Abstract
Pharmacological targeting of mitochondrial ion channels is emerging as a promising approach to eliminate cancer cells; as most of these channels are differentially expressed and/or regulated in cancer cells in comparison to healthy ones, this strategy may selectively eliminate the former. Perturbation of ion fluxes across the outer and inner membranes is linked to alterations of redox state, membrane potential and bioenergetic efficiency. This leads to indirect modulation of oxidative phosphorylation, which is/may be fundamental for both cancer and cancer stem cell survival. Furthermore, given the crucial contribution of mitochondria to intrinsic apoptosis, modulation of their ion channels leading to cytochrome c release may be of great advantage in case of resistance to drugs triggering apoptotic events upstream of the mitochondrial phase. In the present review, we give an overview of the known mitochondrial ion channels and of their modulators capable of killing cancer cells. In addition, we discuss state-of-the-art strategies using mitochondriotropic drugs or peptide-based approaches allowing a more efficient and selective targeting of mitochondrial ion channel-linked events.
Keywords: Mitochondria, Ion channels, Cancer, Drug targeting, Channel interactions
1. Introduction
Mitochondrial involvement in cancer physiology is multi-faceted (reviews e.g. Refs. [[1], [2], [3], [4], [5]]). All other aspects aside, these organelles have a number of tightly regulated ion channels in both the outer and inner membranes [6]. These pores are emerging as important oncological targets, since their pharmacological modulation may directly affect the release of cytochrome c, which is the point of no return during mitochondrial apoptosis. Many chemotherapeutic drugs trigger programmed cell death by inducing damage to DNA and subsequent activation of p53-dependent pathways that lead to migration of pro-apoptotic Bcl-2 family members to the outer mitochondrial membrane (MOM) and subsequent MOM permeabilization (MOMP) leading to cytochrome c release from the mitochondrial inter-membrane space. By acting on mitochondrial channels, especially on those of the inner membrane (MIM), it is possible to bypass p53-related events. This is of great importance, since many types of cancer develop chemo-resistance due to mutations in p53 and/or upregulation of anti-apoptotic proteins or downregulation of pro-apoptotic proteins. MOM channels were shown to play a crucial role for MOMP, via hetero-oligomerization with pro-apoptotic Bax and Bak or anti-apoptotic Bcl-2 [7,8], while MIM ion channels modulate sensitivity to apoptotic stimuli by affecting the mitochondrial membrane potential. An alteration of the MIM potential (Δψ) (around −180 mV) changes the driving force for calcium entry into the matrix: above a critical threshold, the matrix-accumulated calcium triggers opening of the so-called mitochondrial permeability transition pore (MPTP) and this leads to swelling of the organelle, rupture of the MOM and release of pro-apoptotic factors from the inter-membrane space.
MPTP opening can be aided also by oxidative stress, which develops as a consequence of either depolarization or hyperpolarization of the MIM [9]. Mitochondria constitute the redox hub of any cell, and the major source of the reactive oxygen species (ROS) which play such diverse and fundamental roles in cancer (e.g.: revs [1,10,11]). Many “mitocans”, i.e. compounds that contrast cancer by acting on mitochondria [[12], [13], [14], [15]], kill cancerous cells because they elicit a ROS production incompatible with the cell's survival (for reviews see e.g. Refs. [12,14,16,17]). Cancerous cells are especially sensitive to hyper-generation of ROS because they are under oxidative stress to start with [18,19], and this strategy is achieving practical relevance in the clinic (e.g. Refs. [20,21]).
For the above reasons, over the past few decades, more and more groups have focused on the pharmacological targeting of MPTP but also of other channels, whose modulation may trigger cytochrome c release from mitochondria and/or promote other types of cell death.
While many membrane-permeant drugs acting on mitochondrial channels have been identified over the last years, their proceeding into the clinic is hampered by two main drawbacks: first, many of the MOM and MIM ion channels have multiple localizations within the cells, so the cellular effect cannot be reliably ascribed to the action of the drug on the mitochondrial channel only; second, for many of the channel modulators possible side effects have not been taken into account and their specificity toward cancer cells has not been proven. These difficulties can, a priori, be overcome by using mitochondria-targeted so-called mitochondriotropic drugs and/or specific peptides interacting with the mitochondrial channel. This strategy may also reduce the dose required to exert beneficial effects, therefore likely reducing side-effects.
The present review gives a brief overview of mitochondrial channels and highlights the most promising channel modulator drugs, focusing on those tested also in vivo, and briefly describes the above-mentioned strategies possibly leading to enhanced specificity and efficacy.
2. Mitochondrial ion channels and their pharmacological targeting by small molecules
2.1. Mitochondrial permeability transition pore as a key protagonist in cell death
Arguably, the oncologically most relevant mitochondrial channel may be considered to be the mitochondrial permeability transition pore (MPTP), a large, unselective channel forming in the MIM of mitochondria stressed by excessive ROS and Ca2+, whose activation precipitates cell death (recent reviews: [[22], [23], [24], [25]].) This pore is one of the largest present in any biological membrane, having an estimated equivalent diameter of 2–3 nm when fully open (reviewed in Ref. [26]). The mean diameters of VDAC1 (mitochondrial porin), bacterial porin OmpC and α-hemolysin, for comparison, have been estimated – using similar methods based on solute permeation – at about 1.1, 0.8 and 0.3 nm, respectively [27]. Despite this large size, allowing traffic of molecules with a molecular weight up to 1500 Da, transient openings of MPTP seem to occur under physiological conditions and to be relevant in physiological Ca2+ homeostasis in different experimental settings such as ischemic preconditioning or somatic cell re-programming [[28], [29], [30]]. When the MPTP is open for a longer (seconds) time, it causes permanent MIM depolarization, interruption of ATP synthesis, matrix swelling, MOM rupture and, as a consequence, release of pro-apoptotic proteins from the intermembrane space (see e.g. Refs. [26,31]). Because of this crucial contribution to cell death, the importance of inducing long-lasting openings of MPTP in cancer cells became soon evident. It has been argued that cancer cells are desensitized to stimuli leading to the permeability transition [32,33]. Drugs or conditions causing its sustained opening in cancerous cells (only) would thus represent a potentially very valuable addition to the oncologists’ arsenal.
Several compounds have been identified which appear to directly inhibit the MPTP through its (putative) components [[34], [35], [36]]. Since they act as repressors, they may be more useful in other contexts in which the MPTP plays a role, e.g. ischemic injury. The same holds for the long-known MPTP inhibitor acting through the modulatory component Cyclophilin D, i.e., Cyclosporin A (CSA) [37,38].
Many drugs and natural substances have on the other hand been identified as openers of MPTP, on the basis of their ability to induce, at least in vitro, mitochondrial swelling, a process that can be easily detected by a spectrophotometer. Most of these molecules, with variable chemical structures, were tested also on cancer cells to assess their ability to trigger various forms of cell death. The question arises of how dozens of dissimilar molecules (for recent reviews and lists see Refs. [[39], [40], [41]]) can activate MPTP opening. Pore activation is known to be favoured by MIM depolarization, high ROS levels and matrix Ca2+ overload, while its inhibition can be achieved by acidic matrix pH, various divalent cations and Mg2+/ATP(ADP) and by the high-affinity inhibitor cyclosporine A. The identity of the MPTP is still debated (see, e.g. Refs. [25,41]), but after intensive research and several different hypotheses, the scientific community is finding a consensus on the proposal that the ATP–producing enzyme FoF1 ATP synthase may give rise to MPTP channel activity under specific conditions [[42], [43], [44], [45]]. In particular, a recent structural study identified subunit e, connected to a lipid plug within the c-ring, as the one whose retraction may lead to a gradual disassembly of the c-ring, suggesting pore formation by distanced c subunits [46]. If this model turns out to be correct, the action of a large spectrum of molecules might converge on subunits e or c of this enzyme, so important for life. However, an indirect action of the different molecules on MPTP opening cannot be excluded: as a matter of fact, for most molecules that induce the permeability transition in swelling assays, no information is available at the single channel level in patch clamp experiments. Indeed, as mentioned above, beside calcium and ROS, no other agents are known to directly trigger MPTP opening. Thus, most of the compounds that lead to opening of this pore probably do it indirectly, by triggering ROS release, calcium overload in the mitochondrial matrix or strong membrane depolarization (Fig. 1). One example is provided by AUL12, a Gold(III)-dithiocarbamate complex which inhibits Complex I of the mitochondrial respiratory chain, causes production of ROS and thereby activates mitochondrial kinase GSK3-α/β which promotes opening of the MPTP [47,48].
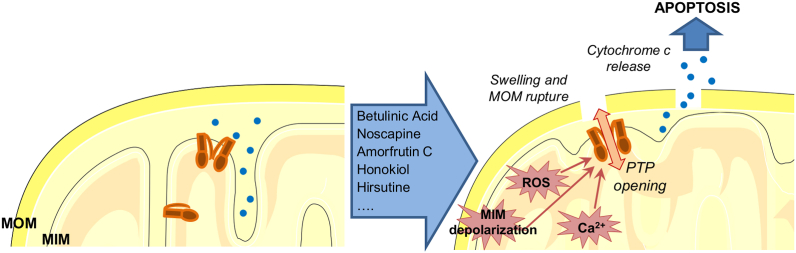
Fig. 1.
Role of the mitochondrial permeability transition pore (MPTP) and its activators in cell death. MPTP can be activated indirectly by different drugs (only those exerting a beneficial effect against cancer in vivo are indicated in the box) eliciting changes in inner membrane potential or causing ROS production or leading to calcium overload in the matrix. MPTP opening leads to swelling, rupture of MOM that contributes to cytochrome c release, a process required for apoptosome formation and subsequent activation of effector caspases. See text (par. 2.1) for further details.
Despite the identification of a number of compounds acting indirectly on MPTP, only few studies have provided in vivo evidence for the usefulness of such a strategy and reached the clinical trial stage. One of the best-investigated drugs is the triterpenoid betulinic acid, which, among many other cellular effects, triggers ROS production and induces CSA-sensitive cytochrome c release via MPTP, even in Bax/Bak-deficient cells. Betulinic acid is highly efficient against tumor cells of different origins as well as against tumor cells that are resistant to other, classical chemotherapeutic agents. It spares instead untransformed cells [49] (for recent review see Ref. [50]). Interestingly, betulinic acid has recently been shown to inhibit voltage-gated calcium channels [51], however whether it acts analogously on mitochondrial calcium channels is still unknown. This drug shows a satisfactory selectivity index for pathological versus healthy cells even at high doses and is effective, at least in vitro, against many types of cancer including melanoma, cancers of the gastrointestinal tract, pancreatic cancer, myeloid leukemia, with IC50 values ranging between 1 and 13.0 μg/mL [52]. Due also to early recognition - it was shown to be effective against melanoma in mice, without causing adverse effects, in 1995 [53] - this compound entered phase I and II clinical trials against dysplastic nevi, which can develop into melanoma. Unfortunately, the trial was stopped due to funding issues (Clinicaltrials.gov).
Another promising drug acting via MTPT is noscapine, an opium phthalide isoquinoline alkaloid that shows no toxicity in animals and humans and was recently employed in two clinical trials on refractory multiple myeloma and on chemo-resistant chronic lymphocytic leukemia. This agent was recently shown to induce apoptosis in chemo-resistant colon cancer cells in vitro [54], by causing mitochondrial membrane depolarization, through a still-undefined mechanism, ultimately leading to MPTP opening. It was tested in two clinical trials against myeloma and low-grade non-Hodgkin's lymphoma, terminated because of lack of response and funding problems, respectively (Clinicaltrials.gov). A similar mechanism of action was proposed for Amorfrutin C, isolated from indigo bush, which reduced proliferation of different types of cancer cells such as colon cancer and breast cancer [55]. This drug however has not entered the clinical phase yet.
Honokiol, from magnolia, is another example of a drug able to induce death of a variety of cancer cells by triggering MPTP opening and to overcome Bcl-2 and Bcl-xL-mediated apoptotic resistance. Honokiol, like Amorfrutin B, activates PPARγ (peroxisome proliferator-activated receptor gamma). It was shown to efficiently kill apoptosis-resistant B-cell chronic lymphocytic leukemia (B-CLL) cells as well as cells from chemoresistant multiple myeloma patients [56]. Together with other active compounds, it is currently in clinical trial for testing against papilloma virus-linked cervical lesions.
Plant-derived hirsutine has recently been shown to exert anti-cancer activity in a lung cancer xenograft mouse model. It acts through a signalling cascade leading to GSK3β dephosphorylation and MPTP opening [57]. GSK3β was shown to modulate activity of the MPTP-regulatory component, cyclophilin (CyP) D, a matrix chaperone that favors pore opening [58]. Importantly, hirsutine in vivo was not toxic for normal tissues.
2.2. Mitochondrial calcium transport systems allow matrix calcium overload that triggers MPTP opening
For most cell types, addition of 50–100 μM Ca2+ to isolated mitochondria is sufficient to induce MPTP opening in both swelling assays and patch clamp experiments. Such a high concentration can be reached in the mitochondrial matrix (where generally it is about 10 μM) by activation of entry pathways or block of exit routes for Ca2+. Calcium uptake into mitochondria takes place prevalently through the mitochondrial calcium uniporter complex (MCUC) [[59], [60], [61], [62], [63], [64]], although other calcium channels were also found to be active in the MIM. In particular, current candidates include the transient receptor potential TRPC3 [65] and vanilloid type1 (TRPV1) [66] channels and the mitochondrial ryanodine receptor (mRyR1) [67,68]. Calcium exit instead is mediated prevalently by NCLX, the mitochondrial Ca2+/Na+ antiporter [69].
MCUC and TRP calcium-permeable channels show different expression levels in healthy cells compared to various types of cancer cells (for recent reviews see Refs. [[70], [71], [72]]). TRPC3 is specifically inhibited by Pyr3 (Ethyl-1-(4-(2,3,3-trichloroacrylamide)phenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate), which has been shown to depolarize the MIM and boost ROS production when applied together with dexamethasone [73]. In the case of TRPV1, a recent study linked apoptosis induction by capsaicin to mitochondrial calcium overload, although whether mitochondrial TRPV1 is responsible for the effect of this channel agonist has not been specifically addressed [74]. As to MCUC, a small heterocyclic compound, AG311, (5-[(4-methylphenyl)thio]-9H-pyrimido[4,5-b]indole-2,4-diamine) was shown to depolarize mitochondria and proposed to exert its tumor growth-retarding effect by activating MCUC [75]. However, the same group identified in their subsequent work complex I of the respiratory chain as the target of AG311. In any case, the drug was not able to reduce the growth of breast cancer in a murine orthotopic xenograft model when applied alone [76]. A further candidate to activate MCUC is kaempferol, a plant flavonoid, observed in a few studies to increase mitochondrial calcium uptake [[77], [78], [79]]. However, caution must be exerted and proof of direct channel activation would be welcome, especially since this agent inhibits other channels, e.g. TRPC5 [80], and activates the large conductance calcium-dependent K+ channel [81], which also has a mitochondrial counterpart.
In addition to direct activation of the pore-forming component of MCUC, one valid possibility would be that of modulating the activity of its regulators, including the EF-hand calcium binding proteins MICU1 and MICU2, widely expressed in different tissues. Recent structural data suggest that the MICU1-MICU2 dimer blocks the channel pore at low cytosolic Ca2+ concentrations, while at increasing concentrations the dimer detaches from the complex [82]. Thus, any molecule detaching this dimer from MCU may facilitate calcium overload. To our knowledge such a compound has not been identified yet. On the other hand, recent data provide evidence that MICU1 potentiates MCU activity by increasing the channel's open probability when cytosolic Ca2+ binds to the EF hands [83,84]. 1 mM spermine was also reported to activate MCU channel [85], but to our knowledge no studies employing genetic deletion of MCU addressed the question of whether apoptosis of cancer cells can be triggered by spermine via its action on MCU. It has been suggested instead that oxidative deamination of spermine might produce ROS and consequent MPTP opening [86].
Matrix calcium is known to be required for the correct metabolism of mitochondria [87]. Interestingly, a recent work provided evidence that in the presence of the MPTP inhibitor CSA, MCU activity promotes cancer cell proliferation [88]. Upregulation of MCU expression contributes to ROS production and cancer cell migration [[89], [90], [91]]. Therefore, since MPTP opening is often prevented by specific signalling pathways in cancer cells, block of MCU might be a strategy in the case of cells that are resistant to MPTP inducers. In this respect, the recent discoveries of a specific MCU inhibitor, mitoxantrone [92], and of a membrane-permeant inhibitory Ruthenium complex named Ru265 [93] as well as of MICU1 modulators MCU-i4 and MCU-i11 [94] (for a recent review see Ref. [95]) may be of relevance, even if they have not yet been applied in the context of cancer (mitoxantrone however was developed as an anti-cancer agent and is/has been tested as such in several clinical trials). A compound named DS16570511 has also been proposed as MCUC inhibitor, but its ability to directly block the channel has not been demonstrated [96]. As pointed out by Hajnoczky and colleagues [95], these inhibitors, except MCUi11, are expected to be toxic for healthy cells as well, since they depolarize the mitochondrial membrane and cause side effects. A further inhibitor, KB-R7943, may be taken into consideration, since it has recently been shown to alleviate P. aeruginosa–triggered inflammatory responses in vivo [97], and it may have a similar anti-inflammatory effect in the tumor micro-environment, therefore negatively affecting tumor growth. However, this drug is also a potent inhibitor of the plasma membrane (PM) Na+/Ca2+ exchanger NCX1 [98] (contra [99]) and its tumor-reducing action observed in a murine prostate xenograft model has been ascribed to its effect on the PM exchanger [100] rather than to inhibition of the mitochondrial channel.
Ca2+ exit from the matrix through MIM-located NCLX can be blocked by the benzodiazepine CGP37157 [69,101]. Surprisingly, a lack of mitochondrial calcium overload and a protective effect were observed in models of neuronal injury using this drug, apparently due to its ability to block voltage-dependent calcium channels of the plasma membrane as well [102]. On the other hand, genetic deletion of NCLX in brown adipose tissue led to calcium overload upon adrenergic stimulation and to the opening of the permeability transition pore [103]. This finding is in accordance with previous studies showing that CGP37157 enhanced the sensitivity of cancer cells to TRAIL-induced death [104,105].
2.3. Voltage-dependent anion channels as a pathway in the MOM for metabolite and calcium entry
VDAC1 and VDAC2, the two major isoforms of mitochondrial porin [106], are involved in cell death in various ways. For example, VDAC1 plays a role in matrix Ca2+ overload, since it provides the Ca2+-permeable pathway in the MOM [8,107]. VDAC1 can oligomerize in a Ca2+-dependent process involving its N-terminus. These oligomers are seemingly implicated in the release of pro-apoptotic factors such as apoptosis-inducing factor (AIF) and cytochrome c [108]. VDAC1 as well as VDAC2 regulate cell death also by interacting with anti-apoptotic members of the Bcl-2 family contributing to MOM permeabilization [109,110], while interaction of VDAC1 with hexokinase (HK) is instrumental for cancer cell survival [108,111]. In addition, VDAC3, capable of sensing the oxidative state in the mitochondrial inter-membrane space [112], is emerging as potential marker for disease (for review see Ref. [113]). VDAC1 and VDAC3 can also interact with anti-apoptotic Mcl-1, promoting release of ROS and calcium transfer into the matrix, which in turn enhance migration [114]. In summary, VDAC isoforms contribute to cancer cell progression in different ways (for recent review see Ref. [115]). However, while many drugs affecting VDAC1 have been identified, those specifically acting on the other isoforms are less investigated. A recent work identified WEHI-9625, a novel tricyclic sulfone small molecule, that was able to bind to VDAC2 and inhibit Bak-triggered apoptosis [116], suggesting that an activator rather than an inhibitor of VDAC2 would be useful to eliminate cancer cells.
Compounds that decrease the conductance (i.e., the slope of the current/voltage relationship; roughly reflecting in this case the permeability of the water-admitting channel) of VDAC1 (Fig. 2) were instead shown to exert a selective pro-apoptotic and cytotoxic activity against cancer cells. For example, cyathin-R, a cyathane diterpenoid, decreases channel conductance as well as cancer growth in vivo, by promoting apoptosis even in Bax/Bak-deficient cells [117]. In addition, Cyathin-R promoted VDAC1 oligomerization, that was linked to apoptosis induction, since VDAC1-interacting molecules such as DIDS and its analogs (H2DIDS, SITS, DPC), which reduce VDAC1 oligomerization, inhibited cell death [118]. Avicins, a class of plant stress-induced triterpenoid metabolites, also displayed anti-cancer and anti-inflammatory properties (see e.g. Refs. [119,120]). Avicins however, in addition to VDAC1, target various proteins, such as tubulin and topoisomerase. They trigger cytochrome c release by inhibiting respiration and by reducing the efficiency of antioxidant systems, leading to enhanced oxidative stress. Another class of drugs that could be of value for the treatment and/or prevention of malignancy are cannabidiols (CBD), which block VDAC1 activity and exhibit strong in vitro anti-tumor effects against numerous cancer cells types [121,122]. Curcumin has also been shown to affect VDAC1 function by interacting with its N-terminal domain [123]. Likewise, the N-terminal part of VDAC can be targeted also by the bioactive components of the herb Rheum officinale Baill, capable of inducing apoptosis in many tumoral cell lines [124]. While many studies addressed the effect of the above drugs in cell lines or xenografts, only few investigated orthotopic models. Among all these drugs modulating VDAC activity, only curcumin entered the clinical phase and is under investigation either alone or in combination for various diseases, including invasive breast cancer and non-small cell lung cancer (clinicaltrial.gov). A clinical trial involving cannabidiol in biochemically recurrent prostate cancer has just been launched (NCT04428203).
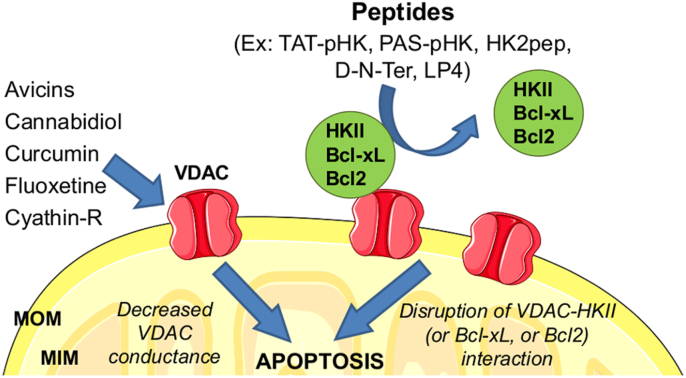
Fig. 2.
Role oftheVoltage-dependent anion channels (VDAC) andtheirpharmacological modulation in cell death. Decrease of the conductance of VDAC (especially of the VDAC1 isoform) by different drugs (left part) triggers apoptosis. In addition, disruption of the interaction of VDAC with the soluble, cytosolic glycolytic enzyme hexokinase II (HKII) or with membrane-inserted anti-apoptotic proteins Bcl-2 or Bcl-xL by specific targeting peptides (right part) equally leads to cell death. See text (par. 2.3 and 4.1) for further details.
2.4. Mitochondrial potassium channels as regulators of membrane potential and ROS release
Surprisingly, in various tissues the mitochondrial inner membrane was demonstrated to host several types of potassium channels. All of them, except the ATP-dependent potassium channel, have multiple localizations within the cells. In most cases the mitochondrial channels show pharmacological and biophysical properties indistinguishable from those of the counterparts located in the PM [6]. The question arises of what physiological relevance can be ascribed to the observed potassium channel redundancy in the MIM. One plausible explanation is that the presence of different channels, regulated by diverse intracellular factors, may offer a stimulus-dependent fine-tuning of mitochondrial function. In fact, some of the MIM K+ channels, such as Kv1.3 [125], Kv1.5 [126] and Kv7.4 [127] are classical voltage-dependent channels, while another class of MIM channels are triggered by calcium: these are the small- [128], intermediate- [129], and big- [130] conductance calcium-activated potassium channels (referred to as SKCa, IKCa and BKCa, respectively). Still another channel is the one gated by ATP and therefore named mitoKATP (see e.g. Refs. [[131], [132], [133], [134]]). The two-pore channel TASK-3 is sensitive to acidic pH [135,136]. In addition, MIM harbours the sodium-activated potassium channel mitoSLO2 [137]. Recent proteomic and biochemical data suggest that the hyperpolarization-activated cationic HCN3 functions as K+ channel in kidney mitochondria [138]. Finally, a splicing isoform of the renal medullary K+ channel ROMK2 has been shown to contribute to mitoKATP activity, at least in some tissues [133,134].
Regardless of the molecular nature of the MIM potassium channels, they have all been linked to the regulation of mitochondrial membrane potential (ΔΨ), as flux of potassium ions driven by the electrochemical gradient into the matrix leads to depolarization and, viceversa, block of these channels leads to hyperpolarization. As a consequence, ΔpH and calcium influx may also be modulated by K+ channel activation or inhibition. In addition, matrix volume is affected by K+ channel function and both hyperpolarization and depolarization favour ROS production [9]. Indeed, activation of MIM K+ channels exerts a protective role in various pathological contexts while their inhibition has been linked to cell death. This latter observation is of relevance, as many of these channels are highly expressed in the plasma membrane of cancer cells, and this apparently correlates with expression in mitochondria. The biophysical and pharmacological properties of these channels have recently been reviewed [39,139], therefore here we focus only on the channels whose modulation has been shown to affect cancer cell behaviour either in vitro or in vivo.
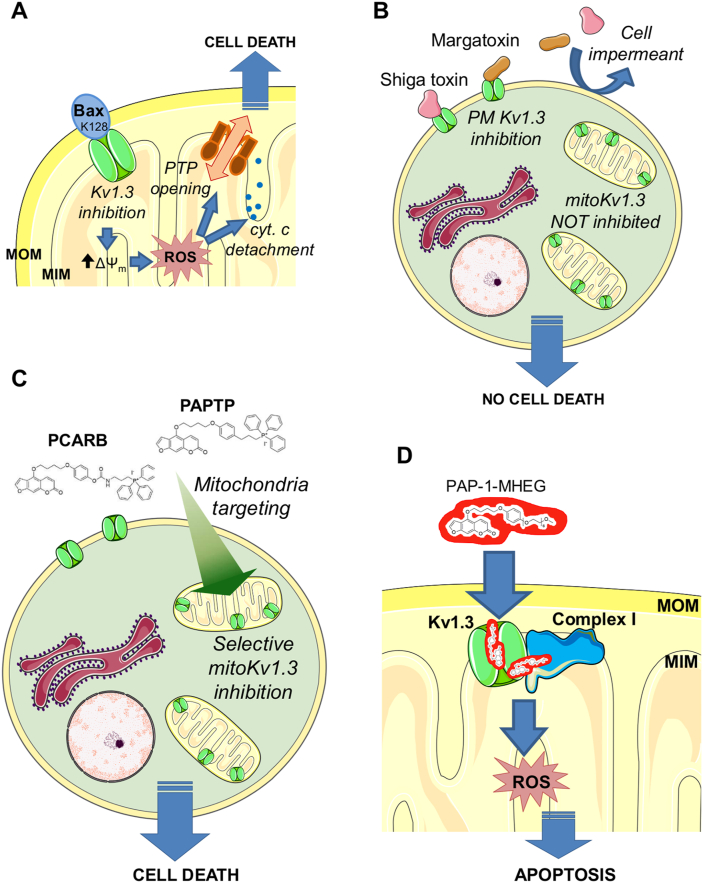
MitoKv1 channels are among the most promising targets. MitoKv1 inhibitors Psora-4 and PAP-1 are members of a family of psoralenic inhibitors originally developed by Wulff, Chandy and coworkers [140,141], among which PAP-1 was selected for extensive testing due to the favourable combination of affinity and selectivity for Kv1.3 over other channels of the family. While considered as a potential drug for autoimmune disorders [142], the membrane permeability of PAP-1 provided our group with a tool to inhibit the mitochondrial population of the channel (mitoKv1.3) [125], which had been found to be involved in cell death mediated by Bax [143]. The mechanism envisioned involves “plugging” of the channel vestibule by a specific, positively charged amino acid residue of Bax (K128) once the pro-apoptotic protein has inserted into the MOM [144] (Fig. 3A). This interaction mimics that of peptide blockers of Kv pores. In fact, treating isolated mitochondria with Bax, with the peptide toxin inhibitors Margatoxin (MgTx), ShK, or with Psora-4 produced the same phenomena: a transient hyperpolarization of the MIM (attributed to block of the channel-mediated depolarizing K+ uptake), a marked production of ROS, consequent activation of the MPTP, and cytochrome c release [143]. Expression of Kv1.3 was found to correlate with sensitivity to chemotherapy in a number of cancer cell lines [145], and PAP-1, Psora-4 and clofazimine - the latter another permeant inhibitor of the channel - turned out to induce apoptosis, acting in a sense like a pharmacological stand-in for Bax. Please note that MgTx and ShK, two membrane-impermeant Kv1.3 blockers, do not affect cell survival or mitochondrial physiology when added to intact cells (Fig. 3B). Clofazimine [146], a drug used in the clinic and exerting also various Kv1.3-independent effects [147], was tested in vivo and found to significantly reduce melanoma and pancreatic ductal adenocarcinoma (PDAC) in murine orthotopic tumor models [148,149]. Silencing of the channel prevented the apoptosis-inducing effect of the three drugs, indicating that Kv1.3-dependent pathways are involved.
Fig. 3.
Role of the voltage-dependent potassium channel Kv1.3 and its pharmacological modulation in cell death. A) The mitochondrial counterpart of the plasmamembrane-located Kv1.3 channel (mtKv1.3) can be blocked by membrane-inserted Bax, through the specific K128 residue. The block of channel activity causes an initial hyperpolarization followed by ROS release and MPTP opening. B) Membrane-impermeant toxin inhibitors of Kv1.3 capable of reaching only the plasma-membrane-located Kv1.3 are unable to trigger cell death, indicating that mtKv1.3 is the channel whose function is relevant for apoptosis. C) Membrane-permeant, mitochondriotropic inhibitors PAPTP and PCARBTP (indicated as PCARB) efficiently trigger the same series of events shown in A), leading to cell death of cancer cells in vitro and in orthotopic tumor models. D) The more soluble derivative of the small molecule inhibitor of Kv1.3 (and of mtKv1.3) PAP-1, named PAP-1-MHEG promotes ROS production by acting on mtKv1.3 that is strictly interacting with the respiratory chain complex I, thereby amplifying the ROS-producing effect of the drug that may transfer electrons from complex I to oxygen. The See text (par. 2.4 and 3.2) for details.
Among the other MIM K+ channels, mitochondrial IKCa is of interest in the context of cancer, since its membrane-permeant inhibitors TRAM-34 and clotrimazole regulate oxidative phosphorylation in PDAC cells [150] and sensitize melanoma cells to apoptotic stimuli, by triggering release of mitochondrial ROS [151]. Likewise, changes of mitoTASK-3 expression/functionality have been linked to alterations of mitochondrial bioenergetics and morphology [152]. However, there is no clear evidence that targeting the mitochondrial channel is relevant for apoptosis induction, since no systematic studies have been carried out comparing the effect of membrane-impermeant and membrane-permeant inhibitors. In addition, recent evidence raises the possibility that even modulation of PM channels might affect mitochondrial ROS production and morphology [153] or mitochondrial membrane potential [154] by still unclarified mechanisms. In particular, Pardo and colleagues observed that lack or inhibition of the Kv10.1 channel of the PM by a membrane-impermeant specific antibody induced mitochondrial fragmentation and ROS production [153], while in the other work block of the ASIC1a acid-sensitive channel with membrane-impermeant Psalmotoxin-1 blocked mitochondrial Ca2+ signals [154].
Altogether, MIM K+ channels remain excellent oncological targets. However, due to i) multiple localization of these proteins within the cell; ii) lack of mitoK+ channel-specific K+ channel inhibitors; iii) the observation that PM-located K+ channels may also affect mitochondrial fitness; iv) the often-observed off-target effects of many inhibitors [139], caution has to be taken when interpreting the effect of K+ channel modulators on cell survival/death. In particular, it would be desirable to assess in each case the cellular effects of channel-modulating drugs in isogenic systems either expressing or lacking the channel protein.
2.5. Anion channels of the inner membrane – a further way to regulate ROS and MPTP opening?
Several members of the Chloride Intracellular Channel (CLIC) family have been located at mitochondria. CLICs are emerging as biomarkers in several diseases (for review see Ref. [155]) including cancer [155,156]. In electrophysiological recordings using artificial bilayer membranes, CLICs form redox- and pH-sensitive channels. While CLIC4 is located in the MOM, CLIC1 [157] and CLIC5 [158] were found in the MIM, also by patch clamp. CLIC5, the first CLIC identified in the MIM, was shown to regulate the production of mitochondrial ROS by respiratory chain complexes, thus in principle CLIC5 modulators could also be exploited for cancer treatment. Indeed, high expression of CLIC1 was correlated with high ROS production. The other way around, inhibition of CLIC1 reduced ROS release and migration of gastric cancer cells [159]. On the other hand, the absence of CLIC5 specifically in cardiac mitochondria increased ROS release [158]. Interestingly, the connection between ROS production and CLICs as well as the above-mentioned channels may also be relevant in inflammation [160].
CLICs are inhibited by indanyloxyacetic acid-94 (IAA-94), but this is not a specific drug for this subset of channels, since IAA-94 inhibits also, e.g., the volume-regulated chloride channels of the plasma membrane [161]. In addition, mitochondrial CLIC family members are present also in several other intracellular membranes [155]. Therefore, more work is needed to identify mitochondrial CLIC-specific modulators.
3. Mitochondriotropic drugs
3.1. Chemical modification strategies of anti-cancer drugs to target them to mitochondria
With the (current) exceptions of MCUC and the K(ATP) channel recently identified by Paggio et al. [132], all known mitochondrial channels are present also in other cell compartments/membranes (for a tabulation see Ref. [40]). As far as is known, differences – if any - between the various populations of these channels are not such as to allow the development of small molecules specifically interacting with those residing in only one of multiple locations. In general, however, selective targeting of subcellular compartments – i.e., in our case, concentrating a drug at mitochondria - is desirable from various points of view (rev., e.g. Ref. [162]). Besides potentially reducing off-target effects and drug dosage, targeting mitochondria means exploiting their connection to cell death(s) and the peculiar roles the organelles have in cancer [2,40]. Selectively targeting mitochondrial channels is thus expected to maximize damage to cancer while minimizing damage to healthy cells.
The most popular strategy to address a “cargo” to mitochondria is to take advantage of the high transmembrane potential the organelles maintain. Positively charged, membrane-permeant objects (drugs, prodrugs, nanovehicles, markers, probes) will accumulate into mitochondria as they tend to reach electrochemical equilibrium. Since the plasma membrane of cells is also polarized, the cytoplasm serves as a “first stage” for the accumulation (roughly 10-fold), which can be as high as 1000-fold (theoretically even higher, depending on the value of the transmembrane potential) when comparing the mitochondrial matrix and the extracellular space. Mitochondria may be hyperpolarized in cancer cells [[163], [164], [165]], thus favouring the concentration of mitochondriotropic drugs in diseased cells and partially explaining the selectivity of their cytotoxic effect. The standard modification of a molecule to drive it into mitochondria consists in attaching to it a lipophilic (i.e., membrane-permeant) moiety carrying a permanent, delocalized charge. Since its invention by Skulachev and coworkers [166], and especially since its intelligent exploitation by Michael Murphy, Robin Smith and coworkers, triphenylphosphonium has been the most commonly used such group (reviews e.g. Refs. [[167], [168], [169], [170], [171]]).
A huge number of compounds have been modified by attachment of a TPP group, due to its efficiency and to the relative ease of introduction, generally by a nucleophilic substitution on a suitable precursor. In many cases this has been done with an eye to cancer therapy (revs: [165,168,[172], [173], [174], [175]]. To cite just a few examples, this approach has been explored with polyphenols (e.g.: resveratrol and quercetin [176]; honokiol [177], lonidamine [178], glycyrrhetinic acid [179], vitamin E succinate [180], the metal chelator desferrioxamine [181], metformin [182], topoisomerase blocker doxorubicin [183], the alkylating agent chlorambucil [184], microtubule-disrupting paclitaxel [185], Hsp90 inhibitors [186], platinum complexes [187], estrogen receptor modulator tamoxifen [188]. A particularly thorough study has been carried out with the triterpenoids betulin and betulinic acid [189]. The most noteworthy application may well be the development by Murphy's group of the mitochondria-targeted antioxidant MitoQ(10), a ubiquinone derivative covalently attached to triphenylphosphonium through an aliphatic chain (optimally 10 methylene units long) [190,191]. This compound has proven highly successful: a PubMed search for “MitoQ” returns 335 items (December 2020). Many studies have been carried out in in vivo models and in humans (e.g. Refs. [192,193]). MitoQ is mentioned in 19 entries in the clinical trials inventory (https://clinicaltrials.gov; December 2020). It has been suggested that it may be useful against Covid-19 [194]. Skulachev's group has developed analogous compounds based on plastoquinones [195].
It should be pointed out that such modifications result in new molecules which may well have somewhat different properties – besides their preferential localization – with respect to the parent compounds. Thus, for example, they may have a decreased affinity for their targets (e.g. Ref. [180]), an issue not addressed in many studies. In addition, the length of alkyl spacers introduced to link the TPP group to the molecule core influences, e.g., the pharmacokinetics of betulin derivatives [179] and the behaviour of MitoQ [191].
Triphenylphosphonium has its drawbacks, the major one probably being its tendency to bind to biomembranes, a consequence of charge and of the lipophilicity which makes it such a serviceable tool (e.g. Ref. [196]). The charged moiety tends to associate with the negatively charged phospholipid headgroups, while the remaining part of the molecule, if sufficiently lipophilic, can insert into the membrane core. Binding is obviously also influenced by the specific properties of each derivative [181]. The accumulation of TPP-decorated molecules at the MIM and matrix may not only enhance their effectiveness at the mitochondrial level, but also limit other, possibly unwanted or confusing, interactions or processes in other cellular districts. This obviously applies to interactions with non-mitochondrial channels. Another example is provided by mito-targeted vitamin E succinate (mitoVES) a mitocan which efficiently kills cancer cells by ROS generation upon interaction with Complex II [180,197,198]. MitoVES reaches the mitochondria even in cancer cells that develop resistance to its untagged parent compound, VES, by upregulating the ATP-binding cassette transporter ABCA1 of the plasma membrane [199].
The bulky triphenylphosphonium group may also have an impact on the interaction of the active portion of the construct and its target protein. An example is again provided by mitoQ [10], which is a good substrate for Complex II of the mitochondrial respiratory chain, but not for Complexes I and III, for steric reasons [196]. The presence of TPP may lead to unexpected effects: for example, just-mentioned mitoVES at sub-apoptotic doses suppressed expression of mitochondrial DNA transcripts, proliferation and mitochondrial biogenesis in cancer cells [200]. Its “parent” compound, α-tocopheryl succinate, did not produce the same effects.
A few papers have reported that some TPP-containing compounds, including some simple alkyl derivatives, can act as uncouplers and/or inhibitors of the respiratory chain (e.g. Refs. [[182], [183], [184]]). This is not a general behaviour exhibited by all such compounds, and, when present, can account in part for cytotoxic anti-cancer effects. TPP is not, in any case, the only possible choice for a mitochondria-targeting group. Other positive moieties, e.g. pyridinium, quinolinium and guanidine, have been tested (for review see Ref. [185]). As expected, mito-targeting generally increased the efficacy of the mitocans that were so modified, or actually created them.
3.2. Mitochondriotropic compounds targeting mitochondrial ion channels
Mitochondriotropic compounds targeting MOM or MIM channels are still only a few, all of them obtained by linking the parent drug to a TPP group. From the observations with psoralenic compounds (sect. 2.4) it was just a short logical step to mitochondriotropic derivatives of channel modulators [[201], [202], [203], [204]]. The two major variants produced had the TPP moiety linked to the PAP-1 core either by an alkoxy ether linkage, essentially stable under physiological conditions, or by a linker comprising a carbamate moiety, which could undergo hydrolysis and thus conferred pro-drug characteristics to the construct (Fig. 3C). These constructs proved effective – much more so than the parent PAP-1 – both in vitro and in vivo. Remarkably, they acted selectively on cancer cells, sparing normal cells and tissues. At the cellular level the details of their action were similar to those already observed for PAP-1: a transient increase of the mitochondrial transmembrane potential, production of reactive oxygen species (ROS), downstream mitochondrial depolarization and morphological changes - attributed to onset of the MPT on the basis of the effect of CSA, the established inhibitor of the latter - and cytochrome c release [201]. In vivo, both derivatives drastically reduced tumor growth in murine melanoma and PDAC models (about 80% and 60% reduction of endpoint tumor volume, respectively), even when administered alone at 5 nmol/mg bw in a straightforward protocol consisting in 4 alternate-day i. p. injections. About 90% melanoma volume reduction was achieved by administering PAPTP along with cisplatin, pointing to the possibility of synergy with other chemotherapeutic drugs. Indeed, one of the attractive features of these compounds is that they act independently of the upper part of the mitochondrial apoptotic cascade, i.e., regardless of the status of p53 and proteins of the Bcl-2 family [148,149,201]. ROS production was a key feature of the path to apoptosis: in vitro, death of tumoral cells was prevented by radical scavangers (NAC, mitoTEMPO, PEG-CAT/PEG-SOD); in vivo, tumor reduction was not obtained if the mice were pre-treated with the anti-oxidant NAC before receiving the mitochondriotropic drugs [201].
The key role of ROS also provides an explanation for the salvation of healthy cells: it is proposed that a threshold exists for oxidative stress above which the cell dies. As mentioned, cancerous cells sustain a higher baseline stress, associated with higher proliferation rates, hypoxia and mitochondrial reprogramming [11,14,17,18,168], and therefore can be more readily pushed above the death threshold by the additional stress caused by the drugs (a reasoning that applies also to other mitocans eliciting ROS production). This model was confirmed by experiments in which the susceptibility of cancerous cells to PAPTP and PCARBTP was lowered by antioxidants, whereas that of Kv1.3-expressing TEM lymphocytes was increased by introducing an additional redox stress in the form of a low concentration of an independent mitochondriotropic pro-oxidant (Q7BTPI [205]). mtKv1.3 inhibition and ROS production appear to be synergic, since cancerous cells not expressing the channel (leukemic K562 cells) withstood the combination of PAPTP and Q7BTPI [201].
ROS generation can be an auto-reinforcing phenomenon [206], since mitochondria that have undergone the MPT generate ROS in the presence of electron donors, yet the question arises of what the origin of the process is. Increased mitochondrial ROS formation has long been known to be associated with mitochondrial hyperpolarization, due to “redox backpressure” along the respiratory chain (e.g. Refs. [[207], [208], [209]]). Given the observed initial increase in MIM transmembrane potential, it is logical to think of this type of mechanism as the origin of ROS production. A recent study by our group [210] has however opened a new perspective, envisioning the psoralenic ring system of these compounds as a redox-cycling system. The paper describes a non-mitochondriotropic derivative of PAP-1, namely PAP-1-MHEG, designed to remedy a shortcoming of PAP-1 derivatives, namely their low aqueous solubility. While solubility was improved, the compound turned out to inhibit respiration at Complex I of the Respiratory Chain, but only in cells expressing Kv1.3 (Fig. 3D). Expression of the channel was also necessary for the compound to cause death of cancerous cells (healthy ones were spared), which it did more efficiently than PAP-1, but less efficiently than PAPTP. Abundant generation of ROS was involved. To clarify matters we then synthesized compounds corresponding to portions of PAP-1-MHEG and studied their effects. We were thus led to the unexpected conclusion that mtKv1.3 and Complex I physically interact. Kv1.3 blockade is carried out by the alkyl-O-phenyl portion, which is in agreement with the conclusions reached in previous studies by other groups [211,212]. Channel block does lead to ROS production, in agreement with our previous conclusions, but with a lower rate than that elicited by the complete PAP-1-MHEG molecule. Complex I inhibition is due to the oligoethyleneglycol side-arm of PAP-1-MHEG: without it, there was no inhibition; on the other hand, that portion of the molecule by itself was inert, even at high concentrations. Coherently, PAP-1 and PAPTP, which have no such appendage, do not inhibit Complex I. The interpretation is that the OEG chain must be appropriately positioned by virtue of the interaction of the rest of PAP-1-MHEG with Kv1.3, which is apposed to Complex I. A similar reasoning presumably applies to ROS generation: if the furocoumarinic ring system was missing, the channel was inhibited but ROS production was relatively modest. On the other hand, 5-methoxypsoralene (5-MOP), i.e. the ring system without appendages, also induced only a comparatively weak production of ROS. It seems therefore that the psoralenic moiety must not only be present, but it must be appropriately positioned thanks to the interaction of the molecule with the mtKv1.3-complex I assembly. Our hypothesis is that its position overlaps that normally engaged by ubiquinone in the Q module of complex I [213,214], which is located in the portion of Complex I extending into the matrix. There, the aromatic, delocalized system could mediate an abnormal transfer of single electrons to oxygen, generating superoxide. Furocoumarins are known to engage in this type of redox chemistry [215] even though they are better known for photoinduced excitation and ionization.
The molecular details of these putative interactions and processes remain to be worked out, but some considerations can already be made. The modes of binding of PAP-1 to Kv1 channels have been defined in experimental and in silico studies [141,211,212]. The latter were based on the published structure of channel Kv1.2 [216], since that of Kv1.3 has not yet been determined and Kv1.2 is highly homologous. Zimin and co-workers [211] concluded that the channel is blocked by an assembly comprising two (but possibly up to four) PAP-1 molecules and a K+ ion, and their calculations detailed a set of 4 loci constituting the binding site and likely to interact with the various parts of the PAP-1 molecule. In the predicted structure, the two furocoumarins are located deep into the channel structure, making contact with their carbonyl moiety with a K+ ion in the conduit of the channel (which is what actually blocks the conduit), while the alkylphenoxy side arms protrude towards the exterior between segments S5 and S6. This model accommodates the notion of an interaction of the oligoethyleneglycol chain with Complex I, but it is hardly compatible with the idea that the furocoumarin ring system may reach out of the Kv1.3 tetramer. However, the molecular dynamics simulations conducted by Jorgensen et al. [212] led to the identification of a total of six possible binding sites for PAP-1 on the Kv1.2 tetramer. Of these, three seem to be the most often engaged: site II corresponds essentially to the inner site identified by Zimin et al.; site VI corresponds to a previously identified side-pocket accommodating small molecule inhibitors at the level of the S4–S5 linker; site III is located at the extremity of the channel structure protruding into the cytoplasm in the plasma membrane channel - and into the mitochondrial matrix in the mitochondrial one, which has the same in-out orientation [143]. Site III thus seems a likely candidate as an additional binding site for PAP-1 and derivatives, providing a foothold from which the molecule could conceivably extend towards Complex I. Importantly, according to the simulations by Jorgensen and colleagues, site III could accommodate either the psoralenic moiety or the phenoxyalkyl branch. Thus, in this picture, channel inhibition and ROS generation would depend on binding to two distinct sites. Obviously, even a low time-averaged occupation of the matrix site (site III) could in principle be sufficient to account for the extra ROS generation observed. The physiological functions, if any, fulfilled by this association between an ion channel and a complex of the respiratory chain remain to be investigated. It is however emerging that other mitochondrial channels also interact with key proteins of the oxphos system (see below).
Another mitochondria-targeted inhibitor of a mitochondrial channel has been synthesized and is being tested in our labs (Bachmann M et al., in press). This is mitoIN-THPP (IsoNipecotate-THPP) a 5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine (THPP)-containing compound and an inhibitor of TASK-3 (Twik-related Acid-Sensitive K channel-3) (see above). The parent molecule is “12f” in Coburn et al., 2012 [217], which was reported to inhibit TASK-3 with an IC50 of 74 nM. The molecule contains in this case a hydrolysable ester linkage, and it may therefore be considered a mitochondriotropic pro-drug of IN-THPP. Indeed, cell esterases cleave the ester bond releasing IN-THPP-COOH. Both IN-THPP and mitoIN-THPP are cytotoxic for different human and mouse melanoma cells (which express TASK-3) in the tens-of-μM range, with the mitochondriotropic variant being more effective, by a factor of 2–4 in the various lines. Cytotoxicity depends on TASK-3 expression, and is associated with ROS production, mitochondrial depolarization, fragmentation of the mitochondrial network and decrease of cellular [ATP]. These phenomena are not induced by the TPP-containing part of the molecule freed upon hydrolysis of the ester bond, and thus can be ascribed to the THPP part, and to TASK-3 inhibition (given the requirement for its expression). ATP decrease is associated with AMPK activation, which in turn is presumably involved in the decrease of migration capacity of melanoma cells, as measured by the wound scratch assay.
4. Modulation of cancer cell fate by peptides targeting mitochondrial ion channel interactions
Peptides (short sequences of amino acids, up to perhaps 30 monomers, found in nature or man-made) have been one of the most active fields of pharmacological research since at least the 1990's. They attract interest because they are efficacious, versatile, and convenient. Their efficacy stems from their composition: they are made of the same building blocks as all proteins, and are therefore recognizable with relative ease by active or binding sites designed by co-evolution to interact with segments of proteins. Versatility follows from the huge number of variations available: if one considers the 20 standard (“L”) natural amino acids (a set that can be expanded by considering D isomers and a variety of unnatural amino acids), one can theoretically build 20N different sequences, where N is the number of monomeric units. Exploiting this variability and selecting the useful sequences is achieved by screening large random libraries selecting effective peptides thanks to phage [218,219], yeast [220], bacterial [221] and other forms of display/biopanning technology. Once an effective sequence is identified, it can be readily produced in the lab by the convenient, generally standard methods of solid phase peptide synthesis.
From a pharmacological point of view, there are two major ways to exploit this resource: one can use peptides to modulate pathophysiological processes, or to selectively deliver an attached “cargo” to a specific target. So-called mitochondria-penetrating peptides (e.g. Refs. [222,223]), a subfamily of cell-penetrating peptides [224,225], have in fact been used to deliver drugs to mitochondria, at least in vitro. For example, Kelley's group coupled chlorambucil to a peptide consisting of three repeated doublets of cyclohexylphenylalanine and d-arginine. The authors verified the mitochondrial localization of a version linked to a fluorescent dye, and were able to confirm the in vivo efficacy of the modified drug (although with mechanistic alterations) [184,226]. The same approach was used with doxorubicin [227]. Peptide-driven delivery to mitochondria has been discussed in recent reviews (e.g. Refs. [226,228]).
Many other functions aside, peptides can be immunomodulatory [229], impacting inflammation [230] and cancer [231]. In oncology, peptides are under consideration for anti-cancer vaccination (e.g. Refs. [229,232]) and chemotherapy (e.g. Refs. [233,234]). Peptide toxins are produced by all kingdoms of life, and several are used or are considered for use as drugs [235]. Some of these target ion channels known to be present also in mitochondria. The best known are the high-affinity peptide inhibitors of K+ channels of the Kv, KCa, K2P families [[235], [236], [237], [238]]. These peptides may help in the characterization of the various conductances found in the MIM, but they are not in use to modulate intracellular channels in cultured cells, ex-vivo preparations or in vivo, because they do not permeate cell membranes. The current state of pharmacological technology might well allow this obstacle to be overcome by including these toxins in constructs or nanovehicles comprising cell-penetrating and possibly mitochondria-targeting peptides. However the efficiency and selectivity of these targeting tools at present is not such as to eliminate off-target effects, which would be expected to be severe. Thus, there is to our knowledge no literature on the use of this class of peptides to modulate mitochondrial channels in a pathophysiological context.
4.1. Modulating VDAC1-hexokinase interaction with small peptides
It is however emerging that at least some mitochondrial ion channels interact, molecularly and functionally, with other components of the mitochondrial membranes or of the cellular milieu [138,210,[239], [240], [241]]. These interactions may in principle be disrupted via competition by appropriate peptides.
The foremost example of this strategy, as applied to mitochondrial channels in the context of cancer, involves VDAC1 (Fig. 2), the mitochondrial porin, whose expression, not coincidentally, is upregulated in many cancers [242]. Aerobic glycolysis, a hallmark of cancer, entails positioning of hexokinase I and II at the MOM, where it has immediate access to freshly produced ATP and can direct it to the production of glucose-6-phosphate [243,244]. Detachment of HKII results in cell death [245,246]. Hexokinase binds to VDAC1 and is preferentially localized at the junction complexes of mitochondria and endoplasmic reticulum (Mitochondria-Associated-Membranes; MAMs) [245,247]. In cancer cells up to 80% of HKII is found at MAMs. Its detachment precipitates cell death via activation of the permeability transition [245,246]. The N-terminal of HKII [248] (in particular His5 [249]) makes contact with VDAC1. In fact, peptides copying the N-terminal part competed with HKII for its binding site on VDAC1 and induced death of cancer cells [248] (Fig. 2). The sequence - MIASHLLAYFFTELN-amide (pHK) [248] – permeates cell membranes only poorly, and in most studies it has therefore been used as part of constructs comprising cell-penetrating sequences. Pastorino et al. [248] used the Antennipedia sequence (alias penetratin; their peptide is: RQIKIWFQNRRMKWKK-MIASHLLAYFFTELN-amide). Chiara and coworkers [246] preferred the TAT sequence, building a TAT-pHK peptide (peptide: MIASHLLAYFFTELNβA-GYGRKKRRQRRRG (TAT-HK)). In their recent work Aihua Zou and coworkers fused pHK with the cell-penetrating sequence KVLKQRAKKK and added a lipid chain to obtain amphiphilic nanostructures which could kill A549 cells, (a human non-small cell lung cancer line) at concentrations in the 10−5 M range [250]. Woldetsadik et al. [251] instead covalently linked pHK to the penetrating sequence GKPILFF (PAS), obtaining analogous results with HeLa and CHO–K1 cells. A recent paper by Ciscato and colleagues describes a peptide (HK2pep) comprising the pHK linked to a polycationic cell-penetrating peptide shielded in turn by a polyanionic stretch with which it pairs up to minimize undesirable interactions. The two segments carrying opposite charges are connected by a sequence which can be cleaved by metallo matrix proteases (MMP) 2 and 9, characteristically abundant in many tumor types (HK2pep: MIASHLLAYFFTELN-bA-RRRRRRRRR-PLGLAG-Ahx-EEEEEEEE). Cleavage of the connecting sequence by these enzymes frees the active part of the molecule – clHKpep, i.e., pHK plus the cationic stretch (MIASHLLAYFFTELN-bA-RRRRRRRRR-PLG) - which enters cells. There it elicits Ca2+ release from the ER, Ca2+ entry through PM Ca2+ channels, Ca2+ uptake by mitochondria, onset of the MPT, and calpain-mediated cell death [245]. Activation of this pathway appears to depend on a marked localization of HKII at MAMs. The cleaved construct was effective against B-CLL cells, and HK2pep worked upon injection into the tumoral mass in allograph models of colon and breast cancer expressing MMP2/9.
Various parts of VDAC1 participate in HK binding [248,252,253] (three cytosol-exposed glutamate residues, E72, E188, and E202 are crucial), which can nonetheless be disrupted also by peptides derived from VDAC1, in particular by a sequence containing E72 [254]. The interaction between VDAC1 and HK is modulated by GSK3β, which disrupts it by phosphorylating VDAC1 [255,256]. Activation of the kinase can thus be a strategy against cancer (e.g. Refs. [257,258]). Cyclophilin D, the already-mentioned mitochondrial matrix peptidyl-prolyl isomerase, upregulated in many cancers [259], which positively regulates the permeability transition [37,38], also stabilizes VDAC-HK interactions [260].
VDAC1 binds many other proteins of relevance in the oncological context (rev [261]) – including anti-apoptotic Bcl-2 and Bcl-xL, which are upregulated in many cancers and act to prevent chemotherapy-induced cell death. As is the case for VDAC1-HK binding, peptides based on VDAC1 sequences disrupt these interactions and have a pro-apoptotic effect (Fig. 2). Shoshan-Barmatz's group has developed various forms of two peptides for this approach. In both cases the amino acids were, in most constructs, in the “unnatural” D conformation to enhance their stability and effectiveness. The D-N-Ter peptide based on the N-terminal sequence ((M)AVPPTYADGLGKSARDVFTKGYGFGL) was joined to the Antp sequence (KKWKMRRNQFWIKIQR) because of the known involvement of the N-terminal of VDAC1 in binding these proteins. It interfered with HK, Bcl-2 and Bcl-xL binding and antagonized their anti-apoptotic effects [262,263]. The other peptide, named “LP4”, consistingof an N-terminal sequence SWTWE and a C-terminal sequence KWTWK, forms a tryptophan zipper and a β-hairpin. This peptide was fused either to Antp (RQIKIWFQNRRMKWKK), to a “mini” version of it (KRRMKWKK), to TAT (RKKRRQRRRGG) or to the transferrin receptor (TfR)-recognizing peptide HAIYPRH [264], with the amino acids in either the L or D configuration [265,266]. Various modifications of these constructs were also implemented. The peptides entered cells and induced detachment of the target proteins from VDAC1. They were tested against cells from patients of B-chronic lymphocytic leukemia (CLL; CD5+/CD19+ cells) and MEC-1 cells, which were killed, while normal peripheral blood lymphocytes were unaffected. The most effective conjugate proved to be the Min-Antp-LP4 construct, with an IC50 of 0.3 μM for CLL lymphocytes (vs. 0.6 μM for its enantiomeric version and 0.7 μM for Antp-LP4). Retro-Tf-D-LP4 caused apoptosis of cancer liver-derived cells and antagonized tumor growth in three murine liver cancer models [267] and in subcutaneous and orthotopic glioblastoma models upon intravenous injection [268].
VDAC1 also binds pro-apoptotic proteins: Bax and Bak, Bim, Bid - whose interactions can also be antagonized by suitable peptides [263,268]. In addition, one can envision to act on the interaction of the other channels with their partners. For example, MCU activity may be enhanced by interrupting its interaction with the gatekeeper MICU proteins even at low cytoplasmic calcium concentration, by acting on critical sequences that can now be identified thanks to the resolved structure of the holocomplex MCU/EMRE/MICU [64].
5. Conclusion and future perspectives
While nowadays the advantages of pharmacologically targeting mitochondrial metabolism/function of cancer cells are universally recognized, only few studies have addressed the specific modulation of mitochondrial channels using mitochondriotropic drugs or cell-penetrating peptides. Nonetheless, the drugs obtained so-far display an excellent, generalizable action, as they are able to efficiently reduce growth of different types of tumors in vivo. The fact that many channels are expressed in tumors in a differential manner with respect to healthy cells apparently ensures a layer of selectivity, since many of the drugs/peptides discussed here do not exert toxic side-effects. Despite great advances in this field, especially during the last decade, many cancer-relevant questions remain open. For example, it has not been systematically investigated how some PM-located channels are targeted also to mitochondria and whether the distribution of some of the channels to MIM is a specific feature of cancer cells. A lot remains to be understood about the interactomes of MOM and MIM channels as well, since the examples known so-far indicate that disruption/modification of such interactions may be very useful to drive cancer cells towards death. In this respect it is interesting to note that basically every respiratory chain complex was shown to be physically (and possibly functionally) coupled to various ion channels. Whether such association is compulsory for cell survival is still unknown.
In terms of future perspectives, hopefully mitochondrial ion channels will become preferred targets of various groups working on cancer. Clinical trials involving betulinic acid, noscapine and honokiol (activators of the MPTP), mitoxantrone (MCU inhibitor), cannabidiol, are referred to in the text (sect. 2). Other compounds mentioned, e.g. capsaicin and kaempferol, are employed in clinical trials, but not as chemotherapeutic agents. Difficulties in crossing biomembranes (requiring fusion with permeating peptides; sect. 4.1), rapid proteolytic degradation in vivo (requiring the use of “tricks” such as the incorporation of D-aminoacids or packaging in nanovehicles) and relatively high costs presumably account for the lack of clinical trials employing peptides targeting the interactions of VDAC with hexokinase or anti-apoptotic proteins. Unfortunately, to date none of the mitochondriotropic ion channel-modulators have reached the clinical trial phase, as to our knowledge large-scale toxicity studies have not been performed yet. Further developments in the field of mitochondriotropic channel modulators may envision a way to activate drug/peptide-channel interaction through events triggered by e.g. tumor-directed gamma-radiation used in radiotherapy or hypoxic conditions prevailing inside solid tumors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to all colleagues who participated in the works performed in our laboratories and we thank all our external collaborators. We also thank for the continuous support the Italian Association for Cancer Research (IG 2017, Id.20286)). Italian Association for Multiple Sclerosis FISM/AISM (Prot. 284/18/F14) and the CNR InterOmics project (GLIOMICS) are also acknowledged for support.
References
- 1.Grasso D., Zampieri L.X., Capelôa T., Van de Velde J.A., Sonveaux P. Mitochondria in cancer. Cell stress. 2020;4(6):114–146. doi: 10.15698/cst2020.06.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth K.G., Mambetsariev I., Kulkarni P., Salgia R. The mitochondrion as an emerging therapeutic target in cancer. Trends Mol. Med. 2020;26(1):119–134. doi: 10.1016/j.molmed.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peruzzo R., Costa R., Bachmann M., Leanza L., Szabò I. Mitochondrial metabolism, contact sites and cellular calcium signaling: implications for tumorigenesis. Cancers. 2020;12(9) doi: 10.3390/cancers12092574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasan K., Werner M., Chandel N.S. Mitochondrial metabolism as a target for cancer therapy. Cell Metabol. 2020;32(3):341–352. doi: 10.1016/j.cmet.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakhle J., Rodriguez A.M., Vignais M.L. Multifaceted roles of mitochondrial components and metabolites in metabolic diseases and cancer. Int. J. Mol. Sci. 2020;21(12) doi: 10.3390/ijms21124405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szabo I., Zoratti M. Mitochondrial channels: ion fluxes and more. Physiol. Rev. 2014;94(2):519–608. doi: 10.1152/physrev.00021.2013. [DOI] [PubMed] [Google Scholar]
- 7.Shoshan-Barmatz V., De Pinto V., Zweckstetter M., Raviv Z., Keinan N., Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Aspect. Med. 2010;31(3):227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Naghdi S., Hajnóczky G. VDAC2-specific cellular functions and the underlying structure. Biochim. Biophys. Acta. 2016;1863(10):2503–2514. doi: 10.1016/j.bbamcr.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malinska D., Mirandola S.R., Kunz W.S. Mitochondrial potassium channels and reactive oxygen species. FEBS Lett. 2010;584(10):2043–2048. doi: 10.1016/j.febslet.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Hornsveld M., Dansen T.B. The hallmarks of cancer from a redox perspective. Antioxidants Redox Signal. 2016;25(6):300–325. doi: 10.1089/ars.2015.6580. [DOI] [PubMed] [Google Scholar]
- 11.Ippolito L., Giannoni E., Chiarugi P., Parri M. Mitochondrial redox hubs as promising targets for anticancer therapy. Frontiers in oncology. 2020;10:256. doi: 10.3389/fonc.2020.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohlena J., Dong L.F., Ralph S.J., Neuzil J. Anticancer drugs targeting the mitochondrial electron transport chain. Antioxidants Redox Signal. 2011;15(12):2951–2974. doi: 10.1089/ars.2011.3990. [DOI] [PubMed] [Google Scholar]
- 13.Neuzil J., Dong L.F., Rohlena J., Truksa J., Ralph S.J. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion. 2013;13(3):199–208. doi: 10.1016/j.mito.2012.07.112. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen C., Pandey S. Exploiting mitochondrial vulnerabilities to trigger apoptosis selectively in cancer cells. Cancers. 2019;11(7) doi: 10.3390/cancers11070916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biasutto L., Dong L.F., Zoratti M., Neuzil J. Mitochondrially targeted anti-cancer agents. Mitochondrion. 2010;10(6):670–681. doi: 10.1016/j.mito.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Ralph S.J., Rodriguez-Enriquez S., Neuzil J., Moreno-Sanchez R. Bioenergetic pathways in tumor mitochondria as targets for cancer therapy and the importance of the ROS-induced apoptotic trigger. Mol. Aspect. Med. 2010;31(1):29–59. doi: 10.1016/j.mam.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Modica-Napolitano J.S., Weissig V. Treatment strategies that enhance the efficacy and selectivity of mitochondria-targeted anticancer agents. Int. J. Mol. Sci. 2015;16(8):17394–17421. doi: 10.3390/ijms160817394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12(12):931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 19.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 20.Huang G., Pan S.T. ROS-mediated therapeutic strategy in chemo-/radiotherapy of head and neck cancer. Oxidative medicine and cellular longevity. 2020;2020:5047987. doi: 10.1155/2020/5047987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui Q., Wang J.Q., Assaraf Y.G., Ren L., Gupta P., Wei L. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 2018;41:1–25. doi: 10.1016/j.drup.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Carraro M., Carrer A., Urbani A., Bernardi P. Molecular nature and regulation of the mitochondrial permeability transition pore(s), drug target(s) in cardioprotection. J. Mol. Cell. Cardiol. 2020;144:76–86. doi: 10.1016/j.yjmcc.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Bonora M., Patergnani S., Ramaccini D., Morciano G., Pedriali G., Kahsay A.E. Physiopathology of the permeability transition pore: molecular mechanisms in human pathology. Biomolecules. 2020;10(7) doi: 10.3390/biom10070998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui Y., Pan M., Ma J., Song X., Cao W., Zhang P. Recent progress in the use of mitochondrial membrane permeability transition pore in mitochondrial dysfunction-related disease therapies. Mol. Cell. Biochem. 2020 doi: 10.1007/s11010-020-03926-0. [DOI] [PubMed] [Google Scholar]
- 25.Winquist R.J., Gribkoff V.K. Targeting putative components of the mitochondrial permeability transition pore for novel therapeutics. Biochem. Pharmacol. 2020;177:113995. doi: 10.1016/j.bcp.2020.113995. [DOI] [PubMed] [Google Scholar]
- 26.Zoratti M., Szabo I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995;1241(2):139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 27.Aksoyoglu M.A., Podgornik R., Bezrukov S.M., Gurnev P.A., Muthukumar M., Parsegian V.A. Size-dependent forced PEG partitioning into channels: VDAC, OmpC, and α-hemolysin. Proc. Natl. Acad. Sci. U.S.A. 2016;113(32):9003–9008. doi: 10.1073/pnas.1602716113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal A., Wu P.H., Hughes E.G., Fukaya M., Tischfield M.A., Langseth A.J. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron. 2017;93(3):587–605. doi: 10.1016/j.neuron.2016.12.034. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ying Z., Xiang G., Zheng L., Tang H., Duan L., Lin X. Short-term mitochondrial permeability transition pore opening modulates histone lysine methylation at the early phase of somatic cell reprogramming. Cell Metabol. 2018;28(6):935–945. doi: 10.1016/j.cmet.2018.08.001. e5. [DOI] [PubMed] [Google Scholar]
- 30.Hausenloy D., Wynne A., Duchen M., Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109(14):1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 31.Di Lisa F., Bernardi P. Mitochondrial function and myocardial aging. A critical analysis of the role of permeability transition. Cardiovasc. Res. 2005;66(2):222–232. doi: 10.1016/j.cardiores.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Rasola A., Sciacovelli M., Chiara F., Pantic B., Brusilow W.S., Bernardi P. Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition. Proc. Natl. Acad. Sci. U.S.A. 2010;107(2):726–731. doi: 10.1073/pnas.0912742107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasola A., Bernardi P. The mitochondrial permeability transition pore and its adaptive responses in tumor cells. Cell Calcium. 2014;56(6):437–445. doi: 10.1016/j.ceca.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Šileikytė J., Devereaux J., de Jong J., Schiavone M., Jones K., Nilsen A. Second-generation inhibitors of the mitochondrial permeability transition pore with improved plasma stability. ChemMedChem. 2019;14(20):1771–1782. doi: 10.1002/cmdc.201900376. [DOI] [PubMed] [Google Scholar]
- 35.Antonucci S., Di Sante M., Sileikyte J., Deveraux J., Bauer T., Bround M.J. A novel class of cardioprotective small-molecule PTP inhibitors. Pharmacol. Res. 2020;151:104548. doi: 10.1016/j.phrs.2019.104548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Šileikytė J., Forte M. The mitochondrial permeability transition in mitochondrial disorders. Oxidative medicine and cellular longevity. 2019;2019:3403075. doi: 10.1155/2019/3403075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broekemeier K.M., Dempsey M.E., Pfeiffer D.R. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J. Biol. Chem. 1989;264(14):7826–7830. [PubMed] [Google Scholar]
- 38.Crompton M., Ellinger H., Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem. J. 1988;255(1):357–360. [PMC free article] [PubMed] [Google Scholar]
- 39.Leanza L., Checchetto V., Biasutto L., Rossa A., Costa R., Bachmann M. Pharmacological modulation of mitochondrial ion channels. Br. J. Pharmacol. 2018;176:4258–4283. doi: 10.1111/bph.14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parrasia S., Mattarei A., Furlan A., Zoratti M., Biasutto L. Small-molecule modulators of mitochondrial channels as chemotherapeutic agents. Cell. Physiol. Biochem. : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2019;53(S1):11–43. doi: 10.33594/000000192. [DOI] [PubMed] [Google Scholar]
- 41.Chinopoulos C. Mitochondrial permeability transition pore: back to the drawing board. Neurochem. Int. 2018;117:49–54. doi: 10.1016/j.neuint.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Giorgio V., von Stockum S., Antoniel M., Fabbro A., Fogolari F., Forte M. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. U.S.A. 2013;110(15):5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urbani A., Giorgio V., Carrer A., Franchin C., Arrigoni G., Jiko C. Purified F-ATP synthase forms a Ca(2+)-dependent high-conductance channel matching the mitochondrial permeability transition pore. Nat. Commun. 2019;10(1):4341. doi: 10.1038/s41467-019-12331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mnatsakanyan N., Park H.-A., Jing W., Llaguno M.C., Murtishi B., Latta M. Mitochondrial megachannel resides in monomeric ATP synthase. Biophys. J. 2019;116(3):156a. doi: 10.1038/s41467-019-13766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerle C. Mitochondrial F-ATP synthase as the permeability transition pore. Pharmacol. Res. 2020;160:105081. doi: 10.1016/j.phrs.2020.105081. [DOI] [PubMed] [Google Scholar]
- 46.Pinke G., Zhou L., Sazanov L.A. Cryo-EM structure of the entire mammalian F-type ATP synthase. Nat. Struct. Mol. Biol. 2020;27(11):1077–1085. doi: 10.1038/s41594-020-0503-8. [DOI] [PubMed] [Google Scholar]
- 47.Nardon C., Chiara F., Brustolin L., Gambalunga A., Ciscato F., Rasola A. Gold(III)-pyrrolidinedithiocarbamato derivatives as antineoplastic agents. ChemistryOpen. 2015;4(2):183–191. doi: 10.1002/open.201402091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiara F., Gambalunga A., Sciacovelli M., Nicolli A., Ronconi L., Fregona D. Chemotherapeutic induction of mitochondrial oxidative stress activates GSK-3α/β and Bax, leading to permeability transition pore opening and tumor cell death. Cell Death Dis. 2012;3(12):e444. doi: 10.1038/cddis.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mullauer F.B., Kessler J.H., Medema J.P. Betulinic acid induces cytochrome c release and apoptosis in a Bax/Bak-independent, permeability transition pore dependent fashion. Apoptosis : an international journal on programmed cell death. 2009;14(2):191–202. doi: 10.1007/s10495-008-0290-x. [DOI] [PubMed] [Google Scholar]
- 50.Hordyjewska A., Ostapiuk A., Horecka A., Kurzepa J. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochemistry Rev. 2019;18(3):929–951. [Google Scholar]
- 51.Bellampalli S.S., Ji Y., Moutal A., Cai S., Wijeratne E.M.K., Gandini M.A. Betulinic acid, derived from the desert lavender Hyptis emoryi, attenuates paclitaxel-, HIV-, and nerve injury-associated peripheral sensory neuropathy via block of N- and T-type calcium channels. Pain. 2019;160(1):117–135. doi: 10.1097/j.pain.0000000000001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seca A.M.L., Pinto D. Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018;19(1) doi: 10.3390/ijms19010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pisha E., Chai H., Lee I.S., Chagwedera T.E., Farnsworth N.R., Cordell G.A. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995;1(10):1046–1051. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- 54.Tian X., Liu M., Huang X., Zhu Q., Liu W., Chen W. Noscapine induces apoptosis in human colon cancer cells by regulating mitochondrial damage and warburg effect via PTEN/PI3K/mTOR signaling pathway. OncoTargets Ther. 2020;13:5419–5428. doi: 10.2147/OTT.S232137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weidner C., Rousseau M., Micikas R.J., Fischer C., Plauth A., Wowro S.J. Amorfrutin C induces apoptosis and inhibits proliferation in colon cancer cells through targeting mitochondria. J. Nat. Prod. 2016;79(1):2–12. doi: 10.1021/acs.jnatprod.5b00072. [DOI] [PubMed] [Google Scholar]
- 56.Ishitsuka K., Hideshima T., Hamasaki M., Raje N., Kumar S., Hideshima H. Honokiol overcomes conventional drug resistance in human multiple myeloma by induction of caspase-dependent and -independent apoptosis. Blood. 2005;106(5):1794–1800. doi: 10.1182/blood-2005-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang R., Li G., Zhang Q., Tang Q., Huang J., Hu C. Hirsutine induces mPTP-dependent apoptosis through ROCK1/PTEN/PI3K/GSK3beta pathway in human lung cancer cells. Cell Death Dis. 2018;9(6):598. doi: 10.1038/s41419-018-0641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rasola A., Sciacovelli M., Pantic B., Bernardi P. Signal transduction to the permeability transition pore. FEBS Lett. 2010;584(10):1989–1996. doi: 10.1016/j.febslet.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Stefani D., Raffaello A., Teardo E., Szabo I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476(7360):336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Stefani D., Patron M., Rizzuto R. Structure and function of the mitochondrial calcium uniporter complex. Biochim. Biophys. Acta. 2015;1853(9):2006–2011. doi: 10.1016/j.bbamcr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baughman J.M. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marchi S., Vitto V.A.M., Danese A., Wieckowski M.R., Giorgi C., Pinton P. Mitochondrial calcium uniporter complex modulation in cancerogenesis. Cell Cycle. 2019;18(10):1068–1083. doi: 10.1080/15384101.2019.1612698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vultur A., Gibhardt C.S., Stanisz H., Bogeski I. The role of the mitochondrial calcium uniporter (MCU) complex in cancer. Pflueg. Arch. Eur. J. Physiol. 2018;470(8):1149–1163. doi: 10.1007/s00424-018-2162-8. [DOI] [PubMed] [Google Scholar]
- 64.Fan M., Zhang J., Tsai C.W., Orlando B.J., Rodriguez M., Xu Y. Structure and mechanism of the mitochondrial Ca(2+) uniporter holocomplex. Nature. 2020;582(7810):129–133. doi: 10.1038/s41586-020-2309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang B., Xiong S., Lin S., Xia W., Li Q., Zhao Z. Enhanced mitochondrial transient receptor potential channel, canonical type 3-mediated calcium handling in the vasculature from hypertensive rats. Journal of the American Heart Association. 2017;6(7) doi: 10.1161/JAHA.117.005812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao R., Tsang S.Y. Versatile roles of intracellularly located TRPV1 channel. J. Cell. Physiol. 2017;232(8):1957–1965. doi: 10.1002/jcp.25704. [DOI] [PubMed] [Google Scholar]
- 67.Beutner G., Sharma V.K., Giovannucci D.R., Yule D.I., Sheu S.S. Identification of a ryanodine receptor in rat heart mitochondria. J. Biol. Chem. 2001;276(24):21482–21488. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- 68.Ryu S.Y., Beutner G., Kinnally K.W., Dirksen R.T., Sheu S.S. Single channel characterization of the mitochondrial ryanodine receptor in heart mitoplasts. J. Biol. Chem. 2011;286(24):21324–21329. doi: 10.1074/jbc.C111.245597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palty R., Silverman W.F., Hershfinkel M., Caporale T., Sensi S.L., Parnis J. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. U.S.A. 2010;107(1):436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cui C., Merritt R., Fu L., Pan Z. Targeting calcium signaling in cancer therapy. Acta Pharm. Sin. B. 2017;7(1):3–17. doi: 10.1016/j.apsb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bustos G., Cruz P., Lovy A., Cardenas C. Endoplasmic reticulum-mitochondria calcium communication and the regulation of mitochondrial metabolism in cancer: a novel potential target. Frontiers in oncology. 2017;7:199. doi: 10.3389/fonc.2017.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marchi S., Giorgi C., Galluzzi L., Pinton P. Ca(2+) fluxes and cancer. Mol. Cell. 2020;78(6):1055–1069. doi: 10.1016/j.molcel.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 73.Abdoul-Azize S., Buquet C., Vannier J.P., Dubus I. Pyr3, a TRPC3 channel blocker, potentiates dexamethasone sensitivity and apoptosis in acute lymphoblastic leukemia cells by disturbing Ca(2+) signaling, mitochondrial membrane potential changes and reactive oxygen species production. Eur. J. Pharmacol. 2016;784:90–98. doi: 10.1016/j.ejphar.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 74.Xu S., Cheng X., Wu L., Zheng J., Wang X., Wu J. Capsaicin induces mitochondrial dysfunction and apoptosis in anaplastic thyroid carcinoma cells via TRPV1-mediated mitochondrial calcium overload. Cell. Signal. 2020;75:109733. doi: 10.1016/j.cellsig.2020.109733. [DOI] [PubMed] [Google Scholar]
- 75.Bastian A., Thorpe J.E., Disch B.C., Bailey-Downs L.C., Gangjee A., Devambatla R.K. A small molecule with anticancer and antimetastatic activities induces rapid mitochondrial-associated necrosis in breast cancer. J. Pharmacol. Exp. Therapeut. 2015;353(2):392–404. doi: 10.1124/jpet.114.220335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bastian A., Matsuzaki S., Humphries K.M., Pharaoh G.A., Doshi A., Zaware N. AG311, a small molecule inhibitor of complex I and hypoxia-induced HIF-1α stabilization. Canc. Lett. 2017;388:149–157. doi: 10.1016/j.canlet.2016.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Montero M., Lobaton C.D., Hernandez-Sanmiguel E., Santodomingo J., Vay L., Moreno A. Direct activation of the mitochondrial calcium uniporter by natural plant flavonoids. Biochem. J. 2004;384(Pt 1):19–24. doi: 10.1042/BJ20040990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bermont F., Hermant A., Benninga R., Chabert C., Jacot G., Santo-Domingo J. Targeting mitochondrial calcium uptake with the natural flavonol kaempferol, to promote metabolism/secretion coupling in pancreatic β-cells. Nutrients. 2020;12(2) doi: 10.3390/nu12020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tadić V., Adam A., Goldhammer N., Lautenschlaeger J., Oberstadt M., Malci A. Investigation of mitochondrial calcium uniporter role in embryonic and adult motor neurons from G93A(hSOD1) mice. Neurobiol. Aging. 2019;75:209–222. doi: 10.1016/j.neurobiolaging.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 80.Naylor J., Minard A., Gaunt H.J., Amer M.S., Wilson L.A., Migliore M. Natural and synthetic flavonoid modulation of TRPC5 channels. Br. J. Pharmacol. 2016;173(3):562–574. doi: 10.1111/bph.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu Y.C., Leung S.W., Leung G.P., Man R.Y. Kaempferol enhances endothelium-dependent relaxation in the porcine coronary artery through activation of large-conductance Ca(2+) -activated K(+) channels. Br. J. Pharmacol. 2015;172(12):3003–3014. doi: 10.1111/bph.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y., Han Y., She J., Nguyen N.X., Mootha V.K., Bai X.C. Structural insights into the Ca(2+)-dependent gating of the human mitochondrial calcium uniporter. eLife. 2020;9 doi: 10.7554/eLife.60513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patron M., Checchetto V., Raffaello A., Teardo E., Vecellio Reane D., Mantoan M. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol. Cell. 2014;53(5):726–737. doi: 10.1016/j.molcel.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garg V., Paranjpe I., Unsulangi T., Suzuki J., Milescu L.S., Kirichok Y. The mechanism of MICU-dependent gating of the mitochondrial Ca2+ uniporter. bioRxiv. 2020 doi: 10.7554/eLife.69312. 2020.04.04.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Michels G., Khan I.F., Endres-Becker J., Rottlaender D., Herzig S., Ruhparwar A. Regulation of the human cardiac mitochondrial Ca2+ uptake by 2 different voltage-gated Ca2+ channels. Circulation. 2009;119(18):2435–2443. doi: 10.1161/CIRCULATIONAHA.108.835389. [DOI] [PubMed] [Google Scholar]
- 86.Grancara S., Ohkubo S., Artico M., Ciccariello M., Manente S., Bragadin M. Milestones and recent discoveries on cell death mediated by mitochondria and their interactions with biologically active amines. Amino Acids. 2016;48(10):2313–2326. doi: 10.1007/s00726-016-2323-z. [DOI] [PubMed] [Google Scholar]
- 87.Rizzuto R., De Stefani D., Raffaello A., Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012;13(9):566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 88.Marchi S., Vitto V.A.M., Patergnani S., Pinton P. High mitochondrial Ca(2+) content increases cancer cell proliferation upon inhibition of mitochondrial permeability transition pore (mPTP) Cell Cycle. 2019;18(8):914–916. doi: 10.1080/15384101.2019.1598729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tosatto A., Sommaggio R., Kummerow C., Bentham R.B., Blacker T.S., Berecz T. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1alpha. EMBO Mol. Med. 2016;8(5):569–585. doi: 10.15252/emmm.201606255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cui C., Yang J., Fu L., Wang M., Wang X. Progress in understanding mitochondrial calcium uniporter complex-mediated calcium signalling: a potential target for cancer treatment. Br. J. Pharmacol. 2019;176(9):1190–1205. doi: 10.1111/bph.14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ren T., Zhang H., Wang J., Zhu J., Jin M., Wu Y. MCU-dependent mitochondrial Ca(2+) inhibits NAD(+)/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene. 2017;36(42):5897–5909. doi: 10.1038/onc.2017.167. [DOI] [PubMed] [Google Scholar]
- 92.Arduino D.M., Wettmarshausen J., Vais H., Navas-Navarro P., Cheng Y., Leimpek A. Systematic identification of MCU modulators by orthogonal interspecies chemical screening. Mol. Cell. 2017;67(4):711–723. doi: 10.1016/j.molcel.2017.07.019. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Woods J.J., Nemani N., Shanmughapriya S., Kumar A., Zhang M., Nathan S.R. A selective and cell-permeable mitochondrial calcium uniporter (MCU) inhibitor preserves mitochondrial bioenergetics after hypoxia/reoxygenation injury. ACS Cent. Sci. 2019;5(1):153–166. doi: 10.1021/acscentsci.8b00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Marco G., Vallese F., Jourde B., Bergsdorf C., Sturlese M., De Mario A. A high-throughput screening identifies MICU1 targeting compounds. Cell Rep. 2020;30(7):2321–2331. doi: 10.1016/j.celrep.2020.01.081. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Márta K., Hasan P., Rodríguez-Prados M., Paillard M., Hajnóczky G. Pharmacological inhibition of the mitochondrial Ca(2+) uniporter: relevance for pathophysiology and human therapy. J. Mol. Cell. Cardiol. 2020;S0022-2828(20):30293–30303. doi: 10.1016/j.yjmcc.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kon N., Murakoshi M., Isobe A., Kagechika K., Miyoshi N., Nagayama T. DS16570511 is a small-molecule inhibitor of the mitochondrial calcium uniporter. Cell death discovery. 2017;3:17045. doi: 10.1038/cddiscovery.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rimessi A., Pozzato C., Carparelli L., Rossi A., Ranucci S., De Fino I. Pharmacological modulation of mitochondrial calcium uniporter controls lung inflammation in cystic fibrosis. Science advances. 2020;6(19) doi: 10.1126/sciadv.aax9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Santo-Domingo J., Vay L., Hernández-Sanmiguel E., Lobatón C.D., Moreno A., Montero M. The plasma membrane Na+/Ca2+ exchange inhibitor KB-R7943 is also a potent inhibitor of the mitochondrial Ca2+ uniporter. Br. J. Pharmacol. 2007;151(5):647–654. doi: 10.1038/sj.bjp.0707260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Namekata I., Odaka R., Hamaguchi S., Tanaka H. KB-R7943 inhibits the mitochondrial Ca(2+) uniporter but not Na(+)-Ca(2+) exchanger in cardiomyocyte-derived H9c2 cells. Biol. Pharm. Bull. 2020;43(12):1993–1996. doi: 10.1248/bpb.b20-00747. [DOI] [PubMed] [Google Scholar]
- 100.Long Z., Chen B., Liu Q., Zhao J., Yang Z., Dong X. The reverse-mode NCX1 activity inhibitor KB-R7943 promotes prostate cancer cell death by activating the JNK pathway and blocking autophagic flux. Oncotarget. 2016;7(27):42059–42070. doi: 10.18632/oncotarget.9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cox D.A., Conforti L., Sperelakis N., Matlib M.A. Selectivity of inhibition of Na(+)-Ca2+ exchange of heart mitochondria by benzothiazepine CGP-37157. J. Cardiovasc. Pharmacol. 1993;21(4):595–599. doi: 10.1097/00005344-199304000-00013. [DOI] [PubMed] [Google Scholar]
- 102.Ruiz A., Alberdi E., Matute C. CGP37157, an inhibitor of the mitochondrial Na+/Ca2+ exchanger, protects neurons from excitotoxicity by blocking voltage-gated Ca2+ channels. Cell Death Dis. 2014;5(4):e1156. doi: 10.1038/cddis.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Assali E.A., Jones A.E., Veliova M., Acín-Pérez R., Taha M., Miller N. NCLX prevents cell death during adrenergic activation of the brown adipose tissue. Nat. Commun. 2020;11(1):3347. doi: 10.1038/s41467-020-16572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaddour-Djebbar I., Choudhary V., Brooks C., Ghazaly T., Lakshmikanthan V., Dong Z. Specific mitochondrial calcium overload induces mitochondrial fission in prostate cancer cells. Int. J. Oncol. 2010;36(6):1437–1444. doi: 10.3892/ijo_00000629. [DOI] [PubMed] [Google Scholar]
- 105.Ohshima Y., Takata N., Suzuki-Karasaki M., Yoshida Y., Tokuhashi Y., Suzuki-Karasaki Y. Disrupting mitochondrial Ca2+ homeostasis causes tumor-selective TRAIL sensitization through mitochondrial network abnormalities. Int. J. Oncol. 2017;51(4):1146–1158. doi: 10.3892/ijo.2017.4096. [DOI] [PubMed] [Google Scholar]
- 106.Messina A., Reina S., Guarino F., De Pinto V. VDAC isoforms in mammals. Biochim. Biophys. Acta. 2012;1818(6):1466–1476. doi: 10.1016/j.bbamem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 107.De Stefani D., Bononi A., Romagnoli A., Messina A., De Pinto V., Pinton P. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ. 2012;19(2):267–273. doi: 10.1038/cdd.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shoshan-Barmatz V., Krelin Y., Shteinfer-Kuzmine A. VDAC1 functions in Ca(2+) homeostasis and cell life and death in health and disease. Cell Calcium. 2018;69:81–100. doi: 10.1016/j.ceca.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 109.Brahimi-Horn M.C., Ben-Hail D., Ilie M., Gounon P., Rouleau M., Hofman V. Expression of a truncated active form of VDAC1 in lung cancer associates with hypoxic cell survival and correlates with progression to chemotherapy resistance. Canc. Res. 2012;72(8):2140–2150. doi: 10.1158/0008-5472.CAN-11-3940. [DOI] [PubMed] [Google Scholar]
- 110.Yamagata H., Shimizu S., Nishida Y., Watanabe Y., Craigen W.J., Tsujimoto Y. Requirement of voltage-dependent anion channel 2 for pro-apoptotic activity of Bax. Oncogene. 2009;28(40):3563–3572. doi: 10.1038/onc.2009.213. [DOI] [PubMed] [Google Scholar]
- 111.Camara A.K.S., Zhou Y., Wen P.C., Tajkhorshid E., Kwok W.M. Mitochondrial VDAC1: a key gatekeeper as potential therapeutic target. Front. Physiol. 2017;8:460. doi: 10.3389/fphys.2017.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reina S., Checchetto V., Saletti R., Gupta A., Chaturvedi D., Guardiani C. VDAC3 as a sensor of oxidative state of the intermembrane space of mitochondria: the putative role of cysteine residue modifications. Oncotarget. 2016;7(3):2249–2268. doi: 10.18632/oncotarget.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reina S., Guarino F., Magri A., De Pinto V. VDAC3 as a potential marker of mitochondrial status is involved in cancer and pathology. Frontiers in oncology. 2016;6:264. doi: 10.3389/fonc.2016.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang H., Shah K., Bradbury N.A., Li C., White C. Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death Dis. 2014;5:e1482. doi: 10.1038/cddis.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bachmann M., Pontarin G., Szabo I. The contribution of mitochondrial ion channels to cancer development and progression. Cell. Physiol. Biochem. : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2019;53(S1):63–78. doi: 10.33594/000000198. [DOI] [PubMed] [Google Scholar]
- 116.van Delft M.F., Chappaz S., Khakham Y., Bui C.T., Debrincat M.A., Lowes K.N. A small molecule interacts with VDAC2 to block mouse BAK-driven apoptosis. Nat. Chem. Biol. 2019;15(11):1057–1066. doi: 10.1038/s41589-019-0365-8. [DOI] [PubMed] [Google Scholar]
- 117.Huang L., Han J., Ben-Hail D., He L., Li B., Chen Z. A new fungal diterpene induces VDAC1-dependent apoptosis in bax/bak-deficient cells. J. Biol. Chem. 2015;290(39):23563–23578. doi: 10.1074/jbc.M115.648774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Keinan N., Tyomkin D., Shoshan-Barmatz V. Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis. Mol. Cell Biol. 2010;30(24):5698–5709. doi: 10.1128/MCB.00165-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haridas V., Li X., Mizumachi T., Higuchi M., Lemeshko V.V., Colombini M. Avicins, a novel plant-derived metabolite lowers energy metabolism in tumor cells by targeting the outer mitochondrial membrane. Mitochondrion. 2007;7(3):234–240. doi: 10.1016/j.mito.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 120.Wang H., Haridas V., Ju Gutterman, Xu Z.X. Natural triterpenoid avicins selectively induce tumor cell death. Commun. Integr. Biol. 2010;3(3):205–208. doi: 10.4161/cib.3.3.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Massi P., Solinas M., Cinquina V., Parolaro D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol. 2013;75(2):303–312. doi: 10.1111/j.1365-2125.2012.04298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rimmerman N., Ben-Hail D., Porat Z., Juknat A., Kozela E., Daniels M.P. Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: a novel mechanism for cannabinoid-induced cell death. Cell Death Dis. 2013;4(12):e949. doi: 10.1038/cddis.2013.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tewari D., Ahmed T., Chirasani V.R., Singh P.K., Maji S.K., Senapati S. Modulation of the mitochondrial voltage dependent anion channel (VDAC) by curcumin. Biochim. Biophys. Acta. 2015;1848(1 Pt A):151–158. doi: 10.1016/j.bbamem.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 124.Li Q., Qiao P., Chen X., Wang J., Bian L., Zheng X. Affinity chromatographic methodologies based on immobilized voltage dependent anion channel isoform 1 and application in protein-ligand interaction analysis and bioactive compounds screening from traditional medicine. J. Chromatogr. A. 2017;1495:31–45. doi: 10.1016/j.chroma.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 125.Szabo I., Bock J., Jekle A., Soddemann M., Adams C., Lang F. A novel potassium channel in lymphocyte mitochondria. J. Biol. Chem. 2005;280(13):12790–12798. doi: 10.1074/jbc.M413548200. [DOI] [PubMed] [Google Scholar]
- 126.Leanza L., Zoratti M., Gulbins E., Szabo I. Induction of apoptosis in macrophages via Kv1.3 and Kv1.5 potassium channels. Curr. Med. Chem. 2012;19(31):5394–5404. doi: 10.2174/092986712803833281. [DOI] [PubMed] [Google Scholar]
- 127.Testai L., Barrese V., Soldovieri M.V., Ambrosino P., Martelli A., Vinciguerra I. Expression and function of Kv7.4 channels in Rat cardiac mitochondria: possible targets for cardioprotection. Cardiovasc. Res. 2016;110(1):40–50. doi: 10.1093/cvr/cvv281. [DOI] [PubMed] [Google Scholar]
- 128.Dolga A.M., Netter M.F., Perocchi F., Doti N., Meissner L., Tobaben S. Mitochondrial small conductance SK2 channels prevent glutamate-induced oxytosis and mitochondrial dysfunction. J. Biol. Chem. 2013;288(15):10792–10804. doi: 10.1074/jbc.M113.453522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Marchi U., Sassi N., Fioretti B., Catacuzzeno L., Cereghetti G.M., Szabo I. Intermediate conductance Ca2+-activated potassium channel (KCa3.1) in the inner mitochondrial membrane of human colon cancer cells. Cell Calcium. 2009;45(5):509–516. doi: 10.1016/j.ceca.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 130.Xu W., Liu Y., Wang S., McDonald T., Van Eyk J.E., Sidor A. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298(5595):1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 131.Kowaltowski A.J., Seetharaman S., Paucek P., Garlid K.D. Bioenergetic consequences of opening the ATP-sensitive K(+) channel of heart mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2001;280(2):H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 132.Paggio A., Checchetto V., Campo A., Menabo R., Di Marco G., Di Lisa F. Identification of an ATP-sensitive potassium channel in mitochondria. Nature. 2019;572(7771):609–613. doi: 10.1038/s41586-019-1498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Foster D.B., Ho A.S., Rucker J., Garlid A.O., Chen L., Sidor A. Mitochondrial ROMK channel is a molecular component of mitoK(ATP) Circ. Res. 2012;111(4):446–454. doi: 10.1161/CIRCRESAHA.112.266445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Laskowski M., Augustynek B., Bednarczyk P., Żochowska M., Kalisz J., O'Rourke B. Single-channel properties of the ROMK-pore-forming subunit of the mitochondrial ATP-sensitive potassium channel. Int. J. Mol. Sci. 2019;20(21) doi: 10.3390/ijms20215323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Toczyłowska-Mamińska R., Olszewska A., Laskowski M., Bednarczyk P., Skowronek K., Szewczyk A. Potassium channel in the mitochondria of human keratinocytes. J. Invest. Dermatol. 2014;134(3):764–772. doi: 10.1038/jid.2013.422. [DOI] [PubMed] [Google Scholar]
- 136.Rusznák Z., Bakondi G., Kosztka L., Pocsai K., Dienes B., Fodor J. Mitochondrial expression of the two-pore domain TASK-3 channels in malignantly transformed and non-malignant human cells. Virchows Arch. : an international journal of pathology. 2008;452(4):415–426. doi: 10.1007/s00428-007-0545-x. [DOI] [PubMed] [Google Scholar]
- 137.Wojtovich A.P., Sherman T.A., Nadtochiy S.M., Urciuoli W.R., Brookes P.S., Nehrke K. SLO-2 is cytoprotective and contributes to mitochondrial potassium transport. PloS One. 2011;6(12) doi: 10.1371/journal.pone.0028287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.León-Aparicio D., Salvador C., Aparicio-Trejo O.E., Briones-Herrera A., Pedraza-Chaverri J., Vaca L. Novel potassium channels in kidney mitochondria: the hyperpolarization-activated and cyclic nucleotide-gated HCN channels. Int. J. Mol. Sci. 2019;20(20) doi: 10.3390/ijms20204995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wrzosek A., Augustynek B., Żochowska M., Szewczyk A. Mitochondrial potassium channels as druggable targets. Biomolecules. 2020;10(8) doi: 10.3390/biom10081200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vennekamp J., Wulff H., Beeton C., Calabresi P.A., Grissmer S., Hansel W. Kv1.3-blocking 5-phenylalkoxypsoralens: a new class of immunomodulators. Mol. Pharmacol. 2004;65(6):1364–1374. doi: 10.1124/mol.65.6.1364. [DOI] [PubMed] [Google Scholar]
- 141.Bodendiek S.B., Mahieux C., Hansel W., Wulff H. 4-Phenoxybutoxy-substituted heterocycles—a structure-activity relationship study of blockers of the lymphocyte potassium channel Kv1.3. Eur. J. Med. Chem. 2009;44(5):1838–1852. doi: 10.1016/j.ejmech.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chandy K.G., Wulff H., Beeton C., Pennington M., Gutman G.A., Cahalan M.D. K+ channels as targets for specific immunomodulation. Trends Pharmacol. Sci. 2004;25(5):280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Szabo I., Bock J., Grassme H., Soddemann M., Wilker B., Lang F. Mitochondrial potassium channel Kv1.3 mediates Bax-induced apoptosis in lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 2008;105(39):14861–14866. doi: 10.1073/pnas.0804236105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Szabo I., Soddemann M., Leanza L., Zoratti M., Gulbins E. Single-point mutations of a lysine residue change function of Bax and Bcl-xL expressed in Bax- and Bak-less mouse embryonic fibroblasts: novel insights into the molecular mechanisms of Bax-induced apoptosis. Cell Death Differ. 2011;18(3):427–438. doi: 10.1038/cdd.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Leanza L., O'Reilly P., Doyle A., Venturini E., Zoratti M., Szegezdi E. Correlation between potassium channel expression and sensitivity to drug-induced cell death in tumor cell lines. Curr. Pharmaceut. Des. 2014;20(2):189–200. doi: 10.2174/13816128113199990032. [DOI] [PubMed] [Google Scholar]
- 146.Ren Y.R., Pan F., Parvez S., Fleig A., Chong C.R., Xu J. Clofazimine inhibits human Kv1.3 potassium channel by perturbing calcium oscillation in T lymphocytes. PloS One. 2008;3(12) doi: 10.1371/journal.pone.0004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cholo M.C., Steel H.C., Fourie P.B., Germishuizen W.A., Anderson R. Clofazimine: current status and future prospects. J. Antimicrob. Chemother. 2012;67(2):290–298. doi: 10.1093/jac/dkr444. [DOI] [PubMed] [Google Scholar]
- 148.Leanza L., Henry B., Sassi N., Zoratti M., Chandy K.G., Gulbins E. Inhibitors of mitochondrial Kv1.3 channels induce Bax/Bak-independent death of cancer cells. EMBO Mol. Med. 2012;4(7):577–593. doi: 10.1002/emmm.201200235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zaccagnino A., Manago A., Leanza L., Gontarewitz A., Linder B., Azzolini M. Tumor-reducing effect of the clinically used drug clofazimine in a SCID mouse model of pancreatic ductal adenocarcinoma. Oncotarget. 2017;8(24):38276–38293. doi: 10.18632/oncotarget.11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kovalenko I., Glasauer A., Schockel L., Sauter D.R., Ehrmann A., Sohler F. Identification of KCa3.1 channel as a novel regulator of oxidative phosphorylation in a subset of pancreatic carcinoma cell lines. PloS One. 2016;11(8) doi: 10.1371/journal.pone.0160658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bauer D., Werth F., Nguyen H.A., Kiecker F., Eberle J. Critical role of reactive oxygen species (ROS) for synergistic enhancement of apoptosis by vemurafenib and the potassium channel inhibitor TRAM-34 in melanoma cells. Cell Death Dis. 2017;8(2) doi: 10.1038/cddis.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nagy D., Gonczi M., Dienes B., Szoor A., Fodor J., Nagy Z. Silencing the KCNK9 potassium channel (TASK-3) gene disturbs mitochondrial function, causes mitochondrial depolarization, and induces apoptosis of human melanoma cells. Arch. Dermatol. Res. 2014;306(10):885–902. doi: 10.1007/s00403-014-1511-5. [DOI] [PubMed] [Google Scholar]
- 153.Hernández-Reséndiz I., Pacheu-Grau D., Sánchez A., Pardo L.A. Inhibition of Kv10.1 channels sensitizes mitochondria of cancer cells to antimetabolic agents. Cancers. 2020;12(4) doi: 10.3390/cancers12040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Savic Azoulay I., Liu F., Hu Q., Rozenfeld M., Ben Kasus Nissim T., Zhu M.X. ASIC1a channels regulate mitochondrial ion signaling and energy homeostasis in neurons. J. Neurochem. 2020;153(2):203–215. doi: 10.1111/jnc.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gururaja Rao S., Patel N.J., Singh H. Intracellular chloride channels: novel biomarkers in diseases. Front. Physiol. 2020;11:96. doi: 10.3389/fphys.2020.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Suh K.S., Mutoh M., Gerdes M., Yuspa S.H. CLIC4, an intracellular chloride channel protein, is a novel molecular target for cancer therapy. J. Invest. Dermatol. Symp. Proc. 2005;10(2):105–109. doi: 10.1111/j.1087-0024.2005.200402.x. [DOI] [PubMed] [Google Scholar]
- 157.Yang J.Y., Jung J.Y., Cho S.W., Choi H.J., Kim S.W., Kim S.Y. Chloride intracellular channel 1 regulates osteoblast differentiation. Bone. 2009;45(6):1175–1185. doi: 10.1016/j.bone.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 158.Ponnalagu D., Gururaja Rao S., Farber J., Xin W., Hussain A.T., Shah K. Molecular identity of cardiac mitochondrial chloride intracellular channel proteins. Mitochondrion. 2016;27:6–14. doi: 10.1016/j.mito.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 159.Zhao W., Lu M., Zhang Q. Chloride intracellular channel 1 regulates migration and invasion in gastric cancer by triggering the ROS-mediated p38 MAPK signaling pathway. Mol. Med. Rep. 2015;12(6):8041–8047. doi: 10.3892/mmr.2015.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ponnalagu D., Singh H. Insights into the role of mitochondrial ion channels in inflammatory response. Front. Physiol. 2020;11:258. doi: 10.3389/fphys.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Szabo I., Lepple-Wienhues A., Kaba K.N., Zoratti M., Gulbins E., Lang F. Tyrosine kinase-dependent activation of a chloride channel in CD95-induced apoptosis in T lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 1998;95(11):6169–6174. doi: 10.1073/pnas.95.11.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Biasutto L., Mattarei A., La Spina M., Azzolini M., Parrasia S., Szabò I. Strategies to target bioactive molecules to subcellular compartments. Focus on natural compounds. Eur. J. Med. Chem. 2019;181:111557. doi: 10.1016/j.ejmech.2019.07.060. [DOI] [PubMed] [Google Scholar]
- 163.Modica-Napolitano J.S., Aprille J.R. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv. Drug Deliv. Rev. 2001;49(1–2):63–70. doi: 10.1016/s0169-409x(01)00125-9. [DOI] [PubMed] [Google Scholar]
- 164.Davis S., Weiss M.J., Wong J.R., Lampidis T.J., Chen L.B. Mitochondrial and plasma membrane potentials cause unusual accumulation and retention of rhodamine 123 by human breast adenocarcinoma-derived MCF-7 cells. J. Biol. Chem. 1985;260(25):13844–13850. [PubMed] [Google Scholar]
- 165.Kafkova A., Trnka J. Mitochondria-targeted compounds in the treatment of cancer. Neoplasma. 2020;67(3):450–460. doi: 10.4149/neo_2020_190725N671. [DOI] [PubMed] [Google Scholar]
- 166.Bakeeva L.E., Grinius L.L., Jasaitis A.A., Kuliene V.V., Levitsky D.O., Liberman E.A. Conversion of biomembrane-produced energy into electric form. II. Intact mitochondria. Biochim. Biophys. Acta. 1970;216(1):13–21. doi: 10.1016/0005-2728(70)90154-4. [DOI] [PubMed] [Google Scholar]
- 167.Murphy M.P., Smith R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 168.Zielonka J., Joseph J., Sikora A., Hardy M., Ouari O., Vasquez-Vivar J. Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem. Rev. 2017;117(15):10043–10120. doi: 10.1021/acs.chemrev.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Murphy M.P. Targeting lipophilic cations to mitochondria. Biochim. Biophys. Acta. 2008;1777(7–8):1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 170.Smith R.A., Hartley R.C., Murphy M.P. Mitochondria-targeted small molecule therapeutics and probes. Antioxidants Redox Signal. 2011;15(12):3021–3038. doi: 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- 171.Guzman-Villanueva D., Mendiola M.R., Nguyen H.X., Yambao F., Yu N., Weissig V. Conjugation of triphenylphosphonium cation to hydrophobic moieties to prepare mitochondria-targeting nanocarriers. Methods Mol. Biol. 2019;2000:183–189. doi: 10.1007/978-1-4939-9516-5_12. [DOI] [PubMed] [Google Scholar]
- 172.Kalyanaraman B., Cheng G., Hardy M., Ouari O., Lopez M., Joseph J. A review of the basics of mitochondrial bioenergetics, metabolism, and related signaling pathways in cancer cells: therapeutic targeting of tumor mitochondria with lipophilic cationic compounds. Redox biology. 2018;14:316–327. doi: 10.1016/j.redox.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Battogtokh G., Cho Y.Y., Lee J.Y., Lee H.S., Kang H.C. Mitochondrial-targeting anticancer agent conjugates and nanocarrier systems for cancer treatment. Front. Pharmacol. 2018;9:922. doi: 10.3389/fphar.2018.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dong L., Gopalan V., Holland O., Neuzil J. Mitocans revisited: mitochondrial targeting as efficient anti-cancer therapy. Int. J. Mol. Sci. 2020;21(21) doi: 10.3390/ijms21217941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dong L., Neuzil J. Targeting mitochondria as an anticancer strategy. Canc. Commun. 2019;39(1):63. doi: 10.1186/s40880-019-0412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Biasutto L., Mattarei A., Sassi N., Azzolini M., Romio M., Paradisi C. Improving the efficacy of plant polyphenols. Anti Canc. Agents Med. Chem. 2014;14(10):1332–1342. doi: 10.2174/1871520614666140627150054. [DOI] [PubMed] [Google Scholar]
- 177.Pan J., Lee Y., Cheng G., Zielonka J., Zhang Q., Bajzikova M. Mitochondria-targeted honokiol confers a striking inhibitory effect on lung cancer via inhibiting complex I activity. iScience. 2018;3:192–207. doi: 10.1016/j.isci.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cheng G., Zhang Q., Pan J., Lee Y., Ouari O., Hardy M. Targeting lonidamine to mitochondria mitigates lung tumorigenesis and brain metastasis. Nat. Commun. 2019;10(1):2205. doi: 10.1038/s41467-019-10042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jin L., Dai L., Ji M., Wang H. Mitochondria-targeted triphenylphosphonium conjugated glycyrrhetinic acid derivatives as potent anticancer drugs. Bioorg. Chem. 2019;85:179–190. doi: 10.1016/j.bioorg.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 180.Dong L.F., Jameson V.J., Tilly D., Cerny J., Mahdavian E., Marín-Hernández A. Mitochondrial targeting of vitamin E succinate enhances its pro-apoptotic and anti-cancer activity via mitochondrial complex II. J. Biol. Chem. 2011;286(5):3717–3728. doi: 10.1074/jbc.M110.186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Alta R.Y.P., Vitorino H.A., Goswami D., Terêsa Machini M., Espósito B.P. Triphenylphosphonium-desferrioxamine as a candidate mitochondrial iron chelator. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2017;30(5):709–718. doi: 10.1007/s10534-017-0039-5. [DOI] [PubMed] [Google Scholar]
- 182.Boukalova S., Stursa J., Werner L., Ezrova Z., Cerny J., Bezawork-Geleta A. Mitochondrial targeting of metformin enhances its activity against pancreatic cancer. Mol. Canc. Therapeut. 2016;15(12):2875–2886. doi: 10.1158/1535-7163.MCT-15-1021. [DOI] [PubMed] [Google Scholar]
- 183.Liu H.N., Guo N.N., Wang T.T., Guo W.W., Lin M.T., Huang-Fu M.Y. Mitochondrial targeted doxorubicin-triphenylphosphonium delivered by hyaluronic acid modified and pH responsive nanocarriers to breast tumor: in vitro and in vivo studies. Mol. Pharm. 2018;15(3):882–891. doi: 10.1021/acs.molpharmaceut.7b00793. [DOI] [PubMed] [Google Scholar]
- 184.Fonseca S.B., Pereira M.P., Mourtada R., Gronda M., Horton K.L., Hurren R. Rerouting chlorambucil to mitochondria combats drug deactivation and resistance in cancer cells. Chem. Biol. 2011;18(4):445–453. doi: 10.1016/j.chembiol.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 185.Hosseinifar N., Goodarzi N., Sharif A.A.M., Amini M., Esfandyari-Manesh M., Dinarvand R. Preparation and characterization of albumin nanoparticles of paclitaxel-triphenylphosphonium conjugates: new approach to subcellular targeting. Drug Res. 2020;70(2–03):71–79. doi: 10.1055/a-1016-6889. [DOI] [PubMed] [Google Scholar]
- 186.Zhang Z., Banerjee M., Davis R.E., Blagg B.S.J. Mitochondrial-targeted Hsp90 C-terminal inhibitors manifest anti-proliferative activity. Bioorg. Med. Chem. Lett. 2019;29(22):126676. doi: 10.1016/j.bmcl.2019.126676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhu Z., Wang Z., Zhang C., Wang Y., Zhang H., Gan Z. Mitochondrion-targeted platinum complexes suppressing lung cancer through multiple pathways involving energy metabolism. Chem. Sci. 2019;10(10):3089–3095. doi: 10.1039/c8sc04871a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rohlenova K., Sachaphibulkij K., Stursa J., Bezawork-Geleta A., Blecha J., Endaya B. Selective disruption of respiratory supercomplexes as a new strategy to suppress her2(high) breast cancer. Antioxidants Redox Signal. 2017;26(2):84–103. doi: 10.1089/ars.2016.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Grymel M., Zawojak M., Adamek J. Triphenylphosphonium analogues of betulin and betulinic acid with biological activity: a comprehensive review. J. Nat. Prod. 2019;82(6):1719–1730. doi: 10.1021/acs.jnatprod.8b00830. [DOI] [PubMed] [Google Scholar]
- 190.Kelso G.F., Porteous C.M., Coulter C.V., Hughes G., Porteous W.K., Ledgerwood E.C. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem. 2001;276(7):4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 191.James A.M., Cochemé H.M., Smith R.A., Murphy M.P. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J. Biol. Chem. 2005;280(22):21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- 192.Adlam V.J., Harrison J.C., Porteous C.M., James A.M., Smith R.A., Murphy M.P. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. Faseb. J. : official publication of the Federation of American Societies for Experimental Biology. 2005;19(9):1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 193.Smith R.A., Murphy M.P. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. N. Y. Acad. Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 194.Ouyang L., Gong J. Mitochondrial-targeted ubiquinone: a potential treatment for COVID-19. Med. Hypotheses. 2020;144:110161. doi: 10.1016/j.mehy.2020.110161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Skulachev V.P. A biochemical approach to the problem of aging: “megaproject” on membrane-penetrating ions. The first results and prospects. Biochemistry Biokhimiia. 2007;72(12):1385–1396. doi: 10.1134/s0006297907120139. [DOI] [PubMed] [Google Scholar]
- 196.James A.M., Sharpley M.S., Manas A.R., Frerman F.E., Hirst J., Smith R.A. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J. Biol. Chem. 2007;282(20):14708–14718. doi: 10.1074/jbc.M611463200. [DOI] [PubMed] [Google Scholar]
- 197.Rodríguez-Enríquez S., Hernández-Esquivel L., Marín-Hernández A., Dong L.F., Akporiaye E.T., Neuzil J. Molecular mechanism for the selective impairment of cancer mitochondrial function by a mitochondrially targeted vitamin E analogue. Biochim. Biophys. Acta. 2012;1817(9):1597–1607. doi: 10.1016/j.bbabio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 198.Yan B., Stantic M., Zobalova R., Bezawork-Geleta A., Stapelberg M., Stursa J. Mitochondrially targeted vitamin E succinate efficiently kills breast tumour-initiating cells in a complex II-dependent manner. BMC Canc. 2015;15:401. doi: 10.1186/s12885-015-1394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Prochazka L., Koudelka S., Dong L.F., Stursa J., Goodwin J., Neca J. Mitochondrial targeting overcomes ABCA1-dependent resistance of lung carcinoma to α-tocopheryl succinate. Apoptosis : an international journal on programmed cell death. 2013;18(3):286–299. doi: 10.1007/s10495-012-0795-1. [DOI] [PubMed] [Google Scholar]
- 200.Truksa J., Dong L.F., Rohlena J., Stursa J., Vondrusova M., Goodwin J. Mitochondrially targeted vitamin E succinate modulates expression of mitochondrial DNA transcripts and mitochondrial biogenesis. Antioxidants Redox Signal. 2015;22(11):883–900. doi: 10.1089/ars.2013.5594. [DOI] [PubMed] [Google Scholar]
- 201.Leanza L., Romio M., Becker K.A., Azzolini M., Trentin L., Manago A. Direct pharmacological targeting of a mitochondrial ion channel selectively kills tumor cells in vivo. Canc. Cell. 2017;31(4):516–531. doi: 10.1016/j.ccell.2017.03.003. e10. [DOI] [PubMed] [Google Scholar]
- 202.Venturini E., Leanza L., Azzolini M., Kadow S., Mattarei A., Weller M. Targeting the potassium channel Kv1.3 kills glioblastoma cells. Neurosignals. 2017;25(1):26–38. doi: 10.1159/000480643. [DOI] [PubMed] [Google Scholar]
- 203.Mattarei A., Romio M., Managò A., Zoratti M., Paradisi C., Szabò I. Novel mitochondria-targeted furocoumarin derivatives as possible anti-cancer agents. Frontiers in oncology. 2018;8:122. doi: 10.3389/fonc.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bergermann T., Born L., Ferguson F., Latkovic P., Scheul A., Sonnenschein N. Inhibition of PI-3-K and AKT amplifies Kv1.3 inhibitor-induced death of human T leukemia cells. Cell. Physiol. Biochem. : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2019;53(S1):1–10. doi: 10.33594/000000187. [DOI] [PubMed] [Google Scholar]
- 205.Sassi N., Biasutto L., Mattarei A., Carraro M., Giorgio V., Citta A. Cytotoxicity of a mitochondriotropic quercetin derivative: mechanisms. Biochim. Biophys. Acta. 2012;1817(7):1095–1106. doi: 10.1016/j.bbabio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 206.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94(3):909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Korshunov S.S., Skulachev V.P., Starkov A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416(1):15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 208.Kadenbach B., Ramzan R., Vogt S. High efficiency versus maximal performance—the cause of oxidative stress in eukaryotes: a hypothesis. Mitochondrion. 2013;13(1):1–6. doi: 10.1016/j.mito.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 209.Robb E.L., Hall A.R., Prime T.A., Eaton S., Szibor M., Viscomi C. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018;293(25):9869–9879. doi: 10.1074/jbc.RA118.003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Peruzzo R., Mattarei A., Azzolini M., Becker-Flegler K.A., Romio M., Rigoni G. Insight into the mechanism of cytotoxicity of membrane-permeant psoralenic Kv1.3 channel inhibitors by chemical dissection of a novel member of the family. Redox biology. 2020;37:101705. doi: 10.1016/j.redox.2020.101705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zimin P.I., Garic B., Bodendiek S.B., Mahieux C., Wulff H., Zhorov B.S. Potassium channel block by a tripartite complex of two cationophilic ligands and a potassium ion. Mol. Pharmacol. 2010;78(4):588–599. doi: 10.1124/mol.110.064014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jorgensen C., Darre L., Vanommeslaeghe K., Omoto K., Pryde D., Domene C. In silico identification of PAP-1 binding sites in the Kv1.2 potassium channel. Mol. Pharm. 2015;12(4):1299–1307. doi: 10.1021/acs.molpharmaceut.5b00023. [DOI] [PubMed] [Google Scholar]
- 213.Wirth C., Brandt U., Hunte C., Zickermann V. Structure and function of mitochondrial complex I. Biochim. Biophys. Acta. 2016;1857(7):902–914. doi: 10.1016/j.bbabio.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 214.Cabrera-Orefice A., Yoga E.G., Wirth C., Siegmund K., Zwicker K., Guerrero-Castillo S. Locking loop movement in the ubiquinone pocket of complex I disengages the proton pumps. Nat. Commun. 2018;9(1):4500. doi: 10.1038/s41467-018-06955-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pasciak E.M., Rittichier J.T., Chen C.H., Mubarak M.S., VanNieuwenhze M.S., Peters D.G. Electroreductive dimerization of coumarin and coumarin analogues at carbon cathodes. J. Org. Chem. 2015;80(1):274–280. doi: 10.1021/jo502272g. [DOI] [PubMed] [Google Scholar]
- 216.Chen X., Wang Q., Ni F., Ma J. Structure of the full-length Shaker potassium channel Kv1.2 by normal-mode-based X-ray crystallographic refinement. Proc. Natl. Acad. Sci. U.S.A. 2010;107(25):11352–11357. doi: 10.1073/pnas.1000142107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Coburn C.A., Luo Y., Cui M., Wang J., Soll R., Dong J. Discovery of a pharmacologically active antagonist of the two-pore-domain potassium channel K2P9.1 (TASK-3) ChemMedChem. 2012;7(1):123–133. doi: 10.1002/cmdc.201100351. [DOI] [PubMed] [Google Scholar]
- 218.Smith G.P. Phage display: simple evolution in a petri dish (Nobel Lecture) Angew. Chem. 2019;58(41):14428–14437. doi: 10.1002/anie.201908308. [DOI] [PubMed] [Google Scholar]
- 219.Winter G. Harnessing evolution to make medicines (Nobel Lecture) Angew. Chem. 2019;58(41):14438–14445. doi: 10.1002/anie.201909343. [DOI] [PubMed] [Google Scholar]
- 220.Linciano S., Pluda S., Bacchin A., Angelini A. Molecular evolution of peptides by yeast surface display technology. MedChemComm. 2019;10(9):1569–1580. doi: 10.1039/c9md00252a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang L., Huang Y., Lindstrom A.R., Lin T.Y., Lam K.S., Li Y. Peptide-based materials for cancer immunotherapy. Theranostics. 2019;9(25):7807–7825. doi: 10.7150/thno.37194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kelley S.O., Stewart K.M., Mourtada R. Development of novel peptides for mitochondrial drug delivery: amino acids featuring delocalized lipophilic cations. Pharmaceut. Res. 2011;28(11):2808–2819. doi: 10.1007/s11095-011-0530-6. [DOI] [PubMed] [Google Scholar]
- 223.Lu P., Bruno B.J., Rabenau M., Lim C.S. Delivery of drugs and macromolecules to the mitochondria for cancer therapy. J. Contr. Release : official journal of the Controlled Release Society. 2016;240:38–51. doi: 10.1016/j.jconrel.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 224.Vale N., Duarte D., Silva S., Correia A.S., Costa B., Gouveia M.J. Cell-penetrating peptides in oncologic pharmacotherapy: a review. Pharmacol. Res. 2020:105231. doi: 10.1016/j.phrs.2020.105231. [DOI] [PubMed] [Google Scholar]
- 225.Kardani K., Milani S.H.S.A., Bolhassani A. Cell penetrating peptides: the potent multi-cargo intracellular carriers. Expet Opin. Drug Deliv. 2019;16(11):1227–1258. doi: 10.1080/17425247.2019.1676720. [DOI] [PubMed] [Google Scholar]
- 226.Jean S.R., Ahmed M., Lei E.K., Wisnovsky S.P., Kelley S.O. Peptide-mediated delivery of chemical probes and therapeutics to mitochondria. Acc. Chem. Res. 2016;49(9):1893–1902. doi: 10.1021/acs.accounts.6b00277. [DOI] [PubMed] [Google Scholar]
- 227.Chamberlain G.R., Tulumello D.V., Kelley S.O. Targeted delivery of doxorubicin to mitochondria. ACS Chem. Biol. 2013;8(7):1389–1395. doi: 10.1021/cb400095v. [DOI] [PubMed] [Google Scholar]
- 228.Kim S., Nam H.Y., Lee J., Seo J. Mitochondrion-targeting peptides and peptidomimetics: recent progress and design principles. Biochemistry. 2020;59(3):270–284. doi: 10.1021/acs.biochem.9b00857. [DOI] [PubMed] [Google Scholar]
- 229.Cai J., Li X., Du H., Jiang C., Xu S., Cao Y. Immunomodulatory significance of natural peptides in mammalians: promising agents for medical application. Immunobiology. 2020;225(3):151936. doi: 10.1016/j.imbio.2020.151936. [DOI] [PubMed] [Google Scholar]
- 230.Prasad S.V., Fiedoruk K., Daniluk T., Piktel E., Bucki R. Expression and function of host defense peptides at inflammation sites. Int. J. Mol. Sci. 2019;21(1) doi: 10.3390/ijms21010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Roudi R., Syn N.L., Roudbary M. Antimicrobial peptides as biologic and immunotherapeutic agents against cancer: a comprehensive overview. Front. Immunol. 2017;8:1320. doi: 10.3389/fimmu.2017.01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tornesello A.L., Tagliamonte M., Tornesello M.L., Buonaguro F.M., Buonaguro L. Nanoparticles to improve the efficacy of peptide-based cancer vaccines. Cancers. 2020;12(4) doi: 10.3390/cancers12041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mikaelian A.G., Traboulay E., Zhang X.M., Yeritsyan E., Pedersen P.L., Ko Y.H. Pleiotropic anticancer properties of scorpion venom peptides: rhopalurus princeps venom as an anticancer agent. Drug Des. Dev. Ther. 2020;14:881–893. doi: 10.2147/DDDT.S231008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yavari B., Mahjub R., Saidijam M., Raigani M., Soleimani M. The potential use of peptides in cancer treatment. Curr. Protein Pept. Sci. 2018;19(8):759–770. doi: 10.2174/1389203719666180111150008. [DOI] [PubMed] [Google Scholar]
- 235.Herzig V., Cristofori-Armstrong B., Israel M.R., Nixon S.A., Vetter I., King G.F. Animal toxins - nature's evolutionary-refined toolkit for basic research and drug discovery. Biochem. Pharmacol. 2020;181:114096. doi: 10.1016/j.bcp.2020.114096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tajti G., Wai D.C.C., Panyi G., Norton R.S. The voltage-gated potassium channel K(V)1.3 as a therapeutic target for venom-derived peptides. Biochem. Pharmacol. 2020;181:114146. doi: 10.1016/j.bcp.2020.114146. [DOI] [PubMed] [Google Scholar]
- 237.Díaz-García A., Varela D. Voltage-gated K(+)/Na(+) channels and scorpion venom toxins in cancer. Front. Pharmacol. 2020;11:913. doi: 10.3389/fphar.2020.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Norton R.S., Chandy K.G. Venom-derived peptide inhibitors of voltage-gated potassium channels. Neuropharmacology. 2017;127:124–138. doi: 10.1016/j.neuropharm.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 239.Huang G., Docampo R. The mitochondrial calcium uniporter interacts with subunit c of the ATP synthase of trypanosomes and humans. mBio. 2020;11(2) doi: 10.1128/mBio.00268-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Caterino M., Ruoppolo M., Mandola A., Costanzo M., Orrù S., Imperlini E. Protein-protein interaction networks as a new perspective to evaluate distinct functional roles of voltage-dependent anion channel isoforms. Mol. Biosyst. 2017;13(12):2466–2476. doi: 10.1039/c7mb00434f. [DOI] [PubMed] [Google Scholar]
- 241.Messina A., Reina S., Guarino F., Magri A., Tomasello F., Clark R.E. Live cell interactome of the human voltage dependent anion channel 3 (VDAC3) revealed in HeLa cells by affinity purification tag technique. Mol. Biosyst. 2014;10(8):2134–2145. doi: 10.1039/c4mb00237g. [DOI] [PubMed] [Google Scholar]
- 242.Shoshan-Barmatz V., Ben-Hail D., Admoni L., Krelin Y., Tripathi S.S. The mitochondrial voltage-dependent anion channel 1 in tumor cells. Biochim. Biophys. Acta. 2015;1848(10 Pt B):2547–2575. doi: 10.1016/j.bbamem.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 243.Mathupala S.P., Heese C., Pedersen P.L. Glucose catabolism in cancer cells. The type II hexokinase promoter contains functionally active response elements for the tumor suppressor p53. J. Biol. Chem. 1997;272(36):22776–22780. doi: 10.1074/jbc.272.36.22776. [DOI] [PubMed] [Google Scholar]
- 244.Mathupala S.P., Ko Y.H., Pedersen P.L. Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin. Canc. Biol. 2009;19(1):17–24. doi: 10.1016/j.semcancer.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ciscato F., Filadi R., Masgras I., Pizzi M., Marin O., Damiano N. Hexokinase 2 displacement from mitochondria-associated membranes prompts Ca(2+) -dependent death of cancer cells. EMBO Rep. 2020;21(7) doi: 10.15252/embr.201949117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chiara F., Castellaro D., Marin O., Petronilli V., Brusilow W.S., Juhaszova M. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PloS One. 2008;3(3) doi: 10.1371/journal.pone.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bantug G.R., Fischer M., Grählert J., Balmer M.L., Unterstab G., Develioglu L. Mitochondria-endoplasmic reticulum contact sites function as immunometabolic hubs that orchestrate the rapid recall response of memory CD8(+) T cells. Immunity. 2018;48(3):542–555. doi: 10.1016/j.immuni.2018.02.012. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pastorino J.G., Shulga N., Hoek J.B. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 2002;277(9):7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 249.Bryan N., Raisch K.P. Identification of a mitochondrial-binding site on the N-terminal end of hexokinase II. Biosci. Rep. 2015;35(3) doi: 10.1042/BSR20150047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Liu D., Angelova A., Liu J., Garamus V.M., Angelov B., Zhang X. Self-assembly of mitochondria-specific peptide amphiphiles amplifying lung cancer cell death through targeting the VDAC1-hexokinase-II complex. J. Mater. Chem. B. 2019;7(30):4706–4716. doi: 10.1039/c9tb00629j. [DOI] [PubMed] [Google Scholar]
- 251.Woldetsadik A.D., Vogel M.C., Rabeh W.M., Magzoub M. Hexokinase II-derived cell-penetrating peptide targets mitochondria and triggers apoptosis in cancer cells. Faseb. J. : official publication of the Federation of American Societies for Experimental Biology. 2017;31(5):2168–2184. doi: 10.1096/fj.201601173R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Abu-Hamad S., Zaid H., Israelson A., Nahon E., Shoshan-Barmatz V. Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: mapping the site of binding. J. Biol. Chem. 2008;283(19):13482–13490. doi: 10.1074/jbc.M708216200. [DOI] [PubMed] [Google Scholar]
- 253.Shoshan-Barmatz V., Zakar M., Rosenthal K., Abu-Hamad S. Key regions of VDAC1 functioning in apoptosis induction and regulation by hexokinase. Biochim. Biophys. Acta. 2009;1787(5):421–430. doi: 10.1016/j.bbabio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 254.Al Jamal J.A. Involvement of porin N,N-dicyclohexylcarbodiimide-reactive domain in hexokinase binding to the outer mitochondrial membrane. Protein J. 2005;24(1):1–8. doi: 10.1007/s10930-004-0600-2. [DOI] [PubMed] [Google Scholar]
- 255.Pastorino J.G., Hoek J.B., Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Canc. Res. 2005;65(22):10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- 256.Pastorino J.G., Hoek J.B. Regulation of hexokinase binding to VDAC. J. Bioenerg. Biomembr. 2008;40(3):171–182. doi: 10.1007/s10863-008-9148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Guo Y., Wei L., Zhou Y., Lu N., Tang X., Li Z. Flavonoid GL-V9 induces apoptosis and inhibits glycolysis of breast cancer via disrupting GSK-3β-modulated mitochondrial binding of HKII. Free Radic. Biol. Med. 2020;146:119–129. doi: 10.1016/j.freeradbiomed.2019.10.413. [DOI] [PubMed] [Google Scholar]
- 258.Xue Y.N., Yu B.B., Li J.L., Guo R., Zhang L.C., Sun L.K. Zinc and p53 disrupt mitochondrial binding of HK2 by phosphorylating VDAC1. Exp. Cell Res. 2019;374(1):249–258. doi: 10.1016/j.yexcr.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 259.Bigi A., Beltrami E., Trinei M., Stendardo M., Pelicci P.G., Giorgio M. Cyclophilin D counteracts P53-mediated growth arrest and promotes Ras tumorigenesis. Oncogene. 2016;35(39):5132–5143. doi: 10.1038/onc.2016.42. [DOI] [PubMed] [Google Scholar]
- 260.Machida K., Ohta Y., Osada H. Suppression of apoptosis by cyclophilin D via stabilization of hexokinase II mitochondrial binding in cancer cells. J. Biol. Chem. 2006;281(20):14314–14320. doi: 10.1074/jbc.M513297200. [DOI] [PubMed] [Google Scholar]
- 261.Shoshan-Barmatz V., Krelin Y., Chen Q. VDAC1 as a player in mitochondria-mediated apoptosis and target for modulating apoptosis. Curr. Med. Chem. 2017;24(40):4435–4446. doi: 10.2174/0929867324666170616105200. [DOI] [PubMed] [Google Scholar]
- 262.Arzoine L., Zilberberg N., Ben-Romano R., Shoshan-Barmatz V. Voltage-dependent anion channel 1-based peptides interact with hexokinase to prevent its anti-apoptotic activity. J. Biol. Chem. 2009;284(6):3946–3955. doi: 10.1074/jbc.M803614200. [DOI] [PubMed] [Google Scholar]
- 263.Arbel N., Shoshan-Barmatz V. Voltage-dependent anion channel 1-based peptides interact with Bcl-2 to prevent antiapoptotic activity. J. Biol. Chem. 2010;285(9):6053–6062. doi: 10.1074/jbc.M109.082990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lee J.H., Engler J.A., Collawn J.F., Moore B.A. Receptor mediated uptake of peptides that bind the human transferrin receptor. Eur. J. Biochem. 2001;268(7):2004–2012. doi: 10.1046/j.1432-1327.2001.02073.x. [DOI] [PubMed] [Google Scholar]
- 265.Prezma T., Shteinfer A., Admoni L., Raviv Z., Sela I., Levi I. VDAC1-based peptides: novel pro-apoptotic agents and potential therapeutics for B-cell chronic lymphocytic leukemia. Cell Death Dis. 2013;4:e809. doi: 10.1038/cddis.2013.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Shteinfer-Kuzmine A., Amsalem Z., Arif T., Zooravlov A., Shoshan-Barmatz V. Selective induction of cancer cell death by VDAC1-based peptides and their potential use in cancer therapy. Molecular oncology. 2018;12(7):1077–1103. doi: 10.1002/1878-0261.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Pittala S., Krelin Y., Shoshan-Barmatz V. Targeting liver cancer and associated pathologies in mice with a mitochondrial VDAC1-based peptide. Neoplasia. 2018;20(6):594–609. doi: 10.1016/j.neo.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shteinfer-Kuzmine A., Arif T., Krelin Y., Tripathi S.S., Paul A., Shoshan-Barmatz V. Mitochondrial VDAC1-based peptides: attacking oncogenic properties in glioblastoma. Oncotarget. 2017;8(19):31329–31346. doi: 10.18632/oncotarget.15455. [DOI] [PMC free article] [PubMed] [Google Scholar]