Abstract
Reactive oxygen species (ROS) carry out prime physiological roles as intracellular signaling agents, yet pathologically high concentrations of ROS cause irreversible damage to biomolecules, alter cellular programs and contribute to various diseases. While decades of intensive research have identified redox-related patterns and signaling pathways, very few addressed how the glycosylation machinery senses and responds to oxidative stress. A common trait among ROS and glycans residing on glycoconjugates is that they are both highly dynamic, as they are quickly fine-tuned in response to stressors such as inflammation, cancer and infectious diseases. On this account, the delicate balance of the redox potential, which is tightly regulated by dozens of enzymes including NOXs, and the mitochondrial electron transport chain as well as the fluidity of glycan biosynthesis resulting from the cooperation of glycosyltransferases, glycosidases, and nucleotide sugar transporters, is paramount to cell survival. Here, we review the broad spectrum of the interplay between redox changes and glycosylation with respect to their principle consequences on human physiology.
Keywords: Glycosylation, Reactive oxygen species, Hypoxia, Golgi, Endoplasmic reticulum, CGD
1. Introduction
Reactive oxygen species (ROS) are highly reactive compounds formed by redox reactions inside and outside of cells. Cellular ROS production can be either nonenzymatic or enzymatic (for review see Ref. [1]). ROS are considered to be proper radicals such as the superoxide anion (O2−•), the hydroxyl radical (·OH), the peroxyl radical (ROO·), and the alkoxyl radical (RO·) or non-radicals such as hydrogen peroxide (H2O2), organic hydroperoxides (ROOH), singlet molecular oxygen (1O2), and hypochlorus (HOCl) and hypobromous (HOBr) acids [[2], [3], [4]]. ROS can be both, detrimental to cells especially under conditions leading to oxidative stress, and essential cellular signaling molecules modulating physiological responses.
Another fundamental cellular component are polysaccharides or glycans. Complex glycan chains can be found on proteins and lipids to which they are added by glycosylation and where they contribute to their correct folding, proper enzymatic activity and molecular interactions [5]. There exist up to 16 protein and lipid glycosylation pathways which are defined by distinct linkages and initiating enzymes. This diversity greatly expands the glycoproteome and glycolipidome and extends their functions and their interactions [6]. Studies related to glyco-enzyme deficiencies in diseases increased our understanding of the roles of glycosylation in physiology, yet full and reliable characterization and readouts of glycans on macromolecules remain challenging.
Cellular glycan profiles are heterogeneous and vary depending on the cell's state. They are stage specific during development and can be over- or under-represented on the cell's surface in response to molecular cues or a stressor. In addition, glycans are tissue specific whereby each cell type generally has distinct glyco-profiles that can vary depending on different stages or in disease. Therefore, glycans are considered as biological switches which alter molecular functions and turn on or off multiple signaling pathways [6] making them excellent biological readouts and assessment tools for scientists and clinical researchers.
Recently, the connection between redox signaling and functional changes of glycans was described with the term “Glyco-redox” [7]. In this review, we extend this view and centralize recent glyco-redox studies in an attempt to close the gap between two cardinal fields, glycobiology and redox biology which allows us to evaluate their inter-related functions and dysfunctions and to reflect on novel therapeutic approaches for the treatment of related diseases.
2. ROS as signaling molecules in cellular homeostasis
In the cell, endogenous ROS sources reside within different cellular compartments. ROS levels vary with the majority of ROS produced by the mitochondrial electron transport chain (ETC: complex I, II and III) [8], localized NADPH oxidases (NOX1 to 5) [[9], [10], [11], [12]], dual oxidases (DUOX1 and 2), superoxide dismutases (SOD1, 2 and 3) [11], and oxidation of polyunsaturated fatty acids [13]. Cellular ROS levels are tightly balanced by a system consisting of antioxidants such as glutaredoxin, peroxiredoxin, thioredoxin, and glutathione as well as antioxidant enzymes such as glutathione reductase (GR), and glutathione peroxidases (GPX) complemented by exogenous low molecular weight substances such as Vitamin E, or Vitamin C which altogether ensure that ROS levels are maintained within a physiological range necessary for intracellular signaling [14]. In addition to ROS generators and antioxidants, membrane proteins from the aquaporin (AQP) family contribute to ROS regulation by controlling their transport, thus providing ROS gradients in the cell [15,16]. A disbalance between prooxidants and antioxidants in favor of the oxidants is commonly referred to as oxidative stress (for reviews see Refs. [1,17,18]).
ROS signaling or redox signaling is highly dynamic, tissue specific, reversible and affects multiple levels. Thereby, ROS control a large spectrum of cellular functions [[19], [20], [21], [22], [23]] including regulation of enzyme activity, protein folding, transcription factor targeting, as well as coding and non-coding RNA regulation by targeting cysteines, tyrosines, and iron-sulfur (Fe–S) clusters. For instance, redox-sensitive targets may include among others transcription factors such as NF-κB, hypoxia-inducible factors (HIFs), and nuclear factor E2-related factor 2 (NRF2). Thereby, cytosolic H2O2 could increase NF-κB signaling by oxidizing and destabilizing its inhibitor IκB [24,25], while nuclear H2O2 inhibits it by oxidizing discrete cysteines in the DNA-binding region of NF-κB [26]. Such differential effect is crucial for the regulation of the inflammatory response and is an example for the specificity and the spatiotemporal activity of ROS. Under hypoxia, ROS can stabilize HIFs in certain cell types by inhibiting HIF prolyl-hydroxylases, which promotes changes in cellular programs such as replication, survival and stress adaptation [27,28]. Other redox-sensitive targets include energy sensing and metabolic regulators such as AMP-activated protein kinase (AMPK) [29,30] and forkhead box proteins (FOXO) [31,32], the glycolytic glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [33] or the cell cycle regulator p53 [34], which are elucidated in more detail in previous excellent reviews [4,14]. While redox signaling regulates important cellular functions, dysregulated ROS production and subsequent oxidative stress lead to imbalances in various cellular processes that can result in diseases including neurodevelopmental disorders [35], circadian rhythm disruption [36], neuro-degeneration such as Parkinson's and Alzheimer's disease [37], cardiovascular dysfunction [[38], [39], [40]], impaired immune response and autoimmune diseases [[41], [42], [43]], metabolic diseases such as diabetes [44,45] as well as premature ageing and/or cell death.
3. Glycans and cellular homeostasis
The importance of glycans for cellular homeostasis is highlighted by the existence of congenital disorders of glycosylation (CDG) where mutations or absence of genes encoding enzymes involved in the glycan biosynthesis process have serious implications on development and health [46,47]. The proper biological roles of glycans are numerous and there are far too many examples to be covered in this review. To state a few, other than their role in protein folding and protein-protein interactions [48,49], they are essential for multicellular development [50], being involved in the acrosomal reaction during fertilization [51] as well as in the inflammatory response of cells by modulating leukocyte recruitment [52]. Glycans serve as binding sites for commensal and pathogenic bacteria as well as viruses [53,54]. Altered glycans are also hallmarks of cancer and in many cancers they are used to evade immune recognition [55,56]. For instance, abnormalities and truncations of glycans are observed in the O-linked glycome accompanied by an increased synthesis of Tn, T and sialyl-T antigens. On the whole, hypersialylation (N- and O-) is associated with a poor cancer prognosis and is observed in aggressive and highly metastatic cancers [[55], [56], [57], [58], [59], [60]].
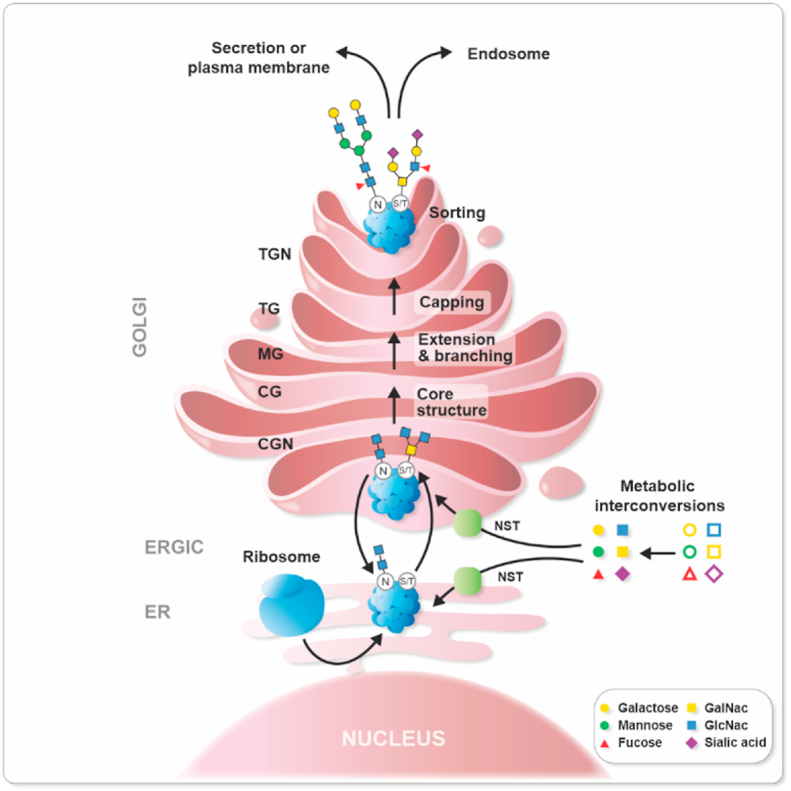
Glycan biosynthesis requires first cellular uptake of simple sugars whereby they are activated by kinases and processed by transferases to become nucleotide sugars (e.g., UDP-Galactose, GDP-Fucose, or CMP-Sialic acid). These sugars are then imported into the endoplasmic reticulum (ER) and the Golgi via nucleotide sugar transporters (NST) (Fig. 1). Within the ER and Golgi they function primarily as donors in sequential glycosyltransferase reactions which form an elongated and branched glycan polymer (Fig. 1). Often, glycosidases trim the glycan chains by removing monosaccharides, hence forming intermediates that can be reused by glycosyltransferases. Around 17 monosaccharides are found in mammals and combining them together generates a large variety of glycan structures where stereochemical α or β conjugations add even more to structures’ complexity. A total of 10^12 combinations of glycan structures is thought to exist. The complete repertoire of these sugars is called the glycome and is considered to be a third language of life beyond nucleic acids and proteins. Subsequently, the glycans produced can be used for the biosynthesis of glycolipids, glycoproteins, and proteoglycans.
Fig. 1.
Glycoprotein formation. (A) First, cytosolic enzymatic reactions convert monosaccharides to “active sugars”, which are then translocated into the ER and Golgi via nucleotide sugar transporters (NST). The most frequently occurring glycans are N-glycans and O-glycans. In N-linked glycans the glycosylation process starts in the lumen of the ER where an oligosaccharyltransferase attaches a monosaccharide precursor to an amide nitrogen of an asparagine or arginine residue residing in a specific consensus sequence of the nascent protein. Further trimming of the glycan structures and control of misfolded glycoproteins in the ER is performed before the protein exits the ER-via the Golgi intermediate compartment (ERGIC). The O-linked glycosylation is a form of glycosylation that occurs in the ER or the Golgi where different sugars can be added to hydroxyl groups present in the amino acids serine, threonine, tyrosine, hydroxylysine, or hydroxyproline; alternatively oxygen present in lipids such as ceramide can be used. In the Golgi lumen, activated sugars are used as donors in sequential reactions with glycosyltransferases (GTs) which together with glycosidases (Gly) extend, and branch the glycan by involving the cis Golgi (CG), and medial Golgi (MG) before they are capped with terminal sugars such as sialic acid. Finally, the glycoprotein is sorted into a vesicle through the trans Golgi (TG) and the trans Golgi network (TGN) and transported to its final destination.
Among glycolipids are glycosphingolipids. These subtypes of glycolipids are ubiquitous, required for embryonic development and depending on the attached sugar chains exert different functions at different stages of development [50,61]. For instance, globosides are predominant in the early embryonic stages but gangliosides, a family of sialic acid-containing glycosphingolipids, are expressed during neural development and participate in neural signaling processes [62].
While glycoproteins such as collagens, mucins, transferrin, and immunoglobulins consist of oligosaccharide chains that are covalently attached to proteins, proteoglycans as chondroitin sulfate, dermatan sulfate, heparan sulfate, or keratan sulfate are made of a core protein to which one or more linear polysaccharide chains made of disaccharide building blocks [63] known as glycosaminoglycans (GAGs) are covalently attached. In both, the glycosylation, i.e. the covalent attachment to the respective protein occurs mainly via two types of glycosylation: N-linked glycosylation and O-linked glycosylation where oligosaccharides are commonly attached to a nitrogen atom of an asparagine or to an oxygen atom of serine or threonine, respectively. In addition, other hydroxyl group containing amino acids such as tyrosine, hydroxylysine, or hydroxyproline as well as oxygen present in lipids such as ceramide can be used for O-linked glycosylation. There are major differences between N- and O-linked glycosylations such as the implicated enzymes, the consensus sites, synthesis localization and glycan chain type and complexity which are elucidated in more detail elsewhere [5].
4. Glycosylation and glycan profiles under oxidative stress
As glycan synthesis occurs in the ER and Golgi it is reasonable to assume that redox changes in these compartments affect glycosylation reactions. By using a genetically encoded redox sensitive probe it was recently shown that the redox status of mitochondria, ER and Golgi is different and that the lumen of the Golgi has even a more oxidizing environment than the ER [1]. This is in agreement with the use of high oxidizing power for tail-to-tail disulfide bond formation during the assembly of secretory proteins from oligomeric or multimeric precursors in the Golgi [64,65]. This process is likely supported by members of the Quiescin-Sulfhydryl Oxidase (QSOX) family. These proteins represent an ancient fusion between PDI-like thioredoxin (TRX) domains and ERV-like oxidase domains. As a result, they can efficiently couple disulfide bond generation with reduction of O2 to H2O2. In addition, ROS generated during these processes may be used by the glutaredoxins, GRX6 and GRX7 which in baker's yeast, are located in the cis-Golgi thereby reducing disulfide bonds by the use of reduced glutathione. GRX6 and GRX7 represent closely related monothiol glutaredoxins that are found in this compartment [66].
Apart from disulfide bond formation ROS in ER and Golgi appear to affect the identity of glycans displayed on proteins and lipids via direct and indirect mechanisms.
It is intriguing that ROS or hypoxia-mediated glycosylation changes have not yet been detected in the initial synthesis of N-glycans in the ER. However, the recent finding that the redox-sensitive selenoprotein T was shown to function as a new oligosaccharyltransferase and to be indispensable for ER homeostasis as well as for maturation and secretion of glycohormones from endocrine glands [67] suggests that hypoxia and/or ROS could affect this process. However, further studies are needed to clarify whether and how hypoxia and/or ROS may affect ER-associated glycosylation events.
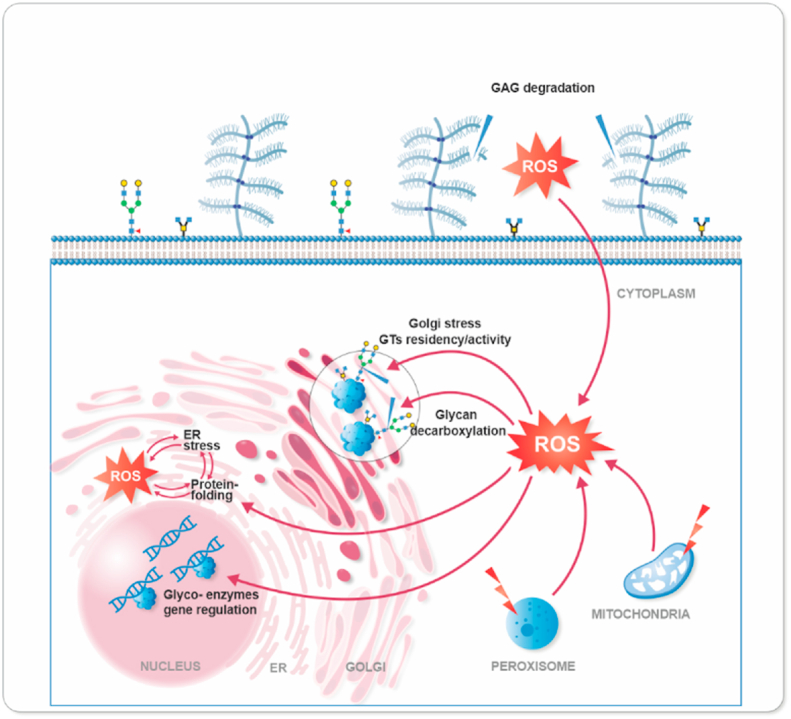
Overall, it appears that changes in ROS levels within the cell in general, but also in both, the ER and Golgi, can affect the identity of glycans displayed on proteins and lipids either by the direct interaction of ROS with carbohydrate chains or indirectly by altering protein-protein interactions, molecular signaling and genetic feedback (Fig. 2).
Fig. 2.
The glyco-redox interplay. ROS, from exogenous sources or formed in different cellular compartments either as signal molecules or as more harmful byproducts of metabolic activities, can affect glycosylation in different ways. This can involve direct action on glycosylated proteins, e.g., on glycosidic bonds, or in a more indirect manner via the regulation of glycosylation enzyme activity, or their gene regulation.
5. Direct
Glycosaminoglycans (GAGs) are an integral component of the extracellular matrix and are present throughout the entire human body with highest concentrations in skin, cartilage and bones [5,68]. As parts of proteoglycans, GAGs are involved in a wide array of biological processes and defects in GAG synthesis contribute to inflammatory diseases, connective tissue disorders and abnormal brain development [69]. GAGs can be modified or degraded by a number of ROS (Fig. 2). For instance, low reactivity ROS such as O2−• and H2O2 were shown to degrade GAGs and proteoglycan aggregates in a direct manner as well as in an indirect fashion via activation of latent collagenases and inhibition of de novo proteoglycan synthesis [[70], [71], [72]]. ROS that are more reactive such as HOCl and·OH depolymerized GAGs by acting directly on thiols and amino groups or, for example, on the glycosidic linkage between the glucuronic acid and the N-acetyl-glucosamine in hyaluronic acid, respectively [73,74] (reviewed in Ref. [69]). ROS and hypoxia-mimicking agents were found to directly affect cell surface localized proteoglycans (i.e. glycosaminoglycans) and N-linked oligosaccharides (i.e. N-glycans), thereby they were able to induce cleavage of the proteo-, and N-glycans in an N-acetyl-amino-sugar-specific manner by employing transition metals such as iron [75]. As a consequence, cell-cell or cell-extracellular matrix interactions were affected in a manner that coincided with inflammatory responses, atherosclerosis, and cancers [7].
6. Indirect
While the relationship between ROS and N-linked glycosylation is yet to be defined, ROS effects on O-linked glycans and GAGs as well as O-GlcNAc signaling are established [76]. Several studies have shown enhanced protein O-GlcNAcylation in response to oxidative distress [[77], [78], [79], [80], [81], [82]]. Possible reasons behind this phenomenon would be increased O-GlcNAc transferase expression and decrease in O-GlcNacase expression and/or activity (Fig. 3). Indeed, the O-GalNAc synthesis seems to be increased in response to oxidative stress in breast cancer due to high expression of GalNAc-transferase 6 (GalNT6) and translocation of both GalNT2 and GalNT6 from cis-Golgi towards the trans-Golgi cisternae [83]. In addition, the GDP-fucose transporter was found to be regulated by TGF-β1 and the transcription factor SP-1, which themselves are under redox control [84].
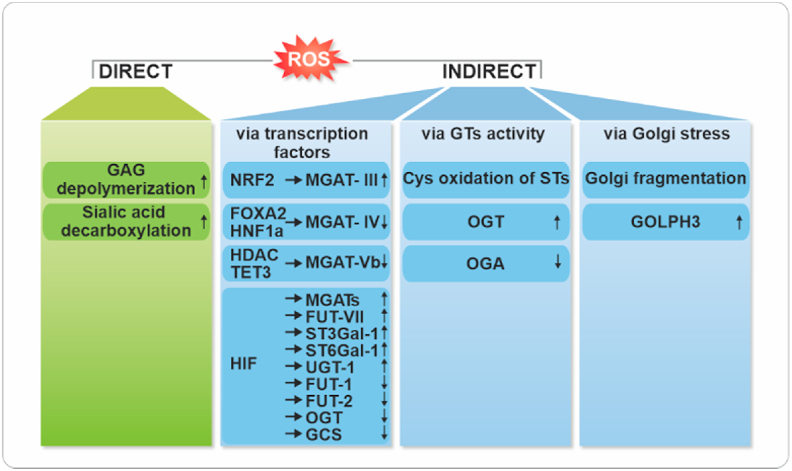
Fig. 3.
Summary of the direct and indirect effects of the redox system on glycan biosynthesis. Abbreviation: FUT: Fucosyltransferase, HIF: Hypoxia-inducible Factor, HDAC: Histone deactylase, HNF: Hepatocyte Nuclear Factors, GAG: Glycosaminoglycans, GCS: Glucosylceramide Synthase, GOLPH3: Golgi Phosphoprotein 3, MGAT: N-acetylglucosaminyltransferase, NRF2: Nuclear Factor Erythroid 2-Related Factor 2), OGT: O-GlcNAc Transferase, OGA: O-GlcNAcase, ST: Sialyltransferase, ST3GAL-1: Alpha-2,3-Sialyltransferase, ST6GAL-1: Alpha-2,6-Sialyltransferase, TET3: Methylcytosine Dioxygenase, UGT: Glucuronosyltransferase.
Whether the synthesis of other glycans containing O-mannose is influenced by ROS or oxidative stress is currently unknown as no reports are available; hence, the connections are yet to be established. However, the redox-stress mediated increase in bisecting GlcNAcs by upregulation of N-acetylglucosaminyltransferase III (MGAT-III) via Nrf2 [7] and reduced expression of N-acetylglucosaminyltransferase IV (MGAT-IV) [85] via FOXA2 and HNF1a, would reduce complex type glycans and sialic acid capping and favor high mannose type glycans which are found to be highly prevalent in cancers [86,87](Fig. 3). Since hypoxia, which is prevalent in most solid cancers, can alter the redox state of a cell, it is ultimately linked to changes in glycosylation. However, the mechanisms involved appear to be multiple and the details therein are still not fully understood. A recent study employing redox screens was able to show that hypoxia inhibits terminal sialylation of N- and O-linked glycans. This redox state change caused loss of two surface-exposed disulfide bonds in the catalytic domain of the α-2,6-sialyltransferase (ST6Gal-I). Mutagenesis of the selected cysteine residues in ST6Gal-I also rendered the enzyme inactive. Consequently, ST6Gal-I loss of function was accompanied by the inability to form a heteromer with B4GalT-I, an enzyme adding the preceding galactose to complex N-glycans and an advantage in proliferation and migration. Interestingly, structure comparisons revealed that similar disulfide bonds reside also in other sialyltransferases such as ST3Gal-I, suggesting that redox switches at discrete disulfide bonds of sialyltransferases contribute to catalytic activation and cooperative action of those enzymes [88].
As mentioned, the redox response is linked to the hypoxia response in which hypoxia-inducible factors (HIF-1,-2 and -3) are major regulators. HIFs appear to regulate hundreds of genes required to sustain a plethora of processes necessary for cellular homeostasis [89]. Surprisingly, the number of HIF-regulated genes among the about 250 genes needed to make all glycan structures in mammalian cells is so far rather small. Among those are genes encoding enzymes that make nucleotide sugars in the cytoplasm [90], as well as in the medial-Golgi such as MGAT-II, MGAT-III, MGAT-V, MGAT-Vb, and the trans-Golgi localized ST6Gal-1 [7]. Oxidative stress was shown to increase the transcription of fucosyltransferase VII (FUT7), sialyltransferase ST3Gal-I and transporters for UDP-Galactose, sialic acid (Sialin) and glucuronosyltransferase (UGT1) [91]. Further, HIF-1α suppressed the α1,2-fucosyltransferase genes (FUT1 and FUT2) in the pancreatic cancer cell lines Pa-Tu-8988S and Pa-Tu-8988T [92]. Likewise, the UDP-glucuronosyltransferases [93], the O-GlcNAc transferase (OGT) [94], and glucosylceramide synthase (GCS) [95] were downregulated by HIF-1α (Fig. 3). Thus, ROS, hypoxia and HIFs can modulate many glycosylation pathways via altering the availability of nucleotide sugars, the level of their transporters and the repertoire of transferases expressed by the cells.
7. Glyco-redox feedback
Vice versa, some factors that regulate the cellular response to ROS or are themselves redox sensitive, such as hypoxia/HIF [96,97], KEAP/NRF2 [[98], [99], [100], [101], [102]], FOXO [[103], [104], [105]], NF-κB [106] and p53 [107,108], can modulate or be modulated by the levels of O-GlcNAc glycosyltransferase (OGT) and O-GlcNAcase and their crosstalk with related molecules [109,110]. Furthermore, O-GlcNAcylation on discreet residues of RelA/p65 and IKKβ was found to increase the transactivation of NF-κB RelA/p65 [111,112]. Similar modifications were found in other transcription factors and co-factors such as N-glycosylation of nuclear factor 1 C-type (NFIC) [113], O-GlcNAc and N-glycosylation of cAMP response element-binding protein (CREB) [114,115], O-GlcNAcylation of c-JUN [116], O-GlcNAcylation Ets transcription factor (ELF1) [117], O-glycosylation NOTCH [118], and O-GlcNAcylation of Yes-associated protein (YAP) [119,120]. Indicating that they play important regulatory roles in cellular signaling, metabolism and tumorigenesis.
8. Glyco-redox in cardiovascular diseases
Oxidative stress occurs during heart and vascular dysfunction caused by various pathologies including hypertension, heart insults, atherosclerosis, inflammation, and obesity, as well as during senescence. These processes are also characterized by heart and vascular remodeling, fibrosis, calcification and altered expression of matrix metalloproteinases (MMPs) affecting both contractility and endothelial functions.
It is well established that NADPH oxidases (NOX) and ROS production have a principal role in cardiovascular diseases (reviewed in Ref. [121]). However, the glyco-redox interplay here is still in its infancy. Yet, some studies have demonstrated how glycans can influence ROS levels and induce cardiovascular damage. This was especially shown in the case of O-linked glycans which are present on signaling proteins involved in vascular functions, such as glutathione peroxidase 1 (GPX-1) under hyperglycemic conditions in rat vascular smooth muscle cells [122]. Thereby, it was shown that increased levels of O-GlcNAc led to endothelial dysfunction by increasing O2−• levels. Although the increased O2−• levels correlated with the expression of the NADPH oxidases NOX1 and NOX4 when aortic segments and vascular smooth muscle cells were treated with 1,5-hydroximolactone (PUGNac), an inhibitor of N-acetylhexosaminidases which artificially increases O-GlcNAc levels [123], mechanistic details about NOXs' involvement are still missing. In contrast, another study shows that increased O-GlcNAc levels, due to PUGNAc treatment or OGT transfection of neonatal rat cardiac myocytes, confer a protective effect by inhibiting Ca2+ overload and ROS production. As a consequence, cells were protected from mitochondrial permeability transition pore (mPTP) opening and loss of mitochondrial membrane potential [124]. Another recent study showed that experimentally induced oxidative stress achieved by application of H2O2 and buthionine sulfoximine (BSO) involves matrix metalloproteases (MMPs) and histone deacetylases (HDACs); both are known to be associated with cardiovascular diseases via the modification of endothelial cell surface glycan structures such as syndecans. Syndecans are heparan sulfate proteoglycans which bind to a variety of ligands and are involved in tissue repair. Although the effect of ROS is not direct and details remain unknown, it appeared that MMP and HDAC inhibitors attenuated H2O2 and BSO stimulated shedding and degradation of syndecans and that of the extracellular superoxide dimutase SOD3 from the endothelial glycocalyx [125]. Similar observations pointing to a correlation between oxidative stress and glycocalyx shedding through MMPs have been reported also in other studies [[126], [127], [128], [129], [130]] suggesting a common, yet unknown, mechanism. Furthermore, oxidative stress has been shown not only to affect glycans but also glycan binding lectins. Lectins are a family of carbohydrate-binding proteins; they recognize glycans on surfaces of cells and pathogenic glycoconjugates, which make them indispensable for cell-cell recognition and immunity. The lectin pathway is part of the immune complement system and is activated by mannose-binding lectins (MBL). Oxidative stress induced by reoxygenation of hypoxic HUVEC cells induced high levels of vascular endothelial MBL and cytokeratin 1 (CK1). Binding of MBL to the kinase CK1 triggered the lectin complement pathway (LCP) and C3 deposition which normally contributes to cardiovascular dysfunction and disease [131].
9. Glyco-redox in inflammation and immunity
ROS-mediated glycan changes closely associate with many inflammatory diseases and immune disorders. A vicious cycle appears to exist in the onset of type-2 diabetes, a disease that is due to its association with a high fat diet characterized by hyperglycemia and insulin resistance that lead to oxidative stress and inflammation. Chronic elevation of free fatty acids and increased production of ROS in mitochondria also attenuate insulin secretion in pancreatic β-cells, and thereby trigger the onset of type-2 diabetes [85]. Thereby, the excess of both glucose and free fatty acids together with high ROS levels, in particular in pancreatic beta-cells, were shown to inhibit the transcription factors FOXA2 and HNF1a. Both would normally induce expression of MGAT-IVa, a glycosyltransferase needed for the synthesis of a glucose transporter 2 (GLUT-2)-N‐glycan complex that provides GLUT-2 plasma membrane retention by enabling lectin‐glycan binding. Although the lectin-glycan binding is part of the immune complement system, impaired MGAT-IVa expression is not only affecting the complement pathway but also impairs GLUT‐2 endocytosis that will lead to subsequent diminished glucose transport into pancreatic β-cells and reduced insulin secretion, thus linking type-2 diabetes and inflammation.
Members of the redox machinery such as NOX1, which has been associated with the innate immune system, and immune diseases appear to display also an aberrant glycosylation in the presence of ROS. For instance, hypoglycosylation of neutrophilic NOX1, i.e., gp91phox, has been detected in patients suffering from neutrophil dysfunction and congenital neutropenia due to mutations in the glucose-6-phosphatase 3 gene, also known as glucose-6-phosphatase beta [132]. In that study, mass spectrometry analyses of the neutrophils' N- and O-glycomes showed truncated and immature complex-type N-glycans, reduced core 2 antennae O-glycans and deficiency in sialylated and lewis X epitopes. Although the exact mechanism for the hypoglycosylation remains unknown, it is thought that glycosylation may protect NOX1 from the content of phagocytic vacuoles, including ROS, or that glycosylation of NOX1 is necessary for heterodimer formation with p22phox [132,133]. Moreover, the cytochrome b558 complex, consisting of NOX1 and p22phox have been shown to be heavily glycosylated in Epstein Barr virus immortalized B-cells which prevents b558 to be fully active through a conformational constraint [134]. At the same time, glycosylation of B-cell-produced immunoglobulins such as IgG and IgA is fundamental for their function and is necessary for Fc receptors binding [135]. Immunoglobulins' sialylation is associated with reduced inflammation [136,137] while autoimmune disease patients usually lack galactose and sialic acid and exhibit truncated glycans on their IgGs and IgAs [138,139].
Another integral and potent component of macrophages is mannose binding lectin (MBL), which usually binds to mannoses on bacteria and viruses. It exhibits reduced levels under high oxidative stress on macrophages in the lungs due to chronic obstructive pulmonary disease (COPD) [140]. Structural analyses showed that oxidized MBL cannot form higher order oligomeric structures and disrupts macrophage activity. Although MBL is also known to be glycosylated [141], no connection to MBL oligomerization has been shown in this study. These examples show that hypo or hyperglycosylation could alter the lifetime and activity of crucial molecules involved in the immune response.
10. Glyco-redox in cancer and other redox-associated diseases
It is well established that enhanced ROS levels and oxidative stress are associated with tumorigenesis. High levels of ROS in cancer are a consequence of a changed metabolic activity, altered mitochondrial homeostasis and differential gene expressions. DNA damage, protein and lipid oxidation all increase the metastatic and proliferative cellular profiles which in turn can lead to further generation of ROS and activation of oncogenic pathways [1,142]. Glycosylation changes in cancer have been well documented especially changes in the tumor glycocalyx and their effects on adhesiveness, metastasis, immune evasion, growth factors and signaling pathways [6,143](Fig. 2). However, the glyco-redox link in cancer is not clearly defined. Hypoxia is considered a hallmark of cancer and HIF-1α promotes malignancy by triggering gene expressions involved in angiogenesis, glycolysis and proliferation [144]. High levels of α2-6 sialylation, ST6Gal-I levels and ROS in cancers have been associated with poor prognosis and both were shown to increase HIF-1α levels [145,146]. Thus, there exist a cancer-associated interplay between redox, HIF-1α and ST6Gal-I, whereby a tumor in a hypoxic microenvironment protects itself by upregulating HIF-1α and α2-6 sialylation which in turn increase cell migration, reduce cell-cell adhesion and promote cancer cell survival and invasivness [147]. Another possible link between redox stress and glycosylation in cancers can be found through Golgi stress (onco-Golgi) [148]. Golgi stress is manifested by Golgi fragmentation which has been shown to cause glycosyltransferases mislocalization and relocation, loss of oligomerization and activity and subsequently aberrant glycosylation (reviewed in Ref. [149]). For instance N-acetylcysteine (NAC) inhibiting compounds such as Geoditin, SOD1 mutations and microtubule oxidative damage, all contribute to Golgi damage and fragmentation (reviewed in Ref. [150]). In addition, inhibition of NOX1 and NOX4 by NOXi inhibitor combined with Golgi stressing compounds such as Brefeldin A (BFA) partially rescued Golgi fragmentation in HeLa or MDA-MB-231 cells [151]. Even though Golgi fragmentation in cancers have been attributed to altered Golgi pH [149], it is thus safe to propose that accumulation of ROS in cancers can distort the Golgi structure and integrity which usually results in glycosylation defects. Golgi fragmentation is not only seen in cancers but also in other diseases namely neurodegenerative diseases that are believed to be triggered by oxidative stress such as the Alzheimer's disease [152] in which decrease in sialylation and increase in high mannose glycan chains are observed [[153], [154], [155]]. Moreover, it has been shown recently that Golgi phosphoprotein-3 (GOLPH3), an enzyme implicated in the secretory pathway, is responsible for recycling of specific glycosyltransferases and retention is highly overexpressed in human cancer samples [156]. Interestingly, GOLPH3 was also linked to ROS as it was reported to be upregulated in neural cells upon oxygen-glucose deprivation and reoxygenation [157]. This indicates that GOLPH3 could act as a Golgi oxidative stress sensor. Consequently, its elevated expression in cancer could then increase oncogenic-related glycosyltransferases such as globoside synthase-3 (Gb3) [156]. The latter is known to contribute to uncontrolled cellular growth by modulating receptor tyrosine kinases and integrin receptors [158]. These findings are linked to changes in core fucosylation of growth factors such as TGF-β1 and increased MMP levels that are implicated in several cancers, such as hepatocellular, gastric, pancreatic, prostate, and colorectal cancers [159,160]. Thereby, the addition of fucose in α-1,6-linkage to the innermost N-acetyl glucosamine of N-glycans carried out by FUT8 seems to control TGF-β1 signaling and MMP function. Moreover, changes in core fucosylation would not only affect growth of cancer cells, but also normal development as knockout of FUT8 in mice leads to impaired lung development and to an emphysema-like phenotype [159,160].
To name a few more examples of the glyco-redox interrelation; there is accumulating evidence that glycans such as sialic acids and O-GlcNAcs are responsible for maintaining kidney functions and protection against ROS induced damage and which have been illustrated in multiple studies [[161], [162], [163], [164], [165], [166], [167], [168], [169], [170]]. For instance, N-acetylneuraminic acid has been shown to consume H2O2 and hydroxyl radicals in cells and consequently become oxidized into a decarboxylated product, 4-(acetylamino)-2,4-dideoxy-d-glycero-d-galacto-octonic acid (ADOA) [167,168]. Aberrant glycans also correlate with higher oxidative stress in diabetic and hypertensive patients and patients suffering from myopathies and neurodegenerative diseases [171,172].
11. Conclusion
Given their pan distribution on proteins and lipids as well as their diverse roles in development and basic cellular functions, glycans are involved in almost all essential pathways. ROS not only can trigger subtle genetic reprogramming and switch on or off signaling pathways affecting glycan synthesis but can also directly interact and degrade saccharide moieties. However, their interrelationship comes at a cost, that is, aberrant glycan synthesis gives rise to many congenital and complex disorders. Vice versa, glycans affect the redox-proteome by altering redox-proteins folding, interactions and activities. Although the relationship between glycans, ROS and oxidative stress is dynamic and extensive, it lacks still knowledge about detailed mechanisms involved.
12. Perspectives
The glyco-redox field is still in its infancy and requires a novel outlook to understand regulatory mechanisms and the effects ROS can cause through glycans or vice versa. From an omics perspective and with the robust development of mass spectrometry techniques, a glyco-redox MS concept could be developed to map the glycome, glycoproteome and the redoxome together in diseases [173,174]. Consequently, it would be necessary to investigate molecular mechanisms especially with the evolution of fluorescent, electron microscopes and single cell techniques [175,176]. In short, bridging the gap between glycobiology and redox biology will most likely reveal unexplored biological mechanisms that will clarify the relationship between the stressors such as free radicals and glycosylation-based diseases and promote the search for novel medical interventions.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors are grateful to all scientists who excellently contributed to the field and whose work could not be cited due to space limitations. Work in the TK lab was supported by the Academy of Finland SA296027, the Jane and Aatos Erkko Foundation, the Finnish Cancer Foundation, the Sigrid Jusélius Foundation, the University of Oulu, and Biocenter Oulu. Biocenter Oulu is a member of Biocenter Finland. We thank Maria Larsson, DO illustration! studio for the artwork.
References
- 1.Samoylenko A., Hossain J.A., Mennerich D., Kellokumpu S., Hiltunen J.K., Kietzmann T. Nutritional countermeasures targeting reactive oxygen species in cancer: from mechanisms to biomarkers and clinical evidence. Antioxidants Redox Signal. 2013;19:2157–2196. doi: 10.1089/ars.2012.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halliwell B., Gutteridge J.M.C. Oxford University Press; Oxford, New York: 2015. Free Radicals in Biology and Medicine. [Google Scholar]
- 3.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 4.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 5.Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P. 2017. Essentials of Glycobiology, the Consortium of Glycobiology Editors, La Jolla, California. [Google Scholar]
- 6.Schjoldager K.T., Narimatsu Y., Joshi H.J., Clausen H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020;21:729–749. doi: 10.1038/s41580-020-00294-x. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi N., Kizuka Y., Takamatsu S., Miyoshi E., Gao C. Glyco-redox, a link between oxidative stress and changes of glycans: lessons from research on glutathione, reactive oxygen and nitrogen species to glycobiology. Arch. Biochem. Biophys. 2016;595:72–80. doi: 10.1016/j.abb.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Brand M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Bedard K., Krause K. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 10.Knock G.A. NADPH oxidase in the vasculature: expression, regulation and signalling pathways; role in normal cardiovascular physiology and its dysregulation in hypertension. Free Radic. Biol. Med. 2019;145:385–427. doi: 10.1016/j.freeradbiomed.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 11.Parascandolo A., Laukkanen M.O. Carcinogenesis and reactive oxygen species signaling: interaction of the NADPH oxidase NOX1–5 and superoxide dismutase 1–3 signal transduction pathways. 2018;30:443–486. doi: 10.1089/ars.2017.7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higdon A., Diers A.R., Oh J.Y., Landar A., Darley-Usmar V.M. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem. J. 2012;442:453–464. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henzler T., Steudle E. Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H(2)O(2) across water channels. J. Exp. Bot. 2000;51:2053–2066. doi: 10.1093/jexbot/51.353.2053. [DOI] [PubMed] [Google Scholar]
- 16.Bienert G.P., Chaumont F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta. 2014;1840:1596–1604. doi: 10.1016/j.bbagen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Sies H. Academic Press; London: 1985. Oxidative Stress. [Google Scholar]
- 18.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro L., Tórtora V., Mansilla S., Radi R. Aconitases: non-redox iron-sulfur proteins sensitive to reactive species. Acc. Chem. Res. 2019;52:2609–2619. doi: 10.1021/acs.accounts.9b00150. [DOI] [PubMed] [Google Scholar]
- 20.Zeida A., Trujillo M., Ferrer-Sueta G., Denicola A., Estrin D.A., Radi R. Catalysis of peroxide reduction by fast reacting protein thiols. Chem. Rev. 2019;119:10829–10855. doi: 10.1021/acs.chemrev.9b00371. [DOI] [PubMed] [Google Scholar]
- 21.Poole L.B. The basics of thiols and cysteines in redox biology and chemistry, Free Radic. Biol. Med. 2015;80:148–157. doi: 10.1016/j.freeradbiomed.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dustin C.M., Heppner D.E., Lin M.J., van der Vliet A. Redox regulation of tyrosine kinase signalling: more than meets the eye. J. Biochem. 2020;167:151–163. doi: 10.1093/jb/mvz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truong T.H., Carroll K.S. Redox regulation of protein kinases. Crit. Rev. Biochem. Mol. Biol. 2013;48:332–356. doi: 10.3109/10409238.2013.790873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira-Marques V., Marinho H.S., Cyrne L., Antunes F. Role of hydrogen peroxide in NF-kappaB activation: from inducer to modulator. Antioxidants Redox Signal. 2009;11:2223–2243. doi: 10.1089/ars.2009.2601. [DOI] [PubMed] [Google Scholar]
- 25.Schreck R., Rieber P., Baeuerle P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halvey P.J., Hansen J.M., Johnson J.M., Go Y., Samali A., Jones D.P. Selective oxidative stress in cell nuclei by nuclear-targeted D-amino acid oxidase. Antioxidants Redox Signal. 2007;9:807–816. doi: 10.1089/ars.2007.1526. [DOI] [PubMed] [Google Scholar]
- 27.Waypa G.B., Smith K.A., Schumacker P.T. O2 sensing, mitochondria and ROS signaling: the fog is lifting, Mol. Aspect. Med. 2016;47–48:76–89. doi: 10.1016/j.mam.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandel N.S., McClintock D.S., Feliciano C.E., Wood T.M., Melendez J.A. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1 alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 29.Hinchy E.C., Gruszczyk A.V., Willows R., Navaratnam N., Hall A.R. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. J. Biol. Chem. 2018;293:17208–17217. doi: 10.1074/jbc.RA118.002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu G.Y., Sabatini D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020;21:183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eijkelenboom A., Burgering B.M.T. FOXOs: signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 32.Klotz L., Steinbrenner H. Cellular adaptation to xenobiotics: interplay between xenosensors, reactive oxygen species and FOXO transcription factors. Redox Biol. 2017;13:646–654. doi: 10.1016/j.redox.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peralta D., Bronowska A.K., Morgan B., Dóka É., Van Laer K. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation, Nat. Chem. Biol. 2015;11:156–163. doi: 10.1038/nchembio.1720. [DOI] [PubMed] [Google Scholar]
- 34.Liu B., Chen Y., St Clair D.K. ROS and p53: a versatile partnership. Free Radic. Biol. Med. 2008;44:1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oswald M.C.W., Garnham N., Sweeney S.T., Landgraf M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018;592:679–691. doi: 10.1002/1873-3468.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pei J., Li X., Li W., Gao Q., Zhang Y. Diurnal oscillations of endogenous H2O2 sustained by p66Shc regulate circadian clocks. Nat. Cell Biol. 2019;21:1553–1564. doi: 10.1038/s41556-019-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sbodio J.I., Snyder S.H., Paul B.D. Redox mechanisms in neurodegeneration: from disease outcomes to therapeutic opportunities. Antioxidants Redox Signal. 2019;30:1450–1499. doi: 10.1089/ars.2017.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Handy D.E., Loscalzo J. Responses to reductive stress in the cardiovascular system. Free Radic. Biol. Med. 2017;109:114–124. doi: 10.1016/j.freeradbiomed.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Incalza M.A., D'Oria R., Natalicchio A., Perrini S., Laviola L., Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Münzel T., Camici G.G., Maack C., Bonetti N.R., Fuster V., Kovacic J.C. Impact of oxidative stress on the heart and vasculature: Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 2017;70:212–229. doi: 10.1016/j.jacc.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah A.A., Sinha A.A. Oxidative stress and autoimmune skin disease. Eur. J. Dermatol. 2013;23:5–13. doi: 10.1684/ejd.2012.1884. [DOI] [PubMed] [Google Scholar]
- 42.Smallwood M.J., Nissim A., Knight A.R., Whiteman M., Haigh R., Winyard P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018;125:3–14. doi: 10.1016/j.freeradbiomed.2018.05.086. [DOI] [PubMed] [Google Scholar]
- 43.Kirkham P.A., Barnes P.J. Oxidative stress in COPD. Chest. 2013;144:266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 44.Watson J.D. Type 2 diabetes as a redox disease. Lancet. 2014;383:841–843. doi: 10.1016/S0140-6736(13)62365-X. [DOI] [PubMed] [Google Scholar]
- 45.Haeusler R.A., McGraw T.E., Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018;19:31–44. doi: 10.1038/nrm.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grunewald S., Matthijs G., Jaeken J. Congenital disorders of glycosylation: a review. Pediatr. Res. 2002;52:618–624. doi: 10.1203/00006450-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Reily C., Stewart T.J., Renfrow M.B., Novak J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019;15:346–366. doi: 10.1038/s41581-019-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moremen K.W., Tiemeyer M., Nairn A.V. Vertebrate protein glycosylation: diversity, synthesis and function. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roth J., Zuber C., Park S., Jang I., Lee Y. Protein N-glycosylation, protein folding, and protein quality control. Mol. Cell. 2010;30:497–506. doi: 10.1007/s10059-010-0159-z. [DOI] [PubMed] [Google Scholar]
- 50.Russo D., Capolupo L., Loomba J.S., Sticco L., D'Angelo G. Glycosphingolipid metabolism in cell fate specification. J. Cell Sci. 2018;131 doi: 10.1242/jcs.219204. [DOI] [PubMed] [Google Scholar]
- 51.Dube D.H., Bertozzi C.R. Glycans in cancer and inflammation–potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 52.Renkonen J., Tynninen O., Häyry P., Paavonen T., Renkonen R. Glycosylation might provide endothelial zip codes for organ-specific leukocyte traffic into inflammatory sites. Am. J. Pathol. 2002;161:543–550. doi: 10.1016/S0002-9440(10)64210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carlin A.F., Uchiyama S., Chang Y.C., Lewis A.L., Nizet V., Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crocker P.R., Paulson J.C., Varki A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 55.Munkley J., Elliott D.J. Hallmarks of glycosylation in cancer. Oncotarget. 2016;7:35478–35489. doi: 10.18632/oncotarget.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinho S.S., Reis C.A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Canc. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 57.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7:599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moriyama H., Nakano H., Igawa M., Nihira H. T antigen expression in benign hyperplasia and adenocarcinoma of the prostate. Urol. Int. 1987;42:120–123. doi: 10.1159/000281868. [DOI] [PubMed] [Google Scholar]
- 59.Wolf M.F., Ludwig A., Fritz P., Schumacher K. Increased expression of Thomsen-Friedenreich antigens during tumor progression in breast cancer patients. Tumour Biol. 1988;9:190–194. doi: 10.1159/000217561. [DOI] [PubMed] [Google Scholar]
- 60.Lloyd K.O., Burchell J., Kudryashov V., Yin B.W., Taylor-Papadimitriou J. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J. Biol. Chem. 1996;271:33325–33334. doi: 10.1074/jbc.271.52.33325. [DOI] [PubMed] [Google Scholar]
- 61.D'Angelo G., Capasso S., Sticco L., Russo D. Glycosphingolipids: synthesis and functions. FEBS J. 2013;280:6338–6353. doi: 10.1111/febs.12559. [DOI] [PubMed] [Google Scholar]
- 62.Russo D., Della Ragione F., Rizzo R., Sugiyama E., Scalabrì F. Glycosphingolipid metabolic reprogramming drives neural differentiation. EMBO J. 2018;37 doi: 10.15252/embj.201797674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Funderburgh J.L. Keratan sulfate: structure, biosynthesis, and function. 2000;10:951–958. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- 64.Wagner D.D. Cell biology of von Willebrand factor. Annu. Rev. Cell Biol. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- 65.Chiu J., Hogg P.J. Allosteric disulfides: sophisticated molecular structures enabling flexible protein regulation. J. Biol. Chem. 2019;294:2949–2960. doi: 10.1074/jbc.REV118.005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mesecke N., Spang A., Deponte M., Herrmann J.M. A novel group of glutaredoxins in the cis-Golgi critical for oxidative stress resistance. Mol. Biol. Cell. 2008;19:2673–2680. doi: 10.1091/mbc.E07-09-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamieh A., Cartier D., Abid H., Calas A., Burel C. Selenoprotein T is a novel OST subunit that regulates UPR signaling and hormone secretion. EMBO Rep. 2017;18:1935–1946. doi: 10.15252/embr.201643504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parsons B.J. Oxidation of glycosaminoglycans by free radicals and reactive oxidative species: a review of investigative methods, Free Radic. Res. 2015;49:618–632. doi: 10.3109/10715762.2014.985220. [DOI] [PubMed] [Google Scholar]
- 69.Fuchs B., Schiller J. Glycosaminoglycan degradation by selected reactive oxygen species. Antioxidants Redox Signal. 2014;21:1044–1062. doi: 10.1089/ars.2013.5634. [DOI] [PubMed] [Google Scholar]
- 70.Burkhardt H., Schwingel M., Menninger H., Macartney H.W., Tschesche H. Oxygen radicals as effectors of cartilage destruction. Direct degradative effect on matrix components and indirect action via activation of latent collagenase from polymorphonuclear leukocytes. Arthritis Rheum. 1986;29:379–387. doi: 10.1002/art.1780290311. [DOI] [PubMed] [Google Scholar]
- 71.Auer D.E., Ng J.C., Seawright A.A. Effect of palosein (superoxide dismutase) and catalase upon oxygen derived free radical induced degradation of equine synovial fluid. Equine Vet. J. 1990;22:13–17. doi: 10.1111/j.2042-3306.1990.tb04195.x. [DOI] [PubMed] [Google Scholar]
- 72.Roberts C.R., Roughley P.J., Mort J.S. Degradation of human proteoglycan aggregate induced by hydrogen peroxide. Protein fragmentation, amino acid modification and hyaluronic acid cleavage. Biochem. J. 1989;259:805–811. doi: 10.1042/bj2590805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schiller J., Arnhold J., Arnold K. NMR studies of the action of hypochlorous acid on native pig articular cartilage. Eur. J. Biochem. 1995;233:672–676. doi: 10.1111/j.1432-1033.1995.672_2.x. [DOI] [PubMed] [Google Scholar]
- 74.Baker M.S., Green S.P., Lowther D.A. Changes in the viscosity of hyaluronic acid after exposure to a myeloperoxidase-derived oxidant. Arthritis Rheum. 1989;32:461–467. doi: 10.1002/anr.1780320416. [DOI] [PubMed] [Google Scholar]
- 75.Eguchi H., Ikeda Y., Ookawara T., Koyota S., Fujiwara N. Modification of oligosaccharides by reactive oxygen species decreases sialyl lewis x-mediated cell adhesion. Glycobiology. 2005;15:1094–1101. doi: 10.1093/glycob/cwj003. [DOI] [PubMed] [Google Scholar]
- 76.Chen P., Chi J., Boyce M. Functional crosstalk among oxidative stress and O-GlcNAc signaling pathways. Glycobiology. 2018;28:556–564. doi: 10.1093/glycob/cwy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zachara N.E., O'Donnell N., Cheung W.D., Mercer J.J., Marth J.D., Hart G.W. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells, J. Biol. Chem. 2004;279:30133–30142. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 78.Zachara N.E., Hart G.W. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim. Biophys. Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 79.Groves J.A., Lee A., Yildirir G., Zachara N.E. Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperones. 2013;18:535–558. doi: 10.1007/s12192-013-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reeves R.A., Lee A., Henry R., Zachara N.E. Characterization of the specificity of O-GlcNAc reactive antibodies under conditions of starvation and stress. Anal. Biochem. 2014;457:8–18. doi: 10.1016/j.ab.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kátai E., Pál J., Poór V.S., Purewal R., Miseta A., Nagy T. Oxidative stress induces transient O-GlcNAc elevation and tau dephosphorylation in SH-SY5Y cells. J. Cell Mol. Med. 2016;20:2269–2277. doi: 10.1111/jcmm.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee A., Miller D., Henry R., Paruchuri V.D.P., O'Meally R.N. Combined antibody/lectin enrichment identifies extensive changes in the O-GlcNAc sub-proteome upon oxidative stress. J. Proteome Res. 2016;15:4318–4336. doi: 10.1021/acs.jproteome.6b00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kurze A., Buhs S., Eggert D., Oliveira-Ferrer L., Müller V. Immature O-glycans recognized by the macrophage glycoreceptor CLEC10A (MGL) are induced by 4-hydroxy-tamoxifen, oxidative stress and DNA-damage in breast cancer cells. Cell Commun. Signal. 2019;17:107. doi: 10.1186/s12964-019-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu Y., Ma A., Liu L. Transforming growth factor β signaling upregulates the expression of human GDP-fucose transporter by activating transcription factor Sp1. PloS One. 2013;8:e74424. doi: 10.1371/journal.pone.0074424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ohtsubo K., Chen M.Z., Olefsky J.M., Marth J.D. Pathway to diabetes through attenuation of pancreatic beta cell glycosylation and glucose transport. Nat. Med. 2011;17:1067–1075. doi: 10.1038/nm.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Leoz M.L.A., Young L.J.T., Joo An H., Kronewitter S.R., Kim J. High-mannose glycans are elevated during breast cancer progression. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M110.002717. M110.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park D.D., Phoomak C., Xu G., Olney L.P., Tran K.A. Metastasis of cholangiocarcinoma is promoted by extended high-mannose glycans. Proc. Natl. Acad. Sci. U. S. A. 2020;117:7633–7644. doi: 10.1073/pnas.1916498117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hassinen A., Khoder-Agha F., Khosrowabadi E., Mennerich D., Harrus D. A Golgi-associated redox switch regulates catalytic activation and cooperative functioning of ST6Gal-I with B4GalT-I, Redox Biol. 2019;24:101182. doi: 10.1016/j.redox.2019.101182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Semenza G.L. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 90.Shirato K., Nakajima K., Korekane H., Takamatsu S., Gao C. Hypoxic regulation of glycosylation via the N-acetylglucosamine cycle. J. Clin. Biochem. Nutr. 2011;48:20–25. doi: 10.3164/jcbn.11-015FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koike T., Kimura N., Miyazaki K., Yabuta T., Kumamoto K. Hypoxia induces adhesion molecules on cancer cells: a missing link between Warburg effect and induction of selectin-ligand carbohydrates, Proc. Natl. Acad. Sci. U. S. A. 2004;101:8132–8137. doi: 10.1073/pnas.0402088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Belo A.I., van Vliet S.J., Maus A., Laan L.C., Nauta T.D. Hypoxia inducible factor 1α down regulates cell surface expression of α1,2-fucosylated glycans in human pancreatic adenocarcinoma cells. FEBS Lett. 2015;589:2359–2366. doi: 10.1016/j.febslet.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 93.Kato R., Matsura A., Kamiya R., Oishi C., Kagawa Y. Effect of hypoxia on UDP-glucuronosyl transferase mRNA expression in human hepatocarcinoma functional liver celL 4 cell line. Pharmazie. 2016;71:152–153. [PubMed] [Google Scholar]
- 94.Liu H., Wang Z., Yu S., Xu J. Proteasomal degradation of O-GlcNAc transferase elevates hypoxia-induced vascular endothelial inflammatory response†. Cardiovasc. Res. 2014;103:131–139. doi: 10.1093/cvr/cvu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao H., Miller M., Pfeiffer K., Buras J.A., Stahl G.L. Anoxia and reoxygenation of human endothelial cells decrease ceramide glucosyltransferase expression and activates caspases. Faseb. J. 2003;17:723–724. doi: 10.1096/fj.02-0806fje. [DOI] [PubMed] [Google Scholar]
- 96.Ngoh G.A., Watson L.J., Facundo H.T., Jones S.P. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40:895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferrer C.M., Lynch T.P., Sodi V.L., Falcone J.N., Schwab L.P. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol. Cell. 2014;54:820–831. doi: 10.1016/j.molcel.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chu C., Lo P., Yeh Y., Hsu P., Peng S. O-GlcNAcylation regulates EZH2 protein stability and function. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1355–1360. doi: 10.1073/pnas.1323226111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Andres L.M., Blong I.W., Evans A.C., Rumachik N.G., Yamaguchi T. Chemical modulation of protein O-GlcNAcylation via OGT inhibition promotes human neural cell differentiation. ACS Chem. Biol. 2017;12:2030–2039. doi: 10.1021/acschembio.7b00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang A.C., Jensen E.H., Rexach J.E., Vinters H.V., Hsieh-Wilson L.C. Loss of O-GlcNAc glycosylation in forebrain excitatory neurons induces neurodegeneration. Proc. Natl. Acad. Sci. U. S. A. 2016;113:15120–15125. doi: 10.1073/pnas.1606899113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arambašić J., Mihailović M., Uskoković A., Dinić S., Grdović N. Alpha-lipoic acid upregulates antioxidant enzyme gene expression and enzymatic activity in diabetic rat kidneys through an O-GlcNAc-dependent mechanism. Eur. J. Nutr. 2013;52:1461–1473. doi: 10.1007/s00394-012-0452-z. [DOI] [PubMed] [Google Scholar]
- 102.Dinić S., Arambašić J., Mihailović M., Uskoković A., Grdović N. Decreased O-GlcNAcylation of the key proteins in kinase and redox signalling pathways is a novel mechanism of the beneficial effect of α-lipoic acid in diabetic liver. Br. J. Nutr. 2013;110:401–412. doi: 10.1017/S0007114512005429. [DOI] [PubMed] [Google Scholar]
- 103.Hanover J.A., Forsythe M.E., Hennessey P.T., Brodigan T.M., Love D.C. A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11266–11271. doi: 10.1073/pnas.0408771102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee J., Kim K., Lee J., Paik Y. Regulation of dauer formation by O-GlcNAcylation in Caenorhabditis elegans. J. Biol. Chem. 2010;285:2930–2939. doi: 10.1074/jbc.M109.022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Love D.C., Ghosh S., Mondoux M.A., Fukushige T., Wang P. Dynamic O-GlcNAc cycling at promoters of Caenorhabditis elegans genes regulating longevity, stress, and immunity. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7413–7418. doi: 10.1073/pnas.0911857107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen P., Smith T.J., Wu J., Siesser P.F., Bisnett B.J. Glycosylation of KEAP1 links nutrient sensing to redox stress signaling. EMBO J. 2017;36:2233–2250. doi: 10.15252/embj.201696113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang W.H., Kim J.E., Nam H.W., Ju J.W., Kim H.S. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat. Cell Biol. 2006;8:1074–1083. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- 108.Shtraizent N., DeRossi C., Nayar S., Sachidanandam R., Katz L.S. MPI depletion enhances O-GlcNAcylation of p53 and suppresses the Warburg effect. Elife. 2017;6 doi: 10.7554/eLife.22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peternelj T.T., Marsh S.A., Strobel N.A., Matsumoto A., Briskey D. Glutathione depletion and acute exercise increase O-GlcNAc protein modification in rat skeletal muscle. Mol. Cell. Biochem. 2015;400:265–275. doi: 10.1007/s11010-014-2283-0. [DOI] [PubMed] [Google Scholar]
- 110.Groves J.A., Maduka A.O., O'Meally R.N., Cole R.N., Zachara N.E. Fatty acid synthase inhibits the O-GlcNAcase during oxidative stress. J. Biol. Chem. 2017;292:6493–6511. doi: 10.1074/jbc.M116.760785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang W.H., Park S.Y., Nam H.W., Kim D.H., Kang J.G. NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17345–17350. doi: 10.1073/pnas.0806198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Allison D.F., Wamsley J.J., Kumar M., Li D., Gray L.G. Modification of RelA by O-linked N-acetylglucosamine links glucose metabolism to NF-κB acetylation and transcription. Proc. Natl. Acad. Sci. U. S. A. 2012;109:16888–16893. doi: 10.1073/pnas.1208468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kane R., Murtagh J., Finlay D., Marti A., Jaggi R. Transcription factor NFIC undergoes N-glycosylation during early mammary gland involution. J. Biol. Chem. 2002;277:25893–25903. doi: 10.1074/jbc.M202469200. [DOI] [PubMed] [Google Scholar]
- 114.Chan C., Mak T., Chin K., Ng I.O., Jin D. N-linked glycosylation is required for optimal proteolytic activation of membrane-bound transcription factor CREB-H. J. Cell Sci. 2010;123:1438–1448. doi: 10.1242/jcs.067819. [DOI] [PubMed] [Google Scholar]
- 115.Lamarre-Vincent N., Hsieh-Wilson L.C. Dynamic glycosylation of the transcription factor CREB: a potential role in gene regulation. J. Am. Chem. Soc. 2003;125:6612–6613. doi: 10.1021/ja028200t. [DOI] [PubMed] [Google Scholar]
- 116.Chen Y., Zhu G., Liu Y., Wu Q., Zhang X. O-GlcNAcylated c-Jun antagonizes ferroptosis via inhibiting GSH synthesis in liver cancer. Cell. Signal. 2019;63:109384. doi: 10.1016/j.cellsig.2019.109384. [DOI] [PubMed] [Google Scholar]
- 117.Tsokos G.C., Nambiar M.P., Juang Y. Activation of the Ets transcription factor Elf-1 requires phosphorylation and glycosylation: defective expression of activated Elf-1 is involved in the decreased TCR zeta chain gene expression in patients with systemic lupus erythematosus. Ann. N. Y. Acad. Sci. 2003;987:240–245. doi: 10.1111/j.1749-6632.2003.tb06054.x. [DOI] [PubMed] [Google Scholar]
- 118.Varshney S., Stanley P. Multiple roles for O-glycans in Notch signalling. FEBS Lett. 2018;592:3819–3834. doi: 10.1002/1873-3468.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang X., Qiao Y., Wu Q., Chen Y., Zou S. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis, Nat. Commun. 2017;8:15280. doi: 10.1038/ncomms15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jackson S.P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988;55:125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- 121.Guzik T.J., Touyz R.M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70:660–667. doi: 10.1161/HYPERTENSIONAHA.117.07802. [DOI] [PubMed] [Google Scholar]
- 122.Yang W.H., Park S.Y., Ji S., Kang J.G., Kim J. O-GlcNAcylation regulates hyperglycemia-induced GPX1 activation. Biochem. Biophys. Res. Commun. 2010;391:756–761. doi: 10.1016/j.bbrc.2009.11.133. [DOI] [PubMed] [Google Scholar]
- 123.Souza-Silva L., Alves-Lopes R., Silva Miguez J., Dela Justina V., Neves K.B. Glycosylation with O-linked β-N-acetylglucosamine induces vascular dysfunction via production of superoxide anion/reactive oxygen species. Can. J. Physiol. Pharmacol. 2018;96:232–240. doi: 10.1139/cjpp-2017-0225. [DOI] [PubMed] [Google Scholar]
- 124.Ngoh G.A., Watson L.J., Facundo H.T., Jones S.P. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40:895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ali M.M., Mahmoud A.M., Le Master E., Levitan I., Phillips S.A. Role of matrix metalloproteinases and histone deacetylase in oxidative stress-induced degradation of the endothelial glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H647–H663. doi: 10.1152/ajpheart.00090.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lipowsky H.H., Lescanic A. The effect of doxycycline on shedding of the glycocalyx due to reactive oxygen species. Microvasc. Res. 2013;90:80–85. doi: 10.1016/j.mvr.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lipowsky H.H., Gao L., Lescanic A. Shedding of the endothelial glycocalyx in arterioles, capillaries, and venules and its effect on capillary hemodynamics during inflammation. Am. J. Physiol. Heart Circ. Physiol. 2011;301:2235. doi: 10.1152/ajpheart.00803.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nieuwdorp M., Mooij H.L., Kroon J., Atasever B., Spaan J.A.E. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55:1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 129.Marechal X., Favory R., Joulin O., Montaigne D., Hassoun S. Endothelial glycocalyx damage during endotoxemia coincides with microcirculatory dysfunction and vascular oxidative stress. Shock. 2008;29:572–576. doi: 10.1097/SHK.0b013e318157e926. [DOI] [PubMed] [Google Scholar]
- 130.Singh A., Ramnath R.D., Foster R.R., Wylie E.C., Fridén V. Reactive oxygen species modulate the barrier function of the human glomerular endothelial glycocalyx. PloS One. 2013;8:e55852. doi: 10.1371/journal.pone.0055852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Collard C.D., Montalto M.C., Reenstra W.R., Buras J.A., Stahl G.L. Endothelial oxidative stress activates the lectin complement pathway: role of cytokeratin 1. Am. J. Pathol. 2001;159:1045–1054. doi: 10.1016/S0002-9440(10)61779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hayee B., Antonopoulos A., Murphy E.J., Rahman F.Z., Sewell G. G6PC3 mutations are associated with a major defect of glycosylation: a novel mechanism for neutrophil dysfunction. Glycobiology. 2011;21:914–924. doi: 10.1093/glycob/cwr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qureshi O.S., Paramasivam A., Yu J.C.H., Murrell-Lagnado R.D. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J. Cell Sci. 2007;120:3838–3849. doi: 10.1242/jcs.010348. [DOI] [PubMed] [Google Scholar]
- 134.Paclet M., Coleman A.W., Burritt J., Morel F. NADPH oxidase of Epstein–Barr-virus immortalized B lymphocytes. 2001;268:5197–5208. doi: 10.1046/j.0014-2956.2001.02455.x. [DOI] [PubMed] [Google Scholar]
- 135.Kobata A. The N-linked sugar chains of human immunoglobulin G: their unique pattern, and their functional roles, Biochim. Biophys. Acta. 2008;1780:472–478. doi: 10.1016/j.bbagen.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 136.Kaneko Y., Nimmerjahn F., Ravetch J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 137.Anthony R.M., Nimmerjahn F., Ashline D.J., Reinhold V.N., Paulson J.C., Ravetch J.V. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nishie T., Miyaishi O., Azuma H., Kameyama A., Naruse C. Development of immunoglobulin A nephropathy- like disease in beta-1,4-galactosyltransferase-I-deficient mice. Am. J. Pathol. 2007;170:447–456. doi: 10.2353/ajpath.2007.060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Parekh R.B., Dwek R.A., Sutton B.J., Fernandes D.L., Leung A. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 140.Tran H.B., Ahern J., Hodge G., Holt P., Dean M.M. Oxidative stress decreases functional airway mannose binding lectin in COPD. PloS One. 2014;9:e98571. doi: 10.1371/journal.pone.0098571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Colley K.J., Baenziger J.U. Identification of the post-translational modifications of the core-specific lectin. The core-specific lectin contains hydroxyproline, hydroxylysine, and glucosylgalactosylhydroxylysine residues, J. Biol. Chem. 1987;262:10290–10295. [PubMed] [Google Scholar]
- 142.Gupta S.C., Hevia D., Patchva S., Park B., Koh W., Aggarwal B.B. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxidants Redox Signal. 2012;16:1295–1322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pinho S.S., Reis C.A. Glycosylation in cancer: mechanisms and clinical implications. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 144.Semenza G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lacher S.E., Levings D.C., Freeman S., Slattery M. Identification of a functional antioxidant response element at the HIF1A locus. Redox Biol. 2018;19:401–411. doi: 10.1016/j.redox.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jones R.B., Dorsett K.A., Hjelmeland A.B., Bellis S.L. The ST6Gal-I sialyltransferase protects tumor cells against hypoxia by enhancing HIF-1α signaling. J. Biol. Chem. 2018;293:5659–5667. doi: 10.1074/jbc.RA117.001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lin S., Kemmner W., Grigull S., Schlag P.M. Cell surface alpha 2,6 sialylation affects adhesion of breast carcinoma cells. Exp. Cell Res. 2002;276:101–110. doi: 10.1006/excr.2002.5521. [DOI] [PubMed] [Google Scholar]
- 148.Petrosyan A. Onco-golgi: is fragmentation a gate to cancer progression? Biochem Mol Biol J. 2015;1 doi: 10.21767/2471-8084.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kellokumpu S. Golgi pH, ion and redox homeostasis: how much do they really matter? Front Cell Dev Biol. 2019;7:93. doi: 10.3389/fcell.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jiang Z., Hu Z., Zeng L., Lu W., Zhang H. The role of the Golgi apparatus in oxidative stress: is this organelle less significant than mitochondria? Free Radic. Biol. Med. 2011;50:907–917. doi: 10.1016/j.freeradbiomed.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 151.Alborzinia H., Ignashkova T.I., Dejure F.R., Gendarme M., Theobald J. Golgi stress mediates redox imbalance and ferroptosis in human cells. 2018;1:1–15. doi: 10.1038/s42003-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Santos R., Ruiz de Almodóvar C., Bulteau A., Gomes C.M. Neurodegeneration, neurogenesis, and oxidative stress. Oxid Med Cell Longev. 2013;2013:730581. doi: 10.1155/2013/730581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Joshi G., Bekier M.I., Wang Y. Golgi fragmentation in Alzheimer's disease. Front. Neurosci. 2015;9 doi: 10.3389/fnins.2015.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schedin-Weiss S., Winblad B., Tjernberg L.O. The role of protein glycosylation in Alzheimer disease. 2014;281:46–62. doi: 10.1111/febs.12590. [DOI] [PubMed] [Google Scholar]
- 155.Wang J., Grundke-Iqbal I., Iqbal K. Glycosylation of microtubule–associated protein tau: an abnormal posttranslational modification in Alzheimer's disease. 1996;2:871–875. doi: 10.1038/nm0896-871. [DOI] [PubMed] [Google Scholar]
- 156.Rizzo R., Russo D., Kurokawa K., Sahu P., Lombardi B. 2019. Retrograde transport of Golgi enzymes by GOLPH3 across maturing cisternae regulates glycan assembly on sphingolipids and cell growth; p. 870477. [Google Scholar]
- 157.Li T., You H., Mo X., He W., Tang X. GOLPH3 mediated Golgi stress response in modulating N2A cell death upon oxygen-glucose deprivation and reoxygenation injury. Mol. Neurobiol. 2016;53:1377–1385. doi: 10.1007/s12035-014-9083-0. [DOI] [PubMed] [Google Scholar]
- 158.Hakomori S. The glycosynapse. Proc. Natl. Acad. Sci. Unit. States Am. 2002;99:225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang X., Inoue S., Gu J., Miyoshi E., Noda K. Dysregulation of TGF-beta 1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15791–15796. doi: 10.1073/pnas.0507375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang X., Gu J., Miyoshi E., Honke K., Taniguchi N. Phenotype changes of Fut8 knockout mouse: core fucosylation is crucial for the function of growth factor receptor(s) Methods Enzymol. 2006;417:11–22. doi: 10.1016/S0076-6879(06)17002-0. [DOI] [PubMed] [Google Scholar]
- 161.Kerjaschki D., Sharkey D.J., Farquhar M.G. Identification and characterization of podocalyxin–the major sialoprotein of the renal glomerular epithelial cell. J. Cell Biol. 1984;98:1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mohos S.C., Skoza L. Glomerular sialoprotein. Science. 1969;164:1519–1521. doi: 10.1126/science.164.3887.1519. [DOI] [PubMed] [Google Scholar]
- 163.Holthöfer H., Virtanen I., Pettersson E., Törnroth T., Alfthan O. Lectins as fluorescence microscopic markers for saccharides in the human kidney. Lab. Invest. 1981;45:391–399. [PubMed] [Google Scholar]
- 164.Murata F., Tsuyama S., Suzuki S., Hamada H., Ozawa M., Muramatsu T. Distribution of glycoconjugates in the kidney studied by use of labeled lectins 1. J. Histochem. Cytochem. 1983;31:139–144. doi: 10.1177/31.1A_SUPPL.6186720. [DOI] [PubMed] [Google Scholar]
- 165.Gelberg H., Healy L., Whiteley H., Miller L.A., Vimr E. In vivo enzymatic removal of alpha 2-->6-linked sialic acid from the glomerular filtration barrier results in podocyte charge alteration and glomerular injury. Lab. Invest. 1996;74:907–920. [PubMed] [Google Scholar]
- 166.Muchitsch E., Pichler L., Schwarz H.P., Ulrich W. Effects of human alpha-1-acid glycoprotein on aminonucleoside-induced minimal change nephrosis in rats. Nephron. 1999;81:194–199. doi: 10.1159/000045276. [DOI] [PubMed] [Google Scholar]
- 167.Iijima R., Takahashi H., Namme R., Ikegami S., Yamazaki M. Novel biological function of sialic acid (N-acetylneuraminic acid) as a hydrogen peroxide scavenger. FEBS Lett. 2004;561:163–166. doi: 10.1016/S0014-5793(04)00164-4. [DOI] [PubMed] [Google Scholar]
- 168.Ogasawara Y., Namai T., Yoshino F., Lee M., Ishii K. Sialic acid is an essential moiety of mucin as a hydroxyl radical scavenger. FEBS Lett. 2007;581:2473–2477. doi: 10.1016/j.febslet.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 169.Pawluczyk I.Z.A., Ghaderi Najafabadi M., Patel S., Desai P., Vashi D. Sialic acid attenuates puromycin aminonucleoside-induced desialylation and oxidative stress in human podocytes. Exp. Cell Res. 2014;320:258–268. doi: 10.1016/j.yexcr.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 170.Hu J., Chen R., Jia P., Fang Y., Liu T. Augmented O-GlcNAc signaling via glucosamine attenuates oxidative stress and apoptosis following contrast-induced acute kidney injury in rats. Free Radic. Biol. Med. 2017;103:121–132. doi: 10.1016/j.freeradbiomed.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 171.Cho A., Christine M., Malicdan V., Miyakawa M., Nonaka I. Sialic acid deficiency is associated with oxidative stress leading to muscle atrophy and weakness in GNE myopathy. Hum. Mol. Genet. 2017;26:3081–3093. doi: 10.1093/hmg/ddx192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shahvali S., Shahesmaeili A., Sanjari M., Karami-Mohajeri S. The correlation between blood oxidative stress and sialic acid content in diabetic patients with nephropathy, hypertension, and hyperlipidemia. Diabetol Int. 2020;11:19–26. doi: 10.1007/s13340-019-00395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Steentoft C., Vakhrushev S.Y., Joshi H.J., Kong Y., Vester-Christensen M.B. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32:1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Held J.M. Redox systems biology: harnessing the sentinels of the cysteine redoxome. Antioxidants Redox Signal. 2020;32:659–676. doi: 10.1089/ars.2019.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen B., Legant W.R., Wang K., Shao L., Milkie D.E. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science. 2014;346:1257998. doi: 10.1126/science.1257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shah S., Lubeck E., Zhou W., Cai L. In situ transcription profiling of single cells reveals spatial organization of cells in the mouse Hippocampus. Neuron. 2016;92:342–357. doi: 10.1016/j.neuron.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]