Abstract
Coronavirus disease 2019 (COVID-19) is associated with a significant morbidity and mortality in patients with cirrhosis. There is a significantly higher morbidity and mortality due to COVID-19 in patients with decompensated cirrhosis as compared to compensated cirrhosis, and in patients with cirrhosis as compared to noncirrhotic chronic liver disease. The fear of COVID-19 before or after liver transplantation has lead to a significant reduction in liver transplantation numbers, and patients with decompensated cirrhosis remain at risk of wait list mortality. The studies in liver transplantation recipients show that risk of mortality due to COVID-19 is generally driven by higher age and comorbidities. The current review discusses available literature regarding outcomes of COVID-19 in patients with cirrhosis and outcomes in liver transplant recipients.
Keywords: SARS-CoV-2, immunosuppression, liver diseases, nash, mortality
Abbreviations: ACE, angiotensin-converting enzyme related carboxypeptidase receptors; ACLF, acute-on chronic liver failure; ALI, acute liver injury; ALT, alanine transaminase; AST, aspartate aminotransferase; CLD, chronic liver disease; COVID-19, Coronavirus disease 2019; HCWs, health care workers; HR, hazard ratio; LFT, liver function tests; LT, liver transplantation; MELD, model for end-stage liver disease; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; OR, Odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–related coronavirus disease 2019 (COVID-19) is a recent pandemic that has overwhelmed the healthcare system in many countries due to large number of patients getting admitted and requiring intensive care. There is no approved treatment for this disease at present. The COVID-19 is associated with significant morbidity and mortality in patients with liver disease when compared to general population.1, 2, 3 Several studies have shown a significant risk of mortality in patients with cirrhosis and in liver transplantation recipients.2, 3, 4 The severity of presentation and risk of mortality is more in patients with decompensated cirrhosis.5,6 COVID-19 had lead to a significant decrease in number of liver transplant surgeries being performed, which would lead to an increased wait list mortality in these patients.7 The present review discusses possible pathogenesis of COVID-19 associated liver injury, and mortality due to COVID-19 in patients with no liver disease, in patients with noncirrhotic chronic liver disease, in patients with cirrhosis and in liver transplantation (LT) recipients.
Pathogenesis of liver injury by SARS-CoV-2 and incidence of raised liver function tests in patients with COVID-19
SARS-CoV-2 enters liver cells through angiotensin-converting enzyme-related carboxypeptidase (ACE2) receptors. ACE2 is mainly expressed on cholangiocytes and less on hepatocytes. Both these cells help in regeneration of the liver after liver injury. The infection of the cholangiocytes and progenitor cell population may lead to decreased regenerative capabilities of the liver. The liver injury in COVID-19 might be partly due to direct injury of cholangiocytes, and deterioration of liver function may happen due to impaired regeneration.8,9 Patients with cirrhosis are more predisposed to acute liver injury due to upregulated expression of ACE2 protein (thus facilitating more viral entry into cells). Patients with decompensated cirrhosis have more expression of ACE2 protein as compared to patients with compensated cirrhosis, thus making them more susceptible to acute liver injury by virus, in presence of already impaired regeneration capacity due to baseline-decompensated cirrhosis.10,11
Abnormal liver function tests (LFT) are common in patients with COVID-19 in absence of chronic liver disease. A systematic review by Ghoshal et al showed that 10.5%–53% of patients with COVID-19 had raised liver enzymes, although jaundice was uncommon as the total bilirubin was raised in only 5–18% patients. A reduction in serum albumin is also reported by several studies. Patients with more severe COVID-19 associated disease had liver function test abnormalities more often.12 Izcovich et al conducted a systematic review of 207 studies and found several variables that provided prognostic information about severe disease and/or mortality. High blood aspartate aminotransferase (AST), decrease in albumin and high blood bilirubin are associated with severe disease.13 Patients with abnormal LFTs have a worse prognosis as compared to those with normal LFT. Wu et al also looked at prognosis of abnormal LFTs in a meta-analysis by 45 studies,14 the pooled incidence of any abnormal liver biochemistry at admission was 27.2% and during hospitalization 36%. At admission, abnormal albumin was the most common finding (39.8%) followed by gamma-glutamyl transferase (35.8%), AST (21.8%), alanine transaminase (ALT) (20.4%), total bilirubin (8.8%) and alkaline phosphatase (4.7%). During hospitalization, abnormal ALT, AST and total bilirubin were present in 38.4%, 28.1% and 23.2%, respectively. Severe and or critical patients and nonsurvivors had a higher incidence of abnormal liver biochemical indicators. It should be noted that abnormal LFTs might be related to drugs or secondary to ischemia (if present) in addition to direct effects of COVID-19.14
Although patients with noncirrhotic chronic liver disease (CLD) may not have a higher mortality, patients with nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) may have more disease severity/mortality due to frequent association with diabetes, obesity, and metabolic syndrome.15,16
It is important to understand that these studies mainly detect case fatality rate (diagnosed cases) and not infection fatality rate (as asymptomatic or mild cases may not get tested). A systematic review of 73 studies (n = 24,299) showed that 3% patients with COVID-19 were suffering from underlying CLD. The presence of CLD was significantly associated with more severe infection (pooled odds ratio [OR] 1.48) and overall mortality (pooled OR 1.78). Moreover, there was a nonsignificant trend for increased ICU and invasive mechanical ventilation requirement in patients with CLD.2 In a multicentric United States study, the presence of CLD and NAFLD was independently associated with ICU stay and mechanical ventilation. The presence of cirrhosis was a significant predictor of mortality (adjusted OR 12.5, 95% confidence interval [CI] 2.16–72.5).17
Patients with liver disease, in particular patients with NASH, may have higher comorbidities then non-liver disease patients. Singh et al compared 250 patients with liver disease (NASH being the most common sitology) to 2530 non-liver disease patients in a multicentric study. The patients in the liver disease group were older and had higher comorbidities, including hypertension in 68% and diabetes in 48%. The authors found that the patients with liver disease were at significantly increased risk for mortality (RR, 2.8; 95% CI, 1.9–4.0; P < 0.001) after 1:1 propensity score matching.18 Ioannou et al identified 88,747 patients tested for COVID-19 in the Veterans Affairs national healthcare system. The following groups were studied: no cirrhosis-SARS-CoV-2 negative (C0–S0, n = 75,315), no cirrhosis-SARS-CoV-2 positive (C0–S1, n = 9826); cirrhosis-SARS-CoV-2 negative (C1–S0, n = 3301); cirrhosis-SARS-CoV-2 positive (C1–S1, n = 305). The 30-day mortality and ventilation rates were 5.2% and 3.6% in C1–S0, and 17.1% and 13.0% in C1–S1. The patients with cirrhosis and a positive test for SARS-CoV-2 were more likely to undergo mechanical ventilation and mortality, risk being 4.1 times and 3.5 times, respectively. Higher age, decompensation, and high model for end-stage liver disease (MELD) score were predictors of mortality in patients with cirrhosis and SARS-CoV-2. Cirrhosis was associated with a 1.7 times increase in mortality in patients with SARS-CoV-2 infection.19
Mortality: noncirrhotic chronic liver disease versus liver cirrhosis
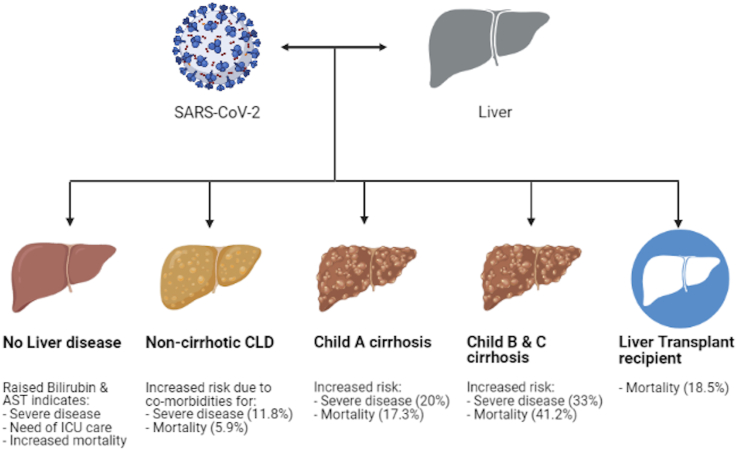
The risk of mortality increases with increasing severity of liver disease as shown in Figure 1. Studies showing outcomes of COVID-19 in patients with cirrhosis are shown in Table 1 (references3,5,6,19, 20, 21, 22).
Figure 1.
Mortality in COVID-19, data based on references5,22 and Table 2.
Table 1.
Outcomes of COVID-19 in Patients with Cirrhosis.
| Author (ref.) | n | Mortality/results | Comments |
|---|---|---|---|
| Shalimar20 | 26 cirrhotics | 11/26 (42.3%) died | Requirement of mechanical ventilation independently predicted mortality (hazard ratio 13.68) |
| Sarin5 | 43 cirrhotics versus 185 CLD- no cirrhosis | 7/43 (16.3%) cirrhosis vs 5/185 (2.7%) CLD- no cirrhosis | Nearly twice mortality in decompensated cirrhosis (versus compensated), Child-Turcotte Pugh score of 9 or more at presentation predicted high mortality AUROC 0.94, HR = 19.2 (95 CI 2.3–163.3), P < 0.001) |
| Iavarone3 | 50 cirrhotics | 30-day mortality rate of 34% (n = 17) | Higher mortality in patients with respiratory failure and in those with worsening liver function |
| Ioannou19 | 305 cirrhotics | 55 (18%) | Higher age, decompensation, and high MELD score were predictors of mortality |
| Moon6 | 103 cirrhotics, 49 non-cirrhotic CLD | Mortality occurred in 12.2% of patients with CLD without cirrhosis, 23.9% with Child-Pugh class A cirrhosis, 43.3% with Child-Pugh class B cirrhosis, and 63.0% with Child-Pugh class C cirrhosis | Child-Pugh class B and C cirrhosis remained associated with death after adjusting for baseline characteristics |
| Kim21 | 867 patients (including 134 compensated and 93 decompensated cirrhotics) | 19/134 (14.1%) of compensated, 38/93 (40.8%) of decompensated, 9/22 (40.9%) of patients with hepatocellular carcinoma | Alcohol related liver disease, decompensated cirrhosis, and HCC predicted higher overall mortality |
| Marjot22 | 386 cirrhosis, 359 without cirrhosis | Mortality was 8% in without cirrhosis, 32% in patients with cirrhosis | Mortality 19%, 35% and 51% and Child-Pugh class A, B and C respectively |
Sarin et al studied acute liver injury (ALI) and its impact on outcomes in patients with noncirrhotic chronic liver disease and with cirrhosis. The authors defined ALI as any one of the following: total bilirubin level of ≥3 mg/dl, acute increase in ALT, AST, SAP, gamma-glutamyl transpeptidase ≥2 times upper normal limit and prothrombin time-international normalized ratio of ≥1.5 with previously normal liver parameters. The study included 228 patients; 185 had a diagnosis of CLD without cirrhosis and 43 were suffering from cirrhosis. In patients with CLD without cirrhosis; diabetes (OR = 2.1, P = 0.01) and in patients with cirrhosis; obesity (OR = 8.1, P = 0.002) predisposed more to liver injury than those without these risk factors. Forty-three percent of CLD noncirrhotic presented as ALI, while 20% of cirrhotics presented with either acute-on-chronic liver failure [11.6%) or acute decompensation (9%). A Child-Turcotte-Pugh score of 9 or more at presentation predicted mortality (area under curve 0.94, sensitivity 85.7% and specificity 94.4%, hazard ratio [HR] 19.2 [95 CI 2.3–163.3], P < 0.001). The liver injury was progressive in 57% of patients with decompensated cirrhosis and 43% died. A rising bilirubin and AST/ALT ratio predicted mortality in patients with cirrhosis.5 Marjot et al compared 386 patients with and 359 without cirrhosis and COVID-19 (data from two international registries). The authors compared this data with non-CLD patients with COVID-19 from a UK hospital network. Mortality was 8% in those without cirrhosis, compared to 32% in patients with cirrhosis. Mortality increased with Child-Turcotte-Pugh severity; 19%, 35% and 51% in patient s with class A, B and C, respectively. Higher age, presence of cirrhosis (more risk in CTP B or C), and alcohol-related liver disease were risk factors for mortality.22
Mortality in patients with COVID-19 and cirrhosis is driven mainly by severity of liver disease. An international registry showed that 47 patients of 152 patients died. The nonsurvivor group had significantly high prevalence of cirrhosis (versus no cirrhosis) and decompensated cirrhosis (Child class B or C 25.7% in survivors versus 63.9% in non-survivors). A total of 43% (13/30) of patients in Child class B and 62.9% (17/27) patients in Child class C died. The mortality rate was 12.2% (6 out of 49) for noncirrhotic CLD, 23.9% (11 out of 46) for Child class A cirrhosis and 52.5% for decompensated cirrhosis (30 out of 57). MELD was also significantly high in nonsurvivors. On multivariate analysis, higher age, body mass index >30 kg/m2, and Child class B and C were significantly associated with mortality.6 In another multicenter retrospective study, patients with cirrhosis and severe acute COVID-19, Iavarone et al demonstrated that a 30-day mortality rate of 34% (17 out of 50) in patients with cirrhosis, which was significantly higher than a comparative cohort of cirrhosis and bacterial infections (17%) and patients without cirrhosis (18%). The severity of lung and liver diseases predicted mortality.3 Other predictors of mortality due to COVID-19 in patients suffering from cirrhosis include need of mechanical ventilation (thus severe pulmonary disease) and Charlson comorbidity -index.20,23
Mortality in patients with COVID-19 related acute-on-chronic liver failure
COVID-19 can cause decompensation or worsening of baseline cirrhosis. Iavarone et al showed that severe COVID-19 in patients with cirrhosis leads to significant increase of bilirubin, prothrombin time, and creatinine, also albumin decrease significantly. Patients with a MELD score ≥15 increased from 13% to 26% (P = 0.037), acute-on-chronic liver failure (ACLF) and acute liver injury occurred in 14 (28%) and 10 (20%) patients.3 In the study by Moon et al, 25% (39 out of 152) had new decompensation event after diagnosis of COVID-19 and 24 of these patients died.6 ACLF related to COVID-19 is common and is associated with significant risk of mortality. In the study by Shalimar et al, 9 patients had ACLF, all of whom died. The mortality in COVID-19 related ACLF was significantly higher than historical controls with ACLF.20 In an Indian study of 57 patients with cirrhosis and COVID-19, 20 (35%) presented as ACLF. The patients in the ACLF group had significantly prolonged hospital stay severe COVID-19 illness, need for intensive care unit, and higher mortality (30% versus 5%). Patients who died in the ACLF group had significantly higher Chronic Liver Failure Consortium (CLIF C) score, CLIF C organ failure score, and ACLF grade.24 The study by Marjot et al found that 50% (89 out of 189) of patients with cirrhosis and acute hepatic decompensation developed ACLF. Among patients with cirrhosis, the mortality was higher in patients with ACLF than in those without ACLF (65% versus 22%).22
Liver transplantation during the COVID-19 pandemic
Liver transplantation (LT) remains the only definitive treatment for patients with decompensated cirrhosis. COVID-19 has affected LT in multiple ways; patients with cirrhosis are at risk of wait list mortality due to COVID-19 infection or due to delay in a timely transplant. In addition, there is risk of COVID-19 infection after transplant. A patient remains at risk of getting infection at hospital as virus remains infectious from several hours to few days on various surfaces,25 or from an infected health care worker (HCW). If the patient with cirrhosis or liver transplant is suffering from COVID-19 also, it carries risk of infecting HCWs. The situation of HCWs getting infected becomes more complex if some patients with COVID-19 remain asymptomatic, and there is a risk of spreading infection in the incubation period (World Health Organization report)26 and the sensitivity of COVID polymerase chain reaction (PCR) is approximately 70%, which may lead to the under diagnosis of COVID-19 and potential exposure of HCWs to an infected but negative PCR patient.27
Various societies have suggested guidelines for this situation, which suggest deferring hospital visits and LT in stable patients.28, 29, 30 Number of LT performed has decreased in the COVID-19 era, both deceased donor and living donor LTs.7,31,32 As a result, LT is being done for more sick patients as compared to earlier. We compared LT in the COVID-19 era and in the same period of 2019. While a total of 39 LTs were performed from March 15th to June 10th in 2019, the number of LTs decreased to 23 (59% of 2019) in 2020. The adult patients with cirrhosis had significantly higher MELD score in year 2020 (19.8 ± 7.0 versus 16.1 ± 5.6 in 2019), P = 0.034.7
Mortality in liver transplant recipients versus nontransplant patients
Table 2 discusses outcomes of COVID-19 in liver transplantation recipients (references4,33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46). Belli et al reported data from 36 centers across Europe. The study included 243 adult LT recipients suffering from symptomatic COVID-19. Thirty-nine recipients (16%) were managed as outpatients, 84% were hospitalized. Forty-nine patients (20.2%) died due to respiratory failure as the major cause. Following factors predicted mortality on multivariable analysis: age >70 (HR, 4.16; 95% CI, 1.78–9.73) and tacrolimus use (protective effect, HR, 0.55; 95% CI, 0.31–0.99). In a model excluding age, both diabetes and chronic kidney disease were significantly associated with mortality.37
Table 2.
Outcomes of LT Recipients with COVID-19 Infection.
| Author reference | n | Outcomes, comments |
|---|---|---|
| Belli33 | 243 | European Liver and Intestine Transplantation Association registry, 49 (20.2%) died, higher age (>70 years) predicted mortality, use of tacrolimus was protective. After excluding age, diabetes and chronic kidney disease significantly associated with mortality |
| Polak4 | 244 | 36 (14.7%) died, Internet-based survey |
| Webb34 | 39 | Data from registry, 9 (23%) died, 4 of death happened in patients transplanted < 2 years back, 4 of died had diabetes and hypertension, 3 were obese |
| Rabiee35 | 112 | 25 (22.3%) died, LT recipients had lower acute liver injury when compared to age- and sex-matched CLD, reduction of immunosuppression was not associated with liver injury/mortality. ALI significantly associated with mortality |
| Lee36 | 38 | 7 died (18% overall, 29% of hospitalized) |
| Dumortier37 | 104 | 20 died, age independently associated with mortality |
| Becchetti38 | 57 | 7 died (12%), 5 of 7 mortalities happened in cases with history of cancers |
| Colmenero39 | 111 | 31.5% had severe disease, 20 (18%) mortality |
| Webb40 | 151 | 28 (19%) died, when compared to matched nontransplant population, mortality was not high in transplant recipients |
| Dhampalwar41 | 12 | 1 died |
| Case reports or small series32,42, 43, 44, 45, 46 | 27 | 9 died |
| Total | 1138 | 211 died (18.5%) |
A prospective Spanish study of 111 LT recipients with COVID-19 infection showed 18% mortality in LT recipients, this mortality rate was lower than matched general population. Thirty-five patients (31.5%) had severe COVID-19. The use of mycophenolate at baseline was an independent predictor of severe COVID-19 disease, this effect was not observed with calcineurin inhibitors or everolimus.39 Another study by Webb et al also did not find a higher mortality in LT recipients with COVID-19; mortality in LT recipients was similar to matched population without liver transplantation.40 The authors compared adult LT recipients with severe COVID-19 (n = 151) from a multicenter database (18 countries) to matched patients (n = 627). The mortality was 19% in transplant cohort versus 27% in control cohort, P = 0.046. The authors found that increased age and comorbidities were related to mortality.40
Rabiee et al compared 112 adult LT recipients with COVID-19 to age- and sex-matched 375 CLD with COVID-19. The mortality rate was 22.3% in LT recipients, 72.3% were hospitalized and 26.8% were admitted to the intensive care unit. A reduction in immunosuppression was not associated with ALI or mortality. The ALI was significantly associated with mortality (P = 0.007; OR, 6.91) and ICU admission (P = 0.007; OR, 7.93) in LT recipients.35 In French solid organ transplant registry, 104 patients were diagnosed with COVID-19 at a median of 92.8 months (interquartile range 40–194 months) after LT. One-third suffered from severe COVID-19, and the 30-day mortality was 20% (28.1% for hospitalized patients). Multivariate analysis showed age to be independently associated with mortality.37
Mortality in early versus late COVID-19 infection after LT
An important issue with LT during the pandemic is recipient outcome in case of COVID-19 infection in the early post-surgery period. There is scarce data on outcomes of early COVID-19 after LT. As discussed previously, studies have shown that LT recipients with higher age and comorbidities have higher mortality; relation of mortality to time since transplantation is not established. Mortality in LT recipients due to COVID-19 infection is a complex interplay of comorbidities and immunosuppression. Bhoori et al described 3 mortalities in LT recipients due to COVID-19. All these mortalities happened in recipients with a post-transplant follow-uP > 10 years. All 3 males were older than 65 years, had obesity, diabetes, hyperlipidemia, and hypertension. Three patients had COVID-19 at <2 years after LT, all did well.43 We published data of 12 LT recipients with COVID-19.41 One of these patient (8.3%) died; the patient who died was a 60-year-old male with comorbidities of diabetes, hypertension, metabolic syndrome, and chronic rejection. He underwent LT 82 months back.41 Although patients with longer follow-up after LT (thus on less immunosuppression) been shown to have higher mortality in some studies, the finding is biased by higher age and higher chances of having comorbidities in LT recipients with long-term follow-up. Several small series have shown mortality in early period after LT. Maggi et al also showed that one of two LT recipients died postoperatively due to COVID-19.32 Recently two centers have reported outcomes of early COVID-19 after LT. Massoumi et al described 5 patients with early COVID-19; 3 were mild cases while 2 were moderate cases.45
Waisberg et al described their experience of 7 patients with early COVID-19 (range 9–39 days) after LT. Three of these patients had severe disease and 2 died. This series had several important differences from series by Massoumi et al. The patients were older and had more comorbidities, and most of the patients were diagnosed with COVID-19 at the index hospitalization.46
A systematic review of 12 studies (n = 517 hospitalized LT recipients with COVID-19) found the following presenting symptoms: fever (71%), cough (62%), dyspnea (48%), and diarrhea (28%). There was a higher mortality risk in age group >60–65 years (OR 4.26; 95% CI, 2.14–8.49). Duration since transplant did not affect outcome.47
While immunosuppression may attenuate inflammatory response to COVID-19, it may also increase virological injury and risk of secondary infections and may prolong viral shedding. Mortality in LT recipients appears to be driven by higher age and comorbidities rather than by higher or lower immunosuppression.
The COVID-19 is associated with a significant risk of more severe disease presentation and mortality in patients with cirrhosis. The risk of mortality is more in patients with decompensated cirrhosis. There is limited data of COVID-19 in liver transplant recipients, which suggests that mortality after LT depends on higher age and comorbidities. Given the current situation of a significant number of patients with COVID-19 in the community, we propose to defer elective liver transplantation for relatively stable patients who can wait for several months. As sick patients will have higher mortality during a long waiting period or in case of COVID-19 infection, emergency liver transplantation should not be deferred.
Credit authorship contribution statement
NSC, NS: conceptualization; NSC, SW: draft writing; NS, ASS: critical revision.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
None.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., et al. China medical treatment expert group for covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovalic A.J., Satapathy S.K., Thuluvath P.J. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14:612–620. doi: 10.1007/s12072-020-10078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iavarone M., D'Ambrosio R., Soria A., et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polak W.G., Fondevila C., Karam V., et al. Impact of COVID-19 on liver transplantation in Europe: alert from an early survey of European liver and intestine transplantation association and European liver transplant registry. Transpl Int. 2020;33:1244–1252. doi: 10.1111/tri.13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarin S.K., Choudhury A., Lau G.K., et al. APASL COVID Task Force APASL COVID liver injury spectrum study (APCOLIS study- NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; the APCOLIS study (APASL COVID-19 liver injury spectrum study) Hepatol Int. 2020;14:690–700. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon A.M., Webb G.J., Aloman C., et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020;73:705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soin A.S., Choudhary N.S., Yadav S.K., et al. Restructuring living donor liver transplantation at a high volume center during the COVID-19 pandemic. J Clin Exp Hepatol. 2020 doi: 10.1016/j.jceh.2020.09.009. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chai X., Hu L., Zhang Y., et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-ncov infection. bioRxiv. 2020 doi: 10.1101/2020.02.03.931766. 02.03.931766. [DOI] [Google Scholar]
- 9.Kumar P., Sharma M., Kulkarni A., Rao P.N. Pathogenesis of liver injury in coronavirus disease 2019. J Clin Exp Hepatol. 2020;10:641–642. doi: 10.1016/j.jceh.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paizis G., Tikellis C., Cooper M.E., et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–1806. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grace J.A., Casey S., Burrell L.M., Angus P.W. Proposed mechanism for increased COVID-19 mortality in patients with decompensated cirrhosis. Hepatol Int. 2020;14:884–885. doi: 10.1007/s12072-020-10084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghoshal U.C., Ghoshal U., Dhiman R.K. Gastrointestinal and hepatic involvement in severe acute respiratory syndrome coronavirus 2 infection: a review. J Clin Exp Hepatol. 2020;10:622–628. doi: 10.1016/j.jceh.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izcovich A., Ragusa M.A., Tortosa F., et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PloS One. 2020;15 doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y., Li H., Guo X., et al. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int. 2020;14:621–637. doi: 10.1007/s12072-020-10074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai Q., Huang D., Ou P., et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 16.Ji D., Qin E., Xu J., et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashemi N., Viveiros K., Redd W.D., et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: a multicentre United States experience. Liver Int. 2020;40:2515–2521. doi: 10.1111/liv.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh S., Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology. 2020;159:768–771. doi: 10.1053/j.gastro.2020.04.064. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ioannou G.N., Liang P.S., Locke E., et al. Cirrhosis and SARS-CoV-2 infection in US Veterans: risk of infection, hospitalization, ventilation and mortality. Hepatology. 2020 Nov 21 doi: 10.1002/hep.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shalimar Elhence A., Vaishnav M., Kumar R., Pathak P., Soni K.D., et al. Poor outcomes in patients with cirrhosis and corona virus disease-19. Indian J Gastroenterol. 2020;39:285–291. doi: 10.1007/s12664-020-01074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D., Adeniji N., Latt N., et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.09.027. S1542-3565(20)31288-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marjot T., Moon A.M., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajaj J.S., Garcia-Tsao G., Biggins S.W., et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2020 doi: 10.1136/gutjnl-2020-322118. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar P., Sharma M., Sulthana S.F., Kulkarni A., Rao P.N., Reddy D.N. SARS-CoV-2 related Acute on chronic liver failure (S-ACLF) J Clin Exp Hepatol. 2020 doi: 10.1016/j.jceh.2020.12.007. 10.1016/j.jceh.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coronavirus disease 2019 (COVID-19) situation report – 73. World health organisation. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200402-sitrep-73-covid-19.pdf?sfvrsn=5ae25bc7_6.
- 27.Fernández-Barat L., López-Aladid R., Torres A. The value of serology testing to manage SARS-CoV-2 infections. Eur Respir J. 2020;56:2002411. doi: 10.1183/13993003.02411-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fix O.K., Hameed B., Fontana R.J., et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology. 2020;72:287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saigal S., Gupta S., Sudhindran S., et al. Liver transplantation and COVID-19 (Coronavirus) infection: guidelines of the liver transplant Society of India (LTSI) Hepatol Int. 2020;14:429–431. doi: 10.1007/s12072-020-10041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boettler T., Newsome P.N., Mondelli M.U., et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100–113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyarsky B.J., Po-Yu Chiang T., Werbel W.A., et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20:1809–1818. doi: 10.1111/ajt.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maggi U., De Carlis L., Yiu D., et al. The impact of the COVID-19 outbreak on liver transplantation programs in Northern Italy. Am J Transplant. 2020;20:1840–1848. doi: 10.1111/ajt.15948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belli L.S., Fondevila C., Cortesi P.A., et al. ELITA-ELTR COVID-19 registry. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with covid-19: results from the ELITA/ELTR multi-center European study. Gastroenterology. 2021;160:1151–1163. doi: 10.1053/j.gastro.2020.11.045. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webb G.J., Moon A.M., Barnes E., Barritt A.S., Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5:643–644. doi: 10.1016/S2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabiee A., Sadowski B., Adeniji N., et al. COLD Consortium Liver injury in liver transplant recipients with coronavirus disease 2019 (COVID-19): U.S. Multicenter experience. Hepatology. 2020 doi: 10.1002/hep.31574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee B.T., Perumalswami P.V., Im G.Y., Florman S., Schiano T.D., COBE Study Group COVID-19 in liver transplant recipients: an initial experience from the US epicenter. Gastroenterology. 2020;159:1176–1178. doi: 10.1053/j.gastro.2020.05.050. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dumortier J., Duvoux C., Roux O., et al. French Solid Organ Transplant COVID Registry; Groupe de Recherche Français en Greffe de Foie (GReF2). Covid-19 in liver transplant recipients: the French SOT COVID registry. Clin Res Hepatol Gastroenterol. 2021;45:101639. doi: 10.1016/j.clinre.2021.101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becchetti C., Zambelli M.F., Pasulo L., et al. COVID-LT group COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832–1840. doi: 10.1136/gutjnl-2020-321923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colmenero J., Rodríguez-Perálvarez M., Salcedo M., et al. Epidemiological pattern, incidence and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2020;74:148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webb G.J., Marjot T., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008–1016. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhampalwar S., Saigal S., Choudhary N., et al. Outcomes of coronavirus disease 2019 in living donor liver transplant recipients. Liver Transplant. 2020;26:1665–1666. doi: 10.1002/lt.25909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-Ruiz M., Andrés A., Loinaz C., et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20:1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhoori S., Rossi R.E., Citterio D., Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varghese J., Malleeswaran S., Patcha R.V., Appusamy E., Karnan P., Kapoor D., Venugopal K., Kedarisetty C.K., Singh B., Rao P.S., Yalakanti R.B., Mohanka R., Shrimal A., Nikam V., Kumar K., Shenvi S.D., Venugopal B.P., Heaton N.D. A multicentric experience on living donor liver transplantation in coronavirus disease 2019 hotspots in India. Liver Transpl. 2021;27:1334–1338. doi: 10.1002/lt.25957. Epub 2021 Feb 15. PMID: 33253477; PMCID: PMC7753810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Massoumi H., Rocca J., Frager S., Kinkhabwala M. Changes in liver transplant center practice in response to coronavirus disease 2019: unmasking dramatic center-level variability. Liver Transplant. 2020;26:1198–1199. doi: 10.1002/lt.25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waisberg D.R., Abdala E., Nacif L.S., Ducatti L., Haddad L.B., Martino R.B., Pinheiro R.S., Arantes R.M., Galvao F.H., Gouveia L.N., Terrabuio D.R., Darce G.F., Rocha-Santos V., Andraus W., Carneiro-D'Albuquerque L.A. Coronavirus disease 2019 in the early postoperative period of liver transplantation: is the outcome really so positive? Liver Transpl. 2021;27:1357–1359. doi: 10.1002/lt.25933. Epub 2020 Dec 9. PMID: 33166024. [DOI] [PubMed] [Google Scholar]
- 47.Jayant K., Reccia I., Virdis F., et al. COVID-19 in hospitalized liver transplant recipients: an early systematic review and meta-analysis. Clin Transplant. 2021 doi: 10.1111/ctr.14246. [DOI] [PMC free article] [PubMed] [Google Scholar]