Abstract
Background
The health impact of antimicrobial resistance (AMR) has not been included in the Global Burden of Disease (GBD) report, as reliable data have been lacking. AMR burden estimates have been derived from models combining incidence and/or prevalence data from national and/or international surveillance systems and mortality estimates from clinical studies. Depending on utilized empirical data, statistical methodology and applied endpoints, the validity and reliability of results can differ substantially.
Objectives
We assessed comprehensiveness, and internal and external validity of studies estimating the clinical impact of infections caused by the priority antibiotic resistant pathogens monitored by the WHO Global Antimicrobial Resistance Surveillance System.
Data sources
Ovid MEDLINE, January 1950 to March 2019, In-Process and other non-indexed citations were searched.
Study eligibility criteria
Studies reporting mortality, length of hospital stay, duration of the disease until remission and/or death, complications, hospital re-admissions, and follow-up beyond hospital discharge were eligible.
Methods
The literature was searched according to the Cochrane recommendations and reported according to Preferred Reporting Items for Systematic Reviews.
Results
Two-hundred and eighty-six studies out of 3529 were eligible. Studies derived mainly from high-income countries (215, 75%) and relied on data from retrospective (226, 79%), single-centre (201, 70%), cohort studies (243, 85%). The health impact was mostly limited to all-cause mortality (128, 45%) with heterogeneity in timing of assessment; attributable length of hospital stay was seldom adjusted for pre-infection admission time and a few studies had enough follow-up for assessing long-term sequelae. Overall, adjustment for confounding has shown a substantial improvement. Data on health state definitions and duration of diseases are generally lacking, precluding calculation of disability-adjusted life years, critical for application of the GBD study methodology.
Conclusion
Efforts to improve harmonization, representativeness, quality of AMR surveillance data and cohort studies to determine AMR attributable mortality and morbidity are urgently required. Policy makers need accurate and detailed burden estimates to inform prioritization of resource allocation, and to select the most effective intervention strategies to halt the AMR crisis.
Keywords: Antimicrobial resistance, Burden of disease, Methodology, Mortality, Surveillance
Introduction
Assessment of the health impact of antimicrobial resistance (AMR) is paramount for public health, since it determines the relevance of specific interventions, policy and investment decisions. Incidence and prevalence data from surveillance systems, together with AMR attributable mortality and other clinical outcomes, have been used to produce estimates of AMR burden at national and global levels [[1], [2], [3], [4]]. Epidemiological data based on surveillance of clinical isolates of infected patients, essential for appropriate estimation of AMR burden, are mainly available from high-income countries (HICs), and seldom include appropriate denominator data [5,6]. Moreover, studies evaluating the clinical impact of AMR differ significantly depending on data source, study setting, statistical methodology, type of endpoints and study quality [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]]. This selective availability of surveillance data together with the large heterogeneity and bias in the evaluation of clinical outcomes has led to imprecise estimates of the health impact of AMR in healthcare and community settings, in particular when performing global assessments [[21], [22], [23], [24]].
The most comprehensive evaluation of the burden of diseases is performed by the Global Burden of Disease (GBD) study, which assesses and quantifies the impact of diseases on global health stratified by age, sex and region [25]. Parameters needed for estimating the burden of disease using disability-adjusted life years (DALYs) include the incidence or prevalence of the disease, related complications with consequent disabilities (expressed as disability weights) and the years of life lost due to premature death. Despite the inclusion of many causative agents of infectious diseases, antimicrobial resistant bacteria, with the only exception of multidrug resistant tuberculosis, have never been included in the GBD study [26,27]. Consequently, the impact of AMR cannot be estimated or compared with other health problems, and prioritization of public health measures to control AMR lacks evidence.
To determine the quality of the required clinical data we systematically verified the availability of the data needed to DALY parameters to quantify the burden of disease, and we assessed the methodologies applied in these studies focusing on the health impact of drug-resistant infections caused by the target pathogens of the World Health Organization (WHO) Global Antimicrobial Resistance Surveillance and Use System (GLASS).
Materials and methods
We performed a systematic review of studies according to the Cochrane recommendations and reported according to PRISMA (please see supplementary material) [28]. The study protocol is available online (https://www.medizin.uni-tuebingen.de/de/das-klinikum/einrichtungen/kliniken/medizinische-klinik/innere-medizin-1/forschung/infektiologie).
The following PICO questions were applied as inclusion criteria:
-
-
Population: all patients with infections, stratified by bloodstream infections (BSIs) versus others, due to antibiotic resistant strains of GLASS pathogens: Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii (the most common among Acinetobacter spp.), Staphylococcus aureus, Streptococcus pneumoniae, Salmonella spp., Shigella spp. and Neisseria gonorrhoeae). Resistance patterns included resistance to penicillins, quinolones, cephalosporins, carbapenems, and polymyxin (details in the definitions section). No age or setting restrictions were applied.
-
-
Intervention: analysis of the health impact of infections caused by resistant strains of GLASS pathogens with no study design restriction.
-
-
Comparison: either infections due to antibiotic-susceptible bacteria or no infection.
-
-
Outcome: all-cause mortality (7-, 14-, 21-, 30-day or long-term mortality (>30 days), or in-hospital), attributable mortality (classified as defined by the authors), age- or gender-stratified mortality, length of hospital stay (LOS), duration of the disease until remission and/or death, complications during hospital stay, hospital readmissions, and clinical outcomes beyond hospital discharge. No restriction to study design was set.
We searched Ovid MEDLINE In-Process (January 1950 up to March 2019); only English published articles were considered for inclusion (search terms in the supplementary material). Data sources also included existing databases from the WHO-funded pathogen priority list for research and development of new antibiotics and the IMI-funded DRIVE-AB project (Driving Reinvestment in research and development for antibiotics and advocating their responsible use) [29,30]. Reasons for study exclusion were documented in a log file.
Countries were stratified according to the WHO classification of regions (African Region, AFR; Region of the Americas, AMER; Eastern Mediterranean Region, EMR; European Region, EUR; South-East Asia Region, SEAR; and Western Pacific Region, WPR) and the 2017–2018 World Bank Country (WBC) economic classification (high income, HIC; upper middle income, UMIC; low middle income, LMIC; low income, LIC) [31,32]. Extended-spectrum β-lactamase (ESBL)-producing bacteria were defined as resistant to third-generation cephalosporins by phenotypic and/or genetic methods. Carbapenem resistance was defined as phenotypic genotypic resistance, to at least one carbapenem agent. Multidrug resistance (MDR) was defined as non-susceptibility to one agent in at least three or more antibiotic classes (carbapenems, extended spectrum cephalosporins, aminoglycosides, antipseudomonal fluoroquinolones, antipseudomonal penicillin + β-lactamase inhibitors); extensively drug-resistant (XDR) was defined as non-susceptibility to one agent in all but two of the antimicrobial drug classes reported above [33].
Two authors screened all titles and abstracts to identify studies that potentially met the inclusion criteria and a third author randomly screened 10% of the data extraction for quality control. Any eligibility issues were resolved by discussion after evaluation of the full text article. Information collected included authors, journal, year of publication, country, patients' population (paediatric defined as <14 years old and elderly as >65 years old), resistance patterns, types and site of acquisition of infection (hospital acquired infections, HAIs; healthcare associated infections, HCAIs; and community acquired infections, CAIs), assessment of comorbidities and illness severity (classification of aggregate measures in the supplementary material), study design, type of comparison, statistical methodology (sample size calculation, descriptive statistic, adjustment for confounders, analysis accounting for matching, regression analysis, survival analysis, multistate models, competing risks) and methodological quality of the study. The reporting of parameters needed for estimating DALYs, incidence/prevalence of disease, related complications and the years of life lost due to premature death were assessed as well. Two authors independently reviewed the quality of the studies according to the New Castle–Ottawa scales for case–control and cohort studies (please see supplementary material) [34]. The scale comprises three sections which addresses study design and selection bias (four items), comparability (two items) and assessment of outcome/ascertainment of exposure (three items). A star-based rating system was used to indicate the quality of a study where, for each item met, one star was assigned to the study to the maximum possible score nine. Low quality was given for a score <2 in the selection domain and a score ≤1 in the outcome domain. For the assessment of comparability, no points were given if no control for confounders was performed; a score of 1 was assigned in case of controlled analysis (medium quality ranking) and one more point was gained if variable adjustments were specified (good quality ranking). Data were coded in binary categorical variable, extracted into a standard data table using Microsoft Excel and analysed using descriptive statistics.
Results
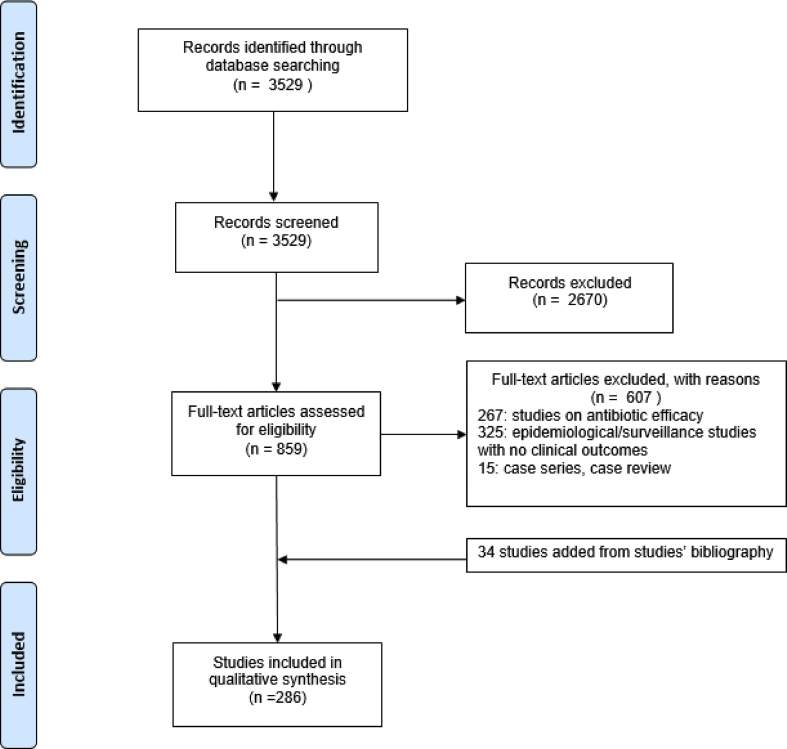
We found 3529 articles. After title screening and abstract reading, 859 full text papers (24%) were reviewed and 34 extra papers were retrieved from the studies' references. Overall, 286/859 (33%) studies were eligible for inclusion (supplementary material). The flow chart illustrates the selection procedure (Fig. 1).
Fig. 1.
PRISMA flow diagram of study identification and selection process of the 286 studies.
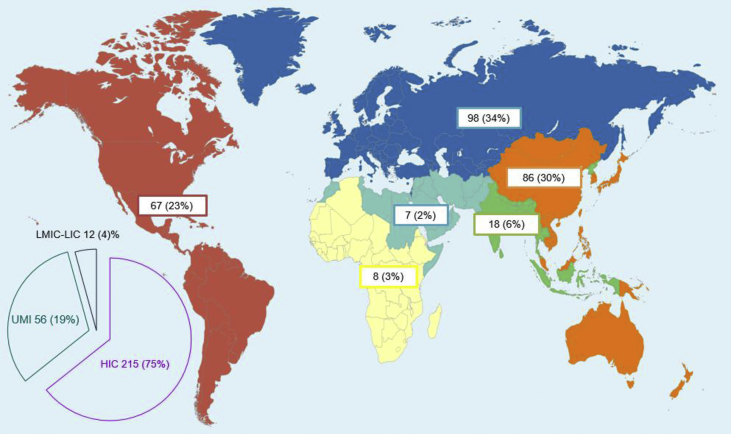
The majority of the studies were carried out in HICs (215, 75%) (Fig. 2). The antibiotic-resistant bacteria most frequently targeted were in order: ESBL and carbapenem resistant E. coli and K. pneumoniae (114/286, 40%), with the majority of the studies performed in EUR (43/114, 38%) and WPR (38/114, 33%); methicillin resistant S. aureus (MRSA; 69/286, 24%) and resistant S. pneumoniae (53/286, 18%), whose studies were mainly conducted in the AMER (24/69, 35%) and EUR (22/53, 41%), respectively; resistant A. baumannii accounted for 13% of the studies (39/286), especially from WPR and EUR (both 12/39, 31%). Salmonella spp. and Shigella spp. represented 4% of the studies (11/286), of which 36% (4/11) were performed in SEAR. No study assessed the health impact of antibiotic-resistant N. gonorrhoeae (Table 1; supplementary material).
Fig. 2.
World map distribution of the 286 studies included in the assessment of the burden of drug-resistant infections in humans due to the World Health Organization (WHO) Global Antimicrobial Resistance Surveillance System (GLASS) priority bacteria according to the WHO regional groupings and to the World Bank Classification of countries. Regions' colours are the same used by the WHO. WHO classification: African Region in yellow; Region of the Americas in red; South-East Asia Region in green; European Region in blue; Eastern Mediterranean Region in turquoise and Western Pacific Region in orange. World Bank Classification: HIC: High-income countries; LIC: Low-income countries; LMIC: Low middle-income countries; UMIC: Upper middle-income countries. Two studies conducted in different countries could not be included in the WHO regional grouping while three studies were conducted in countries with different income and were not included in the World Bank Classification grouping.
Table 1.
Main epidemiological characteristics of 286 studies assessing the burden of infections caused by the Global Antimicrobial Resistance Surveillance System target pathogens, by study design
| Cohort studies (%) N = 243 |
Case–control studies (%) N = 43 |
||
|---|---|---|---|
| Population | Paediatric, adults, elderly | 27 (11) | 2 (5) |
| Paediatric | 41 (17) | 5 (12) | |
| Adults and elderly | 175 (72) | 36 (84) | |
| Special population included (cancer, transplants, HIV, burned) | 29 (12) | 5 (12) | |
| Setting distinction | HAI vs. CAI vs. HCAI | 96 (39) | 25 (58) |
| Bacteria | Staphylococcus aureus | 62 (26) | 7 (16) |
| Streptococcus pneumoniae | 49 (20) | 4 (9) | |
| Escherichia coli and Klebsiella pneumoniae | 87 (36) | 27 (63) | |
| Acinetobacter baumannii | 34 (14) | 5 (12) | |
| Salmonella spp. and Shigella spp. | 11 (5) | NA | |
| Comparisona | Resistant vs. susceptible | 143 (59) | 31 (72) |
| Resistant vs. susceptible versus uninfected | 5 (2) | 8 (19) | |
| Resistant vs. uninfected | 4 (2) | 2 (5) | |
| Survivors vs. non-survivors | 55 (23) | 2 (5) | |
| Infectionsb | Bloodstream infections | 200 (82) | 39 (91) |
| Other infectionsc | 42 (17) | 4 (9) | |
Details about the studies can be found in the supplementary material. HAI, hospital-acquired infection; CAI, community-acquired infections; HCAI, healthcare-associated infection; NA, not available.
Among cohort studies other types of comparison were: treatment group (9; 4%); clinical characteristics (5; 2%); different resistant pathogens (4; 2%); colonised patients with the same resistant pathogen (1 study); studies without comparison (17; 7%).
One study did not define the types of infection.
Urinary tract infection, low respiratory tract infection, central nervous system infection, intra-abdominal infection, skin and soft tissue infection, bone and joint infection.
The overall study populations consisted mainly of adults and elderly people (211, 74%), while only 46 studies (16%) focused on paediatric patients; studies on high-risk patients (cancer, transplanted populations, HIV, burned) were also rarely represented 34 (12%). Setting distinction was specified in 42% of the studies (121/282); the majority focused on BSIs (239, 83%) (Table 1). Studies in HICs and UMICs looked predominantly at the health impact of infections in adults and elderly population (167, 77%, and 39, 69%, respectively), while those performed in LMICs and LICs mainly targeted paediatric patients (9, 75%) (supplementary material).
Study design and methodology
Two hundred and forty-three studies (85%) were cohort and 43 (15%) case–control studies reporting health outcomes; of which only 85/286 studies (30%) were multicentre and 60/286 prospective (21%) (supplementary material). The outcomes measured included mortality (283/286, 99%), LOS (174/286, 61%), complications (225/286, 78%) and clinical outcomes beyond discharge (43/286, 15%). Mortality was mostly measured between day 21 and day 30 from infection (123/283, 43%), followed by assessment at day 14 (32/283, 11%) (Table 2). It was most commonly defined as all-cause mortality (128/283, 45%). One third of the studies (87/283, 31%) did not specify their mortality outcome measure and only a selected number (33/283, 12%) stratified mortality by age. Sixty-one studies indicated that they determined AMR-attributable mortality (21%); LOS was analysed mainly as total days of hospitalization (121/174, 70%). Very few studies accounted for timing of event or correctly focused on LOS after the infection (46/174, 26%) (Table 2). Overall, 223 studies (78%) had a comparison, mainly resistant versus susceptible strains (174/223, 78%) and only 6% (13/223) resistant versus susceptible versus uninfected (Table 1).
Table 2.
Assessment of mortality by World Bank Classification, length of hospital stay, and follow-up duration beyond discharge in 286 studies estimating the health burden of antibiotic resistant infections caused by the Global Antimicrobial Resistance Surveillance System target pathogens
| Mortality | Overall |
Country by WBC (%)a |
|||
|---|---|---|---|---|---|
|
N (%) N = 283/286 |
HIC (%) N = 212/215 |
UMIC (%) N = 56/56 |
LMIC and LIC (%) N = 12/12 |
||
| Definitions | No definition | 87 (31) | 66 (31) | 13 (23) | 7 (58) |
| Overall | 128 (45) | 94 (44) | 31 (55) | 3 (25) | |
| Attributable | 61 (21) | 46 (22) | 13 (23) | 1 (8) | |
| In hospital | 88 (31) | 65 (31) | 19 (34) | 3 (25) | |
| Stratified by age | 33 (12) | 19 (9) | 9 (16) | 5 (42) | |
| Timeline assessment |
6–7 days | 27 (9) | 21 (10) | 5 (9) | NA |
| 14 days | 32 (11) | 22 (10) | 8 (14) | 1 (8) | |
| 21–30 days | 123 (43) | 97 (46) | 25 (45) | NA | |
| >30 days |
28 (10) |
22 (10) |
5 (9) |
NA |
|
|
In-hospital length of stay |
N = 174/286 |
N = 134/215 |
N = 35/56 |
N = 3/12 |
|
| Before infectionb | 67 (38) | 48 (36) | 16 (46) | 2 (67) | |
| After infectionb | 46 (26) | 36 (27) | 8 (23) | 2 (67) | |
| Total LOSb |
121 (70) |
94 (70) |
23 (66) |
3 (100) |
|
|
Follow-up duration after diagnosis of infection |
N = 43/286 |
N = 35/215 |
N = 5/56 |
N = 1/12 |
|
| 1–3 monthsc | 27 (63) | 20 (57) | 4 (80) | 1 (100) | |
| 6 months – 1 yearc | 9 (21) | 8 (23) | 1 (20) | NA | |
| >1 yearc | 7 (16) | 7 (20) | NA | NA | |
Details about the studies can be found in the supplementary material. The following definitions for attributable mortality were used: crude mortality rates of cases minus crude mortality rate of controls; death occurring within 14 days of the first positive blood culture without any other plausible causes; death of patients with persisting clinical evidence of active infection, excluding other causes of mortality; death within 2 weeks of the last positive blood culture in the absence of known non-infectious causes of death; death occurring while receiving antibiotics for the index infection, without any other obvious cause of death; death within 1 week of a positive culture result; mortality occurring during the admission period of the index infection; assessed by clinicians; clinical evidence of active infection and positive cultures, or when death occurred as the result of organ failure that developed or deteriorated during the onset of infection; death in patients who failed to respond to therapy and in patients who died as the result of an acute event involving any of the sites of infection or of an unknown cause; positive blood cultures at the time of death or death within 14 days of the documentation of the index infection without any other explanation; culture positive at the time of death or death within 14 days of the first day index infection without an alternate explanation as determined by the study investigators. HIC, high-income country; LIC, low-income country; LMIC, low–middle-income country; UMIC, upper middle-income countries; WBC, World Bank Classification; NA, not available.
Three studies were conducted in countries with different income so they were not included in the WBC grouping.
Percentages refer to the studies out of the total that measured length of hospital stay.
Percentages refer to the studies out of the total that performed follow-up beyond 30 days.
Severity of illness and comorbidities were measured using aggregate measures in 165 studies (58%); commonly applied scores were Charlson comorbidity index (77/165, 46%), APACHE (51/165, 31%), Pitt bacteraemia (31/165, 19%) and McCabe–Jackson (23/165, 14%). Definitions of appropriate therapy were specified in half (156/286, 54%) of the studies with a minority distinguishing between empiric (52/286, 18%) and targeted (26/286, 9%) therapy. The appropriateness was based on the in vitro susceptibility pattern of the isolate (149/156, 95%), data on timing of therapy from the infection diagnosis (36/156, 23%) were rarely included (supplementary material).
Although adjusted results were reported in the majority of the studies (185/286, 65%), only one third (88/286, 31%) specified the variables used. Sample size estimation and/or justification was reported in a third of the studies (113/286, 39%). The type of adjustment analysis was less frequently specified in studies from non-HICs (9/68, 13% vs. 78/215, 36% in HICs) (supplementary material). Analysis were performed using mostly regression models (232/286, 81%), survival analysis (98/286, 34%) of which 11 (11%) were multistate models (Table 3). Since 1990, there has been an overall substantial improvement in the number of studies adjusting for confounding using matching or multivariate regression methods. Among the 23 studies from the 1990s (18, 78% from HICs), six (26%) used regression models and one (4%) survival analysis; among the 263 studies performed after 2000, regression analysis models were applied by the majority (169, 64%) and survival analysis in 58 studies (22%). The 11 studies adopting multistate models were performed after 2010 (mainly from HICs; nine studies, 81%).
Table 3.
Methodologic assessment in 243 cohort and 43 case-control studies
| Indicators | Cohort (%) N = 243 |
Case-control (%) N = 43 |
|---|---|---|
| Sample size assumption/justification | 89 (37) | 24 (56) |
| Ascertainment of exposure | 243 (100) | NA |
| History of previous infection with resistant bacteria | 34 (14) | 5 (12) |
| Hospital controls | NA | 29 (67) |
| Community controls | NA | 3 (7) |
| No description of controls | NA | 11 (25) |
| Descriptive statistics | 243 (100) | 43 (100) |
| Matching technique | 14 (6) | 25 (58) |
| Analysis accounting for matching | 6/14 (43) | 10/25 (40) |
| Multivariable regression model | 199 (82) | 33 (77) |
| Variables adjustments specified | 67/199 (34) | 21/33 (64) |
| Use of clinical scores to control for confoundinga | 141 (58) | 24 (56) |
| Survival analysis | 82 (34) | 16 (37) |
| Multistate models | 9/82 (11) | 2/16 (12) |
| Univariate results | 175 (72) | 36 (84) |
| Adjusted results | 159 (65) | 26 (60) |
| Reporting of significant results only (p < 0.05) | 38 (16) | 5 (12) |
| Follow-up after discharge | 37 (15) | 6 (14) |
Details about the studies can be found in the supplementary material. NA, not applicable.
Clinical scores: Charlson Comorbidity Index, Glasgow Coma Scale (GCS), McCabe–Jackson, American Society Anesthesiology (ASA) Severity Score, Acute Physiology and Chronic Health Evaluation (APACHE), Simplified Acute Physiology Score (SAPS), Sequential Organ Failure Assessment (SOFA), Pediatric Risk Mortality (PRISM), Pneumonia Severity Index (PSI).
DALY variables' assessment
Regarding information required to calculate AMR-related DALYs, gender-related mortality was reported in all studies; age-related mortality was rarely provided for more composite age stratifications (33/283, 12%). Complications were available in 225/286 studies (78%), mainly limited to the assessment of hemodynamic instability and respiratory failure during hospitalization (supplementary material). A few studies reported duration of clinical symptoms until remission (28, 10%). None of the studies assessed specific health-states or health state duration, which are critical for DALY calculations. Only one study evaluated the productivity loss due to premature death (supplementary material). As an example, Table 4 describes the availability of the DALY variables in the subgroup of 39 studies analysing the burden caused by carbapenem-resistant A. baumannii (tables for the other pathogens in supplementary material).
Table 4.
Assessment of variables needed for the calculation of the disability adjusted life years (DALYs) in 39 studies analysing the health burden of infections caused by carbapenem-resistant Acinetobacter baumannii
| Available variable | Data missing | |
|---|---|---|
| Mortality (%) | ||
| N = 39 | ||
| Overall | 21 (54) | Long-term attributable mortality Gender-stratified mortality |
| Attributable | 10 (26) | |
| In hospital | 12 (31) | |
| No definition | 8 (20) | |
| 21–30-day mortality | 21 (54) | |
| Mortality stratified by age | 3 (8) | |
| Health states and their complications (%) | ||
| N = 39 | ||
| Health state | NA | Definition of most relevant non-fatal health outcomes |
| Duration of health states | ||
|
Acute complications Available in 35 out of 39 studies |
Prevalence and/or incidence of health states and associated mortality and complications Duration of health states |
|
| Need for intensive care unit | 9 (26) | |
| Hemodynamic instability | 10 (28) | |
| Renal impairment | 8 (23) | |
| Neurological impairment | 1 (3) | |
|
Health state duration (%) Available in 30 out of 39 studies | ||
| Clinical symptoms duration | NA | Agreement on duration of clinical symptoms Definition of appropriate follow-up An alternative to length of stay as indicator for disease duration could be the length of antibiotic therapy |
| Length of stay before infection | 19 (63) | |
| Length of stay after infection | 11 (37) | |
| Total length of stay | 12 (40) | |
| 1–3 months follow-up | 1 (3) | |
NA, not available.
Quality of studies
The Newcastle–Ottawa scale judges a study on the selection of study groups, the comparability of the groups and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies respectively. The median score (IQR) of all the studies, comprising the three domains, was 6 (7–5) out of a maximum of 9 points. In the selection domain the median (IQR) was 3 (3–3); in the comparability domain the median (IQR) was 1 (2–1); in the outcome domain the median (IQR) was 2 (2–2).
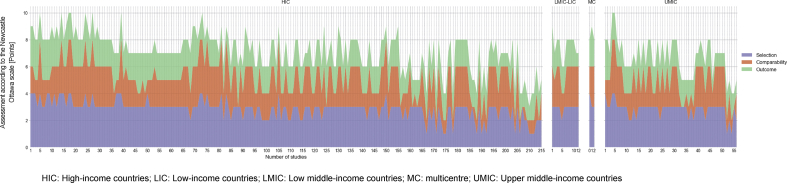
For case–control studies, overall median score (IQR) was 6 (6–5); 84% (36) had a low level score in the selection domain (median 2; IQR 2–1), 72% (31) a good score for the comparability domain (median 2; IQR 2–1) and 97% (42) a medium score for outcome ascertainment (median 2; IQR 2–2). Among the cohort studies, overall median score (IQR) was 6 (9–5); 190 (66%) had a medium quality score within selection domain (median 3; IQR 4–3), 132 (54%) had a medium score in the comparability domain (median 1; IQR 2–1) and 69% (167) a medium score in the outcome domain (median 2; IQR 3–2) (Fig. 3, Fig. 4).
Fig. 3.
Quality assessment according to the Newcastle-Ottawa scale of 286 studies evaluating the burden of drug-resistant infections in humans due to the WHO Global Antimicrobial Resistance Surveillance System (GLASS) priority resistant bacteria.
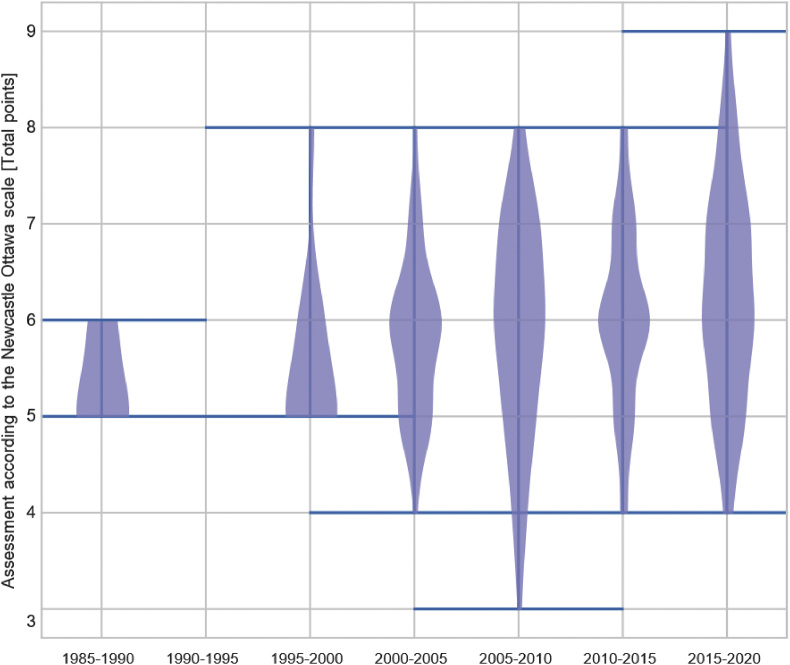
Fig. 4.
Trends over the time of the quality assessment score according to the Newcastle-Ottawa scale of 286 studies evaluating the burden of drug-resistant infections in humans due to the WHO Global Antimicrobial Resistance Surveillance System (GLASS) priority resistant bacteria.
Discussion
Our systematic review shows that the assessment of the health impact of AMR mainly relies on data from single centre, retrospective cohort studies performed in HICs with a medium study quality. Studies mostly analyse all-cause mortality, with large heterogeneity and timing of assessment, and target HAI (particularly BSIs) with a very few distinguish between CAIs and HCAIs. LOS is mainly evaluated as total days of hospitalization with only a few studies correctly indicating LOS before and after infection. Infection's complications are seldom recorded, and mostly include those occurring during hospitalization. Follow-up beyond discharge for assessment of possible chronic sequelae is rarely performed and clinical outcomes (except mortality) are not stratified by age or gender rendering calculation of DALYs impossible.
Although we observed an improvement in the methodology used over the years, mainly pertained to increased awareness of the role of confounders and the consequent adoption of more appropriate techniques, still a remarkable proportion of studies does not clearly report how they adjusted for time dependent bias, comorbidities or severity of illness [35]. Very few studies correctly acknowledged timing of infection when calculating the effect of AMR on LOS, which is used to evaluate infections economic burden on the healthcare system [12]. A previous systematic review has shown that only a third of studies published since 2006 used time-varying methods when analysing differential hospital stay between patients with and without HAI [36]. This becomes very relevant when assessing the attributable LOS due to AMR infections as ignoring the time at which infection occurs would lead to inaccurate hospitalization estimates [16], with substantial implications on cost analysis.
The adjustment for confounding in studies assessing the burden of infections caused by the GLASS pathogens is fragmentary as well. Studies do not consistently define how comorbidities and severity of illness are classified, or when they were measured. The majority applies aggregate measures such as the Charlson comorbidity index, which are not originally designed and validated as predictors in the AMR context [13].
Currently available evidence does not provide all clinical data (age and gender-specific attributable mortality proportions, frequency of attributable health states and their duration) needed to calculate DALYs for antibiotic resistant infections. Despite all studies reporting mortality, the lack of its uniform assessment in terms of definition and timeline, preclude their aggregation and reduce their applicability for burden models. Apart from clinical outcome data, DALYs also require population-based incidence and/or prevalence data. While hospital-based AMR surveillance data are generally available in HICs, little to no information is available from LMICs. Moreover, recent reviews on AMR surveillance underlined how surveillance systems are still very heterogeneous among countries [6,37].
Our review has limitations. Search terms failed to include studies describing data of both resistant and non-resistant pathogens, meaning we excluded information from countries, such as those with low AMR prevalence, where these type of studies are more frequent. We also retrieved only studies written in English. However, study quality was already relatively low, despite focusing on high-quality journals publishing in English, so we do not think extension of our selection would have resulted in different conclusions. We targeted the GLASS pathogens and consequently we excluded studies on other resistant pathogens, which might have provided more accurate estimates. However, the chosen resistant bacteria are also included in the WHO priority list of antibiotic resistant bacteria, and were selected based on their frequency in causing infections in the hospital and community, their carriage in food-producing animals and in the food chain, justifying the need to include them in AMR burden estimations [29].
The way forward
A major challenge of the DALYs concept is the need of epidemiological data linked to detailed clinical information [25]. This is currently difficult to obtain for AMR because most of the data are still generated at laboratory level, and they reflect the bacterial population without any epidemiological insight of the patient's population. Such information is even more dispersed in less resourced countries lacking of hospital information systems, national registries or web-based surveillance systems; the disability weights for different health-states, corresponding to the possible outcomes, can partially be borrowed from infections caused by susceptible pathogens but need to be re-defined depending on the type of resistant bacteria and the most common complications deriving from that infection.
Notwithstanding the current limitations, the DALY approach would generate comprehensive estimates of the AMR health impact, which could be used for policy purposes and resource mobilisation.
First, the representativeness of prevalence and incidence data needs to be enhanced, and data collection needs to be harmonized to enable benchmarking between countries. Many stakeholders are taking strong actions in order to ameliorate the quality of AMR data generated at local, national and global level by providing guidance, protocols and laboratory tools [[38], [39], [40], [41]]. In 2015, the WHO, in alignment with their AMR Global Action Plan, launched the GLASS system which promotes a comprehensive, regular collection of demographic, clinical and microbiological data through a standardized approach [42,43]. GLASS is currently finalizing a protocol to estimate testing coverage at hospital level to minimize sampling bias and developing a protocol to improve representativeness of AMR surveillance data at the national level based on testing coverage capacity.
Second, definitions of the most relevant health states for infections caused by resistant bacteria are also needed to inform AMR DALY metrics. This implies an agreement on definitions and method of assessment of LOS, mortality (crude and attributable), complications and duration of follow-up to assess short and long-term complications.
Third, new studies need to be designed to estimate the public health impact of AMR and avoid the key limitations identified in the current literature. The feasibility to generate estimates of AMR burden in LMICs has been recently demonstrated by a prospective cohort study on the clinical outcomes of BSIs caused by carbapenem-resistant Enterobacteriaceae [44]. In the meantime, WHO-GLASS has developed a master protocol to foster harmonized approach for estimating attributable mortality due to AMR across countries [45]. In this initial step, the protocol focuses on hospital-onset ESBL E. coli and MRSA BSIs, the two resistant pathogens contributing to the 41% of the total DALYs in Europe [2], but it allows for local adaptation and inclusion of other syndromes and pathogens as per local priorities. The primary outcome is in-hospital mortality, an easily available and objective endpoint, which does not require follow-up beyond hospital discharge. Thirty-day mortality (measured as 30 days after infection, with follow-up beyond hospital discharge) has been added as an optional outcome measure. To assess the impact of drug resistance, defined as the excess mortality among patients, the protocol proposes comparison between patients with a drug-resistant infection and two comparator groups: patients with the same drug-susceptible infection, and patients without such an infection. By considering both scenarios, the upper and lower limit of the impact of AMR can be determined. In order to avoid time-dependent bias, the use of incidence density matching to select the comparator groups, and survival methods considering time-varying variables and competing events are suggested for the statistical analysis. The impact of AMR on long-term sequelae and their duration have not yet been included in this first step, but shall be considered in the next phase. Assessment of the burden of AMR in the community setting, or in healthcare centres lacking diagnostic stewardship are remaining challenges that will need to be addressed in different ways.
A call for action
Current estimations of AMR health burden are based on substandard evidence and may have contributed either to magnify the direct clinical burden caused by partially correct analysis of clinical data and related chosen outcomes or to underestimate the indirect public health burden, mainly derived from the lack of knowledge of the AMR impact at social level in terms of both productivity loss and mid- to long-term clinical consequences. Key actions to promote accurate and detailed burden estimates to ensure efficient use of limited resources should include implementation of harmonized AMR surveillance schemes, preferably in the healthcare as well as community settings; implementation of high-quality, prospective studies to generate empirical data about the impact of AMR and improvement of modelling approaches for the estimation of AMR burden. This could guide the uptake of effective interventions within the AMR strategic research agendas, giving us a chance to halt the ever rising prevalence of drug-resistant pathogens (Table 5).
Table 5.
PICO (Population, Intervention, Comparison, Outcome) considerations for future studies aiming to estimate the burden of antimicrobial resistance
| Population | No age restrictions should be applied and a detailed age stratification (neonates, children, adolescents, adults and elderly) and gender stratification is warranted. A precise distinction of the setting (hospital, outpatient, community, long-term care facility) is warranted. |
| Intervention | Prospective cohort studies focusing on infections caused by one of the priority resistant bacteria.a A starting point could be the burden analysis of bloodstream infections within the hospital setting because these are serious indicator infections with a straightforward definition, for which the WHO protocol could serve as a guide [45]. |
| Comparison | Two types of comparisons for patients with a drug-resistant infection:
|
| Outcomes of interest | Major endpoint should be attributable mortality, defined as the excess mortality among patients with drug-resistant infection when compared to patients with a drug-susceptible infection, or without such an infection, adjusted for the influence of confounding factors. Primary outcomes:
|
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18:318–27.
Transparency declaration
For all experts, advice was provided in their personal capacity. The views in this report do not necessarily reflect, and should not be interpreted as, the official position of any agency or institution. SH reports consulting fees by Sandoz. Role of the funding source: The WHO supported the systematic reviews and data analysis and WHO employees (C.P., B.T.) contributed to the study design and writing of the report. The corresponding author had access to all data and had final responsibility for the decision to submit for publication.
Acknowledgements
This work receives support from the WHO AMR Surveillance Unit (WCCPRD5575378 2017/719969). The development of the core database has been funded by the DRIVE-AB project (grant agreement number 115618). We thank Anna Gòrska for the graphical representation of the data.
Editor: M. Leeflang
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.01.004.
Author contributions
B.T. and C.P. developed the research goals and aims of the study. E.T. and M.D.P. wrote the first draft of the study protocol. B.T., C.P. and S.H. reviewed the study protocol. M.D.P., N.D.S. and S.R. extracted and managed the data. E.T., B.T., C.P., S.H., M.D.K. and M.D.P. reviewed and discussed the survey results. E.T. and M.D.P. wrote the first draft of the Article. E.T., B.T., C.P., S.H., M.D.K. and M.D.P. provided feedback, commented on, and reviewed the manuscript.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.United Kingdom Department of Health WT . 2016. Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance.https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf Available from: [Google Scholar]
- 2.Cassini A., Hogberg L.D., Plachouras D., Quattrocchi A., Hoxha A., Simonsen G.S. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . 2019. Biggest threats and data.https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf Available from: [Google Scholar]
- 4.European Centre for Disease Prevention and Control . 2020. Annual surveillance reports on antimicrobial resistance.https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/report Available from: [Google Scholar]
- 5.Laxminarayan R., Duse A., Wattal C., Zaidi A.K., Wertheim H.F., Sumpradit N. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 6.Tacconelli E., Sifakis F., Harbarth S., Schrijver R., van Mourik M., Voss A. Surveillance for control of antimicrobial resistance. Lancet Infect Dis. 2018;18:e99–e106. doi: 10.1016/S1473-3099(17)30485-1. [DOI] [PubMed] [Google Scholar]
- 7.Abat C., Rolain J.M., Dubourg G., Fournier P.E., Chaudet H., Raoult D. Evaluating the clinical burden and mortality attributable to antibiotic resistance: the disparity of empirical data and simple model estimations. Clin Infect Dis. 2017;65:S58–S63. doi: 10.1093/cid/cix346. [DOI] [PubMed] [Google Scholar]
- 8.Blot S., Depuydt P., Vandewoude K., De Bacquer D. Measuring the impact of multidrug resistance in nosocomial infection. Curr Opin Infect Dis. 2007;20:391–396. doi: 10.1097/QCO.0b013e32818be6f7. [DOI] [PubMed] [Google Scholar]
- 9.De Angelis G., Murthy A., Beyersmann J., Harbarth S. Estimating the impact of healthcare-associated infections on length of stay and costs. Clin Microbiol Infect. 2010;16:1729–1735. doi: 10.1111/j.1469-0691.2010.03332.x. [DOI] [PubMed] [Google Scholar]
- 10.de Kraker M.E., Stewardson A.J., Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans S.R., Harris A.D. Methods and issues in studies of CRE. Virulence. 2017;8:453–459. doi: 10.1080/21505594.2016.1213473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limmathurotsakul D., Dunachie S., Fukuda K., Feasey N.A., Okeke I.N., Holmes A.H. Improving the estimation of the global burden of antimicrobial resistant infections. Lancet Infect Dis. 2019;19:e392–e398. doi: 10.1016/S1473-3099(19)30276-2. [DOI] [PubMed] [Google Scholar]
- 13.McGregor J.C., Kim P.W., Perencevich E.N., Bradham D.D., Furuno J.P., Kaye K.S. Utility of the Chronic Disease Score and Charlson Comorbidity Index as comorbidity measures for use in epidemiologic studies of antibiotic-resistant organisms. Am J Epidemiol. 2005;161:483–493. doi: 10.1093/aje/kwi068. [DOI] [PubMed] [Google Scholar]
- 14.Munoz-Price L.S., Frencken J.F., Tarima S., Bonten M. Handling time-dependent variables: antibiotics and antibiotic resistance. Clin Infect Dis. 2016;62:1558–1563. doi: 10.1093/cid/ciw191. [DOI] [PubMed] [Google Scholar]
- 15.Naylor N.R., Atun R., Zhu N., Kulasabanathan K., Silva S., Chatterjee A. Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob Resist Infect Control. 2018;7:58. doi: 10.1186/s13756-018-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson R.E., Nelson S.D., Khader K., Perencevich E.L., Schweizer M.L., Rubin M.A. The magnitude of time-dependent bias in the estimation of excess length of stay attributable to healthcare-associated infections. Infect Control Hosp Epidemiol. 2015;36:1089–1094. doi: 10.1017/ice.2015.129. [DOI] [PubMed] [Google Scholar]
- 17.Rottier W.C., Ammerlaan H.S., Bonten M.J. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum β-lactamase-producing Enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother. 2012;67:1311–1320. doi: 10.1093/jac/dks065. [DOI] [PubMed] [Google Scholar]
- 18.Stewardson A.J., Allignol A., Beyersmann J., Graves N., Schumacher M., Meyer R. The health and economic burden of bloodstream infections caused by antimicrobial-susceptible and non-susceptible Enterobacteriaceae and Staphylococcus aureus in European hospitals, 2010 and 2011: a multicentre retrospective cohort study. Euro Surveill. 2016;21:30319. doi: 10.2807/1560-7917.ES.2016.21.33.30319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wernli D., Jørgensen P.S., Harbarth S., Carroll S.P., Laxminarayan R., Levrat N. Antimicrobial resistance: the complex challenge of measurement to inform policy and the public. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schechner V., Temkin E., Harbarth S., Carmeli Y., Schwaber M.J. Epidemiological interpretation of studies examining the effect of antibiotic usage on resistance. Clin Microbiol Rev. 2013;26:289–307. doi: 10.1128/CMR.00001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosgrove S.E., Carmeli Y. The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis. 2003;36:1433–1437. doi: 10.1086/375081. [DOI] [PubMed] [Google Scholar]
- 22.Vardakas K.Z., Rafailidis P.I., Konstantelias A.A., Falagas M.E. Predictors of mortality in patients with infections due to multi-drug resistant Gram negative bacteria: the study, the patient, the bug or the drug? J Infect. 2013;66:401–414. doi: 10.1016/j.jinf.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Gandra S., Barter D.M., Laxminarayan R. Economic burden of antibiotic resistance: how much do we really know? Clin Microbiol Infect. 2014;20:973–980. doi: 10.1111/1469-0691.12798. [DOI] [PubMed] [Google Scholar]
- 24.Tacconelli E., Pezzani M.D. Public health burden of antimicrobial resistance in Europe. Lancet Infect Dis. 2019;19:4–6. doi: 10.1016/S1473-3099(18)30648-0. [DOI] [PubMed] [Google Scholar]
- 25.Murray C.J., Lopez A.D. Global mortality, disability, and the contribution of risk factors: global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 26.Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 30.Bettiol E., Hackett J., Harbarth S. Stimulating research and development of new antibiotics while ensuring sustainable use and access: further insights from the DRIVE-AB project and others. J Law Med Ethics. 2018;46:5–8. doi: 10.1177/1073110518782910. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization Definition of regional groupings 2020. https://www.who.int/healthinfo/global_burden_disease/definition_regions/en/ Available from:
- 32.World Bank . 2017–2018. World Bank country and lending groups.https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups Available from: [Google Scholar]
- 33.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 34.Wells G.A., Shea B., O'Connell D., Peterson J., Welch V., Losos M. 2014. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available from: [Google Scholar]
- 35.Thom K.A., Shardell M.D., Osih R.B., Schweizer M.L., Furuno J.P., Perencevich E.N. Controlling for severity of illness in outcome studies involving infectious diseases: impact of measurement at different time points. Infect Control Hosp Epidemiol. 2008;29:1048–1053. doi: 10.1086/591453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manoukian S., Stewart S., Dancer S., Graves N., Mason H., McFarland A. Estimating excess length of stay due to healthcare-associated infections: a systematic review and meta-analysis of statistical methodology. J Hosp Infect. 2018;100:222–235. doi: 10.1016/j.jhin.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organisation . 2014. Antimicrobial resistance: global report on surveillance.https://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf;jsessionid=E08D93283CAC1D741A07FFECFCC30341?sequence=1 Available at: [Google Scholar]
- 38.Oxford Uo . 2020. Big data institute.https://www.bdi.ox.ac.uk/ Available from: [Google Scholar]
- 39.Evaluation IfHMa . 2020. Global research on AntiMicrobial resistance (GRAM) project.http://www.healthdata.org/gram Available from: Global Research on AntiMicrobial resistance (GRAM) Project. [Google Scholar]
- 40.United Kingdom Aid Programme DoHaSC . 2018. Fleming fund.http://www.flemingfund.org/about-us/ Available from: [Google Scholar]
- 41.The Center For Disease Dynamics EaP Antibiotic Resistance. Coordinating a global response to counter the emergence of antibiotic-resistant bacteria. https://cddep.org/research-area/antibiotic-resistance/ Available from:
- 42.World Health Organization . 2015. Global antimicrobial resistance surveillance system manual for early implementation. Available from: https://apps.who.int/iris/bitstream/handle/10665/188783/9789241549400_eng.pdf?sequence=1. [Google Scholar]
- 43.Tornimbene B., Eremin S., Escher M., Griskeviciene J., Manglani S., Pessoa-Silva C.L. WHO global antimicrobial resistance surveillance system early implementation 2016–17. Lancet Infect Dis. 2018;18:241–242. doi: 10.1016/S1473-3099(18)30060-4. [DOI] [PubMed] [Google Scholar]
- 44.Stewardson A.J., Marimuthu K., Sengupta S., Allignol A., El-Bouseary M., Carvalho M.J. Effect of carbapenem resistance on outcomes of bloodstream infection caused by Enterobacteriaceae in low-income and middle-income countries (PANORAMA): a multinational prospective cohort study. Lancet Infect Dis. 2019;19:601–610. doi: 10.1016/S1473-3099(18)30792-8. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organisation GLASS method for estimating attributable mortality of antimicrobial resistant bloodstream infections. https://www.who.int/publications/i/item/9789240000650 Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.