Abstract
Allergic bronchopulmonary aspergillosis (ABPA) is a severe form of asthma in which structural airway destruction occurs due to a hypersensitivity reaction to fungi. A 25-year-old man without any major features of asthma had lung infiltration with dilatation of the central bronchus, high-attenuation mucus with histological eosinophilic invasion, fungi detected on cultures, and positive Aspergillus-specific immunoglobulin E (IgE) and precipitating antibody of Aspergillus, with a significant elevation of blood eosinophils and slightly increased total IgE. He recovered rapidly with systemic corticosteroid therapy without recurrence over 1-year follow-up and an increased forced expiratory volume in one second, which supported the possibility of ABPA without any major features of asthma.
Keywords: allergic bronchopulmonary aspergillosis, asthma, diagnostic criteria, ABPA, ABPM, allergic bronchopulmonary
Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is one of the important phenotypes of severe asthma induced by a hypersensitivity reaction to fungi, especially Aspergillus species (1). The clinical features of ABPA are the presence of asthma or cystic fibrosis (CF) with increased immunoglobulin E (IgE) levels, positive Aspergillus-specific IgE and precipitating antibodies, chest X-ray infiltrates with central bronchiectasis, and Aspergillus species-containing mucus plugs (2). We herein report a case of an ABPA patient diagnosed by the new criteria in Japan without major features of asthma, which indicates the possibility of phenotypes different from the ordinary form of ABPA, and who recovered with rapid systemic corticosteroid treatment without recurrence at 1 year. This report will contribute to avoiding a delay in the diagnosis and the start of treatment for ABPA.
Case Report
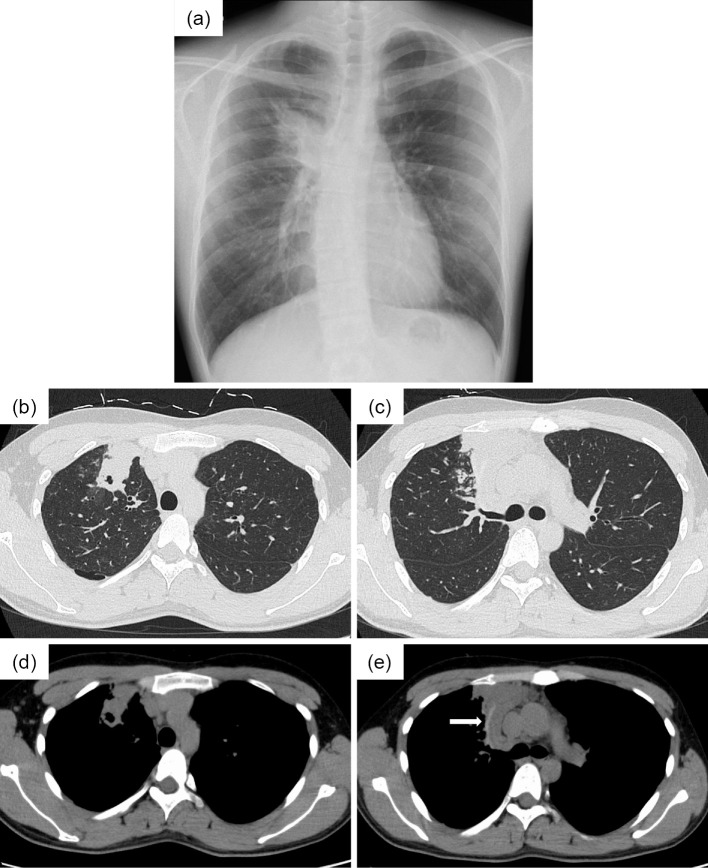
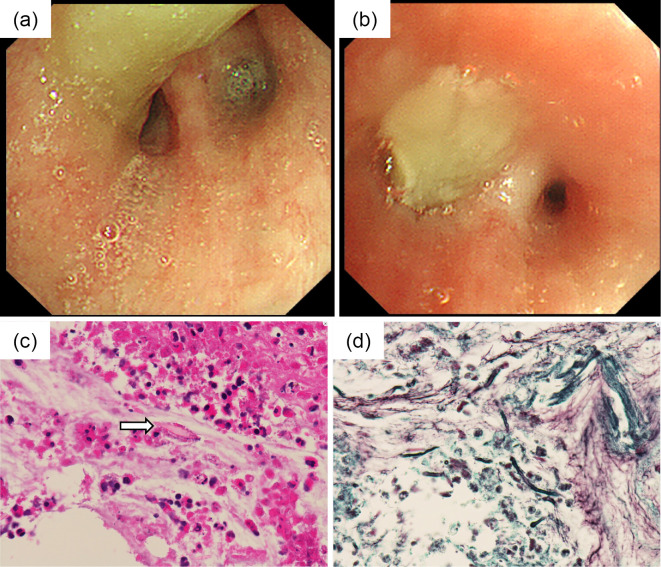
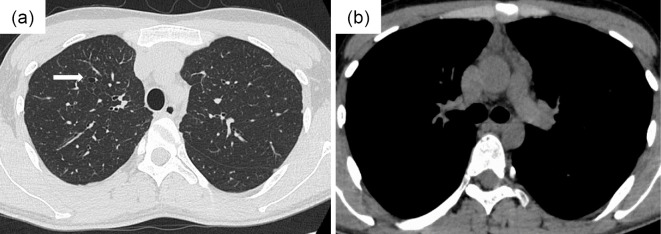
A 25-year-old Japanese man who had fever, general fatigue, and a productive cough with yellow sputum for 2 weeks and an abnormal chest X-ray was referred to a pulmonologist. He had no medical history of asthma and no history of asthma-related symptoms such as wheezing or paroxysmal respiratory symptoms including cough, chest tightness, or dyspnea. He had no history of other respiratory diseases, tobacco smoking, or taking any drugs. He did not have a history of dust exposure, contact with birds, or changes in his living circumstances. On physical examination, his temperature was 37.4℃, and he had no hypoxemia, abnormal respiratory sounds, or a skin rash. Chest radiography showed infiltration of the right upper lung field (Fig. 1a), and chest computed tomography (CT) showed lung infiltration and nodules with dilatation of the central bronchus and high-attenuation mucus (Fig. 1b-e). CT density of the high-attenuation mucus was 96 Hounsfield units (HU), which was higher than that of the paraspinal muscle at 57 HU in the mediastinal window. On laboratory examination, the white blood cell count was 5,700/μL, with 12.3% eosinophils, and total IgE was 257 IU/mL. C-reactive protein was slightly elevated at 0.75 IU/dL. Other results including liver enzymes, anti-neutrophil cytoplasmic antibody, β-D glucan, and Aspergillus galactomannan antigen were normal. Aspergillus-specific IgE and precipitating antibody of Aspergillus were positive. Bronchoscopy showed a mucus plug in the central bronchus (Fig. 2a, b), and the histological findings of the mucus indicated infiltration of eosinophils with Charcot-Leyden crystals and mycotic mycelium on Grocott staining (Fig. 2c, d). Aspergillus flavus was detected in the cultured specimen, with no other pathogens. Pulmonary function testing showed a forced vital capacity (FVC) at 4.30 L, a forced expiratory volume in one second (FEV1) at 3.76 L, and %FEV1 at 87.4%, with a linear appearance of the flow volume curve, which indicated no peripheral airflow limitation (Fig. 3). Fractional exhaled nitric oxide (FeNO) was increased at 78 ppb. ABPA without major features of asthma was diagnosed, according to the new criteria in Japan (3) (Table), and treatment with systemic corticosteroids at 30 mg/day was started. The treatment was discontinued at 12 weeks with gradual tapering, and his productive cough recovered with a resolution of the chest X-ray abnormalities and the elevations of blood eosinophil and IgE levels. Chest CT findings also showed the disappearance of infiltration, nodules, and high-attenuation mucus, and remaining localized bronchiectasis in the right upper lung field (Fig. 4a, b). The results of pulmonary function tests such as FEV1 were not altered, at 3.79 L, by the treatment. Currently, he has had no recurrence for 1 year without receiving any systemic corticosteroid treatment.
Figure 1.
Findings on chest X-ray and computed tomography (CT) before systemic corticosteroid treatment. (a) Chest radiography shows infiltration of the right upper lung field. (b, c) Chest CT shows lung infiltration and nodules with dilatation of the central bronchus on the pulmonary window setting and (d, e) high-attenuation mucus (arrow) on the mediastinal window setting.
Figure 2.
Luminal findings on bronchoscopy and the histological findings of the mucus plug. (a, b) Bronchoscopy shows a mucus plug in the central bronchus. (c) Histological findings of the mucus plug show the infiltration of eosinophils with Charcot-Leyden crystals (arrow) and (d) mycotic mycelium on Grocott staining.
Figure 3.
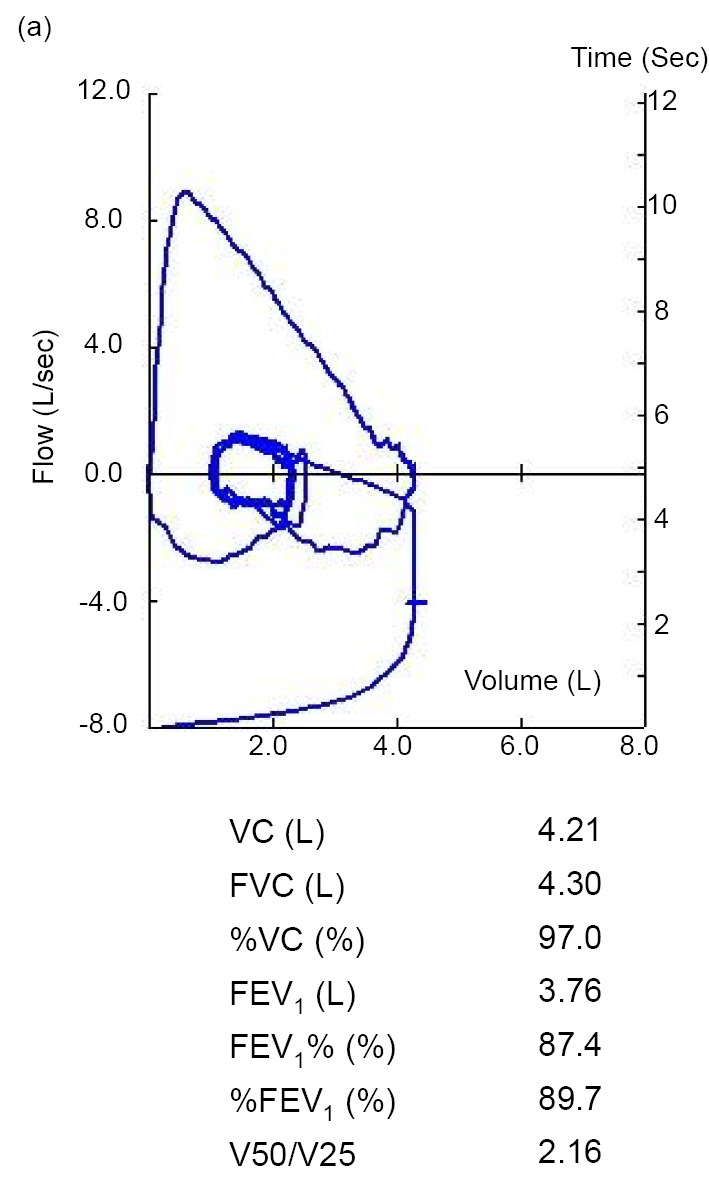
The results of pulmonary function testing including the flow-volume curve before systemic corticosteroid treatment. The flow curve and parameters of lung function are in the normal ranges. VC: vital capacity, FVC: forced vital capacity, FEV1: forced expiratory volume in one second
Table.
New Clinical Diagnostic Criteria in Japan (3).
| 1) | History of asthma or symptoms associated with asthma |
| 2) | Blood eosinophils ≥ 500/μL |
| 3) | Blood total IgE ≥ 417 IU/mL |
| 4) | Positive findings of the type 1 mycosis skin test or specific IgE |
| 5) | Positive findings of mycosis-specific precipitating antibody or specific IgG |
| 6) | Detection of mycosis in sputum or bronchoscopy specimen |
| 7) | Positive findings of mycotic mycelium by Grocott staining of the mucus plug |
| 8) | Dilatation of the central bronchus on chest CT |
| 9) | Current or historical mucus plug existence on chest CT or bronchoscopy |
| 10) | Presence of high-attenuation mucus on chest CT |
ABPM is diagnosed if more than 6 criteria are satisfied in the patient.
IgE: immunoglobulin E, IU: international units, IgG: immunoglobulin G, CT: computed tomography
Figure 4.
Findings on chest computed tomography (CT) after systemic corticosteroid treatment. (a, b) Chest CT shows disappearance of infiltration, nodules, and high-attenuation mucus and remaining localized dilatation of the central bronchus (arrow) in the right upper lung field.
Discussion
ABPA is a pulmonary disorder caused by immunological reactions against antigens of fungi, especially the Aspergillus species. It manifests clinically as allergic asthma (and cystic fibrosis, which is certainly rare in Japan) with structural airway destruction caused by continuous eosinophilic inflammation (4). To avoid a decline in the lung function, an early and accurate diagnosis of ABPA is necessary. However, the diagnostic criteria still remain controversial because of global heterogeneities in the clinical characteristics and the low frequency of definite diagnosis (4,5). Recently, Asano advocated a new clinical guideline for allergic bronchopulmonary mycosis (ABPM) in the Japan Agency for Medical Research and Development (3) while referring to the ordinary diagnostic criteria (4-6). The criteria are shown in Table, and ABPM is diagnosed if more than 6 of the criteria are satisfied (3). The present case met these criteria, except for the presence of asthma and a high blood level of total IgE; thus, the patient was diagnosed as having ABPA without any major features of asthma. Importantly, the ordinary criteria did not provide a definite diagnosis of ABPA because of the high weighting of the presence of asthma and a high blood level of total IgE, but not of other specific features, including the presence of high-attenuation mucus and the detection of fungi in cultured specimens, which might delay the diagnosis and start of treatment (2,4).
Generally, ABPA is one of the severe phenotypes of asthma that indicates poor control and is a risk factor for accelerated decline of lung function (1). Thus, physicians normally suspect the possibility of ABPA in patients with uncontrolled asthma. The present case satisfied the new diagnostic criteria of ABPM, except for the presence of asthma; in fact, the patient had no history of asthma, even of asthma-related symptoms such as wheezing or paroxysmal respiratory symptoms including cough, chest tightness, and dyspnea, which might have delayed the diagnosis and start of treatment. Previous studies reported the existence of ABPA/ABPM patients without asthma, supporting the present case. For example, Ishiguro et al. analyzed 42 ABPM patients and evaluated clinical criteria considering sensitivity and specificity for the diagnosis of ABPM. In their study, 14 patients (33.3%) with ABPM who satisfied their modified diagnostic criteria did not have asthma (6). Additionally, Oguma et al. performed a multicenter nationwide survey in Japan to identify the clinical features of ABPA and clarified the characteristics of 499 patients with physician-diagnosed ABPA. They showed that 81%, but not 100%, of all cases of ABPA had asthma as a predisposing condition (7). The present case did not have any episodes of asthma, with a normal range of pulmonary function tests without peripheral airflow limitation on the flow volume curve. Moreover, no airway reversibility was observed, with alteration of FEV1 at -30 mL (-0.7%) after bronchodilator administration. Furthermore, systemic corticosteroid treatment, which is pivotal treatment for ABPA (8) and asthma (9) improved the clinical abnormalities of ABPA, such as the chest radiological abnormalities and the elevations of blood eosinophil and IgE levels. However, the pulmonary functions, especially FEV1, were not altered compared to the data before systemic corticosteroid treatment, which supported the absence of the main features of asthma in the present case.
One of the parameters that we should consider as a clinical feature of ABPM in the present case is the serum total IgE concentration. The criteria for the diagnosis of ABPM suggest a total serum IgE level ≥416 IU/mL (2,3), but the present case had a level of 257 IU/mL, which did not change at several different time points and was lower than in typical cases of ABPA. There is increasing evidence that serum IgE reflects the disease pathophysiology and activity of ABPA as one of the biomarkers. Agarwal et al. showed that, in patients with ABPA, total IgE decreased with the recovery of the clinical findings after treatment and then increased during exacerbations (10). Additionally, in a Japanese survey, ABPA patients with central bronchiectasis who experienced recurrence/flare showed a significantly higher serum IgE level than those who did not experience recurrence/flare, which suggested that a lower serum IgE level is associated with a better prognosis of ABPA (7). The present case with a lower serum IgE level responded to systemic corticosteroid rapidly, with no recurrences after treatment was stopped for 3 months, which was consistent with these previous data (7,10). Notably, the recommended duration of corticosteroid treatment is reported to be 6 to 12 months (11). Recently, a randomized trial showed that shortening the treatment duration of medium-dose corticosteroid is equally effective, with a reduction of the specific adverse effects (8). The present case was treated by systemic corticosteroid and tapered, taking into account the need to reduce corticosteroid-specific adverse effects and carefully observe for disease recurrence. Consequently, the total treatment duration was 3 months without recurrence, which might support the mild clinical characteristics of ABPA without major features of asthma. In addition, Glancy reported 11 ABPA patients without clinical asthma with low serum IgE levels, as in the present case, and high serum IgE levels, which indicated that the present ABPA case with low serum IgE levels (12) might be one of the clinical phenotypes of ABPA without the major features of asthma.
There is one limitation associated with this report. The airway hyperresponsivity test, which is one of the important examinations for detecting comorbid asthma, was not performed because informed consent was not obtained. Thus, the existence of mild allergic asthma could not be completely ruled out, although it was possible to exclude moderate to severe asthma in the present case.
Conclusion
We herein described a case of an ABPA patient without major features of asthma diagnosed by the new criteria in Japan. This form of ABPA with a low serum IgE level recovered with rapid induction of systemic corticosteroid treatment and had no recurrence at 1 year, which might be one of the clinical features of ABPA.
Author's disclosure of potential Conflicts of Interest (COI).
Koichiro Takahashi: Honoraria, Nippon Boehringer Ingelheim and Astrazeneca; Research funding, Nippon Boehringer Ingelheim.
References
- 1. Porsbjerg C, Menzies-Gow A. Co-morbidities in severe asthma: Clinical impact and management. Respirology 22: 651-661, 2017. [DOI] [PubMed] [Google Scholar]
- 2. Greenberger PA, Bush RK, Demain JG, Luong A, Slavin RG, Knutsen AP. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2: 703-708, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asano K, Hebisawa A, Ishiguro T, et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J Allergy Clin Immunol. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 4. Rosenberg M, Patterson R, Mintzer R, Cooper BJ, Roberts M, Harris KE. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med 86: 405-414, 1977. [DOI] [PubMed] [Google Scholar]
- 5. Agarwal R, Maskey D, Aggarwal AN, et al. Diagnostic performance of various tests and criteria employed in allergic bronchopulmonary aspergillosis: a latent class analysis. PLoS One 8: e61105, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ishiguro T, Takayanagi N, Uozumi R, et al. Diagnostic criteria that can most accurately differentiate allergic bronchopulmonary mycosis from other eosinophilic lung diseases: a retrospective, single-center study. Respir Investig 54: 264-271, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Oguma T, Taniguchi M, Shimoda T, et al. Allergic bronchopulmonary aspergillosis in Japan: a nationwide survey. Allergol Int 67: 79-84, 2018. [DOI] [PubMed] [Google Scholar]
- 8. Agarwal R, Aggarwal AN, Dhooria S, et al. A randomised trial of glucocorticoids in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J 47: 490-498, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 55: 1900588, 2020. [DOI] [PubMed] [Google Scholar]
- 10. Agarwal R, Aggarwal AN, Sehgal IS, Dhooria S, Behera D, Chakrabarti A. Utility of IgE (total and Aspergillus fumigatus specific) in monitoring for response and exacerbations in allergic bronchopulmonary aspergillosis. Mycoses 59: 1-6, 2016. [DOI] [PubMed] [Google Scholar]
- 11. Agarwal R, Gupta D, Aggarwal AN, Behera D, Jindal SK. Allergic bronchopulmonary aspergillosis: lessons from 126 patients attending a chest clinic in north India. Chest 130: 442-448, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Glancy JJ, Elder JL, McAleer R. Allergic bronchopulmonary fungal disease without clinical asthma. Thorax 36: 345-349, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]