Abstract
Background
Up-to-date estimates of the burden of norovirus, a leading cause of acute gastroenteritis (AGE) in the United States, are needed to assess the potential value of norovirus vaccines in development. We aimed to estimate the rates, annual counts, and healthcare charges of norovirus-associated ambulatory clinic encounters, Emergency Department (ED) visits, hospitalizations, and deaths in the United States.
Methods
We analyzed administrative data on AGE outcomes from July 1, 2001 through June 30, 2015. Data were sourced from IBM® MarketScan® Commercial and Medicare Supplemental Databases (ambulatory clinic and ED visits), the Healthcare Utilization Project National Inpatient Sample (NIS; hospitalizations), and the National Center for Health Statistics multiple-cause-of-mortality (MCM) data (deaths). Outcome data (ambulatory clinic and ED visits, hospitalizations, or deaths) were summarized by month, age group, and setting. Healthcare charges were estimated based on insurance claims. Monthly counts of cause-unspecified gastroenteritis-associated outcomes were modeled as functions of cause-specified outcomes, and model residuals were analyzed to estimate norovirus-associated outcomes. Healthcare charges were estimated by applying average charges per cause-unspecified gastroenteritis encounter to the estimated number of norovirus encounters.
Results
We estimate 900 deaths (95% Confidence Interval [CI]: 650 – 1100), 110,000 hospitalizations (95%CI: 80,000 – 145,000), 470,000 ED visits (95% CI: 348,000 – 610,000), and 2.3 million ambulatory clinic encounters (95% CI: 1.7 – 2.9 million) annually due to norovirus, with an associated $430 – 740 million in healthcare charges.
Conclusions
Norovirus causes a substantial health burden in the United States each year, and an effective vaccine could have important public health impact.
SUMMARY:
Using administrative data, norovirus was estimated to cause approximately 900 deaths, 109,000 hospitalizations, 465,000 ED visits, and 2,270,000 ambulatory clinic encounters annually. Rates of outpatient encounters were highest among children, whereas hospitalization and death rates were highest among the elderly.
Introduction
Noroviruses, a diverse group of viruses within the family Caliciviridae [1, 2], are a leading cause of acute gastroenteritis (AGE) worldwide[3]. Typical norovirus symptoms include diarrhea, vomiting, and abdominal cramps. Although illness is usually self-limiting, it can cause severe outcomes (hospitalization or death) in vulnerable populations, such as young children and older adults[4]. Given that a candidate norovirus vaccine recently completed a phase 2b trial, up-to-date estimates of U.S. norovirus burden are needed to assess the potential value of vaccination and to provide baseline data from which future vaccine impact can be determined[5]. A previous study estimated the U.S. burden of norovirus based on synthesis of various studies examining data for the period ranging from 1993 to 2010[4]. However, norovirus burden is dynamic, as circulating norovirus strains vary each year, and the emergence of new strains is often associated with increased outbreaks[6, 7]. Furthermore, an increasing trend in norovirus AGE hospitalization rates was seen during 1997 – 2006, suggesting that previous estimates may underrepresent the current burden. Using three different administrative datasets for years 2001 – 2015, we provide updated estimates of U.S. norovirus burden and its associated healthcare charges, focusing on four distinct outcomes: ambulatory clinic encounters, Emergency Department (ED) visits, hospitalizations, and deaths.
Methods
Data sources
Ambulatory clinic encounters and ED visit data were sourced from two IBM MarketScan® Research Databases: Commercial and Medicare Supplemental [8]. Hospitalization data were obtained from the National Inpatient Sample of the Healthcare Cost and Utilization Project (NIS-HCUP), a nationally representative database of community hospital discharges [9]. Deaths were identified from the National Center for Health Statistics (NCHS) multiple-cause-of-mortality dataset. For each outcome during July 2001– June 2015, data were summarized by month and year and stratified by age group: <5 years, 5 – 17 years, 18 – 64 years, 65 – 74 years, 75 – 84 years, and 85+ years. Given smaller sample sizes, only three age groups were used for calculation of mortality rates: <5 years, 5 – 64 years, and 65+ years. Because all data were fully de-identified and no interaction occurred with human subjects, this study was deemed non-research and thus exempt from Institutional Review Board review.
Definitions
We used International Classification of Diseases, Revision 9, Clinical Modification (ICD-9-CM) codes to identify cause-unspecified and cause-specified AGE-coded ambulatory clinic encounters, ED visits, and hospital discharges. We used International Classification of Diseases, Revision 10 (ICD-10) codes from the National Center for Health Statistics multiple cause-of-mortality dataset to identify AGE-coded deaths. Cause-specified AGE included bacterial, viral (not including norovirus), and parasitic causes (Table 1). Cause-unspecified AGE codes included ICD-9-CM codes such as 009.0 (Infectious colitis, enteritis, and gastroenteritis) and 787.91 (diarrhea not otherwise specified), and their ICD-10 equivalents (Appendix Table 1). In a sensitivity analysis, we tested a revised definition of AGE that also included outcomes with only a vomiting-specific code (ICD-9-CM: 787.01, 787.03; ICD-10: R11.1) and no other AGE-associated codes.
Table 1:
Mean Annual Encounters (in 10,000s) and Deaths (counts) Associated with Acute Gastroenteritis (AGE) by Pathogen Category—United States, 2001 – 2015*
| Cause | ICD-9-CM Codes | ICD-10 Codes | Estimated Ambulatory Clinic Encounters† N (%) |
Estimated Emergency Department Visits† N (%) |
Estimated Hospitalizations N (%) |
Deaths‡
N (%) |
|---|---|---|---|---|---|---|
| Cause-unspecified AGE | 009.0–009.3, 558.9, 787.91, 008.69, 008.8 | A09, K52.9, A08.3-A08.4, A08.8, K55.0₤, K55.9₤ | 1376.6 (94.5%) | 202.2 (93.4%) | 90.2 (73.7%) | 6089 (38.1%) |
| Cause-specified** AGE | 79.8 (5.5%) | 14.4 (6.6%) | 32.3 (26.4%) | 9890 (61.9%) | ||
| Viral | 008.61–008.67 | A08.0–A08.2 | 9.4 (0.6%) | 2.0 (0.9%) | 1.8 (1.5%) | 22 (0.1%) |
| Bacterial | 001.0–01.9, 002.0–002.9, 003.0–003.1, 003.3–003.9, 004.0–004.9, 005.0–005.9, 008.0–008.5 | A00.0–A05.9 | 72.9 (5%) | 13.1 (6%) | 30.1 (24.6%) | 9843 (61.6%) |
| Parasitic | 006.0–006.2, 006.8–006.9, 007.0–007.9 | A06.0–A07.9 | 3.9 (0.3%) | 0.3 (0.1%) | 0.3 (0.2%) | 27 (0.2%) |
| All-cause AGE | 1456.4 (100%) | 216.6 (100%) | 122.4 (100%) | 15,979 (100%) | ||
Abbreviations: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification. ICD-10, International Classification of Diseases, Tenth Revision.
From July 1 2001 through June 30 2015.
Multiple codes may be assigned to a given record; thus, the sum of cases for each specific cause exceeds the subtotals for “cause unspecified” and “cause specified.” However, if any cause-specified code was included in the record, the encounter was categorized as such.
Extrapolated to the population using census data.
Raw numbers presented (i.e., not in 10,000).
Included only for children <5 in years 2001 – 2004.
Statistical Methods for Estimation of Norovirus
Due to limited routine use of norovirus diagnostic testing, healthcare encounters are rarely coded for norovirus-specific gastroenteritis; as a result, ICD codes are too insensitive to directly calculate burden[11, 12]. We therefore applied a previously developed method to estimate norovirus-attributable outcomes from available data on all AGE-related outcomes[13–15]. For each outcome and age group, we modeled the monthly counts of cause-unspecified gastroenteritis as a function of monthly counts for known pathogens. Models also included a term for time, i.e. sequential month of study, to account for secular trends. The negative binomial distribution was used, and models were fitted on the natural scale to facilitate interpretation of estimated coefficients. Predictors included monthly counts of age group-specific rotavirus, C. difficile, and non-C. difficile bacterial infections, as well as monthly counts of parasitic infections summed across all ages. The form of the final models is shown below, where E(CUi,j) denotes the expected count of cause-unspecified gastroenteritis in age group i during year-month j. Additional details are given in Supplemental Methods. Estimated parameters are shown in Appendix Table 2.
| Ambulatory Clinic Encounters, ED Visits, Hospitalizations, and Mortality in ages ≥65 years: | E(CUi, j)=β0i + β1iRotai,j + β2iC.difficilei, j + β3iOtherBacti,j + β4iParaall,j + β5i Timej |
| Mortality in ages <5 and 5–64 years: | E(CUi, j)=β0i + β1iRotaall,j + β2iC.difficileall, j + β3iOtherBactall,j + β4iParaall,j + β5i Timej |
The second step of estimation involved analysis of model residuals. We calculated the minimum residual by seasonal year (July – June), and then subtracted this value from the monthly residuals to obtain estimates of norovirus-associated outcomes. Finally, norovirus-coded outcomes were added to model-estimated norovirus outcomes to generate the final values, hereafter referred to as “estimated norovirus.” For mortality estimates, additional calculations were performed on model residuals due to less distinct seasonality in some age groups. More details are given in Supplemental Methods.
Extrapolation to the Population
For ambulatory clinic encounters and ED visits, rates were calculated by dividing the number of estimated cases by the monthly MarketScan populations from July 2001 to July 2015 for each age group. These rates were applied to census data (for the full U.S. population) to estimate the total number of norovirus-associated visits for each setting. Norovirus counts for hospitalizations were estimated as described above, and these counts applied to census data to generate rate estimates. Similarly, mortality rates were calculated by applying the number of estimated deaths to census data, by age group. Confidence intervals (CI) were derived from the 95% confidence intervals of the modeled unspecified AGE cases. Lifetime risks were calculated for each outcome using age-specific estimated norovirus rates (Table 2), assuming a life expectancy of 78.6 years [18].
Table 2:
Mean Annual Counts and Rates of All-cause Acute Gastroenteritis (AGE) and Estimated Norovirus (NV)* by Outcome and Age Group—United States, Seasonal Years 2001/2002 – 2014/2015
| All-Cause AGE | Estimated* NV | ||||
|---|---|---|---|---|---|
| Counts in 10K† | Rate per 10K PY‡ | Counts in 10K† (95% CI) | Rate per 10K PY‡ (95% CI) | % of All-Cause AGE | |
| Ambulatory Clinic Encounters | |||||
| <5 years | 240.9 | 1209.3 | 56.1 (44.0 – 71.0) | 281.4 (221.0 – 356.2) | 23.3 |
| 5 – 17 years | 166.3 | 309.6 | 53.3 (41.8 – 66.4) | 99.2 (77.9 – 123.6) | 32.0 |
| 18 – 64 years | 756.1 | 397.4 | 75.6 (58.2 – 95.5) | 39.7 (30.6 – 50.2) | 10.0 |
| 65 – 74 years | 119.4 | 561.1 | 16.0 (11.7 – 21.3) | 75.2 (55.0 – 100.0) | 13.4 |
| 75 – 84 years | 112.4 | 855.5 | 18.5 (13.2 – 24.6) | 140.8 (100.2 – 187.6) | 16.5 |
| 85+ years | 61.2 | 1172.2 | 7.9 (5.6 – 10.6) | 151.3 (107.3 – 202.5) | 12.9 |
| All ages | 1456.4 | 479.8 | 227.3 (174.5 – 289.4) | 74.9 (57.5 – 95.3) | 15.6 |
| ED Visits | |||||
| <5 years | 30.3 | 151.9 | 9.2 (7.2 – 11.8) | 46.2 (36.1 – 59.4) | 30.4 |
| 5 – 17 years | 24.5 | 45.6 | 7.4 (5.5 – 9.7) | 13.8 (10.2 – 18.0) | 30.1 |
| 18 – 64 years | 124.8 | 65.6 | 24.0 (17.8 – 31.6) | 12.6 (9.4 – 16.6) | 19.2 |
| 65 – 74 years | 14.0 | 65.9 | 2.2 (1.6 – 3.0) | 10.5 (7.6 – 13.9) | 16.0 |
| 75 – 84 years | 14.2 | 108.4 | 2.1 (1.4 – 2.8) | 15.7 (11.0 – 21.4) | 14.5 |
| 85+ years | 8.8 | 167.5 | 1.7 (1.2 – 2.1) | 31.7 (23.6 – 41.0) | 18.9 |
| All ages | 216.6 | 71.4 | 46.5 (34.8 – 61.0) | 15.3 (11.5 – 20.1) | 21.5 |
| Hospitalizations | |||||
| <5 years | 9.9 | 49.6 | 2.5 (2.0 – 3.2) | 12.5 (10.0 – 15.9) | 25.3 |
| 5 – 17 years | 4.5 | 8.4 | 0.7 (0.5 – 1.0) | 1.3 (0.9 – 1.8) | 15.8 |
| 18 – 64 years | 51.2 | 26.9 | 3.1 (2.1 – 4.2) | 1.6 (1.1 – 2.2) | 6.0 |
| 65 – 74 years | 20.0 | 94.1 | 1.4 (1.0 – 1.8) | 6.5 (4.6 – 8.6) | 6.9 |
| 75 – 84 years | 22.7 | 172.9 | 1.8 (1.3 – 2.4) | 13.7 (9.7 – 18.2) | 7.9 |
| 85+ years | 14.1 | 270.2 | 1.5 (1.1 – 2.0) | 28.5 (20.9 – 37.5) | 10.6 |
| All ages | 122.4 | 40.3 | 10.9 (8.0 – 14.5) | 3.6 (2.6 – 4.8) | 8.9 |
| Mortality†‡ | |||||
| <5 years | 472 | 23.7 | 36 (28 – 44) | 1.8 (1.4 – 2.2) | 7.6 |
| 5 – 64 years | 2031 | 8.3 | 85 (68 – 104) | 0.3 (0.3 – 0.4) | 4.2 |
| 65+ years | 13476 | 339.9 | 744 (560 – 959) | 18.8 (14.1 – 24.2) | 5.5 |
| All ages | 15979 | 52.6 | 865 (656 – 1107) | 2.8 (2.2 – 3.6) | 5.4 |
Defined as model-estimated plus coded norovirus.
For mortality, total actual AGE and estimated norovirus counts presented.
For mortality, rates per million PY. Abbreviations: AGE – Acute Gastroenteritis; CI – confidence interval; NV – Norovirus; K – thousands; PY – Person-Years.
Economic analysis
A secondary aim of this analysis was to estimate norovirus-associated healthcare charges in the United States. The median charges for cause-unspecified gastroenteritis encounters were calculated by age group and outcome for each year, and updated to 2016 U.S. dollars. The mean was taken across years and applied to the estimated average total annual number of norovirus-associated healthcare encounters across the study period to generate a total estimate for norovirus-associated healthcare charges.
Results
Over the course of the 14-year study period, the majority of AGE-coded outcomes were cause-unspecified (ranging from 38% for deaths to 95% for ambulatory clinic encounters) (Table 1). Within cause-specified AGE outcomes, bacterial illnesses, particularly C. difficile, accounted for the bulk of the diagnoses (Appendix Table 3). Norovirus-coded outcomes accounted for <0.25% of all-cause AGE (Appendix Table 4).
Ambulatory Clinic Encounters and ED Visits
We estimated a mean annual rate of 75 (95% Confidence Interval [CI]: 58– 95) norovirus-associated ambulatory clinic encounters per 10,000 person-years (PY) and 15 (95% CI: 12 – 20) norovirus-associated ED visits per 10,000 PY (Table 2), across all ages, equivalent to 2.3 million ambulatory clinic encounters and 470,000 ED visits annually. Estimated norovirus rates for each of these outcomes were disproportionately high in children <5 and adults ≥85 years of age. Among children aged <5 years, the annual estimated norovirus ambulatory clinic encounter and ED visit rates were 281 (95% CI: 221 – 356) per 10,000 PY and 46 (95% CI: 36 – 59) per 10,000 PY, accounting for 23% and 30% of AGE-associated ambulatory clinic encounters and ED visits, respectively. Among adults 85+ years, the annual estimated norovirus ambulatory clinic encounter rate was 151 (95% CI: 107 – 203) per 10,000 PY, while the estimated norovirus-associated ED visit rate was 32 (95% CI: 24 – 41) per 10,000 PY, accounting for 13% and 19% of AGE-associated ambulatory clinic and ED visits, respectively.
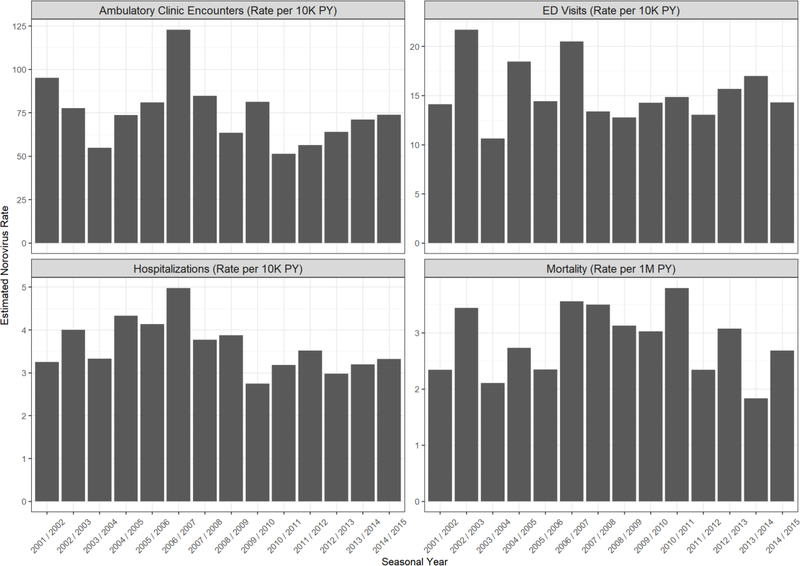
Estimated norovirus ED visit rates spiked in 2002/03, a year during which a new norovirus strain emerged and was associated with increased outbreak activity[21, 22]; a spike was also noted for ED visit rates in 2004/05, a year during which another new norovirus strain emerged[23] (Figure 1). Both ambulatory clinic visit rates and ED visit rates spiked in 2006/07, another year during which a new norovirus strain emerged and was associated with increased outbreak activity[21, 24]. A clear winter seasonality was noted in most age groups for both settings; however, in age groups 65–74 and 75–84, annual median estimated norovirus ambulatory clinic encounters did not have a pronounced winter seasonality (Appendix Figures 1, 2).
Figure 1: Estimated Rates of Norovirus Encounters by Seasonal Year—United States, 2001/2002 – 2014/2015.
Estimated annual rates of norovirus-related encounters varied by seasonal year as well as outcome.
Hospitalizations
We estimated a mean annual rate of 3.6 (95% CI: 2.6 – 4.8) norovirus-associated hospitalizations per 10,000 PY, accounting for 9% of all-cause AGE hospitalizations and equivalent to 110,000 hospitalizations per year (Table 2). Estimated norovirus hospitalization rates were disproportionately high in the youngest and oldest age groups. Among children <5 years old, the annual estimated norovirus hospitalization rate was 13 per 10,000 (95% CI: 10 – 16), accounting for 25% of all-cause AGE hospitalizations in this age group. Among adults ≥85 years old, the annual estimated norovirus hospitalization rate was 29 per 10,000 (95% CI: 21 – 38), accounting for 11% of all-cause AGE hospitalizations in this age group. Estimated norovirus hospitalization rates spiked in 2002/03 and 2006/07 seasonal years and exhibited a pronounced winter seasonality in all age groups. (Figure 1, Appendix Figures 1, 2)
Deaths
We estimated a mean annual rate of 2.8 (95% CI: 2.2 – 3.6) norovirus deaths per million PY, making up 5% of all-cause AGE deaths and equivalent to 900 norovirus deaths annually (Table 2). Norovirus deaths were highest among adults aged ≥65 years (19 per million PY; 95% CI: 14 – 24) and accounted for 86% of estimated norovirus deaths across all ages. Estimated norovirus mortality rates increased in 2002/03 and 2006/07, as seen in hospitalizations. Mortality rates remained elevated until 2011/12, when rates again decreased (Figure 1). Estimated norovirus mortality rates in adults ≥65 years old exhibited a modest winter seasonality, with pronounced and elevated peaks in the 2002/03, 2006/07, and 2012/13 seasonal years; though a new norovirus strain emerged in 2012/13, it was not associated with increased outbreak activity[25] (Appendix Figures 1, 2).
Healthcare Charges
Norovirus-associated ambulatory clinic and ED visits resulted in an average of $225 million and $237 million annually in U.S. healthcare charges, respectively. Compared to other age groups, adults aged 18 to 64 years carried the highest mean charge per norovirus ambulatory clinic and ED visit ($111 and $586, respectively), and had the highest total estimated national charges for norovirus ambulatory clinic and ED visits ($84 million and $141 million, respectively). (Table 3) On average, norovirus hospitalizations resulted in $106 million annually in U.S. healthcare charges; over half of these charges were associated with children <5 years of age, as this age group had the highest mean charge per hospitalization ($2513).
Table 3:
Estimated healthcare burden and medical charges associated with norovirus in the United States, July 2001 – June 2015 (in 2016 U.S. dollars)
| Estimated Total Annual NV Counts in 10,000s (95% CI) | Mean Charge per Encounter (SD) | Estimated Total Annual Charges in MM of 2016 U.S.$ (95% CI) | |
|---|---|---|---|
| Ambulatory Clinic Encounters | |||
| <5 years | 56.1 (44.0 – 71) | $84.08 ($3.86) | $47.2 ($37 – $59.7) |
| 5 – 17 years | 53.3 (41.8 – 66.4) | $87.47 ($4.38) | $46.6 ($36.6 – $58.1) |
| 18 – 64 years | 75.6 (58.2 – 95.5) | $110.73 ($4.31) | $83.7 ($64.4 – $105.8) |
| 65 – 74 years | 16.0 (11.7 – 21.3) | $101.63 ($5.49) | $16.3 ($11.9 – $21.6) |
| 75 – 84 years | 18.5 (13.2 – 24.6) | $97.63 ($7.43) | $18.1 ($12.9 – $24) |
| 85+ years | 7.9 (5.6 – 10.6) | $92.62 ($8.23) | $7.3 ($5.2 – $9.8) |
| All ages | 227.3 (174.5 – 289.4) | $98.89 ($5.82) | $224.8 ($172.6 – $286.2) |
| ED Visits | |||
| <5 years | 9.2 (7.2 – 11.8) | $410.56 ($91.27) | $37.8 ($29.6 – $48.4) |
| 5 – 17 years | 7.4 (5.5 – 9.7) | $481.22 ($112.86) | $35.6 ($26.5 – $46.7) |
| 18 – 64 years | 24.0 (17.8 – 31.6) | $586.06 ($154.05) | $140.7 ($104.3 – $185.2) |
| 65 – 74 years | 2.2 (1.6 – 3) | $327.55 ($123.68) | $7.2 ($5.2 – $9.8) |
| 75 – 84 years | 2.1 (1.4 – 2.8) | $286.79 ($113.09) | $6.0 ($4.0 – $8.0) |
| 85+ years | 1.7 (1.2 – 2.1) | $272.38 ($102.93) | $4.6 ($3.3 – $5.7) |
| All ages | 46.5 (34.8 – 61) | $508.68 ($130.21) | $236.5 ($177 – $310.3) |
| Hospitalizations | |||
| <5 years | 2.5 (2 – 3.2) | $2513.13 ($565.66) | $62.8 ($50.3 – $80.4) |
| 5 – 17 years | 0.7 (0.5 – 1) | $2291.48 ($159.91) | $16 ($11.5 – $22.9) |
| 18 – 64 years | 3.1 (2.1 – 4.2) | $1116.32 ($360.17) | $34.6 ($23.4 – $46.9) |
| 65 – 74 years | 1.4 (1.0 – 1.8) | $538.9 ($60.72) | $7.5 ($5.4 – $9.7) |
| 75 – 84 years | 1.8 (1.3 – 2.4) | $492.28 ($26.51) | $8.9 ($6.4 – $11.8) |
| 85+ years | 1.5 (1.1 – 2) | $505.65 ($39.08) | $7.6 ($5.6 – $10.1) |
| All ages | 10.9 (8.0 – 14.5) | $967.52 ($222.86) | $105.5 ($77.4 – $140.3) |
Abbreviations: NV – Norovirus; CI – Confidence Interval; SD – Standard Deviation; MM – Million
Conclusions
Based on this comprehensive analysis of three distinct national administrative databases, we estimate that norovirus is associated with approximately 900 deaths (2.8 per 1,000,000 PY), 109,000 hospitalizations (3.6 per 10,000 PY), 465,000 ED visits (15.3 per 10,000 PY), and 2,270,000 ambulatory clinic encounters (74.9 per 10,000 PY) annually in the United States, resulting in nearly $600 million in medical charges each year. The norovirus rate estimates produced in this study confirm and expand on previously published work. Prior studies using the same databases and modeling methods estimated 800 deaths, 71,000 hospitalizations, 400,000 ED visits, and 1,693,000 ambulatory clinic encounters due to norovirus anually in the United States[13–15]—our estimates for ambulatory clinic encounters, ED visits, hospitalizations, and deaths were somewhat higher but generally comparable. Similar modeling studies performed using administrative databases from the UK, Taiwan, and Canada have also resulted in comparable norovirus burden estimates: 49 outpatient visits per 10,000 PY and 2 – 7 hospitalizations per 10,000 PY[26–28], while estimates from Japan were somewhat higher at 389 outpatient visits and 13 hospitalizations per 10,000 PY[29]. Our estimates are also aligned with norovirus burden estimates generated using alternative methods. Passive surveillance conducted within specific U.S. healthcare systems have estimated the norovirus burden as 56 to 64 ambulatory clinic encounters per 10,000 PY[11, 30], while passive and active surveillance in European countries have estimated a norovirus burden in the range of 9 – 54 outpatient visits per 10,000 PY, 1.2 – 3.4 hospitalizations per 10,000 PY, and 0.5 – 4.0 deaths per 1,000,000 PY[31–35].
The present study was also consistent with prior observations that the elderly may be more vulnerable to severe outcomes from norovirus[36]: we estimated that 86% of annual norovirus-associated deaths and 43% of norovirus-associated hospitalizations in the U.S. occur in adults aged ≥65 years. In contrast, the incidence of norovirus ambulatory clinic encounters and ED visits was highest in children <5 years of age, highlighting the disproportionate burden of norovirus in the outpatient setting among young children. On average, ~20–25% of norovirus-associated ambulatory clinic and ED visits annually occurred in children aged <5 years, and the annual estimated norovirus ambulatory clinic and ED visit rates in this age group were approximately threefold higher than the respective annual rates across all ages combined. Estimated norovirus-associated hospitalization rates were also high in this age group, again nearly threefold higher than the estimated rate across the age spectrum. These J- and U-shaped patterns, with the highest rates of norovirus-associated outcomes seen in the pediatric and elderly populations, are also consistent with previous research in high-resource settings using diverse methods[26, 27, 29, 32, 34].
Our norovirus estimates were consistent with known norovirus epidemiology: deaths and healthcare encounters demonstrated a wintertime (December – February) seasonality, with rates peaking in December and January for each outcome, across most age groups. Norovirus healthcare encounters spiked with the emergence of new genogroup II, type 4 (GII.4) variant norovirus strains associated with increased outbreak activity (2002/03 and 2006/07)[21, 24, 37]. Overall, we did not see similar spikes during the 2009/10 and 2012/13 seasonal years (except for mortality in 2012/13), during which new GII.4 norovirus variants also emerged; outbreak activity during those seasonal years did not reach the levels seen in 2002/03 or 2006/07[25, 38]. Because genotype data were not available for norovirus-coded outcomes, we were unable to verify these patterns even with limited data.
Several limitations of this analysis should be considered. Certain assumptions in the methods may have led to overestimation of the norovirus burden. For instance, other viruses with similar seasonality to norovirus (such as rotavirus, astrovirus, or sapovirus) may have been mistakenly attributed to norovirus if they were not specifically coded. In the case of rotavirus, this would have been most likely in age groups that are not often tested for rotavirus, but who may still be affected (e.g., older individuals). On the other hand, other aspects of the method may have led to underestimation of the norovirus burden. First, in the analysis of residuals, we assumed that there was one month in each seasonal year where there were no norovirus-associated outcomes. However, it is known that norovirus circulates year-round in the United States[7]. Second, ICD codes are not fully sensitive for AGE[39]; the resulting smaller overall AGE envelope could have led to an underestimate of norovirus. Third, although norovirus can present with only vomiting (no diarrhea), we did not include encounters with the corresponding ICD codes (vomiting in the absence of diarrheal / general gastroenteritis codes). When we included these codes into our overall gastroenteritis counts as a sensitivity analysis, we found that our estimates of norovirus were non-negligibly increased for several outcomes (Appendix Table 5); however, since these estimates were, in some cases, far in excess of the 20% increase that might be expected based on previous research[40], we suspect that our estimation methods may not reliably be extended to vomit-only outcomes, given differences in the underlying distribution of causes of vomit-only presentations. It is also possible that our method could have misattributed background trends to norovirus, or vice-versa, if there were secular trends in overall norovirus rates over time. However, given the absence of a norovirus vaccine or novel therapeutic, a decreasing secular trend seems unlikely. If there were an increasing secular trend in norovirus, this method would tend to underestimate the burden. Unfortunately, we were not able to extend this analysis beyond 2015; given the importance of relatively consistent trends in ICD-coded outcomes to the analysis method, we felt it prudent to end the study period at the switch from ICD-9-CM to ICD-10-CM. Despite these limitations, the robustness of the estimates to variations in model parameters (Appendix Table 6) and the consistency of results with known norovirus seasonality as well as existing literature lend confidence to these estimates.
Other limitations stem from the use of the MarketScan data, which is not nationally representative. Because MarketScan includes only individuals with employer-sponsored commercial insurance or employer-sponsored Medicare Supplemental plans (< 60% of the full U.S. population), if these persons seek care for AGE at different rates than non-included individuals (e.g., uninsured or Medicaid-covered persons), then our results for ambulatory clinic and ED visits may not be a good representation of the full U.S. population. For instance, if un- or underinsured persons are more likely to present to an ED and less likely to present to an ambulatory care clinic for AGE, then our ambulatory care numbers may be overestimates, while our ED numbers may be underestimates of the national burden. However, it is reassuring to note that estimates of norovirus hospitalization rates were similar across many age groups in both NIS-HCUP and MarketScan despite population differences (Appendix Table 7), and analysis suggests that norovirus estimates would remain within the calculated confidence limits even if rates were adjusted for differences in database populations.
Lastly, the financial data obtained from MarketScan represent total charges billed to payers and not actual settled costs, and could overestimate the healthcare spending associated with norovirus; however, since these numbers do not account for lost productivity (work absenteeism) or premature death, the total economic burden is likely underestimated[41]. Differences in charges between norovirus-associated and other cause-unspecified gastroenteritis treatment could also affect our estimates of healthcare charges, as could differences in charges for individuals represented within the MarketScan databases as compared to the general U.S. population.
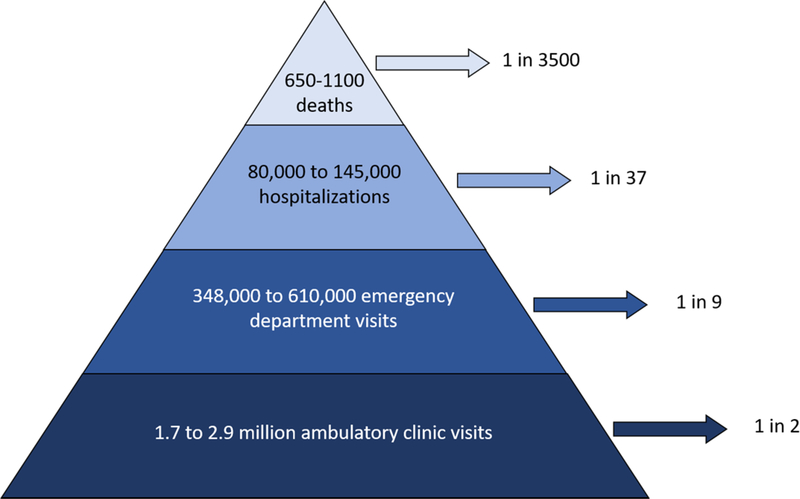
Norovirus causes a significant burden in the United States, causing one in two Americans to have an ambulatory clinic visit, 1 in 9 to go the emergency department, 1 in 37 to be hospitalized, and 1 in 3500 to die from norovirus illness at some point within their lifetime (Figure 2). The burden of outpatient encounters is highest among children, while the elderly experience the highest rates of norovirus-associated hospitalizations and deaths. With several norovirus vaccines in development[5, 42, 43], these findings provide a benchmark against which a future vaccine can be evaluated, as well as an indication of which populations should be targeted for greatest public health impact. Active surveillance of norovirus is warranted to refine burden estimates and provide more detail and precision, particularly with regards to specific norovirus genotypes and economic costs.
Figure 2: The disease burden of norovirus in the United States.
Ranges of total annual deaths, hospitalizations, emergency department visits, and ambulatory clinic encounters are presented within the pyramid and the corresponding estimated lifetime risk is noted with arrows.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to acknowledge the advice of Dr. Ismael Ortega-Sánchez as regards the economic applications of this paper.
FUNDING SOURCE: This work was performed as part of the official duties of the authors as government employees, and no outside funding was sought or obtained.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Vinjé J Advances in Laboratory Methods for Detection and Typing of Norovirus. Journal of Clinical Microbiology 2015; 53(2): 373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chhabra P, de Graaf M, Parra GI, et al. Updated classification of norovirus genogroups and genotypes. J Gen Virol 2019; 100(10): 1393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirk MD, Pires SM, Black RE, et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med 2015; 12(12): e1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall AJ, Lopman BA, Payne DC, et al. Norovirus disease in the United States. Emerg Infect Dis 2013; 19(8): 1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopman BA, Steele D, Kirkwood CD, Parashar UD. The Vast and Varied Global Burden of Norovirus: Prospects for Prevention and Control. PLoS Med 2016; 13(4): e1001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng DP, Widdowson MA, Glass RI, Vinje J. Molecular epidemiology of genogroup II-genotype 4 noroviruses in the United States between 1994 and 2006. J Clin Microbiol 2010; 48(1): 168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon JL, Barclay L, Collins NR, et al. Genetic and Epidemiologic Trends of Norovirus Outbreaks in the United States from 2013 to 2016 Demonstrated Emergence of Novel GII.4 Recombinant Viruses. J Clin Microbiol 2017; 55(7): 2208–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.HL G, Stella C Health Research Data for the Real World: The MarketScan Databases (white paper).
- 9.HCUP. Healthcare Utilization Project: NIS Overview. Available at: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed August 21.
- 10.Esposito DH, Holman RC, Haberling DL, et al. Baseline estimates of diarrhea-associated mortality among United States children before rotavirus vaccine introduction. The Pediatric infectious disease journal 2011; 30(11): 942–7. [DOI] [PubMed] [Google Scholar]
- 11.Hall AJ, Rosenthal M, Gregoricus N, et al. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004–2005. Emerg Infect Dis 2011; 17(8): 1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardemil CV, O’Leary S, Beaty B, et al. Primary care physician knowledge, attitudes and diagnostic testing practices for norovirus and acute gastroenteritis. Pediatric Academic Societies Meeting. Baltimore, MD, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gastanaduy PA, Hall AJ, Curns AT, Parashar UD, Lopman BA. Burden of norovirus gastroenteritis in the ambulatory setting--United States, 2001–2009. J Infect Dis 2013; 207(7): 1058–65. [DOI] [PubMed] [Google Scholar]
- 14.Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis 2012; 55(2): 216–23. [DOI] [PubMed] [Google Scholar]
- 15.Lopman BA, Hall AJ, Curns AT, Parashar UD. Increasing rates of gastroenteritis hospital discharges in US adults and the contribution of norovirus, 1996–2007. Clin Infect Dis 2011; 52(4): 466–74. [DOI] [PubMed] [Google Scholar]
- 16.Bridged-race postcensal estimates of the United States resident population for April 2010, July 1, 2010-July 1, 2016, by county, age, sex, race, and Hispanic origin. National Center for Health Statistics. [Google Scholar]
- 17.Bridged-Race intercensal estimates of the resident population of the United States for July 1, 2000–July 1, 2009, by county, age, sex, race, and Hispanic origin. National Center for Health Statistics. [Google Scholar]
- 18.Life Expectancy. Available at: https://www.cdc.gov/nchs/fastats/life-expectancy.htm.
- 19.Bureau of Economic Analysis. U.S. Department of Commerce. [Google Scholar]
- 20.Dunn A, Grosse SD, Zuvekas SH. Adjusting Health Expenditures for Inflation: A Review of Measures for Health Services Research in the United States. Health Serv Res 2018; 53(1): 175–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease C, Prevention. Norovirus activity--United States, 2002. MMWR Morb Mortal Wkly Rep 2003; 52(3): 41–5. [PubMed] [Google Scholar]
- 22.Widdowson MA, Cramer EH, Hadley L, et al. Outbreaks of acute gastroenteritis on cruise ships and on land: identification of a predominant circulating strain of norovirus--United States, 2002. J Infect Dis 2004; 190(1): 27–36. [DOI] [PubMed] [Google Scholar]
- 23.Bull RA, Tu ET, McIver CJ, Rawlinson WD, White PA. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J Clin Microbiol 2006; 44(2): 327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease C, Prevention. Norovirus activity--United States, 2006–2007. MMWR Morb Mortal Wkly Rep 2007; 56(33): 842–6. [PubMed] [Google Scholar]
- 25.Leshem E, Wikswo M, Barclay L, et al. Effects and Clinical Significance of GII.4 Sydney Norovirus, United States, 2012–2013. Emerging Infectious Diseases 2013; 19(8): 1231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verstraeten T, Cattaert T, Harris J, Lopman B, Tam CC, Ferreira G. Estimating the Burden of Medically Attended Norovirus Gastroenteritis: Modeling Linked Primary Care and Hospitalization Datasets. J Infect Dis 2017; 216(8): 957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burke RM, Shih SM, Yen C, et al. Burden of Severe Norovirus Disease in Taiwan, 2003–2013. Clin Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morton VK, Thomas MK, McEwen SA. Estimated hospitalizations attributed to norovirus and rotavirus infection in Canada, 2006–2010. Epidemiol Infect 2015; 143(16): 3528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang CH, Sakaguchi M, Weil J, Verstraeten T. The incidence of medically-attended norovirus gastroenteritis in Japan: Modelling using a medical care insurance claims database. PLoS One 2018; 13(3): e0195164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grytdal SP, DeBess E, Lee LE, et al. Incidence of Norovirus and Other Viral Pathogens That Cause Acute Gastroenteritis (AGE) among Kaiser Permanente Member Populations in the United States, 2012–2013. PLoS One 2016; 11(4): e0148395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips G, Tam CC, Conti S, et al. Community incidence of norovirus-associated infectious intestinal disease in England: improved estimates using viral load for norovirus diagnosis. Am J Epidemiol 2010; 171(9): 1014–22. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien SJ, Donaldson AL, Iturriza-Gomara M, Tam CC. Age-Specific Incidence Rates for Norovirus in the Community and Presenting to Primary Healthcare Facilities in the United Kingdom. J Infect Dis 2016; 213 Suppl 1: S15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verhoef L, Koopmans M, W VANP, et al. The estimated disease burden of norovirus in The Netherlands. Epidemiol Infect 2013; 141(3): 496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowalzik F, Binder H, Zoller D, et al. Norovirus Gastroenteritis among Hospitalized Patients, Germany, 2007–2012. Emerg Infect Dis 2018; 24(11): 2021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernard H, Hohne M, Niendorf S, Altmann D, Stark K. Epidemiology of norovirus gastroenteritis in Germany 2001–2009: eight seasons of routine surveillance. Epidemiol Infect 2014; 142(1): 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burke RM, Shah MP, Wikswo ME, et al. The Norovirus Epidemiologic Triad: Predictors of Severe Outcomes in US Norovirus Outbreaks, 2009–2016. J Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siebenga JJ, Vennema H, Zheng DP, et al. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001–2007. J Infect Dis 2009; 200(5): 802–12. [DOI] [PubMed] [Google Scholar]
- 38.Yen C, Wikswo ME, Lopman BA, Vinje J, Parashar UD, Hall AJ. Impact of an emergent norovirus variant in 2009 on norovirus outbreak activity in the United States. Clin Infect Dis 2011; 53(6): 568–71. [DOI] [PubMed] [Google Scholar]
- 39.Pindyck T, Hall AJ, Tate JE, et al. Validation of acute gastroenteritis-related ICD-CM codes in pediatric and adult U.S. populations. Clin Infect Dis 2019; (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007–2015. Geneva, Switzerland: World Health Organization, 2015. [Google Scholar]
- 41.Bartsch SM, Lopman BA, Hall AJ, Parashar UD, Lee BY. The potential economic value of a human norovirus vaccine for the United States. Vaccine 2012; 30(49): 7097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riddle MS, Chen WH, Kirkwood CD, MacLennan CA. Update on vaccines for enteric pathogens. Clin Microbiol Infect 2018; 24(10): 1039–45. [DOI] [PubMed] [Google Scholar]
- 43.Mattison CP, Cardemil CV, Hall AJ. Progress on norovirus vaccine research: public health considerations and future directions. Expert Rev Vaccines 2018; 17(9): 773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.