Introduction
Coronavirus Disease 2019 (COVID-19) has posed unique challenges to nephrology. COVID-19–associated acute kidney injury occurs in as high as 36.6% of patients,S1 with the most common etiology being acute tubular injury due to hemodynamic changes and a proinflammatory milieu. In addition to acute tubular injury, patients with COVID-19 can also develop a variety of glomerular pathologies, including primary podocytopathies and immune-mediated glomerular diseases (membranous nephropathy, lupus nephritis, anti–glomerular basement membrane disease).1 Here, we report another pattern of tubular pathology related to COVID-19, osmotic injury associated with antiviral therapy.
Case Presentation
A 33-year-old African American obese man with past medical history of poorly controlled type 2 diabetes mellitus and hypertension presented with left-sided abdominal pain for 2 days. His nasopharyngeal swab polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2 was positive. He was febrile, 38.1 °C (100.6 °F), tachycardic, 106 beats per minute, and blood pressure of 140/79 mm Hg. Respiratory rate of 18 per minute, and oxygen saturation of 98% on room air. He was not in acute distress and was noted to have dry mucous membranes, clear respiratory sounds, and no pedal edema. Computed tomography showed ground glass opacities in both lung bases compatible with COVID-19. Initial laboratory values showed acute kidney injury and hyponatremia (Table 1). The patient was admitted for management of COVID-19 pneumonia and acute kidney injury.
Table 1.
Laboratory values on admission and during the hospital course
| Laboratory | Reference range | Unit | Hospital day |
||
|---|---|---|---|---|---|
| 0 | 5 | 7 | |||
| Sodium | 136–145 | mmol/l | 131 | 123 | 130 |
| Potassium | 3.4–5.1 | mmol/l | 4.6 | 4.9 | 4.7 |
| Chloride | 98–107 | mmol/l | 93 | 89 | 95 |
| Bicarbonate | 22–31 | mmol/l | 25 | 23 | 22 |
| Urea | 6–23 | mg/dl | 30 | 31 | 38 |
| Creatinine | 0.5–1.2 | mg/dl | 1.86 | 1.98 | 2.38 |
| Glucose | 70–100 | mg/dl | 488 | 355 | 198 |
| eGFR | >59 | ml/min per 1.73 m2 | 46 | 43 | 35 |
| Calcium | 8.8–10.7 | mg/dl | 9 | 8.1 | 8.5 |
| Magnesium | 1.7–2.6 | mg/dl | 1.8 | ||
| Albumin | 3.5–5.2 | g/dl | 3.8 | 2.8 | 2.8 |
| Total bilirubin | 0.0–1.0 | mg/dl | 0.4 | 0.3 | 0.2 |
| AST | 10–50 | U/l | 12 | 96 | 191 |
| ALT | 10–50 | U/l | 17 | 130 | 200 |
| ALP | 35–130 | U/l | 85 | 70 | 78 |
| Ferritin | 30–400 | μg/l | 291 | 1881 | |
| LDH | 135–225 | U/l | 186 | ||
| D-dimer | <500 | ng/ml | 276 | 536 | |
| PT-INR | 0.9–1.1 | - | 1.1 | ||
| Serum osmolality | 280 – 296 | mOsm/kg water | 278 | ||
| Hemoglobin | 13.5–18.0 | g/dl | 11.2 | 10.4 | 11 |
| Hematocrit | 40.0–54.0 | % | 33.9 | 30.4 | 31.7 |
| White blood cell | 4–10 | K/μl | 5.59 | 4.47 | 4.7 |
| Platelet | 150–450 | K/μl | 213 | 193 | 201 |
| Abs neutrophil | 1.92–7.6 | K/μl | 3.88 | 3.99 | 3.99 |
| Abs lymphocyte | 0.72–4.10 | K/μl | 0.91 | 1.37 | 1.37 |
Abs, absolute; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; PT-INR, prothrombin time and international normalized ratio.
Hospital Course
After fluid resuscitation, creatinine further rose to 1.98 mg/dl, and hyponatremia worsened to 123 mmol/dl (127 mmol/dl, corrected with glucose). Urine studies suggested the syndrome of inappropriate antidiuretic hormone secretion (Table 2). The 24-hour urine collection confirmed the nephrotic range proteinuria: total protein of 12.8 g, with 8.6 g being albumin. The patient was obese (body mass index 34.1) and had poorly controlled diabetes type 2 since age 20; hemoglobin A1c on admission was 12.1%, but his urine microalbumin was 677 mg/g creatinine 1 year before the admission, with associated baseline creatinine of 1.58 mg/dl. Renal ultrasound showed bilateral echogenic cortices with slightly enlarged kidneys (right kidney at 13.1 cm and left kidney at 13.4 cm) without evidence of hydronephrosis, and renal arteries and veins were patent.
Table 2.
Urine analysis and urine chemistry
| Laboratory | Reference range | Unit | Hospital day |
||
|---|---|---|---|---|---|
| 0 | 5 | 7 | |||
| Bilirubin | Negative | Negative | |||
| Blood | Negative | 2+ | |||
| Clarity | Clear | Clear | |||
| Color | Yellow | Yellow | |||
| Glucose | Negative | 3+ | |||
| Ketones | Negative | Negative | |||
| Leukocyte esterase | Negative | Negative | |||
| Nitrites | Negative | Negative | |||
| pH | 4.5–8.0 | 5 | |||
| Protein | Negative | 3+ | |||
| Specific gravity | 1.003–1.035 | 1.013 | |||
| Urobilinogen | Negative | Negative | |||
| Red blood cell | 0–2 | /hpf | 3 | ||
| White blood cell | <10 | /hpf | 1 | ||
| Bacteria | Negative | /hpf | 1+ | ||
| Squamous cells | Negative | /hpf | Negative | ||
| Hyaline casts | 0–2 | /lpf | 6 | ||
| Granular casts | none | /lpf | 4 | ||
| Urine osmolality | 150–1150 | mOsm/kg water | 304 | ||
| Urine sodium | mmol/l | 30 | |||
| Urine protein | 0–15 | mg/dl | 648 | 715 | |
| Urine albumin | 0.0–2.0 | mg/dl | 394 | 479 | |
| Urine creatinine | mg/dl | 52 | |||
| UPCR | g/g | 12.5 | |||
| UACR | mg/g | 7576 | |||
| Total urine protein | 0–165 | mg/24 h | 12,870 | ||
| Total urine albumin | 0–30 | mg/24 h | 8622 | ||
| Urine volume | ml/24 h | 1800 | |||
hpf, high-power field; lpf, low-power field; UACR, urine albumin to creatinine ratio; UPCR, urine protein to creatinine ratio.
On hospital day 6, the patient developed worsened bilateral opacities on chest radiograph with associated hypoxemia. A course of remdesivir and dexamethasone was started. Remdesivir 200 mg was given i.v. for 1 dose, followed by 100 mg i.v. per day for 4 additional doses. Dexamethasone (10 mg per day i.v.) was given concomitantly for 6 doses. Blood glucose during the hospitalization was well-controlled by insulin. Additional laboratory values revealed total complement >95 unit/ml (reference 42–95 units/ml), C3 115 units/ml (reference 90–180 units/ml), C4 56 units/ml (reference 10–40 units/ml). Serology for anti-neutrophil cytoplasmic antibodies, anti-myeloperoxidase, anti-proteinase-3, anti-PLA2R antibodies, HIV, hepatitis B surface antigen, and hepatitis C virus antibodies were all negative. Serum protein electrophoresis did not reveal evidence of M-spike. The serum-free light chain (FLC) ratio was 1.08 (kappa FLC 101.2 mg/l, lambda FLC 93.5 mg/l). The patient did not receive osmotic agents that are associated with osmotic tubulopathy, such as, but not limited to, i.v.Ig, mannitol, low-molecular weight dextrans, radiologic contrast media, hydroxyethyl starch, excess glucose, methanol, and gelatin.
A kidney biopsy was performed to determine the etiology of proteinuria.
Kidney Biopsy
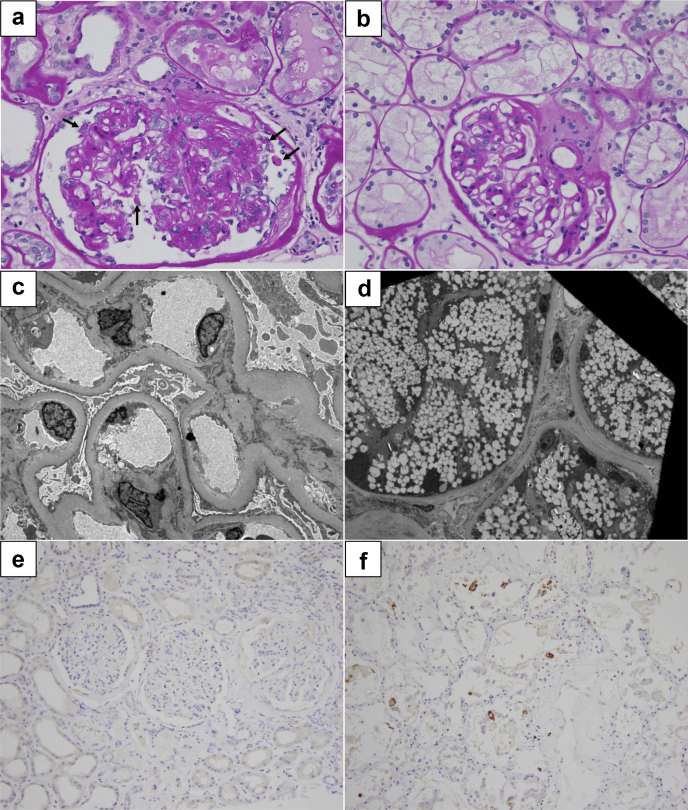
The sample consisted of renal cortex with 31 glomeruli, of which 3 were globally sclerosed, 2 were segmentally sclerosed, and 11 were hypoperfused. The nonsclerosed glomeruli were enlarged. Although a classic example of a collapsing lesion is not seen on multiple levels examined, 2 glomeruli showed features strongly suggestive of segmental capillary collapse, with proliferating epithelial cells filling the Bowman space (Figure 1a). The mesangium was found to be expanded by the extracellular matrix, with few periodic acid-Schiff–positive Kimmelstiel-Wilson nodules. Nonatrophic tubules were enlarged and showed mild distension of the lumen. The tubular epithelium was focally flattened with multifocal loss of the brush border of the proximal tubular epithelium. Tubular epithelial cells revealed diffuse prominent vacuolization (Figure 1b). Several tubules contained necrotic cellular debris and periodic acid-Schiff–positive organizing casts. The interstitium contained mild inflammation in areas of atrophy, which were composed of mononuclear cells and rare eosinophils, neutrophils, and plasma cells. Approximately 70% of the renal cortex showed tubular atrophy and interstitial fibrosis.
Figure 1.
(a) Glomerulus with features suggestive of collapsing glomerulopathy; there is segmental collapse of capillaries and increased number of epithelial cells within the Bowman space. Several epithelial cells also reveal coarse protein reabsorption granules (arrows) (periodic acid-Schiff, original magnification ×40). (b) Proximal tubules with diffuse microvesicular degeneration. The glomerulus reveals moderate expansion of the mesangium by matrix (periodic acid-Schiff, original magnification ×40). (c) The electron microscopy reveals markedly thickened glomerular basement membranes and diffuse effacement of podocyte foot processes (original magnification ×4100). (d) Proximal tubular epithelial cells show extensive vacuolization of the cytoplasm (original magnification ×1640). (e) The renal cortical tissue is negative for severe acute respiratory syndrome coronavirus 2 antigens by immunoperoxidase staining (original magnification ×20). (f) Positive control for severe acute respiratory syndrome coronavirus 2 antigens by immunoperoxidase staining. Lung tissue obtained at autopsy from a patient who died of Coronavirus Disease 2019 (original magnification ×20).
Ultrastructural examination revealed near-global and diffuse effacement of glomerular visceral epithelial cell foot processes (Figure 1c) and prominent cytoplasmic vacuolization of many proximal tubular epithelial cells (Figure 1d). Immunofluorescence and electron microscopy showed no evidence of paraprotein or immune complex deposition.
The immunohistochemical stain for severe acute respiratory syndrome coronavirus 2spike protein was negative in the kidney (Figure 1e).
Follow-up
The patient’s oxygen requirement improved, and creatinine continued to improve to 1.65 mg/dl during the course of i.v. remdesivir therapy. The patient declined genetic testing of APOL1 risk alleles.
Discussion
Our case highlights 2 histopathologic findings developing in the setting of severe acute respiratory syndrome coronavirus 2 infection: collapsing glomerulopathy and osmotic tubulopathy. Collapsing glomerulopathy is one of the characteristic glomerular pathologies reported in COVID-19, as seen also in other viral illnesses, such as HIV and parvovirus B19, with Apolipoprotein L1 risk alleles being one of the risk factors (see Supplementary discussion).S2-S5 In our patient, although the kidney biopsy showed a significant nodular diabetic glomerulosclerosis, the extent of proteinuria (12 g/d) was much more than what could be explained solely by the progression of diabetic kidney disease. The patient had only 677 mg/g of albuminuria 1 year before this presentation, and COVID-19 infection likely worked as a second hit to cause diffuse podocyte injury, which resulted in massive proteinuria.
Osmotic tubulopathy is a finding characterized by cytoplasmic vacuolization, seen when proximal tubules become overwhelmed by the load of indigestible carbohydrates, such as sucrose, dextrans, hydroxyethyl starch, mannitol, and iodinated contrast media.2 Remdesivir is a nucleotide analog that is used to treat patients with COVID-19 both in severe and mild disease. Due to its limited water solubility, remdesivir formulation contains sulfobutylether-β-cyclodextrin (SBECD) as a solubilizing carrier agent, and we postulate that this contributed to osmotic tubulopathy in our patient.
First-generation cyclodextrin carrier agents, α- and β-cyclodextrin, are cyclic oligosaccharides consisting of a macrocyclic ring of glucose subunits joined by α-1,4 glycosidic bonds, and are exclusively excreted by the kidney. Due to their cyclic structure, they are resistant to hydrolysis by amylases and are associated with renal toxicity due to osmotic tubulopathy.3
SBECD is a second-generation cyclodextrin, engineered to enhance ionization properties by adding a sulfobutyl ether group.4 Preclinical animal study suggested that, although cytosolic vacuolation in kidney tubular epithelial cells was observed at as low as 160 mg/kg in rats, serum creatinine remained normal, and the histologic changes were reversible within 1 month after the last dose.5 In a clinical safety study, healthy male volunteers received up to 200 mg/kg of SBECD without acute kidney injury.
The post-marketing studies of voriconazole provide the best available real-world safety data of SBECD, as its i.v. formulation contains 3.2 g of SBECD per 200 mg voriconazole. Pharmacokinetics studies in patients receiving renal replacement therapy showed that standard loading dose of voriconazole with SBECD up to 192 mg/kg per day did not accumulate while on dialysis or continuous renal replacement therapy (further discussion in Supplementary Material).6, 7, 8
Currently, there are 2 formulations of remdesivir available for clinical use. The solution form and lyophilized form contain 6 g and 3 g of SBECD per 100 mg of remdesivir, respectively. Given our patient's low estimated glomerular filtration rate of 43 ml/min per 1.73 m2, we chose the lyophilized form of remdesivir, and he received a total of 600 mg of remdesivir, corresponding to a cumulative 18 g of SBECD over 5 days before the kidney biopsy. The exposure of 3 to 6 g of SBECD per day is well below the safety threshold of 250 mg/kg per day (i.e., for our patient, 25 g/d). A recent study looked at the safety of remdesivir in 11 patients with severe acute kidney injury, and reported no significant increase in serum creatinine after remdesivir treatment,S6 and another study also suggested the safety of remdesivir in 20 patients with estimated glomerular filtration rate <30 ml/min.9
Our patient’s serum creatinine continued to improve after initiation of remdesivir; however, the significant vacuolization in proximal tubules is a clear and unmistakable structural sign of tubular injury. The differential diagnoses of vacuolar change seen in this case include the Armanni-Ebstein lesions characterized by larger cytoplasmic vacuolation in proximal tubules in the outer medullary regions, seen in the setting of extremely high glucosuria, and changes due to toxic or ischemic injury. Our patient had poorly controlled diabetes and had significant glucosuria at the time of presentation, which could have been a contributory factor for osmotic tubular injury. Creatinine did not seem to serve as a sensitive enough biomarker for tubular injury in our case, or hyperfiltration mechanism might have compensated and masked the tubular injury.S7
Conclusion
In addition to collapsing glomerulopathy, the treatment of COVID-19 infection with remdesivir may be associated with osmotic proximal tubulopathy (Table 3). The precise mechanism of the osmotic tubulopathy is unclear in our case, but is probably multifactorial, and clinicians should remain cautious in the use of remdesivir in patients with severe acute kidney injury or advanced chronic kidney disease.
Table 3.
Teaching points
|
|
Disclosures
All the authors declared no competing interests.
Patient Consent
The authors declare that they have obtained consent from the patient discussed in the report.
Author Contributions
All authors participated in the clinical care and assembling of the data and contributed to the intellectual content and the writing of the case report. The authors reviewed and approved the final version of the manuscript.
Footnotes
Supplementary Material
Supplementary References
Supplementary Discussion
References
- 1.Kudose S., Batal I., Santoriello D. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickenmann M., Oettl T., Mihatsch M.J. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis. 2008;51:491–503. doi: 10.1053/j.ajkd.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Frank D.W., Gray J.E., Weaver R.N. Cyclodextrin nephrosis in the rat. Am J Pathol. 1976;83:367–382. [PMC free article] [PubMed] [Google Scholar]
- 4.Lilly C.M., Welch V.L., Mayer T. Evaluation of intravenous voriconazole in patients with compromised renal function. BMC Infect Dis. 2013;13:14. doi: 10.1186/1471-2334-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luke D.R., Tomaszewski K., Damle B., Schlamm H.T. Review of the basic and clinical pharmacology of sulfobutylether-beta-cyclodextrin (SBECD) J Pharm Sci. 2010;99:3291–3301. doi: 10.1002/jps.22109. [DOI] [PubMed] [Google Scholar]
- 6.Luke D.R., Wood N.D., Tomaszewski K.E., Damle B. Pharmacokinetics of sulfobutylether-β-cyclodextrin (SBECD) in subjects on hemodialysis. Nephrol Dial Transplant. 2012;27:1207–1212. doi: 10.1093/ndt/gfr472. [DOI] [PubMed] [Google Scholar]
- 7.Neofytos D., Lombardi L.R., Shields R.K. Administration of voriconazole in patients with renal dysfunction. Clin Infect Dis. 2012;54:913–921. doi: 10.1093/cid/cir969. [DOI] [PubMed] [Google Scholar]
- 8.Kiser T.H., Fish D.N., Aquilante C.L. Evaluation of sulfobutylether-β-cyclodextrin (SBECD) accumulation and voriconazole pharmacokinetics in critically ill patients undergoing continuous renal replacement therapy. Crit Care. 2015;19:32. doi: 10.1186/s13054-015-0753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pettit NN, Pisano J, Nguyen CT, et al. Remdesivir use in the setting of severe renal impairment: a theoretical concern or real risk [e-pub ahead of print]? Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1851. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.