ABSTRACT
Background
Gestational diabetes mellitus (GDM) is a growing public health concern and maternal obesity and poor dietary intakes could be implicated. Dietary polyphenols and fiber mitigate the risk of diabetes and its complications, but little is known about their efficacy in preventing GDM.
Objectives
We examined the effects of whole blueberry and soluble fiber supplementation on primary outcomes of cardiometabolic profiles in women at high risk of developing GDM.
Methods
Women (n = 34; mean ± SD age: 27 ± 5 y; BMI: 35.5 ± 4.0 kg/m2; previous history of GDM ∼56%; Hispanic ∼79%) were recruited in early pregnancy (<20 weeks of gestation) and randomly assigned to 1 of the following 2 groups for 18 wk: intervention (280 g whole blueberries and 12 g soluble fiber per day) and standard prenatal care (control). Both groups received nutrition education and maintained 24-h food recalls throughout the study. Data on anthropometrics, blood pressure, and blood samples for biochemical analyses were collected at baseline (<20 weeks), midpoint (24–28 weeks), and end (32–36 weeks) of gestation. Diagnosis of GDM was based on a 2-step glucose challenge test (GCT). Data were analyzed using a mixed-model ANOVA.
Results
Maternal weight gain was significantly lower in the dietary intervention than in the control group at the end of the trial (mean ± SD: 6.8 ± 3.2 kg compared with 12.0 ± 4.1 kg, P = 0.001). C-reactive protein was also lower in the intervention than in the control group (baseline: 6.1 ± 4.0 compared with 6.8 ± 7.2 mg/L; midpoint: 6.1 ± 3.7 compared with 7.5 ± 7.3 mg/L; end: 5.5 ± 2.2 compared with 9.5 ± 6.6 mg/L, respectively, P = 0.002). Blood glucose based on GCT was lower in the intervention than in the control (100 ± 33 mg/dL compared with 131 ± 40 mg/dL, P < 0.05). Conventional lipids (total, LDL, and HDL cholesterol and triglycerides) did not differ between groups over time. No differences were noted in infant birth weight.
Conclusions
Whole blueberry and soluble fiber supplementation may prevent excess gestational weight gain and improve glycemic control and inflammation in women with obesity.
This trial was registered at clinicaltrials.gov as NCT03467503.
Keywords: blueberries, soluble fiber, gestational weight gain, gestational diabetes, C-reactive protein
Introduction
Gestational diabetes mellitus (GDM), defined as diabetes with its onset or first recognition during pregnancy, is the most common medical complication of pregnancy and childbirth (1). Globally, it represents a significant public health burden (2). Estimates of its prevalence vary over time, also according to diagnostic criteria, methods of ascertainment, and ethnicity, as previously discussed (3) and reviewed (4). In the United States the prevalence is ∼9% (5), but using more stringent criteria of the International Association of Diabetes in Pregnancy Study Group (6), prevalence has been reported to be as high as 24% in certain European countries (7). GDM is associated with increased risk of future type 2 diabetes (T2D) not only for the mother, but also, decades later, for the child (8, 9). GDM also poses immediate risks to the pregnancy, including pre-eclampsia, complications at delivery, abnormal infant birth weight, and impaired development (10, 11). Obesity is especially prevalent among African Americans and Hispanics (12), and has known associations with diabetes and GDM (13). Thus, strategies to optimize maternal gestational weight gain and diet hold promise for the prevention of GDM. Dietary intervention targeting specific ethnic groups, such as African American (14, 15) and Hispanic (8) women who carry a higher burden of GDM risk and elevated risks of future T2D, is much needed.
Diabetes medical nutrition therapy has been defined as the use of nutrients and whole foods in the management of the disease. It remains a cornerstone of GDM management, although the evidence base on optimal diets and foods to reduce risks and adverse outcomes of GDM remains inconclusive and warrants more trials in high-risk women (16). In a meta-analysis of 18 randomized controlled trials on dietary modifications in GDM, low-glycemic-index diets or manipulation of dietary fats and proteins revealed a decrease in maternal hyperglycemia and risks of macrosomia. However, owing to large variations in dietary exposure and participant characteristics in the reported studies, challenges remain in reaching a consensus on the best dietary practices to decrease risks of GDM (17). Similarly, a larger Cochrane review of 19 clinical trials showed protective effects of the “Dietary Approaches to Stop Hypertension” (DASH) diet in reducing cesarean delivery rates and identified the need for further dietary studies relating to GDM (18). Plant-based diets and food groups have been associated with reduced risk of GDM in observational studies (19, 20), but clinical trials are few and inconclusive. The “Mediterranean diet” has been shown to reduce risks of GDM in women who are habituated to this dietary pattern, but findings cannot be generalized to other ethnic groups (21–23). Much of the observed benefits of plant-based diets can be attributed to food groups such as fruits, vegetables, whole grains, legumes, and nuts that are high in micronutrients, fiber, and a wide array of dietary bioactive compounds including polyphenols. Polyphenolic flavonoids that are largely present in functional foods, such as dark-colored berries, grapes, tea, olives, and whole grains, have been associated with decreased risks of diabetes in observational studies (24–26) as well as in experimental models of diabetes (27–29). These plant-based foods deserve attention in GDM, especially in high-risk women with low habitual consumption of fruits and vegetables and their constituent bioactive compounds.
An assessment of nutritional status in pregnant women based on the US NHANES revealed a significant percentage of women do not meet the dietary recommendations for several micronutrients, such as vitamins A, C, and E, folate, iron, calcium, and magnesium, even with the use of dietary supplements (30). In another population-based study of pregnant women in the United States, dietary quality was shown to be significantly inadequate, and this was especially so among non-Hispanic black and Hispanic women (31). Furthermore, based on reported data from US cohorts, it has become urgent to address the low intake of whole fruits, and the high intake of fruit juice, among pregnant women—factors that increase risks of hyperglycemia, weight gain, and consequent GDM (31, 32). Our group has previously reported studies on the beneficial metabolic effects of dietary whole berries, such as blueberries (33), cranberries (34), and strawberries (35), in nonpregnant adults with metabolic syndrome or T2D. Berries are a rich source of several polyphenols, fiber, and vitamins, and have low glycemic indexes (36–39). Blueberries are among the commonly consumed berries: they can improve insulin sensitivity and reduce risk of diabetes in human and animal studies, through effects attributed to their high polyphenol content (39–41). Dietary fiber, especially soluble fiber, can also reduce risk of diabetes (42, 43). Thus, based on a clear need of dietary research in GDM, we aimed to examine the effects of combined dietary supplementation of whole blueberries and soluble fiber on cardiometabolic profiles in minority women at high risk of GDM. Our intervention was implemented in early pregnancy (<20 weeks of gestation), and women were then studied at 2 additional visits in middle (24–28 weeks of gestation) and late pregnancy (32–36 weeks of gestation). We examined the hypothesis that dietary blueberry and fiber supplementation prevents excess gestational weight gain and improves risk of GDM in obese women.
Methods
Participants and criteria
We conducted a randomized controlled trial (NCT03467503) between April 2018 and March 2020 at the prenatal care clinic of the Department of Obstetrics and Gynecology at the University of Nevada at Las Vegas (UNLV) School of Medicine. All participants were consented and enrolled in the study by the nurse practitioner in early gestation (<20 weeks of gestation) and were followed for a mean ± SD of 18 ± 3 wk, i.e., until late pregnancy or delivery. The study was approved by the UNLV Institutional Review Board. Adult women at high risk of GDM were enrolled in the study if they had BMI (in kg/m2) ≥30 and singleton pregnancy with the following options: previous history of GDM and/or family history of diabetes. Exclusion criteria were multiple pregnancy, current use of medications that may influence glucose metabolism (metformin, glucocorticoids, immunosuppressants, antipsychotics), major fetal abnormality on the 11- to 13-wk ultrasound scan, unwillingness or inability to provide written informed consent, or significant underlying medical disorder as assessed by the study physician (e.g., anemia, renal disorders, pregestational hypertension, or diabetes). Women were also excluded if they were allergic to berries and dietary fiber supplements, or were unwilling to make dietary changes, or were vegetarian or consuming any other special diet not consumed habitually. Randomization was performed using a sequence of randomly generated numbers using SAS version 9.4 (SAS Institute Inc.). Health and medical history, anthropometric measurements, blood pressure recordings, and blood draws were conducted at baseline (<20 weeks of gestation), and at 2 follow-up visits in the second (24–28 weeks of gestation) and third trimesters (32–36 weeks of gestation) at the clinic. In addition to these visits, participants made biweekly short visits to the clinic when they met the registered dietitian (RD) and nurse practitioner, received food supplies and nutrition education, and submitted 24-h diet recalls.
Intervention and control groups
Participants in the dietary intervention group were provided with biweekly supplies of frozen blueberries in cooler bags and soluble fiber as partially hydrolyzed guar gum (Nutrisource® Fiber, Nestlé Heath Science) weighed out in individual Ziplock bags, with instructions to consume 2 cups (280 g) of frozen blueberries as a snack and 12 g soluble fiber daily. Participants were instructed not to consume fruit juice during the study, to add the blueberries to their diet as a mid-morning, afternoon, or evening snack to be consumed by itself and not in combination with any other food items, and to add the fiber to their meals in soups, gravies, and shakes. The 2 cups (280 g) of frozen blueberries purchased from the local grocery store in Las Vegas in bulk packages provided the following daily nutrients: 160 kcal, 38 g total carbohydrates, 8 g total fiber, 8 mg vitamin C, 3 mg sodium, 168 mg K, 1600 mg total polyphenols, and 700 mg anthocyanins (44, 45). The fiber supplement provided a total of 12 g soluble fiber only. The control group were also seen biweekly by the study nurse and RD to receive standard prenatal care. Both groups received handouts on nutrition education based on the USDA Dietary Guidelines for Americans (DGA) for pregnant women (46), and recommendations were based on a balance of carbohydrates, fat, and protein, and 5 food groups (46).
Habitual dietary intake and physical activity assessment
All study participants were asked to maintain a 24-h food recall which they submitted to the RD during their biweekly visits and discussed any changes they made in their habitual diet. All participants were otherwise required to maintain their usual diet and level of physical activity throughout the study. Dietary analyses were conducted by the study RD or a trained dietetic assistant using ESHA's Food Processor® Nutrition Analysis software for energy, nutrients, and food group intakes for each participant. Each participant was asked whether they exercised regularly and for how many minutes. To be classed as “physically active,” participants needed to perform habitual exercises such as regular walks, jogging, total body workout at a gym, swimming, yoga, pilates, fitness, exercise ball workouts, or home gymnastics as recommended by the American College of Obstetrics and Gynecology (ACOG) (47). Exercises had to be done regularly (at least twice a week) and 1 training should last for ≥15 min. Participants who did not regularly perform any of the aforementioned activities or did not exercise at all were classed as “physically inactive.”
Compliance
Each participant received weekly phone calls from the study team to ensure the timely consumption of blueberries and fiber in the dietary intervention group and to discuss general dietary concerns in the control group. Participants in both groups were reminded to maintain their biweekly 24-h food recalls. In addition, participants in the intervention group were asked to store and return any unused blueberries and fiber supplements. Plasma chlorogenic acid was measured in the intervention group as a marker of blueberry consumption using published methods (48). The incidence and persistence of any side effects in the intervention group, such as gastrointestinal symptoms and headaches, were recorded.
Glucose challenge test and GDM diagnosis
Women were screened between 24 and 28 weeks of gestation using the 2-step criteria: first step, nonfasting 50-g oral glucose challenge test (GCT); then, if positive: second step, 100-g GCT administered in the fasting state. GDM diagnosis was confirmed if a participant exceeded threshold levels in both criteria. This screening protocol is widely used in US institutions and recommended by the ACOG (49, 50).
Anthropometric measures and blood pressure
Maternal body weight (kg) and systolic and diastolic blood pressure (mm Hg) were measured at baseline, and at 24–28 and 32–36 weeks of gestation during the trial, by the study nurse. Body weight was measured using a digital scale in light clothing and no shoes. Systolic and diastolic blood pressure were measured using a Spot Vital Signs Device (Welch Allyn). At each visit, participants were asked to lie down and relax for ∼8–10 min, after which 3 blood pressure measurements were recorded at intervals of 5–8 min; mean values were recorded.
Biochemical analyses
At each visit (baseline, midpoint, and end) freshly drawn blood samples were sent to Quest Diagnostics (Las Vegas) for analyses of serum glucose and conventional lipid profiles, and insulin, liver, and kidney function tests using automated diagnostic equipment (Abbott Architect Instruments) via enzymatic colorimetric methods that used commercially available kits according to the manufacturer's protocols. C-reactive protein (CRP) was assayed by ultrasensitive nephelometry (Dade Behring). Serum glycated hemoglobin (HbA1c) was analyzed with the use of a DCA 2000+ Analyzer (Bayer). Insulin resistance was evaluated by HOMA-IR and was calculated as follows: [fasting insulin (mU/L) × fasting glucose (mmol/L)]/22.5 (51). NMR-determined lipoprotein subclass profile was performed in first-thaw plasma specimens using a 400-MHz proton NMR analyzer at LipoScience Inc. as described previously (52). In addition, sera were stored at −80°C for the subsequent analyses of IL-6 and adiponectin using a quantitative sandwich enzyme immunoassay technique (R&D Systems), and of plasma chlorogenic acid as a marker of blueberry compliance. The average intra-assay CVs for IL-6 and adiponectin were 3.5% and 4.8%, respectively.
Statistical analyses
For each measure, descriptive statistics were examined to identify outliers: none were found. For baseline demographics and characteristics, continuous variables were expressed as means ± SDs and discrete variables as percentages. Our primary objective was to examine differences in maternal body weight and cardiometabolic profiles between the intervention and control groups at baseline (<20 weeks of gestation), 24–28 weeks of gestation (when a GCT was completed), and between 32 and 36 weeks of gestation. We employed a 2 × 3-factor repeated-measures ANOVA (MIXED procedure; group: intervention, control; time: baseline, midpoint, and end) to examine the main effects of group, time, and whether overall changes in time differed between the 2 groups (interaction). Baseline values were included as covariates for each outcome variable. In addition, differences in final maternal weight gain between the 2 groups at the end of the trial were determined by an independent-samples t test, and the differences in the likelihood of GDM between the 2 groups were assessed by a χ2 test. We also examined differences in our secondary variables of habitual dietary nutrients and food group intakes using a mixed-model ANOVA for main and interaction effects. Effect size measures for primary outcome variables were calculated using partial η2. We further adjusted for familywise Type I error based on the procedures described by Benjamini and Hochberg (53) and results are presented with and without adjustments for multiple hypotheses. Because this is an exploratory and feasibility study in pregnant women with obesity, the assumptions used in the sample size calculation were based on our previously published report on the effects of green tea on body weight in obese nonpregnant adults (54). From our previous dietary intervention study in adults with the metabolic syndrome, we expected a mean ± SD difference in maternal body weight of 2.36 ± 0.6 kg with a sample size of 12 in each group within 8 wk in the present study to achieve 80% power at an α level of 0.05. Sensitivity analysis was conducted for maternal body weight data by imputing the missing data of subjects lost to follow-up (n = 11) using the multiple imputation method (number of simulations = 10) and redoing the mixed-model ANOVA (55). All P values were 2-tailed and main effects and interaction effects were considered if P was <0.05. Analyses were performed using SPSS version 26.0 (SPSS).
Results
Enrollment and baseline characteristics
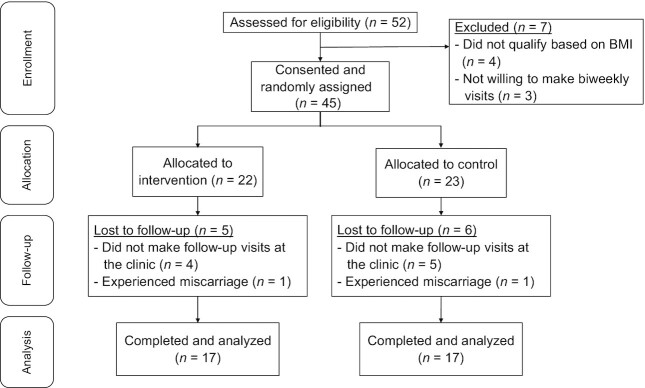
Of the 52 women who were contacted based on initial interest in the study, 45 provided consent. Of these, 9 changed providers and did not attend for follow-up visits after screening, 2 experienced miscarriage, and 34 completed all study visits (Figure 1). As Table 1 shows, at baseline all women had high risk of GDM for reasons as follows: all participants had obesity (BMI ≥30), >50% had a history of prior GDM, >25% had a family history of diabetes, and a high proportion of participants were of Hispanic origin (self-reported; >75%). Baseline characteristics did not significantly differ between the dietary intervention and control groups. Overall, compliance was 95% in the intervention group based on the unused supply of blueberries and soluble fiber. All participants were 100% compliant to biweekly visits throughout the study which did not differ between the 2 groups. The intervention was well tolerated with no reported side effects. Plasma chlorogenic acid (3-chlorogenic acid) was detectable as a biomarker of compliance in all participants in the intervention group. Mean ± SD plasma chlorogenic acid concentrations observed at baseline, midpoint, and end of the trial, ∼12 h after the last blueberry dose (except baseline), were <8.0 nmol/L, 15.9 ± 5.1 nmol/L, and 16.5 ± 5.4 nmol/L, respectively, and concentrations were nondetectable in control samples at these visits (<8.0 nmol/L).
FIGURE 1.
Summary of the flow of participants.
TABLE 1.
Baseline maternal characteristics1
| Variable | Intervention (blueberry + soluble fiber) | Control (standard prenatal care) |
|---|---|---|
| Age, y | 27 ± 5.3 | 27 ± 5.0 |
| Gestational age at start of intervention, wk | 16.0 ± 3.8 | 14.5 ± 4.4 |
| Body weight at start of intervention, kg | 88 ± 10 | 92 ± 12 |
| BMI at start of intervention, kg/m2 | 35 ± 4.2 | 36 ± 4.2 |
| Hemoglobin, g/dL | 12.7 ± 1.0 | 12.6 ± 1.0 |
| Hematocrit, % | 38 ± 3.6 | 37 ± 3.3 |
| RBC, 106/μL | 4.3 ± 0.4 | 4.2 ± 0.3 |
| Platelets, 103/μL | 307 ± 62 | 288 ± 58 |
| Plasma total protein, g/dL | 6.7 ± 0.5 | 6.8 ± 0.3 |
| Plasma albumin, g/dL | 3.8 ± 0.3 | 3.7 ± 0.3 |
| Plasma ALT, U/L | 15 ± 6.2 | 21 ± 19.3 |
| Plasma AST, U/L | 20 ± 7.3 | 23 ± 14.0 |
| Race | ||
| African American, % | 18 | 23 |
| Hispanic, % | 82 | 76 |
| Prenatal vitamin users, % | 29 | 41 |
| History of GDM, % | 59 | 53 |
| Family history of diabetes, % | 35 | 27 |
| Nulliparous, % | 29 | 24 |
n = 17 in each group. Values are mean ± SD unless otherwise indicated. ALT, alanine transaminase; AST, aspartate transaminase; GDM, gestational diabetes mellitus.
Body weight and blood pressure
Maternal body weight increased throughout the 18-wk trial but, overall, women in the intervention group gained less weight than those in the control group (Table 2). Final weight gained (end minus baseline weight) measured at a mean gestational age of between 33 and 34 weeks of gestation was also significantly lower in the intervention (mean ± SD: 6.8 ± 3.2 kg) than in the control group (12.0 ± 4.1 kg) (Supplemental Figure 1) (all P < 0.05). Systolic blood pressure did not differ significantly between the groups with increasing gestational age, whereas mean diastolic blood pressure was significantly lower in the intervention than in the control group (Table 2) (P < 0.05).
TABLE 2.
Cardiometabolic variables in pregnant women with obesity at risk of gestational diabetes that were or were not supplemented with blueberries and soluble fiber for 18 wk of pregnancy1
| Variable by group | Baseline2 | Midpoint3 | End4 | P value5 (group) | P value5 (time) | P value5 (interaction) |
|---|---|---|---|---|---|---|
| Body weight, kg | 0.13 | <0.001 | 0.001 | |||
| Intervention | 88 ± 10 | 92 ± 10 | 95 ± 11 | |||
| Control | 92 ± 12 | 97 ± 12 | 103 ± 11 | |||
| Systolic blood pressure, mm Hg | 0.16 | 0.30 | 0.30 | |||
| Intervention | 124 ± 11 | 120 ± 14 | 116 ± 16 | |||
| Control | 122 ± 14 | 127 ± 9 | 123 ± 11 | |||
| Diastolic blood pressure, mm Hg | 0.01 | 0.04 | 0.47 | |||
| Intervention | 74 ± 10 | 76 ± 7 | 73 ± 8 | |||
| Control | 74 ± 9 | 81 ± 5 | 78 ± 7 | |||
| Serum glucose, mg/dL | 0.13 | 0.02 | 0.27 | |||
| Intervention | 81 ± 10 | 85 ± 10 | 84 ± 19 | |||
| Control | 82 ± 15 | 95 ± 22 | 95 ± 25 | |||
| Serum HbA1c, % | 0.05 | 0.03 | 0.01 | |||
| Intervention | 4.6 ± 0.5 | 4.3 ± 0.7 | 4.5 ± 0.8 | |||
| Control | 4.5 ± 0.5 | 5.0 ± 0.6 | 5.2 ± 0.7 | |||
| Serum insulin, μIU/mL | 0.92 | 0.003 | 0.37 | |||
| Intervention | 23.1 ± 17.4 | 29.4 ± 20.1 | 35.1 ± 20.5 | |||
| Control | 20.2 ± 17.5 | 33.7 ± 18.9 | 32.1 ± 17.4 | |||
| HOMA-IR | 0.88 | 0.04 | 0.96 | |||
| Intervention | 4.4 ± 3.8 | 5.9 ± 5.8 | 6.6 ± 5.8 | |||
| Control | 4.3 ± 4.2 | 6.3 ± 4.1 | 7.0 ± 3.3 | |||
| Serum total cholesterol, mg/dL | 0.11 | <0.001 | 0.14 | |||
| Intervention | 202 ± 47 | 220 ± 45 | 227 ± 43 | |||
| Control | 175 ± 30 | 198 ± 30 | 216 ± 28 | |||
| Serum LDL cholesterol, mg/dL | 0.14 | <0.001 | 0.86 | |||
| Intervention | 111 ± 37 | 123 ± 35 | 129 ± 35 | |||
| Control | 95 ± 20 | 108 ± 19 | 117 ± 23 | |||
| Serum HDL cholesterol, mg/dL | 0.37 | 0.07 | 0.18 | |||
| Intervention | 62 ± 16 | 64 ± 15 | 62 ± 15 | |||
| Control | 56 ± 10 | 58 ± 10 | 61 ± 13 | |||
| Serum triglycerides, mg/dL | 0.12 | 0.005 | 0.81 | |||
| Intervention | 183 ± 78 | 212 ± 69 | 221 ± 71 | |||
| Control | 157 ± 42 | 177 ± 39 | 196 ± 61 | |||
| Serum CRP, mg/L | 0.27 | 0.08 | 0.002 | |||
| Intervention | 6.1 ± 4.0 | 6.1 ± 3.7 | 5.5 ± 2.2 | |||
| Control | 6.8 ± 7.2 | 7.5 ± 7.3 | 9.5 ± 6.6 | |||
| Serum adiponectin, μg/mL | 0.42 | 0.06 | 0.70 | |||
| Intervention | 9.3 ± 5.2 | 9.9 ± 5.3 | 10.7 ± 6.3 | |||
| Control | 8.2 ± 3.9 | 8.8 ± 3.7 | 8.9 ± 4.2 | |||
| Serum IL-6, pg/mL | 0.37 | <0.001 | 0.08 | |||
| Intervention | 22.4 ± 7.4 | 23.5 ± 5.3 | 25.1 ± 6.5 | |||
| Control | 18.8 ± 7.3 | 20.5 ± 6.3 | 26.0 ± 7.2 |
n = 17 in each group. Values are mean ± SD unless otherwise indicated. Intervention: blueberry + soluble fiber; control: standard prenatal care. CRP, C-reactive protein; HbA1c, glycated hemoglobin.
Gestational week 16.0 ± 3.8 for intervention and 14.5 ± 4.4 for control.
Gestational week 26.5 ± 1.3 for intervention and 26.0 ± 1.2 for control.
Gestational week 34.0 ± 1.3 for intervention and 33.0 ± 2.3 for control.
P from mixed-model ANOVA.
Serum glucose, insulin, HbA1c, and lipids
Serum glucose and insulin and the HOMA-IR increased during pregnancy but did not differ between the groups. GCT measured between 24 and 28 weeks of gestation revealed a significantly lower 1-h postprandial blood glucose with a 50-g oral glucose load in the intervention (mean ± SD: 100 ± 33 mg/dL) than in the control (131 ± 40 mg/dL) (all P < 0.05). Based on subsequent positive tests in the 2-step GCT, 3 (18%) and 5 (29%) GDM diagnoses occurred in the intervention and control, respectively, but this difference was not significant between the 2 groups (P = 0.42). HbA1c changed over time and there was an interaction with treatment such that the concentration was lower in the intervention than in the control group over the course of the study (Table 2) (P < 0.05). Serum total and LDL cholesterol, and triglycerides, increased significantly with gestational age but did not differ between the groups, whereas serum HDL cholesterol revealed no significant changes between the groups over time.
Biomarkers of inflammation
Serum CRP revealed an interaction with treatment such that the concentration was lower in the intervention than in the control group over the course of the study (Table 2) (P < 0.05). Serum IL-6 increased significantly over time, but no significant group effect was noted. Serum adiponectin revealed no significant changes between the groups over time. Effect size data are reported for maternal body weight, blood pressure, markers of glycemic control and inflammation, and conventional lipids (serum total, LDL, and HDL cholesterol and triglycerides) (Supplemental Table 1).
NMR-derived lipid profiles
Among the NMR-derived lipoprotein subclasses, concentrations of total and small VLDL particles were significantly lower in the intervention than in the control group but did not change over time (Table 3) (P < 0.05). Among LDL subclasses, the concentration of total LDL particles increased significantly over the course of the study. Small LDL particle concentration did not change but was lower in the intervention than in the control group (Table 3) (P < 0.05). Among HDL subclasses, concentration of total HDL particles significantly decreased with gestational age in both groups (Table 3) (P < 0.05), and no changes were noted in large, medium, and small HDL particles. Finally, the mean size of VLDL, LDL, and HDL particles did not differ over time or between the groups. Effect size data are reported for all NMR variables (Supplemental Table 2).
TABLE 3.
Plasma NMR-derived lipid particle concentrations and size in pregnant women with obesity at risk of gestational diabetes that were or were not supplemented with blueberries and soluble fiber for 18 wk of pregnancy1
| Variable by group | Baseline2 | Midpoint3 | End4 | P value5 (group) | P value5 (time) | P value5 (interaction) |
|---|---|---|---|---|---|---|
| Plasma total VLDL and chylomicron particles, nmol/L | 0.04 | 0.09 | 0.25 | |||
| Intervention | 39.2 ± 22.5 | 44.2 ± 20.4 | 54.4 ± 43.4 | |||
| Control | 52.2 ± 18.4 | 52.9 ± 19.9 | 55.0 ± 23.3 | |||
| Plasma large VLDL and chylomicron particles, nmol/L | 0.78 | 0.62 | 0.68 | |||
| Intervention | 4.7 ± 4.4 | 3.8 ± 3.0 | 4.5 ± 3.5 | |||
| Control | 4.2 ± 1.8 | 4.3 ± 2.5 | 4.7 ± 2.7 | |||
| Plasma medium VLDL particles, nmol/L | 0.31 | 0.34 | 0.62 | |||
| Intervention | 16.0 ± 11.7 | 16.3 ± 11.7 | 21.1 ± 16.0 | |||
| Control | 21.7 ± 12.4 | 18.7 ± 9.5 | 24.5 ± 16.6 | |||
| Plasma small VLDL particles, nmol/L | 0.02 | 0.78 | 0.15 | |||
| Intervention | 20.2 ± 11.1 | 25.5 ± 14.8 | 24.1 ± 17.0 | |||
| Control | 29.6 ± 12.5 | 27.7 ± 12.4 | 25.8 ± 12.6 | |||
| Plasma total LDL particles, nmol/L | 0.69 | 0.004 | 0.48 | |||
| Intervention | 1220 ± 325 | 1300 ± 361 | 1390 ± 386 | |||
| Control | 1120 ± 323 | 1150 ± 326 | 1260 ± 345 | |||
| Plasma IDL particles, nmol/L | 0.38 | 0.07 | 0.78 | |||
| Intervention | 173 ± 132 | 194 ± 145 | 217 ± 147 | |||
| Control | 124 ± 62 | 147 ± 104 | 157 ± 97 | |||
| Plasma large LDL particles, nmol/L | 0.14 | 0.49 | 0.75 | |||
| Intervention | 673 ± 396 | 736 ± 247 | 710 ± 308 | |||
| Control | 408 ± 283 | 473 ± 358 | 527 ± 405 | |||
| Plasma total small LDL particles, nmol/L | 0.03 | 0.07 | 0.56 | |||
| Intervention | 348 ± 452 | 417 ± 476 | 517 ± 496 | |||
| Control | 722 ± 390 | 690 ± 463 | 826 ± 463 | |||
| Plasma total HDL particles, μmol/L | 0.48 | 0.03 | 0.41 | |||
| Intervention | 34.5 ± 7.0 | 33.5 ± 7.2 | 31.8 ± 8.1 | |||
| Control | 33.5 ± 6.7 | 32.7 ± 7.7 | 32.2 ± 5.8 | |||
| Plasma large HDL particles, μmol/L | 0.83 | 0.60 | 0.76 | |||
| Intervention | 13.2 ± 4.3 | 14.5 ± 3.3 | 14.2 ± 3.7 | |||
| Control | 13.5 ± 3.9 | 13.1 ± 3.4 | 12.9 ± 3.3 | |||
| Plasma medium HDL particles, μmol/L | 0.62 | 0.21 | 0.22 | |||
| Intervention | 4.9 ± 5.4 | 4.5 ± 3.8 | 5.3 ± 3.2 | |||
| Control | 5.5 ± 5.9 | 5.5 ± 6.2 | 2.9 ± 1.9 | |||
| Plasma small HDL particles, μmol/L | 0.76 | 0.44 | 0.32 | |||
| Intervention | 16.1 ± 5.5 | 15.7 ± 6.1 | 14.5 ± 5.3 | |||
| Control | 16.0 ± 5.7 | 14.3 ± 5.1 | 16.2 ± 4.5 | |||
| Plasma VLDL size, nm | 0.48 | 0.83 | 0.73 | |||
| Intervention | 53.0 ± 6.2 | 53.4 ± 6.3 | 53.0 ± 6.8 | |||
| Control | 51.6 ± 4.3 | 52.7 ± 5.6 | 52.3 ± 5.8 | |||
| Plasma LDL size, nm | 0.07 | 0.18 | 0.46 | |||
| Intervention | 20.0 ± 1.5 | 20.8 ± 0.5 | 20.8 ± 0.8 | |||
| Control | 20.3 ± 1.3 | 20.4 ± 0.5 | 20.5 ± 0.6 | |||
| Plasma HDL size, nm | 0.98 | 0.26 | 0.73 | |||
| Intervention | 10.0 ± 0.6 | 10.2 ± 0.4 | 10.2 ± 0.3 | |||
| Control | 10.5 ± 1.5 | 10.0 ± 0.3 | 10.0 ± 0.3 |
n = 17 for each group. Values are mean ± SD unless otherwise indicated. Intervention: blueberry + soluble fiber; control: standard prenatal care.
Gestational week 16.0 ± 3.8 for intervention and 14.5 ± 4.4 for control.
Gestational week 26.5 ± 1.3 for intervention and 26.0 ± 1.2 for control.
Gestational week 34.0 ± 1.3 for intervention and 33.0 ± 2.3 for control.
P from mixed-model ANOVA.
Habitual dietary intake and physical activity
Dietary caloric intake significantly increased with gestational age, but did not differ significantly between the groups; however, intake of carbohydrates was significantly lower, and intake of proteins was significantly higher, in the intervention than in the control group over time (Table 4) (all P < 0.05). Total fat intake increased significantly with gestational age in both groups, but overall did not differ between them. Among the micronutrients, dietary intakes of vitamins E and C and calcium, but not iron, significantly increased with gestational age, but none of these differed between the intervention and control groups (Table 4) (all P < 0.05). No significant differences were noted in habitual intake of dietary fiber, as distinct from the fiber supplement, and total servings of fruits and vegetables remained similar over time and between the 2 groups. Also, based on self-reported physical activity data, all the women were classified as “physically inactive” and the status did not differ with increasing gestational age or by group allocation.
TABLE 4.
Background daily dietary nutrient and food group intakes in pregnant women with obesity at risk of gestational diabetes that were or were not supplemented with blueberries and soluble fiber for 18 wk of pregnancy1
| Variable by group | Baseline2 | Midpoint3 | End4 | P value5 (group) | P value5 (time) | P value5 (interaction) |
|---|---|---|---|---|---|---|
| Energy, kcal/d | 0.06 | <0.001 | 0.46 | |||
| Intervention | 2060 ± 155 | 2190 ± 183 | 2470 ± 186 | |||
| Control | 2130 ± 179 | 2320 ± 174 | 2590 ± 159 | |||
| Carbohydrate, g/d | 0.005 | <0.001 | 0.005 | |||
| Intervention | 255 ± 21 | 259 ± 22 | 285 ± 26 | |||
| Control | 265 ± 27 | 286 ± 26 | 321 ± 29 | |||
| Fat, g/d | <0.001 | <0.001 | 0.23 | |||
| Intervention | 82 ± 9 | 78 ± 9 | 88 ± 10 | |||
| Control | 75 ± 7 | 90 ± 8 | 100 ± 8 | |||
| Protein, g/d | 0.38 | <0.001 | 0.01 | |||
| Intervention | 92 ± 20 | 108 ± 21 | 133 ± 14 | |||
| Control | 82 ± 13 | 89 ± 11 | 105 ± 15 | |||
| Vitamin E, mg/d | 0.06 | 0.001 | 0.49 | |||
| Intervention | 9 ± 2 | 10 ± 3 | 11 ± 2 | |||
| Control | 11 ± 3 | 11 ± 2 | 12 ± 4 | |||
| Vitamin C, mg/d | 0.33 | 0.01 | 0.61 | |||
| Intervention | 45 ± 13 | 46 ± 12 | 49 ± 12 | |||
| Control | 41 ± 11 | 43 ± 10 | 46 ± 10 | |||
| Iron, mg/d | 0.46 | 0.15 | 0.88 | |||
| Intervention | 20 ± 3 | 20 ± 2 | 21 ± 3 | |||
| Control | 19 ± 5 | 19 ± 5 | 20 ± 4 | |||
| Calcium, mg/d | 0.19 | 0.01 | 0.22 | |||
| Intervention | 869 ± 103 | 897 ± 70 | 902 ± 75 | |||
| Control | 829 ± 116 | 846 ± 90 | 879 ± 70 | |||
| Fiber,6 g/d | 0.21 | 0.05 | 0.46 | |||
| Intervention | 16 ± 4 | 16 ± 3 | 19 ± 4 | |||
| Control | 19 ± 6 | 18 ± 6 | 20 ± 3 | |||
| Fruits,7 cups/d | 0.23 | 0.18 | 0.45 | |||
| Intervention | 0.8 ± 0.6 | 0.8 ± 0.5 | 1.1 ± 0.5 | |||
| Control | 1.0 ± 0.3 | 1.0 ± 0.2 | 1.3 ± 0.7 | |||
| Vegetables, cups/d | 0.18 | 0.23 | 0.53 | |||
| Intervention | 1.2 ± 0.7 | 1.3 ± 0.8 | 1.0 ± 0.5 | |||
| Control | 1.1 ± 0.3 | 1.4 ± 1.2 | 1.5 ± 0.8 |
n = 17 for each group. Values are means ± SDs unless otherwise indicated. Intervention: blueberry + soluble fiber; control: standard prenatal care.
Gestational week 16.0 ± 3.8 for intervention and 14.5 ± 4.4 for control.
Gestational week 26.5 ± 1.3 for intervention and 26.0 ± 1.2 for control.
Gestational week 34.0 ± 1.3 for intervention and 33.0 ± 2.3 for control.
P from mixed-model ANOVA.
Excluding supplemental fiber in the intervention group.
Excluding supplemental blueberries in the intervention group.
Infant birth weight and mode of delivery
Infant birth weight was not significantly different between the intervention and control groups (3407 ± 552 g compared with 3740 ± 580 g, respectively, P = 0.11). The number of vaginal deliveries was significantly higher in the intervention than in the control (n = 14 compared with n = 7, respectively), whereas cesarean delivery rates were lower (n = 3 compared with n = 10, respectively, overall P = 0.03).
In the final, most rigorous analysis, accounting for multiple comparisons for familywise type I error and adjusting the α level to 0.003 for the cardiometabolic and NMR variables, maternal body weight and serum CRP remained significantly lower in the intervention than in the control group with advancing gestational age (interaction effect). Sensitivity analysis of maternal body weight following the multiple imputation method revealed results similar to the main analysis: significant time and interaction effects (P < 0.001).
Discussion
In women at high risk of GDM, we observed multiple beneficial effects of dietary supplementation with combined blueberries and soluble fiber, beginning mid-pregnancy. Gestational weight gain was normalized: women in the intervention group gained 15 lb, which is within the recommended range of the Institute of Medicine (IOM) (11–20 lb for obese women), whereas those in the control group gained 26 lb, substantially exceeding IOM recommendations (56). Women in the intervention group also experienced a reduction in blood glucose after a standard glucose challenge compared with the control group, and significant reductions in atherogenic NMR-based lipoprotein subclasses, specifically total and small VLDL particles and small LDL particles. Maternal serum CRP concentrations increased significantly in the control group with advancing gestational age compared with the intervention. All these changes represent improved metabolic health and a reduction in risk factors for GDM, and for other adverse outcomes of pregnancy such as pre-eclampsia, among the intervention group. This food-based strategy is safe, simple, and may be more feasible than interventions involving nutrition education or dietary advice, measures that have yielded largely null or modest results in previous studies of GDM (18).
Gestational weight gain, especially in obese women, is one of the main modifiable risk factors for GDM, mediated by concomitant impaired glycemia, insulin resistance, and inflammation (57). Among the fruits and vegetables that merit investigation in the prevention of diabetes and GDM, blueberries stand out as a rich source of polyphenolic flavonoids, phenolic acids, several micronutrients, and fiber; they are also low in total calories (58). Prior observational studies have shown that habitual blueberry consumption is inversely associated with development of T2D in nonpregnant adults (25, 59). Of special relevance to our study are the findings of a meta-analysis by Guo et al. (60) in which habitual intake of dietary berries in prospective cohorts was associated with an 18% risk reduction of T2D in nonpregnant adults. Keeping in view the high risk of developing T2D in later life among women with GDM (8), dietary supplementation of blueberries in the gestational phase may offer some protection.
Clinical trials have revealed that blueberries can improve insulin resistance and normalize postprandial hyperglycemia in adults at elevated risk of cardiometabolic disease (40, 61). Our findings of improved gestational weight gain, glycemic control, lipid profiles, and CRP in women with high-risk pregnancies are consistent with those of clinical trials showing that the DASH diet (62, 63) and the Mediterranean diet (21, 23) were beneficial in GDM. In a 4-wk feeding trial reported from Iran, the DASH diet was shown to decrease HbA1c and improve conventional lipid profiles in overweight and obese women diagnosed with GDM (63). In addition, the diet was associated with improvements in biomarkers of oxidative stress, but not in CRP (62). Neither article reported any difference in maternal body weight after the DASH intervention. A larger study of normal and overweight women in early gestation, randomly assigned to the Mediterranean diet (with pistachio nuts and olive oil) or a control diet, revealed a significant decrease in GDM incidence and gestational weight gain with the intervention (21).
Interventions targeting early pregnancy as implemented by Assaf-Balut et al. (21) based on the Mediterranean diet and by our group may be more effective in preventing than reversing or treating GDM. Beneficial effects observed with blueberries have been attributed to antioxidant and anti-inflammatory effects of constituents, to effects on glucose and lipid metabolism including inhibition of enzymes such as α-glucosidase and maltase, and to effects on glucose transporters, all contributing to improved glycemic control (60). Berries and their anthocyanins have also been shown to increase expression of adenosine monophosphate–activated protein kinase which decreases insulin resistance and hepatic synthesis of cholesterol and triglycerides (64). Another important mechanism underlying the antiobesity effects of blueberry polyphenols is their role as a prebiotic in enhancing the growth of specific beneficial bacteria, such as Bifidobacterium and Muribaculaceae species as reported in high-fat diet–induced obese mice (65, 66). Similar gut microbiome modulation effects have also been observed with soluble fiber supplementation in reducing weight gain in obese mice (67, 68). Further clinical trials are needed to examine the effects of blueberries on the gut microbiome as a potential mechanism in reducing obesity and GDM risk.
Dietary fiber, especially soluble fiber, has been associated with improved glycemic control in randomized controlled trials in nonpregnant adults (69), as well as in observational studies of habitual fiber-rich food intakes in GDM (70). In their prospective cohort study, Zhang et al. (70) reported that a habitual dietary fiber supplement of 10 g/d was associated with a 26% risk reduction of GDM, and an increase of 5 g/d of cereal or fruit fiber was associated with a 23% decreased risk of GDM. However, clinical data on fiber supplementation in GDM are scarce. In a 12-wk intervention focusing on increasing habitual fiber intake as well as providing 12 g supplemental fiber in overweight and obese pregnant women, gestational weight gain was significantly lower in the fiber group than in the usual prenatal care group (71). However, this study did not report any data on maternal glycemic control. We report the role of 12 g/d of soluble fiber, together with ∼8 g total fiber from whole blueberries, in improving gestational weight gain, glycemic control, and CRP concentrations in obese women. Adding this amount of fiber supplementation to the habitually low fiber intake of our study participants helped them meet the USDA DGA fiber guidelines for optimal health and prevention of diseases (46). Dietary fiber has been shown to decrease appetite and energy intake and thereby facilitate weight loss, delay gastric emptying, slow glucose absorption, and exert beneficial effects on glucose homeostasis (72, 73). These mechanisms may contribute to the overall lower amount of gestational weight gained and better glycemic control in our intervention than in the control group, although caloric intake over time was not significantly different between the 2 groups.
Dyslipidemia, especially elevated NMR-derived VLDL subclasses, has been associated with GDM risks in obese pregnant women (74), and elevated NMR-derived LDL subclasses have been previously reported to be associated with pre-eclampsia by our group (75). We noted no incidence of pre-eclampsia in our study. NMR-derived lipid profiles reveal atherogenic properties of lipids in greater detail that are often not detectable in conventional lipid assays as observed in our study. Elevated triglyceride-rich lipoprotein subclasses in GDM in obese women have been linked to insulin resistance, and a subsequent decrease in lipoprotein lipase activity and reduced clearance (76). Our findings that blueberries and fiber improve these atherogenic lipoprotein subclasses in obese insulin-resistant pregnant women are of clinical utility and merit further attention in dietary studies of GDM.
Our study has several strengths. Firstly, we administered a prospective intervention early in gestation that was continued for the next 18–20 wk; in comparison, most other reported studies of dietary intervention began after GDM diagnosis at 24–28 weeks of gestation. Early pregnancy intervention is critical for favorable modification of maternal weight gain and glycemia and may promote sustained compliance as observed in our study. Secondly, we recruited minority women at high risk of GDM, with obesity, previous history of GDM, and/or family history of diabetes, thereby supporting the efficacy of our intervention among those who carry the greatest burden of GDM in the US population. Thirdly, we measured biomarkers of inflammation, such as maternal CRP, IL-6, and adiponectin, at 3 different time points, and these have not been reported in other dietary studies of GDM. Fourthly, we assessed habitual maternal diet throughout the study, and whereas intake of macronutrients, especially carbohydrates, changed with gestational age, overall caloric intake did not differ between the groups over time, thereby attributing our main findings to the effects of the blueberry and fiber intervention. Finally, our intervention of dietary blueberries and soluble fiber was shown to be safe with no adverse effects in the mother, as well as no significant effects on infant birth weight. These findings are of special relevance to Hispanic and African American women with low intakes of fruits and vegetables mostly due to low purchasing power and home availability or inadequate access to fresh foods (77, 78). Nutrition therapy in high-risk pregnancies in these women must consider supplementation of foods, such as fiber and polyphenol-rich blueberries and other berries which may elicit favorable compliance as seen in our study. These findings may also be extended to food assistance programs for women, infants, and children that must emphasize the consumption of berries and fiber and work closely with primary care physicians in integrating these dietary strategies into effective prenatal care for the prevention and management of GDM.
Our study also has certain limitations. Firstly, we recorded maternal variables of interest at 3 time points corresponding to <20 weeks of gestation, between 24 and 28, and between 32 and 36 weeks of gestation, but did not account for any change in maternal weight gained or changes in biochemical measures beyond 36 weeks of gestation until delivery. Secondly, our study did not carry the power to detect differences in GDM incidence between the 2 groups, although we observed a lower number of GDM cases in the intervention than in the control, indicating a positive aspect of the intervention. Thirdly, our findings in obese minority women with high-risk pregnancies may not be applicable to women of optimal weight and other ethnicities who also carry GDM risks. Fourthly, based on the nature of our food-based intervention, participants were not completely blinded, and we addressed this issue by administering group-specific consent forms to minimize bias. All study personnel were otherwise blinded during the analyses of blood samples and dietary data. Fifthly, we used a combined intervention, blueberries and supplemental fiber, and cannot disentangle their individual contributions. We did not account for prepregnancy or postpartum maternal weight and health status that also play an important role in diabetes risks in the mother and infant.
After adjustments for familywise type I error and based on an adjusted P < 0.003, maternal body weight and CRP remained significant whereas other variables did not meet this threshold. We consider this stringent level of significance to be quite conservative because many of the cardiometabolic markers are interdependent. For this reason, we presented data in the tables without formal α-level adjustment. We also emphasize that our findings are biologically plausible and identify maternal indexes responsive to a feasible dietary intervention.
In conclusion, our study shows that blueberry and soluble fiber supplementation was well tolerated and improved classical risk factors for GDM, especially excess maternal weight gain and CRP, in obese women. Although nutrition education was provided to both groups as part of standard prenatal care, the specific food supplementation appeared to be more effective in improving maternal risks. These findings warrant investigation in larger trials that must also include women with pregestational diabetes and postpartum hyperglycemia to address the role of bioactive-rich foods in reducing diabetes complications of pregnancy.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful for the support of our nurse practitioners Ms. Marie Mitchell and Ms. Christina Clignett for assisting with participant recruitment and follow-ups. The authors’ responsibilities were as follows—AB, JLE, PP, and JMA: designed the study and obtained funding; DF: performed the statistical analyses; AB and PP: supervised the consenting, recruitment, and follow-ups; AB: supervised the blinded clinical sample and dietary data analyses; TJL: helped with the study conception, design, and manuscript preparation; and all authors: read and approved the final manuscript.
Notes
Supported by NIH National Institute of General Medical Sciences grant GM103440 (to AB) and the School of Integrated Health Sciences Dean's Faculty Development Award at University of Nevada at Las Vegas (to AB).
Author disclosures: AB is a member of the Journal’s Editorial Board. The authors report no conflicts of interest.
Supplemental Tables 1 and 2 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ACOG, American College of Obstetrics and Gynecology; CRP, C-reactive protein; DASH, Dietary Approaches to Stop Hypertension; DGA, Dietary Guidelines for Americans; GCT, glucose challenge test; GDM, gestational diabetes mellitus; HbA1c, glycated hemoglobin; IOM, Institute of Medicine; RD, registered dietitian; T2D, type 2 diabetes; UNLV, University of Nevada at Las Vegas.
Contributor Information
Arpita Basu, Department of Kinesiology and Nutrition Sciences, University of Nevada at Las Vegas, Las Vegas, NV, USA.
Du Feng, School of Nursing, University of Nevada at Las Vegas, Las Vegas, NV, USA.
Petar Planinic, Department of Obstetrics & Gynecology, School of Medicine, University of Nevada at Las Vegas, Las Vegas, NV, USA.
Jeffrey L Ebersole, School of Dental Medicine, University of Nevada at Las Vegas, Las Vegas, NV, USA.
Timothy J Lyons, Division of Endocrinology, Medical University of South Carolina, Charleston, SC, USA.
James M Alexander, Department of Obstetrics & Gynecology, School of Medicine, University of Nevada at Las Vegas, Las Vegas, NV, USA.
References
- 1. Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21(Suppl 2):B161–7. [PubMed] [Google Scholar]
- 2. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. [DOI] [PubMed] [Google Scholar]
- 3. Azar M, Stoner JA, Dao HD, Stephens L, Goodman JR, Maynard J, Lyons TJ. Epidemiology of dysglycemia in pregnant Oklahoma American Indian women. J Clin Endocrinol Metab. 2015;100:2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao F, Luo H, Jones K, Nicholson W, Bell RA. Gestational diabetes and health behaviors among women: National Health and Nutrition Examination Survey, 2007–2014. Prev Chronic Dis. 2018;15:E131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mayo K, Melamed N, Vandenberghe H, Berger H. The impact of adoption of the International Association of Diabetes in Pregnancy Study Group criteria for the screening and diagnosis of gestational diabetes. Am J Obstet Gynecol. 2015;212:224.e1–9. [DOI] [PubMed] [Google Scholar]
- 7. Egan AM, Vellinga A, Harreiter J, Simmons D, Desoye G, Corcoy R, Adelantado JM, Devlieger R, Van Assche A, Galjaard Set al. . Epidemiology of gestational diabetes mellitus according to IADPSG/WHO 2013 criteria among obese pregnant women in Europe. Diabetologia. 2017;60:1913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casagrande SS, Linder B, Cowie CC. Prevalence of gestational diabetes and subsequent type 2 diabetes among U.S. women. Diabetes Res Clin Pract. 2018;141:200–8. [DOI] [PubMed] [Google Scholar]
- 9. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–6. [DOI] [PubMed] [Google Scholar]
- 10. Yang X, Hsu-Hage B, Zhang H, Zhang C, Zhang Y, Zhang C. Women with impaired glucose tolerance during pregnancy have significantly poor pregnancy outcomes. Diabetes Care. 2002;25:1619–24. [DOI] [PubMed] [Google Scholar]
- 11. Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, Lowe LP, Trimble ER, Coustan DR, Hadden DRet al. . The Hyperglycemia and Adverse Pregnancy Outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35:780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scheinker D, Valencia A, Rodriguez F. Identification of factors associated with variation in US county-level obesity prevalence rates using epidemiologic vs machine learning models. JAMA Netw Open. 2019;2:e192884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shai I, Jiang R, Manson JE, Stampfer MJ, Willett WC, Colditz GA, Hu FB. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006;29:1585–90. [DOI] [PubMed] [Google Scholar]
- 14. Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care. 2012;35:1492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bossick AS, Peters RM, Burmeister C, Kakumanu N, Shill JE, Cassidy-Bushrow AE. Antenatal inflammation and gestational diabetes mellitus risk among pregnant African-American women. J Reprod Immunol. 2016;115:1–5. [DOI] [PubMed] [Google Scholar]
- 16. Moreno-Castilla C, Mauricio D, Hernandez M. Role of medical nutrition therapy in the management of gestational diabetes mellitus. Curr Diab Rep. 2016;16:22. [DOI] [PubMed] [Google Scholar]
- 17. Yamamoto JM, Kellett JE, Balsells M, García-Patterson A, Hadar E, Solà I, Gich I, van der Beek EM, Castañeda-Gutiérrez E, Heinonen Set al. . Gestational diabetes mellitus and diet: a systematic review and meta-analysis of randomized controlled trials examining the impact of modified dietary interventions on maternal glucose control and neonatal birth weight. Diabetes Care. 2018;41:1346–61. [DOI] [PubMed] [Google Scholar]
- 18. Han S, Middleton P, Shepherd E, Van Ryswyk E, Crowther CA. Different types of dietary advice for women with gestational diabetes mellitus. Cochrane Database Syst Rev. 2017;2:CD009275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M. Plant-based and plant-rich diet patterns during gestation: beneficial effects and possible shortcomings. Adv Nutr. 2015;6:581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schoenaker DA, Mishra GD, Callaway LK, Soedamah-Muthu SS. The role of energy, nutrients, foods, and dietary patterns in the development of gestational diabetes mellitus: a systematic review of observational studies. Diabetes Care. 2016;39:16–23. [DOI] [PubMed] [Google Scholar]
- 21. Assaf-Balut C, García de la Torre N, Durán A, Fuentes M, Bordiú E, Del Valle L, Familiar C, Ortolá A, Jiménez I, Herraiz MAet al. . A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): a randomized controlled trial: the St. Carlos GDM prevention study. PLoS One. 2017;12:e0185873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olmedo-Requena R, Gómez-Fernández J, Amezcua-Prieto C, Mozas-Moreno J, Khan KS, Jiménez-Moleón JJ. Pre-pregnancy adherence to the Mediterranean diet and gestational diabetes mellitus: a case-control study. Nutrients. 2019;11:1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de la Torre NG, Assaf-Balut C, Jiménez Varas I, Del Valle L, Durán A, Fuentes M, Del Prado N, Bordiú E, Valerio JJ, Herraiz MAet al. . Effectiveness of following Mediterranean diet recommendations in the real world in the incidence of gestational diabetes mellitus (GDM) and adverse maternal-foetal outcomes: a prospective, universal, interventional study with a single group. The St Carlos Study. Nutrients. 2019;11:1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacques PF, Cassidy A, Rogers G, Peterson JJ, Meigs JB, Dwyer JT. Higher dietary flavonol intake is associated with lower incidence of type 2 diabetes. J Nutr. 2013;143:1474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B, Willett W, Hu FB, Sun Q, van Dam RM. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr. 2012;95:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tresserra-Rimbau A, Castro-Barquero S, Vitelli-Storelli F, Becerra-Tomas N, Vázquez-Ruiz Z, Díaz-López A, Corella D, Castañer O, Romaguera D, Vioque Jet al. . Associations between dietary polyphenols and type 2 diabetes in a cross-sectional analysis of the PREDIMED-Plus trial: role of body mass index and sex. Antioxidants (Basel). 2019;8:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roopchand DE, Kuhn P, Rojo LE, Lila MA, Raskin I. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacol Res. 2013;68:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anhe FF, Varin TV, Le Barz M, Pilon G, Dudonne S, Trottier J, St-Pierre P, Harris CS, Lucas M, Lemire Met al. . Arctic berry extracts target the gut–liver axis to alleviate metabolic endotoxaemia, insulin resistance and hepatic steatosis in diet-induced obese mice. Diabetologia. 2018;61:919–31. [DOI] [PubMed] [Google Scholar]
- 29. Jurado-Ruiz E, Álvarez-Amor L, Varela LM, Berná G, Parra-Camacho MS, Oliveras-Lopez MJ, Martínez-Force E, Rojas A, Hmadcha A, Soria Bet al. . Extra virgin olive oil diet intervention improves insulin resistance and islet performance in diet-induced diabetes in mice. Sci Rep. 2019;9:11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bailey RL, Pac SG, Fulgoni VL 3rd, Reidy KC, Catalano PM. Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Netw Open. 2019;2:e195967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bodnar LM, Simhan HN, Parker CB, Meier H, Mercer BM, Grobman WA, Haas DM, Wing DA, Hoffman MK, Parry Set al. . Racial or ethnic and socioeconomic inequalities in adherence to national dietary guidance in a large cohort of US pregnant women. J Acad Nutr Diet. 2017;117:867–77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shin D, Lee KW, Song WO. Dietary patterns during pregnancy are associated with risk of gestational diabetes mellitus. Nutrients. 2015;7:9369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, Aston CE, Lyons TJ. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. 2010;140:1582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schell J, Betts NM, Foster M, Scofield RH, Basu A. Cranberries improve postprandial glucose excursions in type 2 diabetes. Food Funct. 2017;8:3083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Basu A, Betts NM, Nguyen A, Newman ED, Fu D, Lyons TJ. Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J Nutr. 2014;144:830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blumberg JB, Basu A, Krueger CG, Lila MA, Neto CC, Novotny JA, Reed JD, Rodriguez-Mateos A, Toner CD. Impact of cranberries on gut microbiota and cardiometabolic health: proceedings of the Cranberry Health Research Conference 2015. Adv Nutr. 2016;7:759S–70S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Basu A, Nguyen A, Betts NM, Lyons TJ. Strawberry as a functional food: an evidence-based review. Crit Rev Food Sci Nutr. 2014;54:790–806. [DOI] [PubMed] [Google Scholar]
- 38. Cásedas G, Les F, Gómez-Serranillos MP, Smith C, López V. Anthocyanin profile, antioxidant activity and enzyme inhibiting properties of blueberry and cranberry juices: a comparative study. Food Funct. 2017;8:4187–93. [DOI] [PubMed] [Google Scholar]
- 39. Rocha DMUP, Caldas APS, da Silva BP, Hermsdorff HHM, Alfenas RdCG. Effects of blueberry and cranberry consumption on type 2 diabetes glycemic control: a systematic review. Crit Rev Food Sci Nutr. 2019;59:1816–28. [DOI] [PubMed] [Google Scholar]
- 40. Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. 2010;140:1764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu W, Mao Y, Schoenborn J, Wang Z, Tang G, Tang X. Whole blueberry protects pancreatic beta-cells in diet-induced obese mouse. Nutr Metab. 2019;16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342:1392–8. [DOI] [PubMed] [Google Scholar]
- 43. Riccardi G, Rivellese AA. Effects of dietary fiber and carbohydrate on glucose and lipoprotein metabolism in diabetic patients. Diabetes Care. 1991;14:1115–25. [DOI] [PubMed] [Google Scholar]
- 44. USDA, Agricultural Research Service . FoodData Central. [Internet]. 2019. [Cited 2020 Nov 30]. Available from: https://fdc.nal.usda.gov. [Google Scholar]
- 45. USDA database for the flavonoid content of selected foods, Release 3. [Internet]. 2013; with revisions May 2014. [Cited 2020 Nov 30]. Available from: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav_R03-1.pdf. [Google Scholar]
- 46. US Department of Health and Human Service (US DHHS) , US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed. [Internet]. Washington (DC): US DHHS and USDA; 2015. [Cited 2019 Jan 18]. Available from: https://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 47. ACOG Committee Opinion No. 650: physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol. 2015;126:e135–42. [DOI] [PubMed] [Google Scholar]
- 48. Zhong S, Sandhu A, Edirisinghe I, Burton-Freeman B. Characterization of wild blueberry polyphenols bioavailability and kinetic profile in plasma over 24-h period in human subjects. Mol Nutr Food Res. 2017;61:1700405. [DOI] [PubMed] [Google Scholar]
- 49. O'Sullivan JB, Mahan CM, Charles D, Dandrow RV. Screening criteria for high-risk gestational diabetic patients. Am J Obstet Gynecol. 1973;116:895–900. [DOI] [PubMed] [Google Scholar]
- 50. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–73. [DOI] [PubMed] [Google Scholar]
- 51. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. [DOI] [PubMed] [Google Scholar]
- 52. Lyons TJ, Jenkins AJ, Zheng D, Klein RL, Otvos JD, Yu Y, Lackland DT, McGee D, McHenry MB, Lopes-Virella Met al. . Nuclear magnetic resonance-determined lipoprotein subclass profile in the DCCT/EDIC cohort: associations with carotid intima-media thickness. Diabetic Med. 2006;23:955–66. [DOI] [PubMed] [Google Scholar]
- 53. Keselman HJ, Cribbie R, Holland B. Controlling the rate of type I error over a large set of statistical tests. Br J Math Stat Psychol. 2002;55:27–39. [DOI] [PubMed] [Google Scholar]
- 54. Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, Aston CE, Lyons TJ. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr. 2010;29:31–40. [DOI] [PubMed] [Google Scholar]
- 55. Thabane L, Mbuagbaw L, Zhang S, Samaan Z, Marcucci M, Ye C, Thabane M, Giangregorio L, Dennis B, Kosa Det al. . A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol. 2013;13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Beyerlein A, Schiessl B, Lack N, von Kries R. Optimal gestational weight gain ranges for the avoidance of adverse birth weight outcomes: a novel approach. Am J Clin Nutr. 2009;90:1552–8. [DOI] [PubMed] [Google Scholar]
- 57. Fasshauer M, Blüher M, Stumvoll M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2014;2:488–99. [DOI] [PubMed] [Google Scholar]
- 58. Basu A, Rhone M, Lyons TJ. Berries: emerging impact on cardiovascular health. Nutr Rev. 2010;68:168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Dam RM, Naidoo N, Landberg R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: review of recent findings. Curr Opin Lipidol. 2013;24:25–33. [DOI] [PubMed] [Google Scholar]
- 60. Guo X, Yang B, Tan J, Jiang J, Li D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2016;70:1360–7. [DOI] [PubMed] [Google Scholar]
- 61. Castro-Acosta ML, Lenihan-Geels GN, Corpe CP, Hall WL. Berries and anthocyanins: promising functional food ingredients with postprandial glycaemia-lowering effects. Proc Nutr Soc. 2016;75:342–55. [DOI] [PubMed] [Google Scholar]
- 62. Asemi Z, Samimi M, Tabassi Z, Sabihi SS, Esmaillzadeh A. A randomized controlled clinical trial investigating the effect of DASH diet on insulin resistance, inflammation, and oxidative stress in gestational diabetes. Nutrition. 2013;29:619–24. [DOI] [PubMed] [Google Scholar]
- 63. Asemi Z, Tabassi Z, Samimi M, Fahiminejad T, Esmaillzadeh A. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: a randomised clinical trial. Br J Nutr. 2013;109:2024–30. [DOI] [PubMed] [Google Scholar]
- 64. Wei X, Wang D, Yang Y, Xia M, Li D, Li G, Zhu Y, Xiao Y, Ling W. Cyanidin-3-O-β-glucoside improves obesity and triglyceride metabolism in KK-Ay mice by regulating lipoprotein lipase activity. J Sci Food Agric. 2011;91:1006–13. [DOI] [PubMed] [Google Scholar]
- 65. Jiao X, Wang Y, Lin Y, Lang Y, Li E, Zhang X, Zhang Q, Feng Y, Meng X, Li B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J Nutr Biochem. 2019;64:88–100. [DOI] [PubMed] [Google Scholar]
- 66. Morissette A, Kropp C, Songpadith J-P, Junges Moreira R, Costa J, Mariné-Casadó R, Pilon G, Varin TV, Dudonné S, Boutekrabt Let al. . Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2020;318:E965–E80. [DOI] [PubMed] [Google Scholar]
- 67. Wang H, Hong T, Li N, Zang B, Wu X. Soluble dietary fiber improves energy homeostasis in obese mice by remodeling the gut microbiota. Biochem Biophys Res Commun. 2018;498:146–51. [DOI] [PubMed] [Google Scholar]
- 68. Van Hul M, Karnik K, Canene-Adams K, De Souza M, Van den Abbeele P, Marzorati M, Delzenne NM, Everard A, Cani PD. Comparison of the effects of soluble corn fiber and fructooligosaccharides on metabolism, inflammation, and gut microbiome of high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2020;319:E779–E91. [DOI] [PubMed] [Google Scholar]
- 69. Jovanovski E, Khayyat R, Zurbau A, Komishon A, Mazhar N, Sievenpiper JL, Blanco Mejia S, Ho HVT, Li D, Jenkins ALet al. . Should viscous fiber supplements be considered in diabetes control? Results from a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2019;42:755–66. [DOI] [PubMed] [Google Scholar]
- 70. Zhang C, Liu S, Solomon CG, Hu FB. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care. 2006;29:2223–30. [DOI] [PubMed] [Google Scholar]
- 71. Hull HR, Herman A, Gibbs H, Gajewski B, Krase K, Carlson SE, Sullivan DK, Goetz J. The effect of high dietary fiber intake on gestational weight gain, fat accrual, and postpartum weight retention: a randomized clinical trial. BMC Pregnancy Childbirth. 2020;20:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liese AD, Schulz M, Fang F, Wolever TM, D'Agostino RB Jr, Sparks KC, Mayer-Davis EJ. Dietary glycemic index and glycemic load, carbohydrate and fiber intake, and measures of insulin sensitivity, secretion, and adiposity in the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2005;28:2832–8. [DOI] [PubMed] [Google Scholar]
- 73. McIntosh M, Miller C. A diet containing food rich in soluble and insoluble fiber improves glycemic control and reduces hyperlipidemia among patients with type 2 diabetes mellitus. Nutr Rev. 2009;59:52–5. [DOI] [PubMed] [Google Scholar]
- 74. White SL, Pasupathy D, Sattar N, Nelson SM, Lawlor DA, Briley AL, Seed PT, Welsh P, Poston L. Metabolic profiling of gestational diabetes in obese women during pregnancy. Diabetologia. 2017;60:1903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Basu A, Alaupovic P, Wu M, Jenkins AJ, Yu Y, Nankervis AJ, Hanssen KF, Scholz H, Henriksen T, Lorentzen Bet al. . Plasma lipoproteins and preeclampsia in women with type 1 diabetes: a prospective study. J Clin Endocrinol Metab. 2012;97:1752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Alvarez JJ, Montelongo A, Iglesias A, Lasunción MA, Herrera E. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J Lipid Res. 1996;37:299–308. [PubMed] [Google Scholar]
- 77. Kahr MK, Suter MA, Ballas J, Ramin SM, Monga M, Lee W, Hu M, Shope CD, Chesnokova A, Krannich Let al. . Geospatial analysis of food environment demonstrates associations with gestational diabetes. Am J Obstet Gynecol. 2016;214:110.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bryant M, Stevens J, Wang L, Tabak R, Borja J, Bentley ME. Relationship between home fruit and vegetable availability and infant and maternal dietary intake in African-American families: evidence from the Exhaustive Home Food Inventory. J Am Diet Assoc. 2011;111:1491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.