Abstract
Purpose
Severe acute exacerbations of chronic obstructive pulmonary disease (AECOPD) that require hospitalization and emergency department visits are associated with considerable morbidity and mortality. Respiratory viral infection is an important cause of severe AECOPD. We evaluated the incidence and prognostic factors of viral infection in severe AECOPD.
Patients and Methods
We performed a retrospective study of 262 cases of severe AECOPD in 192 patients who required hospitalization and emergency department visits at a tertiary teaching hospital in Daegu, Korea. A multiplex polymerase chain reaction panel using a nasopharyngeal swab sample was performed to detect viral infection.
Results
Viral infection was detected in 108 events (41.2%) from 96 patients. The most common virus was rhinovirus/enterovirus (27.5%), followed by influenza virus (22.5%), respiratory syncytial virus (13.3%), parainfluenza virus (12.5%), coronavirus (12.5%), metapneumovirus (7.5%), and adenovirus (4.2%). Virus-positive exacerbations, compared to virus-negative exacerbations, had a higher frequency of symptoms of rhinopharyngitis, higher neutrophil count and C-reactive protein (CRP) level, and lower eosinophil count. Multivariate analysis demonstrated that elevated CRP levels (odds ratio [OR], 2.76; 95% confidence interval [CI], 1.24–6.15), symptoms of rhinopharyngitis (OR, 1.98; 95% CI, 1.03–3.78), low eosinophil count (OR, 1.74; 95% CI, 1.03–2.92), and inhaled corticosteroid (ICS) use (OR, 1.70; 95% CI 1.04–2.80) were associated with viral infection in severe AECOPD.
Conclusion
The incidence of viral infection in severe AECOPD was 41.2%, and the most commonly detected virus was rhinovirus/enterovirus. Increased CRP level, symptoms of rhinopharyngitis, low eosinophil count, and use of ICS were associated with viral infection in severe AECOPD.
Keywords: chronic obstructive pulmonary disease, exacerbation, viral infection, multiplex polymerase chain reaction, risk factors
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, progressive disease with persistent respiratory symptoms and airflow limitation.1 COPD is a major cause of morbidity and mortality worldwide and is expected to be the third leading cause of death in 2030.2 Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are related to increased hospital admission, morbidity, and mortality3 and cause accelerated lung function decline.4 Respiratory infections, including bacterial and viral infections, are known to be important triggers of AECOPD.5 As detection of viral infection using serology and culture has low sensitivity,6 the role of viral infection in AECOPD has been underestimated compared to that of bacterial infection. However, with recent advances in polymerase chain reaction (PCR) techniques, the viral detection rate has reached 56%.7 As the detection rate of virus increases, interest in the effect of viral infection on AECOPD has also increased. Rhinovirus, influenza virus, and respiratory syncytial virus (RSV) are the most commonly identified viruses in AECOPD.8 Although there have been several studies on respiratory viruses causing AECOPD,8,9 they could not sufficiently demonstrate the overall prevalence since viral infection has geographic and seasonal variation.
A previous study showed that CRP level and body temperature were associated with viral detection.10 Kwak et al reported female sex was related with high risk of viral infection.11 But few studies have evaluated the risk factors of viral infection in patients with AECOPD. But Few studies have evaluated the risk factors of viral infection in patients with AECOPD.
Therefore, this study aimed to evaluate the prevalence of respiratory viral infection using a highly sensitive PCR method and investigate the risk factors for viral infection in patients with severe AECOPD requiring hospital admission.
Method
Study Design and Participants
We performed a retrospective study of patients with AECOPD who were hospitalized at Yeungnam University Medical Center (a 930-bed, university-affiliated, tertiary referral hospital in Daegu, South Korea) between January 2017 and December 2018. During the study period, all COPD patients (>40 years) admitted to the hospital who had multiplex PCR results for viral identification were eligible for inclusion.
Exclusion criteria were as follows: (1) patients without a multiplex PCR test performed within 24 hour of hospitalization and (2) patients admitted with bronchial asthma or dyspnea of other origin (heart failure, pulmonary edema, or pulmonary embolism).
The primary outcome was the prevalence of respiratory viral infection in AECOPD. The secondary outcome was the risk factors of viral infection.
This study was conducted in accordance with the tenets of the Declaration of Helsinki and was reviewed and approved by the Institutional Review Board of Yeungnam University Hospital (YUH IRB 2021–01-008). Written informed consent was waived because of the retrospective study design, which is in compliance with the institutional and national policies concerning research approvals. Patient’s records were anonymized before analysis to maintain their confidentiality.
Data Collection and Definitions
According to the definition of AECOPD mentioned above, three pulmonologists (JHA, JHJ, and JGJ) made the diagnosis of COPD exacerbation. Patient electronic medical records were reviewed by all authors, and demographic, clinical, and spirometric data were collected.
Laboratory findings, including complete blood counts with differentials, blood chemistry, inflammation markers such as C-reactive protein (CRP) and procalcitonin levels, and multiplex PCR for respiratory virus results, were reviewed. Nasopharyngeal swabs were obtained at admission (within 24 hour). A multiplex PCR panel (FilmArray Respiratory Panel; BioFire Diagnostics, Inc., Salt Lake City, UT, USA), which target 17 viruses, including adenovirus, coronaviruses (OC43, 229E, NL63, and HKU1), human metapneumovirus (hMPV), human rhinovirus and enterovirus, influenza A (including subtypes H1N1, H3N2, and the 2009-H1N1) and B viruses, parainfluenza viruses (1,2,3, and 4), and RSV, was used to detect respiratory viral infections.
The diagnosis and severity of COPD was made according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria, which defines COPD as a post-bronchodilator forced expiratory volume in one second (FEV1) to forced vital capacity ratio of <0.7.1 AECOPD was defined according to the GOLD guidelines as an acute worsening of respiratory symptoms (dyspnea, increased sputum volume, and purulence) that was beyond daily variation and needed additional treatment.12 Frequent exacerbations were defined if acute exacerbation occurred twice or more for 1 year. Respiratory viral detection was defined as detection of any respiratory virus in nasopharyngeal swab samples by multiplex PCR. Nasopharyngeal symptoms included the presence of increased rhinorrhea, nasal congestion, or sore throat. Use of inhaled corticosteroid (ICS) was defined as having been prescribed ICS for at least 3 months.
Statistical Analysis
Continuous variables are expressed as means ± standard deviation (SD), and these were compared using a Student’’s s t-test or Mann–Whitney U-test. Categorical variables were compared using chi-squared test or Fisher’s exact test. Continuous data were categorized using logistic regression analysis. Multivariable logistic regression analyses were performed to identify independent risk factors for viral detection in patients with severe AECOPD, as measured by the odds ratios (ORs) with 95% confidence intervals (CIs). In all analyses, a p-value <0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software (ver. 24.0; SPSS Inc., Chicago, IL, USA).
Results
Study Population
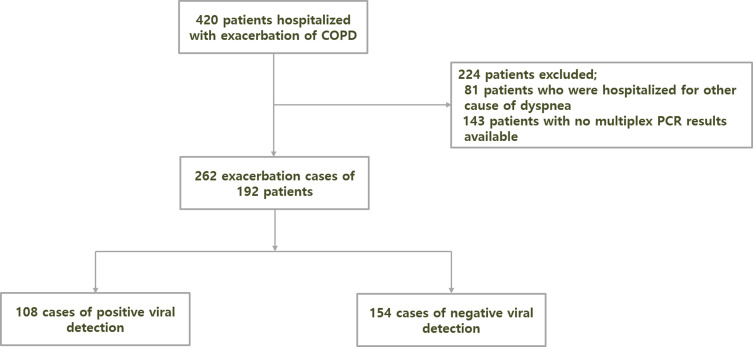
During the study period, 420 patients with AECOPD were hospitalized. After application of the exclusion criteria, 262 cases from 192 patients were included (Figure 1). Table 1 shows the baseline characteristics of the study population. The mean patient age was 76.5±7.9 years, and 155 (80.7%) were males. Most patients had a smoking history (92.7%). The mean FEV1 after bronchodilator was 69.1%, and 56 (29.7%) patients had a history of severe AECOPD in the previous year.
Figure 1.
Flowchart of participant enrollment. A total of 420 patients were hospitalized with acute exacerbation of chronic obstructive pulmonary disease. After excluding patients who were hospitalized for other causes of dyspnea and those with no multiplex polymerase chain reaction (PCR) results available, 262 final exacerbation cases from 192 patients were enrolled in the study.
Table 1.
Characteristics of Enrolled Patients
| Variables | Number of Patients (N=192) |
|---|---|
| Age (years) | 76.5±7.9 |
| Sex, n (%) | |
| Male | 155 (80.7) |
| Female | 37 (19.3) |
| Body mass index (kg/m2) | 21.5±4.5 |
| Smoking, n (%) | |
| Current smoker | 56 (29.2) |
| Ex-smoker | 122 (63.5) |
| Never smoker | 13 (7.3) |
| Comorbidities, n(%) | |
| Coronary artery disease | 18 (9.4) |
| Heart failure | 17 (8.9) |
| Chronic kidney disease | 10 (5.2) |
| Cerebrovascular disease | 18 (9.4) |
| Diabetes mellitus | 31 (16.1) |
| Hypertension | 67 (34.9) |
| Spirometry after bronchodilator | |
| FVC | 69.1±16.2 |
| FEV1 | 49.3±18.8 |
| Ratio of FEV1 to FVC | 47.5±13.3 |
| Maintenance inhaler medication | |
| LAMA | 24 (12.5) |
| LAMA+LABA | 53 (27.6) |
| LAMA+LABA+ICS | 58 (30.2) |
| ICS+LABA | 26 (13.5) |
| No use | 31 (16.1) |
| History of acute exacerbation in the last year | 56 (29.7) |
Note: Data are presented as means ± standard deviations or numbers (percentages).
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV, forced expiratory volume in one second; FVC, forced vital capacity; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2 agonist; ICS, inhaled corticosteroid.
Detection of Respiratory Viruses in Severe AECOPD
In total, 108 (41.2%) out of 262 cases tested positive for respiratory viruses, cases with multiple viral detection were 11. The most common viruses were rhinovirus/enterovirus (30.1%), followed by influenza virus (25.0%), RSV (14.8%), parainfluenza virus (13.9%), coronavirus (13.9%), hMPV (8.3%), and adenovirus (4.6%) (Table 2).
Table 2.
Respiratory Viruses Identified in Severe Chronic Obstructive Pulmonary Disease Exacerbation Events
| Pathogen | No. of Virus Positive Events (N=108) |
|---|---|
| Rhino/enterovirus | 33 (30.1) |
| Influenza | 27 (25.0) |
| Influenza A | 18 (16.7) |
| Flu A/H1 | 0 (0) |
| Flu A/H1-2009 | 7 (6.5) |
| Flu A/H3 | 12 (11.1) |
| Influenza B | 10 (9.3) |
| Parainfluenza | 15 (13.9) |
| Parainfluenza 1 | 6 (5.6) |
| Parainfluenza 2 | 0 (0) |
| Parainfluenza 3 | 5 (4.6) |
| Parainfluenza 4 | 4 (3.7) |
| Coronavirus | 15 (13.9) |
| Coronavirus 229E | 3 (2.8) |
| Coronavirus HKU1 | 1 (0.9) |
| Coronavirus OC43 | 7 (6.5) |
| Coronavirus NL63 | 4 (3.7) |
| Metapneumovirus | 9 (8.3) |
| Respiratory syncytial virus | 16 (14.8) |
| Adenovirus | 5 (4.6) |
| Multiple virus infection | 11 (10.2) |
Note: Data are presented as numbers (percentages).
Difference of Variables Between Groups with Positive and Negative Viral Detection in Severe AECOPD
Comparison between the two groups showed no difference in age, sex, smoking status, vital signs on admission, FEV1, severity of COPD, and clinical outcome (Table 3). Nasopharyngeal symptoms were more prevalent in the virus-positive exacerbation group than in the virus-negative group. The use of ICS was more common in the virus-positive exacerbation group than in the virus-negative exacerbation group. In laboratory tests, mean neutrophil counts and CRP levels were significantly higher and eosinophil count was significantly lower in the virus-positive exacerbation group than those in the virus-negative exacerbation group (Table 4).
Table 3.
Comparison of Clinical Data Between Virus-Positive Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD) and Virus-Negative AECOPD Groups
| Variables | All Events (N=262) |
Respiratory Virus – Positive Events (108) |
Respiratory Virus – Negative Events (154) |
P-value |
|---|---|---|---|---|
| Age | 75.4±7.5 | 76.3±7.9 | 76.5±7.2 | 0.804 |
| Male, n (%) | 220 (84.0) | 89 (82.4) | 131 (85.1) | 0.564 |
| Current smoker, n (%) | 73 (27.9) | 26 (24.1) | 47 (30.5) | 0.252 |
| Body mass index (Kg/m2) | 21.3±4.2 | 21.1±3.4 | 21.4±4.6 | 0.619 |
| Symptoms at admission | 0.483 | |||
| Duration of symptom prior to admission | 4.2±3.4 | 4.4±3.3 | 4.1±3.5 | |
| Nasopharyngeal symptoms | 52 (19.8) | 29 (26.9) | 23 (14.9) | 0.017 |
| Increased cough | 69 (26.9) | 29 (28.4) | 40 (26.0) | 0.874 |
| Increased sputum production | 67 (25.6) | 34 (27.6) | 33 (21.4) | 0.066 |
| Dyspnea | 237 (90.5) | 100 (97.7) | 137 (89.0) | 0.21 |
| ICS use | 118 (45) | 57 (52.8) | 61 (39.6) | 0.035 |
| Frequent exacerbation | 45 (17.2) | 14 (13.0) | 31 (20.1) | 0.130 |
| Vital sign on admission | ||||
| Systolic blood pressure | 127.1±24.3 | 127.3±26.7 | 127.0±22.5 | 0.921 |
| Diastolic blood pressure | 78.2±15.6 | 77.6±16.7 | 78.6±14.9 | 0.596 |
| Mean blood pressure | 110.8±20.7 | 110.7±22.8 | 110.9±19.1 | 0.955 |
| Heart rate | 95.2±21.5 | 95.5±23.1 | 94.9±20.4 | 0.829 |
| Respiration rate | 21.8±4.0 | 22.0±5.3 | 21.7±2.8 | 0.543 |
| Body temperature | 37.4±0.8 | 37.5±0.8 | 37.3±0.8 | 0.104 |
| Spirometry after bronchodilator | ||||
| FVC (% of predicted value) | 69.1±16.2 | 68.0±17.1 | 68.2±15.5 | 0.890 |
| FEV1 (% of predicted value) | 49.3±18.8 | 48.0±19.5 | 47.3±18.0 | 0.761 |
| Ratio of FEV1 to FVC (%) | 47.5±13.3 | 47.1±14.0 | 46.2±13.7 | 0.617 |
| Severity of COPD, n (%) | 0.499 | |||
| GOLD 1 | 16(6.2) | 8(7.5) | 8(5.2) | |
| GOLD 2 | 91(35) | 38(35.8) | 53(44.2) | |
| GOLD 3 | 106(40.8) | 38(35.8) | 68(44.2) | |
| GOLD 4 | 47(18.1) | 22(20.8) | 25(16.2) | |
| Bacterial infection | 56 (21.4) | 22 (23.1) | 34 (22.1) | 0.740 |
| Outcomes | ||||
| Hospital stay, day | 11.4±8.2 | 11.7±9.4 | 11.2±7.3 | 0.610 |
| ICU admission | 20 | 6 (5.6) | 14 (9.1) | 0.289 |
| In hospital death | 13 (5.0) | 6 (5.6) | 7 (4.5) | 0.711 |
| Readmission in 3 months | 68 (25.5) | 22 (28.0) | 46 (29.9) | 0.084 |
Note: Data are presented as means ± standard deviations or numbers (percentages).
Abbreviations: ICS, inhaled corticosteroid; FEV, forced expiratory volume in one second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICU, intensive care unit.
Table 4.
Comparison of Laboratory Findings Between Virus-Positive and Virus-Negative Events
| Variables | All Events (N=262) |
Respiratory Virus – Positive Events (108) |
Respiratory Virus – Negative Events (154) |
P-value |
|---|---|---|---|---|
| Complete blood count (normal range) White blood cells, ×109/L) |
11.5±5.1 |
12.3±6.2 |
11.0±4.2 |
0.063 |
| >10 | 152 (58.3) | 63 (58.3) | 89 (58.6) | 0.930 |
| Neutrophil count, ×109/L (1.8–6.3) | 8.9±4.4 | 9.6±4.8 | 8.4±4.2 | 0.036 |
| >4.8, n (%) | 207 (79.3) | 92 (85.2) | 115 (75.2) | 0.049 |
| Eosinophil count,/uL (50–500) | 163.2±212.2 | 124.80±171.6 | 190.1±233.4 | 0.010 |
| >100, n (%) | 120 (45.8) | 40 (37) | 80 (51.8) | 0.017 |
| Eosinophil percent | 1.7±2.4 | 1.3±2.0 | 2.0±2.5 | 0.032 |
| >2%, n (%) | 71 (37.2) | 22 (20.6) | 49 (31.8) | 0.044 |
| Blood chemistry (normal range) | ||||
| Albumin, g/dL (3.5–5) | 3.5±0.8 | 3.5±0.5 | 3.5±0.9 | 0.763 |
| Creatinine | 1.1±0.7 | 1.1±0.8 | 1.0±0.7 | 0.157 |
| Infection markers (normal range) | ||||
| C-reactive protein, mg/dL (0–0.5) | 7.5±10.2 | 9.2±13.1 | 6.4±7.5 | 0.043 |
| >0.5, n (%) | 220 (84.3) | 98 (91.6) | 122 (79.2) | 0.007 |
| Procalcitonin, ng/mL (0–0.5) | 0.6±1.6 | 0.6±1.5 | 0.6±1.6 | 0.88 |
Note: Data are presented as means ± standard deviations or numbers (percentages).
Factors Associated with Viral Detection in Severe AECOPD
To clarify the factors associated with viral detection in severe AECOPD cases, we investigated several variables including age, sex, nasopharyngeal symptoms, use of ICS, eosinophil count, CRP level, and absolute neutrophil count (Table 5). The univariable analysis revealed that nasopharyngeal symptoms, use of ICS, eosinophil count <100/uL, and CRP level >0.5mg/dL were significantly associated with viral detection. In the multivariable logistic analysis, nasopharyngeal symptoms (OR, 1.98; 95% CI, 1.03–3.78; p = 0.019), use of ICS (OR, 1.69; 95% CI, 1.01–2.84; p = 0.036), eosinophil count <100/uL (OR, 1.74; 95% CI, 1.03–2.92; p = 0.018), and CRP level >0.5 (OR, 2.76; 95% CI, 1.24–6.15; p = 0.009) were found to be associated with viral detection in severe AECOPD. This association was consistently maintained after adjusting for COPD severity and frequent exacerbation (Supplementary Table 1).
Table 5.
Factors Associated with Viral Detection in Severe Chronic Obstructive Pulmonary Disease Exacerbation Based on Multivariable Logistic Regression Analysis
| Univariable | Multivariable Modela | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age >70 | 0.89 (0.47–1.7) | 0.732 | 0.878 (0.43–1.80) | 0.723 |
| Female, sex | 1.22 (0.63–2.36) | 0.564 | 1.40 (0.68–2.87) | 0.357 |
| Nasopharyngeal symptoms | 2.09 (1.13–3.87) | 0.019 | 1.98 (1.03–3.78) | 0.040 |
| Use of ICS | 1.70 (1.04–2.80) | 0.036 | 1.69 (1.01–2.84) | 0.047 |
| Eosinophil<100/uL | 1.84 (1.12–3.04) | 0.018 | 1.74 (1.03–2.92) | 0.038 |
| CRP>0.5 mg/dL | 2.86 (1.302–6.27) | 0.009 | 2.76 (1.24–6.15) | 0.013 |
| ANC>4.8, ×109/L | 1.90 (0.997–3.622) | 0.051 | 1.41 (0.71–2.80) | 0.332 |
Note: aAdjusted for age, sex, rhinopharyngitis symptom, ICS use, eosinophil count, CRP level, ANC.
Abbreviations: ANC, absolute neutrophil count; CI, confidence interval; CRP, C-reactive protein; ICS, inhaled corticosteroid; OR, odds ratio.
Discussion
In our study, we analyzed the prevalence of viral infections and risk factors associated with viral infection in a large number of patients with severe AECOPD who required hospitalization. The main findings of this study were as follows: (1) The overall prevalence of viral infection was 41.2%; (2) the most commonly detected viral pathogen was rhinovirus/enterovirus; (3) the nasopharyngeal symptoms, use of ICS, low eosinophil count, and elevated CRP levels were associated with viral detection.
The prevalence of viral infection was 41.2% in our study. The rate of viral detection varies depending on the diagnostic method used. Previous studies using serology or viral culture reported that the prevalence of viral infection was 9% in patients with AECOPD.6 However, the advent of PCR has improved the detection rate of viral infection. Kherad et al reported that viral detection was observed in approximately half of patients with AECOPD.13 The rates of viral detection by PCR in AECOPD patients are variable. Previous studies have reported a detection rate of 37–51% in patients hospitalized with AECOPD,13–16 which is consistent with our results. The viral detection rate in some studies performed in Asia was relatively low, 22.1% and 28.1%.11,17 These differences might be caused by several factors, such as geographic differences, influenza vaccination rate, seasonal variation of the study period, difference of PCR techniques, or the timing and method of sampling.11,17,18 This study was conducted over 2 years, and we collected a relatively large number of nasopharyngeal samples within 24 hour of hospitalization. These points might explain the higher prevalence of viral detection in our study than that in other Asian studies.
A systemic review analyzing 24 studies on viral infections in AECOPD reported that human rhinovirus, influenza virus, RSV, and coronaviruses were the most commonly detected viruses,18 which was consistent with our results, except for parainfluenza virus. In our study, rhinovirus/enterovirus was the most commonly detected virus, which was consistent with the previous result.11,13,15,19 In the case of influenza virus, our result showed a higher detection rate compared to that in previous studies11,19,20 but was consistent with those of several studies.17,21 As the influenza virus detection rate could be related to seasonal variation and vaccine usage, differences between studies in the detection rate of this virus is inevitable. The incidence of RSV infection was consistent with those of several studies.19,22 RSV is an important pathogen of respiratory infection in elderly and high-risk adults23 and is commonly detected in patients with AECOPD.16 hMPV is a virus that is not commonly associated with AECOPD.16 However, Hamelin et al reported that hMPV is associated with COPD exacerbation.24 Our results also support the role of hMPV in AECOPD.
Symptoms and inflammatory markers can be used to predict viral infection. Nasopharyngeal symptoms (such as nasal congestion, increased rhinorrhea, sore throat) and fever are frequently present in patients with viral infection leading to COPD exacerbation.10,13,19,20,22,25 In our study, patients with viral infection had a higher frequency of nasopharyngeal symptoms than those without viral infection, indicating that nasopharyngeal symptoms might have a predictive value for viral infection.
CRP is an acute-phase systemic inflammatory biomarker that is associated with AECOPD.26 The relationship between CRP level and viral infection remains unclear. Some studies did not show an association between CRP levels and viral infection.11,13,21 However, Clark et al studied the relationship between serum CRP level and the rate of detection of virus and bacteria, and reported that CRP level was associated with viral detection,10 which was consistent with our results.
The interesting points of this present study were the association of viral detection with use of ICS and eosinophil count. The GOLD guideline recommend the use of ICS in combination with bronchodilators for patients with a history of asthma, blood eosinophil count >300 cells/µL, and severe or frequent AECOPD despite long-acting bronchodilator therapy.27 However, the ICS use is associated with an increased risk of pneumonia and pulmonary tuberculosis infection.28–30 Until now, the relationship between the use of ICS and risk of viral infection remains unclear. The “Toward a Revolution in COPD Health (TORCH)” study showed that the ICS use increased the risk of upper respiratory tract infection as well as pneumonia.31 Thomas et al reported that usage of glucocorticosteroids increases viral replication in the respiratory tract in vitro and in vivo.32 In our data, the use of ICS increased the risk of severe COPD exacerbation, suggesting that ICS use is associated with viral infection. Further prospective studies should be performed to evaluate the association between ICS use and viral infection.
Previous studies reported that blood eosinophilia was associated with an increased risk of AECOPD33,34 and is a predictive biomarker of response to inhaled or systemic corticosteroid.35 Until now, no clear relationship between eosinophil count and respiratory viral infection has been established. MacDonald et al reported that low eosinophil counts were associated with infection.36 Drake et al showed the antiviral effects of eosinophils in vivo and in vitro, and their results suggested that eosinophils enhance viral clearance and contribute to innate immune responses.37 However, there is no clear evidence of an association between eosinophil count and viral infection in a clinical study. Although our results showed that low eosinophil count was associated with viral infection, a prospective randomized controlled study will be required to confirm this.
Poor prognosis of AECOPD includes readmission to hospital and in-hospital mortality. Recent study reported comorbidities (heart failure, renal failure, depression, and alcohol use), previous exacerbation and hospitalization, and longer duration of admission were associated with risk factors for readmission.38 Although one study showed frequency of AECOPD is related to the frequency of the common cold,39 the relationship between the viral infection and poor prognosis in AECOPD is unclear. Previous studies showed that there was no difference in hospital days, in hospital death, and readmission,11,13,40 which is consistent with our result. Further evaluation about impact of viral infection on poor prognosis of AECOPD will be warranted.
Our study has some limitations. First, it was a retrospective and single-center study; thus, our data were not representative of the Korean population. Furthermore, we enrolled only patients who underwent PCR test among severe AECOPD patients requiring hospitalization, so there may be some selection bias. Second, influenza virus infection is associated with vaccination, but we did not evaluate the vaccination status because of our retrospective study design. Third, bacterial infection is a potential confounding factor in this study because CRP levels can be elevated in bacterial infection associated AECOPD. However, since there was no difference in bacterial infection rate between the virus positive exacerbation group and the virus negative exacerbation group, we carefully speculate the effect of bacterial infection on CRP level in our study could be insignificant. Finally, because the PCR method cannot discriminate between active and latent viral infections, viral detection did not confirm whether AECOPD was due to an active viral infection.
Conclusion
Viral infection was common among patients with severe AECOPD. The commonly detected viruses were rhinovirus and influenza. Our study showed the risk factors associated with viral detection in patients with severe AECOPD. To confirm these findings, further studies are needed.
Funding Statement
This work was supported by the Yeungnam University Research Fund (2020).
Abbreviations
AECOPD, acute exacerbations of chronic obstructive pulmonary disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; FEV1, forced expiratory volume in one second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; hMPV, human metapneumovirus; ICS, inhaled corticosteroid; OR, odds ratio; PCR polymerase chain reaction; RSV, respiratory syncytial virus; SD, standard deviation.
Ethics Approval and Informed Consent
This study was conducted in accordance with all relevant tenets of the Declaration of Helsinki. The protocol was reviewed and approved by the institutional review board of our hospital (no. YUH IRB 2021-01-008). Written informed consent was waived because of the retrospective study design, which is in compliance with the institutional and national policies concerning research approvals. Patient’s records were anonymized before analysis to maintain their confidentiality.
Disclosure
The authors declare no competing interests.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi: 10.1016/s0140-6736(07)61382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dransfield MT, Kunisaki KM, Strand MJ, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(3):324–330. doi: 10.1164/rccm.201605-1014OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi: 10.1164/rccm.201104-0597OC [DOI] [PubMed] [Google Scholar]
- 6.Boixeda R, Rabella N, Sauca G, et al. Microbiological study of patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease (AE-COPD) and the usefulness of analytical and clinical parameters in its identification (VIRAE study). Int J Chron Obstruct Pulmon Dis. 2012;7:327–335. doi: 10.2147/copd.S30568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi: 10.1136/thorax.58.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan A, Chandra S, Agarwal D, et al. Prevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: a systematic review. Respirology. 2010;15(3):536–542. doi: 10.1111/j.1440-1843.2010.01722.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi J, Oh JY, Lee YS, et al. Bacterial and viral identification rate in acute exacerbation of chronic obstructive pulmonary disease in Korea. Yonsei Med J. 2019;60(2):216–222. doi: 10.3349/ymj.2019.60.2.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark TW, Medina MJ, Batham S, Curran MD, Parmar S, Nicholson KG. C-reactive protein level and microbial aetiology in patients hospitalised with acute exacerbation of COPD. Eur Respir J. 2015;45(1):76–86. doi: 10.1183/09031936.00092214 [DOI] [PubMed] [Google Scholar]
- 11.Kwak HJ, Park DW, Kim JE, et al. Prevalence and risk factors of respiratory viral infections in exacerbations of chronic obstructive pulmonary disease. Tohoku J Exp Med. 2016;240(2):131–139. doi: 10.1620/tjem.240.131 [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117(5Suppl 2):398s–401s. doi: 10.1378/chest.117.5_suppl_2.398s [DOI] [PubMed] [Google Scholar]
- 13.Kherad O, Kaiser L, Bridevaux PO, et al. Upper-respiratory viral infection, biomarkers, and COPD exacerbations. Chest. 2010;138(4):896–904. doi: 10.1378/chest.09-2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McManus TE, Marley A-M, Baxter N, et al. Respiratory viral infection in exacerbations of COPD. Respir Med. 2008;102(11):1575–1580. doi: 10.1016/j.rmed.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zwaans WA, Mallia P, van Winden ME, Rohde GG. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease—a systematic review. J Clin Virol. 2014;61(2):181–188. doi: 10.1016/j.jcv.2014.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jafarinejad H, Moghoofei M, Mostafaei S, Salimian J, Azimzadeh Jamalkandi S, Ahmadi A. Worldwide prevalence of viral infection in AECOPD patients: a meta-analysis. Microb Pathog. 2017;113:190–196. doi: 10.1016/j.micpath.2017.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko FW, Ip M, Chan PK, et al. Viral etiology of acute exacerbations of COPD in Hong Kong. Chest. 2007;132(3):900–908. doi: 10.1378/chest.07-0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hewitt R, Farne H, Ritchie A, Luke E, Johnston SL, Mallia P. The role of viral infections in exacerbations of chronic obstructive pulmonary disease and asthma. Ther Adv Respir Dis. 2016;10(2):158–174. doi: 10.1177/1753465815618113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi: 10.1164/ajrccm.164.9.2105011 [DOI] [PubMed] [Google Scholar]
- 20.McManus TE, Marley AM, Baxter N, et al. Respiratory viral infection in exacerbations of COPD. Respir Med. 2008;102(11):1575–1580. doi: 10.1016/j.rmed.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin T, Zhu Z, Mei Z, et al. Analysis of viral infection and biomarkers in patients with acute exacerbation of chronic obstructive pulmonary disease. Clin Respir J. 2018;12(3):1228–1239. doi: 10.1111/crj.12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosseini SS, Ghasemian E, Jamaati H, Tabaraie B, Amini Z, Cox K. Association between respiratory viruses and exacerbation of COPD: a case-control study. Infect Dis (Lond). 2015;47(8):523–529. doi: 10.3109/23744235.2015.1022873 [DOI] [PubMed] [Google Scholar]
- 23.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951 [DOI] [PubMed] [Google Scholar]
- 24.Hamelin ME, Côtù S, Laforge J, et al. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clinical Infectious Diseases. 2005;41(4):498–502. doi: 10.1086/431981 [DOI] [PubMed] [Google Scholar]
- 25.Biancardi E, Fennell M, Rawlinson W, Thomas PS. Viruses are frequently present as the infecting agent in acute exacerbations of chronic obstructive pulmonary disease in patients presenting to hospital. Intern Med J. 2016;46(10):1160–1165. doi: 10.1111/imj.13213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309(22):2353–2361. doi: 10.1001/jama.2013.5732 [DOI] [PubMed] [Google Scholar]
- 27.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis m, and prevention of chronic obstructive pulmonary disease. 2020. Available from: www.goldcopd.org/. Accessed April30, 2021.
- 28.Dong YH, Chang CH, Wu FL, et al. Use of inhaled corticosteroids in patients with COPD and the risk of TB and influenza: a systematic review and meta-analysis of randomized controlled trials. a systematic review and meta-analysis of randomized controlled trials. Chest. 2014;145(6):1286–1297. doi: 10.1378/chest.13-2137 [DOI] [PubMed] [Google Scholar]
- 29.Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176(2):162–166. doi: 10.1164/rccm.200611-1630OC [DOI] [PubMed] [Google Scholar]
- 30.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070 [DOI] [PubMed] [Google Scholar]
- 31.Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070 [DOI] [PubMed] [Google Scholar]
- 32.Thomas BJ, Porritt RA, Hertzog PJ, Bardin PG, Tate MD. Glucocorticosteroids enhance replication of respiratory viruses: effect of adjuvant interferon. Sci Rep. 2014;4:7176. doi: 10.1038/srep07176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan MC, Yeung YC, Yu ELM, Yu WC, Eosinophil B. Risk of exacerbation in chronic obstructive pulmonary disease patients: a retrospective cohort analysis. Int J Chron Obstruct Pulmon Dis. 2020;15:2869–2877. doi: 10.2147/copd.S268018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193(9):965–974. doi: 10.1164/rccm.201509-1869OC [DOI] [PubMed] [Google Scholar]
- 35.Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. The Lancet Respiratory Medicine. 2015;3(6):435–442. doi: 10.1016/S2213-2600(15)00106-X [DOI] [PubMed] [Google Scholar]
- 36.MacDonald MI, Osadnik CR, Bulfin L, et al. Low and high blood eosinophil counts as biomarkers in hospitalized acute exacerbations of COPD. Chest. 2019;156(1):92–100. doi: 10.1016/j.chest.2019.02.406 [DOI] [PubMed] [Google Scholar]
- 37.Drake MG, Bivins-Smith ER, Proskocil BJ, et al. Human and mouse eosinophils have antiviral activity against parainfluenza virus. Am J Respir Cell Mol Biol. 2016;55(3):387–394. doi: 10.1165/rcmb.2015-0405OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alqahtani JS, Njoku CM, Bereznicki B, et al. Risk factors for all-cause hospital readmission following exacerbation of COPD: a systematic review and meta-analysis. Eur Respir Rev. 2020;29(156):190166. doi: 10.1183/16000617.0166-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurst JR, Donaldson GC, Wilkinson TMA, Perera WR, Wedzicha JA. Epidemiological relationships between the common cold and exacerbation frequency in COPD. Eur Respir J. 2005;26(5):846–852. doi: 10.1183/09031936.05.00043405 [DOI] [PubMed] [Google Scholar]
- 40.Choi J, Oh JY, Lee YS, et al. Pseudomonas aeruginosa infection increases the readmission rate of COPD patients. Int J Chronic Obstruct Pulmonary Dis. 2018;13:3077–3083. doi: 10.2147/COPD.S173759 [DOI] [PMC free article] [PubMed] [Google Scholar]