Abstract
Cancer cells display abnormal metabolic activity as a result of activated oncogenes and loss of tumor suppressor genes. The Warburg Effect is a common metabolic feature of cancer that involves a preference for aerobic glycolysis over oxidative phosphorylation to generate ATP and building blocks for biosynthesis. However, emerging evidence indicates that mitochondrial metabolic pathways are also reprogrammed in cancer and play vital roles in bioenergetics, biosynthesis, and managing redox homeostasis. The mitochondria act a central hub for metabolic pathways that generate ATP and building blocks for lipid, nucleic acid and protein biosynthesis. However, mitochondrial respiration is also a leading source of reactive oxygen species that can damage cellular organelles and trigger cell death if levels become too high. In general, cancer cells are reported to have higher levels of reactive oxygen species than their non-cancerous cells of origin, and therefore must employ diverse metabolic strategies to prevent oxidative stress. However, mounting evidence indicates that the metabolic profiles between proliferative and disseminated cancer cells are not the same. In this review, we will examine mitochondrial metabolic pathways, such as glutaminolysis, that proliferative and disseminated cancer cells utilize to control their redox status.
Keywords: Glutaminolysis, Mitochondria metabolism, Redox homeostasis, Cancer progression
1. Introduction
Cancer cells have long been known to exhibit abnormal metabolic activity in comparison to their tissue of origin. One of the most well-known abnormal metabolic characteristics of cancer cells is the Warburg effect: a state of highly elevated glucose uptake and preference of glycolysis, rather than mitochondrial oxidative phosphorylation (OXPHOS) for ATP production, even in the presence of sufficient oxygen [1]. Although the effects of glucose addiction and glycolytic dependency are still being actively researched, the Warburg effect has been demonstrated to benefit proliferative cancer cells through rapid ATP generation and simultaneous flux through the pentose phosphate pathway (PPP) to support redox homeostasis and biosynthesis [2]. While the Warburg effect is commonly seen in proliferative cancer cells, evidence now suggests that the metabolic phenotypes between proliferative primary cancer cells and disseminated cancer cells are not the same [3].
In most epithelial and endothelial cells, detachment from the extracellular matrix (ECM) perturbs multiple processes important for cellular viability and initiates apoptotic cell death called anoikis [75]. In recent years, several studies have demonstrated a strong connection between alterations in cellular metabolism and anoikis induction following matrix detachment [[4], [5], [6]]. For instance, it is well-established that the mitochondria are a focal point of apoptotic signaling, since both the extrinsic and intrinsic pathways of apoptosis converge on the mitochondria to induce mitochondrial outer membrane permeabilization (MOMP) and liberate cytochrome c: a critical point in the execution of apoptosis [7]. A direct example of the link between cell metabolism and apoptosis lies in the dual roles of cytochrome c. This small hemeprotein, which is associated with the inner mitochondrial membrane (IMM), plays an important role in bioenergetics and cellular ATP production because it mediates the transfer of an electron from respiratory complex III to complex IV in the mitochondrial electron transport chain (ETC). Cytochrome c also plays an essential role in the execution of apoptosis by activating caspases [8]. While the Bcl-2 family proteins are well-established regulators of cytochrome c in apoptosis by mediating its mitochondrial release into the cytosol, it is also important to mention that the apoptotic activity of cytochrome c is known to be directly inhibited by reduced intracellular glutathione (GSH), an important antioxidant that acts on cytochrome c to keep it in its reduced state [9]. In addition to classical caspase-induced cell death, ECM-disengagement greatly disrupts cellular metabolic activity, including but not limited to diminished glucose uptake, reduced pentose phosphate pathway (PPP) flux and elevated production of reactive oxygen species (ROS) to potentiate oxidative stress [63]. These metabolic disturbances impair the cell's ability to maintain sufficient bioenergetics and redox homeostasis, thereby contributing to anoikis susceptibility. Therefore, mitigating oxidative stress is likely crucial for anoikis resistance following cancer cell ECM detachment. In fact, some evidence now suggests detached cancer cells upregulate metabolic pathways that support ROS detoxification and efficient bioenergetics and ATP production in conditions of low nutrients [10,11]. Several well-characterized oncogenic mutations are known to affect downstream signaling pathways that alter metabolic activity in cancer. Therefore, it is highly plausible that oncogene-mediated metabolic reprogramming promotes anoikis resistance and survival in the circulatory system during cancer metastasis.
2. Reactive oxygen species are a double-edged sword in cancer
Reactive oxygen species (ROS) are naturally produced as metabolic byproducts from aerobic mitochondrial metabolism under normal physiological conditions [12]. For instance, the ETC complexes generate superoxide anion when single electrons react with oxygen. In fact, it is estimated that up to 5% of oxygen used for mitochondrial respiration is converted to superoxide anions [13]. It is important to note that ROS have dual functions within cells. For instance, a moderate increase in ROS can activate signaling pathways important for cell proliferation, differentiation, and survival. Whereas, excessive amounts of ROS can cause oxidative damage to cellular proteins, lipids and nucleic acids to a point that triggers cell death. In addition, ROS are involved in the execution of programmed cell death through the peroxidation of cardiolipin, which liberates cytochrome c from the inner mitochondrial membrane [14]. As such, ROS play vital roles in the induction of apoptosis and anoikis. Cancer cells have long been known to exhibit higher levels of ROS than their non-cancerous counterparts, and ROS production is further increased in cells that have undergone matrix detachment, and supplementation of detached cells with antioxidants has been shown to suppress anoikis in certain cell types [15,16]. Therefore, cancer cells must adapt multiple metabolic strategies to balance efficient energy production and redox status to avoid increases in ROS that lead to oxidative stress and cell death.
Although it may seem counterintuitive, extensive evidence has indicated that chronic elevations in intracellular ROS levels below a certain threshold can actually promote cancer cell survival, proliferation, anoikis resistance and metastatic spread. For example, sublethal administration of hydrogen peroxide significantly upregulates Cav-1 which leads to anoikis resistance and anchorage-independent growth through increased Akt signaling in melanoma and lung carcinoma cells [17,18]. Of note, many cancers have chronically elevated intracellular ROS levels due to constitutive activation of ROS-producing enzymes such as 5-lipoxygenase and NADPH oxidase (NOX) [19]. For instance, metastatic prostate cancer cells often exhibit increased intracellular ROS via constitutive activation of 5-lipoxygenase, which promotes Src-mediated ligand-independent firing of pro-survival EGFR signaling [20]. Activation of Akt and EGFR signaling through ROS-induced Cav-1 and Src activity following matrix detachment may play a direct role in anoikis resistance through negative regulation of pro-apoptotic Bcl-2 family proteins, such as degradation of Bim [20]. Indeed, treatment of aggressive prostate cancer cells with antioxidants erases anoikis resistance from Src-induced ligand-independent EGFR activation by restoring pro-apoptotic signaling [20]. In agreement, additional evidence suggests that elevated intracellular ROS can promote the activation of Src kinase and downstream ERK and PI3K/Akt signaling pathway to promote cell survival by inhibiting pro-apoptotic Bim and Bad [[21], [22], [23]].
Additional research has shown that cancer cells may utilize chronic ROS to inhibit anoikis through other mechanisms as well. For instance, expression of angiopoietin-like 4 protein has been shown to promote anoikis resistance in cancer by interacting with β1 and β5 integrins and stimulating NOX-mediated O2− production to mimic anchorage conditions [24]. In addition, chronic moderate elevations of intracellular ROS may promote the expression of anti-apoptotic proteins such as Bcl-xL, c-FLIP and XIAP through activation of the transcription factor NF-κB [25]. In summary, slightly elevated ROS levels can promote pro-growth and anti-apoptotic signaling, but cancer cells must employ antioxidant defenses and modulate metabolic pathways to keep ROS levels from rising too high and causing oxidative stress.
3. Glutaminolysis and glutamate-mediated redox regulation in cancer
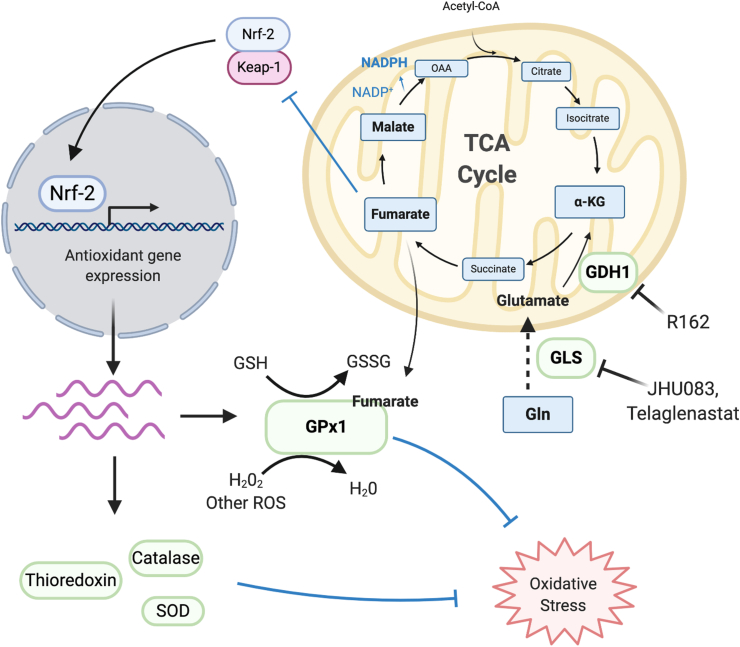
Glutamine is the most abundant amino acid in blood plasma and serves as the major source of reduced nitrogen for cells. In addition to an increased demand for glucose, cancer cells also demonstrate abnormally high rates of glutamine consumption and glutaminolysis [26]. Glutaminolysis, a mitochondrial metabolic pathway characterized by initial deamination of glutamine by glutaminase (GLS), produces ammonia and glutamate. Glutamate is then further metabolized by glutamate dehydrogenase (GLUD1, GDH1) to its product, alpha ketoglutarate (⍺-KG): an important TCA intermediate used to fuel ATP production and anabolic biosynthesis of amino acids, lipids and nucleotides [27,28]. In addition, glutaminolysis is also involved in the production of antioxidant molecules to protect cells against oxidative stress [27,29,30]. For instance, reduced glutathione (GSH) is a tripeptide containing glutamate, glycine and cysteine, and is therefore heavily dependent on glutaminolysis for glutamate production and import of cysteine's precursor, cystine, via the XC− antiporter (xCT/SLC7A11) [31]. In the context of cancer, xCT is known to be upregulated through the RAS-RAF-MEK-ERK signaling pathway and is essential for oncogenic Kras-mediated cellular transformation by mitigating oxidative stress through the GSH antioxidant defense system [32]. Furthermore, xCT expression and environmental cystine are both necessary for increased glutamine TCA anaplerosis and glutaminase dependence often observed in cancer cells [33], and depletion of xCT is reported to subject pancreatic cancer cells to ferroptosis: a cell death outcome resulting from the accumulation of lipid ROS [34]. Glutamine anaplerosis contributes to the regeneration of other antioxidant molecules as well. For instance, the conversion of glutamine to oxaloacetate (OAA) by malate dehydrogenase in the TCA cycle reduces NADP + back to NADPH, which can then act either as a directly operating antioxidant on mitochondrial electron transport chain (ETC)-derived O2− and other ROS, or as an indirectly operating antioxidant through the re-reduction of glutathione disulfide (GSSG) to GSH via glutathione reductase (Fig. 1) [35,36].
Fig. 1.
Glutaminolysis promotes ROS detoxification and redox homeostasis in cancer through TCA cycle intermediates. Upregulated GDH1 and other enzymes involved in glutaminolysis maintain increased levels of ⍺-KG and downstream TCA metabolites such as fumarate and malate. Fumarate modulates cysteine residues on Keap-1 to promote antioxidant gene expression through the Nrf-2 transcription factor, and also enhances the antioxidant activity of GPx1. Conversion of malate to oxaloacetate via malate dehydrogenase regenerates NADP + to NADPH, which can directly detoxify mitochondrial ROS or maintain intracellular pools of reduced glutathione.
Many cancers harbor oncogenic alterations that drive both increased glutamine uptake and metabolism. For instance, c-myc is a transcription factor commonly amplified in cancer that is known to drive glutamine import by upregulating expression of glutamine transporters such as system N transporter 2 (SN2) and alanine-serine-cysteine transporter 2 (ASCT2) [37]. Of note, ASCT2 plays a critical role in leukemia initiation and maintenance driven by activation of the oncogene MLL-AF9 or deletion of Pten [38]. In addition, c-myc activity also induces expression of several enzymes that participate in glutaminolysis, including glutaminase [39] and carbamoyl-phosphate synthetase 2 [40]. The deletion or inactivation of tumor suppressor genes in cancer can also promote glutaminolysis. For instance, deletion of the Rb gene leads to unchecked E2F transcription factor activity, which promotes both the uptake and usage of glutamine through increased mRNA expression of ASCT2 and GLS1 [41]. In recent years, research has demonstrated that proliferative tumor cells utilize metabolites produced through glutaminolysis as cofactors to modulate the activity of ROS-scavenging enzymes and mitigate oxidative stress. For example, GDH1 is upregulated in many types of cancer and is important for redox homeostasis. Specifically, the metabolic product of GDH1, ⍺-KG, is further metabolized into fumarate in the TCA cycle. Fumarate then binds to and enhances the activity of glutathione peroxidase 1 (GPx1), an enzyme that uses reduced glutathione to detoxify cellular ROS [42]. Moreover, pharmacological and genetic inhibition of GDH1 results in decreased proliferation of lung cancer, breast cancer and leukemia, but not of non-cancerous human cells [42]. This suggests that glutaminolysis may be more important for redox regulation specifically in cancer cells.
Although the majority of research surrounding glutaminolysis in cancer has focused on its role in enhancing proliferation of cancer cells and tumor growth, emerging evidence suggests that glutaminolysis plays a pivotal role in promoting the metastatic progression of cancer. Farris et al. first identified that GLUD1 and its product ⍺-KG are increased during the epithelial to mesenchymal transition (EMT), which is a key driver of metastasis, and are critical for suppressing hydrogen peroxide generation and protecting epithelial cells from ROS-induced anoikis [43]. The transcription factor grainyhead-like 2 (GRHL2) is reported to downregulate GLUD1 expression and sensitize cells to anoikis [43], while the transcription factor pleomorphic adenoma gene 1 (PLAG1) is reported to drive GLUD1 expression after matrix detachment and promote anoikis resistance [44]. Additional evidence demonstrates that GLUD1 can confer anoikis resistance by sustaining ATP production in cancer cells that are not able to properly monitor their bioenergetic state in nutrient-poor conditions. For instance, approximately one-third of non-small cell lung cancers exhibit loss of the tumor suppressor liver kinase B1 (LKB1), a deficiency that is associated with metastasis and poor prognosis [45]. LKB1 activates the master metabolic sensor, AMPK, through direct phosphorylation of its ⍺ subunit (AMPK⍺) at Thr 172. However, AMPK can also by activated by other kinases, including TGF-β-activated kinase 1 (TAK1) and calcium/calmodulin-dependent protein kinase kinase 2 (CamKK2) [46,47]. Importantly, AMPK contributes to cell survival under conditions of metabolic stress by phosphorylating and inhibiting the mTOR pathway, which promotes energy-consuming protein synthesis [46,48]. Therefore, AMPK activity may contribute to anoikis resistance in cancer cells under certain circumstances, such as in nutrient-deprived conditions post-ECM detachment. Indeed, evidence now demonstrates that, in LKB1-deficient lung cancer, glutaminolysis contributes to anoikis resistance and metastatic potential by reactivating AMPK signaling through GLUD1 and its metabolic product ⍺-KG [44]. Specifically, binding of ⍺-KG to CamKK2 enhances the recruitment and subsequent activation of CAMKK2's substrate, AMPK, thereby sustaining mTOR pathway inhibition, balancing energy status and eventually restoring anti-anoikis signaling [44]. In agreement, other studies have also demonstrated that LKB1- and CamKK2-mediated AMPK signaling contributes to anoikis resistance under detachment-induced metabolic stress [49]. The differential contributions of GLUD1 and its metabolic product ⍺-KG in both redox and energy homeostasis implies that the role of ⍺-KG in cell metabolism and anoikis protection may depend on cell type and discrete cellular metabolic conditions.
In summary, a growing body of research highlights glutamine and glutaminolysis as indispensable for TCA anaplerosis, maintenance of bioenergetics and regulation of redox status in diverse types of cancer. Unsurprisingly, small molecule inhibitors and other drugs that can target glutamine metabolism have gained considerable interest as anti-cancer therapies. Glutamine blockade with the pro-drug form of the glutaminase inhibitor glutamine antagonist 6-diazo-5-oxo-l-norleucine (DON) has recently been shown to disrupt glycolytic cancer metabolism and increase effector T cell oxidative metabolism to increase anti-tumor immunity (Fig. 1) [50]. Additionally, targeting GDH1 with the pupurin analog R162 attenuates proliferation and tumor growth in lung, breast and leukemia cancer cells (Fig. 1) [42]. Several preclinical studies have demonstrated that the glutaminase inhibitor Telaglenastat (CB-839) exhibits anti-tumor activity as a single agent in triple negative breast cancer cells [51], in combination with radiation therapy in NSCLC cell lines [52], and in combination with proteasome inhibitors in multiple myeloma (Fig. 1) [53]. The combination of Telaglenastat and standard-of-care chemoimmunotherapy is currently under investigation in clinical trials for non-squamous NSCLC (NCT04265534), multiple myeloma (NCT03798678), and IDH-mutated astrocytoma (NCT03528642). Nevertheless, more clinical research is needed to determine the efficacy of targeting glutamine metabolism in combination with other chemotherapeutic agents and immune checkpoint therapies for cancer.
4. Restriction of glucose oxidation
While the previously discussed disruptions in glucose-derived carbon flux can lead to bioenergetic stress after matrix detachment, matrix-detached cells may benefit from more limited glucose oxidation under certain circumstances. In normal epithelial and endothelial cells, detachment from the ECM leads to increased production of mitochondrial ROS [13,15]. In order to delay anoikis, detached cells must strike a balance between efficiently producing ATP via mitochondrial respiration in nutrient poor conditions and safeguarding against mitochondria-derived ROS overload. It is already well-established that matrix detachment is followed by reduced nutrient uptake, thereby limiting metabolic flux through glycolysis and the TCA cycle [16,54,55]. However, untransformed epithelial cells have been reported to restrict flux of glucose through the TCA cycle after matrix detachment by upregulating pyruvate dehydrogenase kinase 4 (PDHK4) expression to phosphorylate and inhibit pyruvate dehydrogenase (PDH), which is responsible for converting pyruvate to acetyl-CoA that flows into the TCA cycle [54,55]. Likewise, PDHK4 depletion and restoration of PDH activity increases oxidative metabolism of glucose and accelerates both ROS accumulation and downstream anoikis induction in detached cells [54]. Taken together, although normal epithelial cells will generate ROS and eventually undergo anoikis when detached from matrix, this process can be delayed by limiting mitochondrial respiration through already decreased nutrient uptake and negative regulation of PDH activity to limit flux of glucose through the TCA cycle.
While normal epithelial cells are reported to restrict aerobic respiration of mitochondria after detachment, many cancer cells already preferentially limit glucose oxidation in mitochondria due to the Warburg effect. Therefore, the tendency of cancer cells to prefer aerobic glycolysis may give them an inherent survival advantage at suppressing mitochondrial ROS overload soon after detachment. For instance, cancer cells are already known to express high levels of PDHKs under attached conditions to inhibit PDH and divert glucose metabolic flux away from the TCA cycle [54]. Upregulation of PDHK1 gene expression by the transcription factors Myc and hypoxia inducible factor-1 (HIF-1), or activation of PDHK1 by post-translational modification such as tyrosine phosphorylation by diverse oncogenic tyrosine kinases are reported to further decrease pyruvate dehydrogenase complex (PDC) activity [56]. Higher expression of PDHKs is also associated with poor cancer patient survival [57]. Likewise, suppression of PDHK and activation of PDH has been shown to sensitize some cancer cells to anoikis and decreases their metastatic potential by stimulating glucose oxidation and ROS production [54]. In addition, the M2 isoform of pyruvate kinase (PKM2), which is catalytically involved in the final key step of glycolysis and allosterically regulated by distinct tetrameric and dimeric states, is often overexpressed in cancer cells and allows glycolytic metabolites to divert away from the TCA cycle into other metabolic pathways such as the PPP, thereby limiting mitochondrial respiration [58]. Taken together, this evidence demonstrates that although glucose uptake is important for bioenergetics and cell survival after matrix detachment, mitochondrial oxidation of glucose may trigger anoikis through the production and accumulation of ROS. In support of this possibility, treatment with metformin, which binds to complex I of the ETC and suppresses OXPHOS, has been shown to support the detachment and viability of breast cancer cells in vitro [59,60]. However, more research is needed to determine how anoikis resistant cells regulate carbon flux to balance bioenergetic needs and to maintain redox homeostasis.
5. Upregulation of antioxidant defense systems
In normal epithelial cells, detachment from the ECM leads to downregulated expression of EGFR and consequent decrease in cellular signaling through the pro-survival PI3K/Akt pathway [61]. Since activation of PI3K and downstream Akt play essential roles in regulating both glucose uptake and glucose metabolism [62], detached cells often experience a significant reduction in levels of glucose-6-phosphate, an intermediate metabolite which fuels both glycolysis and the PPP, as a consequence of decreased glucose import. This, in turn, limits metabolic flux through the PPP and impaired regeneration of GSH, thereby potentiating an increase in intracellular ROS levels [63]. This is important because increased ROS production after ECM detachment can initiate anoikis if levels become too high [15,16,64]. While limited increases in ROS production can promote pro-survival signaling, ECM-detached cancer cells must be able to maintain adequate antioxidant capacity in order to prevent oxidative stress and initiation of anoikis. Indeed, circulating tumor cells often exhibit higher ROS levels in comparison to the primary tumors they were derived from [65]. However, it is well-established that cancer cells actively engage multiple antioxidant defense systems to cope with high oxidative stress [45].
In addition to limiting the amount of ROS produced from mitochondrial respiration, aspects of the Warburg effect may help to reduce matrix-detachment induced oxidative stress by shunting glucose into the PPP. For instance, aerobic glycolysis in cancer cells diverts more glucose-derived carbon into the oxidative phase of the PPP, which produces antioxidants NADPH and GSH [66]. In addition, acute increases in intracellular concentrations of ROS inhibits the glycolytic enzyme PKM2 through oxidation of Cys358, which in turn diverts glucose flux into the PPP to generate reducing power for ROS detoxification [67]. Inhibition of PDH though overexpression of PDKs in cancer may have similar effects as well [54,56]. While flux through the PPP can contribute to indirect inhibition of anoikis through the detoxification of ROS, it is also worth mentioning that production of reduced glutathione through the PPP can directly inhibit anoikis and apoptosis by keeping cytochrome c in its reduced state, thereby inhibiting its apoptogenic role in caspase activation [9]. However, it is important to mention that many of these aspects Warburg effect have historically been observed in proliferative primary solid tumors. Therefore, diversion of glucose into the PPP by PKM2 and PDHK upregulation needs to be further tested in detached cancer cells to determine whether these mechanisms are main drivers of anoikis resistance.
Evidence suggests that cancer cells are also able to employ reductive carboxylation of glutamine to keep ROS levels in check after ECM detachment. For instance, in anchorage-independent lung cancer tumor spheroids, oxidation of glutamine and glucose is suppressed, while reductive formation of citrate from glutamine-derived ⍺-KG via cytosolic isocitrate dehydrogenase-1 (IDH1) is increased [10]. This reductively produced citrate in the cytosol is then able to enter the mitochondria and participate in the TCA cycle, thereby generating NADPH to combat mitochondria-derived ROS such as O2− [10]. In addition to glutamine, fatty acid oxidation (FAO) also appears to confer anoikis resistance to certain types of cancer through modulation of redox status. For example, colorectal cancer cells have been reported to upregulate the rate-limiting enzyme of fatty acid oxidation, CPT1A, when cultured in suspension and CPT1A expression is also upregulated in colorectal cancer metastases [68]. Specifically, CPT1A-mediated FAO has been demonstrated to suppress anoikis in colorectal cancer by providing acetyl-CoA for the TCA cycle to maintain adequate NADPH/NADP+ and GSH/GSSG ratios [68]. Taken together, this evidence also highlights that cancer cells can utilize nutrients other than glucose to maintain antioxidant defense after matrix detachment.
While NADPH and GSH constitute important antioxidant molecules for the detoxification of intracellular ROS, several antioxidant enzymes may be used by matrix-detached cells in attempts to maintain redox homeostasis as well [69]. Manganese superoxide dismutase (MnSOD), which is localized to the mitochondria, plays an important role in detoxifying superoxide into the less reactive hydrogen peroxide (H2O2), which is then further broken down into water and oxygen. Cell disengagement from the ECM is reported to induce expression of MnSOD to combat detachment-induced elevations in mitochondrial ROS [13]. Accordingly, cells with reduced levels of MnSOD are reported to be hypersensitive to anoikis following matrix detachment due to ROS overload leading to oxidative stress [13]. While chronically elevated ROS levels are often observed in cancer [44], many cancer cells also upregulate antioxidant enzymes to make sure ROS levels do not become high enough to cause cell death [45]. In particular, MnSOD upregulation is reported to suppress anoikis in human mammary epithelial cells and metastatic breast cancer cells through dismutation of mitochondria-produced superoxide radicals [13]. Other studies have also demonstrated that superoxide dismutase and the antioxidant enzyme catalase grant anoikis resistance to breast cancer cells after ECM disengagement by reducing ROS and therefore indirectly supporting ATP production [64]. While MnSOD gene expression is reported to be under transcriptional control of NF-κB [13], its expression, along with other antioxidant genes, has also been reported to be induced by the Nrf-2 transcription factor, which is known to be overactivated via upstream signaling by oncogenic Ras, Raf and Myc [70,71]. Under normal conditions, Nrf-2 is bound to its inhibitor, Keap-1, and remains in the cytoplasm, but increases in ROS from oncogenic growth factor receptor signaling or ECM detachment lead to the dissociation of Nrf-2 from Keap-1, and Nrf-2 is able to enter the nucleus where it binds to antioxidant response elements (ARE) in DNA. This promotes gene expression of several ROS-scavenging enzymes including catalase, thioredoxin, glutathione peroxidase and superoxide dismutase [72]. In addition, metabolites produced by glutaminolysis are able to directly modulate the antioxidant Nrf-2 response as well. Fumarate, which is derived from ⍺-KG, is known to modify cysteine residues on Keap-1 which impairs its ability to repress Nrf-2 by keeping it in the cytosol (Fig. 1) [73,74]. Therefore, glutaminolysis may play a central role in upregulating expression of antioxidant genes to keep ROS levels in check in both adherent and disseminated cancer cells.
6. Conclusion
Eukaryotic cellular metabolism is composed of an incredibly complex network of catabolic and anabolic pathways that are tightly regulated to balance energy production with redox homeostasis and biosynthesis. It is now well-established that epithelial and endothelial cellular detachment from the extracellular matrix causes widespread disturbances in metabolism. Furthermore, cell fate is highly influenced by the availability of nutrients and activity of diverse metabolic pathways that produce ATP, antioxidants, biosynthetic precursors and metabolites used in cell signaling. Therefore, it is unsurprising that mitochondrial metabolic and apoptotic pathways are intertwined. Indeed, preclinical research is now beginning to delineate how cancer cells modulate mitochondrial metabolic pathways in a context-dependent manner to maintain a redox status that promotes tumor growth, apoptosis resistance and survival in circulation. In general, evidence thus far indicates that oncogenic alterations promote diversion of glucose away from the mitochondria and a heavy reliance on glutaminolysis to facilitate rapid ATP production and biosynthesis while simultaneously keeping ROS production in check. Continued research on mitochondrial metabolic reprogramming and its role in maintaining redox homeostasis will be crucial for identifying future cancer therapies.
Authors' contributions
A.C.B drafted the manuscript; A.C.B. and S.K. edited and revised the manuscript; all authors read and approved the final version of the manuscript.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgment
This work was supported in part by NIH grants R01 CA207768 (S.K.), R01 CA175316 (S.K.), and F31 CA246889 (A.C.B.). A.C.B. is an NIH pre-doctoral fellow. S.K. is a Georgia Cancer Coalition Scholar and an American Cancer Society Basic Research Scholar.
References
- 1.Vander Heiden M.G., DeBerardinis R.J. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168(4):657–669. doi: 10.1016/j.cell.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metabol. 2016;23(1):27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber G.F. Metabolism in cancer metastasis. Int. J. Canc. 2016;138(9):2061–2066. doi: 10.1002/ijc.29839. [DOI] [PubMed] [Google Scholar]
- 4.Wang C., Youle R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberg S.E., Chandel N.S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 2015;11(1):9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen J.L., Kornbluth S. The tangled circuitry of metabolism and apoptosis. Mol. Cell. 2013;49(3):399–410. doi: 10.1016/j.molcel.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llambi F., Moldoveanu T., Tait S.W., Bouchier-Hayes L., Temirov J., McCormick L.L., Dillon C.P., Green D.R. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol. Cell. 2011;44(4):517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X., Kim C.N., Yang J., Jemmerson R., Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86(1):147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 9.Vaughn A.E., Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat. Cell Biol. 2008;10(12):1477–1483. doi: 10.1038/ncb1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang L., Shestov A.A., Swain P., Yang C., Parker S.J., Wang Q.A., Terada L.S., Adams N.D., McCabe M.T., Pietrak B. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature. 2016;532(7598):255–258. doi: 10.1038/nature17393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeBleu V.S., O'Connell J.T., Gonzalez Herrera K.N., Wikman H., Pantel K., Haigis M.C., de Carvalho F.M., Damascena A., Domingos Chinen L.T., Rocha R.M. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014;16(10):992–1003. doi: 10.1038/ncb3039. 1001-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stowe D.F., Camara A.K. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxidants Redox Signal. 2009;11(6):1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamarajugadda S., Cai Q., Chen H., Nayak S., Zhu J., He M., Jin Y., Zhang Y., Ai L., Martin S.S. Manganese superoxide dismutase promotes anoikis resistance and tumor metastasis. Cell Death Dis. 2013;4:e504. doi: 10.1038/cddis.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orrenius S., Gogvadze V., Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 15.Li A.E., Ito H., Rovira, Kim K.S., Takeda K., Yu Z.Y., Ferrans V.J., Finkel T. A role for reactive oxygen species in endothelial cell anoikis. Circ. Res. 1999;85(4):304–310. doi: 10.1161/01.res.85.4.304. [DOI] [PubMed] [Google Scholar]
- 16.Schafer Z.T., Grassian A.R., Song L., Jiang Z., Gerhart-Hines Z., Irie H.Y., Gao S., Puigserver P., Brugge J.S. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461(7260):109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halim H., Chanvorachote P. Long-term hydrogen peroxide exposure potentiates anoikis resistance and anchorage-independent growth in lung carcinoma cells. Cell Biol. Int. 2012;36(11):1055–1066. doi: 10.1042/CBI20120111. [DOI] [PubMed] [Google Scholar]
- 18.Rungtabnapa P., Nimmannit U., Halim H., Rojanasakul Y., Chanvorachote P. Hydrogen peroxide inhibits non-small cell lung cancer cell anoikis through the inhibition of caveolin-1 degradation. Am. J. Physiol. Cell Physiol. 2011;300(2):C235–C245. doi: 10.1152/ajpcell.00249.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown D.I., Griendling K.K. Nox proteins in signal transduction. Free Radic. Biol. Med. 2009;47(9):1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannoni E., Fiaschi T., Ramponi G., Chiarugi P. Redox regulation of anoikis resistance of metastatic prostate cancer cells: key role for Src and EGFR-mediated pro-survival signals. Oncogene. 2009;28(20):2074–2086. doi: 10.1038/onc.2009.77. [DOI] [PubMed] [Google Scholar]
- 21.Gough D.R., Cotter T.G. Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis. 2011;2:e213. doi: 10.1038/cddis.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peshavariya H., Dusting G.J., Jiang F., Halmos L.R., Sobey C.G., Drummond G.R., Selemidis S. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009;380(2):193–204. doi: 10.1007/s00210-009-0413-0. [DOI] [PubMed] [Google Scholar]
- 23.Parri M., Chiarugi P. Redox molecular machines involved in tumor progression. Antioxidants Redox Signal. 2013;19(15):1828–1845. doi: 10.1089/ars.2012.5040. [DOI] [PubMed] [Google Scholar]
- 24.Zhu P., Tan M.J., Huang R.L., Tan C.K., Chong H.C., Pal M., Lam C.R., Boukamp P., Pan J.Y., Tan S.H. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2(-):H2O2 ratio and confers anoikis resistance to tumors. Canc. Cell. 2011;19(3):401–415. doi: 10.1016/j.ccr.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Karin M., Lin A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002;3(3):221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 26.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. U.S.A. 2007;104(49):19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reitzer L.J., Wice B.M., Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 1979;254(8):2669–2676. [PubMed] [Google Scholar]
- 28.Lu W., Pelicano H., Huang P. Cancer metabolism: is glutamine sweeter than glucose? Canc. Cell. 2010;18(3):199–200. doi: 10.1016/j.ccr.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeBerardinis R.J., Cheng T. Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29(3):313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wise D.R., Thompson C.B. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 2010;35(8):427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo M., Wang Y.Z., Gout P.W. The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J. Cell. Physiol. 2008;215(3):593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- 32.Lim J.K.M., Delaidelli A., Minaker S.W., Zhang H.F., Colovic M., Yang H., Negri G.L., von Karstedt S., Lockwood W.W., Schaffer P. Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. Proc. Natl. Acad. Sci. U.S.A. 2019;116(19):9433–9442. doi: 10.1073/pnas.1821323116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muir A., Danai L.V., Gui D.Y., Waingarten C.Y., Lewis C.A., Vander Heiden M.G. Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. Elife. 2017;6 doi: 10.7554/eLife.27713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badgley M.A., Kremer D.M., Maurer H.C., DelGiorno K.E., Lee H.J., Purohit V., Sagalovskiy I.R., Ma A., Kapilian J., Firl C.E.M. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368(6486):85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biology. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandolfi P.P., Sonati F., Rivi R., Mason P., Grosveld F., Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995;14(21):5209–5215. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wise D.R., DeBerardinis R.J., Mancuso A., Sayed N., Zhang X.-Y., Pfeiffer H.K., Nissim I., Daikhin E., Yudkoff M., McMahon S.B. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. U.S.A. 2008;105(48):18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ni F., Yu W.M., Li Z., Graham D.K., Jin L., Kang S., Rossi M.R., Li S., Broxmeyer H.E., Qu C.K. Critical role of ASCT2-mediated amino acid metabolism in promoting leukaemia development and progression. Nat Metab. 2019;1(3):390–403. doi: 10.1038/s42255-019-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao P., Tchernyshyov I., Chang T.C., Lee Y.S., Kita K., Ochi T., Zeller K.I., De Marzo A.M., Van Eyk J.E., Mendell J.T. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458(7239):762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eberhardy S.R., Farnham P.J. c-Myc mediates activation of the cad promoter via a post-RNA polymerase II recruitment mechanism. J. Biol. Chem. 2001;276(51):48562–48571. doi: 10.1074/jbc.M109014200. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds M.R., Lane A.N., Robertson B., Kemp S., Liu Y., Hill B.G., Dean D.C., Clem B.F. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene. 2014;33(5):556–566. doi: 10.1038/onc.2012.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin L., Li D., Alesi G.N., Fan J., Kang H.B., Lu Z., Boggon T.J., Jin P., Yi H., Wright E.R. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Canc. Cell. 2015;27(2):257–270. doi: 10.1016/j.ccell.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farris J.C., Pifer P.M., Zheng L., Gottlieb E., Denvir J., Frisch S.M. Grainyhead-like 2 reverses the metabolic changes induced by the oncogenic epithelial-mesenchymal transition: effects on anoikis. Mol. Canc. Res. : MCR. 2016;14(6):528–538. doi: 10.1158/1541-7786.MCR-16-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin L., Chun J., Pan C., Kumar A., Zhang G., Ha Y., Li D., Alesi G.N., Kang Y., Zhou L. The PLAG1-GDH1 Axis promotes anoikis resistance and tumor metastasis through CamKK2-AMPK signaling in LKB1-deficient lung cancer. Mol. Cell. 2018;69(1):87–99. doi: 10.1016/j.molcel.2017.11.025. e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Cespedes M., Parrella P., Esteller M., Nomoto S., Trink B., Engles J.M., Westra W.H., Herman J.G., Sidransky D. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Canc. Res. 2002;62(13):3659–3662. [PubMed] [Google Scholar]
- 46.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo Z., Zang M., Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010;6(3):457–470. doi: 10.2217/fon.09.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J., Kundu M., Viollet B., Guan K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sundararaman A., Amirtham U., Rangarajan A. Calcium-oxidant signaling network regulates AMP-activated protein kinase (AMPK) activation upon matrix deprivation. J. Biol. Chem. 2016;291(28):14410–14429. doi: 10.1074/jbc.M116.731257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leone R.D., Zhao L., Englert J.M., Sun I.M., Oh M.H., Sun I.H., Arwood M.L., Bettencourt I.A., Patel C.H., Wen J. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366(6468):1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gross M.I., Demo S.D., Dennison J.B., Chen L., Chernov-Rogan T., Goyal B., Janes J.R., Laidig G.J., Lewis E.R., Li J. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Canc. Therapeut. 2014;13(4):890–901. doi: 10.1158/1535-7163.MCT-13-0870. [DOI] [PubMed] [Google Scholar]
- 52.Boysen G., Jamshidi-Parsian A., Davis M.A., Siegel E.R., Simecka C.M., Kore R.A., Dings R.P.M., Griffin R.J. Glutaminase inhibitor CB-839 increases radiation sensitivity of lung tumor cells and human lung tumor xenografts in mice. Int. J. Radiat. Biol. 2019;95(4):436–442. doi: 10.1080/09553002.2018.1558299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson R.M., Dytfeld D., Reyes L., Robinson R.M., Smith B., Manevich Y., Jakubowiak A., Komarnicki M., Przybylowicz-Chalecka A., Szczepaniak T. Glutaminase inhibitor CB-839 synergizes with carfilzomib in resistant multiple myeloma cells. Oncotarget. 2017;8(22):35863–35876. doi: 10.18632/oncotarget.16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamarajugadda S., Stemboroski L., Cai Q., Simpson N.E., Nayak S., Tan M., Lu J. Glucose oxidation modulates anoikis and tumor metastasis. Mol. Cell Biol. 2012;32(10):1893–1907. doi: 10.1128/MCB.06248-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grassian A.R., Metallo C.M., Coloff J.L., Stephanopoulos G., Brugge J.S. Erk regulation of pyruvate dehydrogenase flux through PDK4 modulates cell proliferation. Genes Dev. 2011;25(16):1716–1733. doi: 10.1101/gad.16771811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hitosugi T., Fan J., Chung T.W., Lythgoe K., Wang X., Xie J., Ge Q., Gu T.L., Polakiewicz R.D., Roesel J.L. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol. Cell. 2011;44(6):864–877. doi: 10.1016/j.molcel.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wigfield S.M., Winter S.C., Giatromanolaki A., Taylor J., Koukourakis M.L., Harris A.L. PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br. J. Canc. 2008;98(12):1975–1984. doi: 10.1038/sj.bjc.6604356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christofk H.R., Vander Heiden M.G., Harris M.H., Ramanathan A., Gerszten R.E., Wei R., Fleming M.D., Schreiber S.L., Cantley L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 59.Owen M.R., Doran E., Halestrap A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000;348 Pt 3:607–614. [PMC free article] [PubMed] [Google Scholar]
- 60.Bizjak M., Malavasic P., Dolinar K., Pohar J., Pirkmajer S., Pavlin M. Combined treatment with Metformin and 2-deoxy glucose induces detachment of viable MDA-MB-231 breast cancer cells in vitro. Sci. Rep. 2017;7(1):1761. doi: 10.1038/s41598-017-01801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reginato M.J., Mills K.R., Paulus J.K., Lynch D.K., Sgroi D.C., Debnath J., Muthuswamy S.K., Brugge J.S. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat. Cell Biol. 2003;5(8):733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 62.Elstrom R.L., Bauer D.E., Buzzai M., Karnauskas R., Harris M.H., Plas D.R., Zhuang H., Cinalli R.M., Alavi A., Rudin C.M. Akt stimulates aerobic glycolysis in cancer cells. Canc. Res. 2004;64(11):3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 63.Buchheit C.L., Rayavarapu R.R., Schafer Z.T. The regulation of cancer cell death and metabolism by extracellular matrix attachment. Semin. Cell Dev. Biol. 2012;23(4):402–411. doi: 10.1016/j.semcdb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Davison C.A., Durbin S.M., Thau M.R., Zellmer V.R., Chapman S.E., Diener J., Wathen C., Leevy W.M., Schafer Z.T. Antioxidant enzymes mediate survival of breast cancer cells deprived of extracellular matrix. Canc. Res. 2013;73(12):3704–3715. doi: 10.1158/0008-5472.CAN-12-2482. [DOI] [PubMed] [Google Scholar]
- 65.Piskounova E., Agathocleous M., Murphy M.M., Hu Z., Huddlestun S.E., Zhao Z., Leitch A.M., Johnson T.M., DeBerardinis R.J., Morrison S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527(7577):186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cairns R.A., Harris I.S., Mak T.W. Regulation of cancer cell metabolism. Nat. Rev. Canc. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 67.Anastasiou D., Poulogiannis G., Asara J.M., Boxer M.B., Jiang J.K., Shen M., Bellinger G., Sasaki A.T., Locasale J.W., Auld D.S. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334(6060):1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y.N., Zeng Z.L., Lu J., Wang Y., Liu Z.X., He M.M., Zhao Q., Wang Z.X., Li T., Lu Y.X. CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene. 2018;37:6025–6040. doi: 10.1038/s41388-018-0384-z. [DOI] [PubMed] [Google Scholar]
- 69.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 70.DeNicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K.H., Yeo C.J., Calhoun E.S. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trachootham D., Lu W., Ogasawara M.A., Nilsa R.D., Huang P. Redox regulation of cell survival. Antioxidants Redox Signal. 2008;10(8):1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang H.C., Nguyen T., Pickett C.B. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. U.S.A. 2000;97(23):12475–12480. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adam J., Hatipoglu E., O'Flaherty L., Ternette N., Sahgal N., Lockstone H., Baban D., Nye E., Stamp G.W., Wolhuter K. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Canc. Cell. 2011;20(4):524–537. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ooi A., Wong J.C., Petillo D., Roossien D., Perrier-Trudova V., Whitten D., Min B.W., Tan M.H., Zhang Z., Yang X.J. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Canc. Cell. 2011;20(4):511–523. doi: 10.1016/j.ccr.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 75.Frisch S.M., Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]