Abstract
Immunizations have proven to be an important tool for public health and for reducing the impact of vaccine preventable diseases. To realize the maximum benefits of immunizations, a coordinated effort between public policy, health care providers and health systems is required to increase vaccination coverage and to ensure high-quality data are available to inform clinical and public health interventions. Immunization information systems (IIS) are confidential, population-based, computerized databases that record all immunization doses administered by participating providers to persons residing within a given geopolitical area. The key output of an IIS is high-quality data for use in targeting and monitoring immunization program activities and providing clinical decision support at the point of care. To be truly effective, IISs need to form a nationwide network and repository of immunization data. Since the early 2000s Centers for Disease Control and Prevention has made strides to help IIS move toward a nationwide network through efforts focused on improving infrastructure and functionality, such as the IIS Minimum Functional Standards, and the IIS Annual Report, a self-reported data collection of IIS progress toward achieving the functional standards. While these efforts have helped immunization programs achieve more functional standards, there is a need to shift focus from infrastructure and functionality improvements to high data quality through objective measurement of IIS performance and evaluating critical outcomes. Additionally, realizing the vision of a nationwide repository of high-quality immunization data requires tackling the many challenges that impact data quality and availability including those related to policy, data sharing, data use, aging IIS technology, sustainability, and participation in the IIS. This paper describes the current state of IIS in the United States, critical challenges impacting the quality of data in IIS, and potential components of a future IIS model to address these challenges.
Keywords: immunization data, immunization information systems, immunization registry
Immunizations are important tools for reducing vaccine preventable diseases and are considered one of the top 10 great public health achievements in the United States.1,2 Immunizations have reduced disease, hospitalizations, deaths, and health care-related costs associated with vaccine prevented diseases.1 The impact of immunizations in the United States primarily targeting children to protect against 9 vaccine preventable diseases show a reduction over time by more than 90% of diseases with most of the diseases either eradicated or achieved reductions of more than 99%.2 In a study to examine the return on investment of childhood immunizations, it showed immunizations resulted in a return 16 times greater than the initial costs.3 To realize the maximum benefits of immunizations in reducing vaccine preventable diseases, a well-coordinated effort with health systems, federal and state public health agencies, providers, and public policies is required.4 Ensuring sufficient immunization coverage levels to keep vaccine preventable diseases in check is dependent on the availability of complete, timely and accurate information for members of a population.4,5 Immunization information systems (IISs) are confidential, population-based, computerized databases that record all immunization doses administered by participating providers to persons residing within a given geopolitical area.
At the point of clinical care, an IIS provides consolidated immunization histories and clinical decision support to providers.
At the population level, an IIS provides aggregate data on vaccinations to guide public health actions to improve vaccination rates, identify populations potentially at risk for vaccine-preventable diseases, and effectively prioritize program resources.
IISs are an invaluable tool to support a patient and their health care provider through the patient’s immunization journey from childhood to adulthood and can help ensure a patient is fully immunized.6,7 Pediatric providers can access an IIS to obtain a patient’s immunization record to ensure a child is up to date on their immunizations and prevent the patient from becoming over- or under-vaccinated. IISs contain a reminder recall function that reminds patients of upcoming vaccinations. The automated reminder function in an IIS is based on an evidence-based strategy for increasing vaccination; however, the effectiveness of the feature is impacted by challenges in implementation and sustainability.8 In 1 study, the effect of reminder recalls in selected states demonstrated that use of statewide reminder recalls did not improve human papillomavirus vaccination rates in New York and this feature minimally improved the rates in Colorado.9 While the effectiveness of reminder recalls should be further evaluated, IISs can learn from successful private sector reminder recall implementations and explore public-private collaborations to remove barriers that prevent the optimal implementation and use of the reminder recall feature.8 IISs are also critical during an outbreak response quickly providing insight on vaccination coverage and pockets of need, which can both be used to inform public health interventions. IISs play a critical role through a lifetime of care for a patient and contain key features providers can use to ensure their patients are appropriately vaccinated.
IISs exist within immunization programs and play a critical role in supporting the programs and providers with vaccine ordering and inventory management, clinical decision support (evaluation and forecasting), reminder/recall capabilities for patient follow-up, consolidated immunization histories, proof of immunity, and vaccination coverage estimates for specific geographic areas and population groups.10 Immunization programs were pioneers in the development of public health registries which have evolved significantly over time to meet changing programmatic needs and take advantage of advances in health information technology. The Community Preventive Services Task Force recommends IIS use as an evidence-based strategy to improve vaccination coverage.11 However, to be most effective for pediatricians and other health care providers, IISs would benefit from forming a nationwide network and repository of immunization data to facilitate access to data across jurisdictions and to stakeholders. Like other public health infrastructure, IISs have had difficulty keeping pace with the rapidly evolving health information technology landscape.12 This article will discuss the current state of IISs in the United States and describe potential opportunities to enhance IISs and improve immunization data in the future for all users of this information including pediatric providers.
Overview
US immunization programs began developing IISs in the early 1990s using a variety of jurisdiction-based approaches including commercial products and homegrown solutions.13 IIS developers and immunization programs broke new ground with the development of complex systems that connected public health, health care, and consumers in new ways. The Centers for Disease Control and Prevention (CDC), in collaboration with partners, published the first complete set of standards for IISs in the early 2000s entitled IIS Minimum Functional Standards, 2001 to 2012.14 These standards included infrastructure and programmatic activities, which focused on laying a strong foundation for future work. Over the years, these standards have evolved, resulting in the version currently in use today, Version 4.1.15 Developed in 2019, the current standards reflect an evolution that is consistent with the increasing complexity of IIS from simple immunization databases in the early years to multi-functional electronic systems capable of bidirectional data exchange with a myriad of other medical and public health information systems including Electronic Health Records (EHRs), Health Information Exchanges, and other IISs.
In 1999, CDC launched the IIS Annual Report (IISAR) and in 2002, it was revised to measure progress toward meeting functional standards.16 The IISAR is an annual assessment of the 64 immunization programs that receive funding under Section 317b of the Public Health Service Act, including 50 states, 5 US cities, the District of Columbia, and 8 territories. It is a self-administered questionnaire completed by jurisdiction immunization program managers to provide a snapshot of IIS-based immunization coverage estimates, provider participation in the IIS, and progress toward meeting functional standards.17 IISAR data provide critical insight needed to set future direction and target funding and resources to the most impactful activities.
Throughout the history of IIS functional standards and measurement efforts, a few key concepts have been a consistent focus: data quality, provision of data to stakeholders, privacy and confidentiality, clinical decision support, data exchange with EHRs, and support of immunization program operations. As more US immunization programs meet core functional standards, the focus of improvement efforts will continue to shift from infrastructure and functionality to IISs producing high-quality data and from self-reported progress to objective measurement. The first step in moving toward outputs is embodied in the IIS Data Quality Blueprint, which was released by CDC in January 2020 (Figure).18 The Blueprint builds upon IIS Functional Standards but shifts the emphasis from system functionality to data quality. High-quality data are essential to health care providers and public health. Health care providers and public health rely on high-quality data from IIS to aid with clinical decisions, ensuring coverage estimates are reliable, and identifying vulnerable populations to target for interventions. While IISs have made significant progress in improving data quality, there are numerous challenges to achieving the performance measures outlined in the Blueprint, including variation in policies and interpretation, inconsistent and aging technology, lack of comprehensive provider participation in the IIS, barriers at the provider level, and less than full implementation of bidirectional data exchange.
Figure.
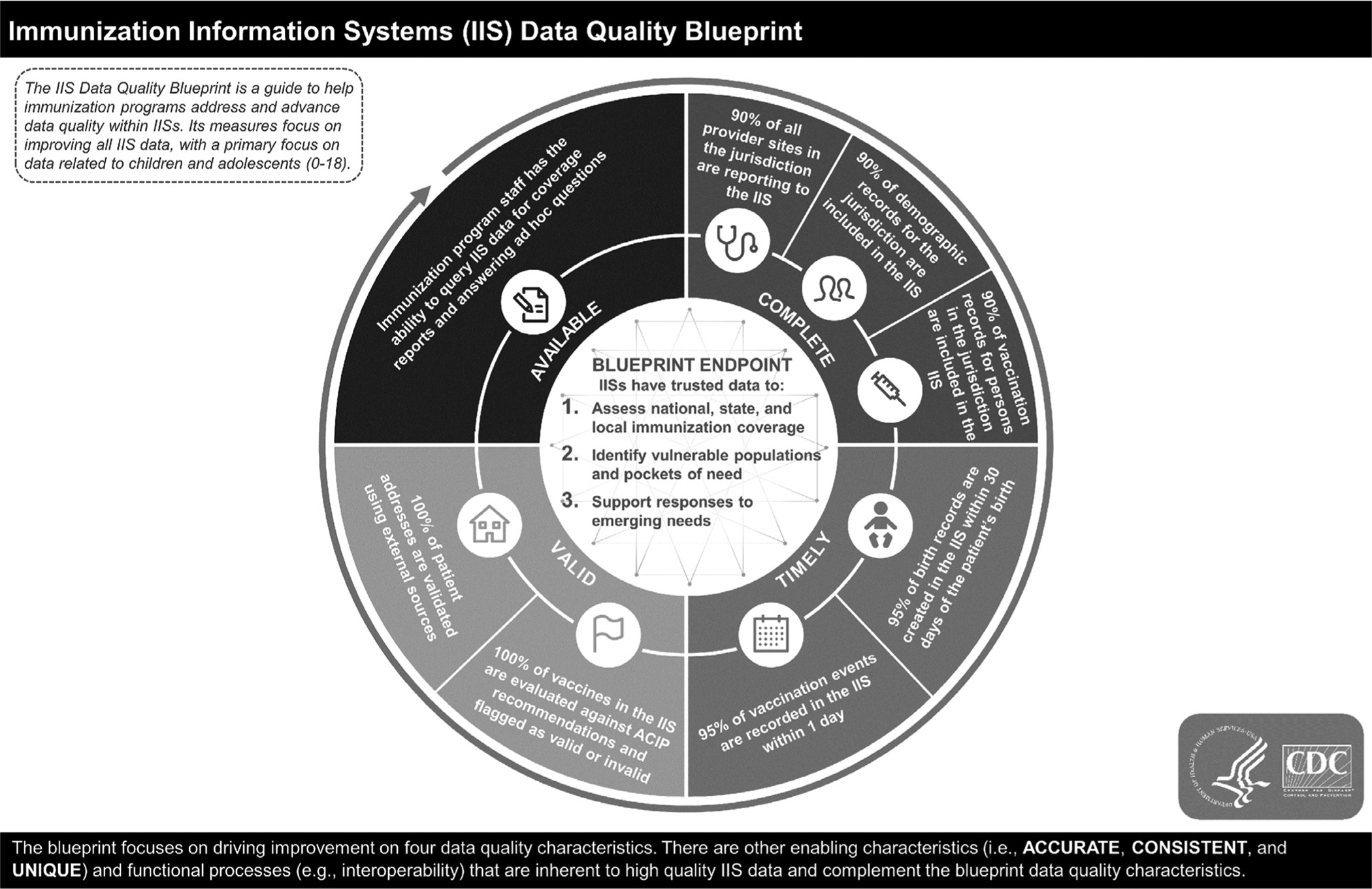
IIS data quality blueprint. IIS indicates immunization information systems.
Challenges in the Current State
Policy
Statutes and policies are often cited as barriers to fully capturing immunization data. There is no national reporting law for immunizations or policy that guides the operation of IISs; state, county, or city statutes and rules govern these systems and vary substantially by jurisdiction. Slightly greater than half (58%) of the jurisdictions in the United States require all immunization providers to report to an IIS and even fewer (39%) require reporting for all age groups. Additionally, even jurisdictions that have reporting regulations or policies do not always have mechanisms in place to enforce them.19 As noted in the IISAR, in these jurisdictions, between 55% and 100% (median 98%) of providers reported immunization data for enrolled patients in the IIS at least once in 2018. In comparison, a median of 92% (range: 52%–100%) of providers reported data in jurisdictions that do not have a requirement to report to the IIS. However, reporting once annually is not the most robust indicator of provider participation in the IIS and may represent significant under-reporting to the IIS for some providers. The Blueprint specifies that 95% of all vaccination events should be reported to the IIS within 1 day. Many states that authorize, but do not require reporting for specific age groups or vaccines, may benefit from an increase in capture of vaccination records in cases where providers have already established interoperability and choose to report all doses regardless of the mandate.
Federal laws like the Health Insurance Portability and Accountability Act (HIPAA) and Family Education Rights and Privacy Act (FERPA) also impact the reporting of immunization data and data quality. HIPAA, which regulates use and disclosure of personal health information, has a public health exception that allows the disclosure of personal health information to public health entities for the purpose of public health surveillance, investigations, and interventions.20 Despite this exception, varying interpretations of HIPAA by providers can limit the amount of information health care providers think they are permitted to share with an IIS. FERPA restricts the ability of schools to share information from a student’s educational record without parental consent. Information on student vaccination status that is provided to schools in order to track compliance with school entry vaccination requirements becomes part of the educational record, preventing schools from sharing these data with their jurisdiction’s IIS. This creates additional burden on providers and the education and public health systems and results in unnecessary costs as 2 separate systems attempt to collect similar data on the same population. While FERPA restricts what data a school can share with an IIS, a school’s access to students’ existing immunization records within an IIS is not restricted. FERPA does contain an exemption in the case of an emergency, but this emergency only allows disclosure without consent due to an “articulable and significant” threat to public health—for example, an outbreak of vaccine-preventable disease.21
Patient consent policies related to IIS data vary and may also impact data quality. Some states require explicit consent of a patient or parent/guardian (ie, opt-in model) for participation of a patient in an IIS, while other states automatically enroll patients in an IIS (ie, opt out model), though the patient (or for children, their parent/guardian) can later choose not to participate. The 64 jurisdictions funded by CDC have a mix of consent policy types ranging from mandatory IIS participation with no ability to opt out to opt-in only participation (Table 1). While policies that make IIS participation optional do not necessarily translate into lower vaccination coverage or efficiencies for the jurisdiction, they may require awardee staff to employ other strategies and expend additional resources to ensure high IIS participation given the constraints. For example, Texas evaluated the program cost of their “opt-in” model to be $2.00 to $2.64 per child depending on the location where consent was obtained compared to an estimated cost of $0.29 per child with an “opt-out” model.22
Table 1.
4:3:1:3:3:1:4 Vaccine Series* Coverage for Children 19 to 35 Months of Age by Participation Policy Type
| IIS Participation Policy Type | No. of Jurisdictions | 4:3:1:3:3:1:4 Vaccine Series Coverage For 19–35 Months of Age (%) | Median 4:3:1:3:3:1:4 Vaccine Series Coverage For 19–35 Months of Age (%) |
|---|---|---|---|
| Mandatory participation with no opt out option | 12 | 18–88 | 67 |
| Mandatory participation with opt out option | 2 | 46, 70 | 58 |
| Implicit consent to participate with opt out option | 45 | 9–77 | 62 |
| Opt in participation only | 4 | 41–75 | 56 |
| N/A— No IIS | 1 | – | – |
IIS indicates immunization information systems.
4:3:1:3:3:1:4 vaccine series refers to: 4 doses of Diphtheria, Tetanus, and Pertussis (DtaP), ≥3 doses of poliovirus vaccine, ≥1 dose of measles-containing vaccine, the full series of Haemophilus influenzae type b vaccine (Hib–≥3 or ≥4 doses, depending on product type), ≥3 doses of Hepatitis B (HepB) vaccine, ≥1 dose of varicella vaccine, and ≥4 doses of Pneumococcal (PCV) vaccine.
Jurisdictional policy variations affect data capture on multiple levels, impacting which patients can be reported to IIS, as well as which data about those patients and their immunization events are reported. These variations may create confusion for providers, especially those working in more than 1 jurisdiction. In 1995, CDC defined recommended core data elements for IISs.23 These elements represent fundamental attributes necessary for identifying individuals and for describing immunization events such as patient birth date and vaccination type. There are instances in which IISs may have incomplete vaccination or demographic data for persons in their jurisdiction. However, some IISs are subject to laws, policies, or interstate data sharing restrictions that limit the full capture of these elements, thereby resulting in lower IIS data quality. In some cases, limits on the capture of all data elements results in records submitted to the IIS that include these elements being rejected, resulting in missing data in the IIS.
One of the most significant policy challenges confronting IISs today is the inability to easily and universally share data among jurisdictions (Table 2). Technology and standards for data exchange between IISs and between IISs and EHRs have been widely adopted; however, current policies do not allow an IIS to share any data without establishing a data sharing agreement between the IIS and each individual entity with which data are to be shared. The inability to seamlessly exchange data across jurisdictions is particularly burdensome for geographically connected states with sizeable populations that receive care in one jurisdiction and live in another, individuals that may reside and receive care in multiple noncontiguous states, or anyone who moves across state lines. Without accurate and comprehensive information, providers and public health are limited in their ability to use IISs to support clinical and public health decisions. Multiple efforts have been made or are underway to streamline data sharing with other IISs. Still, there is no single policy solution that allows for the sharing of immunization data with another IIS.
Table 2.
Key IIS Characteristics Related to Policy, Data Use, and Data Sharing (N = 64)
| Metric | Number of IIS (%) |
|---|---|
| Provider participation | |
| Jurisdictions that reported VFC provider participation in 2018 (N = 61) | 57 (93.4) |
| Percentage of VFC jurisdictions whose provider agreements require VFC providers to report to the IIS (N = 61) | 41 (67.2) |
| IIS to school connectivity | |
| IIS that can identify the number of public elementary schools that were authorized users of the IIS in 2018 | 28 (43.8) |
| IIS that can identify the number of public middle schools and high schools that were authorized users of the IIS in 2018 | 29 (45.3) |
| Data use and sharing | |
| IIS sent at least one QBP (query by parameter) message in production to another jurisdiction’s IIS in 2018 | 5 (7.8) |
| IIS sent at least one VXU (vaccine update) message in production to another jurisdiction’s IIS in 2018 | 6 (9.3) |
| IIS is currently using an external source (ie, SmartyStreets) to verify that patient addresses in their IIS are valid | 28 (43.8) |
| IIS used external sources in 2018 (eg, US Postal Service, Lexis-Nexis) to verify whether patients currently reside at the addresses listed in their IIS record, or to update the record to reflect the current address | 14 (21.9) |
IIS indicates immunization information systems; VFC, vaccines for children.
Varying jurisdictional policies and diverse interpretation of federal laws demonstrate the need for a national IIS policy framework. A framework to require capture of vaccination data through the lifespan and enable data exchange across state lines would support the ability to estimate national vaccination coverage rates, identify pockets of low coverage, and support providers’ needs for complete immunization history. A national policy framework would have added benefits of helping ensure that vaccinations were administered according to Advisory Committee on Immunization Practices recommendations, reducing duplicative vaccination due to lack of records-sharing, and reducing costs incurred by repeat vaccination.
Data Sharing and Use
Data use is another critical aspect on the path to enhanced IIS data quality. To effectively use IIS data, challenges related to patient matching and patient status must be systematically addressed. IISs independently develop identity resolution algorithms to match persons from a variety of sources, such as vital records, medical claims, and providers. Clerical errors in the spelling of names, multiple births, popular name combinations, delays in naming children, and legal name changes all lead to challenges in matching patients. Some jurisdictions use a Master Person Index, which is a database that contains demographic and locating information of registered persons and associates each person with the identifiers for the person from each participating system in the jurisdiction. Use of a Master Person Index allows 1 system to request the identifier for a person that was assigned by another system. That identifier may then be used to request data from that second system and assures a positive match.24 Because there is no national unique patient identifier, systems make matches through a combination of deterministic and probabilistic methods, but none of these algorithms is perfect. When algorithm-based matches are not conclusive, the required human review can be costly. However, many things can be done to improve data matching, including standardizing existing data points. For example, in 2017, the American Immunization Registry Association (AIRA) and CDC collaborated to fund a shared license agreement with a service that allows all IISs to validate, standardize, and geocode their patient and provider addresses at no cost to their programs. This service does not validate the accuracy of the address, but this kind of address cleaning alone can increase match rates by several percentage points.24
The Trusted Exchange Framework and Common Agreement (TEFCA)25 may help to enhance IIS data quality through technical and legal requirements aimed to facilitate exchange of health information across jurisdictions and among trusted partners participating as Qualified Health Information Networks (QHINs). The Trusted Exchange Framework is a set of nonbinding principles intended to foster trust among all data exchange partners. The Common Agreement provides governance required to support and expand a functioning system of connected Health Information Networks (HIN) that meet the needs of individuals, providers, and payers. While TEFCA may help to enhance IIS data quality, realizing the potential benefits of TEFCA in the future of IIS is complex and remaining barriers need to be addressed before IISs are able to participate as a QHIN. For example, one of the prerequisites of a QHIN is all participating HINs applying to be a QHIN must already be connected with existing persons or entities exchanging health information in a live clinical environment. This stipulation hinders an IIS-specific QHIN since, although IISs exchange data with providers/EHRs who are operating in a live clinical environment, IISs themselves operate outside of this clinical setting. Another example that impacts the ability of IISs to realize the benefits of TEFCA involves the need for QHINs to comply with HIPAA covered entity (CE) privacy, security and individual access requirements. This requirement essentially nullifies the “public health exception” that exempts public health agencies from compliance. TEFCA may create opportunities that address data use and sharing challenges impacting IIS data quality but realizing these opportunities requires further assessment of how best to apply the legal and technical requirements across IISs. As previously mentioned, the ability to share data across jurisdictions is critically important for geographically connected jurisdictions where individuals frequently cross boundaries to receive care and for noncontiguous states that share populations. Despite its importance, only 29 states and the District of Columbia allow access to or share data with another IIS. Some jurisdictions’ policies explicitly prohibit the sharing of IIS data with other stakeholders outside the jurisdiction. At least 8 jurisdictions prohibit IISs sharing data with a Health Information Exchange, while 14 jurisdictions prohibit sharing data with other jurisdictions.19 Some jurisdictions require patient consent for data to be shared across state lines. Further, under current regulations, many jurisdictions are required to negotiate Memoranda of Understanding with every other jurisdiction they wish to share data with. The development of these Memoranda of Understanding can be a lengthy process and subject to restrictions that dilute the usefulness of data sharing.
Future State Considerations
Any future model for IIS must include solutions to overcome current challenges and barriers to the high-quality data needed to estimate national vaccination coverage, identify pockets of need, and support responses to emerging threats due to vaccine-preventable diseases. CDC’s 2020 Immunization Program Operations Manual includes a requirement for the 64 CDC funded jurisdictions to make progress in sharing IIS data with CDC. This requirement, along with new technology, policy solutions, and improved data quality, lays the groundwork for a nationwide network and repository of immunization data that provides:
improved infrastructure
standardized implementation
support for IIS data stakeholders
Ways to Improve IIS Infrastructure and Facilitate Data Exchange
The CDC, in collaboration with partners and the Department of Health and Human Services, Office of the Chief Technology Officer, is exploring options to address these challenges by sponsoring a set of pilot projects focused on improving EHRs, IISs, and data exchange between them. The Immunization Gateway (IZ Gateway) is a pilot project designed to overcome policy and technology barriers related to data exchange. The IZ Gateway uses a platform provided by the Association of Public Health Laboratories to simplify the process for provider organizations operating in multiple states to connect with multiple IISs and for IISs to connect with one another to bidirectionally exchange immunization data. The IZ Gateway provides a centralized IT architecture and technical solution that alleviates the need for multiple IIS connections, streamlines the onboarding process, and provides centralized or common legal agreements.
Implementation of the IZ Gateway beyond the pilot phase would substantially change the operational model of IISs today. Currently, vaccine providers connect to their jurisdiction’s IIS. Multistate providers must connect to the IIS in each of the jurisdictions they serve, which requires consideration of differences in state reporting requirements, including variations in age groups, consent type, data elements, and data quality. Widespread implementation of the IZ Gateway would result in a single connection of EHR platforms to the cloud-based hub, as well as each participating IIS, resulting in interjurisdictional data exchange, multistate data exchange, and allowing more complete and timely immunization data. While this does not resolve issues with restrictions related to variations in reporting requirements or business rules in individual jurisdictions, it would centralize and streamline onboarding and address some of the other policy issues. If the IZ Gateway were used to facilitate a nationwide IIS data hub, it could be integrated with an immunization data lake. A data lake is a system or repository of all enterprise data for tasks such as reporting, visualization, and advanced analytics. An immunization data lake would enable CDC and other authorized stakeholders to access de-identified IIS data on an ongoing, timely basis and query, extract, and analyze IIS data from the lake. Interfaces and dashboards could also be developed to visualize immunization coverage and other outcomes from a regional and nationwide perspective.
The IZ Gateway is just one opportunity to improve IIS infrastructure and leverage new technologies and standards to improve data quality, clinical decision support, and electronic data exchange. IISs led efforts to develop a Health Level 7 (HL7)26 messaging standard that is a highly effective mechanism for exchanging data. However, as new models for data exchange, like Fast Healthcare Interoperability Resources,27 gain traction and demonstrate opportunities for better efficiencies, the IIS community must study further benefits of adopting Fast Healthcare Interoperability Resources, but this must be a thoughtful and deliberate process, closely coordinated with the EHR/pharmacy community and all other data exchange partners to avoid disruption to existing connections and balance the data exchange benefits with time and financial constraints.
Ways to Ensure Consistent Implementation and Lower IIS Costs
CDC is also leading initiatives to modernize IIS technology and leverage economies of scale. Because IISs are jurisdiction-based systems, their technology infrastructures vary and are not based on any single platform. This has led to innovation, but also variability in functionality and, in some cases, scalability. CDC is working with jurisdictions and IIS vendors to create consortia that provide opportunities for coordinated development and collaboration among jurisdictions using the same platform. The sustainability and system architecture challenges inherent in uncoordinated development of systems can be mitigated by consistent collaboration and planning among consortium members, allowing for development of shared services and avoiding redundant maintenance, operations, and infrastructure costs.
The Measurement and Improvement initiative, a project of AIRA and the CDC is intended to assess and improve IIS alignment with established standards. The initiative is a 3-stage process that measures IIS functionality and capability for various content areas such as data exchange and clinical decision support by connecting with IIS pre-production or test environments, which allows for independent testing and validation of the IIS. Overall, 58 of 64 IIS jurisdictions have the technological capability to participate in the initiative. Participation is voluntary; as of February 2020, more than 86% (50 of 58) of IIS programs capable of participating are currently participating. To date, AIRA has completed development of the process for testing and discovery, IIS assessment, and validation for 3 of the 5 identified content areas. These include Message and Transport (43 IISs measured), HL7 Submission and Acknowledgement (47 IISs measured), and HL7 Query and Response (43 IISs measured). The Clinical Decision Support content area is currently in the assessment stage, and Data Quality is in the testing and discovery stage. Significant progress has been made in each measurement area since baseline measurement was conducted. For example, the number of IISs meeting one primary measure in the Message and Transport content area has increased from 19 to 39 in 3 years. Similarly, the number of IISs sending standards-conformant HL7 acknowledgment messages has increased 14-fold since measurement began in 2017. Within the first 2 quarters of assessment, the percentage of IISs meeting the CDS measures aimed at supporting IIS alignment with Advisory Committee on Immunization Practices recommendations increased 15% from baseline. As IISs continue to more fully align with standards, systems are more streamlined and interoperable, data quality improves, and systems can achieve greater impact across communities.28
In addition to improving IIS infrastructure and data exchange, it is critical to improve EHRs and their data exchange with IISs. The Immunization Integration Program (IIP) is a multiorganization partnership sponsored by the CDC to recognize EHR products with immunization functionality and to bring together the EHR and IIS communities to jointly solve problems inhibiting bidirectional data exchange between these systems (IIP Collaborative). Together, the recognition program and IIP Collaborative are intended to drive the development and adoption of solutions that enable both clinicians and IISs to have timely access to complete and accurate immunization data, not only to improve decision-making, but also to increase vaccination coverage and reduce vaccine-preventable disease. This year the Collaborative identified priority projects to address:
variability in the way EHRs and IISs communicate about acknowledgment/error messages to ensure clinicians and public health can access the most accurate information to make immunization decisions
challenges in accurately identifying unique individuals within the national network
promoting standardization in data transport methods across EHRs and IISs
Ways to Support the Needs of Immunization Data Stakeholders
Future models must also consider the increasing demands for immunization data by a broader range of stakeholders. CDC is also sponsoring 2 pilot projects related to IIS data access and use. The first project is using a human-centered design approach to solve complex issues related to data sharing. Through this process, a workgroup of stakeholders will define business requirements for an analytical toolkit, data request and approval processes, and data use agreement mechanisms. These requirements will then be used to identify opportunities to use an existing data platform to implement the business requirements. If successful, this project would facilitate key stakeholders having access to IIS data and cross-organization analyses. The second project is focused on testing statistical models to estimate population level vaccination coverage using imperfect IIS data. A panel of national experts convened in 2019 to discuss potential methodologies and considerations that CDC is now testing for feasibility. If successful, the combination of improved IIS data, increased access to data, and enhanced methodologies, analyses, and visualization, will dramatically change public health’s ability to focus interventions on vulnerable populations, monitor and respond to outbreaks of vaccine-preventable disease, and provide comprehensive patient data to clinical providers.
Conclusion
Over the last 2 decades, IISs have continued to evolve and mature and remain pioneers in the world of public health registries. However, there are numerous challenges that must be overcome in order to maximize their utility and provide accurate, timely, and complete information to immunization programs, vaccination providers, and other key stakeholders. Projects to explore and test components of a future state model are underway, but critical policy issues that impede data quality and exchange negatively affect data quality. Tracking outbreaks and identifying pockets of under vaccination in order to target outreach and vaccine availability requires a robust IIS. Ensuring pediatricians and other providers have access to complete immunization histories, accurate immunization forecasts, and clinical decision support that is needed to implement complex vaccination schedules, also requires a robust IIS capable of sharing data with other jurisdictions. The convergence of new technology and emerging threats provides a unique opportunity to invest in and strengthen a nation-wide IIS network.
Financial disclosure:
This article was published as part of a supplement sponsored by the Centers for Disease Control and Prevention.
The authors would like to thank Terence Ng, MPH, CNI Advantage contractor to the CDC, for his assistance with synthesizing data and Rachel Lawley, Deloitte Consulting contractor to the CDC, for her assistance with graphics.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors have no conflicts of interest to disclose.
References
- 1.Orenstein Walter A, Rafi A. Simply put: vaccination saves lives. Proc Natl Acad Sci U S A. 2017;114:4031–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Ten great public health achievements—United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2011;60:619–623. [PubMed] [Google Scholar]
- 3.Sachiko O, Samantah C, Allison P, et al. Return on investment from childhood immunization in low- and middle-income countries, 2011–20. Health Aff (Millwood). 2016;35:199–207. [DOI] [PubMed] [Google Scholar]
- 4.Holly G, David H, Laura P, et al. Immunization information systems to increase vaccination rates: a community guide systematic review. J Public Health Manag Pract. 2015;21:227–248. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf H, Adams M, Rodewald L, et al. Fragmentation of immunization history among providers and parents of children in selected under-served areas. Am J Prev Med. 2002;23:106–112. [DOI] [PubMed] [Google Scholar]
- 6.American Immunization Registry Association. How IIS support a patient’s journey. Available at: https://repository.immregistries.org/files/resources/5caf7b197bf77/how_iis_support_a_patient_s_journey_infographic.pdf. Accessed December 10, 2020.
- 7.American Immunization Registry Association. The Value of IIS. American Immunization Registry Association Available at: https://repository.immregistries.org/files/resources/5caf7b197bf77/value_of_iis_infographic.pdf. Accessed December 10, 2020.
- 8.Fisher MP, Gurfinkel D, Szilagyi PG, et al. Supporting and sustaining centralized reminder/recall for immunizations: qualitative insights from stakeholders. Vaccine. 2019;37:6601–6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szilagyi P, Albertin C, Gurfinkel D, et al. Effect of state immunization information system centralized reminder and recall on HPV vaccination rates. Pediatrics. 2020;145:e20192689. 10.1542/peds.2019-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pabst LJ, Williams W. Immunization information systems. J Public Health Manag Pract. 2015;21:225–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Community Preventive Services Task Force. Recommendation for use of immunization information systems to increase vaccination rates. J Public Health Manag Pract. 2015;21:249–252. [DOI] [PubMed] [Google Scholar]
- 12.Popovich M, Watkins T, Baker B. A model for sustaining and investing in Immunization Information Systems. Online J Public Health Inform. 2019;11:e20. 10.5210/ojphi.v11i2.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Public Health Informatics Institute. Immunization Information Systems: The First Twenty-Five Years: A Commemorative History. Public Health Informatics Institute; 2018. Available at: https://phii.org/sites/default/files/iis_history_spotlight-_technology.pdf. Accessed December 10, 2020. [Google Scholar]
- 14.Centers for Disease Control and Prevention. IIS Minimum Functional Standards, 2001–2012. Centers for Disease Control and Prevention; 2001. Available at: https://www.cdc.gov/vaccines/programs/iis/func-stds-2001.html. Accessed December 10, 2020. [Google Scholar]
- 15.Centers for Disease Control and Prevention. IIS Functional Standards, ver 4.1 Centers for Disease Control and Prevention; 2019. Available at: https://www.cdc.gov/vaccines/programs/iis/functional-standards/func-stds-v4-1.html. Accessed December 10, 2020. [Google Scholar]
- 16.Centers for Disease Control and Prevention. Immunization Information System Annual Report. Centers for Disease Control and Prevention; 2019. Available at: https://www.cdc.gov/vaccines/programs/iis/annual-report-iisar/overview.html. Accessed December 10, 2020. [Google Scholar]
- 17.Murthy N, Rodgers L, Pabst L, et al. Progress in childhood vaccination data in Immunization Information Systems—United States, 2013–2016. MMWR Morb Mortal Wkly Rep. 2017;66:1178–1181. 10.15585/mmwr.mm6643a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Immunization Information Systems Data Quality Blueprint. Centers for Disease Control and Prevention; 2020. Available at: https://www.cdc.gov/vaccines/programs/iis/downloads/Data-Quality-Blueprint-508.pdf. Accessed December 10, 2020. [Google Scholar]
- 19.Martin DW, Lowrey NE, Brand B, et al. IIS: a decade of progress in law and policy. J Public Health Manag Pract. 2015;21:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Overview of the Health Insurance Portability and Accountability Act of 1996. Centers for Disease Control and Prevention; 2018. Available at: https://www.cdc.gov/phlp/publications/topic/hipaa.html. Accessed December 10, 2020. [Google Scholar]
- 21.US Department of Education. Family Educational Rights and Privacy Act (FERPA) and the Disclosure of Student Information Related to Emergencies and Disasters. US Department of Education; 2010. Available at: https://www2.ed.gov/policy/gen/guid/fpco/pdf/ferpa-disaster-guidance.pdf. Accessed December 10, 2020. [Google Scholar]
- 22.Boom JA, Sahni LC, Nelson CS, et al. Immunization information systems opt-in consent: at what cost? J Public Health Manag Pract. 2010;16: E18–25. 10.1097/PHH.0b013e3181cbc4ec. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. IIS Recommended Core Data Elements. Centers for Disease Control and Prevention. 2018. Available at: https://www.cdc.gov/vaccines/programs/iis/core-data-elements.html. Accessed December 10, 2020. [Google Scholar]
- 24.Grannis SJ, Xu H, Vest JR, et al. Evaluating the effect of data standardization and validation on patient matching accuracy. J Am Med Inform Assoc. 2019;26:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Office of the National Coordinator for Health Information Technology. Trusted Exchange Framework and Common Agreement. Office of the National Coordinator for Health Information Technology; 2019. Available at: https://www.healthit.gov/sites/default/files/page/2019-04/FINALTEFCAQTF41719508version.pdf. Accessed December 10, 2020. [Google Scholar]
- 26.Centers for Disease Control and Prevention, American Immunization Registry Association. HL7 2.5.1 Implementation Guide for Immunization Messaging Centers for Disease Control and Prevention and American Immunization Registry Association; 2014. Available at: https://www.cdc.gov/vaccines/programs/iis/technical-guidance/downloads/hl7guide-1-5-2014-11.pdf. Accessed December 10, 2020. [Google Scholar]
- 27.Health Level 7 International. Fast Healthcare Interoperability Resources Brief. HL 7 International; 2015. Available at: https://www.hl7.org/implement/standards/product_brief.cfm?product_id=491. Accessed December 10, 2020. [Google Scholar]
- 28.American Immunization Registry Association. Immunization Information System Measurement and Improvement. American Immunization Registry Association; 2019. Available at: https://www.immregistries.org/measurement-improvement. Accessed December 10, 2020. [Google Scholar]


