Abstract
Background:
We aimed to examine the association between acute pancreatitis, a potential early symptom of pancreatic cancer, and pancreatic cancer stage, treatment, and prognosis.
Methods:
We conducted a cohort study of patients diagnosed with pancreatic cancer during 2004–2017 using population-based registry data from Denmark and Surveillance, Epidemiology, and End Results (SEER) data linked with Medicare claims from the United States (US), which include individuals aged 65+. We ascertained information on acute pancreatitis diagnoses ≤90 days before pancreatic cancer and followed them for a maximum of five years. We assessed overall survival difference at 30 days, six months, and one, three and five years, comparing patients with and without coexistence of acute pancreatitis. Secondary outcomes were cancer stage and treatment.
Results:
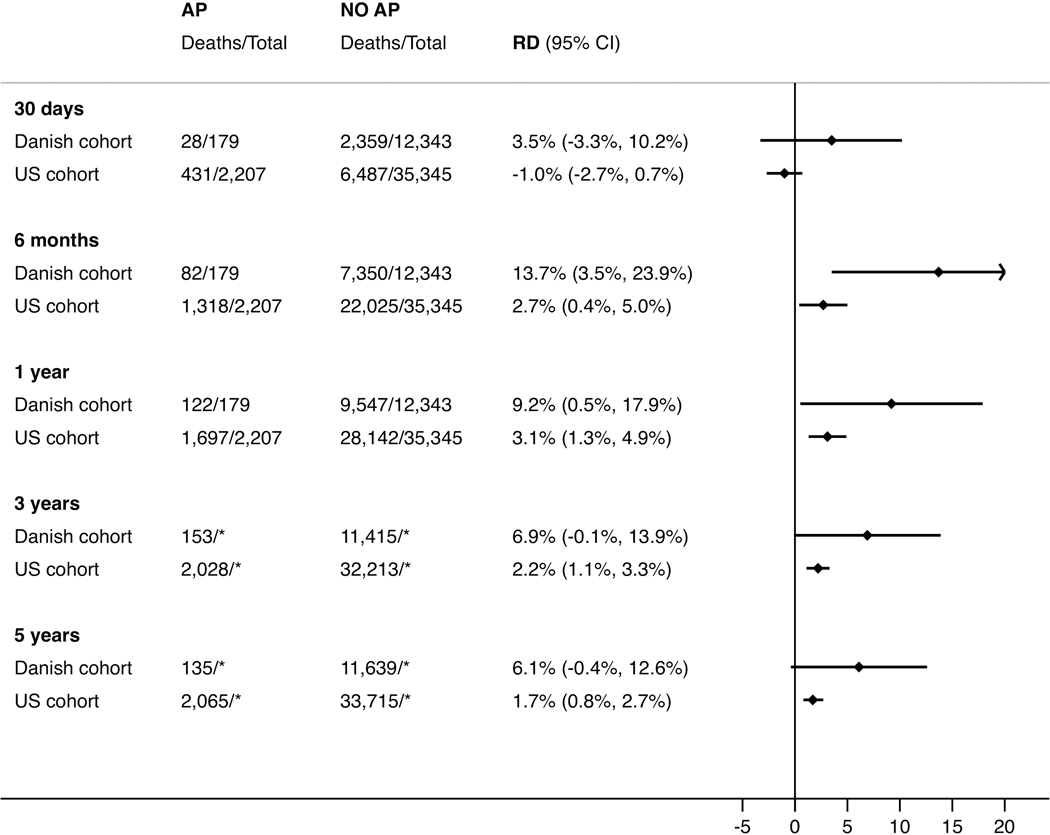
We identified 12,522 Danish and 37,552 US patients with pancreatic cancer (median age 71 and 78 years, respectively). In the Danish cohort, 1.4% had acute pancreatitis before pancreatic cancer vs. 5.9% in the US cohort. After five years of follow-up, the survival difference was 6.1% (95% CI: [−0.4%, 12.6%]) in Danish and 1.7% (95% CI: [0.8%, 2.7%]) in US patients, comparing patients with and without acute pancreatitis. Patients with acute pancreatitis had lower prevalence of metastatic tumors at diagnosis (Denmark: 42.5% vs. 48.7%; US: 34.4% vs. 45.9%) and higher resection frequencies (Denmark: 20.1% vs. 12.1%; US: 16.1% vs.11.3%) than patients without acute pancreatitis.
Conclusions:
Pancreatic cancer patients with acute pancreatitis diagnosed ≤90 days before cancer diagnosis had earlier stage at diagnosis and better survival than patients without acute pancreatitis.
Keywords: Pancreatic cancer, acute pancreatitis, early diagnosis, cohort study
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer-related mortality in the western world and is projected to increase.1, 2 About 15% of pancreatic tumors are resectable at diagnosis, limiting five-year survival to 8%.2, 3 To improve pancreatic cancer prognosis, early detection when the tumor is resectable is essential. This is difficult due to a lack of early disease-specific symptoms.4
Acute pancreatitis may constitute an early and symptom of pancreatic cancer, as around 1% of acute pancreatitis admissions are due to pancreatic cancer.5 Many of these patients are diagnosed with pancreatic cancer within few months after acute pancreatitis diagnosis,6, 7 but some patients experience longer diagnostic delays because of initial misdiagnosis, impeding survival.6, 8 However, the prognostic value of acute pancreatitis on pancreatic cancer prognosis is unclear. Studies have suggested that pancreatic cancer patients with acute pancreatitis may have lower tumor stage,9–11 higher resection frequencies,9, 11 and improved prognosis9, 12 compared with patients without acute pancreatitis. Another study found no differences in tumor stage, treatment, or survival.13 However, all studies had substantial limitations – failing to distinguish between acute and chronic pancreatitis,9 including patients with self-reported pancreatitis,9 or provided imprecise estimates.10–13
Given the limitations of previous research, we examined differences in stage, treatment, and survival in two independent cohorts of pancreatic cancer patients comparing patients with and without acute pancreatitis before pancreatic cancer diagnosis. We hypothesized that acute pancreatitis was associated with an improved survival, likely due to earlier detection.
METHODS
Setting and design
We identified patients diagnosed with pancreatic cancer in Denmark (Danish cohort) and the United States (US cohort) from 1 January 2004. We included patients diagnosed through December 2017 and December 2013 in the two cohorts, respectively (Table, Supplemental Digital Content 1).
Data sources
Danish cohort
We used data from the Danish Cancer Registry (DCR), the Danish National Patient Registry (DNPR), and the Civil Registration System.14–16 These registries contain information on all cancers, comorbidity diagnoses, and vital status for all Danish residents, respectively, and can be linked on individual level (Table, Supplemental Digital Content 2).16
US cohort
We used data from the Surveillance, Epidemiology, and End Results (SEER) Program linked with Medicare claims (Parts A and B). The SEER program covers approximately 34% of the US population and routinely collects information on patient demographics, tumor characteristics, and vital status.17 The SEER population is largely representative of the demographics of the entire US population.18 Medicare is a nationwide, federally funded, health insurance program available for adults aged 65+, and those under age 65 with certain disabilities or end-stage renal disease.19 Medicare has different parts covering inpatient care (Part A), physician and outpatient visits (Part B), and prescription drugs (Part D). Only patients age 66+ years were included in the US cohort analyses.
Study population
From the DCR and SEER Program, we identified all patients diagnosed with pancreatic cancer during the study period (Figure, Supplemental Digital Content 3 and Table, Supplemental Digital Content 4). We excluded patients diagnosed at autopsy or date of death. In the Danish cohort, we excluded patients aged <18 years. In the US cohort, we excluded patients aged <66 years. These patients were Medicare-eligible due to certain disabilities and therefore not largely reflective of the younger population, or had less than 12 months continuous enrollment in Medicare Parts A and B before pancreatic cancer diagnosis. For US patients, we had information on the month but not date of cancer diagnosis. Therefore, we assigned the 15th of the month of cancer diagnosis as the date of diagnosis, excluding patients who died between the 1st and 14th in the month of cancer diagnosis. We used information on the tumor-node-metastasis classification to compute the American Joint Committee on Cancer (AJCC) stage, 7th edition.20
Follow-up and outcome
We followed each patient for a maximum of five years from date of pancreatic cancer diagnosis (index date) until 7 March 2019 (Danish cohort) or 31 December 2014 (US cohort), death, or loss to follow-up/emigration, whichever occurred first.
Acute pancreatitis
We captured any inpatient diagnosis of acute pancreatitis recorded in the 90 days before pancreatic cancer diagnosis in the DNPR (Danish cohort) and Medicare Part A (US cohort). We excluded patients undergoing an endoscopic retrograde cholangio-pancreatography (ERCP) procedure (in-/outpatient) within five days before (but not on) the date of acute pancreatitis (Figure, Supplemental Digital Content 3). In these patients, acute pancreatitis was likely caused by the ERCP and not the tumor. Considering these patients to have acute pancreatitis due to a pancreatic tumor could introduce bias, because the ERCP was likely performed in the diagnostic workup for suspected pancreatic cancer.
Cancer treatment
Information on cancer treatment was ascertained from the DNPR (Danish cohort) and Medicare Parts A and B (US cohort). Treatments are registered according to the Nordic Medico-Statistical Committee Classification of Surgical Procedures21 and Current Procedural Terminology/Healthcare Common Procedure Coding System codes,21–23 respectively (Table, Supplemental Digital Content 5). We captured any treatment (resection or oncological treatment) registered within 30 days before or 120 days after pancreatic cancer diagnosis. We considered treatments initiated within 30 days before pancreatic cancer to allow for late registration of the cancer. We classified treatments as resection +/− oncological treatment (adjuvant/neoadjuvant therapy), oncological treatment alone, and best supportive care (no anti-neoplastic treatment registered).
Comorbidity
For the Danish and US cohort, we identified all diagnoses recorded within one year preceding pancreatic cancer diagnosis from the DNPR and Medicare Parts A and B, respectively. We assessed specific comorbidities (alcohol-/smoking-related diseases, cholecystitis, and cholangitis) and computed a summary score of comorbidity burden using the Gagne Comorbidity Index, excluding any malignancies (Tables, Supplemental Digital Content 6–7).24, 25 We defined three levels of comorbidity: low (score ≤0), moderate (score 1–2), and severe (score >2). For the US cohort, we also computed the patients’ predicted probability of frailty using the Faurot Frailty Score (Table, Supplemental Digital Content 8),21 grouped into three categories based on their probability of dependency in activities of daily living: low (<5%), moderate (5%−19%), and high (≥20%).26–28
Statistical analyses
We tabulated descriptive characteristics for both cohorts. Continuous variables are summarized by medians with interquartile ranges (IQRs) and categorical variables as frequencies and percentages. Using the Kaplan-Meier estimator, we computed survival probabilities at 30 days, six months, and one, three, and five years following pancreatic cancer diagnosis. At these time points, we calculated the difference in survival (or risk difference, RD), as a measure of the absolute survival difference, as the survival probability in patients with acute pancreatitis minus the survival probability in patients without acute pancreatitis. We used bootstrapping with 200 repetitions to derive confidence intervals (CIs) for the RDs. In addition to the overall RD, we calculated RDs for each level of selected variables (age group, sex, race, Gagne comorbidity score, frailty, and AJCC stage) to examine variations across these subgroups. We calculated median survival for patients with and without acute pancreatitis both overall and stratified by treatment, considering treatment as a time-varying covariate to avoid immortal time bias.29 For patients undergoing surgery, we also computed five-year survival from the date of surgery in addition to crude and adjusted (age, sex, year of cancer diagnosis, Gagne Comorbidity Index score, alcohol-/smoking-related diseases, cholecystitis, and cholangitis) hazard ratios (HRs) comparing patients with and without acute pancreatitis. The covariates in the Cox model were chosen based on a directed acyclic graph (Figure, Supplemental Digital Content 9). Proportionality was assessed graphically using log(-log) plot. For both cohorts, we constructed crude and inverse probability weighted survival curves, comparing patients with and without acute pancreatitis. All risk estimates are presented with associated 95% CIs. Statistical analyses were conducted using Stata 15.1 (StataCorp LP, College Station, TX, USA) and SAS software 9.3 (SAS Institute Inc., Cary, NC, USA).
Sensitivity analyses
We conducted five sensitivity analyses. First, we changed the period to capture acute pancreatitis diagnoses from 90 days to 7, 30, 60, or 365 days before pancreatic cancer diagnosis. Second, we restricted our analyses in the Danish cohort to patients aged 66+ and diagnosed in the same period as the US patients to increase comparability between the populations. Third, because capturing of comorbidities in the Danish population was limited using the Gagne Index, we applied the Charlson Index30, 31 (Table, Supplemental Digital Content 10) instead. This may have better capture in a Danish population, as some conditions included in the Gagne Index (e.g. electrolyte disorders and weight loss) may not be recorded in discharge letters from Danish hospitals. Additionally, we applied a 10-year lookback period for these indices in the Danish population. Fourth, we assigned the first and last date in the month of cancer diagnosis as the date of cancer diagnosis in the US cohort. Fifth, we included race and frailty in the adjusted Cox model for the US cohort.
Ethics
This study was approved by the Danish Data Protection Agency (no. 1-16-02-796-17) and the Institutional Review Board of University of North Carolina at Chapel Hill (no. 18–0992).
RESULTS
Characteristics
We identified 12,522 Danish (1.4% with acute pancreatitis) and 37,552 US (5.9% with acute pancreatitis) patients with pancreatic cancer (Table 1). Acute pancreatitis patients were younger and had more comorbidity than patients without acute pancreatitis. Although pancreatic head tumors were more frequent in patients with acute pancreatitis, there was a high proportion of missing data for this variable. US patients had higher comorbidity levels than Danish patients (Table 1 and Table, Supplemental Digital Content 11). Patients with acute pancreatitis were more likely to have stage I-II cancer and to undergo surgical treatment than patients without acute pancreatitis (Table 1).
Table 1.
Descriptive characteristics of the study population, by cohort and presence of acute pancreatitis (AP).
| Danish cohort N=12,522 | US cohort N=37,552 | |||
|---|---|---|---|---|
| AP | No AP | AP | No AP | |
| Total | 179 (1.4%) | 12,343 (98.6%) | 2,207 (5.9%) | 35,345 (94.1%) |
| Age, y, median (IQR) | 65 (56–72) | 71 (63–78) | 77 (71–83) | 78 (72–84) |
| Age group | ||||
| ≤65 years | 93 (52.0%) | 3,911 (31.7%) | - | - |
| 66–70 years | 27 (15.1%) | 2,188 (17.7%) | 479 (21.7%) | 6,438 (18.2%) |
| 71–75 years | 25 (14.0%) | 2,199 (17.8%) | 481 (21.8%) | 7,306 (20.7%) |
| >75 years | 34 (19.0%) | 4,045 (32.8%) | 1,247 (56.5%) | 21,601 (61.1%) |
| Sex | ||||
| Men | 97 (54.2%) | 6,216 (50.4%) | 977 (44.3%) | 15,994 (45.2%) |
| Women | 82 (45.8%) | 6,127 (49.6%) | 1,230 (55.7%) | 19,351 (54.8%) |
| Race | ||||
| White | - | - | 1,730 (78.4%) | 29,307 (82.9%) |
| African-American | - | - | 270 (12.2%) | 3,306 (9.4%) |
| Hispanic | - | - | 95 (4.3%) | 1,181 (3.3%) |
| Other | - | - | 112 (5.1%) | 1,551 (4.4%) |
| Gagne Comorbidity score | ||||
| Low (≤0) | 133 (74.3%) | 9,718 (78.7%) | 311 (14.1%) | 11,972 (33.9%) |
| Moderate (1–2) | 39 (21.8%) | 2,186 (17.7%) | 605 (27.4%) | 10,319 (29.2%) |
| Severe (>2) | 7 (3.9%) | 439 (3.6%) | 1,291 (58.5%) | 13,054 (36.9%) |
| Comorbidity | ||||
| Alcohol-related disease | 13 (7.3%) | 221 (1.8%) | 145 (6.6%) | 964 (2.8%) |
| Smoking-related disease | 18 (10.1%) | 705 (5.7%) | 1,251 (56.7%) | 13,383 (37.9%) |
| Cholecystitis | ≤52 (−) | 165 (1.3%) | 721 (32.7%) | 5,103 (14.4%) |
| Cholangitis | ≤52 (−) | 291 (2.4%) | 196 (8.9%) | 1,165 (3.3%) |
| Frailty | ||||
| Low | - | - | 789 (35.8%) | 15,909 (45.0%) |
| Moderate | - | - | 826 (37.4%) | 12,270 (34.7%) |
| High | - | - | 592 (26.8%) | 7,166 (20.3%) |
| Period of diagnosis | ||||
| 2004–2007 | 35 (19.6%) | 3,291 (26.7%) | 875 (39.7%) | 14,107 (39.9%) |
| 2008–2011 | 43 (24.0%) | 3,613 (29.3%) | 709 (32.1%) | 10,612 (30.0%) |
| 2012–20161 | 101 (56.4%) | 5,439 (44.1%) | 623 (28.2%) | 10,626 (30.1%) |
| Tumor site | ||||
| Head | 89 (51.0%) | 4,731 (38.3%) | 1,389 (62.9%) | 16,781 (47.5%) |
| Body | ≤102 (−) | 960 (7.8%) | 168 (7.6%) | 3,830 (10.8%) |
| Tail | ≤102 (−) | 663 (5.4%) | 131 (5.9%) | 3,987 (11.3%) |
| Other | 14 (6.5%) | 651 (5.3%) | 519 (23.5%) | 10,747 (30.4%) |
| Unknown | 66 (36.9%) | 5,338 (43.2%) | - | - |
| AJCC stage | ||||
| Stage I | 13 (7.3%) | 430 (3.5%) | 231 (10.5%) | 2,630 (7.4%) |
| Stage II | 31 (17.3%) | 1,387 (11.2%) | 627 (28.4%) | 7,553 (21.4%) |
| Stage III | 17 (9.5%) | 1,157 (9.4%) | 167 (7.6%) | 2,538 (7.2%) |
| Stage IV | 76 (42.5%) | 6,015 (48.7%) | 759 (34.4%) | 16,211 (45.9%) |
| Unknown | 42 (23.5%) | 3,354 (27.2%) | 423 (19.2%) | 6,413 (18.1%) |
| Treatment | ||||
| Best supportive care | 79 (44.1%) | 6,396 (51.8%) | 1,212 (54.9%) | 19,204 (54.3%) |
| Oncological treatment | 64 (35.8%) | 4,456 (36.1%) | 639 (30.0%) | 12,140 (34.4%) |
| Resection +/−oncological | 36 (20.1%) | 1,491 (12.1%) | 356 (16.1%) | 4,001 (11.3%) |
2012–2013 for the US cohort.
Numbers collapsed for confidentiality purposes.
Survival
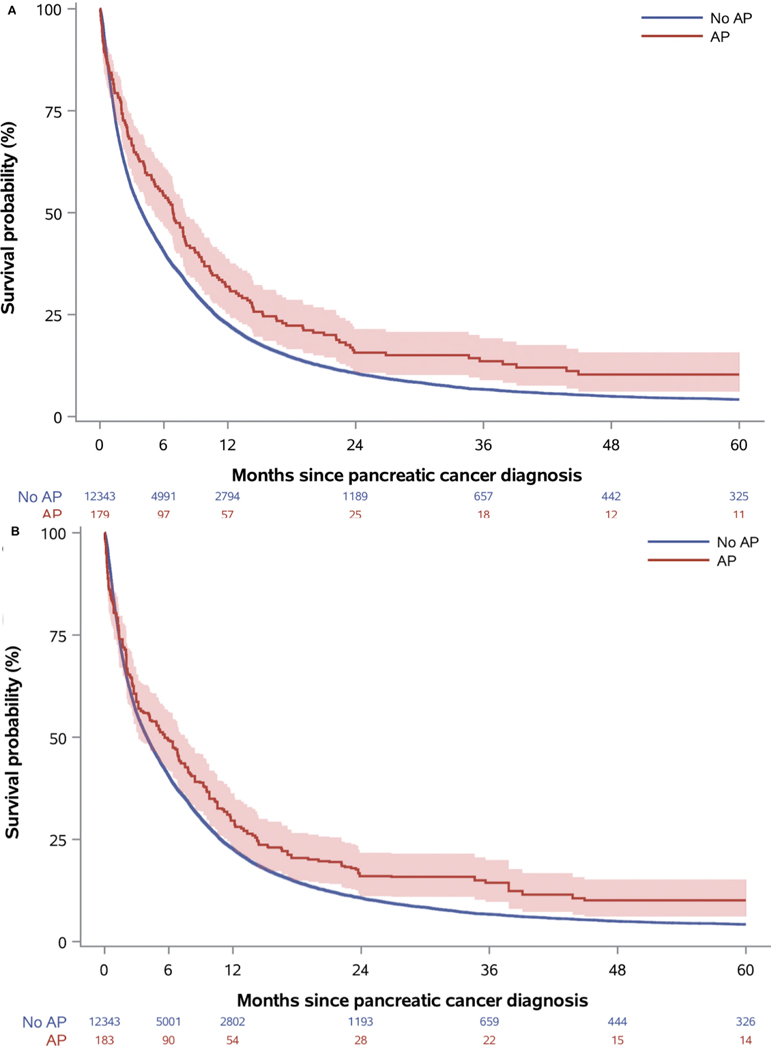
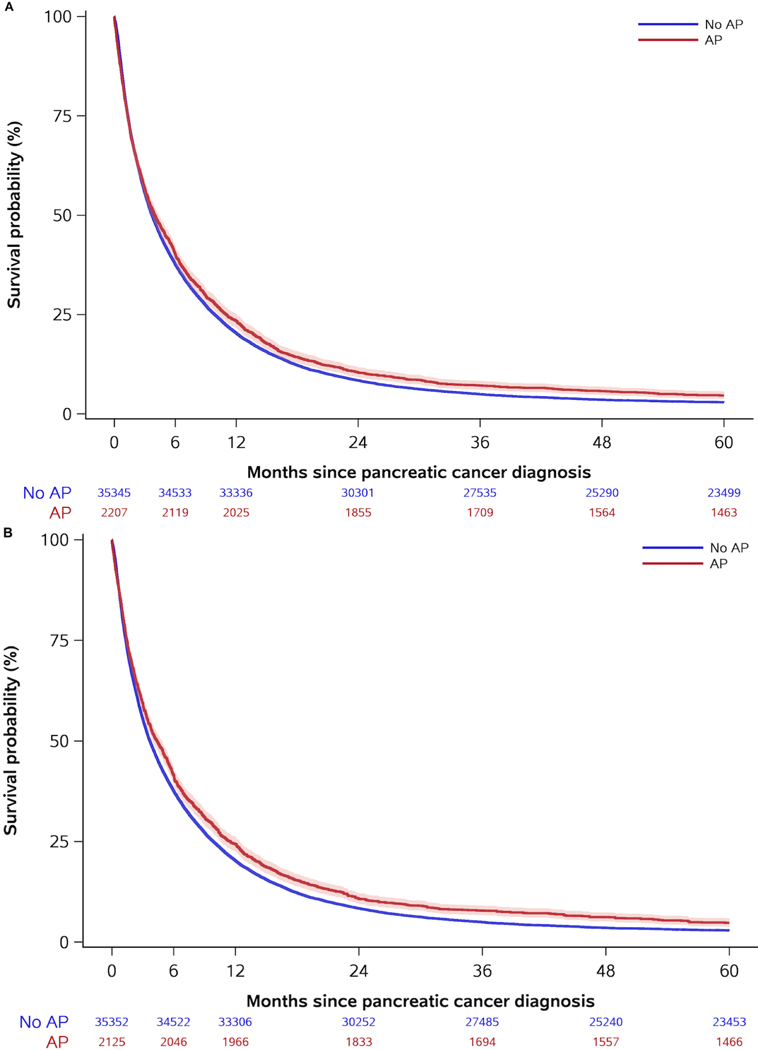
Acute pancreatitis patients had better survival in both cohorts (Table 2 and Figures 1–2). Survival estimates in the Danish cohort had higher magnitude and lower precision than in the US cohort, but confidence intervals were overlapping. After five years, the absolute survival differences were 6.1% (95% CI: [−0.4%, 12.6%]) and 1.7% (95% CI: [0.8%, 2.7%]) in the Danish and US cohort, respectively (Table 2 and Figure 3). Results stratified by baseline characteristics revealed few variations but suggested that the five-year survival difference was most pronounced in US patients with low-stage cancer and Danish patients with low comorbidity (Table, Supplemental Digital Content 12–13). In the Danish cohort, median survival was longer in patients with than without acute pancreatitis with the most pronounced difference among patients undergoing resection (Table 3). After surgery, five-year survival probabilities were higher in patients with acute pancreatitis than in patients without acute pancreatitis in both the Danish (38.3%; 95% CI: [20.7%, 55.7%] vs. 23.8%; 95% CI: [21.4%, 26.2%]) and US (18.7%; 95% CI: [14.7%, 23.7%] vs. 15.1%; 95% CI: [13.9%, 16.4%]) cohorts. The respective crude HRs were 0.72 (95% CI: [0.46, 1.21]) and 0.91 (95% CI: [0.80, 1.03]) in Danish and US patients. After adjustment for potential confounders, the HRs were 0.77 (95% CI: [0.49, 1.20]) and 0.84 (95% CI: [0.74, 0.95]), respectively.
Table 2.
Number of deaths, crude survival probabilities with 95% CIs, and risk differences (RD) at different follow-up times, by cohort and presence of acute pancreatitis (AP).
| Danish cohort | US cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up | AP (n=179) | No AP (n=12,343) | RD | AP (n=2,207) | No AP (n=35,345) | RD | ||||
| N | Survival | N | Survival | N | Survival | N | Survival | |||
| 30 days | 28 | 84.4% (78.2%, 88.9%) |
2,359 | 80.9% (80.2%, 81.6%) |
3.5% (−3.3%, 10.2%) |
431 | 80.5% (78.8%, 82.1%) |
6,487 | 81.6% (81.2%, 82.0%) |
−1.0% (−2.7%, 0.7%) |
| 6 months | 82 | 54.2% (46.7%, 61.2%) |
7,350 | 40.4% (39.6%, 41.3%) |
13.7% (3.5%, 23.9%) |
1,318 | 40.3% (38.2%, 42.3%) |
22,025 | 37.7% (37.2%, 38.2%) |
2.7% (0.4%, 5.0%) |
| 1 year | 122 | 31.8% (25.2%, 38.7%) |
9,547 | 22.6% (21.9%, 23.4%) |
9.2% (0.5%, 17.9%) |
1,691 | 23.4% (21.6%, 25.2%) |
28,142 | 20.4% (20.0%, 20.8%) |
3.1% (1.3%, 4.9%) |
| 3 years | 153 | 13.6% (8.9%, 19.2%) |
11,415 | 6.6% (6.2%, 7.1%) |
6.9% (−0.1%, 13.9%) |
2,028 | 7.1% (6.1%, 8.3%) |
33,213 | 5.0% (4.7%, 5.2%) |
2.2% (1.1%, 3.3%) |
| 5 years | 157 | 10.3% (6.1%, 15.7%) |
11,639 | 4.2% (3.8%, 4.6%) |
6.1% (−0.4%, 12.6%) |
2,065 | 4.6% (3.7%, 5.7%) |
33,715 | 2.9% (2.7%, 3.1%) |
1.7% (0.8%, 2.7%) |
Figure 1. Crude (a) and inverse probability weighted (b) survival curves for the Danish cohort, comparing patients with and without acute pancreatitis (AP).
Note to Figure 1: Curves are shown with corresponding 95% confidence interval bands. Numbers at risk differ for crude and adjusted curves due to the inverse probability weighting.
Figure 2. Crude (a) and inverse probability weighted (b) survival curves for the US cohort, comparing patients with and without acute pancreatitis (AP).
Note to Figure 2: Curves are shown with corresponding 95% confidence interval bands. Numbers at risk differ for crude and adjusted curves due to the inverse probability weighting.
Figure 3. Absolute survival difference (risk difference, RD) in pancreatic cancer patients, comparing patients with and without acute pancreatitis (AP).
Note to Figure 3: *Unable to report total number at risk because we did not have a full five years of follow-up for all patients.
Table 3.
Person-time in years and median overall survival (mOS) in months (IQR), by cohort, treatment, and presence of acute pancreatitis (AP).
| Danish cohort | US cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | AP (n=179) | No AP (n=12,343) | AP (n=2,207) | No AP (n=35,345) | ||||
| Person-time | mOS | Person-time | mOS | Person-time | mOS | Person-time | mOS | |
| All patients | 188 | 6.9 (2.0, 15.3) | 9,269 | 3.9 (1.3, 10.8) | 19,888 | 3.9 (1.3, 11.0) | 282,920 | 3.8 (1.3, 9.8) |
| Best supportive care | 51 | 3.4 (1.4, 8.9) | 2,693 | 2.3 (1.1, 5.4) | 6,298 | 2.7 (1.1, 6.0) | 95,521 | 2.6 (1.2, 5.7) |
| Oncological treatment | 51 | 7.8 (4.2, 13.0) | 3,344 | 6.7 (2.9, 12.0) | 5,481 | 5.7 (2.1, 11.5) | 102,523 | 5.7 (2.4, 11.1) |
| Resection +/− oncological | 86 | 39.1 (12.2, -) | 3,232 | 22.4 (10.5, 55.8) | 8,109 | 16.1 (8.5, 43.7) | 84,876 | 16.1 (7.0, 35.2) |
Sensitivity analyses
Applying different time periods to capture acute pancreatitis diagnoses did not change our survival estimates substantially, except for a time-window of seven days, which decreased survival in Danish patients with acute pancreatitis (Tables, Supplemental Digital Content 14–17). Restricting the Danish population to patients aged 66+ and diagnosed in the same period as the US population resulted in more similar survival estimates in the two cohorts (five-year survival difference: 4.7% in Denmark, 1.7% in the US), but imbalances in baseline characteristics still existed, particularly with respect to comorbidity level (Tables, Supplemental Digital Content 18–20). Applying the Charlson Index instead of the Gagne Index in the Danish population had limited impact on capturing of comorbidity using the one-year lookback (Table, Supplemental Digital Content 20). Using a 10-year lookback naturally increased comorbidity levels, more so for the Charlson than the Gagne Index. Using the Charlson Index reinforced that the survival difference was largest in patients with low comorbidity levels, regardless of lookback period (Table, Supplemental Digital Content 12). The choice of comorbidity index had no measurable effect on the HRs (adjusted HR using the Charlson Index regardless of lookback period: 0.78; 95% CI: [0.50,1.22]). When switching the date of diagnosis to the first day or last day of the month in the US population, results were comparable except for 30-day survival (Table, Supplemental Digital Content 21). Adjusting for race and frailty in the US cohort did not change the estimate (adjusted HR: 0.84; 95% CI: [0.74, 0.95]).
DISCUSSION
In the present study of 50,074 pancreatic cancer patients, acute pancreatitis diagnosed within 90 days before pancreatic cancer diagnosis was associated with lower tumor stage, higher resection frequencies, and better survival. Findings were consistent in direction in both cohorts but more pronounced in Danish than US patients.
Acute pancreatitis can be an early marker of pancreatic cancer. This is due to tumor obstruction of the pancreatic duct, leading to a release of digestive enzymes into the pancreatic interstitial space instead of the duct lumen.32 As acute pancreatitis is a painful condition, patients are likely to seek medical care, which could lead to a cancer diagnosis at an earlier stage. This theory is supported by the higher proportion of stage I-II cancer among patients with acute pancreatitis observed in this study, which also agrees with some previous studies.9–11
In both cohorts, patients with acute pancreatitis had 16–23% lower mortality within five years after surgery compared to patients without acute pancreatitis. The absolute survival estimates were higher in Danish than US patients. A possible explanation for this is differences in baseline characteristics between the two cohorts, as US patients were older and had more registered comorbidities. In Danish patients undergoing resection, those with acute pancreatitis had a five-year survival of 38% and a median survival of 39 months, which agrees with findings from a Taiwanese cohort study of 298 patients.12 However, the estimates were based on few patients and may have occurred by chance. A median survival of 22 months in Danish patients without acute pancreatitis agrees with numbers from international high-volume centres.33, 34 The better survival in patients with than without acute pancreatitis supports the idea that timely diagnosis of pancreatic cancer in patients with acute pancreatitis is beneficial, as this can increase resection frequencies leading to improved survival. However, we also observed small but consistent differences in survival in Danish patients with oncological or no anti-neoplastic treatment, suggesting that some element of lead time may contribute to our findings.35 While this may be true for patients not undergoing resection, we observed a striking median survival difference of 17 months in patients undergoing surgery. Such a difference is unlikely to be explained by lead time bias, but it may be due to chance due to a low number of observations.
Our results largely agree with previous research. Two studies, each with less than 50 patients, reported one-year survival estimates in patients with acute pancreatitis similar to ours.7, 36 Our findings are also partly in agreement with a US-based cohort study of 2,573 pancreatic cancer patients examining 100 patients with self-reported acute or chronic pancreatitis within 10 days before or after pancreatic cancer diagnosis.9 They reported median survival of 450 and 325 days in patients with and without acute pancreatitis, respectively. However, their study was prone to bias. Half of the patients underwent resection, which is higher than the expected 10–20%,3 and they included patients diagnosed with pancreatitis after pancreatic cancer diagnosis, which may have occurred due to resection. In contrast to our findings, a study of 318 Japanese pancreatic cancer patients found no association between acute pancreatitis at diagnosis and tumor location, stage, resection frequencies, or survival.13 Comparison of the published literature is challenging because of varying time periods used to capture acute pancreatitis diagnoses, ranging from ten days to two years.7, 9 However, our sensitivity analyses suggest that the choice of any time-window between 30–365 days before pancreatic cancer diagnosis has little effect on the survival estimates. We did not examine the impact of pancreatitis on survival in patients with acute pancreatitis more than one year before pancreatic cancer. Syed et al. observed that 93% of cancers occurred within a year after pancreatitis diagnosis, increasing to 99% after three years.37 However, they included both acute and chronic pancreatitis diagnoses, and other work has suggested that the risk of cancer in the period 12–24 months after acute pancreatitis is negligible.6
We examined the same hypothesis in two different healthcare systems, entailing some important differences in patient characteristics. Danish patients were younger than US patients, because Medicare is only eligible for patients aged 65+ (or with certain disabilities). This may have contributed to the higher comorbidity levels and lower resection frequencies in the US cohort. However, when restricting analyses on Danish patients to those aged 66+, there were still substantial differences in comorbidity levels between the populations. Although there were no major differences in comorbidity levels depending on the choice of comorbidity index, our findings suggest that the choice of lookback period may have contributed to the differences. However, neither the choice of comorbidity index nor lookback period had any profound effect on our risk estimates. Also, US physicians may have an incentive to record comorbidities on claims data because of their potential impacts on reimbursement. This may also have contributed to the different prevalence of acute pancreatitis in this study. The prevalence of acute pancreatitis and difference between Danish and US patients agree with previous reports.38–41
Some issues warrant consideration. First, it was unknown if acute pancreatitis was a symptom of pancreatic cancer. We used a proxy of 90 days before cancer diagnosis to capture acute pancreatitis diagnoses. This may have introduced misclassification, as acute pancreatitis in this period may have been caused by other factors. However, sensitivity analyses showed that survival estimates are unlikely to be affected by this. Also, we and others have previously shown that acute pancreatitis diagnoses in the DNPR have high validity.42–45 This mitigates concerns of exposure misclassification, although findings from these studies may not be applicable to the entire Danish population, as only diagnoses from three out of five regions were validated. However, pancreatic cancers registered in the Danish Cancer Registry have not been validated. As differentiation between pancreatic adenocarcinoma, cancer of the papilla Vateri, and distal bile duct cancers can be very difficult, some misclassification may be present. Furthermore, as these cancers can differ with respect to prognosis,46 this could have contributed to our findings. Second, information on tumor stage was missing in 20–25% of the patients, and clinical staging of pancreatic cancer is difficult, particularly when inflammation co-exists.47 Thus, tumor stage may be inaccurately determined in patients not undergoing surgery. However, we had complete information on treatment, which is closely correlated to tumor stage, mitigating this concern. Third, we lacked information on performance status, which is an important determinant of treatment allocation and prognosis.48 However, adjustment for a proxy of frailty did not affect our results. Fourth, acute pancreatitis was rare in Danish patients, limiting the precision of our estimates. Fifth, because we considered treatment to be a time-varying exposure, time-varying confounding may have been introduced. While methods exist for dealing with time-varying confounding, this would require clinical information, which is not readily available.
In conclusion, acute pancreatitis diagnosed within 90 days before pancreatic cancer is associated with lower tumor stage, higher resection frequencies, and improved survival. Our study supports that timely cancer detection in acute pancreatitis patients is important and may improve survival.
Supplementary Material
Acknowledgments
Financial support: Jakob Kirkegård is funded by the Danish Cancer Society (Grant no. R124-A7521). No study-specific funding was received.
Footnotes
Potential competing interests: Dr. Lund’s spouse is a full-time, paid employee of GlaxoSmithKline. The remaining authors declare no conflict of interests.
Guarantor of the article: Jakob Kirkegård
REFERENCES
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 2.Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers 2016; 2:16022. [DOI] [PubMed] [Google Scholar]
- 3.Huang L, Jansen L, Balavarca Y, et al. Resection of pancreatic cancer in Europe and USA: an international large-scale study highlighting large variations. Gut 2019; 68:130–139. [DOI] [PubMed] [Google Scholar]
- 4.Chari ST, Kelly K, Hollingsworth MA, et al. Early detection of sporadic pancreatic cancer: summative review. Pancreas 2015; 44:693–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet 2015; 386:85–96. [DOI] [PubMed] [Google Scholar]
- 6.Munigala S, Kanwal F, Xian H, et al. Increased risk of pancreatic adenocarcinoma after acute pancreatitis. Clin Gastroenterol Hepatol 2014; 12:1143–1150. [DOI] [PubMed] [Google Scholar]
- 7.Li S, Tian B. Acute pancreatitis in patients with pancreatic cancer: Timing of surgery and survival duration. Medicine (Baltimore) 2017; 96:e5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swords DS, Mone MC, Zhang C, et al. Initial Misdiagnosis of Proximal Pancreatic Adenocarcinoma Is Associated with Delay in Diagnosis and Advanced Stage at Presentation. J Gastrointest Surg 2015; 19:1813–1821. [DOI] [PubMed] [Google Scholar]
- 9.Dzeletovic I, Harrison ME, Crowell MD, et al. Pancreatitis before pancreatic cancer: clinical features and influence on outcome. J Clin Gastroenterol 2014; 48:801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng Z, Zhang X, Zheng Q, et al. Acute pancreatitis as an early indicator of pancreatic head carcinoma. Hepatogastroenterology 2014; 61:1201–1206. [PubMed] [Google Scholar]
- 11.Sadr-Azodi O, Oskarsson V, Discacciati A, et al. Pancreatic Cancer Following Acute Pancreatitis: A Population-based Matched Cohort Study. Am J Gastroenterol 2018; 113:1711–1719. [DOI] [PubMed] [Google Scholar]
- 12.Thorat A, Huang WH, Yeh TS, et al. Pancreatic ductal adenocarcinoma presenting with acute and chronic pancreatitis as initial presentation: is prognosis better? A comparison study. Hepatogastroenterology 2014; 61:2110–2116. [PubMed] [Google Scholar]
- 13.Minato Y, Kamisawa T, Tabata T, et al. Pancreatic cancer causing acute pancreatitis: a comparative study with cancer patients without pancreatitis and pancreatitis patients without cancer. J Hepatobiliary Pancreat Sci 2013; 20:628–633. [DOI] [PubMed] [Google Scholar]
- 14.Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health 2011; 39:42–45. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014; 29:541–549. [DOI] [PubMed] [Google Scholar]
- 17.Surveillance, Epidemiology, and End Results (SEER). https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf. Date accessed: 8 August 2018.
- 18.SEER Population Characteristics. https://seer.cancer.gov/registries/characteristics.html. Date accessed: 23 April 2019.
- 19.Medicare facts. http://www.cms.gov/Medicare/. Date accessed: 18 February 2019.
- 20.Edge SB, Byrd DR, Compton CG, et al. AJCC Cancer Staging Handbook. New York, NY: Springer, 2010. [Google Scholar]
- 21.NOMESCO Classification of Surgical Procedures. https://norden.diva-portal.org/smash/get/diva2:970547/FULLTEXT01.pdf. Date accessed: 1 November 2018.
- 22.Healthcare Common Procedure Coding System (HCPCS). https://www.cms.gov/Medicare/Coding/MedHCPCSGenInfo/index.html Date accessed: 25 January 2019.
- 23.Common Procedural Terminology (CPT). https://www.ama-assn.org/amaone/cpt-current-procedural-terminology. Date accessed: 25 January 2019.
- 24.Gagne JJ, Glynn RJ, Avorn J, et al. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 2011; 64:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun JW, Rogers JR, Her Q, et al. Adaptation and Validation of the Combined Comorbidity Score for ICD-10-CM. Med Care 2017; 55:1046–1051. [DOI] [PubMed] [Google Scholar]
- 26.Faurot KR, Jonsson Funk M, Pate V, et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf 2015; 24:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuthbertson CC, Kucharska-Newton A, Faurot KR, et al. Controlling for Frailty in Pharmacoepidemiologic Studies of Older Adults: Validation of an Existing Medicare Claims-based Algorithm. Epidemiology 2018; 29:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer SE, Tan HJ, Peacock Hinton S, et al. Comparison of Medicare Claims-based Proxy Measures of Poor Function and Associations With Treatment Receipt and Mortality in Older Colon Cancer Patients. Med Care 2019; 57:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Targownik LE, Suissa S. Understanding and Avoiding Immortal-Time Bias in Gastrointestinal Observational Research. Am J Gastroenterol 2015; 110:1647–1650. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 31.Thygesen SK, Christiansen CF, Christensen S, et al. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol 2011; 11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohler H, Lankisch PG. Acute pancreatitis and hyperamylasaemia in pancreatic carcinoma. Pancreas 1987; 2:117–119. [DOI] [PubMed] [Google Scholar]
- 33.Pugalenthi A, Protic M, Gonen M, et al. Postoperative complications and overall survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Surg Oncol 2016; 113:188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Roessel S, Kasumova GG, Verheij J, et al. International Validation of the Eighth Edition of the American Joint Committee on Cancer (AJCC) TNM Staging System in Patients With Resected Pancreatic Cancer. JAMA Surg 2018; 153:e183617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison AS. The effects of early treatment, lead time and length bias on the mortality experienced by cases detected by screening. Int J Epidemiol 1982; 11:261–267. [DOI] [PubMed] [Google Scholar]
- 36.Mujica VR, Barkin JS, Go VL. Acute pancreatitis secondary to pancreatic carcinoma. Study Group Participants. Pancreas 2000; 21:329–332. [DOI] [PubMed] [Google Scholar]
- 37.Syed A, Babich O, Thakkar P, et al. Defining Pancreatitis as a Risk Factor for Pancreatic Cancer: The Role, Incidence, and Timeline of Development. Pancreas 2019; 48:1098–1101. [DOI] [PubMed] [Google Scholar]
- 38.Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol 2012; 107:1096–1103. [DOI] [PubMed] [Google Scholar]
- 39.Gislason H, Horn A, Hoem D, et al. Acute pancreatitis in Bergen, Norway. A study on incidence, etiology and severity. Scand J Surg 2004; 93:29–33. [DOI] [PubMed] [Google Scholar]
- 40.Appelros S, Borgstrom A. Incidence, aetiology and mortality rate of acute pancreatitis over 10 years in a defined urban population in Sweden. Br J Surg 1999; 86:465–470. [DOI] [PubMed] [Google Scholar]
- 41.Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 2016; 1:45–55. [DOI] [PubMed] [Google Scholar]
- 42.Kirkegård J, Mortensen MR, Johannsen IR, et al. Positive predictive value of acute and chronic pancreatitis diagnoses in the Danish National Patient Registry: A validation study. Scandinavian Journal of Public Health 2018. [DOI] [PubMed] [Google Scholar]
- 43.Floyd A, Pedersen L, Nielsen GL, et al. Secular trends in incidence and 30-day case fatality of acute pancreatitis in North Jutland County, Denmark: a register-based study from 1981–2000. Scand J Gastroenterol 2002; 37:1461–1465. [DOI] [PubMed] [Google Scholar]
- 44.Munch T, Christensen LB, Adelborg K, et al. Positive predictive values of ICD-10 codes to identify incident acute pancreatitis and incident primary malignancy in the Scandinavian national patient registries among women with postmenopausal osteoporosis. Clin Epidemiol 2017; 9:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nojgaard C, Bendtsen F, Matzen P, et al. The aetiology of acute and chronic pancreatitis over time in a hospital in Copenhagen. Dan Med Bull 2010; 57:A4103. [PubMed] [Google Scholar]
- 46.Gonzalez RS, Bagci P, Basturk O, et al. Intrapancreatic distal common bile duct carcinoma: Analysis, staging considerations, and comparison with pancreatic ductal and ampullary adenocarcinomas. Mod Pathol 2016; 29:1358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frampas E, Morla O, Regenet N, et al. A solid pancreatic mass: tumour or inflammation? Diagn Interv Imaging 2013; 94:741–755. [DOI] [PubMed] [Google Scholar]
- 48.Tas F, Sen F, Odabas H, et al. Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol 2013; 18:839–846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.