeabilized in 8% SDS for 10min. Sections were washed twice with PBS between each step. Following SDS treatment, the RNAscope 2.5 HD Assay-Red kit protocol was followed from the probe hybridization step. Following the completion of the in situ protocol, sections were incubated in blocking buffer containing NeuN antibody overnight at 4°C. Sections were then incubated in blocking buffer containing DAPI and secondary antibody for 2hrs at room temperature.
Behavioral analysis
Male mice were tested during the light cycle in a room with lighting maintained at 230 Lux. Animals were transferred to the testing area at least one hour prior to the initiation of experiments. Tracking of mouse behavior was done using EthoVision XT (Noldus) tracking system.
Three-chamber social approach assay
Adult male mice were assayed for sociability using a three-chamber social approach assay. The arena was constructed of white acrylic (50cm × 35cm × 30cm). Wire cups (Spectrum Diversified) were placed in the back left and right corner of the arena beneath water-filled 1L bottles (Nalgene). On day 0, mice were habituated to the arena for 10min. Immediately following habituation, mice were singly housed. On day 1 (Pre), mice were placed in the center of the arena and allowed to freely explore. Following 10min, mice were confined to the center of the arena. An inanimate object (rubber stopper) or a male conspecific were placed beneath the wire cups. Placement of the inanimate object and social target were alternated. Mice were then allowed to freely explore the arena for 10min. Interaction time was defined as time spent in the areas circumscribing the wire cups (<2cm). Sociability was defined as interaction time with the social target divided by total interaction time and expressed as a percentage. For experiments involving LPS injections, mice were injected with either saline (Veh) or LPS (50μg/kg, i.p., L2630, Sigma) on day 2 (Test), four hours prior to testing. For the experiment assaying sociability 72hrs following LPS injection, mice used for 4hr LPS sociability experiments were tested for sociability again at 72hrs.
Three-chamber social approach assay with DREADD manipulation
Adult male Vgat-Cre MIA offspring were bilaterally injected with virus encoding the inhibitory DREADD receptor fused to mCherry into the vLPO. Following >3 weeks of recovery, mice were assayed on the three-chamber social approach assay outlined above. Baseline sociability was assayed on day 1 (Pre). On day 2 and day 3, mice were injected with either Saline (Veh) or CNO (1.5mg/kg i.p., BML-NS105, Enzo Life Sciences), two hours prior to initiation of behavior. Injection order was counterbalanced. Following behavioral experiments, post-mortem histology was used to confirm mCherry expression within the vLPO. For experiments assaying the effect of CNO and LPS injection in Vgat-Cre PBS and MIA offspring that have not undergone surgery, baseline sociability was assessed on day 1. On day 2 and 3 mice received counterbalanced injections of CNO or vehicle. On day 4, mice were injected with LPS.
Three-chamber social approach assay with S1DZ IL-17a administration
Adult male mice were implanted with a cannula into S1DZ bilaterally and allowed to recover for >2 weeks prior to the behavioral experiments. On day 1 (Pre) mice were assayed for sociability. On day 2 (Test), mice were anesthetized briefly using isoflurane and either saline (Veh) or IL-17a (50ng/side in 1μl at a rate of 180nl/min, 7956-ML/CF, R&D) was administered bilaterally into S1DZ through 250μm-projecting injector tips (PlasticsOne). Four hours after vehicle or IL-17a administration, mice were assayed for sociability. Cannula placements were verified using histology.
Three-chamber social approach assay with S1DZ optogenetic inhibition
Enhanced yellow fluorescent protein or halorhodopsin were virally targeted to the S1DZ. After two weeks of recovery mice were allowed to freely explore the arena for 10 min. The following day, the mice were given 3 min of no stimulation (‘off’ session) and 3 min of laser stimulation (‘on’ session) (594 nm, 6mW).
Three-chamber social approach assay with IL-17a blocking antibody
For central cytokine blockade experiments, adult male mice were implanted with a cannula into the lateral ventricle and allowed to recover for >2 weeks prior to the behavioral experiments. On day 1 (Pre), mice were tested for baseline sociability. On day 2 (Test) mice were injected with IL-17a blocking antibody (clone 50104; R&D) or isotype control antibody (IgG2a, clone 54447; R&D). Antibodies were dissolved in saline and administered intracerebroventricularly at 1mg/kg in 500nl at a rate of 180nl/min through 750μm-projecting injector tips (PlasticsOne). Blocking antibody was administered 30min prior to LPS administration. Four hours following LPS administration, mice were assayed for sociability.
Three-chamber social approach assay in mice deficient for IL17R in the S1DZ
IL-17Ra knockout was mediated by viral delivery of Cre-recombinase, expressed under the control of the human synapsin (hSyn) promoter, into the S1DZ of IL-17Rafl/fl MIA offspring. Following at least three weeks of recovery, mice were assayed for sociability. On the next day sociability was assessed four hours following LPS administration.
Marble burying assay
On day 1, mice were tested for their baseline marble burying phenotype. On day 2, four hours prior to beginning the marble burying assay, mice were treated with either LPS or vehicle. Marble burying assay was carried out as described previously8. Mice were placed into testing arenas (arena size: 40cm×20cm×30cm, bedding depth: 3cm) each containing 20 glass marbles (laid out in four rows of five marbles equidistant from one another). At the end of the 15min exploration period mice were carefully removed from the testing cages and the number of marbles buried was recorded. The marble burying index was arbitrarily defined as the following: 1 for marbles covered >50% with bedding, 0.5 for marbles covered <50% with bedding, or 0 for anything less.
Reciprocal social interaction assay
Four hours prior to testing, mice were injected with vehicle or LPS. Two unfamiliar mice of the same treatment and background were placed in a fresh mouse cage and allowed to freely interact for 10min. Videos were acquired using IC Capture (The Imaging Source) at 640×480 aspect ratio and 25fps. Social interaction (close following, push-crawl, nose-nose sniffing, and nose-anus sniffing) was scored by an observer blind to treatment and background31.
Quantification of c-Fos+ cells in the brain after LPS administration
Adult male mice were sacrificed five hours after LPS injection. c-Fos+ cells were quantified using the Cell Counter plugin in Fiji32. All cells were counted within a single coronal section of each respective brain region as defined by the Paxinos and Franklin Mouse Atlas33. Regions quantified include mPFC (PrL and IL) (AP+1.98), S1DZ (AP-0.46), S1BF (AP-0.46), M1 (AP-0.46), M2 (AP-0.46), AuD (AP-1.94), CeA (AP-1.94), and V1 (AP-3.64). Medial prefrontal cortex (mPFC), prelimbic cortex (PrL), infralimbic cortex (IL), primary somatosensory cortex dysgranular zone (S1DZ), primary somatosensory cortex barrel field (S1BF), primary motor cortex (M1), secondary motor cortex (M2), secondary auditory cortex dorsal part (AuD), central amygdala (CeA), primary visual cortex (V1). For experiments testing IL-17a dependence of LPS-induced changes in c-Fos expression, mice were injected i.c.v. with control antibodies or blocking antibody against IL-17a 30min prior to i.p. vehicle or LPS injection. Surgical and injection methods were identical to behavioral experiments.
Enzyme-linked immunosorbent assay (ELISA)
After four hours of vehicle or LPS administration, mice were anesthetized by intraperitoneal injection of fatal plus (100mg/kg). All blood samples were centrifuged at 10,000g for 10min at 4C. All samples were stored at −80 °C until further analysis. Cytokines concentrations in plasma were measured using an ELISA kit (IFN-γ; 430804, TNF-α; 430904, IL-6; 431304, IL-17a; 432504, Biolegend), following the manufacturer’s instructions.
PCR for assaying IL-17Ra knockout
IL17Rafl/fl male mice were bilaterally injected with virus encoding nuclear Cre fused to EGFP or control virus encoding only EYFP into the S1DZ. Following >3 weeks, injection sites were dissected from the primary somatosensory cortex. Single cells were dissociated from brain tissue using a modified version34 of the Papain Dissociation Kit protocol (LK003153, Worthington) and sorted on a BD FACS Aria (BD biosciences) based on EGFP/EYFP expression. RNA was extracted from sorted cells using a Quick-RNA micro-prep kit (Zymo). 20ng of RNA was converted into cDNA using oligodT (Protoscript First Strand CDNA Synthesis Kit, NEB). Il17ra mRNA expression was analyzed using PCR. 1μl of cDNA was diluted in a 20ul reaction volume. Il17ra and Gapdh mRNA expression were assessed using the following primers: Il17ra 5′-AGATGCCAGCATCCTGTACC-3′ and 5′-CACAGTCACAGCGTGTCTCA-3′; Gapdh 5′-GACTTCAACAGCCTCCCACTCTTCC-3′ and 5′-TGGGTGGTCCAGGTTTCTTACTCCTT-3′. Cycling conditions for Il17ra: 95 °C × 5min (1 cycle), 95 °C × 20 s, 60 °C × 30 s, 72 °C × 30s (32 cycles), 72 °C × 5min (1 cycle) and 4 °C hold. Cycling conditions for Gapdh: 95 °C × 5min (1 cycle), 95 °C × 20 s, 60 °C × 30 s, 72 °C × 30s (28 cycles), 72 °C × 5min (1 cycle) and 4 °C hold. Band intensity from gel images were quantified using ImageJ.
In vivo electrophysiology
Electrophysiological experiments were conducted in head-fixed mice trained to walk on a rotating running wheel. Prior to training, mice were implanted with custom crowns permitting head-fixing above the wheel and allowed to recover for one week before training. Mice were trained to walk on the wheel for three 10min sessions daily for at least one week. Following training, mice were implanted with a multi-electrode array targeting the S1DZ and allowed to recover before testing. To assess IL-17a dependence of LPS-induced changes in neural activity, mice were injected i.p. with control antibodies or blocking antibody against IL-17a (1mg/mouse), 30min prior to i.p. vehicle or LPS injection. Baseline neural activity was measured while mice were running on the wheel immediately prior to the first injection. Following the first injection, mice were returned to their home cage. Post-injection (Test) neural activity during wheel running was measured 4hrs after vehicle or LPS injection.
Multi-electrode array construction and implantation
Custom multi-electrode array scaffolds (drive bodies) were designed using 3D CAD software (SolidWorks) and printed in Accura 55 plastic (American Precision Prototyping) as described previously35,36. Prior to implantation, each array scaffold was loaded with 8–24 independently movable micro-drives carrying 12.5μm nichrome (California Fine Wire Company) tetrodes. Electrodes were pinned to custom-designed, 32- or 128-channel electrode interface boards (EIB, Sunstone Circuits) along with a common reference wire (A-M systems).
Electrophysiological recordings and spike sorting
Signals were acquired using a Neuralynx multiplexing digital recording system (Neuralynx) through a combination of 32- and 64-channel digital multiplexing headstages plugged into the EIB of the implant. Signals from each electrode were amplified, filtered between 0.1 Hz and 9 kHz and digitized at 30 kHz. Initial spike sorting was performed using MountainSort, followed by manual quality control using MClust toolbox (http://redishlab.neuroscience.umn.edu/mclust/MClust.html).
Analysis of firing rate
Firing rate was calculated across one second time windows during which animals were walking stably on the rotating wheel averaged across 25–50 trials per condition. The first second after wheel rotation onset was omitted to avoid firing rate changes due to acceleration. Firing rate was sampled with a 1ms bin width passed through a box car filter (100ms). The resulting PSTHs were then smoothed with a 50ms gaussian. To assess the effect of treatment on neural activity, changes in firing rates during wheel running 4hrs after injection were normalized to the pre-injection baseline. Cells showing firing rate below 0.1Hz were excluded from analysis.
Statistics and reproducibility
Statistical analyses were performed using GraphPad Prism. Sample size was chosen based on similar previous studies and not based on statistical methods to predetermine sample size8,15. Within each iteration of an experiment, animals were randomly assigned to groups with approximately balanced sample size. Behavioral results from mice with inaccurate targeting of viral infection or cannula implantations were excluded. Experimenters were blind to subject treatment during data collection and analysis.
Extended Data
Extended Data Figure 1. Cntnap2, Fmr1, and Shank3 mutant mice show variable sociability performance.
a, Sociability performance (MIA n=13, WT n=22, Cntnap2 n=71, Fmr1 n=165, Shank3 n=50; from 30 independent experiments). b, Time spent investigating social (S) versus inanimate (I) objects for mice described in (a). c,d, total interaction time (c) and distance traveled (d) during three-chambered sociability experiments described in (a). Statistics calculated by one-way ANOVA with Dunnett’s post-hoc test (a,c,d) and two-way ANOVA with Dunnett’s post-hoc test (b). Graphs indicate mean ± s.e.m.
Extended Data Figure 2. Further behavioral analyses for sociability performance following LPS treatment in PBS and MIA offspring, and monogenic mutant mice.
a, Time spent investigating social (S) versus inanimate (I) objects (a), total interaction time (b), time spent in social (S), center (C) or inanimate (I) chamber (c), and distance traveled (d) for sociability experiments in Fig 1c (PBS-Veh n=8, PBS-LPS n=9, MIA-Veh n=13, MIA-LPS n=11; from 3 independent experiments). Statistics calculated by two-way ANOVA with Sidak’s (a) Dunnett’s (c) post-hoc test and two-way repeated measures ANOVA with Sidak’s post-hoc test (b,d). Graphs indicate mean ± s.e.m.
Extended Data Figure 3. LPS-induced rescue of MIA behavioral phenotypes is transient, effective in aged mice, and extends beyond three-chambered sociability.
a-e, Sociability measured 72hrs following Veh or LPS injection in PBS and MIA offspring from Fig. 1c. Data expressed as percent sociability (a), time spent investigating social (S) versus inanimate (I) objects (b), total interaction time (c), time spent in social (S), center (C) or inanimate (I) chamber (d), and distance traveled (e) during three-chambered sociability experiments (PBS-Veh n=7, PBS-LPS n=7, MIA-Veh n=8, MIA-LPS n=6; from 2 independent experiments). f-j, Sociability measured before and 4hr after Veh or LPS injection in aged MIA mice (9–12 months). Data expressed as percent sociability (f), time spent investigating social (S) versus inanimate (I) objects (g), total interaction time (h), time spent in social (S), center (C) or inanimate (I) chamber (i), and distance traveled (j) during three-chambered sociability experiments (MIA-Veh n=6, MIA-LPS n=7: from 2 independent experiments). k, Reciprocal social interactions measured upon Veh or LPS treatment in PBS or MIA offspring (PBS-Veh n=9, PBS-LPS n=9, MIA-Veh n=11, MIA-LPS n=11; from 4 independent experiments). l, Marble burying index (% of buried marbles) measured before and 4hr after Veh or LPS treatment in PBS or MIA offspring (PBS-Veh n=12, PBS-LPS n=12, MIA-Veh n=12, MIA-LPS n=11; from 5 independent experiments). Statistics calculated by two-way ANOVA with Sidak’s (a,b,c,e,g), Dunnett’s (d,i), and Tukey’s (k) post-hoc tests, and two-way repeated measures ANOVA with Sidak’s post-hoc test (f,h,j,l). Graphs indicate mean ± s.e.m.
Extended Data Figure 4. Acute increase in body temperature is insufficient to promote sociability.
a, Body temperature profile following Veh or LPS injection in MIA offspring (Veh n=10, LPS n=10; from 4 independent experiments). Initial spike in body temperature is due to handling stress. b-e, Data expressed as time spent investigating social (S) versus inanimate (I) objects (b), total interaction time (c), time spent in social (S), center (C) or inanimate (I) chamber (d), and distance traveled (e) during three-chambered sociability experiments described in Fig. 1g (n=9 for all groups; from 2 independent experiments). f-j, Sociability performance in Vgat-Cre PBS and MIA offspring following Veh, CNO, or LPS treatment. Data expressed as percent sociability (f), time spent investigating social (S) versus inanimate (I) objects (g), total interaction time (h), time spent in social (S), center (C) or inanimate (I) chamber (i), and distance traveled (j) during three-chambered sociability experiments (PBS n=11, MIA n=7; from 2 independent experiments). *P<0.05, **P<0.01 calculated by two-way repeated measures ANOVA with Bonferroni’s (a) and Dunnett’s (f,h,j) post-hoc tests, and two-way ANOVA with Sidak’s (b,g) and Dunnett’s (d,i) post-hoc tests, and one-way repeated measures ANOVA with Tukey’s post-hoc test (c,e). Graphs indicate mean ± s.e.m.
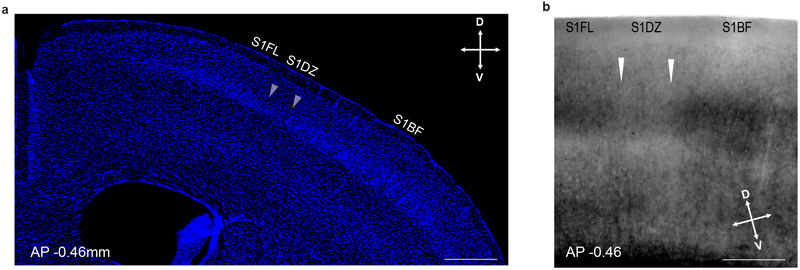
Extended Data Figure 5. Histological identification of S1DZ.
a, Coronal section of the cortex counterstained with DAPI to highlight the abrupt reduction in cell density in layer 4 between the S1DZ and the S1BF at AP −0.46mm. (n=5, from 1 independent experiment). b, Coronal section of the cortex imaged with differential interference contrast further highlighting the reduced layer 4 in the S1DZ at AP −0.46mm (n=3, from 2 independent experiments). White arrows indicate borders of S1DZ. Scare bar represents 500μm (a) and 300μm (b). D: dorsal, V: ventral.
Extended Data Figure 6. LPS treatment in MIA offspring does not have a distinguishable effect on c-Fos expression in other cortical regions analyzed.
a, Full cortical depth of S1DZ c-Fos staining as shown in Fig. 2a for PBS and MIA offspring after Veh or LPS administration (PBS-Veh n=8, PBS-LPS n=9, MIA-Veh n=13, MIA-LPS n=11; from 3 independent experiments). Scare bar represents 200μm. b,c, Representative images (b) and quantification (c) of c-Fos(Green)/NeuN(Red) co-labeled cells within the S1DZ of PBS and MIA offspring (PBS n=4, MIA n=3; from 1 independent experiment). Scale bar represents 50μm. d,e, Representative images (d) and quantification (e) of c-Fos expression in a series of cortical regions and following Veh or LPS injection in PBS or MIA offspring. Sections are stained for c-Fos (Green) and DAPI (Blue). Scale bar represents 200μm. S1BF, Primary somatosensory cortex, barrel field; M1, Primary motor cortex; M2, Secondary motor cortex; mPFC, medial prefrontal cortex (prelimbic and infralimbic cortex); AuD, Primary auditory cortex; V1, Primary visual cortex (For S1BF, M2, M1, and AuD; PBS-Veh n=8, PBS-LPS n=9, MIA-Veh n=13, MIA-LPS n=11. For mPFC; PBS-Veh n=8, PBS-LPS n=9, MIA-Veh n=12, MIA-LPS n=11. For V1; PBS-Veh n=7, PBS-LPS n=8, MIA-Veh n=12, MIA-LPS n=9; from 4 independent experiments). Statistics calculated by unpaired two-tailed t-test (c) and two-way ANOVA with Tukey’s post-hoc test (e). Graphs indicate mean ± s.e.m.
Extended Data Figure 7. Further behavioral analyses of S1DZ optical inhibition-mediated rescue of sociability in monogenic mutant mice.
a, Quantification of c-Fos expressing cells in the S1DZ of monogenic mutant mice (WT n=6, Cntnap2 n=21, Fmr1 n=17, Shank3 n=15: from 5 independent experiments). b, Correlation of c-Fos expression in the S1DZ with severity of sociability deficits across monogenic mutant mice (Cntnap2 n=21, Fmr1 n=17, Shank3 n=15; from 4 independent experiments). Black solid lines represent regression line; grey lines indicate 90% confidence intervals. c, Individual data for experiments in Fig. 2f. d-f, Data expressed as time spent investigating social (S) versus inanimate (I) objects (d), total interaction time (e) and distance traveled (f) during three-chambered sociability experiments described in Fig. 2f (WT-EYFP n=7, WT-eNpHR n=8, Cntnap2-EYFP n=11, Cntnap2-eNpHR n=9, Fmr1-EYFP n=8, Fmr1-eNpHR n=12, Shank3-EYFP n=8, Shank3-eNpHR n=10; from 6 independent experiments). Statistics calculated by one-way ANOVA with Dunnett’s post-hoc test (a), linear regression (b), one-way repeated measures ANOVA with Dunnet’s post-hoc test (c), two-way ANOVA with Sidak’s post-hoc test (d), and two-way repeated measures ANOVA with Sidak’s post-hoc test (e,f). Graphs indicate mean ± s.e.m.
Extended Data Figure 8. Il17ra expression in the S1DZ of PBS and MIA offspring. Further behavioral analyses of S1DZ IL-17a rescue of sociability in MIA offspring and monogenic mutant mice.
a, Representative images of Il17ra expression in the S1DZ of PBS and MIA offspring. Scare bar represents 1mm. b, Quantification of Il17ra expression within the S1DZ of MIA offspring according to cortical layer (n=6; from 2 independent experiments). c, Quantification of overall Il17ra expression in the S1DZ of PBS and MIA offspring (PBS n=8, MIA n=6; from 2 independent experiments). d-h, Further behavioral analyses of experiments described in Fig. 3f; Time spent investigating social (S) versus inanimate (I) objects (e), total interaction time (f), time spent in social (S), center (C) or inanimate (I) chamber (g), and distance traveled (h) (PBS-Veh n=11, PBS-IL-17a n=12, MIA-Veh n=14, MIA-IL-17a n=10, WT-Veh n=11, WT-IL-17a n=11, Cntnap2-Veh n=8, Cntnap2-IL-17a n=10, Fmr1-Veh n=9, Fmr1-IL-17a n=11; from 6 independent experiments). Statistics calculated by unpaired two-tailed t test (c), two-way ANOVA with Sidak’s (e) and Dunnett’s (g) post-hoc tests, and two-way repeated measures ANOVA with Sidak’s post-hoc test (f,h). Graphs indicate mean ± s.e.m.
Extended Data Figure 9. IL-17a is necessary for LPS-induced behavioral rescue and reduction of c-Fos expression in MIA offspring.
a-e, Further behavioral analyses of experiments described in Fig. 4a; Time spent investigating social (S) versus inanimate (I) objects (b), total interaction time (c), time spent in social (S), center (C) or inanimate (I) chamber (d), and distance traveled (e) (PBS-Veh-Isotype n=9, PBS-LPS-Isotype n=11, MIA-Veh-Isotype n=10, MIA-LPS-Isotype n=10, MIA-LPS-αIL-17a n=10; from 7 independent experiments). f, Quantification of c-Fos expressing cells in the S1DZ and CeA following Veh or LPS injection in MIA offspring pretreated i.c.v. with isotype control antibody or blocking antibody against IL-17a (αIL-17a). Statistics calculated by two-way ANOVA with Sidak’s (b) and Dunnett’s (d) post-hoc tests, two-way repeated measures ANOVA with Sidak’s post-hoc tests (c,e), and one-way ANOVA with Dunnett’s post-hoc test (f). Graphs indicate mean ± s.e.m.
Extended Data Figure 10. Further analyses of the necessity of IL-17a for the LPS-induced reduction of firing rate in the S1DZ, and the necessity of S1DZ IL-17Ra expression for the LPS-induced rescue of sociability deficits in MIA offspring.
a-c, Further analyses for experiments described in Fig 4d. a, Example of a head-fixed mouse on the running wheel used during single-unit recording. b, Representative image of a tetrode placement in the S1DZ. Scale bar represents 500μm. c, Firing rate for individual cells before and 4hrs after vehicle or LPS injection in PBS and MIA offspring pretreated with isotype control antibody or blocking antibody against IL-17a (αIL-17a). d-h, Further analyses for experiments described in Fig. 4e–f. Time spent investigating social (S) versus inanimate (I) objects (e), total interaction time (f), time spent in social (S), center (C) or inanimate (I) chambers (g), and distance traveled (h) (IL-17Rafl/fl;EYFP n=9, IL-17Rafl/fl;EGFP:nCre n=10; from 5 independent experiments). i-j, Representative images (i) and corresponding quantification (j) of Il17ra and Gapdh amplicon following PCR using cDNA derived from cells isolated from the cortical region centered on S1DZ of IL-17Rafl/fl;EYFP and IL-17Rafl/fl;EGFP:nCre mice (n=3 for all groups; from 1 experiment). Statistics calculated by two-way ANOVA with Sidak’s post-hoc test (e,g), two-way repeated measures ANOVA with Sidak’s post-hoc tests, (f,h) and unpaired two-tailed t test (j). Graphs indicate mean ± s.e.m.
Acknowledgements
We thank N. Soares, M. Garcia, and Y. Liu for assistance with experiments and B. Noro and M. Trombly for critical reading of the manuscript. This work was supported by the National Institute of Mental Health (1-R01-MH115037-01, G.B.C), Jeongho Kim Neurodevelopmental Research Fund (G.B.C. and J.R.H.), Hock E. Tan and K. Lisa Yang Center for Autism Research (G.B.C. and M.D.R.), Perry Ha (G.B.C.), Simons Center for the Social Brain (G.B.C., J.R.H. and Y.S.Y.), the Simons Foundation Autism Research Initiative (G.B.C. and J.R.H), the Champions of the Brain Weedon Fellowship (G.M.W), and the National Science Foundation Graduate Research Fellowship (#1122374, M.D.R.). We are deeply grateful to Barbara Picower, the JPB Foundation, the Picower Institute for Learning and Memory, Lore McGovern and the McGovern Institute for Brain Research for their mentorship and direct support of this work over the years.
Footnotes
The authors declare no competing financial interests.
Data availability: Source data containing raw data for all experiments are available in the online version of the paper. All other data are available from the corresponding author on reasonable request.
References
- 1.Curran LK et al. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics 120, e1386–e1392 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Grzadzinski R, Lord C, Sanders SJ, Werling D & Bal VH Children with autism spectrum disorder who improve with fever: Insights from the Simons Simplex Collection. Autism Res. 11, 175–184 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Shi L, Fatemi SH, Sidwell RW & Patterson PH Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci 23, 297–302 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SEP, Li J, Garbett K, Mirnics K & Patterson PH Maternal immune activation alters fetal brain development through Interleukin-6. J. Neurosci 27, 10695–10702 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peñagarikano O et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 147, 235–246 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell 78, 23–33 (1994). [PubMed] [Google Scholar]
- 7.Peça J et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 472, 437–442 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yim YS et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 549, 482–487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitrow M Wagner-Jauregg and fever therapy. Med. Hist 34, 294–310 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyall K et al. The Changing Epidemiology of Autism Spectrum Disorders. Annu. Rev. Public Health 38, 81–102 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawley JN Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin. Neurosci. 14, 293–305 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ey E, Leblond CS & Bourgeron T Behavioral profiles of mouse models for autism spectrum disorders. Autism Res. 4, 5–16 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Patterson PH Maternal infection and immune involvement in autism. Trends Mol. Med 17, 389–394 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozak W, Conn CA & Kluger MJ Lipopolysaccharide locomotor activity induces fever and depresses in unrestrained mice. Am. J. Physiol 266, R125–135 (1994). [DOI] [PubMed] [Google Scholar]
- 15.Choi GB et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malkova NV, Yu CZ, Hsiao EY, Moore MJ & Patterson PH Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain. Behav. Immun 26, 607–616 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armbruster BN, Li X, Pausch MH, Herlitze S & Roth BL Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci 104, 5163–5168 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vong L et al. Leptin Action on GABAergic Neurons Prevents Obesity and Reduces Inhibitory Tone to POMC Neurons. Neuron 71, 142–154 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao ZD et al. A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci 114, 2042–2047 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai H, Haubensak W, Anthony TE & Anderson DJ Central amygdala PKC-δ+neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci 17, 1240–1248 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gogolla N et al. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J. Neurodev. Disord 1, 172–181 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selby L, Zhang C & Sun QQ Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci. Lett 412, 227–232 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orefice LL et al. Targeting Peripheral Somatosensory Neurons to Improve Tactile-Related Phenotypes in ASD Models. Cell 178, 867–886.e24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erickson MA & Banks WA Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: Multiplex quantification with path analysis. Brain. Behav. Immun 25, 1637–1648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C et al. IL-17 is a neuromodulator of Caenorhabditis elegans sensory responses. Nature 542, 43–48 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lammert CR et al. Cutting Edge: Critical Roles for Microbiota-Mediated Regulation of the Immune System in a Prenatal Immune Activation Model of Autism. J. Immunol 201, 845–850 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Malki K et al. An alternative pathway of imiquimod-induced psoriasis-like skin inflammation in the absence of interleukin-17 receptor a signaling. J. Invest. Dermatol 133, 441–451 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Tusi BK et al. Population snapshots predict early haematopoietic and erythroid hierarchies. Nature 555, 54–60 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bankhead P et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep 7, 16878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverman JL, Yang M, Lord C & Crawley JN Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci 11, 490–502 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schindelin J et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paxinos G & Franklin KBJ Mouse brain in stereotaxic coordinates. Academic Press (2001). [Google Scholar]
- 34.Hrvatin S et al. Single-cell analysis of experience-dependent transcriptomic states in the mouse visual cortex. Nat. Neurosci 21, 120–129 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halassa MM et al. State-dependent architecture of thalamic reticular subnetworks. Cell 158, 808–821 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunetti M et al. Design and fabrication of ultralight weight, Adjustable multi-electrode probes for electrophysiological recordings in mice. J. Vis. Exp 91, e51675 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]