Abstract
Objective:
Obesity is a major public health challenge, and the US military veteran population is disproportionately affected. Using de-identified records from a local weight management clinic and a national clinical data repository, we assessed obesity pharmacotherapy use and effectiveness for weight loss and obesity comorbidities in this vulnerable population.
Methods:
During the initial year of the local clinic, we found 43 records with monthly follow-up of MOVE! lifestyle intervention augmented by obesity pharmacotherapy. Nationally, we identified over 2 million records of prescribed obesity pharmacotherapy compared to metformin as control. We selected records with detailed documentation of weight trends from 1 year before to 1 year after prescription date for further analysis.
Results:
The most commonly prescribed medications in the local clinic were metformin, liraglutide and combination phentermine/topiramate. On average, we observed −4.0±2.1 kg weight loss over the initial 6 month intervention. In the national cohort, 577,491 records were identified with an obesity or control metformin prescription and adequate weight documentation. The most effective pharmacotherapy in the national cohort was phentermine/topiramate (−0.0931±0.0198 kg/week difference), followed by liraglutide, lorcaserin and orlistat.
Conclusions:
Obesity pharmacotherapy is effective in achieving clinically meaningful weight loss in veterans as part of an integrated care approach.
Keywords: veterans, obesity, pharmacotherapy, virtual cohort, weight loss
INTRODUCTION
Excess weight, comprising the overweight and obese categories, is the most important preventable cause of disease in the United States, and is estimated to affect over 70% of adults in 2013-2014, as well as 2015-2016 (1, 2). Obesity is associated with multiple comorbidities, including joint disease, sleep apnea, hypertension, dyslipidemia, fatty liver disease, type 2 diabetes, coronary artery disease, cerebrovascular disease and many cancers, and it is also associated with all-cause mortality (3, 4, 5). Excess weight disproportionately affects Veterans, at least those who receive primary care through the VA system; in particular, this group has been studied for the VA’s fiscal year 2014 (6). In that study, the estimated prevalence of overweight or obesity in Veterans was 78%, and was accompanied by higher rates of comorbid conditions including, hypertension, dyslipidemia, and type 2 diabetes.
To address this problem, the Veterans Health Administration of the United States Department of Veterans Affairs (VA) implemented a multimodal population-based behavioral weight management program called MOVE!, which combines individual coaching (delivered in person, via telephone and text messaging) and group counseling to provide veterans with lifestyle guidance and nutritional education. The program offers participants food journals, scales, use of a mobile app for diet, exercise, and weight tracking and virtual coaching, as well as access to exercise facilities at participating VA centers (7). Despite provision of intensive behavioral management for weight loss in the form of MOVE!, less than 10% of eligible patients attended any in-person sessions (8, 9). Lack of structure following military service, as well as the misconception that physical activity has more impact than diet for weight loss might be important factors limiting participation in MOVE! (10). Several studies examined the efficacy of the MOVE! program and have shown that participation in the program has been associated with modest short-term weight loss, with an average of 0.95 kg to 1.84 kg at 6 months and 0.13 kg to 3.3 kg at 1 year, and greater weight loss as participation increases (11) (12, 13).
For Veterans who do not meet clinically significant weight loss with behavioral intervention (i.e. MOVE!), the VA DOD Guidelines for Management of Overweight and Obesity call for therapy intensification with pharmacotherapy or bariatric surgery. To address this unmet clinical need at our local medical center, we started the NY-MOVE Endocrinology Weight Management clinic in September 2017. This clinic was designed to provide a multidisciplinary, team centered approach to care. The team is comprised of nutritionists, psychologists, nurses and physicians trained or training in internal medicine, endocrinology and obesity medicine. Referral to the clinic is broadly available to all disciplines at the medical center and based simply on BMI>30, prior or current participation in MOVE!, and willingness to meet monthly with the clinical team. This clinic also provides a powerful setting to perform clinical research to help veterans with obesity comorbidities. We practice motivational interviewing and weight-centric management of type 2 diabetes based on treating glycemia and weight concurrently. In this context, we sought to benchmark our results to those of obesity medicine practices in the VA system.
Multiple new pharmacologic agents and combinations have been approved by the FDA for use in medical weight management and have been shown to be effective compared to placebo (14, 15). Weight management pharmacotherapy is available within the VA system, but the rate of utilization is low and comparable to that in the general population (16). Semla et al. revealed that in 2014-2015, approximately 2500 veterans (2% of veterans) eligible for an anti-obesity drug received a prescription (13). Similarly, in a retrospective cohort study done between 2013 and 2016, Thomas and colleagues showed that 1.1% of VA patients (N=153,939) eligible for an anti-obesity medication through the MOVE program received it (17). A likely reason for the low rates of utilization is that most weight-management medications require prior approval before dispensing. Each medication requires the absence of contraindications, BMI ≥30 kg/m2 (or ≥27 kg/m2 with an obesity-related comorbidity), and prior participation in the VA MOVE! program (or equivalent).
Moreover, there have been few head-to-head trials comparing the effectiveness of weight management pharmacotherapy, particularly in veterans. Grabarczyk (2018), in a retrospective cohort study using the VA Corporate Data Warehouse, compared the effectiveness of anti-obesity medications used in Veterans participating in the MOVE! Program between 2012 and 2016 and showed that the percentage of weight loss from baseline after 20 weeks was similar but higher in patients taking phentermine-topiramate compared to lorcaserin, phentermine, and orlistat (4.1%, 3.6%, 3.6% and 2.1% respectively) (18). Interestingly, in a retrospective case-control study of Veterans participating in the MOVE! program in San Diego, Desalermos et al. (2019) confirmed that the concomitant use of obesogenic medications was associated with worse weight loss outcomes, and patients were 37% less likely to achieve weight loss amounting to 5% or more of total body weight (19).
In the present study, by retrospective examination of de-identified records from a local weight management clinic and a national clinical data repository, we assessed obesity pharmacotherapy use and its real-world effectiveness for weight loss and improvement of comorbid metabolic parameters in this vulnerable population. The primary objective for this study was to assess the rate of weight loss (in kg/week) before and after weight management intervention in subjects from our local clinic, and compare these to the national cohort. Secondary objectives included comparisons of effectiveness between individual anti-obesity agents in this veteran cohort. Of note, this study included lorcaserin, which was marketed during the time period we examined, but has subsequently been taken off the market in the USA due to safety concerns. Since data on the real-world efficacy of lorcaserin may still be of interest, we include it in this report.
METHODS
Retrospective de-identified data collection and analysis for the local and virtual cohort is approved by the VA NY Harbor Healthcare System IRB under protocol 07133. Since its inception in 2017, practitioners at the weight management clinic at the Manhattan campus of the New York Harbor VA Healthcare System have employed a structured format to document data pertinent to the routine clinical care of their patients within their clinical notes. We compiled this data via chart review and found 43 records with monthly follow-up of MOVE lifestyle intervention augmented by obesity pharmacotherapy at 1 year time point. The obesity pharmacotherapies used at the clinic are liraglutide, lorcaserin (while it was on the market), orlistat, and phentermine-topiramate; patients with dysglycemia are also offered a trial of metformin if appropriate, although it is not labeled for weight management. We also noted whether patients were prescribed bupropion, topiramate alone, or empagliflozin. Data was captured in de-identified fashion in a customized RedCap database. The unit of assignment for this study is the individual de-identified subject. This sample size was determined by feasibility considerations for this local pilot study.
To compare data at the level of the national system, we queried the Veterans Affairs Corporate Data Warehouse for records before January 2019. We first queried for unique patients who have been prescribed any medication from a specified list, which included orlistat, metformin, liraglutide, bupropion-naltrexone, lorcaserin and phentermine-topiramate, canagliflozin, dapagliflozin and empagliflozin, with glimepiride, glipizide, glyburide, and pioglitazone as control anti-diabetic agents hypothesized to cause weight gain. We further noted other medication of possible interest which included liraglutide branded for weight loss (“Saxenda”), semaglutide (approved as injectable formulation during the time period studied) and phentermine monotherapy. We noted the date of first prescription for each patient for each agent, and counted single agents as well as combinations that included the single agents of interest. Next, we queried for each patient’s weight as documented within 800 days before or after each medication initiation, which we defined as the first date of release of a medication by a VA pharmacy to a patient. We augmented this data with patient-specific and date-of-first-prescription-specific details including patient sex and prescribing facility. We excluded data labeled as test data, incomplete data, data labeled as erroneous, and a very small number of data points we considered physiologically impossible or extremely implausible.
We analyzed weight data computationally using R, a language and environment for statistical computing, version 3.6.1. We computed weight slopes over specified time periods by ordinary least-squares regression as the covariance of weight and time divided by the variance of time. We computed weight first differences, for each medication initiation event, by starting with the weight at each time point and subtracting the weight at the most recent prior time point; we further scaled these first differences to their respective time intervals. Separately, we transformed each set of weight data into a continuous trend by using simple linear interpolation to estimate the patients’ weight at 30-day intervals based on the nearest recorded data points before and after the interpolated time points, excluding individuals without data documented both before and after the interpolated times.
To estimate glycemic status, we used an approach based on HgbA1c and pharmacy records. Patients were classified based on whether they were prescribed insulin, non-insulin anti-diabetic medications and not insulin, or no anti-diabetic medications in the year prior to initiating the medication under study. Patients with at least two HgbA1cs during that year >=6.5% were moved from the third group to the second group. The remainder of the third group was divided between those who had at least one HgbA1c >=5.7% recorded and those who did not. There criteria were inspired by clinical diagnostic criteria for type 2 diabetes and prediabetes.
RESULTS
Local weight management clinic
During the initial study period in the local weight management clinic, we found 43 records with monthly follow-up of MOVE lifestyle intervention augmented by obesity pharmacotherapy (Tables 1-2). The mean age in our cohort was 54.5±13.7 years; 79% (34) were male and 2 were transgender (1 female to male and 1 male to female); 51.1% (22) were black, 27.9% (12) were white, and 18.6% (8) Hispanic (Table 1). The mean BMI of our clinic patients was 40.2±6.0kg/m2, with 8 (18.6%) having class 1 obesity, 15 (34.8%) having class 2 obesity, and 19 (44.1%) having class 3 obesity. Prediabetes and type 2 diabetes mellitus were present in 38 (88.3%); the hemoglobin A1C mean was 6.4±0.8% (Table 2).
Table 1.
Baseline characteristics of the local clinic and national cohorts
| Clinic Cohort | National Cohort | |||
|---|---|---|---|---|
| Patients | number | (%) | number | (%) |
| 43 | - | 2,051,571 | - | |
| Characteristic | ||||
| Race | ||||
| White | 12 | 28.0% | 1,371,380 | 66.8% |
| Black | 22 | 51.0% | 325,239 | 15.9% |
| Asian | 1 | 2.0% | 16,045 | 0.8% |
| Native American | 0 | 0.0% | 32,334 | 1.6% |
| Missing | 0 | 0.0% | 306,573 | 14.9% |
| Ethnicity | ||||
| Not Hispanic | 35 | 81.0% | 1,710,287 | 83.4% |
| Hispanic | 8 | 19.0% | 125,215 | 6.1% |
| Declined/Unknown/Multiple | 0 | 0.0% | 70,421 | 3.4% |
| Gender/Sex | ||||
| Female | 7 | 16.0% | 104,787 | 5.1% |
| Male | 34 | 79.0% | 1,946,782 | 94.9% |
| FTM | 1 | 2.0% | - | - |
| MTF | 1 | 2.0% | - | - |
| Obesity | ||||
| Normal Weight | - | - | 55,292 | 2.7% |
| Overweight | - | - | 215,699 | 10.5% |
| Class 1 | 8 | 19.0% | 530,166 | 25.8% |
| Class 2 | 15 | 35.0% | 322,680 | 15.7% |
| Class 3 | 19 | 44.0% | 220,069 | 10.7% |
| Dysglycemia | ||||
| Prediabetes/elevated HgbA1c | 19 | 44.0% | 5,951 | 0.3% |
| Type 2 diabetes or medication | 19 | 44.0% | 1,771,992 | 86.4% |
| Type 1 diabetes/on insulin | 0 | 0.0% | 260,079 | 12.7% |
Table 2.
Quantitative characteristics of local clinic and national cohort.
| mean±95% CI | median (IQR) | Range | |
|---|---|---|---|
| Local Clinic Cohort (N=43) | |||
| Age (years) | 54.5±13.7 | 55 (49.5-65.5) | 25-75 |
| BMI (kg/m2) | 40.2±6.0 | 39.2 (35.1-45.5) | 29-53.8 |
| HgbA1c | 6.4±0.8% | 6.2% (4.75-7.1%) | 4.8-8% |
| National Cohort (N=2,051,571) | |||
| Age (years) | 62.1±11.0 | 63 (55-69) | 19-109 |
| BMI (kg/m2) | 33.4±6.45 | 32.6 (28.9–37.1) | 14.2–76.9 |
| HgbA1c | 7.4±1.5% | 7.0% (6.4-8.0%) | 0-21.6% |
CI = confidence interval, IQR = interquartile range
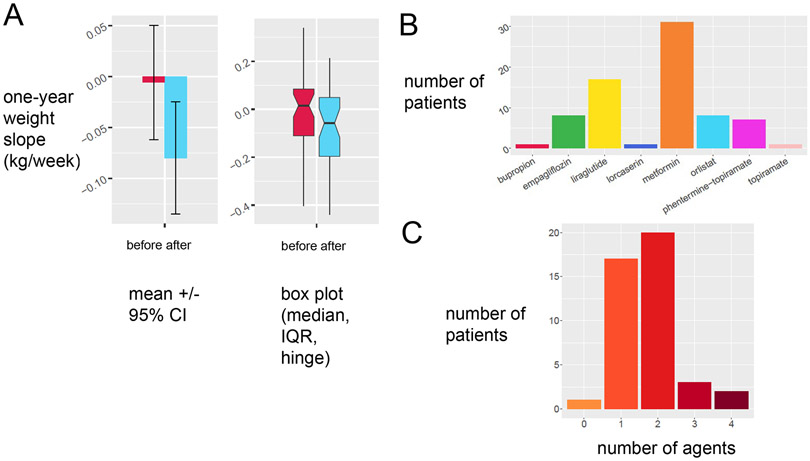
Our combined MOVE! lifestyle intervention with obesity pharmacotherapy was associated a more negative weight trajectory (Figure 1A). In other words, our clinic patients showed downtrending weights in the year subsequent than in the year prior, limited by the small sample size. This finding was present whether weight trajectories were calculated by average weight loss (Figure 1A, left panel) or median weight loss (Figure 1A, right panel). A large fraction of our patients tried two or more agents, either sequentially or in combination (Figure 1B). The most commonly prescribed medications in our local weight management clinic were metformin (31), liraglutide (18), orlistat (9) and combination phentermine-topiramate (8) (Figure 1C). We did not offer bupropion-naltrexone during this time period, but we tracked whether bupropion alone was prescribed.
Figure 1:
Obesity pharmacotherapy augments lifestyle intervention for weight loss in veterans with obesity and comorbidities. A. Retrospective deidentified inspection of records from veterans in our local weight management clinic in subjects with at least 3 data points in 6 months shows the slope of weight 6 months before intervention (red) decreases compared to slope of weight 6 months after the weight management intervention. B. Prescription patterns of the local eight management clinic by specific agent. C. Prescription patterns of the local weight management clinic by number of agents used per subject.
National Data
In the national clinical data repository, we identified 2,051,571 medication initiation events for medications approved for the treatment of obesity (Figure 2). Table 1 describes the baseline characteristics of the national VA cohort in comparison to the local VA cohort. Of note, the national VA cohort had a lower percentage of black (17.1%), Hispanic (6.6%) and female (5.1%) veterans than our local cohort, more closely reflecting the national VA system. The national VA cohort also captures Native American and Native Hawaiian populations that we did not observe in the local cohort. On the other hand, self-identified gender documentation is presently generally absent or unreliable in the national dataset. The national cohort is enriched for patients with class I obesity (32.2%) and Type 2 Diabetes (82.6%), but we find obesity medication prescriptions at normal weight and overweight. Quantitatively, the national cohort was older (mean age 62.1±11.0 years) and leaner (mean BMI 33.4+/−6.4 kg/m2) than our local cohort, with more severe dysglycemia (mean A1c 7.4±.5%). Of these nearly 2 million records, we further filtered those prescribed bupropion-naltrexone (853), liraglutide (34,627), lorcaserin (775), orlistat (22,948), and phentermine-topiramate (1629), all of which are FDA-approved for weight management. We also included metformin (811,206 records) and empagliflozin (9,870 records), agents we considered likely to be associated with weight reduction although not specifically approved for this indication. Due to low patient numbers, we chose not to analyze prescriptions of canagliflozin and dapagliflozin. We selected over 800,000 records with detailed documentation of weight trends from 1 year before to 1 year after prescription date for further analysis and calculated average slope of weight during the intervals before and after the prescription of interest.
Figure 2:
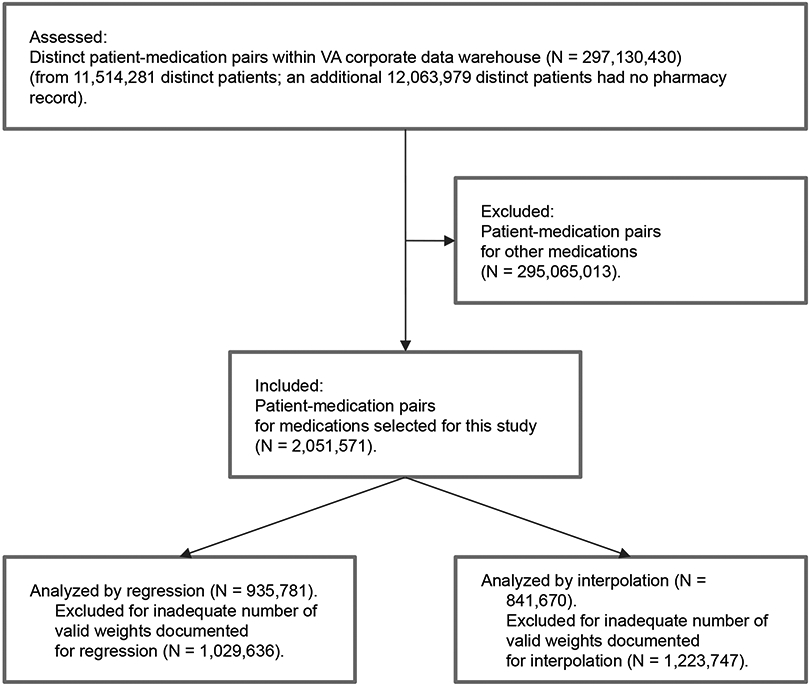
TREND Diagram analysis of a virtual weight management cohort in the VA Corporate Data Warehouse.
Initial survey of the weight trends shows that response to weight-negative medications or obesity medications is heterogeneous. For instance, while metformin is associated with weight maintenance in the Diabetes Prevention Study, we observe a significant number of medication initiation events with negative weight slope before prescription followed by positive weight slope after prescription. On average, records with metformin prescription showed a mean difference of −0.0340 kg/ week (95% CI −0.0390 – −0.0291) following intervention. This tendency is less pronounced in more effective obesity medications such as lorcaserin (Data not shown).
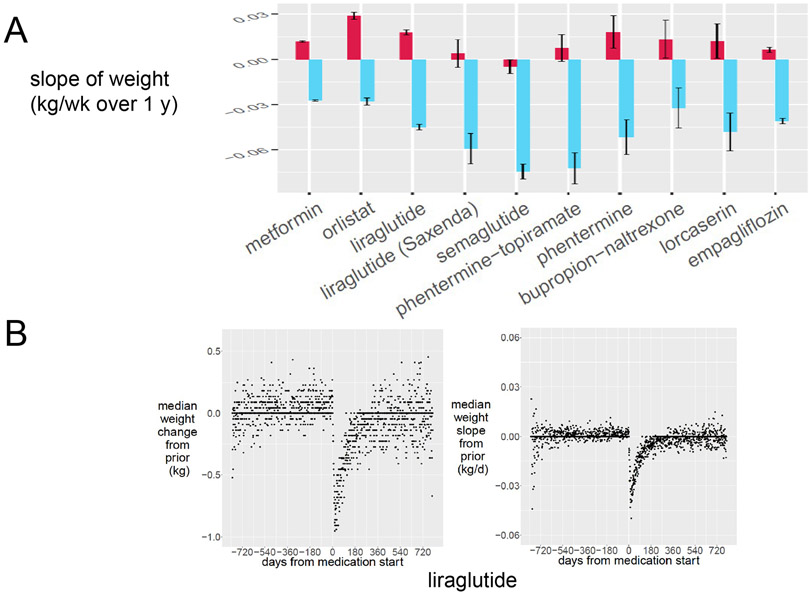
We next compared the average weight slopes for obesity medication initiation events over the 20 years of prescription data available in the VA Informatics and Computational Infrastructure database (Figure 3A). Interestingly, metformin prescription showed a −0.0273±0.0005 kg/ week difference following intervention, and empagliflozin showed a −0.0403±0.0016 kg/ week difference in weight. The most effective obesity pharmacotherapy in the national data set was phentermine-topiramate (mean −0.0931, 95% CI −0.1129– −0.0733 kg/week difference), followed by liraglutide, lorcaserin, orlistat and bupropion-naltrexone (Figure 3A). Phentermine monotherapy was prescribed infrequently, specifically 1,177 instances within the 20 years of prescription data available. While only the liraglutide 3.0 mg dose (Saxenda) is FDA-approved for treatment of obesity, both the 1.8 mg dose (31,830 prescriptions) and 3.0 mg dose (2,797 prescriptions) show similar effects in terms of decreasing weight slope. As expected, phentermine monotherapy, empagliflozin, and (injectable) semaglutide also all showed trends toward weight loss, in contrast with medications like glimepiride and pioglitazone which are not considered to promote weight loss (Supplemental Figure 1). Newer GLP-1 agonists such as lixisenatide and semaglutide similarly show few prescriptions, but semaglutide prescriptions are increasing dramatically, with 3,642 prescriptions since its approval in 2019. We include the detailed slope analysis for all medications surveyed in Supplemental Figure 1. First-difference analysis confirms a weight-loss signal correlated with initiation of each one of these therapies, with Figure 3B highlighting the example of liraglutide. Both median weight in kg (left panel) and median weight slope changes in kg/d (right panel) over 2 years prior and subsequent to liraglutide prescription reflect a negative deflection in their respective curves. This weight loss signal is absent for sulfonylureas and inverted for PPAR-γ agonists.
Figure 3:
Obesity pharmacotherapy treatment alters weight trends in the VA national cohort. (A) For subjects with at least 3 data points in 6 months, the slope of weight 6 months before (red) is compared to the slope of weight 6 months after the designated prescription (blue). Error bars represent the 95% confidence interval (B) Population response from days of medication start to weight negative agent liraglutide in absolute weight (kg, left panel) or slope of weight (kg/d, right panel).
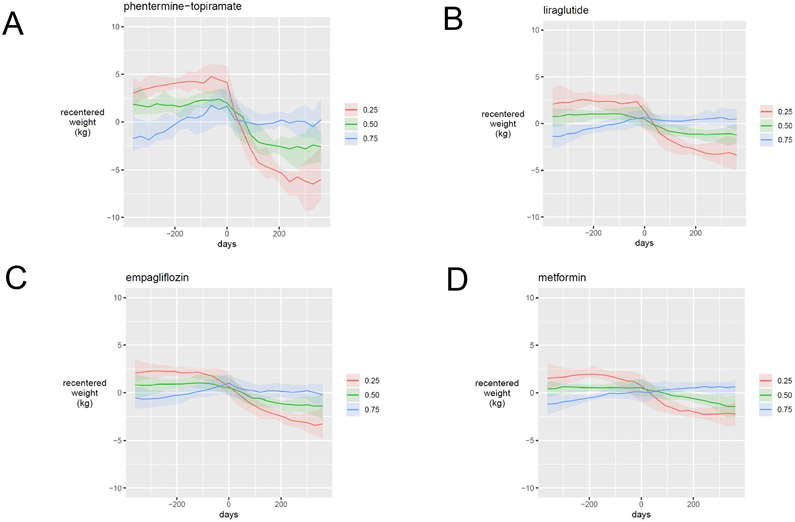
Since weight change is unlikely to remain linear over even a 1- or 2-year interval in most patients, we used linear interpolation to estimate patients’ weights at defined time points, re-centered against each patient’s own time-averaged weight (Figure 4). To compare weight loss in absolute terms for patients and groups of patients, we determined the median re-centered weights at defined time points for subpopulations of interest treated with phentermine-topiramate (Fig 4A), liraglutide (Fig 4B), empagliflozin (Fig 4C) and metformin (Fig 4D). Line plots are ordered based on average change in weight loss rate, with phentermine-topiramate being the greatest and metformin the least. Each panel highlights the median weight trajectories before and after medication initiation for patients in a window around the 25th percentile (red line), 50th percentile (green line) and 75th percentile (blue line) of weight loss. Across all prescriptions, the 25th percentile subjects gained weight after prescription of the selected agent, while the 75th percentile subjects lost as little as 2.5 kg with metformin and as much as 5.0 kg with phentermine-topiramate over the 365 days following prescription. Empagliflozin is not approved for treatment of obesity, but placebo-controlled studies show negative weight trends comparable to those demonstrated in this virtual cohort.
Figure 4:
Weight trends in response to obesity pharmacotherapy are heterogeneous in the national veteran cohort. Recentered weights trends of selected obesity pharmacotherapy agents by response quartile. Subjects with adequate weight trend data were divided in quartiles of response from highest to lowest area under the curve weight change within 6 months of pharmacotherapy administration. The 25th percentile curve (red) represents the best response, 50th percent response curve (green) represents the median and 75 percentile response curve (blue) represents the worst response. Phentermine-topiramate (A) shows the best response and highest variation, followed by liraglutide (B), empagliflozin (C) and metformin (D).
DISCUSSION
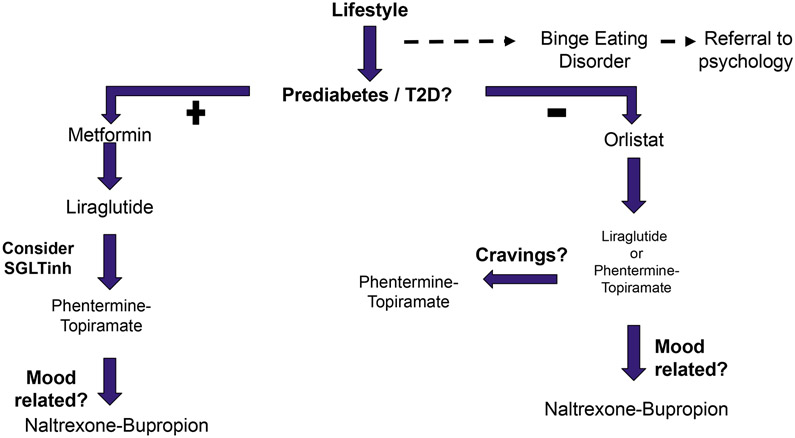
This retrospective analysis of de-identified veteran medical records validated the efficacy of weight management pharmacotherapy in actual clinical practice, while also confirming substantial inter-individual variability within this patient population. In addition to confirming weight loss rates similar to placebo-controlled studies for obesity pharmacotherapy (14), we also observed weight negative effects of diabetes medications such as metformin (1) and empagliflozin (7) in the VA virtual cohort. Selecting among options for medical therapy involves assessing a patient’s goals and preferences and possible (patient-specific) benefits and risks of therapeutic options, as well as costs. Taking into account specific, serious contraindications to specific therapies and the obvious advantages of choosing agents that offer dual benefits (such as to treat obesity as well as diabetes), we suggest a specific algorithm for selecting agents for weight management pharmacotherapy (Figure 5). We exclude lorcaserin from the algorithm because it has been taken off the market in the USA subsequent to our study interval due to safety concerns. Our algorithm, in harmony with guidance for weight management generally and in the VA system specifically, recognizes lifestyle optimization as the foundation of weight management. In addition, we recognize the importance of optimizing the patient’s overall medical regimen to avoid or limit obesogenic therapies and, where possible, to favor agents with a weight benefit instead. With respect to pharmacotherapy specifically intended for weight loss, we similarly propose, after excluding agents with unacceptably high risks, that agents expected to provide a dual benefit be offered first. Specifically, we offer diabetes medications with weight management effects to patients with both prediabetes or Type 2 diabetes mellitus and need for weight management. This part of our pathway overlaps with the American Diabetes Association Standard of Care Recommendations for Obesity Management for the Treatment of Type 2 Diabetes (20). We recognize binge eating disorder responds poorly to weight management pharmacotherapy and should be addressed before such therapy is considered. Next, we propose that the options for weight management with the empirically strongest effects be offered, with a plan to substitute or add agents sequentially if the initial agent is poorly tolerated or not effective, in a manner similar to AACE recommendations (21, 22).
Figure 5:
Proposed algorithm for pharmacological management of obesity in the Veterans Affairs system. For patient with prediabetes and type 2 diabetes comorbidities, we select weight-neutral or weight negative agents in order of safety and effectiveness largely aligned with the 2019 ADA guidelines. For patients without dysglycemia, we select orlistat initially as the safest long term use agent, then move to appetite suppressant agents by order of effectiveness in the virtual cohort.
Based on the information obtained in this study, we propose an algorithm for deployment of obesity pharmacotherapy in the context of medical weight management of veterans presented in Figure 5. This algorithm recognizes the importance of diabetes and prediabetes comorbidities to the management of obesity, and stratifies use of glycemic control agents to this population. After an initial attempt of 12 weeks for a given agent, we follow AACE guidelines in terms of discontinuation, addition or continuation of the selected agent (21, 22). We further use agents with fewer side effects first, for instance orlistat in normoglycemic subjects, before escalating care. Orlistat monotherapy as an initial choice may be somewhat controversial, but given its effectiveness borne out of our analysis and its long history of use with an excellent safety profile, we feel it should be offered first to patients who can tolerate it and potentially benefit. This effort represents a first step in standardizing obesity pharmacotherapy in the Veterans Affairs system beyond patient individualization.
Strengths of our study include characterizing an underserved population with male predominance different from most weight loss studies. Another strength is access to a large numbers of subjects through the VA Corporate Data Warehouse to assess the effects of multiple medications. On the other hand, our studies had some weaknesses. Most importantly these were retrospective analyses in non-randomized, non-blinded patient populations. Moreover, in our analysis of data from the national system, it should not be assumed the patients were prescribed the different agents within similar clinical contexts. While many of the agents we studied are indicated and typically prescribed only for weight management, some of the most commonly used ones – notably liraglutide and metformin – are more frequently prescribed for glycemic management, which is a difference we have not attempted to account for. Specifically for liraglutide doses of 1.8 mg versus 3 mg daily, our analysis was limited by infrequent prescription of the 3 mg dose.
Conclusion
Retrospective analysis of de-identified veteran medical records validates the efficacy of weight management pharmacotherapy in actual clinical practice over a follow-up time of up to 2 years, and also confirms substantial inter-individual variability within the veteran patient population. The insights gained from the rich and detailed data gathered on a limited number of patients within a single research-oriented clinic can complement the insights gained from evaluating the more generic data available on a very large pool of patients within the national system. Models identified and hypotheses generated by these approaches will be valuable starting points for prospective studies.
Supplementary Material
Supplemental Figure 1: Detailed weights trends before and after obesity pharmacotherapy treatment in all agents evaluated. For subjects with at least 3 data points in 6 months, the slope of weight 6 months before (red) is compared to the slope of weight 6 months after the designated prescription (blue). Error bars represent the 95% confidence interval.
STUDY IMPORTANCE QUESTIONS.
-What is already known about this subject?
Veterans are disproportionately affected by obesity and related disease. Obesity pharmacotherapy is underprescribed in this population.
- What are the new findings in your manuscript?
We report on the clinical effectiveness of obesity pharmacotherapy in a local and national cohort of veterans with obesity in regards to weight loss and weight loss rates across 6 months prior to and 6 months after prescription, in addition to MOVE! lifestyle intervention.
- How might your results change the direction of research or the focus of clinical practice?
Our results support continued prescription of obesity pharmacotherapy in veterans with obesity and comorbid disease, and introduce an algorithm for prescription.
ACKNOWLEDGMENTS
We are grateful to our patients and clinic staff without whom this work would not have been possible, as well as to Ira Goldberg for his support and to Louis J. Aronne and Andrew J. Kelly for helpful discussion.
FUNDING
JP has been supported financially by NIH NHLBI institutional training grant T32HL98129. JOA has been supported financially by the Doris Duke Charitable Foundation, the American Heart Association 17-SFRN33490004 and NIH K08 DK117064. JP, JOA conceived the design if the study. FV, AP, MG and JOA contributed clinical data to the local repository. AP, MC, CT, JOA designed the data capture framework for the local repository. JP executed analyses with the national data repository. All authors reviewed and approved the final manuscript.
Footnotes
DISCLOSURES
The authors declare no conflicts of interest relevant to this work.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine 2002;346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Consortium LWGftL, Belle SH, Chapman W, Courcoulas AP, Flum DR, Gagner M, et al. Relationship of body mass index with demographic and clinical characteristics in the Longitudinal Assessment of Bariatric Surgery (LABS). Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2008;4: 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebbert JO, Elrashidi MY, Jensen MD. Managing overweight and obesity in adults to reduce cardiovascular disease risk. Current atherosclerosis reports 2014;16: 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. The New England journal of medicine 2016;375: 794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alemán JO, Iyengar N, Walker J, Milne GL, Correa da Rosa J, Liang Y, et al. Effects of Rapid Weight Loss on Systemic and Adipose Tissue Inflammation and Metabolism in Obese Postmenopausal Women. Journal of the Endocrine Society 2017;1: 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breland JY, Phibbs CS, Hoggatt KJ, Washington DL, Lee J, Haskell S, et al. The Obesity Epidemic in the Veterans Health Administration: Prevalence Among Key Populations of Women and Men Veterans. Journal of general internal medicine 2017;32: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. The New England journal of medicine 2015;373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 8.Kahwati LC, Lance TX, Jones KR, Kinsinger LS. RE-AIM evaluation of the Veterans Health Administration's MOVE! Weight Management Program. Translational behavioral medicine 2011;1: 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Littman AJ, Boyko EJ, McDonell MB, Fihn SD. Evaluation of a weight management program for veterans. Preventing chronic disease 2012;9: E99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jay M, Mateo KF, Squires AP, Kalet AL, Sherman SE. Military service and other socioecological factors influencing weight and health behavior change in overweight and obese Veterans: a qualitative study to inform intervention development within primary care at the United States Veterans Health Administration. BMC obesity 2015;3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das SR, Kinsinger LS, Yancy WS Jr., Wang A, Ciesco E, Burdick M, et al. Obesity prevalence among veterans at Veterans Affairs medical facilities. American journal of preventive medicine 2005;28: 291–294. [DOI] [PubMed] [Google Scholar]
- 12.Noel PH, Copeland LA, Pugh MJ, Kahwati L, Tsevat J, Nelson K, et al. Obesity diagnosis and care practices in the Veterans Health Administration. Journal of general internal medicine 2010;25: 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semla TP, Ruser C, Good CB, Yanovski SZ, Ames D, Copeland LA, et al. Pharmacotherapy for Weight Management in the VHA. Journal of general internal medicine 2017;32: 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism 2015;100: 342–362. [DOI] [PubMed] [Google Scholar]
- 15.Veterans_Affairs UDo. VA/DoD Clinical Practice Guidelines: Management of Obesity and Overweight (OBE) (2014). 2015. [Google Scholar]
- 16.Saxon DR, Iwamoto SJ, Mettenbrink CJ, McCormick E, Arterburn D, Daley MF, et al. Antiobesity Medication Use in 2.2 Million Adults Across Eight Large Health Care Organizations: 2009-2015. Obesity 2019;27: 1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas DD, Waring ME, Ameli O, Reisman JI, Vimalananda VG. Patient Characteristics Associated with Receipt of Prescription Weight-Management Medications Among Veterans Participating in MOVE! Obesity 2019;27: 1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grabarczyk TR. Observational Comparative Effectiveness of Pharmaceutical Treatments for Obesity within the Veterans Health Administration. Pharmacotherapy 2018;38: 19–28. [DOI] [PubMed] [Google Scholar]
- 19.Desalermos A, Russell B, Leggett C, Parnell A, Ober K, Hagerich K, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity 2019;27: 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes A. 8. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes care 2020;43: S89–S97. [DOI] [PubMed] [Google Scholar]
- 21.Mechanick JI, Hurley DL, Garvey WT. Adiposity-Based Chronic Disease as a New Diagnostic Term: The American Association of Clinical Endocrinologists and American College of Endocrinology Position Statement. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2017;23: 372–378. [DOI] [PubMed] [Google Scholar]
- 22.Mechanick JI, Garber AJ, Grunberger G, Handelsman Y, Garvey WT. Dysglycemia-Based Chronic Disease: An American Association of Clinical Endocrinologists Position Statement. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2018;24: 995–1011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Detailed weights trends before and after obesity pharmacotherapy treatment in all agents evaluated. For subjects with at least 3 data points in 6 months, the slope of weight 6 months before (red) is compared to the slope of weight 6 months after the designated prescription (blue). Error bars represent the 95% confidence interval.