Abstract
Global epidemiological studies show that chronic non-communicable diseases such as atherosclerosis and metabolic disorders represent the leading cause of premature mortality and morbidity. Cardiovascular disease such as ischemic heart disease is a major contributor to the global burden of disease and the socioeconomic health costs. Clinical and epidemiological data show an association of typical oxidative stress markers such as lipid peroxidation products, 3-nitrotyrosine or oxidized DNA/RNA bases with all major cardiovascular diseases. This supports the concept that the formation of reactive oxygen and nitrogen species by various sources (NADPH oxidases, xanthine oxidase and mitochondrial respiratory chain) represents a hallmark of the leading cardiovascular comorbidities such as hyperlipidemia, hypertension and diabetes. These reactive oxygen and nitrogen species can lead to oxidative damage but also adverse redox signaling at the level of kinases, calcium handling, inflammation, epigenetic control, circadian clock and proteasomal system. The in vivo footprints of these adverse processes (redox biomarkers) are discussed in the present review with focus on their clinical relevance, whereas the details of their mechanisms of formation and technical aspects of their detection are only briefly mentioned. The major categories of redox biomarkers are summarized and explained on the basis of suitable examples. Also the potential prognostic value of redox biomarkers is critically discussed to understand what kind of information they can provide but also what they cannot achieve.
Keywords: Redox biomarker, Oxidative stress, Cardiovascular disease, Comorbidities
Graphical abstract
Highlights
-
•
Global burden of disease, oxidative stress and cardiovascular disease (CVD).
-
•
Discussion of the different categories of redox biomarkers in CVD.
-
•
Discussion of prognostic value of classical oxidative stress markers in human CVD.
-
•
Discussion of novel redox biomarkers for risk stratification in human CVD.
-
•
Highlighting the additivity of redox biomarkers in human CVD comorbidities.
Abbreviations
- 3-NT
3-nitrotyrosine;
- 4-HNE
4-hydroxynonenal
- 8-iso-PGF2α
8-iso-prostaglandin F2α
- 8-OHdG
8-hydroxy-2-deoxyguanosine;
- 8-OHG
8-hydroxyguanosine (also 8-oxoGuo)
- AF
atrial fibrillation
- AGE
advanced glycation endproduct
- CAD
coronary artery disease
- cGMP
cyclic guanosine monophosphate
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CVD
cardiovascular disease
- GSH
reduced glutathione
- GSSG
oxidized glutathione disulfide;
- HR
hazard ratio
- HIF-1α
hypoxia-inducible factor-1α
- IHD
ischemic heart disease
- I/R
ischemia/reperfusion
- LDL
low-density lipoprotein
- MDA
malondialdehyde
- MI
myocardial infarction
- MPO
myeloperoxidase
- NOS
nitric oxide synthase
- NOX
NADPH oxidase
- OR
odds ratio
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RR
relative risk
- SMD
standardized mean difference
- sNox2-dp
soluble nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) 2-derived peptide;
- SOD
superoxide dismutase
- WHO
World Health Organization
- XO
xanthine oxidase
1. Introduction
Within the last decades the global burden of disease experienced a shift from maternal, perinatal and nutritional causes as well as communicable (infectious) diseases to non-communicable diseases (e.g. atherosclerosis, hypertension and diabetes), which may be best explained by the demographic shift in population, exacerbation of environmental pollution but also improved global nutrition, medication and sanitation [1,2]. The future projections of the World Health Organization (WHO), based on data of the Global Health Observatory, predict a 77% share of non-communicable diseases in the global burden of disease in 2030 [3,4]. In 2016, 44% of all global deaths caused by chronic non-communicable diseases were of cardiovascular origin [5], with ischemic heart disease (IHD) as a leading risk factor and a global prevalence of 128 million people as well as 9 million attributable premature deaths mainly caused by myocardial infarction (MI) and heart failure [6]. Importantly, non-communicable diseases represent or generate comorbidities (e.g. hyperlipidemia, hypertension or diabetes) that have great impact on cardiovascular risk and mortality [7]. These lines of evidence highlight the importance of comorbidities for the assessment of overall cardiovascular risk and the need for more and better biomarkers to characterize and provide early diagnosis of cardiovascular risk factors.
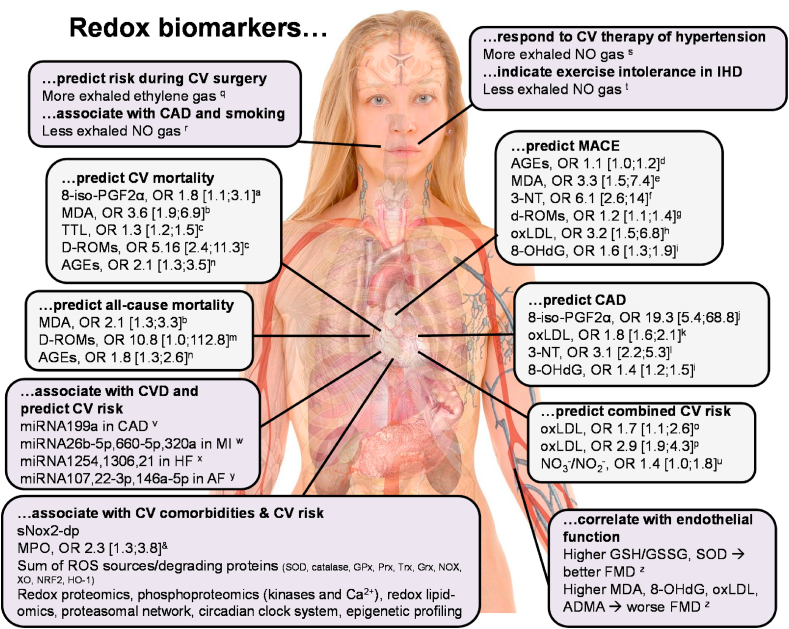
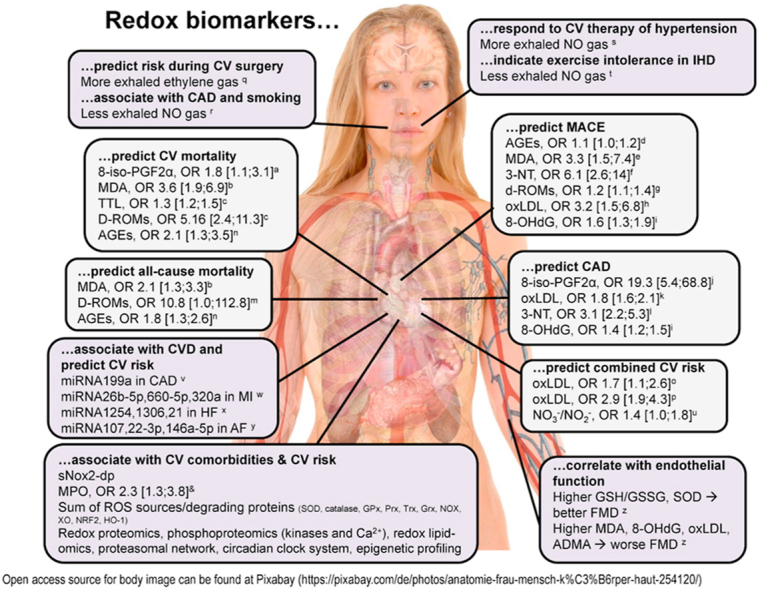
The mentioned comorbidities are all associated with an increased burden of oxidative stress (Fig. 1) [8,9], which is characterized by excessive formation or insufficient break-down of reactive oxygen and nitrogen species (ROS2 and RNS,3 definitions as explained in Ref. [10] and the footnotes below) or inadequate repair of the resulting oxidative damage [11,12]. The resulting accumulation of oxidatively modified biomolecules and dysregulation of cellular redox signaling can be used as a diagnostic tool, in the form of defined redox biomarkers, to assess the magnitude of oxidative damage and alterations in redox pathways [13]. The concept of oxidative stress as a trigger of disease and death dates back to the year 1956, when Harman in 1956 postulated the “free radical theory of aging” [14]. However, also more than 60 years later, we rarely find drugs in clinical use that are by definition acting via an antioxidant mechanism, thereby questioning the “free radical theory of aging” [15,16]. In contrast, most cardiovascular mainstay drugs such as AT1-receptor blockers, angiotensin-converting enzyme inhibitors, statins, beta-blockers and many more possess potent pleiotropic antioxidant properties [17,18].
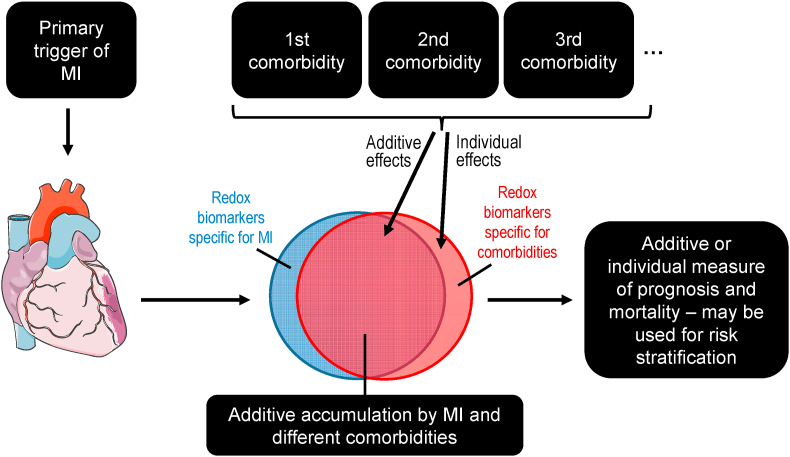
Fig. 1.
Concept of redox biomarkers for comorbidities in myocardial infarction (MI). Identification of specific redox biomarkers for different comorbidities (e.g. hypertension, hyperlipidemia or diabetes) in major cardiovascular events (e.g. MI) would significantly advance redox diagnostic approaches. Currently most redox biomarkers belong to the category of „additive accumulation” that could at least be used as prognostic tools to identify patients with multiple comorbidities representing the group of highest cardiovascular risk. Heart cartoon from Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License.
Although most large clinical trials dedicated to the therapeutic efficacy of classical antioxidants such as vitamins C and E failed to demonstrate a clear benefit for cardiovascular prognosis (for review see Refs. [[19], [20], [21]]), there is clear evidence that most cardiovascular diseases (CVD) are associated with or even triggered by oxidative stress [22,23]. This fact is also well supported by phenotypic changes in animal models of CVD with genetic deletion or overexpression of enzymes involved in the synthesis or degradation of ROS and RNS [18,19,24,25]. Further support for the oxidative stress concept comes from modern approaches using systems biology to identify therapeutic targets based on redox processes within a specific disease and systematic overviews of redox drug candidates in advanced clinical phase II-III trials [26,27]. Overall the discrepancy between the rather disappointing outcome of antioxidant trials and the established presence of redox biomarkers as well as drugable redox targets in CVD highlights the need of better knowledge on sources of ROS and RNS, their interaction, discrimination between beneficial (physiological) redox signaling and detrimental (pathophysiological) oxidative damage pathways [10]. This knowledge gap can be potentially filled by better characterization and application of redox biomarkers, which may be used for personalized or precision medicine approaches to identify patients with high burden of oxidative stress and to apply optimized redox therapeutic strategies [28], as already suggested for cancer and cardiovascular therapy in particular as well as pharmacotherapy guidance and validation of drug efficacy in general [29,30]. In the subsequent paragraphs we will discuss different approaches to identify and quantify ROS and RNS formation in CVD by their footprints in the form of redox biomarkers with a critical assessment of their specificity and prognostic value.
2. Redox biomarkers
2.1. Sources of ROS/RNS and their major role in health and disease
Primary sources of ROS such as NADPH oxidases and the mitochondrial respiratory chain can generate secondary sources of ROS by oxidative conversion of xanthine dehydrogenase (XDH) to xanthine oxidase (XO) or NO-producing endothelial nitric oxide synthase (eNOS) to the uncoupled superoxide-generating enzyme [31]. NADPH oxidases are highly specialized ROS producing protein complexes with almost no other biological function [21]. Mitochondria, under normal conditions, produce ROS as a byproduct of the excessive use of oxygen, transport of electrons from reducing factors along the respiratory complexes and escape of these electrons to molecular oxygen (up to 1% of the total flow of electrons by estimation) [32]. In pathological situations, e.g. upon ischemia/reperfusion (I/R) the (redox) activation of p66Shc or monoamine oxidases (MAO), activation of mitochondrial ATP-sensitive potassium channels and opening of the mitochondrial permeability transition pore (mPTP) can dramatically increase the formation and release of ROS from mitochondria [25]. Also, a ROS-induced ROS formation via redox-based amplification mechanisms was described [33,34]. ROS can exert beneficial and detrimental effects, which makes their therapeutic targeting so difficult and so far mostly unsuccessful (reviewed extensively in Ref. [10]). They can have protective effects via preconditioning-like mechanisms, kill pathogens during host defense or even contribute to essential cellular redox signaling (e.g. in cell differentiation, proliferation and migration). Vice versa, after exceeding a certain threshold, ROS have adverse effects including oxidative damage of various biomolecules, activation of inflammatory and cell death (e.g. apoptotic) pathways. Dysregulated redox signaling, e.g. by dysregulation of protein kinase pathways and calcium handling [35,36], epigenetic homeostasis [[37], [38], [39]], lipid metabolism [36] and vascular function [18,40], is counter-balanced by oxidatively activated antioxidant defense systems like NRF2/KEAP1 or hypoxia-inducible factor-1α (HIF-1α) that control multiple ROS/RNS-degrading enzymes (e.g. heme oxygenase-1 (HO-1), superoxide dismutases (SOD) or thiol-based reductases/peroxidases) [12,26,41]. An example of this kind of dysregulated redox signaling was established for peripartum cardiomyopathy [42]. Redox biomarkers should help to discriminate these fundamentally different properties of ROS/RNS in health and disease enabling the search for novel redox therapeutic drugs.
2.2. What information should redox biomarkers provide and what basic requirements should they fulfill?
As discussed in detail by Frijhoff and colleagues, redox biomarkers should fullfill the following minimal requirements and provide the following minimal level of information [43]: By definition of the WHO, a biomarker represents any substance, structure or functional parameter that can be measured in a biological sample or the whole organism with prognostic value regarding the progression/severity of a disease or the health outcome [44]. In order to add clarity to these criteria, a clinically useful biomarker should be specific for a certain disease (diagnostic), have prognostic value or reflect the disease state or in other words the health risk of an individual. This information will allow monitoring of the therapeutic efficacy of drugs in already diagnosed illness or help to choose the appropriate drug and initiate therapy in so far undiagnosed disease states. For routine use in the clinics, a biomarker should possess a sufficient stability during measurement and storage and should be easily accessible in widely obtainable tissues or preferably in blood. Finally, cost-effectiveness is a major criterion in modern health care systems and reproducibly on a large scale. Criteria for biomarkers in general and redox biomarkers in particular were further refined by Ghezzi and coworkers providing more useful definitions (e.g. for discrimination between passive and active biomarkers) [15,45].
Besides these minimal essential requirements a biomarker should fulfill, it would be also desirable to learn more about the nature of ROS/RNS (helpful for approaches using specific ROS/RNS scavengers) as well as the cellular site and organ system of their formation (helpful to understand the pathophysiological role of ROS/RNS in the respective disease) [10]. However, for the simple conclusion that a certain disease is associated with excessive ROS and RNS formation (oxidative stress), their chemical identity is not required and accordingly sophisticated ROS and RNS detection techniques are not necessary but classical oxidative stress biomarkers (e.g. 3-nitrotyrosine (3-NT)- or 4-hydroxynonenal (4-HNE)-positive proteins or 8-oxo-G-positive RNA) will suffice for qualitative assessment of oxidative stress [46]. This approach may also be enough for simple tests of redox drugs, where it will be sufficient to see whether the oxidative phenotype in diseased animals or challenged cells changes and the tested drug successfully suppresses oxidative stress mediated damage. These tests can be further refined by treatment with specific inhibitors of ROS/RNS sources (e.g. NOX inhibitors, XO inhibitors) or specific scavengers (e.g. SOD mimetics, mitochondria-targeted antioxidants), which will add to further mechanistic insights, even for classical oxidative stress marker-based assays.
2.3. Categories of redox biomarkers
Redox biomarkers can be separated into different categories as also defined in the past (Fig. 2) [10,43]. They comprise the classical oxidative stress markers that in most cases represent endproducts of oxidation reactions such as lipid peroxidation, oxidized amino acids (free or protein-bound), oxidized DNA/RNA bases (free or DNA/RNA-bound) and changes in thiol redox state (simplest example is reduced glutathione (GSH)/oxidized glutathione disulfide (GSSG) ratio). Another category of redox biomarkers relies on expression/activity of enzymes that are involved in the formation (e.g. NOX, XO) or breakdown (e.g. SODs, peroxiredoxins) of ROS/RNS. Also the modulation of reporter molecules for signaling cascades (e.g. phosphorylated vasodilator-stimulated phosphoprotein [P-VASP] as the endtarget of NO/cyclic guanosine monophosphate (cGMP) signaling) or concentrations of endogenous inhibitors (e.g. asymmetric dimethyl arginine as inhibitor of eNOS [47]) as well as eNOS uncoupling surrogates (e.g. S-glutathionylation of eNOS) can serve as redox biomarkers [48]. As these rather traditional redox biomarkers, at least if not a plethora of them is determined in the same sample, provide only limited mechanistic insights regarding ROS/RNS identity or pathophysiology and are considered not specific at all for a certain disease or comorbidity, we will here focus on the evaluation of their prognostic value and their additivity in the presence of different comorbidities in human disease studies. Finally, redox profiling represents an emerging technique to obtain a complete picture of the redox changes that characterize a certain disease. Examples are redox lipidomics that characterizes the landscape of oxidized fatty acid products by means of mass spectroscopy, kinase activity networks using phosphoproteomics or epigenetic profiling. These emerging techniques may yield specific redox profiles of certain disease and also provide detailed mechanistic insights.
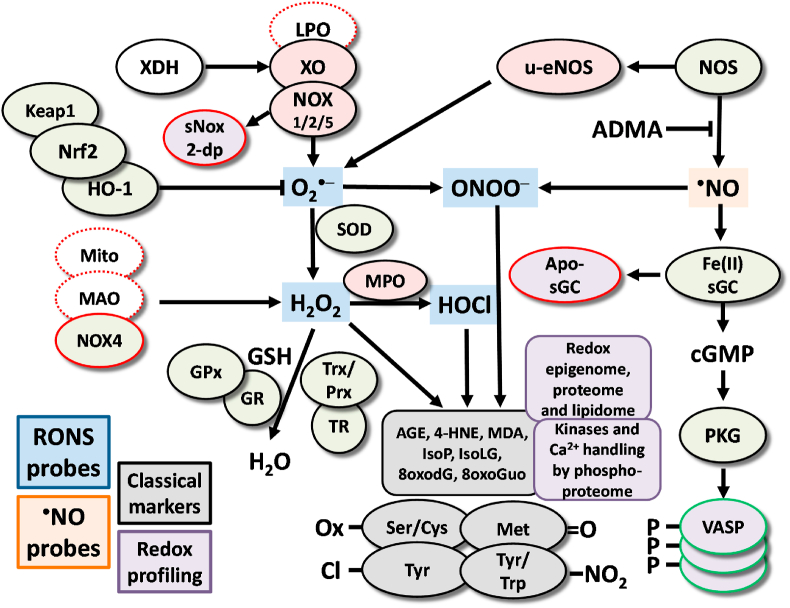
Fig. 2.
Redox pathways associated with putative biomarkers of oxidative stress [10,43]. The processes that lead to oxidative modifications of proteins, lipids and nucleotides are highly complex. Enzymes, such as XO, NOX and NOS, as well as organelles such as mitochondria, can produce ROS and RNS. ROS/RNS from primary sources can trigger secondary ROS sources (e.g. conversion of XDH to XO or uncoupling of eNOS) [31]. ROS can serve as substrates for other enzymes to generate additional types of ROS, such as the generation of HOCl from H2O2 by MPO. Importantly, some ROS sources are solely producing detrimental ROS (filled red color, e.g. NOX1/2/5), some have physiological functions but generate ROS as a side product or in the presence of certain substrates (dashed red line, e.g. LPO, Mito) or even produce mostly beneficial ROS (filled red color with red line, e.g. NOX4). Cellular systems and enzymes, including the SOD, GSH/GPx and Trx/Prx system, counterbalance the production of ROS (filled green color). In addition, increased levels of ROS activate the transcription factor Nrf2 via Keap1 to transcribe genes such as HO-1 that are involved in counteracting these ROS (filled green color). Oxidative stress affects cGMP signaling through its effects on nitric oxide (•NO) production, scavenging and on the •NO producing enzyme eNOS as well as the •NO receptor sGC (by oxidative heme depletion to the apo-sGC) and its target kinase PKG. In principle, all of these mentioned ROS producing, detoxifying and oxidatively modified enzymes and systems represent redox biomarkers as their expression or activity will provide information on the redox balance. ROS and RNS can be directly detected using specific probes (e.g. by chemiluminescence, fluorescence based HPLC or EPR-based methods) [46,57]. ROS and RNS also leave footprints in vivo in the form of classical oxidative stress markers such as oxidized amino acids in proteins or oxidized nucleotides in DNA/RNA as well as free or protein-bound reactive aldehydes from glucotoxicity and lipid peroxidation (filled grey color) [43]. In addition, functional readout of signaling pathways (e.g. redox-regulated kinases and calcium-handling [35,36], epigenetic [[37], [38], [39]] and lipidome profiles [36], P-VASP as the endpoint of the NO/cGMP signaling cascade and novel activity markers such as sNox2-dp or apo-sGC; all filled purple color) can significantly add to our understanding and identification of redox imbalances. For the sake of clarity, we could not show and discuss all existing ROS/RNS sources, species and targets in the present scheme, but focused on the most important ones according to the cited references. Abbreviations: AGE, advanced glycation end products; cGMP, cyclic guanosine monophosphate; EPR, electron paramagnetic resonance; GSH, glutathione; GPx, GSH peroxidase; GR, GSH reductase; H2O2, hydrogen peroxide; HO-1, heme oxygenase-1; HOCl, hypochlorous acid; LPO, lipoxygenases; MAO, monoamine oxidase; MPO, myeloperoxidase; NOS, nitric oxide synthase; NOX, NADPH oxidase; ONOO−, peroxynitrite; PKG, protein kinase G; Prx, peroxiredoxin; P-VASP, phospho(Ser239)-vasodilator-stimulated phosphoprotein; RNS, reactive nitrogen species; ROS, reactive oxygen species; sGC, soluble guanylate cyclase; sNox2-dp, soluble Nox2-derived peptide; SOD, superoxide dismutase; Trx, thioredoxin; TR, Trx reductase; u-eNOS, uncoupled eNOS; XDH, xanthine dehydrogenase; XO, xanthine oxidase. Reused from Ref. [43] and updated from Refs. [10,57,161] with permission under the terms of the Creative Commons Attribution Noncommercial License. Copyright © [43]; Published by Mary Ann Liebert, Inc. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.4. Direct detection of ROS and RNS formation using fluorescence, chemiluminescence or UV/Vis absorption
The direct measurement of ROS and RNS formation represents a redox biomarker, although not easily applicable in large cohort studies as the assays need to be applied to freshly harvested tissue or biological fluid samples (at least the incubation of the biological samples with the probes). These direct ROS and RNS assays can provide information on the involved reactive species and are mostly based on fluorescence or chemiluminescence dyes combined with HPLC- or plate reader-based detection/quantification methods as well as on UV/Vis photometric assays. These techniques will not be discussed further here as they were reviewed in detail in the past. The classical “old” assays such as hydroethidium-dependent fluorescence microtopography in tissue cryo-sections to assess vascular ROS formation, lucigenin-enhanced chemiluminescence in tissue membrane fractions to quantify NADPH oxidase activity and luminol derivative (L-012)-enhanced chemiluminescence in whole blood to determine oxidative burst of leukocytes were critically discussed extensively in the past [46]. The measurement of oxidative burst by L-012-enhanced chemiluminescence in whole blood was also successfully applied in clinical studies and correlated with the walking distance as well as critical limb ischemia in patients with peripheral artery disease [49,50]. The combination of advanced HPLC-based fluorescence assays for the quantification of ROS and RNS formation was described for cell culture [51] that can be also used for specific detection of cellular superoxide generation [52]. In addition there are excellent reviews available discussing the detection of ROS and RNS formation in biological samples [53,54], including statements of the American Heart Association defining first and second choice assays [55].
Other experimental techniques comprise ROS-induced formation of microbubbles that are formed from liposome-encapsulated allylhydrazine, a liquid compound that yields nitrogen and propylene gas, which may be detected by ultrasound by in vivo application to animals [56] and in the future potentially in humans (reviewed in Ref. [9]). Also, L-band electron paramagnetic resonance (EPR) spectroscopy in combination with the most advanced spin traps represents a cutting-edge technique for the in vivo detection of ROS and RNS, which is currently translated from animal to human application [57]. Furthermore, the combination of different techniques such as immuno-spin trapping as reported for trapping of thiyl radicals in proteins or other radicals in DNA by 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and formation of stable spin-adducts with subsequent immunological detection of DMPO-bound proteins or DNA molecules is already used for the detection of ROS and RNS formation, or more precisely their footprints in biological samples [58]. Additionally, the targeting of specific chemiluminescent or fluorescent dyes and/or ROS and RNS scavengers by linking them to site specific antibodies or loading them into nanoparticles might provide the next step in oxidative stress and redox biology research and may be used in humans in the near future (reviewed in Refs. [57,59]). For preclinical research also genetically encoded fluorescent enzymatic probes, e.g. HyPer for hydrogen peroxide detection [60,61] and geNOps for nitric oxide [62,63], will in the future allow the highly specific and timely as well as spatially resolved monitoring of ROS and RNS formation in isolated cells. More recently, the technique of genetically-encoded redox sensors was also translated to the animal level allowing the in vivo monitoring of redox changes in organs, even distinguishing between cytosolic and mitochondrial ROS/RNS formation by encoding a cytosolic or mitochondrial glutaredoxin/green fluorescent protein construct (Grx1-roGFP2) [64,65]. Redox changes in tissues of these animals can be even conserved by alkylation of reduced thiols in the Grx1-roGFP2 construct for later “redox histology” using fluorescence microscopy [64,66].
3. Classical “oxidative stress” biomarkers in human cardiovascular disease
Mounting evidence indicates classical biomarkers of oxidative stress to be a prominent feature and suggestive of disease severity in several CVD phenotypes by displaying increased expression of these markers in CVD compared with control groups. Importantly, these markers are also partly predictive of future cardiovascular events and mortality in initially healthy subjects after adjustment for established cardiovascular risk factors, thereby demonstrating an independent crucial role of oxidative stress in the development and progression of CVD and mortality. However, future studies should address the incremental value of oxidative stress markers beyond established cardiovascular risk factors for the prediction of incident cardiovascular events and related outcomes.
3.1. Lipid peroxidation products 8-isoprostane, 4-hydroxynonenal and malondialdehyde
Malondialdehyde (MDA) is one of the best studied and most abundant (10–20 μM in plasma) endproducts of lipid peroxidation chain reactions [10,43]. Other members of this class of redox biomarkers comprise peroxidation products of cholesterol, phospholipid and polyunsaturated fatty acids including ketones such as 4-HNE [67,68], alcohols such as isoprostanes [69], hydroperoxides such as 1-palmitoyl-2-(5′-oxo-valeroyl)-sn-glycero-3-phosphocholine, oxidized low-density lipoprotein (LDL) [70] and cyclic endoperoxides. Polyunsaturated fatty acids (free or in phospholipids) containing unconjugated olefinic bonds are easily oxidized by ROS/RNS and derived free radicals [71]. Commonly reported examples of oxidized cholesterols (oxysterols) include 6-cholesten-5α-hydroperoxide, 7-ketocholesterol, 7-dehydrocholesterol and 25-hydroxycholesterol [72]. We here focus on 8-isoprostane, 4-HNE and MDA as they are most widely used in the clinical routine, fairly specific ELISAs are commercially available and their formation mechanism is fully characterized [10,43,67]. The gold standard for detection of these products are gas and liquid chromatography in combination with mass spectrometry [73], which can at least be used to validate the results obtained with ELISA in selected samples. Of note, due to their reactivity 4-HNE and MDA (and other reactive lipid peroxidation products) can bind to thiol or amino groups in proteins (or other biomolecules) and thereby modulate the activity of enzymes and may act as toxifiers in disease pathomechanisms or confer redox hormesis via electrophilic activation of antioxidant defense systems. As a note of caution, we would like to mention that MDA and 4-HNE can also be formed by ROS-independent mechanisms such as the fragmentation of prostaglandin endoperoxide (PGH2) or diendoperoxide (generated by oxygenation of arachidonic acid by cyclooxygenase-2 and 5-lipoxygenase) during platelet aggregation or inflammation [74].
A nested case-cohort study in postmenopausal women by Roest et al. showed that elevated levels of urinary 8-iso-prostaglandin F2α (8-iso-PGF2α) were associated with increased cardiovascular mortality due to coronary artery disease (CAD) (141 deaths) and stroke (109 deaths) after a follow-up of 18 years [75]. An odds ratio (OR) of 1.8 (95% CI 1.1–3.1) was observed for the highest quartile of 8-iso-PGF2α levels (Fig. 3A), independent of age, while further adjustment for systolic blood pressure, history of CVD, diabetes, smoking, and body mass index did not attenuate this association. A more recent meta-analysis from 2017, aimed at classifying oxidative damages across 50 human health conditions including 242 studies, displayed small increases in 8-iso-PGF2α levels for hypertension (standardized mean difference (SMD) between affected and control group - Hedges'g 0.4, 95% CI 0.2–0.6), chronic heart failure (g 1.4, 95% CI 1.1–1.7), CAD (g 0.4, 95% CI 0.3–0.5), and ischemic stroke (g 1.2, 95% CI 1.0–1.4) compared to control groups [76]. A case-control study on 93 CAD patients and 93 age- and sex-matched control subjects demonstrated increased levels of urinary 8-iso-PGF2α in CAD patients with multivariable analyses revealing an OR of 19.3 (95% CI 5.4–68.8) for the highest tertile (vs. lowest) of 8-iso-PGF2α after adjustment for CAD risk factors [77]. Similar results were obtained by Wolfram et al. presenting increased levels of plasma, serum, salivary and urinary 8-iso-PGF2α in CAD patients (n = 20) with subgroup analyses indicating positive and negative correlations of 8-iso-PGF2α with New York Heart Association (NYHA) classification stages and left ventricular ejection fraction, respectively [78]. Contrarily, a study from Woodward et al. found no association between 8-iso-PGF2α levels and risk of fatal or non-fatal CAD (OR 1.08, 95% CI 0.91–1.29 per 1-standard deviation (SD) increase in 8-iso-PGF2α levels) for the comparison of 227 coronary cases and 420 control subjects [79].
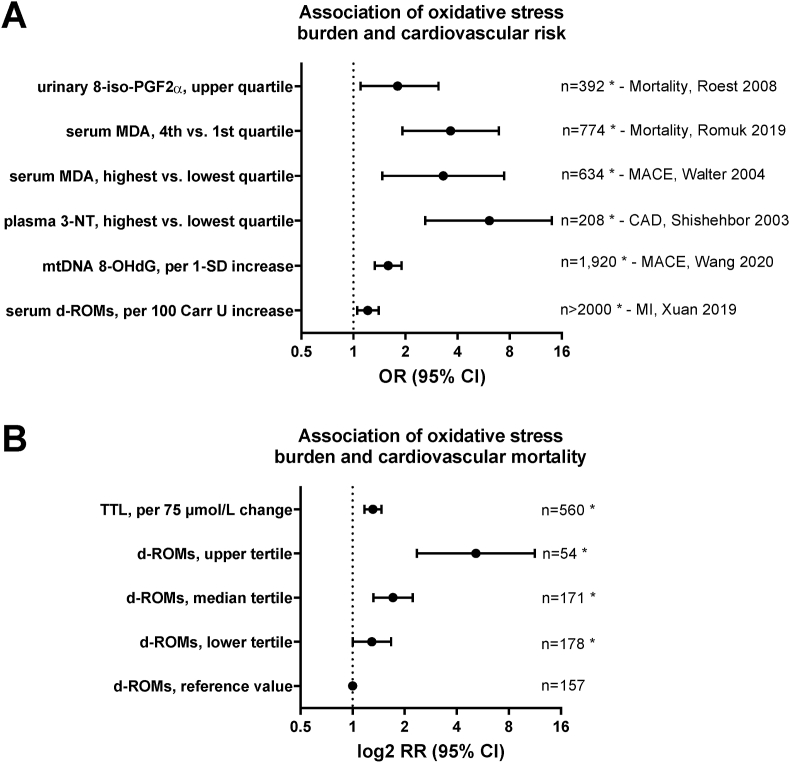
Fig. 3.
Prognostic value of classical oxidative stress markers for cardiovascular risk. (A) Association of markers of lipid peroxidation (isoprostane [8-iso-PGF2α] and malondialdehyde [MDA]) [75,83,85], nitro-oxidative stress (3-nitrotyrosine [3-NT]) [96], oxidative mitochondrial DNA damage (8-hydroxy-deoxyguanosine [8-OHdG]) [120] and derivatives of reactive oxygen metabolites (d-ROMs) [125] with cardiovascular risk in the comparison of healthy subjects with cardiovascular disease patients. (B) Associations of d-ROM levels and total thiol levels (TTL) with cardiovascular disease-specific mortality (adjusted for age and sex). d-ROMs groups [Carratelli Units]: reference, <340; T1, 341–400; T2, 401–500; T3, >500. Graph was generated from tabular data in Ref. [124] with permission and reused from Ref. [48]. Copyright © 2015, Schöttker et al. * indicates significant differences (p < 0.05) versus reference groups.
Nakamura et al. showed 5-fold increased 4-HNE levels in 23 patients with dilated cardiomyopathy compared with 13 control subjects with normal cardiac function [80]. The treatment of 11 subjects for 9 ± 4 months with carvedilol (22 ± 8 mg/d), a beta-blocker with antioxidant capacity, reduced 4-HNE levels by 40% along with functional amelioration of heart failure symptoms. In a study by Mak et al. comparison of 8 patients with chronic heart failure with 8 age-matched patients with normal left ventricular function revealed elevated levels of total aldehydes including 4-HNE in heart failure patients [81]. Barrios et al. reported increased 4-HNE levels in 22 patients with acute MI compared with 20 control subjects without presence of acute CAD. Infarct size was strongly correlated with 4-HNE levels [82].
In 774 chronic heart failure patients, MDA was a significant predictor of death and a combined endpoint of death and heart transplantation at one-year follow-up [83]. After adjustment for a range of clinical and laboratory variables, elevated levels of serum MDA were associated with a two-fold increased risk of death (hazard ratio (HR) 2.103, 95% CI 1.330–3.325) as well as a two-fold increased risk of the combined endpoint (HR 2.000, 95% CI 1.366–2.928 per 1 μmol/L). For the comparison of the highest vs. lowest quartile of baseline MDA levels the relative risk (RR) of death was 3.64 (95% CI, 1.917–6.926) and the RR for the combined endpoint was 2.71 (95% CI 1.551–4.739) (Fig. 3A). In line with this, Radovanovic et al. reported that high plasma MDA levels (>8 μmol/L) in 120 chronic heart failure patients independently increased risk of death in median 13-month follow-up [84]. Patients with MDA levels above the cut-off had an eight times higher risk of death. A large prospective study of 634 patients with stable CAD from the PREVENT trial was able to demonstrate that serum MDA increased the risk of cardiovascular events [85]. For the comparison of the highest vs. lowest quartile of MDA, a RR of 3.30 (95% CI 1.47–7.42) for major vascular events (fatal/non-fatal MI and stroke) (Fig. 3A), a RR of 4.10 (95% CI 2.55–6.60) for non-fatal vascular events (unstable angina) and a RR of 3.84 (95% CI 2.56–5.76) for major vascular procedures (percutaneous interventions and coronary artery bypass grafting) were observed.
A meta-analysis of 12 observational studies aimed at investigating the association between oxidized LDL and risk of atherosclerotic CVD revealed an overall effect size of 1.79 (95% 1.56–2.05) [86]. Among 1371 patients with acute MI, increased levels of oxidized LDL were associated with a combined endpoint of death due to CVD, occurrence of unstable angina pectoris and MI at 6-month follow-up (HR 1.66, 95% CI 1.07–2.57 for highest vs. lowest tertile) [87]. Another study has shown a 215% (95% CI 47–576) increased risk of a major adverse coronary event in 246 patients with CAD in dependence of oxidized LDL levels (for highest vs. lowest quartile) [88]. An association between oxidized LDL and cardiovascular events was also reported in a study of 425 patients with acute coronary syndrome by demonstrating an HR of 2.88 (95% CI 1.93–4.32 per 1 mmol/L increase in oxidized LDL) for acute MI recurrence or acute coronary syndrome-related death at 5-year follow-up [89].
Ethylene in the breathing air of patients undergoing cardiopulmonary bypass surgery allowed real-time monitoring of endogenous lipid peroxidation in the intraoperative setting and was indicative of regional myocardial ischemia and reperfusion [90]. The authors suggest ethylene monitoring as a non-invasive method of risk stratification and prevention of intraoperative complications during cardiopulmonary bypass surgery. However, so far this method requires expensive instruments (mass spectrometry) that may not be affordable and applicable in every small hospital.
3.2. 3-Nitrotyrosine as a footprint of peroxynitrite in vivo formation (or inflammatory activation of the myeloperoxidase/hydrogen peroxide/nitrite pathway) and other NO-related markers
The major nitro-oxidative protein modifications elicited by peroxynitrite (ONOO−) are S-glutathiolation and S-oxidation of cysteine as well as nitration of tyrosine residues. Other modifications are zinc finger oxidation, methionine sulfoxidation and nitration of tryptophan residues by ROS as well as RNS [10,43]. We here focus on 3-NT as there is substantial clinical evidence for its association with disease pathogenesis, fairly specific ELISAs are commercially available and its formation mechanism is well understood. Importantly, most other oxidative protein modifications are not specific for ONOO−. Regarding a summary of the clinical evidence for other protein-based oxidative stress markers, also including protein carbonyl groups, we refer to previous overviews [10,43]. The immunological detection methods can be validated by mass spectrometry-based methods. Importantly, also 3-NT can act as a toxifier in disease pathogenesis by triggering autoimmune reactions (IgGs against 3-NT) that also correlate with CAD health outcomes [91], inactivating important ROS scavenging and vasodilator-producing enzymes such as Mn-SOD [92] and prostacyclin synthase [93], or aggravating the prothrombotic effects of fibrinogen [94,95].
A case-control study (100 cases with established CAD and 108 controls with no clinical history or symptoms suggestive of CAD) by Shishehbor et al. aimed at investigating the association of systemic 3-NT levels and the presence of CAD [96]. The authors demonstrated that in patients with CAD, 3-NT levels were markedly higher compared with control subjects (median values, 9.1 vs. 5.2 μmol/mol). Likewise, prevalence of CAD increased with higher 3-NT quartiles (26% in the lowest vs. 58% in the highest quartile). After multivariable adjustment for the Framingham Global Risk Score and levels of C-reactive protein, increased 3-NT levels were associated with higher odds of CAD (OR 6.1, 95% confidence interval (CI) 2.6–14.0 for highest vs. lowest quartile) (Fig. 3A). Moreover, statin therapy in 35 participants (atorvastatin 10 mg/d) for 12 weeks reduced 3-NT levels by 25%. Concordantly, Pirro et al. were able to demonstrate that the combination of a 4-week rosuvastatin therapy (10 mg/d) and low-fat diet was associated with a pronounced decrease in 3-NT levels as well as arterial stiffness, a well-established marker of CVD risk, in 35 patients with primary hypercholesterolemia (vs. 36 patients with a low-fat diet only) [97]. A study of 120 prediabetic patients by Chu et al. demonstrated increased levels of 2-h 3-NT levels after 75g oral glucose tolerance test in patients with concomitant CAD compared with patients having no CAD [98]. After multivariable adjustment for risk factors and glucose tolerance status, 2-h 3-NT levels in the highest quartile (OR 3.1, 95% CI 2.2–5.3 vs. lowest quartile) remained independently associated with the presence of CAD. Conversely, a recent study by Quidim et al. found that 3-NT levels were not predictive of mortality rates during a follow-up period of four years in a cohort of 342 patients with CAD [99].
Also, other products of nitric oxide-dependent reactions or even NO gas itself were used in the past or may be used in the future to test their diagnostic and prognostic value in CVD. Although nitrotryptophan derivatives were not used so far as markers of nitro-oxidative stress in the clinical setting, their measurement may provide superior insights in the RNS being formed and analytical methods to determine their levels in tissues and biological fluids have been established [100]. Another emerging marker of nitro-oxidative stress that was not applied in clinical studies so far is 8-nitroguanosine-3′,5′-cyclic monophosphate (8-nitro-cGMP), which itself can act as an electrophile and contribute to cellular redox regulation [101,102]. Nitrated fatty acids represent another footprint of RNS reactions in vivo, display potent electrophilic properties and contribute to cellular redox signaling [103,104]. Of note, nitro-fatty acids could not only serve as redox biomarkers but also represent potent therapeutic compounds [105] and analytical methods are in place to detect and quantify these species in biological samples [106]. Recently, the urinary nitrolipidome was quantified using high resolution mass spectrometry with fragmentation analysis [107].
The use of gaseous NO in exhaled air as a redox biomarker is inconclusive so far, as increased NO may be indicative of higher iNOS activity and an inflammatory phenotype, but vice versa may also mirror improved eNOS function and higher endogenous vasodilatory NO production. The exhaled NO concentrations showed a moderate trend of being lower in patients with stable CAD as compared to healthy subjects and was further decreased in both groups among active smokers [108]. Of note, physical exercise decreased exhaled NO concentrations in CAD patients but had only minor effects in healthy subjects, which may indicate dysregulated exercise-induced eNOS activation in the setting of CAD. The link between exhaled NO concentrations and endogenous NO production was supported by a dramatic increase in NO in the breathing air and plasma cGMP levels after infusion of l-arginine in patients with preeclampsia [109]. Therapy of hypertensive patients with the calcium antagonist amlodipine increased exhaled levels of NO in response to exercise [110] and exhaled NO concentrations correlated with exercise intolerance in patients with heart disease [111]. Whereas the measurement of exhaled NO levels as a diagnostic and prognostic tool for assessment of cardiovascular risk is still in its infancy, the application of this assay was already tested on a broader basis in patients with asthma and chronic obstructive pulmonary disease (COPD) to determine the inflammatory status in the lung [112].
The plasma or serum levels of the NO degradation products nitrite and nitrate have only limited diagnostic and prognostic value as they are largely affected by dietary uptake of these anions, especially by certain vegetables. However, in 2885 Framingham Offspring Study subjects, nitrate levels were associated with increased all-cause mortality (HR 1.21, 95% CI 1.03–1.40 per unit increase) at median follow-up of 17.3 years after adjustment common risk factors, whereas no association was observed for incident CVD (HR 1.08, 95% CI 0.89–1.31) [113]. Importantly, in an analysis of 2443 subjects without CVD at baseline, nitrate/nitrite levels were predictive of incident CVD (OR 1.35, 95% CI 1.01–1.80), further showing that nitrate/nitrite measurement added to a better reclassification of individuals at risk of CVD beyond classical cardiovascular risk factors [114].
3.3. Oxidative DNA/RNA damage
Nucleotide oxidation is a highly abundant DNA modification [115] and represents a pre-mutagenic lesion that induces CG:TA transversion mutations, which can also contribute to cancer [116]. The guanine moiety is particularly prone to oxidation under formation of 8-hydroxy-2-deoxyguanosine (8-OHdG) or 8-OHG (8-oxoGuo) and can be used as a marker of DNA and RNA oxidation by Fenton chemistry-derived hydroxyl radicals or peroxynitrite. Levels of free 8-OH(d)G can be measured upon DNA/RNA digestion and hydrolysis from tissues or directly in urine and serum/plasma [10,43]. Whereas commercial ELISAs are available but critically discussed [117], the gold standard for measurement is based on mass spectrometry methods.
A meta-analysis on the association between 8-OHdG levels and CVD (CAD, stroke, peripheral artery disease and carotid atherosclerosis) including five case-control studies and nine prospective studies (810 CVD patients and 1106 control subjects) showed higher levels of 8-OHdG in CVD patients compared with controls (SMD 1.04, 95% CI 0.61–1.47) [118]. In good agreement, a further meta-analysis of six studies (446 heart failure patients and 140 controls) from the same author group showed higher levels of 8-OHdG in heart failure patients than in controls (SMD 0.89, 95% CI 0.68–1.10) with a progressive increase for higher NYHA class [119]. A recent study by Wang et al. from 2020 aimed to investigate whether 8-OHdG may contribute to the incidence of CAD in 1920 patients with type 2 diabetes mellitus [120]. After multivariable adjustment, the authors reported elevated levels of 8-OHdG to be cross-sectionally related to increased odds of obstructive CAD (OR 1.38, 95% CI 1.24–1.52), higher number of diseased vessels with ≥ 50% stenosis (OR 1.29, 95% CI 1.19–1.41), modified Gensini score (OR 1.28, 95% CI 1.18–1.39) and higher levels of C-reactive protein (β 0.18, 95% CI 0.06–0.31). Importantly, increased levels of 8-OHdG were associated with a 59% higher risk of a major adverse cardiovascular and cerebral event in 701 diabetic patients subjected to coronary revascularization at one-year follow-up (hazard ratio (HR) 1.59, 95% CI 1.33–1.90 per 1-SD increase in 8-OHdG levels) (Fig. 3A). A study by Liu et al. found the extent of vascular complications to be associated with elevated RNA damage by measuring 8-HOG (8-oxoGuo) levels in 633 type 2 diabetes mellitus patients compared to 683 controls [121]. Levels of 8-HOG were highest among type 2 diabetes mellitus patients with vascular complications followed by type 2 diabetes mellitus patients without vascular complications and healthy controls, increasing overall with age. In good agreement, in 936 patients with type 2 diabetes mellitus, higher levels of 8-HOG were associated with increased risk of diabetes-related mortality including death from MI, stroke, and peripheral vascular disease at follow-up (HR 1.72, 95% CI 1.11–2.66 for quartile 4 vs. quartile 1) [122]. Furthermore, Broedbaek et al. demonstrated that 8-HOG levels predicted incident CVD in 1381 type 2 diabetes mellitus patients [123].
3.4. Other, less specified classical redox biomarkers such as ROM or TTL
In a meta-analysis from Schöttker et al. serum derivatives of reactive oxygen metabolite (D-ROM) and total thiol (TTL) levels were measured to determine their impact on all-cause and CVD mortality in 10,622 subjects from population-based cohorts from Germany, Poland, Czech Republic and Lithuania [124]. The results revealed that both D-ROM and TTL were independently associated with all-cause (D-ROM: RR 1.20, 95% CI 1.12–1.29 per 1-SD increase; TTL: RR 1.12, 95% CI 1.04–1.21 per 1-SD decrease) and CVD mortality (D-ROM: RR 1.30, 95% CI 1.12–1.51 per 1-SD increase; TTL: RR 1.25, 95% CI 1.09–1.45 per 1-SD decrease) after adjustment for established risk factors (Fig. 3B). A pooled analysis of the same cohorts from Xuan et al. also indicated D-ROM levels to be associated with MI (OR 1.21, 95% CI 1.05–1.40 per 100 Carr units increase) (Fig. 3A) and stroke incidence (OR 1.17, 95% CI 1.01–1.35), while TTL levels were only associated with the incidence of stroke (OR 0.79, 95% CI 0.63–0.99 for quartiles 2–4 vs. quartile 1) [125]. Masaki et al. confirmed the prognostic value of D-ROM by showing that high levels of D-ROM (highest vs. lowest quartile) in 265 patients with treated CVD were associated with increased risk of all-cause mortality (HR 10.791, 95% CI 1.032–112.805) and cardiovascular events (HR 2.651, 95% CI 1.138–6.177) at follow-up [126].
4. Redox-regulated microRNAs in human cardiovascular disease
Beyond the classical oxidative stress biomarkers, epigenetic profiling may represent a promising redox diagnostic strategy in the future, especially as the costs per assay continue to decrease. A group of naturally occurring, small and noncoding RNAs, microRNAs (miRNAs), has been identified as regulators of gene expression that are known to regulate approximately 30% of human genes [127,128]. In the clinical setting, miRNAs are regarded as advantageous biomarkers as they can be easily obtained from peripheral blood samples and are stable at room temperature as well as after being frozen and thawed [129]. A number of miRNA expression levels were shown to change in response to oxidative stress via their respective redox interaction partners comprising NRF2 and SIRT1, both detailed in this review, as well as NF-κB [130,131]. Due to the sheer number of miRNAs described in oxidative-stress-induced heart disease, a selection of prominent miRNAs involved in the onset and progression of CVD will be presented in the next section.
While miRNAs have been recognized as a diagnostic tool which still has to undergo robust clinical evaluation, the future may see multiple ways to therapeutically influence miRNA expression. Antagomirs, oligonucleotides designed to bind and inhibit miRNA [132], as well as miRNA mimics, which are designed to therapeutically re-establish miRNA levels [133], are currently being investigated within clinical trials [134]. Notably, none of the trials involve patients suffering from CVD.
MicroRNAs display a huge potential as a screening marker in CVD while at the same time suffering from an enormous gap of evidence. On the one hand, certain miRNAs were shown to independently predict cardiovascular events in certain pathological conditions (Table 2) while on the other, only a fraction of them has so far been investigated regarding their molecular targets. Further research on the molecular mechanisms of miRNAs in CVD seems warranted as their redox-sensitivity can be assumed from the available data [135], from their own contribution to ROS/RNS formation [136] and from the fact that their levels change in diseases that are associated with oxidative stress.
Table 2.
Selected microRNAs in human CVD.
| miRNA | CVD | Clinical use | Molecular mechanism | Reference |
|---|---|---|---|---|
| 199 | CAD | Associated with MACE reduction | SIRT-1 induction | [140] |
| 21 | Heart failure | Screening parameter | Upregulation of natriuretic peptide B | [147,148] |
| 1254 and 1306‐5p | Heart failure | Association with mortality | Unknown | [145] |
| 26b-5p, 660-5p and 320a | STEMI | MACE prediction | Unknown | [141] |
| 107, 22-3p and 146a-5p | AF | MACE prediction | Unknown | [160] |
MACE: major adverse cardiovascular events; SIRT-1: sirtuin 1; STEMI: ST-elevation myocardial infarction; AF: atrial fibrillation.
4.1. Coronary artery disease and miRNAs 24, 92a and 199a
miRNA-24 is highly expressed in the vasculature [137] and is upregulated in response to vascular injury which in turn indirectly activates the NRF2-pathway by regulating KEAP1 resulting in an overall protective effect [138]. While miRNA-92a overexpression leads to impaired endothelial function by way of reducing heme oxygenase-1 (HO-1) expression, thus promoting the formation of oxidative stress in a murine model for diabetic vasculopathy [139], miRNA-199a expression was found to be lowered in patients with CAD [140]. In these patients, who had undergone coronary artery bypass surgery, miRNA-199a expression levels were found to be reduced in myocardial tissue in those who would be less likely to suffer from major adverse cardiac and cerebrovascular events over the course of three years following surgery [140]. This predictive effect was attributed to the upregulation of SIRT1 in response to lowered miRNA-199a levels.
4.2. Myocardial infarction and miRNAs 26b-5p, 660-5p and 320a
In patients presenting with ST-segment elevation MI (STEMI), a study by Jakob and colleagues found three miRNAs, 26b-5p, 660-5p and 320a, which were associated with cardiac death or recurrent MI within the next year [141]. In this study, miRNA levels in blood samples were shown to independently predict MACE while their predictive value was improved via combination with the clinically used GRACE score. While the miRNAs associated with cardiovascular events in this study are known to be involved in the development of cardiac hypertrophy [142] and cardiomyocyte apoptosis [143], no experimental data are available regarding their redox-sensitivity.
4.3. Heart failure and miRNAs 1254,1306 and 21
Amongst the myriad of microRNAs screened in the serum of patients suffering from heart failure (reviewed in detail by Zhou and colleagues [144]), miRNAs 1254 and 1306 have recently been identified as prognostic markers in the blood of approximately 2200 patients [145]. While in this study, higher levels of miRNAs 1254 and 1306 were associated with the risk for all-cause mortality and hospitalization due to heart failure, the mechanism behind this effect remains unknown [145]. MiRNA-1254 was also predictive of ventricular remodeling in patients with ST-segment-elevation MI [146]. MiRNA-21 was found to be upregulated in murine models for heart failure by Vettori and colleagues [147] as well as in patients suffering from heart failure [[148], [149], [150]], subclinical diabetic cardiomyopathy [151] and diastolic dysfunction [152]. MiRNA-21 is upregulated in multiple types of human cancer [153] and cell-growth in general, and consequently linked to cardiac hypertrophy [154] and vascular neointimal lesion formation [155]. MiRNA-21 was also implicated in suppression of angiogenic progenitor cell function in CAD patients via inhibition of eNOS by asymmetric dimethyl arginine [156]. Of note, treatment with the antagomir antimiRNA-21 prevented myocardial damage and normalized cardiac function in a pig model of I/R injury [157].
4.4. Atrial fibrillation and miRNAs 107, 22-3p and 146a-5p
Within the last two years, miRNAs in atrial fibrillation (AF) have received increased attention. While a number of studies suggest a relationship of certain miRNAs with incident or recurrent AF [158,159], a recent study by Rivera-Caravanca et al. was able to show a correlation between miRNAs 107, 22-3p and 146a-5p and a higher risk of major cardiovascular events during a mean follow-up of 7.6 years [160]. While miRNAs 22-3p and 107 correlated positively with risk (HR 1.07 and 3.66, respectively), a negative correlation was found regarding miRNA 146a-5p (HR 0.86) [160]. For these miRNAs, however, data is lacking on whether they are regulated by oxidative stress.
5. Advanced redox biomarkers
5.1. sNox2-dp as a read-out of NOX2 activation in human cardiovascular/metabolic disease
Although NADPH oxidases are already investigated for therapeutic targeting since more than a decade, there are still no NOX inhibitors in clinical use for the therapy of CVD [161,162]. So far, there are several promising drug candidates for NOX inhibition in clinical phase II and III trials, although not directly for the therapy of CVD but associated comorbidities [27,163].
The molecular proof to demonstrate the direct association of soluble nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) 2-derived peptide (sNox2-dp) with NOX-2 activity was conducted in patients with chronic granulomatous disease4 displaying better endothelial function (measured by flow-mediated dilation, FMD) and lower sNOX2-dp and 8-iso-PGF2α levels [164]. Mechanistically, a burst of ROS was detected in collagen-stimulated platelets and in phorbol ester-stimulated human neutrophils, which was accompanied by a maximum of sNox2-dp release and could be blocked by matrix metallo proteinase-2 inhibition or the NOX-2 inhibitor NOX2ds-tat [165]. The presence of two comorbidities in children, hypercholesterolemia and obesity, resulted in an additive aggravation of 8-isoprostanes and sNox2-dp as well as impairment of endothelial function (FMD) [166]. Higher sNox2-dp as well as oxidized LDL levels were also observed in children with only one risk factor, hypercholesterolemia [167]. Patients with peripheral artery disease had enhanced sNOX2-dp and isoprostane levels but lower NO-derived metabolites and impaired endothelial function (measured by flow-mediated dilation), all of which correlated with more pronounced p47phox translocation to the membrane in patient platelets or white blood cells [168]. Non-alcoholic steatohepatitis (NASH) showed an association with higher levels of 8-isoprostanes and sNox2-dp, which also showed a correlation with histological grading of steatosis and liver inflammation, ballooning and fibrosis [169]. Nonalcoholic fatty liver disease (NAFLD) was associated with higher sNox2-dp and 8-isoprostane levels in patients, also correlating with more severe steatosis and portal inflammation [170] or with markers of infection [171]. Likewise, peripheral endothelial dysfunction (FMD) in patients with NASH also correlated with sNox2-dp and isoprostane levels, all of which was prevented by dietary uptake of polyphenol-rich dark chocolate [172]. Weight loss (≥5%) in patients with metabolic syndrome significantly diminished the serum sNOX2-dp and urinary 8-iso-PGF2α levels and improved the antioxidant status [173].
Patients with COPD and AF showed higher levels of sNox2-dp and 8-isoprostane, which were additively increased in the presence of both risk factors [174]. Patients with obstructive sleep apnoea syndrome (OSAS) had higher urinary 8-iso-PGF2α and serum sNox2-dp levels but lower NO-derived metabolites and impaired endothelial function (measured by flow-mediated dilation), all of which was ameliorated by continuous positive airway pressure therapy (CPAP) [175]. Similar observations were made in children with sleep disordered breathing who showed higher sNox2-dp and 8-iso-PGF2α levels but impaired endothelial function (measured by flow-mediated dilation) [176]. Also, active smokers displayed serum sNox2-dp levels but lower NO-derived metabolites and impaired endothelial function (measured by flow-mediated dilation) [177]. Children exposed to passive smoke also showed similar effects as the active adult smokers [178]. Workers exposed to metal-rich particulate matter with an aerodynamic diameter <10 μm (PM₁₀) had higher urinary 8-iso-PGF2α and 8-OHdG as well as plasma sNOX2-dp levels [179].
5.2. Advanced glycation end products
Advanced glycation end products (AGEs) are a group of highly reactive compounds that are formed by reaction of lipids and proteins with AGE precursors such as (methyl)glyoxal [180]. AGE precursors are generated in the presence of high glucose/fructose levels under oxidative stress conditions as observed in diabetic patients but may also accumulate during the aging process [181] and by dietary uptake [182]. Glycation of lipids and proteins can change the activity and biological properties of these biomolecules but AGEs can also promote crosslinking and further oxidation of biomolecules. AGEs can also bind to their receptor (RAGE) and thereby induce inflammation and oxidative stress [183]. In an elderly population of 2111 U S. subjects free of CVD at baseline, increased levels of carboxymethyl-lysine, a major AGE, were associated with elevated risk of incident cardiovascular events (comprising stroke and/or CAD) after multivariable adjustment (HR 1.11, 95% CI 1.03–1.19 per 1-SD increase) [184]. Likewise, Semba et al. demonstrated increased levels of carboxymethyl-lysine to be associated with increased risk of all-cause (HR 1.84, 95% CI 1.30–2.60 for highest vs. lowest two tertiles) and CVD mortality (HR 2.11, 95% CI 1.27–3.49) in a cohort of 1013 elderly Italian subjects after multivariable adjustment [185]. These observations were also confirmed for subjects without diabetes mellitus. Of note, there are pharmacological inhibitors of the AGE/receptor of AGE (RAGE) axis that may be translated to the clinical setting [186].
5.3. Myeloperoxidase expression and activity
Myeloperoxidase (MPO) expression and activity were proposed as suitable redox biomarkers describing a toxifying mechanism of H2O2 with rather low reactivity in the presence of chloride compared to the more reactive hypochlorite (OCl−) [43,161]. In 1090 patients with acute coronary syndrome, MPO levels were assessed to determine its association with death and MI at 6-month follow-up [187]. The results displayed that elevated MPO levels (>350 μg/L) predicted an increased risk of cardiovascular events (HR 2.25, 95% CI 1.32–3.82), which were also shown to extend the prognostic value derived from traditional biochemical markers. In good agreement with these previous observations, MPO levels predicted increased odds of a major adverse cardiac event at 6-month follow-up in 490 subjects with acute chest pain [188]. Likewise, the activity of MPO was previously measured by 3-chlorotyrosine (or 3-bromotyrosine) concentrations in patients with MI by HPLC with electrochemical detection [189], as well as 2-chloroethidium using liquid chromatography with mass spectrometry quantification [190]. Although the clinical evidence for prognostic value of MPO in CVD is so far moderate, this redox biomarker was proposed as a reliable candidate for future routine use (reviewed in Refs. [161,191]). The importance of MPO as a redox biomarker is supported by several pharmacological MPO inhibitors that are currently investigated within phase II trials [27].
5.4. Redox profiling
In principle any protein involved in ROS/RNS generation or degradation can be used as a redox biomarker and measurement of its expression or activity will provide limited mechanistic insights and will probably also correlate with disease severity or cardiovascular risk. As the number of publications on this topic is huge and even discussion of selected examples would exceed the frame of the present review, we will only add some general considerations here. The expression and activity of antioxidant enzymes such as SODs, catalase, glutathione peroxidases or heme oxygenase-1 represent controversial redox biomarkers as they may be upregulated in one disease and down-regulated in the other. In principle, most antioxidant enzymes are under the control of redox-regulated transcription factors implying that under oxidative stress conditions their expression should be upregulated. However, also expression-suppressing mechanisms may come into play at the epigenetic level or by proteasomal degradation as well as activity of these antioxidant enzymes may be oxidatively inhibited as in the case of Mn-SOD [92]. Indeed, SODs, catalase, glutathione peroxidases are often found down-regulated or inactivated under disease conditions serving as a potential explanation of the observed oxidative stress condition. In contrast, heme oxygenase-1 is often upregulated due to the nature of its regulation via NRF2/KEAP1 that are activated by electrophiles such as ROS, although other regulatory mechanisms exist as well [26]. NRF2/KEAP1 and their antioxidant down-stream targets are therefore suitable redox biomarkers that characterize part of the antioxidant defense system and the process of redox hormesis. Also the activity of dysfunctional redox-sensitive enzymes, e.g. in mitochondria, can be used as a redox biomarker read-out: oxidative inactivation of mitochondrial aldehyde dehydrogenase (ALDH-2) correlated with larger infarct size in mouse models of MI [192] and aconitase activity was associated with lipid oxidation in the human failing heart [193], although both enzymatic activities are so far not regularly used as redox biomarkers in the clinical setting.
In addition, proteasomal activity may represent an attractive readout of redox dysregulation as the proteasomal system is subject to redox regulation [194] and changes in proteasomal activity will ultimately lead to the accumulation of oxidatively modified proteins [195]. Therefore, the determination of markers of proteasome activity by measurement of the concentrations of typical oxidized targets for proteasomal degradation (e.g. protein carbonyls) may provide additional mechanistic insights into dysregulated redox processes. Also impairment of the circadian rhythm is a well-accepted cardiovascular risk factor [196]. Oxidative stress and adverse redox signalling play a central role for the control of the circadian rhythm (also termed “redox control of cellular timekeeping”) [197]. A detailed description of harmful redox modifications within the circadian clock system and the impact of environmental stressors therein can be found in Ref. [198].
Also thiol-based enzymes play an important role for antioxidant defense and cellular redox signaling. Thioredoxins, peroxiredoxins and glutaredoxins play an important role for hydrogen peroxide and organic peroxide degradation but also their expression or activity shows no consistent regulatory readout (up- or down-regulation) for different CVD [199]. Therefore, assessment of a larger number of these redox markers, e.g. by (redox) proteomics [200,201] or transcriptomics, will provide more meaningful insights. The resulting “redox profiles” may show a characteristic pattern of up- and down-regulated antioxidant proteins or mRNA for a specific disease condition. A general description of redox analytical approaches can be found in Refs. [202,203], for redox proteomics of S-glutathionylated proteins in Ref. [204], for H2S-modified proteome (known as the sulfhydrome) in Ref. [205] and for a systems biology approach in Ref. [206].
Redox profiling can be also based on assessment of a metabolic pattern. Determination of the GSH/GSSG ratio is the simplest readout of the cellular or serum/plasma redox state, however, with only limited prognostic implications [43]. Assessment of the trans-sulfuration pathway may be a more meaningful approach in the future [207]. Redox lipidomics (or oxidative phospholipidomics) [208,209] and phosphoproteomics [210] represent smaller subsets of the metabolome and proteome that are optimized to assess altered redox processes or oxidative stress conditions. In addition, the content of extracellular vesicles can provide important information on dysregulated redox processes and represent a useful prognostic tool to assess cardiovascular risk [211].
Finally, redox regulation of epigenetic profiles is a well-known process in the cancer field [212,213]. Epigenetic pathways involve a plethora of redox switches such as direct DNA modification by ROS (e.g. 5-hydroxy-methylcytosine or 8-OHdG), redox control of DNA methyltransferases (DNMTs) or DNA demethylases (e.g. TET) via oxygen/H2O2 or redox-sensitive transcription factors, redox control of histone methyl/acetyltransferases (e.g. SMYD1) as well as demethylases/deacetylases (e.g. Jumonji, sirtuins) [37]. The known redox changes of enzymes involved in epigenetic control in heart disease were also previously summarized in full detail [214]. The above mentioned redox regulation of miRNAs and ncRNAs expression by various mechanisms also adds to this concept [39,136]. In general, the genome stability is redox regulated by various epigenetic and classical pathways [38]. The classical genome integrity maintaining mechanisms comprise the redox control of DNA repair mechanisms, mRNA stability (via GAPDH, AP-1 and HuR) and control of gene expression by redox-sensitive transcription factors such as NRF2, HIF-1α, OxyR and NFkB. Of note, epigenetic changes are implicated in the pathogenesis of CVD [215,216]. In the future, the complete characterization of epigenetic profiles in heart disease may allow conclusions on the contribution of redox signaling pathways or specific ROS/RNS to these epigenetic changes and thereby disease severity/progression.
6. Additivity of redox biomarkers in the presence of multiple comorbidities
According to the proposed concept in Fig. 1, patients with different comorbidities should have additive accumulation of redox biomarkers. As a proof of concept, we here present selected examples. Patients with COPD and AF had elevated sNox2-dp and 8-isoprostane levels that showed an additive increase in the presence of both risk factors (Fig. 4) [174]. 3-NT levels in both abnormal glucose tolerance status and presence of CAD increased compared with the combination of normal glucose tolerance status and the presence of CAD [98]. Smoking, diabetes and body mass index were highly associated with systemic oxidative stress as determined by creatinine-indexed urinary 8-epi-PGF2α levels and a history of CVD [217]. Urinary 8-epi-PGF2α concentrations (normalized to creatinine) were also indicative of the presence of insulin resistance and obesity as envisaged by a dramatic increase in insulin-resistant patients with a body mass index ≥ 30 [218]. There was also an additive accumulation of 8-iso-PGF2α levels in the urine of CAD patients with multiple comorbidities such as obesity, diabetes mellitus, hypercholesterolemia, hypertension and smoking (Fig. 4) [77]. Levels of the AGE marker, carboxymethyl-lysine, in a population-based study were higher in the presence of the comorbidity diabetes and this additive increase was also associated with higher all-cause and CVD mortality of non-diabetic subjects [185]. Plasma levels of 8-iso-PGF2α gradually increased in individuals that were healthy, underwent coronary angiography without coronary artery atherosclerotic lesions or with proven CAD (123.2 ± 9.5, 314.6 ± 40 and 389.6 ± 36.2 pg/ml, respectively) [219]. Importantly, in the patient group, there was a substantial increase in 8-iso-PGF2α concentration in the presence of hypertension as compared to the normotensive group (394.2 ± 42.7 and 232.7 ± 25.1 pg/ml, respectively) and also patients with dyslipidemia displayed higher 8-iso-PGF2α levels (359.1 ± 35.6 and 240.3 ± 34.3 pg/ml). Finally, this study revealed a positive correlation between 8-iso-PGF2α concentrations and age. At the functional level, the presence of the typical cardiovascular comorbidities smoking and hypercholesterolemia aggravated endothelial dysfunction (measured by impaired acetylcholine-dependent forearm blood flow increases by plethysmography) in an additive manner [220]. This trend was also mirrored by the plasma levels of autoantibodies against oxidized LDL, which was significantly elevated in smokers with hypercholesterolemia.
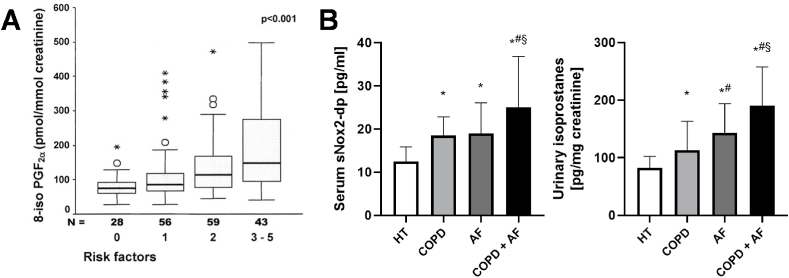
Fig. 4.
Additivity of comorbidities on redox biomarkers levels in patients with cardiovascular disease. (A) Urinary levels of 8-iso-PGF2α showed additive accumulation with risk factors for heart disease (comorbidities such as obesity, diabetes mellitus, hypercholesterolemia, hypertension, and smoking). Median, interquartile range, outliers and extremes of urinary excretion are presented; p-value was calculated for the trend. Reused from Ref. [77] with permission. Copyright © 2004, Wolters Kluwer Health. (B) Levels of sNox2‐dp and urinary isoprostanes in patients with hypertension (HT) and chronic obstructive pulmonary disease (COPD) and/or atrial fibrillation (AF) on top. Data are presented as mean ± SD (n = 33–49). *, p < 0.05 vs. HT; #, p < 0.05 vs. COPD; §, p < 0.05 vs. AF. Graphs were generated from data in Ref. [174] with permission. Copyright © 2019, Mary Ann Liebert Inc.
In a study with 46 patients with rheumatoid arthritis endothelial function, as assessed by FMD and coronary flow reserve, and nitro-oxidative stress, as assessed by 3-NT and MDA, were shown to be related to left ventricular myocardial deformation in rheumatoid arthritis patients [221]. In a double-blind crossover trial in 80 patients with rheumatoid arthritis (60 with CAD and 20 without) patients with CAD had 3-fold higher protein carbonyl, 3-NT, and MDA levels compared to the ones without CAD [222]. In a study of 100 poorly-controlled diabetic patients the reduction of oxidative stress was associated with improved arterial elasticity and myocardial deformation after 12 months of intensive antidiabetic treatment indicating that a decrease of oxidative stress burden was associated with a greater improvement of vascular function and myocardial deformation [223]. In 115 newly diagnosed untreated patients with primary systemic amyloidosis, increased FMD was found to be an independent predictor of all-cause mortality. Increased 3-NT levels were observed in high-risk amyloidosis patients with elevated FMD and hypotension, which may be secondary to increased inflammation and iNOS activity, as exhaled NO gas as well as 3-NT levels were also higher in these patients [224].
A major cardiovascular comorbidity is the aging process and increased (mitochondrial) oxidative stress, impaired endothelial function as well as higher cardiovascular risk in the elderly is well documented [225]. The accumulating oxidative damage with increasing age is also supported by exacerbated lipofuscin concentrations in the lysosomal system of the elderly, where lipofuscin represents a highly aggregated mixture of cross-linked proteins, peroxidized lipids and carbohydrates [226]. Of note, lipofuscins themselves represent a source of ROS and trigger apoptosis, inhibit the proteasomal system and cause mitochondrial dysfunction [227], all of which leads to dysregulated proteostasis and thereby to a loss of a functional proteome due to insufficient removal of damaged/inactive proteins [226]. Despite this appealing concept, at present there are no large scale clinical studies or meta-analyses that link the aging process with accumulation of lipofuscin, whereas accumulation of lipofuscin in the retina seems to be associated with age-related macular degeneration [228]. However, support for an association of oxidative stress and aging comes from a study of 2190 subjects undergoing a health care examination by showing substantial relations to total antioxidant status, total peroxides and autoantibodies against oxidized LDL with age and body mass index [229]. These results were confirmed in smaller studies that demonstrated aging to be correlated with increased oxidative stress or impaired antioxidant defense [[230], [231], [232], [233], [234], [235], [236]]. Moreover, the MARK-AGE project revealed rather moderate associations of markers of oxidative stress or antioxidants with progressing age [237]. Only 3-NT showed a weak correlation with age and higher levels against the middle-aged control group, whereas no comparable trend was found for protein carbonyls and MDA. Only the antioxidant lycopene was lower in all higher age groups, whereas reduced cysteine levels even tended to increase with higher age. The authors concluded that lifestyle and environmental factors have a more pronounced impact on age-related redox biomarkers than previously anticipated [237].Whereas the levels of most redox biomarkers seem to correlate with the progression or severity of a certain disease, so far there are no clear threshold values that could be used for early disease diagnosis, especially since most cardiovascular disease display a multi-comorbidity profile. Therefore, better redox biomarkers or even profiles are needed for a reliable cardiovascular risk stratification and the establishment of redox biomarkers in clinical routine use.
7. Conclusions and outlook
There are basically two major classes of redox biomarkers (Fig. 2). The classical markers of oxidative stress can neither provide specificity for the nature of ROS/RNS nor for certain disease/comorbidities but they have prognostic implications (Fig. 3 and Table 1) and show additivity in the presence of different disease/comorbidities (Fig. 4). The more meaningful redox profiling techniques (e.g. redox proteomics, lipidomics and epigenomics) may help in the future to add the missing specificity to redox diagnostics, to understand and better discriminate beneficial and harmful ROS/RNS processes (e.g. dysregulated kinase, proteasomal, circadian networks) and to identify highly specific redox targets for the development of novel redox drugs. The most promising non-classical redox biomarkers, even when measured as a single parameter, could be NOX enzymes, NRF2 and MPO as they are also subject therapeutic targeting within advanced clinical phase II-III trials on multiple disease categories [26,27]. Combining of these redox biomarkers with functional measurements, e.g. redox-sensitive endothelial function using flow-mediated dilation or acetylcholine-dependent increases in peripheral blood flow by plethysmography [9,238], may further improve their prognostic value. Endothelial function itself also correlates with markers of oxidative stress and redox biomarkers [[239], [240], [241]]. In addition, direct measurement of ROS/RNS formation using specific probes can provide valuable information on the nature, source and disease mechanisms but are hardly applicable to large clinical trials as storage of samples will usually modify the readout due to autoxidation of the probes or inactivation of enzymatic sources in a time-dependent manner. Other protocols for assessment of redox-related biomarkers or biological changes with relevance for preclinical and clinical studies on cardioprotection (e.g. oxygen consumption or mitochondrial membrane potential) were previously summarized in a compendium of practical guidelines [242]. Fig. 5 summarizes the prognostic value of the classical markers of oxidative stress and features of other redox biomarkers. Further support for the prognostic value of redox biomarkers (mostly oxidative stress parameters) comes from multiple studies on pharmacological antioxidant interventions leading to a normalization of these redox biomarkers as well as functional parameters (reviewed in Ref. [18]).
Table 1.
Studies on the association of oxidative stress biomarkers with cardiovascular disease/markers or mortality.
| Biomarker | Study type | Quality level | Sampling/method of detection | Major outcome | Reference |
|---|---|---|---|---|---|
| 3-NT | Case-control study (100 CAD patients, 108 CTR) | +++ | Plasma/stable isotope dilution liquid chromatography–electrospray ionization tandem mass spectrometry-based method using an ion trap mass spectrometer | Increased 3-NT in CAD patients. OR of 4.4 (95% CI 1.8–10.6 for highest vs. lowest quartile) for CAD. | [96] |
| 3-NT | Prospective cohort study (342 CAD patients) | +++ | Serum/colorimetric ELISA | No relationship between 3-NT and mortality rates during 4-year follow-up. | [99] |
| 3-NT | Clinical trial (120 prediabetic patients) | +++ | Plasma/ELISA | 2-h 3-NT after 75 g oral glucose tolerance tests is related to the presence of CAD (OR 3.1, 95% CI 2.2–5.3 for highest vs. lowest quartile). | [98] |
| 3-NT | Animal study | + | Immunohistochemical analysis of rat hearts | Increased 3-NT after ischemia/reperfusion injury in rat hearts. | [243] |
| 3-NT | Animal study | + | Dot blot method analysis of rat hearts | Increased 3-NT after MI in rats. | [244] |
| HNE | Clinical trial (23 patients with dilated cardiomyopathy, 13 CTR) | ++ | Immunohistochemical analysis of endomyocardial biopsies | 5-fold increased HNE in cardiomyopathy patients. Treatment with carvedilol reduces HNE by 40%. | [80] |
| HNE | Clinical trial (22 patients with acute MI, 20 CTR) | ++ | Plasma/high-performance liquid chromatography | Increased HNE in MI patients. Infarct size strongly correlates with HNE. | [245] |
| HNE | Animal study | + | High-performance liquid chromatography analysis of rat hearts | Generation and release of HNE in isolated perfused hearts of normotensive and spontaneously hypertensive rats with cardiac hypertrophy and signs of HF during ischemia and reperfusion. | [246] |
| HNE | Animal study | + | Immunohistochemical and image analysis of rat hearts | Presence of HNE-modified proteins in ischemic rats hearts after transplantation and reperfusion. | [247] |
| 8-iso PGF2α | Case-cohort study (250 cases of death due to CAD and stroke, 142 CTR) | +++ | Urine/liquid chromatography/tandem mass spectrometry | Increased 8-iso PGF2α predicts cardiovascular mortality in postmenopausal women (OR 1.8, 95% CI 1.1–3.1 for highest vs. lowest quartile). | [75] |
| 8-iso PGF2α | Meta-analysis (242 studies) | ++++ | Mixed (plasma, urine, sputum) | Increased 8-iso PGF2α in patients with hypertension (g 0.4, 95% CI 0.2–0.6), chronic HF (g 1.4, 95% CI 1.1–1.7), CAD (g 0.4, 95% CI 0.3–0.5) and ischemic stroke (g 1.2, 95% CI 1.0–1.4). | [76] |
| 8-iso PGF2α | Animal study | + | Serum/enzyme immuno-assay | Increased 8-iso PGF2α in the myocardium of rabbits with HF. 8-iso PGF2α correlates with cardiac function. | [248] |
| 8-iso PGF2α | Animal study | + | Plasma and myocardial tissue/ELISA | Increased 8-iso PGF2α in rats with acute myocardial ischemia. | [249] |
| 8-OHdG | Meta-analysis (14 studies) | ++++ | Mixed (urine and blood) | Increased 8-OHdG in CVD patients (SMD 1.04, 95% CI 0.61–1.47). | [118] |
| 8-OHdG | Meta-analysis (6 studies) | ++++ | Mixed (urine and plasma) | Increased 8-OHdG in HF patients (SMD 0.89, 95% CI 0.68–1.10). | [119] |
| 8-OHdG | Animal study | + | Immunohistochemical and image analysis of rat hearts as well as DNA analysis using ELISA | 8-OHdG is related to cardiac dysfunction after ischemia/reperfusion injury in isolated rat hearts. | [250] |
| 8-OHdG | Animal study | + | Immunohistochemical myocard analysis | Left ventricular dilatation and systolic dysfunction is associated increased 8-OHdG in rabbits after MI. | [251] |
| MDA | Prospective cohort study (774 chronic HF patients) | +++ | Serum/Ohkawa's method | Increased MDA predicts death in chronic HF patients (RR 3.64, 95% CI 1.917–6.926 for highest vs. lowest quartile). | [83] |
| MDA | Prospective cohort study (634 stable CAD patients) | +++ | Serum/TBARS | Increased MDA predicts cardiovascular events in patients with stable CAD (RR 3.30, 95% CI 1.47–7.42 for major vascular events, RR 4.10, 95% CI 2.55–6.60 for non-fatal vascular events and RR 3.84, 95% CI 2.56–5.76 for major vascular procedures). | [85] |
| MDA | Animal study | + | Plasma/ELISA | Increased MDA after myocardial injury induced by sepsis in rats. | [252] |
| MDA | Animal study | + | Immunohistochemical analysis of cardiac tissues | Increased MDA after HF induced by left anterior descending coronary artery ligation rats. | [253] |
| D-ROM | Meta-analysis (10,622 subjects) | ++++ | Serum/FRAP assay | Increased D-ROM predicts all-cause (RR 1.20, 95% CI 1.12–1.29 per 1-SD increase) and CVD mortality (RR 1.30, 95% CI 1.12–1.51) in 10,622 subjects from population-based cohorts. | [124] |
| D-ROM | Pooled case-control study (476 cases of MI and 2380 CTR, 454 cases of stroke and 2270 CTR) | +++ | Serum/FRAP assay | Increased D-ROM predicts MI (OR 1.21, 95% CI 1.05–1.40 per 100 Carr units increase) and stroke (OR 1.17, 95% CI 1.01–1.35). | [125] |
| D-ROM | Animal study | + | Plasma/photometric test | Increased D-ROM in the myocardium of hypoxic rats. | [254] |
| D-ROM | Animal study | + | Serum/fras4 test | Increased D-ROM is related to ventricular remodeling and arrhythmia in rats. | [255] |
| TTL | Meta-analysis (10,622 subjects) | ++++ | Serum/FRAP assay | Increased TTL predicts all-cause (RR 1.12, 95% CI 1.04–1.21 per 1-SD decrease) and CVD mortality (RR 1.25, 95% CI 1.09–1.45) in 10,622 subjects from population-based cohorts. | [124] |
| TTL | Pooled case-control study (476 cases of MI and 2380 CTR, 454 cases of stroke and 2270 CTR) | +++ | Serum/FRAP assay | Increased TTL predicts stroke (OR 0.79, 95% CI 0.63–0.99 for quartiles 2–4 vs. quartile 1). | [125] |
| TTL | Animal study | + | Serum/spectrophotometry | Induction of MI in rats is associated with a decrease in TTL. | [256] |
| TTL | Animal study | + | Cardiac tissue/spectrophotometry | Induction of MI in rats is associated with a decrease in TTL. | [257] |
+: Medium quality (animal study), ++: Good quality (clinical trial/cohort study <100 subjects), +++: Strong quality (clinical trial/cohort study >100 subjects), ++++: Very strong quality (large scale meta-analysis), 3-NT: 3-nitrotyrosine, CAD: Coronary artery disease, CTR: Controls, OR: Odds ratio, CI: Confidence interval, TBARS: Thiobarbituric Acid Reactive Substances, FRAP: Ferric Reducing Antioxidant Power, fras4: Free Radical Analytical System 4, HNE: 4-Hydroxynonenal, MI: Myocardial infarction, HF: Heart failure, 8-iso PGF2α: 8-iso-prostaglandin F2α, g: standardized mean difference - Hedges'g, 8-OHdG: 8-Hydroxy-2-deoxyguanosine, CVD: Cardiovascular disease, MDA: Malondialdehyde, RR: Relative risk, D-ROM: Derivatives of reactive oxygen metabolite, SD: Standard deviation, TTL: Total thiol.
Fig. 5.
Odds ratio (OR) or association of redox biomarkers with health risks or complications. Grey boxes represent classical oxidative stress markers. Purple boxes represent advanced or experimental redox biomarkers. Numbers in square brackets represent the 95% confidence interval. Abbreviations: ADMA, asymmetric dimethylarginine; CV, cardiovascular; CAD, coronary artery disease; CVD, cardiovascular disease; FMD, flow-mediated dilation; IHD, ischemic heart disease; MACE, major cardiovascular events; oxLDL, oxidized low-density lipoprotein. All alphabetical cross references in this figure (a, b, c …) are linked to literature reference numbers in the figure legend. The respective references are as follows: a [75], b [83], c [124], d [184], e [85], f [96], g [125], h [88], i [120], j [77], k [86], l [98], m [126], n [185], o [87], p [89], q [90], r [108], s [110], t [111], u [114], v [140], w [141], x [145,[148], [149], [150], [151], [152]], y [160], z [[239], [240], [241]], & [187]. Open access source for body image can be found at Pixabay (https://pixabay.com/de/photos/anatomie-frau-mensch-k%C3%B6rper-haut-254120/). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Funding
A.D., S.S. and T.M. were supported by vascular biology research grants from the Boehringer Ingelheim Foundation for the collaborative research group “Novel and neglected cardiovascular risk factors: molecular mechanisms and therapeutics”. Further support was provided by the Else-Kröner Fresenius Foundation (2019_A110 to S.D. and 2017_A106 to S.S.), the German Heart Foundation/German Foundation of Heart Research (F/51/19 to S.D.) and the Foundation Heart of Mainz (continuous support to A.D., O.H., S.S. and S.D.). I.A. acknowledges support from the European Union (ERDF) and Greek national funds through the Operational Program “Competitiveness, Entrepreneurship and Innovation”, under the call “RESEARCH – CREATE - INNOVATE” (project code: 5048539). Our research was continuously supported by the European Cooperation in Science and Technology and EU-CARDIOPROTECTION COST-ACTION (CA16225), a funding scheme to enhance scientific networking in Europe. Thomas Münzel is PI of the DZHK (German Center for Cardiovascular Research), Partner Site Rhine-Main, Mainz, Germany.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Throughout this report, the term ROS refers almost exclusively to hydrogen peroxide and superoxide anion radical since both species are involved in redox signaling and are formed by a number of enzymatic sources. Other ROS include the hydroxyl radical or singlet oxygen, which however have most likely no specific roles in redox signaling but rather contribute to unspecific oxidative damage and oxidative stress. A large number of organic peroxides (e.g. lipid peroxides) are also covered by the term ROS but these species are only marginally discussed in the present overview. Other ROS such as hypochlorite are also not in the focus of the present work.
Throughout this report, the term RNS refers almost exclusively to peroxynitrite, peroxynitrous acid and derived free radicals (e.g. hydroxyl radicals and nitrogen dioxide radicals). Peroxynitrite anion has a high specificity for activated thiols and readily reacts with carbon dioxide (the latter supports radical reactions). Peroxynitrous acid also has a high specificity for activated thiols but also reacts with transition metal complexes. RNS also comprises nitric oxide, which upon reaction with oxygen can form nitrogen dioxide radicals or nitrosating species such as N2O3 and has a high affinity for transition metal complexes such as iron(II), all of which contributes to the potent redox signaling properties of nitric oxide. However, in the context of “oxidation of biomolecules” the term RNS does not refer to nitric oxide due to its weak reactivity towards biomolecules in general.
Patients suffering from inborn defects in NADPH function - in the mentioned study gp91phox (x chromosomal recessive CGD) and p47phox (autosomal recessive CGD Type 1).
Contributor Information
Andreas Daiber, Email: daiber@uni-mainz.de.
Thomas Münzel, Email: tmuenzel@uni-mainz.de.
References
- 1.Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H., Amann M., Anderson H.R., Andrews K.G., Aryee M., Atkinson C., Bacchus L.J., Bahalim A.N., Balakrishnan K., Balmes J., Barker-Collo S., Baxter A., Bell M.L., Blore J.D., Blyth F., Bonner C., Borges G., Bourne R., Boussinesq M., Brauer M., Brooks P., Bruce N.G., Brunekreef B., Bryan-Hancock C., Bucello C., Buchbinder R., Bull F., Burnett R.T., Byers T.E., Calabria B., Carapetis J., Carnahan E., Chafe Z., Charlson F., Chen H., Chen J.S., Cheng A.T., Child J.C., Cohen A., Colson K.E., Cowie B.C., Darby S., Darling S., Davis A., Degenhardt L., Dentener F., Des Jarlais D.C., Devries K., Dherani M., Ding E.L., Dorsey E.R., Driscoll T., Edmond K., Ali S.E., Engell R.E., Erwin P.J., Fahimi S., Falder G., Farzadfar F., Ferrari A., Finucane M.M., Flaxman S., Fowkes F.G., Freedman G., Freeman M.K., Gakidou E., Ghosh S., Giovannucci E., Gmel G., Graham K., Grainger R., Grant B., Gunnell D., Gutierrez H.R., Hall W., Hoek H.W., Hogan A., Hosgood H.D., 3rd, Hoy D., Hu H., Hubbell B.J., Hutchings S.J., Ibeanusi S.E., Jacklyn G.L., Jasrasaria R., Jonas J.B., Kan H., Kanis J.A., Kassebaum N., Kawakami N., Khang Y.H., Khatibzadeh S., Khoo J.P., Kok C., Laden F., Lalloo R., Lan Q., Lathlean T., Leasher J.L., Leigh J., Li Y., Lin J.K., Lipshultz S.E., London S., Lozano R., Lu Y., Mak J., Malekzadeh R., Mallinger L., Marcenes W., March L., Marks R., Martin R., McGale P., McGrath J., Mehta S., Mensah G.A., Merriman T.R., Micha R., Michaud C., Mishra V., Mohd Hanafiah K., Mokdad A.A., Morawska L., Mozaffarian D., Murphy T., Naghavi M., Neal B., Nelson P.K., Nolla J.M., Norman R., Olives C., Omer S.B., Orchard J., Osborne R., Ostro B., Page A., Pandey K.D., Parry C.D., Passmore E., Patra J., Pearce N., Pelizzari P.M., Petzold M., Phillips M.R., Pope D., Pope C.A., 3rd, Powles J., Rao M., Razavi H., Rehfuess E.A., Rehm J.T., Ritz B., Rivara F.P., Roberts T., Robinson C., Rodriguez-Portales J.A., Romieu I., Room R., Rosenfeld L.C., Roy A., Rushton L., Salomon J.A., Sampson U., Sanchez-Riera L., Sanman E., Sapkota A., Seedat S., Shi P., Shield K., Shivakoti R., Singh G.M., Sleet D.A., Smith E., Smith K.R., Stapelberg N.J., Steenland K., Stockl H., Stovner L.J., Straif K., Straney L., Thurston G.D., Tran J.H., Van Dingenen R., van Donkelaar A., Veerman J.L., Vijayakumar L., Weintraub R., Weissman M.M., White R.A., Whiteford H., Wiersma S.T., Wilkinson J.D., Williams H.C., Williams W., Wilson N., Woolf A.D., Yip P., Zielinski J.M., Lopez A.D., Murray C.J., Ezzati M., AlMazroa M.A., Memish Z.A. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray C.J., Ezzati M., Flaxman A.D., Lim S., Lozano R., Michaud C., Naghavi M., Salomon J.A., Shibuya K., Vos T., Wikler D., Lopez A.D. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–2066. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 3.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., Alvarado M., Anderson H.R., Anderson L.M., Andrews K.G., Atkinson C., Baddour L.M., Barker-Collo S., Bartels D.H., Bell M.L., Benjamin E.J., Bennett D., Bhalla K., Bikbov B., Bin Abdulhak A., Birbeck G., Blyth F., Bolliger I., Boufous S., Bucello C., Burch M., Burney P., Carapetis J., Chen H., Chou D., Chugh S.S., Coffeng L.E., Colan S.D., Colquhoun S., Colson K.E., Condon J., Connor M.D., Cooper L.T., Corriere M., Cortinovis M., de Vaccaro K.C., Couser W., Cowie B.C., Criqui M.H., Cross M., Dabhadkar K.C., Dahodwala N., De Leo D., Degenhardt L., Delossantos A., Denenberg J., Des Jarlais D.C., Dharmaratne S.D., Dorsey E.R., Driscoll T., Duber H., Ebel B., Erwin P.J., Espindola P., Ezzati M., Feigin V., Flaxman A.D., Forouzanfar M.H., Fowkes F.G., Franklin R., Fransen M., Freeman M.K., Gabriel S.E., Gakidou E., Gaspari F., Gillum R.F., Gonzalez-Medina D., Halasa Y.A., Haring D., Harrison J.E., Havmoeller R., Hay R.J., Hoen B., Hotez P.J., Hoy D., Jacobsen K.H., James S.L., Jasrasaria R., Jayaraman S., Johns N., Karthikeyan G., Kassebaum N., Keren A., Khoo J.P., Knowlton L.M., Kobusingye O., Koranteng A., Krishnamurthi R., Lipnick M., Lipshultz S.E., Ohno S.L., Mabweijano J., MacIntyre M.F., Mallinger L., March L., Marks G.B., Marks R., Matsumori A., Matzopoulos R., Mayosi B.M., McAnulty J.H., McDermott M.M., McGrath J., Mensah G.A., Merriman T.R., Michaud C., Miller M., Miller T.R., Mock C., Mocumbi A.O., Mokdad A.A., Moran A., Mulholland K., Nair M.N., Naldi L., Narayan K.M., Nasseri K., Norman P., O'Donnell M., Omer S.B., Ortblad K., Osborne R., Ozgediz D., Pahari B., Pandian J.D., Rivero A.P., Padilla R.P., Perez-Ruiz F., Perico N., Phillips D., Pierce K., Pope C.A., 3rd, Porrini E., Pourmalek F., Raju M., Ranganathan D., Rehm J.T., Rein D.B., Remuzzi G., Rivara F.P., Roberts T., De Leon F.R., Rosenfeld L.C., Rushton L., Sacco R.L., Salomon J.A., Sampson U., Sanman E., Schwebel D.C., Segui-Gomez M., Shepard D.S., Singh D., Singleton J., Sliwa K., Smith E., Steer A., Taylor J.A., Thomas B., Tleyjeh I.M., Towbin J.A., Truelsen T., Undurraga E.A., Venketasubramanian N., Vijayakumar L., Vos T., Wagner G.R., Wang M., Wang W., Watt K., Weinstock M.A., Weintraub R., Wilkinson J.D., Woolf A.D., Wulf S., Yeh P.H., Yip P., Zabetian A., Zheng Z.J., Lopez A.D., Murray C.J., AlMazroa M.A., Memish Z.A. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Projections of mortality and causes of death, 2016 to 2060. https://www.who.int/healthinfo/global_burden_disease/projections/en/
- 5.WHO Global health observatory data on causes of non-communicable disease in 2016. https://www.who.int/gho/ncd/mortality_morbidity/en/
- 6.Dai H., Much A.A., Maor E., Asher E., Younis A., Xu Y., Lu Y., Liu X., Shu J., Bragazzi N.L. Global, regional, and national burden of ischemic heart disease and its attributable risk factors, 1990-2017: results from the global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes. 2020 doi: 10.1093/ehjqcco/qcaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghoneim S., Dhorepatil A., Shah A.R., Ram G., Ahmad S., Kim C., Asaad I. Non-alcoholic steatohepatitis and the risk of myocardial infarction: a population-based national study. World J. Hepatol. 2020;12:378–388. doi: 10.4254/wjh.v12.i7.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griendling K.K., FitzGerald G.A. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 9.Daiber A., Steven S., Weber A., Shuvaev V.V., Muzykantov V.R., Laher I., Li H., Lamas S., Munzel T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017;174:1591–1619. doi: 10.1111/bph.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egea J., Fabregat I., Frapart Y.M., Ghezzi P., Gorlach A., Kietzmann T., Kubaichuk K., Knaus U.G., Lopez M.G., Olaso-Gonzalez G., Petry A., Schulz R., Vina J., Winyard P., Abbas K., Ademowo O.S., Afonso C.B., Andreadou I., Antelmann H., Antunes F., Aslan M., Bachschmid M.M., Barbosa R.M., Belousov V., Berndt C., Bernlohr D., Bertran E., Bindoli A., Bottari S.P., Brito P.M., Carrara G., Casas A.I., Chatzi A., Chondrogianni N., Conrad M., Cooke M.S., Costa J.G., Cuadrado A., My-Chan Dang P., De Smet B., Debelec-Butuner B., Dias I.H.K., Dunn J.D., Edson A.J., El Assar M., El-Benna J., Ferdinandy P., Fernandes A.S., Fladmark K.E., Forstermann U., Giniatullin R., Giricz Z., Gorbe A., Griffiths H., Hampl V., Hanf A., Herget J., Hernansanz-Agustin P., Hillion M., Huang J., Ilikay S., Jansen-Durr P., Jaquet V., Joles J.A., Kalyanaraman B., Kaminskyy D., Karbaschi M., Kleanthous M., Klotz L.O., Korac B., Korkmaz K.S., Koziel R., Kracun D., Krause K.H., Kren V., Krieg T., Laranjinha J., Lazou A., Li H., Martinez-Ruiz A., Matsui R., McBean G.J., Meredith S.P., Messens J., Miguel V., Mikhed Y., Milisav I., Milkovic L., Miranda-Vizuete A., Mojovic M., Monsalve M., Mouthuy P.A., Mulvey J., Munzel T., Muzykantov V., Nguyen I.T.N., Oelze M., Oliveira N.G., Palmeira C.M., Papaevgeniou N., Pavicevic A., Pedre B., Peyrot F., Phylactides M., Pircalabioru G.G., Pitt A.R., Poulsen H.E., Prieto I., Rigobello M.P., Robledinos-Anton N., Rodriguez-Manas L., Rolo A.P., Rousset F., Ruskovska T., Saraiva N., Sasson S., Schroder K., Semen K., Seredenina T., Shakirzyanova A., Smith G.L., Soldati T., Sousa B.C., Spickett C.M., Stancic A., Stasia M.J., Steinbrenner H., Stepanic V., Steven S., Tokatlidis K., Tuncay E., Turan B., Ursini F., Vacek J., Vajnerova O., Valentova K., Van Breusegem F., Varisli L., Veal E.A., Yalcin A.S., Yelisyeyeva O., Zarkovic N., Zatloukalova M., Zielonka J., Touyz R.M., Papapetropoulos A., Grune T., Lamas S., Schmidt H., Di Lisa F., Daiber A. European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS) Redox biology. 2017;13:94–162. doi: 10.1016/j.redox.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griendling K.K., FitzGerald G.A. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 12.Sies H., editor. Oxidative Stress: Oxidants and Antioxidants. Academic Press; London, UK: 1991. [DOI] [PubMed] [Google Scholar]
- 13.Ho E., Karimi Galougahi K., Liu C.C., Bhindi R., Figtree G.A. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox biology. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 15.Ghezzi P., Floridi L., Boraschi D., Cuadrado A., Manda G., Levic S., D'Acquisto F., Hamilton A., Athersuch T.J., Selley L. Oxidative stress and inflammation induced by environmental and psychological stressors: a biomarker perspective. Antioxidants Redox Signal. 2018;28:852–872. doi: 10.1089/ars.2017.7147. [DOI] [PubMed] [Google Scholar]
- 16.Ghezzi P., Jaquet V., Marcucci F., Schmidt H. The oxidative stress theory of disease: levels of evidence and epistemological aspects. Br. J. Pharmacol. 2017;174:1784–1796. doi: 10.1111/bph.13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steven S., Munzel T., Daiber A. Exploiting the pleiotropic antioxidant effects of established drugs in cardiovascular disease. Int. J. Mol. Sci. 2015;16:18185–18223. doi: 10.3390/ijms160818185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daiber A., Chlopicki S. Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: evidence for redox-based therapies. Free Radic. Biol. Med. 2020;157:15–37. doi: 10.1016/j.freeradbiomed.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Chen A.F., Chen D.D., Daiber A., Faraci F.M., Li H., Rembold C.M., Laher I. Free radical biology of the cardiovascular system. Clin. Sci. (Lond.) 2012;123:73–91. doi: 10.1042/cs20110562. [pii] 10.1042/CS20110562. [DOI] [PubMed] [Google Scholar]
- 20.Gori T., Munzel T. Oxidative stress and endothelial dysfunction: therapeutic implications. Ann. Med. 2011;43:259–272. doi: 10.3109/07853890.2010.543920. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt H.H., Stocker R., Vollbracht C., Paulsen G., Riley D., Daiber A., Cuadrado A. Antioxidants in translational medicine. Antioxidants Redox Signal. 2015;23:1130–1143. doi: 10.1089/ars.2015.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karbach S., Wenzel P., Waisman A., Munzel T., Daiber A. eNOS uncoupling in cardiovascular diseases--the role of oxidative stress and inflammation. Curr. Pharmaceut. Des. 2014;20:3579–3594. doi: 10.2174/13816128113196660748. [DOI] [PubMed] [Google Scholar]
- 23.Munzel T., Gori T., Bruno R.M., Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart J. 2010;31:2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 24.Daiber A., Oelze M., Daub S., Steven S., Schuff A., Kroller-Schon S., Hausding M., Wenzel P., Schulz E., Gori T., Munzel T. Vascular redox signaling, redox switches in endothelial nitric oxide synthase and endothelial dysfunction. In: Laher I., editor. Systems Biology of Free Radicals and Antioxidants. Springer-Verlag; Berlin Heidelberg: 2014. pp. 1177–1211. [Google Scholar]
- 25.Daiber A., Di Lisa F., Oelze M., Kroller-Schon S., Steven S., Schulz E., Munzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 2017;174:1670–1689. doi: 10.1111/bph.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuadrado A., Manda G., Hassan A., Alcaraz M.J., Barbas C., Daiber A., Ghezzi P., Leon R., Lopez M.G., Oliva B., Pajares M., Rojo A.I., Robledinos-Anton N., Valverde A.M., Guney E., Schmidt H. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol. Rev. 2018;70:348–383. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 27.Casas A.I., Nogales C., Mucke H.A.M., Petraina A., Cuadrado A., Rojo A.I., Ghezzi P., Jaquet V., Augsburger F., Dufrasne F., Soubhye J., Deshwal S., Di Sante M., Kaludercic N., Di Lisa F., Schmidt H. On the clinical pharmacology of reactive oxygen species. Pharmacol. Rev. 2020;72:801–828. doi: 10.1124/pr.120.019422. [DOI] [PubMed] [Google Scholar]
- 28.Nogales C., Gronning A.G.B., Sadegh S., Baumbach J., Schmidt H. Network medicine-based unbiased disease modules for drug and diagnostic target identification in ROSopathies. Handbook of experimental pharmacology. 2020. [DOI] [PubMed]
- 29.Schork N.J. Personalized medicine: time for one-person trials. Nature. 2015;520:609–611. doi: 10.1038/520609a. [DOI] [PubMed] [Google Scholar]
- 30.Lee M.S., Flammer A.J., Lerman L.O., Lerman A. Personalized medicine in cardiovascular diseases. Korean Circ J. 2012;42:583–591. doi: 10.4070/kcj.2012.42.9.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz E., Wenzel P., Munzel T., Daiber A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxidants Redox Signal. 2014;20:308–324. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson B.H. The role of manganese superoxide dismutase in health and disease. J. Inherit. Metab. Dis. 1998;21:598–603. doi: 10.1023/a:1005427323835. [DOI] [PubMed] [Google Scholar]
- 33.Zorov D.B., Filburn C.R., Klotz L.O., Zweier J.L., Sollott S.J. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: a mutual interplay. Redox biology. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corcoran A., Cotter T.G. Redox regulation of protein kinases. FEBS J. 2013;280:1944–1965. doi: 10.1111/febs.12224. [DOI] [PubMed] [Google Scholar]
- 37.Kietzmann T., Petry A., Shvetsova A., Gerhold J.M., Gorlach A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br. J. Pharmacol. 2017;174:1533–1554. doi: 10.1111/bph.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mikhed Y., Gorlach A., Knaus U.G., Daiber A. Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair. Redox biology. 2015;5:275–289. doi: 10.1016/j.redox.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miguel V., Lamas S., Espinosa-Diez C. Role of non-coding-RNAs in response to environmental stressors and consequences on human health. Redox biology. 2020:101580. doi: 10.1016/j.redox.2020.101580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gryglewski R.J., Palmer R.M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 41.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sanchez-Perez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox biology. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hilfiker-Kleiner D., Sliwa K. Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat. Rev. Cardiol. 2014;11:364–370. doi: 10.1038/nrcardio.2014.37. [DOI] [PubMed] [Google Scholar]
- 43.Frijhoff J., Winyard P.G., Zarkovic N., Davies S.S., Stocker R., Cheng D., Knight A.R., Taylor E.L., Oettrich J., Ruskovska T., Gasparovic A.C., Cuadrado A., Weber D., Poulsen H.E., Grune T., Schmidt H.H., Ghezzi P. Clinical relevance of biomarkers of oxidative stress. Antioxidants Redox Signal. 2015;23:1144–1170. doi: 10.1089/ars.2015.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO . WHO; Geneva: 2001. Biomarkers in Risk Assessment: Validity and Validation. [Google Scholar]
- 45.Ghezzi P. Environmental risk factors and their footprints in vivo - a proposal for the classification of oxidative stress biomarkers. Redox biology. 2020:101442. doi: 10.1016/j.redox.2020.101442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daiber A., Oelze M., Steven S., Kroller-Schon S., Munzel T. Taking up the cudgels for the traditional reactive oxygen and nitrogen species detection assays and their use in the cardiovascular system. Redox biology. 2017;12:35–49. doi: 10.1016/j.redox.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnabel R., Blankenberg S., Lubos E., Lackner K.J., Rupprecht H.J., Espinola-Klein C., Jachmann N., Post F., Peetz D., Bickel C., Cambien F., Tiret L., Munzel T. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ. Res. 2005;97:e53–59. doi: 10.1161/01.RES.0000181286.44222.61. [DOI] [PubMed] [Google Scholar]
- 48.Daiber A., Xia N., Steven S., Oelze M., Hanf A., Kroller-Schon S., Munzel T., Li H. New therapeutic implications of endothelial nitric oxide synthase (eNOS) function/dysfunction in cardiovascular disease. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dopheide J.F., Doppler C., Scheer M., Obst V., Radmacher M.C., Radsak M.P., Gori T., Warnholtz A., Fottner C., Munzel T., Daiber A., Espinola-Klein C. Critical limb ischaemia is characterised by an increased production of whole blood reactive oxygen species and expression of TREM-1 on neutrophils. Atherosclerosis. 2013;229:396–403. doi: 10.1016/j.atherosclerosis.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 50.Steven S., Daiber A., Dopheide J.F., Munzel T., Espinola-Klein C. Peripheral artery disease, redox signaling, oxidative stress - basic and clinical aspects. Redox biology. 2017;12:787–797. doi: 10.1016/j.redox.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zielonka J., Cheng G., Zielonka M., Ganesh T., Sun A., Joseph J., Michalski R., O'Brien W.J., Lambeth J.D., Kalyanaraman B. High-throughput assays for superoxide and hydrogen peroxide design OF A screening workflow to identify inhibitors OF NADPH oxidases. J. Biol. Chem. 2014;289:16176–16189. doi: 10.1074/jbc.M114.548693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zielonka J., Vasquez-Vivar J., Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat. Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 53.Dikalov S.I., Harrison D.G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxidants Redox Signal. 2014;20:372–382. doi: 10.1089/ars.2012.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Debowska K., Debski D., Hardy M., Jakubowska M., Kalyanaraman B., Marcinek A., Michalski R., Michalowski B., Ouari O., Sikora A., Smulik R., Zielonka J. Toward selective detection of reactive oxygen and nitrogen species with the use of fluorogenic probes--Limitations, progress, and perspectives. Pharmacol. Rep. : PR. 2015;67:756–764. doi: 10.1016/j.pharep.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Griendling K.K., Touyz R.M., Zweier J.L., Dikalov S., Chilian W., Chen Y.R., Harrison D.G., Bhatnagar A., S American heart association council on basic cardiovascular Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: a scientific statement from the American heart association. Circ. Res. 2016;119:e39–75. doi: 10.1161/RES.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perng J.K., Lee S., Kundu K., Caskey C.F., Knight S.F., Satir S., Ferrara K.W., Taylor W.R., Degertekin F.L., Sorescu D., Murthy N. Ultrasound imaging of oxidative stress in vivo with chemically-generated gas microbubbles. Ann. Biomed. Eng. 2012;40:2059–2068. doi: 10.1007/s10439-012-0573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maulucci G., Bacic G., Bridal L., Schmidt H.H., Tavitian B., Viel T., Utsumi H., Yalcin A.S., De Spirito M. Imaging reactive oxygen species-induced modifications in living systems. Antioxidants Redox Signal. 2016;24:939–958. doi: 10.1089/ars.2015.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mason R.P. Imaging free radicals in organelles, cells, tissue, and in vivo with immuno-spin trapping. Redox biology. 2016;8:422–429. doi: 10.1016/j.redox.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howard M.D., Hood E.D., Zern B., Shuvaev V.V., Grosser T., Muzykantov V.R. Nanocarriers for vascular delivery of anti-inflammatory agents. Annu. Rev. Pharmacol. Toxicol. 2014;54:205–226. doi: 10.1146/annurev-pharmtox-011613-140002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bilan D.S., Belousov V.V. HyPer family probes: state of the Art. Antioxidants Redox Signal. 2016;24:731–751. doi: 10.1089/ars.2015.6586. [DOI] [PubMed] [Google Scholar]
- 61.Ermakova Y.G., Bilan D.S., Matlashov M.E., Mishina N.M., Markvicheva K.N., Subach O.M., Subach F.V., Bogeski I., Hoth M., Enikolopov G., Belousov V.V. Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide. Nat. Commun. 2014;5:5222. doi: 10.1038/ncomms6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eroglu E., Gottschalk B., Charoensin S., Blass S., Bischof H., Rost R., Madreiter-Sokolowski C.T., Pelzmann B., Bernhart E., Sattler W., Hallstrom S., Malinski T., Waldeck-Weiermair M., Graier W.F., Malli R. Development of novel FP-based probes for live-cell imaging of nitric oxide dynamics. Nat. Commun. 2016;7:10623. doi: 10.1038/ncomms10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eroglu E., Charoensin S., Bischof H., Ramadani J., Gottschalk B., Depaoli M.R., Waldeck-Weiermair M., Graier W.F., Malli R. Genetic biosensors for imaging nitric oxide in single cells. Free Radic. Biol. Med. 2018;128:50–58. doi: 10.1016/j.freeradbiomed.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swain L., Kesemeyer A., Meyer-Roxlau S., Vettel C., Zieseniss A., Guntsch A., Jatho A., Becker A., Nanadikar M.S., Morgan B., Dennerlein S., Shah A.M., El-Armouche A., Nikolaev V.O., Katschinski D.M. Redox imaging using cardiac myocyte-specific transgenic biosensor mice. Circ. Res. 2016;119:1004–1016. doi: 10.1161/CIRCRESAHA.116.309551. [DOI] [PubMed] [Google Scholar]
- 65.Shokhina A.G., Kostyuk A.I., Ermakova Y.G., Panova A.S., Staroverov D.B., Egorov E.S., Baranov M.S., van Belle G.J., Katschinski D.M., Belousov V.V., Bilan D.S. Red fluorescent redox-sensitive biosensor Grx1-roCherry. Redox biology. 2019;21:101071. doi: 10.1016/j.redox.2018.101071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swain L., Nanadikar M.S., Borowik S., Zieseniss A., Katschinski D.M. Transgenic organisms meet redox bioimaging: one step closer to physiology. Antioxidants Redox Signal. 2018;29:603–612. doi: 10.1089/ars.2017.7469. [DOI] [PubMed] [Google Scholar]
- 67.Castro J.P., Jung T., Grune T., Siems W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017;111:309–315. doi: 10.1016/j.freeradbiomed.2016.10.497. [DOI] [PubMed] [Google Scholar]
- 68.Zarkovic K., Jakovcevic A., Zarkovic N. Contribution of the HNE-immunohistochemistry to modern pathological concepts of major human diseases. Free Radic. Biol. Med. 2017;111:110–126. doi: 10.1016/j.freeradbiomed.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 69.Davies S.S., Roberts L.J. 2nd. F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic. Biol. Med. 2011;50:559–566. doi: 10.1016/j.freeradbiomed.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winklhofer-Roob B.M., Faustmann G., Roob J.M. Low-density lipoprotein oxidation biomarkers in human health and disease and effects of bioactive compounds. Free Radic. Biol. Med. 2017;111:38–86. doi: 10.1016/j.freeradbiomed.2017.04.345. [DOI] [PubMed] [Google Scholar]
- 71.Gianazza E., Brioschi M., Fernandez A.M., Banfi C. Lipoxidation in cardiovascular diseases. Redox biology. 2019;23:101119. doi: 10.1016/j.redox.2019.101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iuliano L. Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem. Phys. Lipids. 2011;164:457–468. doi: 10.1016/j.chemphyslip.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 73.Li L., Zhong S., Shen X., Li Q., Xu W., Tao Y., Yin H. Recent development on liquid chromatography-mass spectrometry analysis of oxidized lipids. Free Radic. Biol. Med. 2019;144:16–34. doi: 10.1016/j.freeradbiomed.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Griesser M., Boeglin W.E., Suzuki T., Schneider C. Convergence of the 5-LOX and COX-2 pathways: heme-catalyzed cleavage of the 5S-HETE-derived di-endoperoxide into aldehyde fragments. J. Lipid Res. 2009;50:2455–2462. doi: 10.1194/jlr.M900181-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roest M., Voorbij H.A., Van der Schouw Y.T., Peeters P.H., Teerlink T., Scheffer P.G. High levels of urinary F2-isoprostanes predict cardiovascular mortality in postmenopausal women. J Clin Lipidol. 2008;2:298–303. doi: 10.1016/j.jacl.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 76.van 't Erve T.J., Kadiiska M.B., London S.J., Mason R.P. Classifying oxidative stress by F2-isoprostane levels across human diseases: a meta-analysis. Redox biology. 2017;12:582–599. doi: 10.1016/j.redox.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwedhelm E., Bartling A., Lenzen H., Tsikas D., Maas R., Brummer J., Gutzki F.M., Berger J., Frolich J.C., Boger R.H. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 2004;109:843–848. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 78.Wolfram R., Oguogho A., Palumbo B., Sinzinger H. Enhanced oxidative stress in coronary heart disease and chronic heart failure as indicated by an increased 8-epi-PGF(2alpha) Eur. J. Heart Fail. 2005;7:167–172. doi: 10.1016/j.ejheart.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Woodward M., Croft Kevin D., Mori Trevor A., Headlam H., Wang Xiao S., Suarna C., Raftery Mark J., MacMahon Stephen W., Stocker R. Association between both lipid and protein oxidation and the risk of fatal or non-fatal coronary heart disease in a human population. Clin. Sci. 2008;116:53–60. doi: 10.1042/cs20070404. [DOI] [PubMed] [Google Scholar]
- 80.Nakamura K., Kusano K., Nakamura Y., Kakishita M., Ohta K., Nagase S., Yamamoto M., Miyaji K., Saito H., Morita H., Emori T., Matsubara H., Toyokuni S., Ohe T. Carvedilol decreases elevated oxidative stress in human failing myocardium. Circulation. 2002;105:2867–2871. doi: 10.1161/01.cir.0000018605.14470.dd. [DOI] [PubMed] [Google Scholar]
- 81.Mak S., Lehotay D.C., Yazdanpanah M., Azevedo E.R., Liu P.P., Newton G.E. Unsaturated aldehydes including 4-OH-nonenal are elevated in patients with congestive heart failure. J. Card. Fail. 2000;6:108–114. [PubMed] [Google Scholar]
- 82.Haramaki N., Ikeda H., Takajo Y., Katoh A., Kanaya S., Shintani S., Haramaki R., Murohara T., Imaizumi T. Long-term smoking causes nitroglycerin resistance in platelets by depletion of intraplatelet glutathione. Arterioscler. Thromb. Vasc. Biol. 2001;21:1852–1856. doi: 10.1161/hq1001.097021. [DOI] [PubMed] [Google Scholar]
- 83.Romuk E., Wojciechowska C., Jachec W., Zemla-Woszek A., Momot A., Buczkowska M., Rozentryt P. Malondialdehyde and uric acid as predictors of adverse outcome in patients with chronic heart failure. Oxidative medicine and cellular longevity. 2019:9246138. doi: 10.1155/2019/9246138. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Radovanovic S., Savic-Radojevic A., Pljesa-Ercegovac M., Djukic T., Suvakov S., Krotin M., Simic D.V., Matic M., Radojicic Z., Pekmezovic T., Simic T. Markers of oxidative damage and antioxidant enzyme activities as predictors of morbidity and mortality in patients with chronic heart failure. J. Card. Fail. 2012;18:493–501. doi: 10.1016/j.cardfail.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 85.Walter M.F., Jacob R.F., Jeffers B., Ghadanfar M.M., Preston G.M., Buch J., Mason R.P., study P. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: a longitudinal analysis of the PREVENT study. J. Am. Coll. Cardiol. 2004;44:1996–2002. doi: 10.1016/j.jacc.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 86.Gao S., Zhao D., Wang M., Zhao F., Han X., Qi Y., Liu J. Association between circulating oxidized LDL and atherosclerotic cardiovascular disease: a meta-analysis of observational studies. Can. J. Cardiol. 2017;33:1624–1632. doi: 10.1016/j.cjca.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 87.Gomez M., Valle V., Aros F., Sanz G., Sala J., Fiol M., Bruguera J., Elosua R., Molina L., Marti H., Covas M.I., Rodriguez-Llorian A., Fito M., Suarez-Pinilla M.A., Amezaga R., Marrugat J., researchers F.g.o., Oxidized L.D.L. Lipoprotein (a) and other emergent risk factors in acute myocardial infarction (FORTIAM study) Rev. Esp. Cardiol. 2009;62:373–382. doi: 10.1016/s1885-5857(09)71664-0. [DOI] [PubMed] [Google Scholar]
- 88.Shimada K., Mokuno H., Matsunaga E., Miyazaki T., Sumiyoshi K., Miyauchi K., Daida H. Circulating oxidized low-density lipoprotein is an independent predictor for cardiac event in patients with coronary artery disease. Atherosclerosis. 2004;174:343–347. doi: 10.1016/j.atherosclerosis.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y.C., Tang Y., Chen Y., Huang X.H., Zhang M., Chen J., Sun Y.G., Li Y.G. Oxidized low-density lipoprotein and C-reactive protein have combined utility for better predicting prognosis after acute coronary syndrome. Cell Biochem. Biophys. 2014;68:379–385. doi: 10.1007/s12013-013-9718-1. [DOI] [PubMed] [Google Scholar]
- 90.Cristescu S.M., Kiss R., Hekkert S., Dalby M., Harren F.J., Risby T.H., Marczin N., Harefield B.s.i. Real-time monitoring of endogenous lipid peroxidation by exhaled ethylene in patients undergoing cardiac surgery. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307:L509–L515. doi: 10.1152/ajplung.00168.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomson L., Tenopoulou M., Lightfoot R., Tsika E., Parastatidis I., Martinez M., Greco T.M., Doulias P.T., Wu Y., Tang W.H., Hazen S.L., Ischiropoulos H. Immunoglobulins against tyrosine-nitrated epitopes in coronary artery disease. Circulation. 2012;126:2392–2401. doi: 10.1161/CIRCULATIONAHA.112.103796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.MacMillan-Crow L.A., Crow J.P., Kerby J.D., Beckman J.S., Thompson J.A. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davis B., Zou M.H. CD40 ligand-dependent tyrosine nitration of prostacyclin synthase in vivo. Circulation. 2005;112:2184–2192. doi: 10.1161/CIRCULATIONAHA.105.553206. [DOI] [PubMed] [Google Scholar]
- 94.Martinez M., Cuker A., Mills A., Lightfoot R., Fan Y., Tang W.H., Hazen S.L., Ischiropoulos H. Nitrated fibrinogen is a biomarker of oxidative stress in venous thromboembolism. Free Radic. Biol. Med. 2012;53:230–236. doi: 10.1016/j.freeradbiomed.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vadseth C., Souza J.M., Thomson L., Seagraves A., Nagaswami C., Scheiner T., Torbet J., Vilaire G., Bennett J.S., Murciano J.C., Muzykantov V., Penn M.S., Hazen S.L., Weisel J.W., Ischiropoulos H. Pro-thrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. J. Biol. Chem. 2004;279:8820–8826. doi: 10.1074/jbc.M306101200. [DOI] [PubMed] [Google Scholar]
- 96.Shishehbor M.H., Aviles R.J., Brennan M.L., Fu X., Goormastic M., Pearce G.L., Gokce N., Keaney J.F., Jr., Penn M.S., Sprecher D.L., Vita J.A., Hazen S.L. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289:1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 97.Pirro M., Schillaci G., Mannarino M.R., Savarese G., Vaudo G., Siepi D., Paltriccia R., Mannarino E. Effects of rosuvastatin on 3-nitrotyrosine and aortic stiffness in hypercholesterolemia. Nutr. Metabol. Cardiovasc. Dis. 2007;17:436–441. doi: 10.1016/j.numecd.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 98.Chu C.S., Lee K.T., Cheng K.H., Lee M.Y., Kuo H.F., Lin T.H., Su H.M., Voon W.C., Sheu S.H., Lai W.T. Postchallenge responses of nitrotyrosine and TNF-alpha during 75-g oral glucose tolerance test are associated with the presence of coronary artery diseases in patients with prediabetes. Cardiovasc. Diabetol. 2012;11:21. doi: 10.1186/1475-2840-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Quidim A.V.L., Bruno T., Leocadio P.C.L., Santos I.S., Alvarez-Leite J.I., Dos Reis Menta P.L., Lotufo P.A., Bensenor I.M., Goulart A.C. The prognostic value of nitrotyrosine levels in coronary heart disease: long-term evaluation in the Acute Coronary Syndrome Registry Strategy (ERICO study) Clin. Biochem. 2019;66:37–43. doi: 10.1016/j.clinbiochem.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 100.Nuriel T., Hansler A., Gross S.S. Protein nitrotryptophan: formation, significance and identification. Journal of proteomics. 2011;74:2300–2312. doi: 10.1016/j.jprot.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nishida M., Nishimura A., Matsunaga T., Motohashi H., Kasamatsu S., Akaike T. Redox regulation of electrophilic signaling by reactive persulfides in cardiac cells. Free Radic. Biol. Med. 2017;109:132–140. doi: 10.1016/j.freeradbiomed.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 102.Fujii S., Akaike T. Redox Signaling by 8-nitro-cyclic guanosine monophosphate: nitric oxide- and reactive oxygen species-derived electrophilic messenger. Antioxidants Redox Signal. 2013;19:1236–1246. doi: 10.1089/ars.2012.5067. [DOI] [PubMed] [Google Scholar]
- 103.Freeman B.A., Baker P.R., Schopfer F.J., Woodcock S.R., Napolitano A., d'Ischia M. Nitro-fatty acid formation and signaling. J. Biol. Chem. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trostchansky A., Rubbo H. Nitrated fatty acids: mechanisms of formation, chemical characterization, and biological properties. Free Radic. Biol. Med. 2008;44:1887–1896. doi: 10.1016/j.freeradbiomed.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 105.Trostchansky A., Bonilla L., Gonzalez-Perilli L., Rubbo H. Nitro-fatty acids: formation, redox signaling, and therapeutic potential. Antioxidants Redox Signal. 2013;19:1257–1265. doi: 10.1089/ars.2012.5023. [DOI] [PubMed] [Google Scholar]
- 106.Melo T., Montero-Bullon J.F., Domingues P., Domingues M.R. Discovery of bioactive nitrated lipids and nitro-lipid-protein adducts using mass spectrometry-based approaches. Redox biology. 2019;23:101106. doi: 10.1016/j.redox.2019.101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salvatore S.R., Rowart P., Schopfer F.J. Mass spectrometry-based study defines the human urine nitrolipidome. Free Radic. Biol. Med. 2020 doi: 10.1016/j.freeradbiomed.2020.10.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nadziakiewicz P., Knapik P., Ziora D., Nowak D. Moderate exercise decreases nitric oxide exhalation in patients with stable coronary artery disease. J. Physiol. Pharmacol. 2006;57(Suppl 4):213–221. [PubMed] [Google Scholar]
- 109.Grunewald C., Carlstrom K., Kumlien G., Ringqvist A., Lundberg J. Exhaled oral and nasal nitric oxide during L-arginine infusion in preeclampsia. Gynecol. Obstet. Invest. 1998;46:232–237. doi: 10.1159/000010040. [DOI] [PubMed] [Google Scholar]
- 110.Kato M., Matsumoto A., Nakajima T., Hirose K., Iwasawa K., Takenaka K., Yamashita H., Sugiura S., Hirata Y., Nagai R. Amlodipine increases nitric oxide production in exhaled air during exercise in patients with essential hypertension. Am. J. Hypertens. 2004;17:729–733. doi: 10.1016/j.amjhyper.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 111.Adachi H., Oshima S., Sakurai S., Toyama T., Hoshizaki H., Taniguchi K., Ito H. Nitric oxide exhalation correlates with ventilatory response to exercise in patients with heart disease. Eur. J. Heart Fail. 2003;5:639–643. doi: 10.1016/s1388-9842(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 112.Tufvesson E., Nilsson E., Popov T.A., Hesselstrand R., Bjermer L. Fractional exhaled breath temperature in patients with asthma, chronic obstructive pulmonary disease, or systemic sclerosis compared to healthy controls. Eur Clin Respir J. 2020;7:1747014. doi: 10.1080/20018525.2020.1747014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maas R., Xanthakis V., Goen T., Muller J., Schwedhelm E., Boger R.H., Vasan R.S. Plasma nitrate and incidence of cardiovascular disease and all-cause mortality in the community: the Framingham offspring study. Journal of the American Heart Association. 2017;6 doi: 10.1161/JAHA.117.006224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hadaegh F., Asgari S., Bozorgmanesh M., Jeddi S., Azizi F., Ghasemi A. Added value of total serum nitrate/nitrite for prediction of cardiovascular disease in middle east caucasian residents in Tehran. Nitric Oxide. 2016;54:60–66. doi: 10.1016/j.niox.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 115.Kasai H., Crain P.F., Kuchino Y., Nishimura S., Ootsuyama A., Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986;7:1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- 116.Larsen E.L., Weimann A., Poulsen H.E. Interventions targeted at oxidatively generated modifications of nucleic acids focused on urine and plasma markers. Free Radic. Biol. Med. 2019;145:256–283. doi: 10.1016/j.freeradbiomed.2019.09.030. [DOI] [PubMed] [Google Scholar]
- 117.Poulsen H.E., Weimann A., Henriksen T., Kjaer L.K., Larsen E.L., Carlsson E.R., Christensen C.K., Brandslund I., Fenger M. Oxidatively generated modifications to nucleic acids in vivo: measurement in urine and plasma. Free Radic. Biol. Med. 2019;145:336–341. doi: 10.1016/j.freeradbiomed.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 118.Di Minno A., Turnu L., Porro B., Squellerio I., Cavalca V., Tremoli E., Di Minno M.N. 8-Hydroxy-2-Deoxyguanosine levels and cardiovascular disease: a systematic review and meta-analysis of the literature. Antioxidants Redox Signal. 2016;24:548–555. doi: 10.1089/ars.2015.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Di Minno A., Turnu L., Porro B., Squellerio I., Cavalca V., Tremoli E., Di Minno M.N. 8-Hydroxy-2-deoxyguanosine levels and heart failure: a systematic review and meta-analysis of the literature. Nutr. Metabol. Cardiovasc. Dis. 2017;27:201–208. doi: 10.1016/j.numecd.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 120.Wang X.B., Cui N.H., Liu X., Liu X. Mitochondrial 8-hydroxy-2'-deoxyguanosine and coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2020;19:22. doi: 10.1186/s12933-020-00998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu X., Gan W., Zou Y., Yang B., Su Z., Deng J., Wang L., Cai J. Elevated levels of urinary markers of oxidative DNA and RNA damage in type 2 diabetes with complications. Oxidative medicine and cellular longevity. 2016:4323198. doi: 10.1155/2016/4323198. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Broedbaek K., Siersma V., Henriksen T., Weimann A., Petersen M., Andersen J.T., Jimenez-Solem E., Hansen L.J., Henriksen J.E., Bonnema S.J., de Fine Olivarius N., Poulsen H.E. Association between urinary markers of nucleic acid oxidation and mortality in type 2 diabetes: a population-based cohort study. Diabetes Care. 2013;36:669–676. doi: 10.2337/dc12-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Broedbaek K., Koster-Rasmussen R., Siersma V., Persson F., Poulsen H.E., de Fine Olivarius N. Urinary albumin and 8-oxo-7,8-dihydroguanosine as markers of mortality and cardiovascular disease during 19 years after diagnosis of type 2 diabetes - a comparative study of two markers to identify high risk patients. Redox biology. 2017;13:363–369. doi: 10.1016/j.redox.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schottker B., Brenner H., Jansen E.H., Gardiner J., Peasey A., Kubinova R., Pajak A., Topor-Madry R., Tamosiunas A., Saum K.U., Holleczek B., Pikhart H., Bobak M. Evidence for the free radical/oxidative stress theory of ageing from the CHANCES consortium: a meta-analysis of individual participant data. BMC Med. 2015;13:300. doi: 10.1186/s12916-015-0537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xuan Y., Bobak M., Anusruti A., Jansen E., Pajak A., Tamosiunas A., Saum K.U., Holleczek B., Gao X., Brenner H., Schottker B. Association of serum markers of oxidative stress with myocardial infarction and stroke: pooled results from four large European cohort studies. Eur. J. Epidemiol. 2019;34:471–481. doi: 10.1007/s10654-018-0457-x. [DOI] [PubMed] [Google Scholar]
- 126.Masaki N., Sato A., Horii S., Kimura T., Toya T., Yasuda R., Namba T., Yada H., Kawamura A., Adachi T. Usefulness of the d-ROMs test for prediction of cardiovascular events. Int. J. Cardiol. 2016;222:226–232. doi: 10.1016/j.ijcard.2016.07.225. [DOI] [PubMed] [Google Scholar]
- 127.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 128.Treiber T., Treiber N., Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019;20:5–20. doi: 10.1038/s41580-018-0059-1. [DOI] [PubMed] [Google Scholar]
- 129.Gilad S., Meiri E., Yogev Y., Benjamin S., Lebanony D., Yerushalmi N., Benjamin H., Kushnir M., Cholakh H., Melamed N., Bentwich Z., Hod M., Goren Y., Chajut A. Serum microRNAs are promising novel biomarkers. PloS One. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wan Y., Cui R., Gu J., Zhang X., Xiang X., Liu C., Qu K., Lin T. Identification of four oxidative stress-responsive MicroRNAs, miR-34a-5p, miR-1915-3p, miR-638, and miR-150-3p, in hepatocellular carcinoma. Oxidative medicine and cellular longevity 2017. 2017:5189138. doi: 10.1155/2017/5189138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Banerjee J., Khanna S., Bhattacharya A. MicroRNA regulation of oxidative stress. Oxidative medicine and cellular longevity. 2017:2872156. doi: 10.1155/2017/2872156. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Krutzfeldt J., Rajewsky N., Braich R., Rajeev K.G., Tuschl T., Manoharan M., Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 133.Wang H.R., Wu M., Yu H., Long S., Stevens A., Engers D.W., Sackin H., Daniels J.S., Dawson E.S., Hopkins C.R., Lindsley C.W., Li M., McManus O.B. Selective inhibition of the K(ir)2 family of inward rectifier potassium channels by a small molecule probe: the discovery, SAR, and pharmacological characterization of ML133. ACS Chem. Biol. 2011;6:845–856. doi: 10.1021/cb200146a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bonneau E., Neveu B., Kostantin E., Tsongalis G.J., De Guire V. How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. EJIFCC. 2019;30:114–127. [PMC free article] [PubMed] [Google Scholar]
- 135.Kura B., Szeiffova Bacova B., Kalocayova B., Sykora M., Slezak J. Oxidative stress-responsive MicroRNAs in heart injury. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miguel V., Cui J.Y., Daimiel L., Espinosa-Diez C., Fernandez-Hernando C., Kavanagh T.J., Lamas S. The role of MicroRNAs in environmental risk factors, noise-induced hearing loss, and mental stress. Antioxidants Redox Signal. 2018;28:773–796. doi: 10.1089/ars.2017.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fu X.M., Zhou Y.Z., Cheng Z., Liao X.B., Zhou X.M. MicroRNAs: novel players in aortic aneurysm. BioMed Res. Int. 2015;2015:831641. doi: 10.1155/2015/831641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang J., Cai W., Fan Z., Yang C., Wang W., Xiong M., Ma C., Yang J. MicroRNA-24 inhibits the oxidative stress induced by vascular injury by activating the Nrf2/Ho-1 signaling pathway. Atherosclerosis. 2019;290:9–18. doi: 10.1016/j.atherosclerosis.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 139.Gou L., Zhao L., Song W., Wang L., Liu J., Zhang H., Huang Y., Lau C.W., Yao X., Tian X.Y., Wong W.T., Luo J.Y., Huang Y. Inhibition of miR-92a suppresses oxidative stress and improves endothelial function by upregulating heme oxygenase-1 in db/db mice. Antioxidants Redox Signal. 2018;28:358–370. doi: 10.1089/ars.2017.7005. [DOI] [PubMed] [Google Scholar]
- 140.Yamac A.H., Huyut M.A., Yilmaz E., Celikkale I., Bacaksiz A., Demir Y., Demir A.R., Erturk M., Bakhshaliyev N., Ozdemir R., Kilic U. MicroRNA 199a is downregulated in patients after coronary artery bypass graft surgery and is associated with increased levels of sirtuin 1 (SIRT 1) protein and major adverse cardiovascular events at 3-year follow-up. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. : international medical journal of experimental and clinical research. 2018;24:6245–6254. doi: 10.12659/MSM.912065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jakob P., Kacprowski T., Briand-Schumacher S., Heg D., Klingenberg R., Stahli B.E., Jaguszewski M., Rodondi N., Nanchen D., Raber L., Vogt P., Mach F., Windecker S., Volker U., Matter C.M., Luscher T.F., Landmesser U. Profiling and validation of circulating microRNAs for cardiovascular events in patients presenting with ST-segment elevation myocardial infarction. Eur. Heart J. 2017;38:511–515. doi: 10.1093/eurheartj/ehw563. [DOI] [PubMed] [Google Scholar]
- 142.Han M., Yang Z., Sayed D., He M., Gao S., Lin L., Yoon S., Abdellatif M. GATA4 expression is primarily regulated via a miR-26b-dependent post-transcriptional mechanism during cardiac hypertrophy. Cardiovasc. Res. 2012;93:645–654. doi: 10.1093/cvr/cvs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ren X.P., Wu J., Wang X., Sartor M.A., Jones K., Qian J., Nicolaou P., Pritchard T.J., Fan G.C. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou S.S., Jin J.P., Wang J.Q., Zhang Z.G., Freedman J.H., Zheng Y., Cai L. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018;39:1073–1084. doi: 10.1038/aps.2018.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bayes-Genis A., Lanfear D.E., de Ronde M.W.J., Lupon J., Leenders J.J., Liu Z., Zuithoff N.P.A., Eijkemans M.J.C., Zamora E., De Antonio M., Zwinderman A.H., Pinto-Sietsma S.J., Pinto Y.M. Prognostic value of circulating microRNAs on heart failure-related morbidity and mortality in two large diverse cohorts of general heart failure patients. Eur. J. Heart Fail. 2018;20:67–75. doi: 10.1002/ejhf.984. [DOI] [PubMed] [Google Scholar]
- 146.de Gonzalo-Calvo D., Cediel G., Bar C., Nunez J., Revuelta-Lopez E., Gavara J., Rios-Navarro C., Llorente-Cortes V., Bodi V., Thum T., Bayes-Genis A. Circulating miR-1254 predicts ventricular remodeling in patients with ST-Segment-Elevation Myocardial Infarction: a cardiovascular magnetic resonance study. Sci. Rep. 2018;8:15115. doi: 10.1038/s41598-018-33491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vettori S., Gay S., Distler O. Role of MicroRNAs in fibrosis. Open Rheumatol. J. 2012;6:130–139. doi: 10.2174/1874312901206010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sygitowicz G., Tomaniak M., Blaszczyk O., Koltowski L., Filipiak K.J., Sitkiewicz D. Circulating microribonucleic acids miR-1, miR-21 and miR-208a in patients with symptomatic heart failure: preliminary results. Arch Cardiovasc Dis. 2015;108:634–642. doi: 10.1016/j.acvd.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 149.Cakmak H.A., Coskunpinar E., Ikitimur B., Barman H.A., Karadag B., Tiryakioglu N.O., Kahraman K., Vural V.A. The prognostic value of circulating microRNAs in heart failure: preliminary results from a genome-wide expression study. J. Cardiovasc. Med. 2015;16:431–437. doi: 10.2459/JCM.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 150.Goren Y., Kushnir M., Zafrir B., Tabak S., Lewis B.S., Amir O. Serum levels of microRNAs in patients with heart failure. Eur. J. Heart Fail. 2012;14:147–154. doi: 10.1093/eurjhf/hfr155. [DOI] [PubMed] [Google Scholar]
- 151.Tao L., Huang X., Xu M., Qin Z., Zhang F., Hua F., Jiang X., Wang Y. Value of circulating miRNA-21 in the diagnosis of subclinical diabetic cardiomyopathy. Mol. Cell. Endocrinol. 2020:110944. doi: 10.1016/j.mce.2020.110944. [DOI] [PubMed] [Google Scholar]
- 152.Dai B., Li H., Fan J., Zhao Y., Yin Z., Nie X., Wang D.W., Chen C. MiR-21 protected against diabetic cardiomyopathy induced diastolic dysfunction by targeting gelsolin. Cardiovasc. Diabetol. 2018;17:123. doi: 10.1186/s12933-018-0767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Krichevsky A.M., Gabriely G. miR-21: a small multi-faceted RNA. J. Cell Mol. Med. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thum T., Galuppo P., Wolf C., Fiedler J., Kneitz S., van Laake L.W., Doevendans P.A., Mummery C.L., Borlak J., Haverich A., Gross C., Engelhardt S., Ertl G., Bauersachs J. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 155.Ji R., Cheng Y., Yue J., Yang J., Liu X., Chen H., Dean D.B., Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ. Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 156.Fleissner F., Jazbutyte V., Fiedler J., Gupta S.K., Yin X., Xu Q., Galuppo P., Kneitz S., Mayr M., Ertl G., Bauersachs J., Thum T. Short communication: asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microRNA-21-dependent mechanism. Circ. Res. 2010;107:138–143. doi: 10.1161/CIRCRESAHA.110.216770. [DOI] [PubMed] [Google Scholar]
- 157.Hinkel R., Ramanujam D., Kaczmarek V., Howe A., Klett K., Beck C., Dueck A., Thum T., Laugwitz K.L., Maegdefessel L., Weber C., Kupatt C., Engelhardt S. AntimiR-21 prevents myocardial dysfunction in a pig model of ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2020;75:1788–1800. doi: 10.1016/j.jacc.2020.02.041. [DOI] [PubMed] [Google Scholar]
- 158.Briasoulis A., Sharma S., Telila T., Mallikethi-Reddy S., Papageorgiou N., Oikonomou E., Tousoulis D. MicroRNAs in atrial fibrillation. Curr. Med. Chem. 2019;26:855–863. doi: 10.2174/0929867324666170920151024. [DOI] [PubMed] [Google Scholar]
- 159.Kapodistrias N., Theocharopoulou G., Vlamos P. A hypothesis of circulating MicroRNAs' implication in high incidence of atrial fibrillation and other electrocardiographic abnormalities in cancer patients. Adv. Exp. Med. Biol. 2020;1196:1–9. doi: 10.1007/978-3-030-32637-1_1. [DOI] [PubMed] [Google Scholar]
- 160.Rivera-Caravaca J.M., Teruel-Montoya R., Roldan V., Cifuentes-Riquelme R., Crespo-Matas J.A., de Los Reyes-Garcia A.M., Aguila S., Fernandez-Perez M.P., Reguilon-Gallego L., Zapata-Martinez L., Garcia-Barbera N., Vicente V., Marin F., Martinez C., Gonzalez-Conejero R. Pilot study on the role of circulating miRNAs for the improvement of the predictive ability of the 2MACE score in patients with atrial fibrillation. J. Clin. Med. 2020;9 doi: 10.3390/jcm9113645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Casas A.I., Dao V.T., Daiber A., Maghzal G.J., Di Lisa F., Kaludercic N., Leach S., Cuadrado A., Jaquet V., Seredenina T., Krause K.H., Lopez M.G., Stocker R., Ghezzi P., Schmidt H.H. Reactive oxygen-related diseases: therapeutic targets and emerging clinical indications. Antioxidants Redox Signal. 2015;23:1171–1185. doi: 10.1089/ars.2015.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wingler K., Hermans J.J., Schiffers P., Moens A., Paul M., Schmidt H.H. NOX1, 2, 4, 5: counting out oxidative stress. Br. J. Pharmacol. 2011;164:866–883. doi: 10.1111/j.1476-5381.2011.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dao V.T., Casas A.I., Maghzal G.J., Seredenina T., Kaludercic N., Robledinos-Anton N., Di Lisa F., Stocker R., Ghezzi P., Jaquet V., Cuadrado A., Schmidt H.H. Pharmacology and clinical drug candidates in redox medicine. Antioxidants Redox Signal. 2015;23:1113–1129. doi: 10.1089/ars.2015.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Loffredo L., Carnevale R., Sanguigni V., Plebani A., Rossi P., Pignata C., De Mattia D., Finocchi A., Martire B., Pietrogrande M.C., Martino S., Gambineri E., Giardino G., Soresina A.R., Martino F., Pignatelli P., Violi F. Does NADPH oxidase deficiency cause artery dilatation in humans? Antioxidants Redox Signal. 2013;18:1491–1496. doi: 10.1089/ars.2012.4987. [DOI] [PubMed] [Google Scholar]
- 165.Nocella C., Cammisotto V., Bartimoccia S., Castellani V., Loffredo L., Pastori D., Pignatelli P., Sanguigni V., Violi F., Carnevale R. A novel role of MMP2 in regulating platelet NOX2 activation. Free Radic. Biol. Med. 2020;152:355–362. doi: 10.1016/j.freeradbiomed.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 166.Loffredo L., Martino F., Carnevale R., Pignatelli P., Catasca E., Perri L., Calabrese C.M., Palumbo M.M., Baratta F., Del Ben M., Angelico F., Violi F. Obesity and hypercholesterolemia are associated with NOX2 generated oxidative stress and arterial dysfunction. J. Pediatr. 2012;161:1004–1009. doi: 10.1016/j.jpeds.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 167.Loffredo L., Pignatelli P., Martino F., Carnevale R., Bartimoccia S., Catasca E., Colantoni C., Zanoni C., Perri L., Violi F. Early increase of NOX2-derived oxidative stress in children: relationship with age. Pediatr. Res. 2013;73:788–793. doi: 10.1038/pr.2013.55. [DOI] [PubMed] [Google Scholar]
- 168.Loffredo L., Carnevale R., Cangemi R., Angelico F., Augelletti T., Di Santo S., Calabrese C.M., Della Volpe L., Pignatelli P., Perri L., Basili S., Violi F. NOX2 up-regulation is associated with artery dysfunction in patients with peripheral artery disease. Int. J. Cardiol. 2013;165:184–192. doi: 10.1016/j.ijcard.2012.01.069. [DOI] [PubMed] [Google Scholar]
- 169.Loffredo L., Zicari A.M., Perri L., Carnevale R., Nocella C., Angelico F., Del Ben M., Mosca A., Zaffina S., Panera N., Alisi A., Duse M., Violi F., Nobili V. Does Nox2 overactivate in children with nonalcoholic fatty liver disease? Antioxidants Redox Signal. 2019;30:1325–1330. doi: 10.1089/ars.2018.7596. [DOI] [PubMed] [Google Scholar]
- 170.Carpino G., Pastori D., Baratta F., Overi D., Labbadia G., Polimeni L., Di Costanzo A., Pannitteri G., Carnevale R., Del Ben M., Arca M., Violi F., Angelico F., Gaudio E. PNPLA3 variant and portal/periportal histological pattern in patients with biopsy-proven non-alcoholic fatty liver disease: a possible role for oxidative stress. Sci. Rep. 2017;7:15756. doi: 10.1038/s41598-017-15943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Baratta F., Pastori D., Bartimoccia S., Cammisotto V., Cocomello N., Colantoni A., Nocella C., Carnevale R., Ferro D., Angelico F., Violi F., Del Ben M. Poor adherence to mediterranean diet and serum lipopolysaccharide are associated with oxidative stress in patients with non-alcoholic fatty liver disease. Nutrients. 2020;12 doi: 10.3390/nu12061732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Loffredo L., Baratta F., Ludovica P., Battaglia S., Carnevale R., Nocella C., Novo M., Pannitteri G., Ceci F., Angelico F., Violi F., Del Ben M. Effects of dark chocolate on endothelial function in patients with non-alcoholic steatohepatitis. Nutr. Metabol. Cardiovasc. Dis. 2017;28:143–149. doi: 10.1016/j.numecd.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 173.Del Ben M., Angelico F., Cangemi R., Loffredo L., Carnevale R., Augelletti T., Baratta F., Polimeni L., Pignatelli P., Violi F. Moderate weight loss decreases oxidative stress and increases antioxidant status in patients with metabolic syndrome. ISRN Obes. 2012:960427. doi: 10.5402/2012/960427. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pastori D., Andreozzi P., Carnevale R., Bartimoccia S., Limaj S., Melandri S., Brunori M., Spallacci G., Violi F., Pignatelli P. Does the coexistence of chronic obstructive pulmonary disease and atrial fibrillation affect Nox2 activity and urinary isoprostanes excretion? Antioxidants Redox Signal. 2019;31:786–790. doi: 10.1089/ars.2019.7811. [DOI] [PubMed] [Google Scholar]
- 175.Del Ben M., Fabiani M., Loffredo L., Polimeni L., Carnevale R., Baratta F., Brunori M., Albanese F., Augelletti T., Violi F., Angelico F. Oxidative stress mediated arterial dysfunction in patients with obstructive sleep apnoea and the effect of continuous positive airway pressure treatment. BMC Pulm. Med. 2012;12:36. doi: 10.1186/1471-2466-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Loffredo L., Zicari A.M., Occasi F., Perri L., Carnevale R., Angelico F., Del Ben M., Martino F., Nocella C., Savastano V., Cesoni Marcelli A., Duse M., Violi F. Endothelial dysfunction and oxidative stress in children with sleep disordered breathing: role of NADPH oxidase. Atherosclerosis. 2015;240:222–227. doi: 10.1016/j.atherosclerosis.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 177.Loffredo L., Carnevale R., Perri L., Catasca E., Augelletti T., Cangemi R., Albanese F., Piccheri C., Nocella C., Pignatelli P., Violi F. NOX2-mediated arterial dysfunction in smokers: acute effect of dark chocolate. Heart. 2011;97:1776–1781. doi: 10.1136/heartjnl-2011-300304. [DOI] [PubMed] [Google Scholar]
- 178.Loffredo L., Zicari A.M., Occasi F., Perri L., Carnevale R., Battaglia S., Angelico F., Del Ben M., Martino F., Nocella C., Farcomeni A., De Castro G., Duse M., Violi F. Passive smoking exacerbates nicotinamide-adenine dinucleotide phosphate oxidase isoform 2-induced oxidative stress and arterial dysfunction in children with persistent allergic rhinitis. J. Pediatr. 2018;202:252–257. doi: 10.1016/j.jpeds.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 179.Bertazzi P.A., Cantone L., Pignatelli P., Angelici L., Bollati V., Bonzini M., Carugno M., Mannucci P.M., Violi F. Does enhancement of oxidative stress markers mediate health effects of ambient air particles? Antioxidants Redox Signal. 2014;21:46–51. doi: 10.1089/ars.2013.5694. [DOI] [PubMed] [Google Scholar]
- 180.Schleicher E.D., Bierhaus A., Haring H.U., Nawroth P.P., Lehmann R. Chemistry and pathobiology of advanced glycation end products. Contrib. Nephrol. 2001:1–9. doi: 10.1159/000060056. [DOI] [PubMed] [Google Scholar]
- 181.Akhter F., Chen D., Akhter A., Yan S.F., ShiDu Yan S. Age-dependent accumulation of dicarbonyls and advanced glycation endproducts (AGEs) associates with mitochondrial stress. Free Radic. Biol. Med. 2020 doi: 10.1016/j.freeradbiomed.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ott C., Jacobs K., Haucke E., Navarrete Santos A., Grune T., Simm A. Role of advanced glycation end products in cellular signaling. Redox biology. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Steven S., Frenis K., Oelze M., Kalinovic S., Kuntic M., Bayo Jimenez M.T., Vujacic-Mirski K., Helmstadter J., Kroller-Schon S., Munzel T., Daiber A. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxidative medicine and cellular longevity. 2019:7092151. doi: 10.1155/2019/7092151. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kizer J.R., Benkeser D., Arnold A.M., Ix J.H., Mukamal K.J., Djousse L., Tracy R.P., Siscovick D.S., Psaty B.M., Zieman S.J. Advanced glycation/glycoxidation endproduct carboxymethyl-lysine and incidence of coronary heart disease and stroke in older adults. Atherosclerosis. 2014;235:116–121. doi: 10.1016/j.atherosclerosis.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Semba R.D., Bandinelli S., Sun K., Guralnik J.M., Ferrucci L. Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J. Am. Geriatr. Soc. 2009;57:1874–1880. doi: 10.1111/j.1532-5415.2009.02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sanajou D., Ghorbani Haghjo A., Argani H., Aslani S. AGE-RAGE axis blockade in diabetic nephropathy: current status and future directions. Eur. J. Pharmacol. 2018;833:158–164. doi: 10.1016/j.ejphar.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 187.Baldus S., Heeschen C., Meinertz T., Zeiher A.M., Eiserich J.P., Munzel T., Simoons M.L., Hamm C.W., Investigators C. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 188.Nicholls S.J., Tang W.H., Brennan D., Brennan M.L., Mann S., Nissen S.E., Hazen S.L. Risk prediction with serial myeloperoxidase monitoring in patients with acute chest pain. Clin. Chem. 2011;57:1762–1770. doi: 10.1373/clinchem.2011.166827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cheng M.L., Chen C.M., Gu P.W., Ho H.Y., Chiu D.T. Elevated levels of myeloperoxidase, white blood cell count and 3-chlorotyrosine in Taiwanese patients with acute myocardial infarction. Clin. Biochem. 2008;41:554–560. doi: 10.1016/j.clinbiochem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 190.Talib J., Maghzal G.J., Cheng D., Stocker R. Detailed protocol to assess in vivo and ex vivo myeloperoxidase activity in mouse models of vascular inflammation and disease using hydroethidine. Free Radic. Biol. Med. 2016;97:124–135. doi: 10.1016/j.freeradbiomed.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 191.Karimi Galougahi K., Antoniades C., Nicholls S.J., Channon K.M., Figtree G.A. Redox biomarkers in cardiovascular medicine. Eur. Heart J. 2015;36:1576–1582. doi: 10.1093/eurheartj/ehv126. 1582a-b. [DOI] [PubMed] [Google Scholar]
- 192.Chen C.H., Budas G.R., Churchill E.N., Disatnik M.H., Hurley T.D., Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [pii] 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Maack C., Kartes T., Kilter H., Schafers H.J., Nickenig G., Bohm M., Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 194.Hohn T.J., Grune T. The proteasome and the degradation of oxidized proteins: part III-Redox regulation of the proteasomal system. Redox biology. 2014;2:388–394. doi: 10.1016/j.redox.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jung T., Hohn A., Grune T. The proteasome and the degradation of oxidized proteins: Part II - protein oxidation and proteasomal degradation. Redox biology. 2014;2:99–104. doi: 10.1016/j.redox.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Crnko S., Du Pre B.C., Sluijter J.P.G., Van Laake L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019;16:437–447. doi: 10.1038/s41569-019-0167-4. [DOI] [PubMed] [Google Scholar]
- 197.Putker M., O'Neill J.S. Reciprocal control of the circadian clock and cellular redox state - a critical appraisal. Mol. Cell. 2016;39:6–19. doi: 10.14348/molcells.2016.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li H., Kilgallen A.B., Munzel T., Wolf E., Lecour S., Schulz R., Daiber A., Van Laake L.W. Influence of mental stress and environmental toxins on circadian clocks: implications for redox regulation of the heart and cardioprotection. Br. J. Pharmacol. 2020;177:5393–5412. doi: 10.1111/bph.14949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ahsan M.K., Lekli I., Ray D., Yodoi J., Das D.K. Redox regulation of cell survival by the thioredoxin superfamily: an implication of redox gene therapy in the heart. Antioxidants Redox Signal. 2009;11:2741–2758. doi: 10.1089/ARS.2009.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Butterfield D.A., Perluigi M. Redox proteomics: a key tool for new insights into protein modification with relevance to disease. Antioxidants Redox Signal. 2017;26:277–279. doi: 10.1089/ars.2016.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Butterfield D.A., Dalle-Donne I. Redox proteomics. Antioxidants Redox Signal. 2012;17:1487–1489. doi: 10.1089/ars.2012.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chen X., Lee J., Wu H., Tsang A.W., Furdui C.M. Mass spectrometry in advancement of redox precision medicine. Adv. Exp. Med. Biol. 2019;1140:327–358. doi: 10.1007/978-3-030-15950-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kumar V., Calamaras T.D., Haeussler D., Colucci W.S., Cohen R.A., McComb M.E., Pimentel D., Bachschmid M.M. Cardiovascular redox and ox stress proteomics. Antioxidants Redox Signal. 2012;17:1528–1559. doi: 10.1089/ars.2012.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fratelli M., Demol H., Puype M., Casagrande S., Eberini I., Salmona M., Bonetto V., Mengozzi M., Duffieux F., Miclet E., Bachi A., Vandekerckhove J., Gianazza E., Ghezzi P. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3505–3510. doi: 10.1073/pnas.052592699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gao X.H., Li L., Parisien M., Wu J., Bederman I., Gao Z., Krokowski D., Chirieleison S.M., Abbott D., Wang B., Arvan P., Cameron M., Chance M., Willard B., Hatzoglou M. Discovery of a redox thiol switch: implications for cellular energy metabolism. Mol. Cell. Proteomics : MCP. 2020;19:852–870. doi: 10.1074/mcp.RA119.001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ghezzi P., Davies K., Delaney A., Floridi L. Theory of signs and statistical approach to big data in assessing the relevance of clinical biomarkers of inflammation and oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 2018;115:2473–2477. doi: 10.1073/pnas.1719807115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rius-Perez S., Perez S., Torres-Cuevas I., Marti-Andres P., Talens-Visconti R., Paradela A., Guerrero L., Franco L., Lopez-Rodas G., Torres L., Corrales F., Sastre J. Blockade of the trans-sulfuration pathway in acute pancreatitis due to nitration of cystathionine beta-synthase. Redox biology. 2020;28:101324. doi: 10.1016/j.redox.2019.101324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Reis A. Oxidative Phospholipidomics in health and disease: achievements, challenges and hopes. Free Radic. Biol. Med. 2017;111:25–37. doi: 10.1016/j.freeradbiomed.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 209.Gruber F., Kremslehner C., Narzt M.S. The impact of recent advances in lipidomics and redox lipidomics on dermatological research. Free Radic. Biol. Med. 2019;144:256–265. doi: 10.1016/j.freeradbiomed.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 210.Skeffington K.L., Bond A.R., Bigotti M.G., AbdulGhani S., Iacobazzi D., Kang S.L., Heesom K.J., Wilson M.C., Stoica S., Martin R., Caputo M., Suleiman M.S., Ghorbel M.T. Changes in inflammation and oxidative stress signalling pathways in coarcted aorta triggered by bicuspid aortic valve and growth in young children. Experimental and therapeutic medicine. 2020;20:48. doi: 10.3892/etm.2020.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Malloci M., Perdomo L., Veerasamy M., Andriantsitohaina R., Simard G., Martinez M.C. Extracellular vesicles: mechanisms in human health and disease. Antioxidants Redox Signal. 2019;30:813–856. doi: 10.1089/ars.2017.7265. [DOI] [PubMed] [Google Scholar]
- 212.Hitchler M.J., Domann F.E. Redox regulation of the epigenetic landscape in cancer: a role for metabolic reprogramming in remodeling the epigenome. Free Radic. Biol. Med. 2012;53:2178–2187. doi: 10.1016/j.freeradbiomed.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hayes P., Knaus U.G. Balancing reactive oxygen species in the epigenome: NADPH oxidases as target and perpetrator. Antioxidants Redox Signal. 2013;18:1937–1945. doi: 10.1089/ars.2012.4895. [DOI] [PubMed] [Google Scholar]
- 214.Hanf A., Oelze M., Manea A., Li H., Munzel T., Daiber A. The anti-cancer drug doxorubicin induces substantial epigenetic changes in cultured cardiomyocytes. Chem. Biol. Interact. 2019;313:108834. doi: 10.1016/j.cbi.2019.108834. [DOI] [PubMed] [Google Scholar]
- 215.Ordovas J.M., Smith C.E. Epigenetics and cardiovascular disease. Nat. Rev. Cardiol. 2010;7:510–519. doi: 10.1038/nrcardio.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.van der Harst P., de Windt L.J., Chambers J.C. Translational perspective on epigenetics in cardiovascular disease. J. Am. Coll. Cardiol. 2017;70:590–606. doi: 10.1016/j.jacc.2017.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Keaney J.F., Jr., Larson M.G., Vasan R.S., Wilson P.W., Lipinska I., Corey D., Massaro J.M., Sutherland P., Vita J.A., Benjamin E.J., Framingham S. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 218.Meigs J.B., Larson M.G., Fox C.S., Keaney J.F., Jr., Vasan R.S., Benjamin E.J. Association of oxidative stress, insulin resistance, and diabetes risk phenotypes: the Framingham Offspring Study. Diabetes Care. 2007;30:2529–2535. doi: 10.2337/dc07-0817. [DOI] [PubMed] [Google Scholar]
- 219.Vassalle C., Botto N., Andreassi M.G., Berti S., Biagini A. Evidence for enhanced 8-isoprostane plasma levels, as index of oxidative stress in vivo, in patients with coronary artery disease. Coron. Artery Dis. 2003;14:213–218. doi: 10.1097/01.mca.0000063504.13456.c3. [DOI] [PubMed] [Google Scholar]
- 220.Heitzer T., Ylä-Herttuala S., Luoma J., Kurz S., Munzel T., Just H., Olschewski M., Drexler H. Cigarette smoking potentiates endothelial dysfunction of forearm resistance vessels in patients with hypercholesterolemia: role of oxidized LDL. Circulation. 1996;93:1346–1353. doi: 10.1161/01.cir.93.7.1346. [DOI] [PubMed] [Google Scholar]
- 221.Ikonomidis I., Tzortzis S., Lekakis J., Paraskevaidis I., Andreadou I., Nikolaou M., Kaplanoglou T., Katsimbri P., Skarantavos G., Soucacos P., Kremastinos D.T. Lowering interleukin-1 activity with anakinra improves myocardial deformation in rheumatoid arthritis. Heart. 2009;95:1502–1507. doi: 10.1136/hrt.2009.168971. [DOI] [PubMed] [Google Scholar]
- 222.Ikonomidis I., Tzortzis S., Andreadou I., Paraskevaidis I., Katseli C., Katsimbri P., Pavlidis G., Parissis J., Kremastinos D., Anastasiou-Nana M., Lekakis J. Increased benefit of interleukin-1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circulation. Cardiovascular imaging. 2014;7:619–628. doi: 10.1161/CIRCIMAGING.113.001193. [DOI] [PubMed] [Google Scholar]
- 223.Lambadiari V., Pavlidis G., Kousathana F., Maratou E., Georgiou D., Andreadou I., Kountouri A., Varoudi M., Balampanis K., Parissis J., Triantafyllidi H., Katogiannis K., Birba D., Lekakis J., Dimitriadis G., Ikonomidis I. Effects of different antidiabetic medications on endothelial glycocalyx, myocardial function, and vascular function in type 2 diabetic patients: one year follow-up study. J. Clin. Med. 2019;8 doi: 10.3390/jcm8070983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stamatelopoulos K., Georgiopoulos G., Athanasouli F., Nikolaou P.E., Lykka M., Roussou M., Gavriatopoulou M., Laina A., Trakada G., Charakida M., Delialis D., Petropoulos I., Pamboukas C., Manios E., Karakitsou M., Papamichael C., Gatsiou A., Lambrinoudaki I., Terpos E., Stellos K., Andreadou I., Dimopoulos M.A., Kastritis E. Reactive vasodilation predicts mortality in primary systemic light-chain amyloidosis. Circ. Res. 2019;125:744–758. doi: 10.1161/CIRCRESAHA.119.314862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mikhed Y., Daiber A., Steven S. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int. J. Mol. Sci. 2015;16:15918–15953. doi: 10.3390/ijms160715918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Korovila I., Hugo M., Castro J.P., Weber D., Hohn A., Grune T., Proteostasis T. Jung. Oxidative stress and aging. Redox biology. 2017;13:550–567. doi: 10.1016/j.redox.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Reeg S., Grune T. Protein oxidation in aging: does it play a role in aging progression? Antioxidants Redox Signal. 2015;23:239–255. doi: 10.1089/ars.2014.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hanus J., Zhao F., Wang S. Current therapeutic developments in atrophic age-related macular degeneration. Br. J. Ophthalmol. 2016;100:122–127. doi: 10.1136/bjophthalmol-2015-306972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wonisch W., Falk A., Sundl I., Winklhofer-Roob B.M., Lindschinger M. Oxidative stress increases continuously with BMI and age with unfavourable profiles in males. Aging Male. 2012;15:159–165. doi: 10.3109/13685538.2012.669436. [DOI] [PubMed] [Google Scholar]
- 230.Gil L., Siems W., Mazurek B., Gross J., Schroeder P., Voss P., Grune T. Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic. Res. 2006;40:495–505. doi: 10.1080/10715760600592962. [DOI] [PubMed] [Google Scholar]
- 231.Pandey K.B., Mehdi M.M., Maurya P.K., Rizvi S.I. Plasma protein oxidation and its correlation with antioxidant potential during human aging. Dis. Markers. 2010;29:31–36. doi: 10.3233/DMA-2010-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Komosinska-Vassev K., Olczyk P., Winsz-Szczotka K., Klimek K., Olczyk K. Plasma biomarkers of oxidative and AGE-mediated damage of proteins and glycosaminoglycans during healthy ageing: a possible association with ECM metabolism. Mechanisms of ageing and development. 2012;133:538–548. doi: 10.1016/j.mad.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 233.Cakatay U., Kayali R., Uzun H. Relation of plasma protein oxidation parameters and paraoxonase activity in the ageing population. Clin. Exp. Med. 2008;8:51–57. doi: 10.1007/s10238-008-0156-0. [DOI] [PubMed] [Google Scholar]
- 234.Samiec P.S., Drews-Botsch C., Flagg E.W., Kurtz J.C., Sternberg P., Jr., Reed R.L., Jones D.P. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic. Biol. Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 235.Giustarini D., Dalle-Donne I., Lorenzini S., Milzani A., Rossi R. Age-related influence on thiol, disulfide, and protein-mixed disulfide levels in human plasma. The journals of gerontology. Series A, Biological sciences and medical sciences. 2006;61:1030–1038. doi: 10.1093/gerona/61.10.1030. [DOI] [PubMed] [Google Scholar]
- 236.Jones D.P., Mody V.C., Jr., Carlson J.L., Lynn M.J., Sternberg P., Jr. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic. Biol. Med. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 237.Weber D., Stuetz W., Toussaint O., Debacq-Chainiaux F., Dolle M.E.T., Jansen E., Gonos E.S., Franceschi C., Sikora E., Hervonen A., Breusing N., Sindlinger T., Moreno-Villanueva M., Burkle A., Grune T. Associations between specific redox biomarkers and age in a large European cohort: the MARK-AGE project. Oxidative medicine and cellular longevity. 2017:1401452. doi: 10.1155/2017/1401452. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Heitzer T., Schlinzig T., Krohn K., Meinertz T., Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 239.Fratta Pasini A., Albiero A., Stranieri C., Cominacini M., Pasini A., Mozzini C., Vallerio P., Cominacini L., Garbin U. Serum oxidative stress-induced repression of Nrf2 and GSH depletion: a mechanism potentially involved in endothelial dysfunction of young smokers. PloS One. 2012;7 doi: 10.1371/journal.pone.0030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jurado-Gamez B., Fernandez-Marin M.C., Gomez-Chaparro J.L., Munoz-Cabrera L., Lopez-Barea J., Perez-Jimenez F., Lopez-Miranda J. Relationship of oxidative stress and endothelial dysfunction in sleep apnoea. Eur. Respir. J. 2011;37:873–879. doi: 10.1183/09031936.00027910. [DOI] [PubMed] [Google Scholar]
- 241.Yilmaz M.I., Saglam M., Caglar K., Cakir E., Sonmez A., Ozgurtas T., Aydin A., Eyileten T., Ozcan O., Acikel C., Tasar M., Genctoy G., Erbil K., Vural A., Zoccali C. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am. J. Kidney Dis. : the official journal of the National Kidney Foundation. 2006;47:42–50. doi: 10.1053/j.ajkd.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 242.Botker H.E., Hausenloy D., Andreadou I., Antonucci S., Boengler K., Davidson S.M., Deshwal S., Devaux Y., Di Lisa F., Di Sante M., Efentakis P., Femmino S., Garcia-Dorado D., Giricz Z., Ibanez B., Iliodromitis E., Kaludercic N., Kleinbongard P., Neuhauser M., Ovize M., Pagliaro P., Rahbek-Schmidt M., Ruiz-Meana M., Schluter K.D., Schulz R., Skyschally A., Wilder C., Yellon D.M., Ferdinandy P., Heusch G. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res. Cardiol. 2018;113:39. doi: 10.1007/s00395-018-0696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang Y., Sun Q., He B., Xiao J., Wang Z., Sun X. Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. Int. J. Cardiol. 2011;148:91–95. doi: 10.1016/j.ijcard.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 244.Oliveira M.S., Tanaka L.Y., Antonio E.L., Brandizzi L.I., Serra A.J., Dos Santos L., Krieger J.E., Laurindo F.R.M., Tucci P.J.F. Hyperbaric oxygenation improves redox control and reduces mortality in the acute phase of myocardial infarction in a rat model. Mol. Med. Rep. 2020;21:1431–1438. doi: 10.3892/mmr.2020.10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Barrios M.J., Munoz G., T R. 315 - 4-hydroxynonenal as oxidative damage index in acute myocardial infarction patients. Free Radic. Biol. Med. 2016;100:S136. [Google Scholar]
- 246.Blasig I.E., Grune T., Schonheit K., Rohde E., Jakstadt M., Haseloff R.F., Siems W.G. 4-Hydroxynonenal, a novel indicator of lipid peroxidation for reperfusion injury of the myocardium. Am. J. Physiol. 1995;269:H14–H22. doi: 10.1152/ajpheart.1995.269.1.H14. [DOI] [PubMed] [Google Scholar]
- 247.Renner A., Sagstetter M.R., Harms H., Lange V., Gotz M.E., Elert O. Formation of 4-hydroxy-2-nonenal protein adducts in the ischemic rat heart after transplantation. J. Heart Lung Transplant. 2005;24:730–736. doi: 10.1016/j.healun.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 248.Wu X.Y., Luo A.Y., Zhou Y.R., Ren J.H. N-acetylcysteine reduces oxidative stress, nuclear factorkappaB activity and cardiomyocyte apoptosis in heart failure. Mol. Med. Rep. 2014;10:615–624. doi: 10.3892/mmr.2014.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yan Q.C., Xiong A.H., Xiao X., Luo Y.T. [Significance of plasma 8-iso-prostaglandin F2alpha level in acute myocardial ischemia and intervention effect of N-acetylcysteine: a study in rats] Di Yi Jun Yi Da Xue Xue Bao. 2003;23:605–607. [PubMed] [Google Scholar]
- 250.Inafuku H., Kuniyoshi Y., Yamashiro S., Arakaki K., Nagano T., Morishima Y., Kise Y. Determination of oxidative stress and cardiac dysfunction after ischemia/reperfusion injury in isolated rat hearts. Ann. Thorac. Cardiovasc. Surg. 2013;19:186–194. doi: 10.5761/atcs.oa.12.01896. [DOI] [PubMed] [Google Scholar]
- 251.Qin F., Simeone M., Patel R. Inhibition of NADPH oxidase reduces myocardial oxidative stress and apoptosis and improves cardiac function in heart failure after myocardial infarction. Free Radic. Biol. Med. 2007;43:271–281. doi: 10.1016/j.freeradbiomed.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 252.Yang C., Wu K., Li S.H., You Q. Protective effect of curcumin against cardiac dysfunction in sepsis rats. Pharm. Biol. 2013;51:482–487. doi: 10.3109/13880209.2012.742116. [DOI] [PubMed] [Google Scholar]
- 253.Sehirli A.O., Koyun D., Tetik S., Ozsavci D., Yiginer O., Cetinel S., Tok O.E., Kaya Z., Akkiprik M., Kilic E., Sener G. Melatonin protects against ischemic heart failure in rats. J. Pineal Res. 2013;55:138–148. doi: 10.1111/jpi.12054. [DOI] [PubMed] [Google Scholar]
- 254.Gyongyosi A., Terraneo L., Bianciardi P., Tosaki A., Lekli I., Samaja M. The impact of moderate chronic hypoxia and hyperoxia on the level of apoptotic and autophagic proteins in myocardial tissue. Oxidative medicine and cellular longevity. 2018:5786742. doi: 10.1155/2018/5786742. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kurokawa S., Niwano S., Niwano H., Ishikawa S., Kishihara J., Aoyama Y., Kosukegawa T., Masaki Y., Izumi T. Progression of ventricular remodeling and arrhythmia in the primary hyperoxidative state of glutathione-depleted rats. Circ. J. 2011;75:1386–1393. doi: 10.1253/circj.cj-10-1089. [DOI] [PubMed] [Google Scholar]
- 256.Boarescu P.M., Chirila I., Bulboaca A.E., Bocsan I.C., Pop R.M., Gheban D., Bolboaca S.D. Effects of curcumin nanoparticles in isoproterenol-induced myocardial infarction. Oxidative medicine and cellular longevity. 2019:7847142. doi: 10.1155/2019/7847142. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Boarescu P.M., Boarescu I., Bocsan I.C., Pop R.M., Gheban D., Bulboaca A.E., Nicula C., Rajnoveanu R.M., Bolboaca S.D. Curcumin nanoparticles protect against isoproterenol induced myocardial infarction by alleviating myocardial tissue oxidative stress, electrocardiogram, and biological changes. Molecules. 2019;24 doi: 10.3390/molecules24152802. [DOI] [PMC free article] [PubMed] [Google Scholar]