Abstract
Rotavirus vaccination has been associated with a short-term increased risk of intussusception. Analysis of insurance claims for 1,858,827 US children with 544 recorded cases of intussusception found a non-significant decrease in intussusception (HR 0.79, 95% CI: 0.57 – 1.09) in fully rotavirus-vaccinated children followed up to 2 years of age.
Keywords: Rotavirus, Rotavirus vaccine, Immunizations, Intussusception, Pediatric gastroenteritis
Background
RotaShield®, the first vaccine against rotavirus, was licensed in the United States in 1998; however, it was withdrawn from the market in 1999 after it was associated with intussusception [1]. Beginning in 2006, two rotavirus vaccines were licensed and recommended for routine immunization of US infants: pentavalent RotaTeq® (RV5; Merck and Company), given at 2, 4, and 6 months of age; and monovalent Rotarix®(RV1; GlaxoSmithKline Biologicals), given at 2 and 4 months of age [2].
While clinical trials did not show a risk [3, 4], post-licensure studies have indicated that both rotavirus vaccines are associated with a temporally limited increase in risk of intussusception, mainly in the first week after vaccination [5]. However, no research has assessed whether longer-term intussusception risk differs by rotavirus vaccination status, which is important to examine for at least two reasons. First, it is possible that rotavirus vaccination might trigger cases of intussusception among vulnerable infants; if vaccination changes the timing rather than the risk of intussusception, the overall intussusception risk would not be expected to increase among vaccinated infants during long-term follow-up [6]. Second, it is possible that rotavirus vaccination could decrease long-term risk of intussusception by preventing natural rotavirus infection, if natural rotavirus infection is a cause of intussusception, as has been suggested [7, 8].
We utilized administrative claims data to examine differences in intussusception risk by rotavirus vaccination status among commercially insured U.S. children followed for up to 2 years of age.
Methods
We abstracted 2006 – 2016 data from the Truven Health MarketScan Commercial Claims and Encounters database, which covers millions of U.S. individuals enrolled in employer-sponsored commercial health insurance plans representing > 100 different payers. Claims and encounter data, and some demographic information, are available at the individual level [9]. This analysis was not considered human subjects research.
The first eligibility criterion was birth date on or after January 1, 2006, so that all included children were eligible to receive the first dose of rotavirus vaccine at 2 months of age (the vaccine was recommended for use in the U.S. in February, 2006) [2]. The date of birth was proxied using the date of the earliest delivery-related claim identified by the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes V30-V39. Secondly, we required continuous enrolment in the insurance plan beginning with the month of birth so that all potential vaccinations or intussusception events were captured. At least 8 weeks of continuous enrollment were required, and children were followed beginning from the month of birth. Current Procedural Terminology (CPT) codes (90680 [RV5] and 90681 [RV1]) were used to determine rotavirus vaccination status and the infant’s age at each dose. Children with improbable dates of vaccination (dose 1 given at <4 weeks of age, dose 2 given <6 weeks, or dose 3 <8 weeks), or who had plausible dates for later doses but missing dates for earlier doses, were excluded. Unvaccinated children were defined by the absences of a rotavirus vaccine CPT code. Once a child received a single dose of either rotavirus vaccine, they were reclassified as “partially vaccinated.” Children were reclassified as “fully vaccinated” once they had received two doses of RV1, three doses of RV5, or 3 doses of a mixed series. Intussusception was defined as discharge ICD code 560.0* (ICD-9) or K56.1 (ICD-10) in any position, and only the first event was considered. In a sensitivity analysis, intussusception discharge codes were required to be in the first diagnostic position. In the absence of an intussusception event, children were censored at 24 months or when no longer enrolled, whichever came first. To increase sensitivity while mitigating the risk of misclassification, children presenting to the emergency room with suspected intussusception were included only if hospitalized within 2 days of presentation; the date of first presentation was used in the analysis.
Survival analysis was employed using time-to-intussusception as the outcome, and age (in weeks) as the time scale. The time-varying nature of the exposure (vaccination status) was accounted for by analyzing data in the counting process format and using extended Cox regression. The model included rotavirus vaccination status as the (time-varying) exposure and controlled for year of birth (to account for secular changes in rotavirus vaccine coverage and rotavirus circulation) and DTaP doses (also time-varying); robust standard errors were calculated and used to generate 95% confidence intervals (CI). Unless otherwise noted, person-time was recorded and analyzed beginning from birth.
Four sensitivity analyses were performed. In the first sensitivity analysis, we excluded children born in states with universal rotavirus vaccination programs to mitigate the possibility of exposure misclassification (i.e., mistakenly classifying children as unvaccinated if their vaccinations were not reflected by an insurance claim); these states are listed in Table 1. In the second sensitivity analysis, we only included only intussusception codes in the first diagnostic position. In a third sensitivity analysis, we included only children who had received at least one dose of DTaP-containing vaccine by the age of six months, since children who do not receive any routine vaccinations may be different from those who do; these analyses included only children surviving intussusception-free until at least six months of age. In the last sensitivity analysis, we ran the third sensitivity analysis but restricted events to intussusceptions in the first diagnostic position. We also compared the age at first rotavirus vaccination dose among children who were partially versus fully vaccinated to see if partially vaccinated children were more likely to have delayed vaccination schedules. We also compared the ages at first and last doses by vaccine type. Data were cleaned and analyzed using SAS version 9.4 (Cary, NC) and the R Environment for Statistical Computing.
Table 1:
Comparison of Results from Primary and Sensitivity Analyses, Hazard Ratio for Intussusception by Rotavirus Vaccination Status (No Rotavirus Vaccination as Referent Category)*
| Full rotavirus vaccination | Partial rotavirus vaccination | |||||
|---|---|---|---|---|---|---|
| Analysis | HR** | 95% CI | P value | HR** | 95% CI | P value |
| Primary analysis | 0.79 | (0.57, 1.09) | 0.13 | 0.89 | (0.66, 1.19) | 0.45 |
| Sensitivity analyses | ||||||
| Excluding children born in states with universal rotavirus vaccination programs*** | 0.84 | (0.59, 1.20) | 0.32 | 0.91 | (0.66, 1.24) | 0.54 |
| Including intussusception in only the first diagnostic position | 0.70 | (0.48, 1.03) | 0.06 | 0.93 | (0.65, 1.33) | 0.67 |
| Including only children receiving at least one dose of DTaP by the age of 6 months (model uses data from children surviving intussusception-free until at least 6 months of age) | 0.70 | (0.49, 1.01) | 0.04 | 0.70 | (0.45, 1.08) | 0.10 |
| Including intussusception in only the first diagnostic position and only children receiving at least one dose of DTaP by the age of 6 months (model uses data from children surviving intussusception-free until at least 6 months of age) | 0.62 | (0.41, 0.95) | 0.02 | 0.85 | (0.51, 1.41) | 0.52 |
All rotavirus models adjust for year of birth and receipt of DTaP doses and use unvaccinated children as the referent. HR=Hazard Ratio.
2007 – 2012: Alaska, Idaho, Maine, Massachusetts, New Hampshire, New Mexico, North Dakota, Oregon, Rhode Island, Vermont, Washington, Wisconsin, Wyoming; 2013 – 2014: Connecticut, Idaho, Maine, Massachusetts, New Hampshire, New Mexico, Rhode Island, South Dakota, Vermont, Washington, Wyoming; 2015 – 2016: Alaska, Connecticut, Idaho, Maine, Massachusetts, New Hampshire, New Mexico, Rhode Island, Vermont, Washington, Wyoming.
Results
Among 1,858,827 children eligible for analysis, intussusception was recorded in any diagnostic position for 544, among whom 385 had the code in the first diagnostic position. Most (406, 74.6%) intussusception cases occurred before 12 months of age. The estimated 2-year risk of intussusception was 0.044% (95% CI 0.040 – 0.048%). Of 1,349,221 children surviving and followed until at least 8 months of age, 906,850 (67.2%) were fully vaccinated, 168,291 (12.5%) were partially vaccinated, and 274,080 (20.3%) were unvaccinated.
The crude survival curve showed that the greatest intussusception hazard was between approximately 2 and 12 months of age. Confidence intervals overlapped for vaccinated, partially vaccinated, and unvaccinated children (Figure 1). Crude and adjusted hazard ratios (HR) were similar, < 1, and non-significant (adjusted HR [aHR] for full vaccination: 0.79, 95% CI: 0.57 – 1.09; aHR for partial vaccination: 0.89 [0.66 – 1.19]). HRs were similar in sensitivity analyses, and the effects were stronger when restricting to intussusceptions in the first diagnostic position or including only children receiving at least one DTaP by 6 months (Table 1). The median age at which the first dose of vaccine was received (9 weeks) was similar among partially and fully vaccinated children, and for children receiving RV1 versus RV5 (median 9 weeks for both). The median age at which the last dose of vaccine was received was 18 weeks for children receiving RV1 and 27 weeks for children receiving RV5.
Figure 1: Unadjusted intussusception hospitalization survival curves.
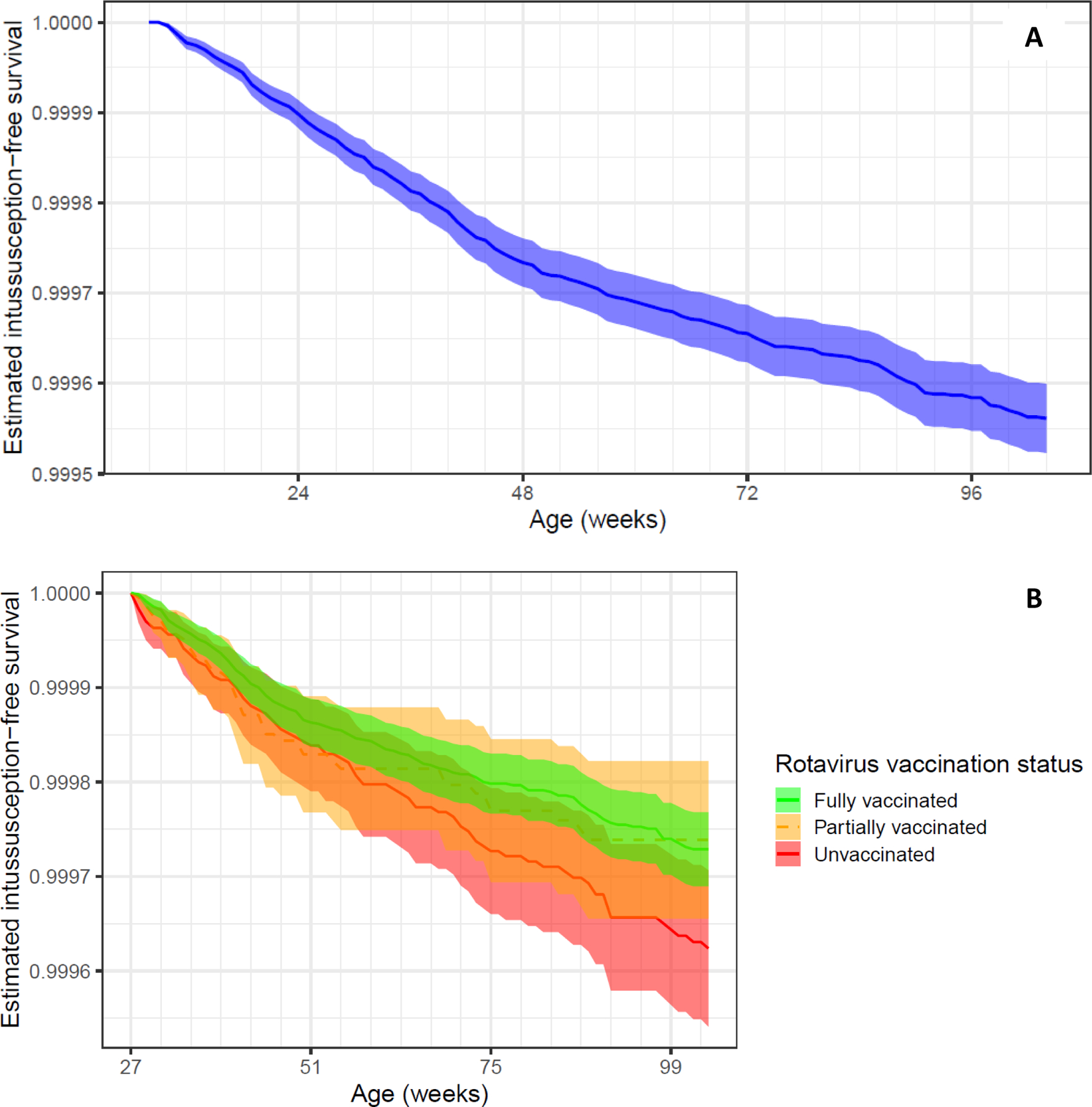
A) Crude survival curve. The plot is shown beginning at 8 weeks and going to 2 years of age. Only children with at least 8 weeks of continuous enrollment were included (N = 1,858,827). B) Crude survival curve, by vaccination status. Intussusception survival curves overlapped among children fully vaccinated (green), as compared to children partially (yellow) or unvaccinated (red) against rotavirus. The plot is shown beginning at 27 weeks (N = 1,463,313), by which time all children are eligible to be fully vaccinated against rotavirus. Rotavirus vaccination status is treated as time-varying.
Discussion
In this longitudinal cohort of commercially insured U.S. children followed up to 2 years of age, full series rotavirus vaccination was not associated with an increased long-term risk of intussusception. In fact, we observed a 21% decrease in the two-year risk of intussusception, even including the period of increased risk immediately following vaccination; though these results did not show statistical significance, they were consistent across sensitivity analyses. Intriguingly, a study of intussusception admissions in the periods before and during Rotashield use also suggested a shift in the age distribution of intussusception cases, with increased incidence in the younger ages (corresponding to the age of vaccination) and decreased incidence in the older ages [6]. More recently, and focusing on second-generation rotavirus vaccines, a population-based ecological analysis of intussusception rates in the U.S. found no significant increase in overall intussusception rates among infants comparing post-vaccine years (2006 and beyond) to pre-vaccine years [10]. These observations are consistent with the hypothesis that rotavirus vaccination is not associated with an overall increased risk of intussusception during infancy and toddlerhood. It might be that rotavirus vaccination triggers intussusception that would have occurred later in childhood or prevents intussusception associated with natural rotavirus infection. If intussusceptions occurring proximal to rotavirus vaccination are more quickly diagnosed and treated, this could lead to improved outcomes relative to later-occurring and later-diagnosed intussusceptions. Additionally, short-term increased risk might be offset by long-term decline, affecting risk-benefit considerations.
This analysis has several strengths. The large population in the MarketScan database—with over 2 million children eligible for analysis, and over 1 million doses of vaccine given—improves our power to detect significant differences in intussusception risk even given the rarity of the event. Including intussusception in any diagnostic position in the analysis also enhanced our ability to detect intussusception cases. The sensitivity analyses helped to elucidate the potential relationship between intussusception and rotavirus vaccination. Excluding children born into states with universal vaccination programs mitigated the possibility of misclassification of vaccination status. Results were similar in the analyses including only intussusception codes in the first diagnostic position, which is reassuring regarding the possibility of misclassification of outcome status. Consistent results among analyses including only children receiving at least one DTaP vaccination also suggest that results were not due to confounding.
Several limitations should be considered when interpreting the present analysis. First, the MarketScan database is not fully representative of the entire U.S. population because it does not include individuals covered by Medicaid or non-employer-sponsored insurance plans. Thus, the included population may differ on factors related to immunization, such as access to care. Second, we did not have data available to control for all possible potential confounders, such as race / ethnicity, so residual confounding may exist. However, the age at which the first dose was received was similar among all vaccinated children, suggesting that fully and partially vaccinated children are somewhat comparable in their vaccine-seeking behavior. Third, ICD-9 codes for intussusception may be neither fully sensitive nor specific, and were not confirmed by chart review. However, previous research has shown that intussusception ICD codes in the inpatient setting have a positive predictive value of 75% [11]. Fourth, the analysis does not include intussusceptions treated only in the emergency department unless the patient was admitted to hospital within 48 hours. Thus, it is possible that some outcome misclassification could have occurred, which could have biased results if associated with rotavirus vaccination status. Last, intussusception is an extremely rare event. As such, our power to detect small associations is limited even given the large size of the MarketScan database.
The present study indicates that rotavirus vaccination is not associated with an increased two-year intussusception risk among commercially insured U.S. children, and might even be associated with an overall reduced risk. The benefits of rotavirus vaccination have already been estimated to outweigh the increased short-term risk of intussusception [12]; the present study provides additional data to support the long-term safety profile of the vaccines and supports continued universal rotavirus vaccination in the U.S.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
CONFLICT OF INTEREST
No authors have any conflicts of interest to declare.
REFERENCES
- 1.Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 2001; 344(8): 564–72. [DOI] [PubMed] [Google Scholar]
- 2.Cortese MM, Parashar UD, CDC. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58(RR-2): 1–25. [PubMed] [Google Scholar]
- 3.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354(1): 11–22. [DOI] [PubMed] [Google Scholar]
- 4.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354(1): 23–33. [DOI] [PubMed] [Google Scholar]
- 5.Kassim P, Eslick GD. Risk of intussusception following rotavirus vaccination: An evidence based meta-analysis of cohort and case-control studies. Vaccine 2017; 35(33): 4276–86. [DOI] [PubMed] [Google Scholar]
- 6.Simonsen L, Morens D, Elixhauser A, Gerber M, Van Raden M, Blackwelder W. Effect of rotavirus vaccination programme on trends in admission of infants to hospital for intussusception. Lancet 2001; 358(9289): 1224–9. [DOI] [PubMed] [Google Scholar]
- 7.Minney-Smith CA, Levy A, Hodge M, et al. Intussusception is associated with the detection of adenovirus C, enterovirus B and rotavirus in a rotavirus vaccinated population. J Clin Virol 2014; 61(4): 579–84. [DOI] [PubMed] [Google Scholar]
- 8.Konno T, Suzuki H, Kutsuzawa T, et al. Human rotavirus infection in infants and young children with intussusception. J Med Virol 1978; 2(3): 265–69. [DOI] [PubMed] [Google Scholar]
- 9.HL G, Stella C Health Research Data for the Real World: The MarketScan Databases (white paper).
- 10.Tate JE, Yen C, Steiner CA, Cortese MM, Parashar UD. Intussusception Rates Before and After the Introduction of Rotavirus Vaccine. Pediatrics 2016; 138(3). [DOI] [PubMed] [Google Scholar]
- 11.Shui IM, Baggs J, Patel M, et al. Risk of intussusception following administration of a pentavalent rotavirus vaccine in US infants. JAMA 2012; 307(6): 598–604. [DOI] [PubMed] [Google Scholar]
- 12.Desai R, Cortese MM, Meltzer MI, et al. Potential intussusception risk versus benefits of rotavirus vaccination in the United States. Pediatr Infect Dis J 2013; 32(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


