Abstract
The prognostic value of Ki-67 in nasopharyngeal carcinoma (NPC) was controversial according to previous studies. We aimed to clarify the association between K-67 expression and survival in NPC through meta-analysis. We conducted a meta-analysis to explore the potential prognostic effect of Ki-67 on overall survival (OS), disease-free survival (DFS), distant metastasis-free survival (DMFS), and local recurrence-free survival (LRFS) in NPC. A total of 13 studies comprising 1314 NPC patients were included. High Ki-67 expression was associated with poor OS (hazard ratio [HR]= 2.70, 95% confidence interval [CI]= 1.97–3.71, P<0.001), DFS (HR = 1.93, 95% CI = 1.49–2.50, P<0.001), and LRFS (HR = 1.86, 95% CI = 1.11–3.12, P=0.019). However, there was no significant association between Ki-67 and DMFS (HR = 1.37, 95% CI = 0.78–2.38, P=0.270). Furthermore, the prognostic role of Ki-67 was maintained throughout different sample sizes, analyses of HR, and study designs for OS and DFS in various subgroups. Elevated Ki-67 expression is a reliable prognostic factor for poorer survival outcomes in NPC.
Keywords: evidence-based medicine, Ki-67, meta-analysis, nasopharyngeal carcinoma, prognostic factors
Introduction
Nasopharyngeal carcinoma (NPC) is a rare cancer that originates from the lining of the nasopharynx [1]. The incidence of NPC is distinguished geographically; it is relatively high in Southeast Asia but low in Western countries [2]. The management of NPC chiefly depends on the disease status. For non-metastatic disease, radiotherapy is the mainstay treatment strategy [3]. For metastatic and locally recurrent disease, chemoradiotherapy and systemic therapies are the current therapeutic modalities [4]. However, over 20% of patients with NPC develop distant metastasis or recurrence after initial treatment, resulting in a poor prognosis [5]. Prognostic markers, such as tumor-node-metastasis (TNM) staging system and Epstein–Barr viral (EBV) DNA load, are widely used for prognostication and are required for the clinical management of patients with NPC. However, these parameters do not provide adequate prognostic information for individual patients. Therefore, there is an urgent need to develop valid prognostic factors for NPC.
Sustaining proliferative signaling is a hallmark of cancer cells, and tumor proliferation markers can provide a prognosis for patients [6]. Ki-67 is one of the most common proliferation markers [7] which can be detected in the cell nuclei during all phases of the cell cycle (G1, S, G2, and mitosis) [8]. Ki-67 has been widely investigated as a prognostic indicator in various cancers, including non-muscle invasive bladder [8], ovarian [9], gastric [10], breast [11], and non-small cell lung cancer [12]. A variety of studies reported the prognostic value of Ki-67 in patients with NPC; however, the results were inconsistent [13–15]. Therefore, we comprehensively and systematically searched for eligible studies to clarify the prognostic role of Ki-67 in patients with NPC.
Materials and methods
Study guidelines and ethics
We performed the present meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [16]. Ethical approval was not necessary because the present study did not involve patient consent.
Literature search
The literature search was conducted from the inception of the present study to 19 June 2020. We retrieved the electronic databases of PubMed, Web of Science, Embase, Scopus, and The Cochrane Library. The following search terms were used: ‘Ki-67,’ ‘Ki67,’ ‘MIB-1,’ ‘prognosis,’ ‘prognostic,’ ‘survival,’ ‘outcome,’ ‘nasopharyngeal carcinoma,’ ‘nasopharyngeal cancer,’ and ‘nasopharyngeal neoplasms.’ We manually examined the reference lists of relevant literature to identify eligible studies.
Selection criteria
The inclusion criteria were as follows: (1) studies evaluating the association between Ki-67 expression and survival in patients with NPC; (2) Ki-67 detection in tumor tissue using immunohistochemistry (IHC); (3) hazard ratios (HRs) and 95% confidence intervals (CIs) for survival outcomes were provided in text or could be calculated; (4) a cutoff value was identified to stratify high and low Ki-67 expression; (5) published in English. Studies that did not meet all the inclusion criteria were excluded. Two reviewers (Y.l.L. and L.Y.) independently evaluated candidate studies, and all disagreements were resolved by consensus.
Data extraction
Two investigators (Y.l.L. and L.Y.) extracted the data of the eligible studies independently with a predefined form. All discrepancies were resolved by discussion with a third investigator (X.L.). Extracted data included the name of the author, year of publication, study location, survival outcomes, TNM stage, treatment method, sample size, study design, and analysis of HR. Overall survival (OS) was the primary endpoint. Disease-free survival (DFS), distant metastasis-free survival (DMFS), and local recurrence-free survival (LRFS) were secondary endpoints.
Quality assessment
The Newcastle–Ottawa Scale (NOS) [17] was employed to assess the quality of the methodology used in the included studies. It contains three domains: selection of patients (0–4 points), comparability of cohorts (0–2 points), and outcome assessment (0–3 points). NOS scores of at least 6 were considered high quality.
Statistical analysis
The association between Ki-67 and OS, DFS, DMFS, and LRFS was evaluated by combining HRs and 95% CIs of included studies. HR > 1 without a 95% CI overlapping 1 indicated that overexpression of Ki-67 was the prognostic risk factor, and HR < 1 without a 95% CI overlapping 1 was a protective factor. Statistical heterogeneity was calculated according to Higgins I2 statistic and Cochran’s Q test. The I2 values > 50% or P of heterogeneity < 0.1 were considered significant heterogeneity, and consequently the random-effects model was adopted; if not, the fixed-effects model was selected. We performed subgroup analyses stratified by clinical variables including geographical region, TNM stage, treatment, sample size, cutoff value, analysis of HR, and study design for OS and DFS. Publication bias was detected using Begg’s rank correlation test and Egger’s linear regression test. Stata statistical software (version 12.0; Stata Corp, College Station, TX, U.S.A.) was used to analyze the extracted data. A P-value <0.05 was considered significant.
Results
Study search
Initially, 542 studies were retrieved from the databases, and 283 studies remained after duplicates were removed. By examining titles and abstracts, 254 studies were discarded, leaving 29 studies for full-text evaluation. Sixteen studies were excluded for the following reasons: no survival analysis (n=13), no data for Ki-67 (n=2), and no IHC method (n=1). Finally, 13 studies that met the inclusion criteria were included in the present meta-analysis [13–15,18–27] (Figure 1).
Figure 1. Flow diagram of the literature identification process.
Characteristics of included studies
The included studies were published between 2000 and 2019 (Table 1). The studies were conducted in six countries/regions, including China (n=8) [19,14,22–27], Tunisia (n=1) [13], Taiwan (n=1) [18], Greece (n=1) [20], Turkey (n=1) [21], and Japan (n=1) [15]. Two studies [19,20] were prospective trials, and eleven studies [13–15,18,21–27] had a retrospective study design. Nine studies [13,14,18,21–26] recruited patients with TNM stages I–IV, two studies [19,27] with TNM stages III–IV, and two studies [20,15] with TNM stages II–IV. A total of 1314 patients were included in the meta-analysis. Five studies provided multivariate HRs [13,14,20,26,27], and eight studies presented univariate HRs [15,18,19,21–25]. The cutoff values of Ki-67 were not uniform between eligible studies: ≥10% (n=4) [14,21,22,27], ≥5% (n=3) [13,19,20], ≥50% (n=3) [15,23,24], ≥25% (n=1) [25], ≥77.5% (n=1) [26], and H-score ≥ median (n=1) [18]. NOS scores of included studies were no less than 6 (high quality), and details for each study are listed in Table 2.
Table 1. Baseline characteristics of studies included in the present meta-analysis.
| Study | Year | Country/ region | Outcome | TNM stage | Treatment | Sample size | Cut-off value for Ki-67 | Analysis of HR | Study design | Detection method | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ben-Haj-Ayed | 2016 | Tunisia | OS, DFS | I–IV | Mixed | 71 | ≥5% | Multivariate | Retrospective | IHC | 7 |
| Chang | 2017 | Taiwan | DFS, DMFS, LRFS | I–IV | Mixed | 124 | H-score ≥ median | Univariate | Retrospective | IHC | 7 |
| Fan | 2019 | China | DMFS | III–IV | Chemoradiotherapy | 147 | ≥5% | Univariate | Prospective | IHC | 8 |
| Fountzilas | 2012 | Greece | OS, DFS | II–IV | Chemoradiotherapy | 141 | ≥5% | Multivariate | Prospective | IHC | 9 |
| Genç | 2000 | Turkey | OS | I–IV | Radiotherapy | 35 | ≥10% | Univariate | Retrospective | IHC | 6 |
| Guan | 2015 | China | OS, DFS | I–IV | Mixed | 58 | ≥10% | Multivariate | Retrospective | IHC | 7 |
| Kijima | 2001 | Japan | OS | II–IV | Radiotherapy | 19 | ≥50% | Univariate | Retrospective | IHC | 6 |
| Lu | 2017 | China | OS, DFS, DMFS, LRFS | I–IV | Chemoradiotherapy | 334 | ≥10% | Univariate | Retrospective | IHC | 8 |
| Shi | 2015 | China | OS | I–IV | Chemoradiotherapy | 55 | ≥50% | Univariate | Retrospective | IHC | 7 |
| You | 2015 | China | OS | I–IV | Mixed | 118 | ≥50% | Univariate | Retrospective | IHC | 7 |
| Zhang | 2016 | China | OS | I–IV | Chemoradiotherapy | 59 | ≥25% | Univariate | Retrospective | IHC | 8 |
| Zhao | 2018 | China | OS, DFS | I–IV | Mixed | 45 | ≥77.5% | Multivariate | Retrospective | IHC | 6 |
| Zhao | 2017 | China | DFS | III–IV | Mixed | 108 | ≥10% | Multivariate | Retrospective | IHC | 8 |
Table 2. Details of NOS scores for studies included in this meta-analysis.
| Study | Year | Selection | Comparability | Outcome | NOS score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non- exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | |||
| Ben-Haj-Ayed | 2016 | ★ | ★ | ★ | ★ | ★ | ★ | - | ★ | 7 |
| Chang | 2017 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | - | 7 |
| Fan | 2019 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | - | 8 |
| Fountzilas | 2012 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Genç | 2000 | ★ | ★ | - | ★ | ★ | ★ | ★ | - | 6 |
| Guan | 2015 | ★ | ★ | ★ | ★ | ★ | ★ | - | ★ | 7 |
| Kijima | 2001 | ★ | ★ | ★ | ★ | ★ | ★ | - | - | 6 |
| Lu | 2017 | ★ | ★ | ★ | ★ | ★★ | ★ | - | ★ | 8 |
| Shi | 2015 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | - | 7 |
| You | 2015 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | - | 7 |
| Zhang | 2016 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | - | 8 |
| Zhao | 2018 | ★ | ★ | ★ | - | ★ | ★ | ★ | - | 6 |
| Zhao | 2017 | ★ | ★ | ★ | ★ | ★★ | ★ | - | ★ | 8 |
Prognostic value of Ki-67 for survival outcomes
The prognostic value of Ki-67 for various survival outcomes, including OS, DFS, DMFS, and LRFS, were analyzed. Ten studies with 935 patients [13–15,20–26] provided HRs and 95% CIs for OS (Figure 2A and Table 3). The pooled results were HR = 2.70, 95% CI = 1.97–3.71, and P<0.001, suggesting that Ki-67 overexpression was associated with poorer OS in NPC. Data from seven studies with 881 patients [13,14,18,20,22,26,27] were aggregated, and the results were HR = 1.93, 95% CI = 1.49–2.50, and P<0.001, which demonstrated the significant prognostic role of Ki-67 in DFS (Figure 2B and Table 2). The correlation between Ki-67 and poor LRFS was also significant (n=2, HR = 1.86, 95% CI = 1.11–3.12, P=0.019; Figure 2D and Table 3). However, there was no significant association between Ki-67 and DMFS (n=3, HR = 1.37, 95% CI = 0.78–2.38, P=0.270; Figure 2C and Table 3).
Figure 2. The forest plots depicting the prognostic value of Ki-67 for NPC.
Forest plots for the relationship between Ki-67 expression and (A) OS, (B) DFS, (C) DMFS, and (D) LRFS in patients with NPC.
Table 3. Summary of the subgroup analysis.
| Subgroups | Studies (n) | Patients (n) | Effects model | HR (95% CI) | P | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2 (%) | P | ||||||
| OS | |||||||
| Total | 10 | 935 | FEM | 2.70 (1.97–3.71) | <0.001 | 0 | 0.461 |
| Geographical region | |||||||
| Asia | 8 | 723 | FEM | 2.89 (2.05–4.08) | <0.001 | 0 | 0.713 |
| Non-Asia | 2 | 212 | REM | 2.16 (0.48–9.66) | 0.314 | 69.1 | 0.072 |
| TNM stage | |||||||
| I–IV | 8 | 775 | FEM | 2.76 (1.97–3.86) | <0.001 | 0 | 0.518 |
| II–IV | 2 | 160 | REM | 2.34 (0.54–10.13) | 0.256 | 59.2 | 0.117 |
| Treatment | |||||||
| Radiotherapy | 2 | 54 | FEM | 1.90 (1.00–3.61) | 0.049 | 0 | 0.358 |
| Chemoradiotherapy | 4 | 589 | FEM | 3.79 (2.28–6.29) | <0.001 | 0 | 0.925 |
| Mixed | 4 | 292 | FEM | 2.39 (1.41–4.03) | 0.001 | 31.5 | 0.223 |
| Sample size | |||||||
| <100 | 7 | 342 | FEM | 2.45 (1.63–3.67) | <0.001 | 20.7 | 0.272 |
| ≥100 | 3 | 593 | FEM | 3.16 (1.90–5.24) | <0.001 | 0 | 0.739 |
| Cutoff value | |||||||
| ≥5% | 2 | 212 | REM | 2.16 (0.48–9.66) | 0.314 | 69.1 | 0.072 |
| ≥10% | 3 | 427 | FEM | 2.79 (1.75–4.47) | <0.001 | 0 | 0.747 |
| ≥50% | 3 | 192 | FEM | 2.52 (1.38–4.59) | 0.003 | 14.8 | 0.309 |
| Others | 2 | 104 | FEM | 4.72 (1.82–12.23) | 0.001 | 0 | 0.533 |
| Analysis of HR | |||||||
| Univariate | 6 | 620 | FEM | 2.74 (1.89–3.97) | <0.001 | 0 | 0.646 |
| Multivariate | 4 | 315 | FEM | 2.61 (1.41–4.82) | 0.002 | 44.2 | 0.146 |
| Study design | |||||||
| Retrospective | 9 | 794 | FEM | 2.61 (1.88–3.61) | <0.001 | 0 | 0.443 |
| Prospective | 1 | 141 | - | 4.96 (1.31–18.81) | 0.019 | - | - |
| DFS | |||||||
| Total | 7 | 881 | FEM | 1.93 (1.49–2.50) | <0.001 | 25.3 | 0.236 |
| Geographical region | |||||||
| Asia | 5 | 669 | FEM | 1.91 (1.45–2.53) | <0.001 | 1.5 | 0.398 |
| Non-Asia | 2 | 212 | REM | 2.48 (0.55–11.19) | 0.237 | 74.7 | 0.047 |
| TNM stage | |||||||
| I–IV | 5 | 632 | FEM | 1.78 (1.33–2.39) | <0.001 | 16.2 | 0.312 |
| II–IV/III–IV | 2 | 249 | FEM | 2.59 (1.46–4.60) | 0.001 | 49.2 | 0.161 |
| Treatment | |||||||
| Chemoradiotherapy | 2 | 475 | REM | 2.72 (0.87–8.50) | 0.085 | 68.1 | 0.077 |
| Mixed | 5 | 406 | FEM | 1.89 (1.36–2.64) | <0.001 | 17.9 | 0.300 |
| Sample size | |||||||
| <100 | 3 | 174 | FEM | 2.26 (1.23–4.17) | 0.009 | 49.5 | 0.138 |
| ≥100 | 4 | 707 | FEM | 1.86 (1.40–2.47) | <0.001 | 20.0 | 0.290 |
| Cutoff value | |||||||
| ≥5% | 2 | 212 | REM | 2.48 (0.55–11.19) | 0.237 | 74.7 | 0.047 |
| ≥10% | 3 | 500 | FEM | 1.97 (1.40–2.79) | <0.001 | 0 | 0.433 |
| Others | 2 | 169 | REM | 2.31 (0.81-6.58) | 0.118 | 56.4 | 0.130 |
| Analysis of HR | |||||||
| Univariate | 2 | 458 | FEM | 1.67 (1.20–2.32) | 0.002 | 0 | 0.787 |
| Multivariate | 5 | 423 | FEM | 2.43 (1.60–3.69) | <0.001 | 33.6 | 0.197 |
| Study design | |||||||
| Retrospective | 6 | 740 | FEM | 1.83 (1.41–2.39) | <0.001 | 0 | 0.419 |
| Prospective | 1 | 141 | - | 5.76 (1.64–20.21) | 0.006 | - | - |
| DMFS | |||||||
| Total | 3 | 605 | REM | 1.37 (0.78–2.38) | 0.270 | 67.4 | 0.047 |
| LRFS | |||||||
| Total | 2 | 458 | FEM | 1.86 (1.11–3.12) | 0.019 | 43.5 | 0.183 |
Subgroup analysis
Subgroup analysis for OS and DFS was carried out to investigate the source of heterogeneity. We used seven variables for subgroup analysis, including geographical region, TNM stage, treatment, sample size, cutoff value, analysis of HR, and study design. High Ki-67 expression remained a significant prognostic factor for OS irrespective of treatment, sample size, analysis of HR, and study design (Table 3; all P<0.05). In addition, Ki-67 overexpression was associated with poor OS in Asian patients (P<0.001), in patients with TNM stage I–IV (P<0.001), and with cutoff values ≥10% (P<0.001) and ≥50% (P=0.003) (Table 3). Elevated Ki-67 expression was predictive of poor DFS in all subgroups of TNM stage, sample size, analysis of HR, and study design (Table 3; all P<0.05). Moreover, high Ki-67 expression was correlated with poor DFS in Asian patients (P<0.001), in patients receiving mixed treatments (P<0.001), and with a Ki-67 cutoff value of ≥10% (P<0.001) (Table 3).
Publication bias
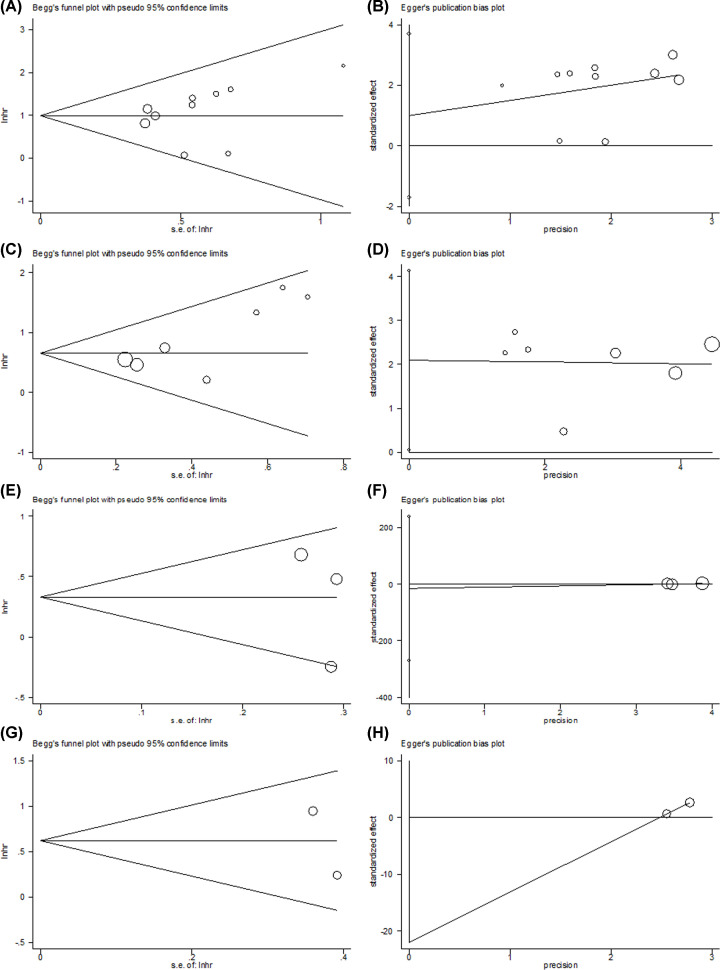
The funnel plots of Begg’ test and Egger’s regression test for the meta-analysis are shown in Figure 3. The funnel plots were visually symmetrical, and Egger’s test suggested non-significant publication bias in this meta-analysis.
Figure 3. Publication bias test through Begg’s funnel plot and Egger’s regression test in this meta-analysis.
(A) Begg’s test for OS, P=0.072. (B) Egger’s test for OS, P=0.419. (C) Begg’s test for DFS, P=0.133. (D) Egger’s test for DFS, P=0.086. (E) Begg’s test for DMFS, P=0.602. (F) Egger’s test for DMFS, P=0.591. (G) Begg’s test for LRFS, P=0.317. (H) Egger’s test for LRFS, P=1.
Discussion
To our knowledge, the present study is the first meta-analysis exploring the prognostic value of Ki-67 in patients with NPC. The prognostic effect of Ki-67 in patients with NPC is controversial based on the results of relevant studies [13–15,18–27]. The current meta-analysis incorporated data from 13 studies comprising 1314 patients and demonstrated that elevated Ki-67 expression was associated with long-term (OS and DFS) and short-term (LRFS) survival outcomes in patients with NPC. Furthermore, the prognostic role of Ki-67 was maintained throughout different sample sizes, analyses of HR, and study designs for OS and DFS in various subgroups. Ki-67 exerts significant prognostic value for Asian patients, and a Ki-67 cutoff value ≥10% showed consistent prognostic efficiency. According to these results, Ki-67 could be used as a reliable prognostic indicator for NPC, particularly in patients of Asian ethnicity.
Ki-67 is a nuclear protein expressed throughout the cell cycle in proliferating cells that has been investigated as a prognostic marker in various cancers [28,8,12]. The current meta-analysis demonstrated the prognostic role of Ki-67 expression in patients with NPC. Notably, a recent meta-analysis explored the prognostic value of hematological parameters in patients with NPC, which included 23 studies comprising 23417 patients and found neutrophil-to-lymphocyte ratio, C-reactive protein-to-albumin ratio, lymphocyte-to-monocyte ratio, plasma fibrinogen level, and Glasgow prognostic score (GPS) to have an impact on prognostication in NPC [29]. That meta-analysis [29] included 23 studies encompassing 23417 patients and demonstrated a series of hematological indexes, including neutrophil-to-lymphocyte ratio, C-reactive protein-to-albumin ratio, lymphocyte-to-monocyte ratio, plasma fibrinogen level, and GPS have impact on prognostication in NPC. Serum-based parameters are easily accessible and cost-effective in clinical practice. Compared with hematological indexes, Ki-67 has several advantages. First, Ki-67 is stable and cannot be significantly affected by the immunological status of patients; whereas Ki-67 is measured using IHC in tumor tissue, hematological markers are derived from blood-based indexes that can be influenced by chronic inflammation and nutritional condition, not just by cancer. Second, Ki-67 protein, a tumor proliferation marker, is comparable in other types of cancers such as head and neck [30], colorectal [31], and non-small cell lung cancer [12].
The cutoff values of Ki-67 to stratify high and low expression were not consistent in previous studies. In the current meta-analysis, a Ki-67 cutoff value ≥10% showed a consistent prognostic effect. In a recent study on colorectal cancer, a cutoff value of 25% for Ki-67 expression was a good classification tool in the AJCC-8 (American Joint Committee on Cancer 8 edition) stratification [31]. Another study indicated that a Ki-67 index of 5% is better than 2% in stratifying G1 and G2 pancreatic neuroendocrine tumors [32]. These studies suggest that the optimal cutoff value of Ki-67 may vary among different solid tumors. As suggested by our meta-analysis, a cutoff of 10% for Ki-67 expression should be validated for NPC in clinical practice.
The limitations of our meta-analysis need to be acknowledged. First, most of the included studies were retrospective, and heterogeneity may have been introduced. Second, some HRs and 95% CIs extracted using the Kaplan–Meier curves were not directly reported in text; therefore, data calculated may not be accurate. Third, the sample sizes for DMFS/LRFS were relatively small, which may compromise the validity of the prognostic significance of Ki-67 for DMFS and LRFS.
Conclusions
In summary, elevated Ki-67 expression is a reliable prognostic factor for poorer survival outcomes in NPC. The prognostic effect of Ki-67 remains stable across different subgroups of patients. Therefore, the Ki-67 index may be an important supplementary tool for the prognosis of patients with NPC.
Abbreviations
- CI
confidence interval
- DFS
disease-free survival
- DMFS
distant metastasis-free survival
- HR
hazard ratio
- IHC
immunohistochemistry
- LRFS
local recurrence-free survival
- NOS
Newcastle–Ottawa Scale
- NPC
nasopharyngeal carcinoma
- OS
overall survival
- TNM
tumor-node-metastasis
Data Availability
All data associated with the present study are included in this published article or are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Foundation of Health Commission of Sichuan Province [grant number 19PJ033].
Author Contribution
Y.l.L. and L.Y. collected the studies and analyzed the data. Y.q.L. and Q.Z. wrote the script. X.L. revised the final paper. All authors read and approved the final manuscript.
References
- 1.Sun X.S., Li X.Y., Chen Q.Y., Tang L.Q. and Mai H.Q. (2019) Future of radiotherapy in nasopharyngeal carcinoma. Br. J. Radiol. 92, 20190209 10.1259/bjr.20190209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perri F., Scarpati G.D., Caponigro F., Ionna F., Longo F., Buonopane S.et al. (2019) Management of recurrent nasopharyngeal carcinoma: current perspectives. Onco Targets Ther. 12, 1583–1591 10.2147/OTT.S188148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y.P., Chan A.T.C., Le Q.T., Blanchard P., Sun Y. and Ma J. (2019) Nasopharyngeal carcinoma. Lancet 394, 64–80 10.1016/S0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- 4.Ng W.T., Corry J., Langendijk J.A., Lee A.W.M., Makitie A., Mendenhall W.M.et al. (2020) Current management of stage IV nasopharyngeal carcinoma without distant metastasis. Cancer Treat. Rev. 85, 101995 10.1016/j.ctrv.2020.101995 [DOI] [PubMed] [Google Scholar]
- 5.Yang H., Wang K., Liang Z., Guo S., Zhang P., Xu Y.et al. (2020) Prognostic role of pre-treatment serum albumin in patients with nasopharyngeal carcinoma: a meta-analysis and systematic review. Clin. Otolaryngol. 45, 167–176 10.1111/coa.13454 [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D. and Weinberg R.A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 7.Avdalyan A.M., Ivanov A.A., Lushnikova E.L., Molodykh O.P. and Vikhlyanov I.V. (2020) The relationship of immunoexpression of Ki-67 and Hsp70 with clinical and morphological parameters and prognosis of papillary thyroid cancer. Bull. Exp. Biol. Med. 168, 688–693 10.1007/s10517-020-04781-1 [DOI] [PubMed] [Google Scholar]
- 8.Stec R., Cierniak S., Lubas A., Brzóskowska U., Syrylo T., Zielinski H.et al. (2020) Intensity of nuclear staining for Ki-67, p53 and survivin as a new prognostic factor in non-muscle invasive bladder cancer. Pathol. Oncol. Res. 26, 1211–1219 10.1007/s12253-019-00678-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabowski J.P., Vila C.M., Richter R., Taube E., Plett H., Braicu E.et al. (2020) Ki67 expression as a predictor of chemotherapy outcome in low-grade serous ovarian cancer. Int. J. Gynecol. Cancer 30, 498–503 10.1136/ijgc-2019-000976 [DOI] [PubMed] [Google Scholar]
- 10.Seo S.H., Kim K.H., Oh S.H., Choi Y., Ahn K.J., Lee J.Y.et al. (2019) Ki-67 labeling index as a prognostic marker in advanced stomach cancer. Ann. Surg. Treat. Res. 96, 27–33 10.4174/astr.2019.96.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanyılmaz G., Yavuz B.B., Aktan M., Karaağaç M., Uyar M. and Fındık S. (2019) Prognostic importance of Ki-67 in breast cancer and its relationship with other prognostic factors. Eur. J. Breast Health 15, 256–261 10.5152/ejbh.2019.4778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D.M., Chen D.M., Zhang C., Chai M., Guan M.J., Wang Z.Y.et al. (2020) Analysis of the relationship between Ki-67 expression and chemotherapy and prognosis in advanced non-small cell lung cancer. Transl. Cancer Res. 9, 3491–3498 10.21037/tcr.2020.03.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Haj-Ayed A., Moussa A., Ghedira R., Gabbouj S., Miled S., Bouzid N.et al. (2016) Prognostic value of indoleamine 2,3-dioxygenase activity and expression in nasopharyngeal carcinoma. Immunol. Lett. 169, 23–32 10.1016/j.imlet.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 14.Guan G.F., Zhang D.J., Wen L.J., Yu D.J., Zhao Y., Zhu L.et al. (2015) Prognostic value of TROP2 in human nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 8, 10995–11004 [PMC free article] [PubMed] [Google Scholar]
- 15.Kijima T., Kinukawa N., Gooding W.E. and Uno M. (2001) Association of Epstein-Barr virus with tumor cell proliferation: clinical implication in nasopharyngeal carcinoma. Int. J. Oncol. 18, 479–485 10.3892/ijo.18.3.479 [DOI] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G. and Grp P. (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Med. 6, e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M.et al. (2009) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://wwwohrica/programs/clinical_epidemiology/oxfordasp [Google Scholar]
- 18.Chang S.L., Chan T.C., Chen T.J., Lee S.W., Lin L.C. and Win K.T. (2017) HOXC6 overexpression is associated with Ki-67 expression and poor survival in NPC patients. J. Cancer 8, 1647–1654 10.7150/jca.18893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan X.J., Xie Y., Chen H.Y., Guo X.B., Ma Y., Pang X.L.et al. (2019) Distant metastasis risk definition by tumor biomarkers integrated nomogram approach for locally advanced nasopharyngeal carcinoma. Cancer Control 26, 10.1177/1073274819883895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fountzilas G., Ciuleanu E., Bobos M., Kalogera-Fountzila A., Eleftheraki A.G., Karayannopoulou G.et al. (2012) Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann. Oncol. 23, 427–435 10.1093/annonc/mdr116 [DOI] [PubMed] [Google Scholar]
- 21.Genç E., Hoşal A.S., Gedikoğlu G., Ozyar E. and Sözeri B. (2000) Prognostic value of p53, proliferating cell nuclear antigen, and Ki-67 expression in undifferentiated nasopharyngeal carcinomas. Otolaryngol. Head Neck Surg. 122, 868–873 10.1016/S0194-5998(00)70016-7 [DOI] [PubMed] [Google Scholar]
- 22.Lu Y., Huang H.X., Kang M., Yi M., Yang H., Wu S.B.et al. (2017) Combined Ki67 and ERCC1 for prognosis in non-keratinizing nasopharyngeal carcinoma underwent chemoradiotherapy. Oncotarget 8, 88552–88562 10.18632/oncotarget.19158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S., Li X.Y., You B., Shan Y., Cao X.L. and You Y.W. (2015) High expression of FGFR4 enhances tumor growth and metastasis in nasopharyngeal carcinoma. J. Cancer 6, 1245–1254 10.7150/jca.12825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.You B., Shan Y., Shi S., Li X.Y. and You Y.W. (2015) Effects of ADAM10 upregulation on progression, migration, and prognosis of nasopharyngeal carcinoma. Cancer Sci. 106, 1506–1514 10.1111/cas.12800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J.P., Liu Y.Y., Deng Y., He J., Lang J.T. and Fan J.P. (2016) Ki67 and nm23 are potential prognostic markers in patients with nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 9, 6350–6356 [Google Scholar]
- 26.Zhao L., Chen H., Hu B., Zhang H. and Lin Q. (2018) Prognostic significance of Ki67 expression and the derived neutrophil–lymphocyte ratio in nasopharyngeal carcinoma. Cancer Manag. Res. 10, 1919–1926 10.2147/CMAR.S167626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y.J., Shen L., Huang X.Q., Jing D., Huang D., Fu J.et al. (2017) High expression of Ki-67 acts a poor prognosis indicator in locally advanced nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 494, 390–396 10.1016/j.bbrc.2017.09.118 [DOI] [PubMed] [Google Scholar]
- 28.Mukai H., Yamaguchi T., Takahashi M., Hozumi Y., Fujisawa T., Ohsumi S.et al. (2020) Ki-67 response-guided preoperative chemotherapy for HER2-positive breast cancer: results of a randomised Phase 2 study. Br. J. Cancer 122, 1747–1753 10.1038/s41416-020-0815-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S., Zhao K., Ding X., Jiang H. and Lu H. (2019) Prognostic significance of hematological markers for patients with nasopharyngeal carcinoma: a meta-analysis. J. Cancer 10, 2568–2577 10.7150/jca.26770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuzul I., Pavic I., Granic M., Kuna T., Kotarac A.K. and Rogulj A.A. (2018) The significance of Ki-67 in head and neck cancers: review article. Res. J. Pharm. Biol. Chem. Sci. 9, 34–38 [Google Scholar]
- 31.Tong G.J., Zhang G.Y., Liu J., Zheng Z.Z., Chen Y., Niu P.P.et al. (2020) Cutoff of 25% for Ki67 expression is a good classification tool for prognosis in colorectal cancer in the AJCC-8 stratification. Oncol. Rep. 43, 1187–1198 10.3892/or.2020.7511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao S.W., Huang C.S., Huang X.T., Chen L.H., Chen W., Cai J.P.et al. (2019) Ki-67 index of 5% is better than 2% in Stratifying G1 and G2 of the World Health Organization Grading System in pancreatic neuroendocrine tumors. Pancreas 48, 795–798 10.1097/MPA.0000000000001331 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data associated with the present study are included in this published article or are available from the corresponding author on reasonable request.