Abstract
Introduction
Oxidative stress and inflammation are known to play a critical role in ageing and chronic disease development and could therefore represent important targets for developing dietary strategies for disease prevention. We aimed to systematically review the results from observational studies and intervention trials published in the last 5 years on the associations between dietary patterns and biomarkers of oxidative stress and inflammation.
Methods
A systematic search of the PubMed, MEDLINE and Web of Science (January 2015 to October 2020) was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Methodological quality of selected studies was evaluated based on the NUTRIGRADE and BIOCROSS assessment tools.
Results
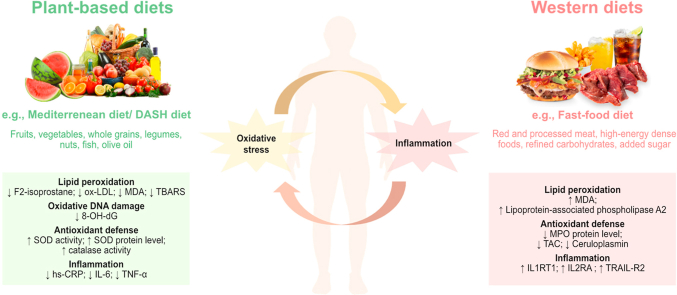
In total, 29 studies among which 16 observational studies and 13 intervention studies were found eligible for review. Overall, results indicated an inverse association between plant-based diets - the Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diet - and oxidative stress and proinflammatory biomarkers. In observational studies, inverse associations were further revealed for the vegetarian diet, the USDA Healthy Eating Index (HEI) - based diet and the paleolithic diet, whereas a positive association was seen for western and fast food diets. Quality assessment suggested that majority of dietary intervention studies (n = 12) were of low to moderate quality.
Conclusions
This study provides evidence that the plant-based dietary patterns are associated with lowered levels of oxidative stress and inflammation and may provide valid means for chronic disease prevention. Future large-scale intervention trials using validated biomarkers are warranted to confirm these findings.
Keywords: Dietary patterns, Oxidative stress, Inflammation, Biomarkers, Systematic review
Graphical abstract
Highlights
-
•
Following plant-based diet was associated with lower levels of oxidative stress and inflammation.
-
•
Mediterranean diet reduced levels of lipid peroxidation and oxidative DNA damage.
-
•
DASH diet lowered levels of lipid peroxidation and increased nitric oxide levels.
-
•
Western diets were associated with higher oxidative stress and inflammation levels.
-
•
The overall quality of dietary intervention studies was low to moderate.
Abbreviations
- ABTs
2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid
- AGEs
Advanced glycation end products
- AHEI
Alternative Healthy Eating Index
- aMED
Alternate Mediterranean diet
- AOPPs
Advanced oxidation protein products
- ARE
Antioxidant response elements
- CEACAM8
Carcinoembryonic antigen-related cell adhesion molecule 8
- CRP
C-reactive protein
- CML
N(6)-carboxymethyllysine
- COWs
Coke oven workers
- CRP
C-reactive protein
- CVD
Cardiovascular disease
- DASH
Dietary Approaches to Stop Hypertension
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- FFQ
Food frequency questionnaire
- FlOP
Fluorescent oxidation product
- FRAP
Ferric reducing ability of plasma
- Gal-4
Galectin-4
- GSH
Glutathione
- HEI
Healthy Eating Index
- HT
Hashimoto thyroiditis
- IL
Interleukin
- IL1RT1
Interleukin-1 receptor type 1
- IL2RA
Interleukin-2 receptor subunit alpha
- MCP-1
Monocyte chemoattractant protein-1
- MDA
Malondialdehyde
- MetS
Metabolic syndrome
- MI
Myocardial infarction
- MIP-1β
Macrophage inflammatory protein-1 beta
- MMP-7
Matrix metalloproteinase-7
- MPO
Myeloperoxidase
- MUFA
Monounsaturated fatty acids
- NAFLD
Non-alcoholic fatty liver disease
- NO Nitric oxide Ox-LDL
Oxidated low-density lipoprotein
- PCA
Principal component analysis
- PON-3
Paraoxonase 3
- PRISMA
Preferred Reporting of Systematic Reviews and Meta-Analyses
- PUFA
Polyunsaturated fatty acids
- Q
Quartile
- RCT
Randomized-controlled trial
- ROS
Reactive oxygen species
- SFA
Saturated fatty acids
- sNox2-dp
Soluble Nox2-derived peptide
- SOD
Superoxide dismutase
- TAC
Total antioxidant capacity
- TBARS
Thiobarbituric acid reactive substances
- TNF-α
Tumor necrosis factor alpha
- TRAIL-R2
Tumor necrosis factor-related apoptosis inducing ligand receptor 2
- UPAR
Urokinase plasminogen activator surface receptor
- 8-OH-dG
8-hydroxy-2-deoxyguanosine
1. Introduction
Oxidative stress is characterized by an imbalance between production and accumulation of oxygen reactive species (ROS) in cells and tissues and the natural ability of organisms to detoxify abundant reactive species, leading to global oxidative damage and cellular aging [1,2]. ROS represent partially reduced metabolites of molecular oxygen generated as products of metabolic reactions or as by-products of various cellular processes, including inflammation [[3], [4], [5]]. Oxidative stress and inflammation are two closely interrelated and interdependent pathophysiological processes. On one hand, ROS initiate intracellular signaling cascade that enhances proinflammatory gene expression [6]; on the other hand, inflammatory cells secrete ROS and immune mediators (i.e. cytokines and chemokines) leading to induced oxidative stress and tissue damage at the site of inflammation [7]. Oxidative stress and inflammation are known to play an important role in ageing and age-related diseases, including cardiovascular diseases (CVDs), neurodegenerative diseases and cancer [[6], [8], [9], [10], [11], [12], [13]]. Understanding the role of nutrition as modifiable determinant of these pathophysiological pathways may therefore hold the key to age-related disease prevention.
Paradoxically, so far, the majority of trials aimed to assess the role of antioxidant nutrients – minerals and vitamins – as preventive targets for chronic diseases, such as CVD and cancer, have not been successful [14,15]. A potential explanation for these disappointing results could be that those studies were based on measurements of individual pro- and anti-oxidant markers and thus may not provide a comprehensive assessment of both the nutritional exposure and the systemic redox status [14]. While previous research has been mostly focused on individual nutrients or single foods, the last decade has been marked by an increased recognition of the importance of the whole diet as opposed to specific nutrients [16]. The dietary pattern approach recognizes that foods are composed by various nutrients and bioactive constituents and are consumed in combinations and may interact with each other in complex ways [17]. Due to this complexity, the associations between single nutrients and age-related diseases and phenotypes may be difficult to explore and interpret. Dietary patterns allow examining combinations of various food components and their complex interactions [18]. Dietary patterns can be defined by posteriori (data-driven or empirical approaches) and a priori guidelines and recommendations (diet quality indices). The posteriori approaches include statistical modeling techniques aimed to disentangle intercorrelated structures dietary items (principal component analysis) or to group individuals into patterns based on their reported mean intakes of foods (cluster analysis). On the other hand, the a priori diet quality indices are useful to assess adherence to dietary guidelines, i.e. Healthy Eating Index [19] or to a particular types of diet such as the Dietary Approaches to Stop Hypertension (DASH) [20] or Mediterranean diet [21]. A number of studies suggested that dietary patterns are associated with cardiometabolic risk factors, inflammatory levels and all-cause mortality in human research [[21], [22], [23]].
The field of biomarker discovery has been booming in recent years leading to new advances in the identification of novel molecules characterizing oxidative stress and inflammation in human research [24]. Likewise, in nutrition research the dietary pattern assessment has been in focus over the last years [23]. So far, the link between dietary patterns in relation to oxidative stress was only partly addressed in a previous systematic review [25]. The newly emerging evidence on link between dietary patterns and various biomarkers representing these pathways has not been evaluated.
We therefore aimed to systematically review and synthesize the results from observational studies and dietary intervention trials published in the last 5 years to clarify the association between dietary patterns and biomarkers characterizing oxidative stress and inflammation.
2. Methods
2.1. Protocol registration
The systematic review followed the requirements of the Preferred Reporting of Systematic Reviews and Meta Analyses (PRISMA) statement [26] and was registered at the International Prospective Register of Systematic Reviews (PROSPERO, Reference number: 212315; https://www.crd.york.ac.uk/prospero/212315).
2.2. Search strategy
A systematic search of the PubMed, MEDLINE and Web of Science (January 2015 to October 2020) was conducted using the following combination of Medical Subject Heading (MeSH) terms and text words, with limitation to English language (“Eat-Lancet diet" [All Fields] OR “planetary health diet" [All Fields] OR “portfolio diet" [All Fields] OR “DASH" [All Fields] OR “Dietary Approaches to Stop Hypertension" [All Fields] OR “Dietary Inflammatory Index" [All Fields] OR “nordic diet" [All Fields] OR “paleolithic diet" [All Fields] OR “plant-based diet" [All Fields] OR “vegetarian diet" [All Fields] OR “vegan diet" [All Fields] OR “Mediterranean diet" [All Fields] OR “dietary pattern*" [All Fields] OR “eating pattern*" [All Fields] OR “food pattern*" [All Fields] OR “diet index" [All Fields] OR “dietary index" [All Fields] OR “diet score" [All Fields] OR “dietary score" [All Fields]) AND (“oxidative damage" [All Fields] OR “oxidative stress" [All Fields] OR “immun*" [All Fields] OR “inflammat*" [All Fields] OR “CRP" [All Fields] OR ″C-reactive protein" [All Fields] OR “IL" [All Fields] OR “interleukin*" [All Fields] OR “TNF" [All Fields] OR “tumor necrosis factor" [All Fields] OR “acute-phase protein*" [All Fields] OR “adipokin*" [All Fields] OR “cytokine*" [All Fields]). The PICOs (Population, Intervention, Comparator, Outcome, Study Design) criteria used to define our research question are listed in Supplementary Table 1.
2.3. Eligibility criteria
Studies were included if they reported on the association (observational studies) or effect (intervention studies) of dietary patterns (as exposure) with biomarkers of oxidative stress and inflammation (as outcome). A primarily focus was put on studies reporting results on biomarkers representing pathways denoting oxidative stress and wherever available simultaneous measurements of inflammatory biomarkers.
The inclusion criteria were as follows: a) assessment of dietary patterns (based on whole foods) as main exposure; b) plasma/serum/urine measurements of biomarkers as main outcome measures; c) enrolled humans at adult and old age; d) analytical epidemiological studies, i.e. observational studies (cross-sectional, case-control or prospective cohort studies) and intervention studies (non-randomized trials, i.e. pre-post studies, and randomized control trials); and e) studies written in English and published in peer-reviewed journals.
The exclusion criteria were: (a) no original research (e.g. reviews, editorials, non-research letters); (b) case reports or case series; (c) ecological studies; (d) lack of data on dietary patterns (e.g., examined only individual nutrients or did not examine all dietary components); (e) no biomarker measurement reported; (f) studies not conducted in humans; (g) studies not conducted in adult population (<18 years old); and (h) studies without reported effect estimates. Additionally, intervention studies were excluded if: a) they used lifestyle interventions in conjunction with diet intervention (e.g., exercise or behavioral management); b) postprandial studies; or c) studies with intervention duration of less than 4 weeks. The latter criteria were applied to allow characterizing potential effect of habitual diet on changes of biomarker concentrations at a longer run rather than acute effects of initial drastic dietary change.
2.4. Selection of studies
Identified records were imported in EndNote referencing software (version X7, 2013; Thomson Reuters) and their titles and abstracts were screened by two independent reviewers (LK and TH). Full-text articles were retrieved if the article was considered eligible, and subjected to a second evaluation by two other independent reviewers (CER and KA). Any discrepancies and disagreements were discussed and resolved by consensus among reviewers. After retrieval of full-text articles, the reference lists of selected articles and other reviews were checked to identify additional potentially relevant articles. Where results from the same study were reported in multiple articles, the most recent article was included to avoid duplication of results.
2.5. Data extraction
Data extraction was performed by two independent reviewers (LK and TH) using a predefined data extraction form and extracted data was verified by a third reviewer (CER). The following information was extracted: first author, publication year and country, study design, sample size, dietary patterns identified (incl. dietary assessment tool and method of identifying dietary patterns), details of intervention and control groups (intervention studies), biomarker measured, and main findings. In addition, data on analytical methods, sample type and participants’ fasting status was recorded for each of the selected studies. When a study provided several estimates with adjustment for different confounders, results were reported based on the one adjusting for the largest number of factors (see Supplementary Table 2 for adjustment models). Discrepancies in data extraction were discussed and resolved by consensus among the reviewers.
2.6. Assessment of quality and risk of bias of included studies
The assessment of the quality of studies was performed by three independent reviewers (CER, LK and TH) using a combination of tools specifically developed for the assessment of nutritional exposures (NUTRIGRADE) [27] and biomarker outcomes (BIOCROSS) [28]. These tools were additionally adapted to consider features of cross-sectional and intervention study designs of the included studies. The specific questions and assigned points of the combined tool for observational and intervention studies are presented in Supplementary Tables 3 and 4, respectively. Each study was graded for a) overall risk of bias pertaining to how the study was conducted and reported, incl. study quality, study limitations, statistical analysis, data interpretation and funding bias (based on NUTRIGRADE) and b) risk of bias related to biomarker assessment and reporting, incl. biomarker measurement, specimen characteristics and assay methods, and laboratory measurements (based on BIOCROSS). The total scores of the study quality assessment tool for cross-sectional studies and intervention studies were 14.5 and 13, respectively. Studies scoring 0–5 were considered low quality, studies scoring 5–10 were considered moderate quality and studies scoring ≥10 points were considered high quality.
3. Results
3.1. Search results
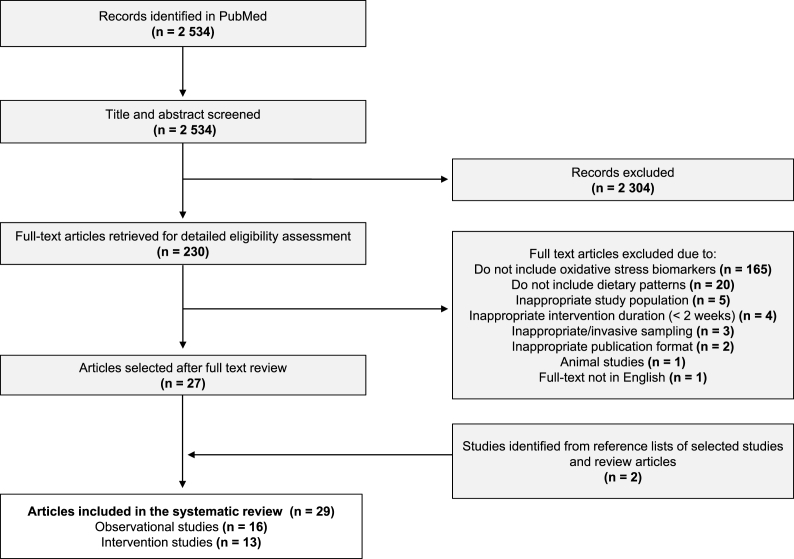
The process of study selection, including identification, screening, eligibility, and inclusion is illustrated in a flowchart presented in Fig. 1. The search strategy retrieved 2534 unique records. Initial screening of title and abstract excluded 2304 citations. 230 records were then selected for full-text detailed evaluation. Following the screening of the full-texts, further 203 articles were excluded because: a) studies did not report on measurements of oxidative stress biomarkers (n = 165); b) did not include information on dietary patterns (n = 20); c) the study included inappropriate study population (n = 5); d) had inappropriate intervention duration (<2 weeks) (n = 4); and e) other reasons (n = 7). Among these, 27 articles were identified as meeting the eligibility criteria. In addition to that, 2 studies were found through manual search of reference lists of selected studies and review articles. Finally, a total of 29 articles have been included in this current systematic review among which 16 observational studies and 13 intervention studies.
Fig. 1.
Flowchart of study selection, including identification, screening, eligibility, and inclusion of studies.
3.2. Study characteristics
The study characteristics of the selected observational and intervention studies are presented in Table 1, Table 2, respectively. Overall, there were 16 cross-sectional studies [[29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]], 5 non-randomized intervention trials [[45], [46], [47], [48], [49]] and 8 RCTs [[50], [51], [52], [53], [54], [55], [56], [57]]. None of the studies applied a prospective observational design to investigate associations between food patterns and changes in biomarker concentrations. Of the 8 RCTs, 6 used a parallel design and 2 used a crossover design [[50], [51], [52], [53], [54], [55], [56], [57]]. The 29 studies were conducted in the following countries: Australia [53,54], Brazil [29], Chile [47], China [43], Cyprus [38], Greece [42,45], Germany [49], Italy [30,31,37,44,46,51], Korea [57], Spain [39,50,56], Sweden [35], United States of America [34,40,41,48,52] and Iran [32,33,36,55]. The study sample sizes ranged from 40 to 2240 participants for observational studies [[29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]], and 20 to 805 participants for intervention studies [[45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]]. The duration of intervention studies ranged from 2 weeks to 5 years with a mean duration of 10 weeks [[45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]]. Participants’ ages ranged from 16 to 80 years at the time of study in the observational studies [[29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]] and 20–80 years in the intervention studies [[45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]]. 5 studies were restricted to women [33,38,41,47,57] and 4 studies included only men [29,37,43,49]. The remaining 20 studies examined both sexes. Most observational studies recruited healthy community-dwelling adults and several studies included also participants with underlying health condition, i.e. metabolic syndrome (MetS) [36], non-alcoholic fatty liver disease (NAFLD) [30], euthyroid Hashimoto thyroiditis (HT) [31] obesity [34] or atrial fibrillation patients [44]. Conversely, majority of intervention studies were conducted in participants with existing health problem such as obesity and MetS [46,47,52,[55], [56], [57]], CVD [50], NAFLD [45,55], whereas five of the 13 studies were conducted in predominantly healthy individuals [48,49,51,53,54].
Table 1.
Summary of observational studies investigating the associations between dietary patterns and biomarkers of oxidative stress and inflammation.
| Authors, Year | Country | Study design, duration | Participants | Dietary pattern/dietary assessment method | Biomarkers | Resultsa Positive association (↑); Inverse association (↓); No association (↔) |
|---|---|---|---|---|---|---|
| Cinegalia et al. [29], 2020 | Brazil | Cross-sectional | 2 groups: 1) N = 44, mean age (sem): 46.8 (1.4) years, healthy omnivorous adults 2) N = 44, mean age (sem): 45.5 (1.2) years; healthy vegetarian adults 100% male |
1) Omnivorous diet 2) Vegetarian diet (reference) |
Plasma: hs-CRP; heme oxygenase-1 levels | Omnivorous diet vs vegetarian diet: ↑ heme oxygenase-1 levels; ↔ hs-CRP |
| Baratta et al. [30], 2020 | Italy | Cross-sectional/PLINIO Study | N = 238, mean age (sd): 53.1 (12.4) years, 57% male, patients with NAFLD | Mediterranean diet /validated dietary questionnaire |
Serum: sNox2-dp | ↓ sNox2-dp |
| Ruggeri et al. [31], 2020 | Italy | Cross-sectional | 2 groups (pooled together): 1) N = 81, age range: 18–66 years, 12% male, patients with euthyroid HT 2) N = 119, age range: 18–65 years, 14% male, without euthyroid HT |
Mediterranean diet /FFQ |
Serum: AGEs; AOPPs Plasma: SOD; Glutathione reductase activity; Glutathione peroxidase activity; Thioredoxin reductase activity; TAC |
↔ AGEs; ↔ AOPPs; ↔ SOD; ↔ Glutathione reductase activity; ↔ Glutathione peroxidase activity; ↔ Thioredoxin reductase activity; ↔ TAC |
| Seyedi et al. [32], 2020 | Iran | Cross-sectional/Tehran Lipid and Glucose Study | N = 470, age range: 40–70 years, 25% male | 1) Western pattern 2) Semi-Mediterranean pattern Healthy pattern (reference) /FFQ |
Lipoprotein-associated phospholipase A2 | 1) Western pattern vs healthy pattern: ↑ Lipoprotein-associated phospholipase A2 (univariate and multivariate models) 2) Semi-Mediterranean pattern vs healthy pattern: ↓ Lipoprotein-associated phospholipase A2 (univariate model); ↔ Lipoprotein-associated phospholipase A2 (multivariate model) |
| Abashzadeh et al. [33], 2020 | Iran | Cross-sectional | N = 320, age range: 20–45 years, 0% male, healthy female nurses | 1) Healthy pattern 2) Traditional pattern 3) Unhealthy pattern /FFQ |
Serum: Ceruloplasmin; protein carbonyl; TAC | 1) Healthy pattern: ↔ Ceruloplasmin; ↔ protein carbonyl; ↔ TAC 2) Traditional pattern: ↔ Ceruloplasmin; ↔ protein carbonyl; ↔ TAC 3) Unhealthy pattern: ↓ Ceruloplasmin; ↓ protein carbonyl; ↔ TAC |
| Crowe-White et al. [34], 2019 | USA | Cross-sectional | N = 133, mean age (sd): 70.4 (4.8) years, 40% male, obese older adults | HEI /3 × 24h dietary recalls |
Serum: Hydrophilic antioxidant capacity; lipophilic antioxidant capacity; TAC; hs-CRP; TNF-α; IL-6 |
↔ Hydrophilic antioxidant capacity; ↔ lipophilic antioxidant capacity; ↔ TAC; ↔ hs-CRP; ↔ TNF-α; ↔ IL-6 |
| Lemming et al. [35], 2019 | Sweden | Cross-sectional/EpiHealth | N = 2240, mean age (sd): 61 (8.4) years, 50% male | 1) Healthy pattern 2) Western pattern 3) Dairy and sandwich pattern 4) Fast food and alcohol pattern /FFQ |
Plasma: MPO protein level; resistin; spondin-2; follistatin; MMP-7; PON-3; Gal-4; TRAIL-R2; IL1RT1; UPAR; CEACAM8; IL2RA |
1) Healthy pattern: ↑ PON-3; ↓ MPO protein level; ↓ spondin-2; ↓ follistatin; ↓ MMP-7; ↓ TRAIL-R2; ↔ resistin; ↔ Gal 4; ↔ IL1RT1; ↔ UPAR; ↔ CEACAM8; ↔ IL2RA 2) Western pattern: ↑ MPO protein level; ↑ resistin; ↑ follistatin; ↑ CEACAM8; ↑ IL1RT1; ↑ TRAIL-R2; ↑ IL2RA; ↑ UPAR; ↓ Gal-4; ↔ spondin-2; ↔ PON-3; ↔ MMP-7; ↔ resistin 3) Dairy and sandwich pattern: ↑ CEACAM8; ↔ spondin-2; ↔ follistatin; ↔ MMP-7; ↔ Gal-4; ↔ TRAIL-R2; ↔ IL1RT1; ↔ UPAR; ↔ IL2RA; ↔ PON-3; ↔ MPO protein level; ↔ resistin 4) Fast food and alcohol pattern: ↑ PON-3; ↓ MPO protein level; ↓ spondin-2; ↓ Gal-4; ↓ IL1RT1; ↓ CEACAM8; ↔ resistin; ↔ follistatin; ↔ TRAIL-R2; ↔ UPAR; ↔ IL2RA; ↔ MMP-7 |
| Mirmiran et al. [36], 2018 | Iran | Cross-sectional/Tehran Lipid and Glucose Study | N = 400, age range: 20–60 years, adults with MetS | 1) Healthy pattern (high in fruits and vegetables) 2) Unhealthy pattern (high in soft drinks, fast foods, organ meats) /FFQ |
Plasma: TAC; MDA |
1) Healthy pattern: ↑ TAC; ↓ MDA 2) Unhealthy pattern: ↓ TAC; ↑ MDA |
| Vanacore et al. [37], 2018 | Italy | Cross-sectional | N = 30 (N = 10 omnivore diet; N = 10 vegan diet; N = 10 vegetarian diet), age range: 20–30 years, 100% male, metabolically healthy adults | 1) Omnivore diet 2) Vegetarian diet 3) Vegan diet /FFQ |
Serum: DPPH; FRAP; total phenol; ABTS; TBARS; nitrite |
1) Omnivore diet vs vegan: ↑ Total phenol; ↔ FRAP; ↔ DPPH; ↔ ABTS; ↔ TBARS; ↔ nitrite 2) Vegetarian diet vs vegan: ↑ Total phenol; ↑ FRAP; ↔ DPPH; ↔ ABTS; ↔ TBARS; ↔ nitrite (↑ nitrite compared to omnivore diet) 3) Vegan diet vs the other diets: ↑ TBARS; ↑ nitrite compared to omnivore diet, ↔ nitrite compared to vegan diet; ↔ DPPH; ↔ ABTS; ↔ total phenol; ↔ FRAP |
| Kakkoura et al. [38], 2017 | Cyprus | Cross-sectional | N = 564, mean age (sd): 55.3 (7.3), 0% male, women without breast cancer | Mediterranean diet /FFQ |
Serum: GSH; Flavin mononucleotide; methionine sulfoxide; homocysteine; cystathionine; total cysteine |
↔ GSH; ↔ flavin mononucleotide; ↔ methionine sulfoxide; ↔ homocysteine; ↔ cystathionine; ↔ total cysteine |
| Aranda et al. [39], 2017 | Spain | Cross-sectional | N = 81, mean age (95% CI): 43.6 (40.1–47.1) years, 43% male, healthy adults | Mediterranean diet with various types of seafood /3-day dietary record |
Plasma: Ox-LDL; F2-isoprostane |
1) Mediterranean diet + white fish (adjusted): ↓ F2-isoprostane; ↔ Ox-LDL 2) Mediterranean diet + oily fish (adjusted): ↔ Ox-LDL; ↔ F2-isoprostane 3) Mediterranean diet + shellfish (adjusted): ↑ Ox-LDL; ↔ F2-isoprostane |
| Whalen et al. [40], 2016 | USA | Cross-sectional/pooled MAPI and MAPII studies | N = 646 (N = 558 with hs-CRP samples; N = 434 with F2-isoprostane samples), age range: 30–74 years | 1) Paleolithic diet 2) Mediterranean diet /FFQ |
Plasma: F2-isoprostane; hs-CRP |
1) Paleolithic diet ↓ F2-isoprostane; ↓ hs-CRP 2) Mediterranean diet: ↓ F2-isoprostane; ↓ hs-CRP |
| Jung et al. [41], 2016 | USA | Cross-sectional/Nurses’ Health Study | N = 1688, age range: 30–55 years, 0% male, women free of cancer and MI | 1) AHEI 2) DASH 3) aMED /FFQ |
Plasma: Fluorescent oxidation products (FlOP_320; FlOP_360; FlOP_400) |
1) AHEI: ↑ FlOP_320; ↑ FlOP_360; ↔ FlOP_400 2) DASH ↑ FlOP_320; ↑ FlOP_360; ↔ FlOP_400 3) aMED: ↑ FlOP_320; ↑ FlOP_360; ↔ FlOP_400 |
| Koloverou et al. [42], 2016 | Greece | Cross-sectional/ATTICA study | N = 191 | Mediterranean diet /FFQ |
Serum: TAC; ox-LDL; CRP; IL-6; TNF-α; serum amyloid A; homocysteine |
↑ TAC; ↓ ox-LDL; ↓ CRP; ↓ IL-6; ↓ TNF-α; ↓ serum amyloid A; ↓ homocysteine |
| Xie et al. [43], 2015 | China | Cross-sectional | N = 51 topside COWs, mean age (sd): 35.5 (8.3) years, 100% male, adults exposed to high levels of toxic chemicals N = 79 other COWs, mean age (sd): 37.8 (8.2) years, 100% male, adults exposed to high levels of toxic chemicals |
1) Rice-noodle pattern 2) Fruit-vegetable pattern 3) High-protein food pattern 4) Snack-sugar pattern /FFQ |
Serum: MDA; SOD protein level; glutathione peroxidase protein level |
In all COWs (Q4 vs Q1 of pattern): 1) Rice-noodle pattern: ↔ MDA; ↔ SOD protein level; ↔ glutathione peroxidase protein level 2) Fruit-vegetable pattern: ↑ SOD protein level; ↑ glutathione peroxidase protein level; ↓ MDA 3) High-protein pattern: ↔ MDA; ↔ SOD protein level; ↔ glutathione peroxidase protein level 4) Snack-sugar pattern: ↔ MDA; ↔ SOD protein level; ↔ glutathione peroxidase protein level |
| Pastori et al. [44], 2015 | Italy | Cross-sectional | N = 709, median age (IQR): 72.6 (8.7) years, 56% male, atrial fibrillation patients | Mediterranean diet /validated short dietary questionnaire |
Serum: sNOX2-dp | ↓ sNOX2-dp |
Results ordered according to type of biomarker (oxidative stress, immune-inflammatory) and according to significance.
Abbreviations: ABTs, 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid; AGEs, advanced glycation end prodcuts; AHEI, Alternative Healthy Eating Index; aMED, Alternate Mediterranean diet; AOPPs, advanced oxidation protein products; ARE, antioxidant response elements; CEACAM8, carcinoembryonic antigenrelated cell adhesion molecule 8; COWs, coke oven workers; CRP, C-reactive protein; DASH, Dietary Approach to Stop Hypertension; DPPH, 1,1-diphenyl-2-picrylhydrazyl; FFQ, food frequency questionnaire; FlOP, fluorescent oxidation product; FRAP, ferric reducing ability of plasma; Gal-4, Galectin-4; HEI, Healthy Eating Index; hs, high sensitivity; HT, hashimoto thyroiditis; IQR, interquartile range; IL, interleukin; ILTR1, interleukin-1 receptor type 1; IL2RA, interleukin-2 receptor subunit alpha; MDA, malondialdehyde; MetS, metabolic syndrome; MI, myocardial infarction; MMP-7, matrix metalloproteinase-7; MPO, myeloperoxidase; NAFLD, non-alcohol fatty liver disease; NO, nitric oxide; ox-LDL; oxidated low density lipoprotein; PON-3, paraoxonase 3; Q, quartile; ROS, reactive oxygen species; sd, standard deviation; sem, standard error of mean; sNOX2-dp, soluble Nox2-derived peptide; SOD, superoxide dismutase; TAC, total antioxidant capacity; TBARS, thiobarbituric acid reactive substances; tGSH, total glutathione; TNF-α, tumor necrosis factor alpha; TRAIL-R2, tumor necrosis factor-related apoptosis inducing ligand receptor 2; UPAR, urokinase plasminogen activator surface receptor.
P<0.05.
Table 2.
Summary of intervention studies investigating the associations between dietary patterns and biomarkers of oxidative stress and inflammation.
| Authors, Year | Country | Study design, duration | Participants | Dietary pattern | Biomarkers | Resultsa Increase in biomarker concentration (↑); Decrease in biomarker concentration (↓); No change (↔) |
|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||
| Yubero-Serrano et al. [50], 2020 | Spain | Parallel RCT, 1 year |
Intervention: N = 418, mean (sd) age: 60.4 (0.5) years, 91% male, coronary heart disease patients Control: N = 387, mean (sd) age: 59.9 (0.5) years, 94% male, coronary heart disease patients |
Intervention: Mediterranean diet Control: Low-fat, high-complex carbohydrate diet |
Serum: Methylglyoxal; hs-CRP |
↓ Methylglyoxal; ↓ hs-CRP |
| Sofi et al. [51], 2018 | Italy | Crossover RCT, 3 months each |
Intervention (2 arms): 1) N = 60, age range: 24–70 years, 18% male, healthy adults 2) N = 58, age range: 21–75 years, 26% male, healthy adults |
Intervention (2-arms): 1) Lacto-ovo vegetarian low-calorie diet 2) Mediterranean low-calorie diet |
Plasma: TBARS; TAC; L-derived ROS; M-derived ROS; G-derived ROS |
1) Vegetarian diet: ↓ TBARS; ↓ L-derived ROS; ↔ M-derived ROS; ↔ G-derived ROS; ↔ TAC 2) Mediterranean diet: ↓ TBARS; ↔ L-derived ROS; ↔ M-derived ROS; ↔ G-derived ROS; ↔ TAC |
| Jaacks et al. [52], 2018 | United States | Parallel RCT, 8 weeks |
Intervention: N = 11, overweight or obese adults Control: N = 9, overweight or obese adults Mean (sd) age: 51.4 (6.6) years, 27% male |
Intervention: Mediterranean diet Control: Habitual US diet |
Plasma: Cysteine (reduced form); cystine (oxidized form); GSH |
↓ Cystine; ↔ cysteine; ↔ GSH |
| Kim et al. [53], 2017 | Australia | Crossover RCT, 4 weeks each |
All participants: N = 51, mean (sd) age: 35.1 (15.6) years, 42% male, people without diabetes |
Intervention (2 arms): 1) Diet high in red and processed meat and refined grains 2) Diet high in whole grains, nuts, legumes, dairy, and devoid of red and processed meat |
Plasma: Fluorescent AGEs; CML; IL-6; hs-CRP |
Post-intervention levels of diet 1 vs diet 2: ↔ Fluorescent AGEs; ↔ CML; ↔ IL-6; ↔ hs-CRP |
| Davis et al. [54], 2017 | Australia | Parallel RCT, 24 weeks |
Intervention: N = 80, mean (sd) age: 71.0 (4.9) years, 42% male, healthy adults Control: N = 72, mean (sd) age: 70.8 (4.7) years, 46% male, healthy adults |
Intervention: Mediterranean diet Control: Habitual Australian diet |
Plasma: F2-Isoprostanes; hs-CRP |
↓ F2-Isoprostanes; ↔ hs-CRP |
| Zade et al. [55], 2016 | Iran | Parallel RCT, 8 weeks |
Intervention: N = 30, mean (sd) age: 39.7 (7.3) years, 50% male Control: N = 30, mean (sd) age: 42.8 (10.6) years, 50% male Overweight and obese patients with NAFLD |
Intervention: DASH diet Control: Calorie restricted diet |
Plasma: MDA; NO; GSH; TAC Serum: hs-CRP |
↑ NO; ↑ GSH; ↓ MDA; ↔ TAC; ↓ hs-CRP |
| Sureda et al. [56], 2016 | Spain | Parallel RCT, 5 years |
Intervention (2 arms): 1) N = 25, 46% male 2) N = 25, 45% male Control: N = 25, 48% male Age range: 55–80 years, people with metabolic syndrome |
Intervention (2 arms): 1) Mediterranean diet + olive oil 2) Mediterranean diet + nuts Control: Low-fat diet |
Total blood: SOD activity Plasma: Catalase activity; MPO activity; MPO protein level; xanthine oxidase activity; xanthine oxidase protein level; SOD protein level; nitrate; nitrite; nitrotyrosine index; carbonylated proteins |
1) Mediterranean diet + olive oil vs control: ↑ SOD activity; ↑ SOD protein level; ↑ catalase activity; ↑ nitrate; ↓ xanthine oxidase activity; ↔ xanthine oxidase protein level; ↔ MPO activity; ↔ MPO protein level; ↔ nitrite; ↔ nitrotyrosine index; ↔ carbonylated proteins 2) Mediterranean diet + nuts vs control: ↑ SOD activity; ↑ SOD protein level; ↑ catalase activity; ↑ nitrate; ↓ xanthine oxidase activity; ↔ xanthine oxidase protein level; ↔ MPO activity; ↔ MPO protein level; ↔ nitrite; ↔ nitrotyrosine index; ↔ carbonylated proteins |
| Choi et al. [57], 2015 | Korea | Parallel RCT, 8 weeks |
Intervention: N = 21, Mean age: 73.0 (3.9) years Control: N = 18, Mean age: 73.8 (5.8) years 0% male, women with abdominal obesity |
Intervention: DASH diet Control: Dietary counselling |
Plasma: TBARS; FRAP |
↓ TBARS; ↔ FRAP |
| Intervention trials (no comparison group) | ||||||
| Kaliora et al. [45], 2019 | Greece | 24 weeks | N = 44, mean (sd) age: 50.4 (10.2) years, 40.9% male, patients with nonfibrotic NAFLD | Intervention: Mediterranean diet | Serum: Ox-LDL; CRP; TNF-α; IL-6; visfatin; leptin |
↓ Ox-LDL; ↓ CRP; ↓ visfatin; ↔ TNF-α; ↔ IL-6; ↔ leptin |
| Luisi et al. [46], 2019 | Italy | 3 months | Two groups: 1) Overweight or obese: N = 18, age range: 20–61 years, 61% male 2) Normal weight: N = 18, age range: 24–71 years, 33% male |
Intervention: Mediterranean diet |
MPO activity; MDA; 8-OH-dG; TNF-α; IL-6; IL-10; adiponectin | 1) Overweight or obese: ↓ MPO activity; ↓ MDA; ↓ 8-OH-dG; ↑ IL-10; ↑ adiponectin; ↓ TNF-α; ↓ IL-6 2) Normal weight: ↓ MPO activity; ↓ MDA; ↓ 8-OH-dG; ↑ adiponectin; ↓ TNF-α; ↓ IL-6; ↔ IL-10 |
| Rodríguez et al. [47], 2015 | Chile | 3 months | N = 47, age range: 25–45 years, 0% male, overweight and obese premenopausal women | Intervention: Mediterranean diet (excluding wine) | Serum: CML |
↓ CML |
| Bloomer at al. [48], 2015 | USA | 21 days | Intervention (3 arms): 1) N = 12, mean (sd) age: 31.1 (4.7) years 2) N = 12, mean (sd) age: 27.9 (3.8) years 3) N = 11, mean (sd) age: 31.5 (4.5) years 16% male, healthy adults |
Intervention (3 arms): 1) Traditional Daniel Fast dietb 2) Modified Daniel Fast dietc 3) Unrestricted vegan diet |
Plasma: MDA; nitrate/nitrite; AOPP; hs-CRP |
1) Traditional Daniel Fast diet: ↔ MDA; ↔ nitrate/nitrite; ↔ AOPP; ↔ hs-CRP 2) Modified Daniel Fast diet: ↔ MDA; ↔ nitrate/nitrite; ↔ AOPP; ↔ hs-CRP 3) Unrestricted vegan diet: ↔ MDA; ↔ nitrate/nitrite; ↔ AOPP; ↔ hs-CRP |
| Parcina et al. [49], 2015 | Germany | 2 weeks | Intervention (3 arms): 1) N = 14, mean (sd) age: 31.9 (6.3) years 2) N = 13, mean (sd) age: 29.1 (5.8) years 3) N = 12, mean (sd) age: 27.4 (5.7) years 100% male, healthy adults |
Intervention (3 arms): 1) Mediterranean diet 2) Habitual German diet 3) Fast food diet |
Serum: 8-OH-dG; MDA; methylglyoxal; homocysteine |
1) Mediterranean diet: ↔ 8-OH-dG; ↔ MDA; ↔ methylglyoxal; ↔ homocysteine 2) Habitual German diet: ↔ 8-OH-dG; ↔ MDA; ↔ methylglyoxal; ↔ homocysteine 3) Fast food diet: ↔ 8-OH-dG; ↔ MDA; ↔ methylglyoxal; ↔ homocysteine |
Abbreviations: AOPP, advanced oxidation protein products; CML, N(6)-carboxymethyllysine; CPT1, carnitine palmitoyl transferase 1; CRP, C-reactive protein; DASH, Dietary Approaches to Stop Hypertension; ELISA, enzyme-linked immunosorbent assay; FRAP, ferric reducing ability of plasma; GSH, glutathione; hs, high-sensitivity; IFN-y, interferon gamma; IL, interleukin; IP-10, interferon gamma induced protein-10; ox-LDL, oxidized low density lipoprotein; MCP-1, monocyte chemoattractant protein-1; MDA, malonyldialdehyde; MIP-1β, macrophage inflammatory protein-1 beta; MPO, myeloperoxidase; NO, nitric oxide; RCT, randomized control trial; ROS, reactive oxygen species; SD, standard deviation; SOD, Superoxide dismutase; TAC, total antioxidant capacity; TBARS, thiobarbituric acid reactive substances; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor; 8-OH-dG, 8-hydroxy-2-deoxyguanosine.
P<0.05.
Traditional Daniel Fats Diet eliminate all processed foods, white flour products, additives, preservatives, sweeteners, flavorings, caffeine, alcohol.
Animal products, including lean meat and milk.
3.3. Dietary patterns
In observational studies, dietary assessment was performed predominantly using validated food frequency questionnaires (FFQs) (n = 11 [[31], [32], [33],[35], [36], [37], [38],[40], [41], [42], [43]]), whereas the remaining studies used dietary records (n = 4 [29,30,39,44]) and repeated 24-h dietary recalls (n = 1 [34]). Majority of observational reports were based on a priori approaches (diet quality scores or indexes) to define dietary patterns (n = 9 [30,31,34,[38], [39], [40], [41], [42],44]). These included the Mediterranean diet score [30,31,[38], [39], [40], [41], [42],44], USDA Healthy Eating Index (HEI) [34,41] and various other dietary patterns such as paleolithic diet [40] and DASH diet [41]. Overall, 5 studies used a posteriori approaches, which included factor/principal component analysis (PCA) to identify data-driven dietary patterns [32,33,35,36,43]. Two studies included participants who were vegetarian, vegan or omnivorous by choice so they did not include a score [29,37]. The dietary components of the identified dietary patterns are summarized in Supplementary Table 5. In intervention studies, Mediterranean diet was again the most commonly used main intervention approach (9 studies [[45], [46], [47],[49], [50], [51], [52],54,56], among which 5 RCTs [[50], [51], [52],54,56]). Further dietary interventions included the DASH diet [55,57], the lacto-ovo vegetarian low-calorie diet [51] and the traditional Daniel Fast diet [48]. 2 studies explored variations of Mediterranean diet whereby specific food components were additionally included [56] or excluded [47]. While most studies focused on evaluation of dietary patterns generally considered healthy, one study explored effect of a western diet (high in red and processed meat and refined grains) so far described to exert unfavorable health effects [53]. In the majority of the RCTs, dietary recommendations were provided for both the intervention and control groups. Most commonly, control groups followed a habitual diet [52,54], habitual diet with dietary counselling [57], or a low-fat/low-calorie diet [50,55,56]. The most common methods for evaluating dietary intervention compliance were dietary questionnaires and diet records.
3.4. Biomarkers evaluated
A summary of evaluated biomarkers for oxidative stress and inflammation used in the selected studies according to their main characteristics and pathophysiological actions is provided in Table 3. A short description of methods for measurement and sample type used in the observational and intervention studies has been summarized in the Supplementary Tables 6 and 7, respectively. Overall, various biomarkers were used as study endpoints to reflect different pathways involved in oxidative stress including both pro-oxidative and antioxidant defense mechanisms [[29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]]. In addition to oxidative stress biomarkers, 14 studies addressed biomarkers of specific immune-inflammatory activation, with C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) being most commonly assessed [29,[33], [34], [35],40,42,45,46,48,50,[53], [54], [55]].
Table 3.
Classification of commonly assessed biomarkers of oxidative stress and inflammation in the studies included in the systematic review.
|
Biological process |
Biomarker | Short description (mechanistic pathway) | Interpretation | Referencesa |
|---|---|---|---|---|
| Reactive oxygen and nitrogen species (RONS) | ||||
| RONS (e.g., ROMs, NO) | Products and byproducts of biological processes that mediate signal transduction and induce oxidative damage. | ↑Oxidative stress | [58,59] | |
| Biomarkers of oxidative damage | ||||
| Lipid peroxidation | Isoprostanes (F2-Isoprostanes) | Formed by free radical-catalyzed oxidation of arachidonic acid. | ↑Oxidative stress | [60] |
| Ox-LDL | Originated from oxidative modification hypothesis of atherosclerosis. | ↑Oxidative stress | [60] | |
| Malondialdehyde (MDA) | Reactive aldehyde derived from lipid peroxidation of various polyunsaturated fatty acids that can from DNA- and protein adducts. | ↑Oxidative stress | [61,62] | |
| TBARS | TBA-reactive substances (reactive carbonyl groups–containing compounds) measured as proxy of MDA levels.b | ↑Oxidative stress | [61,62] | |
| Oxidative damage to proteins/amino acids | Protein carbonyls | Result of oxidative cleavage of protein backbones. | ↑Oxidative stress | [60] |
| Nitrotyrosine | Nitration of tyrosine (free amino acid or within a peptide) induced by RNS. | ↑Oxidative stress | [60] | |
| Oxidative DNA damage | 8-OH-dG | Oxidative stress induced base modification. If not repaired, 8-OH-dG can lead to GC-TA transversion (mutation). | ↑Oxidative stress | [60] |
| Reactive metabolic products and byproducts | ||||
| Metabolic byproducts | Methylglyoxal | Highly reactive dicarbonyl compound that is a by-product of glycolysis and major cell-permeant precursor of AGEs. | ↑Oxidative stress | [63] |
| Glycoxidation - Protein or lipid become glycation | AGEs (e.g., CML) | Heterogeneous group of molecules formed in a nonenzymatic reaction between reducing sugars and amino groups (lipids, DNA, proteins) during normal metabolism. | ↑Oxidative stress | [60] |
| Antioxidant defense mechanisms (detoxification of ROS) | ||||
| Superoxide dismutases | SOD1, 2 and 3 | A family of metalloenzymes that catalyze the dismutation of two molecules of superoxide anion to hydrogen peroxide and molecular oxygen, functioning as powerful antioxidant in the cells. | ↓Oxidative stress | [64] |
| Catalase system | Catalase | Catalyzes the detoxification of hydrogen peroxide. | ↓Oxidative stress | [64] |
| Glutathione system | GSH | The reduced form of the most important low molecular weight antioxidant synthesized in cells involved in detoxification of reactive substances. | ↓Oxidative stress | [65] |
| Glutathione reductase | Detoxifies GSSG, a potentially toxic product of the oxidation of GSH. | ↓Oxidative stress | [65] | |
| Glutathione peroxidase | Catalyzes the detoxification of hydrogen peroxide to water, and lipid peroxides to their corresponding alcohols. | ↓Oxidative stress | [64] | |
| Thioredoxin system | Thioredoxin reductase | NADPH-dependent reducing enzyme in reactions involving thioredoxin. | ↓Oxidative stress | [66] |
| Paraoxonases | PON1, 2 and 3 | Reflect antioxidant activity and play a fundamental role in detoxification of many compounds (e.g., early oxidative products). | ↓Oxidative stress | [67] |
| Heme oxygenases | Heme oxygenase-1 | Antioxidant and anti-inflammatory enzyme that is usually expressed at low levels, but that can be highly upregulated in response to oxidative stress (Nrf2-regulated) | ↑Oxidative stress | [68] |
| Antioxidant capacity | TAC | General term that depicts total antioxidant capacity which can be assessed following various approaches. | ↓Oxidative stress | [69] |
| FRAP | Result of a reaction of sample antioxidants with inorganic oxidants (e.g., Fe3+ or Cu2+), representing rather the reducing capacity. | ↓Oxidative stress | [69] | |
| Immune-inflammatory activation as sources of oxidative stress | ||||
| Immune-system machinery for generating ROS | MPO | Enzyme stored in the granules of neutrophiles that catalyzes the formation of hypochlorous acid from hydrogen peroxide. | ↑Oxidative stress | [70,71] |
| AOPP | Formed mainly by chlorinated oxidants as a result from activity of myeloperoxidase. | ↑Oxidative stress | [72] | |
| sNOX2-dp | Product of NOX2 activation, an enzyme that plays a role in ROS generation by phagocytic leukocytes. | ↑Oxidative stress | [73,74] | |
| Lipoprotein-associated phospholipase A2 | Enzyme secreted from immune cells regulated by cytokines and steroid hormones that can release isoprostanes from esterified phospholipids. | ↑Oxidative stress | [75] | |
| Biomarkers of immune-inflammatory pathways | ||||
| Acute phase reactant | hsCRP | Produced mainly in the liver in response to increase of proinflammatory cytokines that represents a sensitive and nonspecific marker of systemic low-grade inflammation. | ↑Inflammation | [76] |
| Cytokines | IL-6 | Produced in response to infections, tissue injuries, hematopoiesis, and other immune reactions. Its dysregulation plays a role in on chronic inflammatory states and autoimmunity. | ↑Immune activation | [77] |
| TNF-α | Induces inflammation, activation of vascular endothelium, recruitment of immune cells, and tissue destruction and plays a role in chronic inflammation. | ↑Immune activation | [78] | |
| IL-10 | Anti-inflammatory function, with a central role in preventing inflammatory and autoimmune diseases. | ↓Immune activation | [79] | |
| Adipokines | Adiponectin | Plays a role in various aspects of metabolism and exerts anti-inflammatory activity (e.g., inhibiting phagocytic activity and IL-6 and TNF production). | ↓Inflammation | [80] |
| Leptin | Plays a role in various aspects of metabolism and exerts pro-inflammatory and immune activation properties. | ↑Inflammation | [80] | |
Abbreviations: AOPP, advanced oxidation protein products; CML, N(6)-carboxymethyllysine; FRAP, ferric reducing ability of plasma; GSSG, glutathione (oxidized); GSH, glutathione (reduced); hsCRP, high-sensitivity C-reactive protein; IL, interleukin; MDA, malondialdehyde; MPO, myeloperoxidase; NADPH, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide; NOX2, NADPH oxidase 2; ox-LDL, oxidized low-density lipoprotein; PON, paraoxonase; ROMs, reactive oxygen metabolites; RONS, reactive oxygen and nitrogen species; sNOX2-dp, soluble NOX2–derived peptide; SOD, superoxide dismutase; TAC, total antioxidant capacity; TBARS, thiobarbituric acid reactive substances; TNF-α, tumor necrosis factor- α; 8-OH-dG; 8-oxo-2′-deoxyguanosine.
Selected publications describing mechanistic pathways of biomarkers.
TBARS Assay is characterized by low sensitivity and specificity.
3.5. Associations between dietary patterns and biomarkers of interest
An overview of studies reporting results on significant differences between biomarker levels in participants according to identified dietary patterns in observational and intervention studies is presented in Table 4. Overall, reduced concentrations of oxidative stress and proinflammatory biomarkers and increased concentrations of antioxidant and anti-inflammatory biomarkers were reported for the Mediterranean diet, the vegetarian diet, the DASH diet, the USDA HEI diet and the paleolithic diet. Vice versa, studies that explored Western and fast-food diets (based on data from observational studies) reported higher levels of oxidative stress and inflammation biomarkers. Of note, only for the Mediterranean and DASH diet significant results could be seen in both observational and intervention studies. The associations described for the other dietary patterns were based just on data from observational studies [[29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]].
Table 4.
Overview of results on significant differences between biomarker levels in participants according to dietary patterns reported in observational and intervention studies.
| Dietary patterns | Observational studies |
Intervention studies |
||
|---|---|---|---|---|
| Oxidative stress biomarkers | Inflammatory biomarkers | Oxidative stress biomarkers | Inflammatory biomarkers | |
| Mediterranean diet | N = 6/NS = 3 [31,32,38] ↓ F2-isoprostane [39,40] ↓ sNox2-dp [30,44] ↓ Ox-LDL [42] ↑ FlOP_320; ↑ FlOP_360 [41] ↑ TAC [42] ↑ Ox-LDL [39] |
N = 2/NS = 0 ↓ hs-CRP [40] ↓ CRP [42] ↓ IL-6; ↓ TNF-α; ↓ serum amyloid A; ↓ homocysteine [42] |
N = 8/NS = 1 [49] ↓ F2-Isoprostanes [54] ↓ Ox-LDL [45] ↓ MDA (measured via TBARS) [46] ↓ TBARS [51] ↓ 8-OH-dG [46] ↓ Methylglyoxal [50] ↓ CML [47] ↓ MPO activityen ↓ Cystine [52] ↑ nitrate; ↓ xanthine oxidase activity; ↑ SOD activity; ↑ SOD protein level; ↑ catalase activity [56] |
N = 3/NS = 2 [49,54] ↓ hs-CRP [50], ↓ CRP [45] ↓ visfatin [45] ↓ TNF-α; ↓ IL-6 [46] ↑ IL-10; ↑ adiponectin [46] |
| Vegetarian Diet | N = 4/NS = 2 [33] ↓ MDA [36,43] ↓ MPO protein level; ↓ spondin-2; ↓ follistatin; ↓ MMP-7; ↓ TRAIL-R2 [35] ↑ PON-3 [35]; ↑ TAC [36] ↑ Total phenol; ↑ FRAP, ↑ nitrite [37] ↑ SOD protein level; ↑ glutathione peroxidase protein level [43] |
N = 0/NS = 1 [35] | N = 1/NS = 0 ↓ L-derived ROS [51] ↓ TBARS [51] |
– |
| Vegan Diet | N = 1/NS = 0 ↑ TBARS; ↑ nitrite [37] |
– | N = 0/NS = 1 [48] | N = 0/NS = 1 [48] |
| DASH Diet | N = 1/NS = 0 ↑ FlOP_320; ↑ FlOP_360 [41] |
– | N = 2/NS = 0 ↓ MDA [55] ↓TBARS [57] ↑ NO; ↑ GSH [55] |
N = 1/NS = 0 ↓ hs-CRP [55] |
| USDA HEI diet | N = 1/NS = 1 [34] ↑ FlOP_320; ↑ FlOP_360 [41] |
N = 0/NS = 1 [34] | – | – |
| Paleolithic diet | N = 1/NS = 0 ↓ F2-isoprostane [40] |
N = 1/NS = 0 ↓ hs-CRP [40] |
– | – |
| Western diet/Fast food diet | N = 6/NS = 1 [43] ↑ heme oxygenase-1 [29] ↑ Lipoprotein-associated phospholipase A2 [32] ↑ PON-3 [35] ↑ Total phenol [37] ↓ protein carbonyl [33] ↓ MMP-7; ↓ MPO protein level [35] ↓ TAC; ↑ MDA [36] ↓ Ceruloplasmin [33] |
N = 1/NS = 1 [29] ↑ resistin ↑ TRAIL-R2; ↑ UPAR; ↑ IL2RA [35] ↑ IL1RT1 ↑ follistatin; ↑ CEACAM8 (Western diet); ↓ IL1RT1 (Fast food diet) [35] ↓ Gal-4 [35] |
N = 0/NS = 2 [49,53] | N = 0/NS = 1 [49] |
NS = number of studies that found non-significant findings of measured biomarkers in corresponding category (P > 0.05).
Abbreviations: CEACAM8, carcinoembryonic antigenrelated cell adhesion molecule 8; CML, N(6)-carboxymethyllysine; CRP, C-reactive protein; DASH diet, Dietary Approaches to Stop Hypertension diet; FlOP, fluorescent oxidation product; FRAP, ferric reducing ability of plasma; Gal-4, Galectin-4; GSH, glutathione; HEI, Healthy Eating Index; hs, high-sensitivity; IL, interleukin; ILTR1, interleukin-1 receptor type 1; IL2RA, interleukin-2 receptor subunit alpha; ox-LDL, oxidized low density lipoprotein; MDA, malonyldialdehyde; MMP-7, matrix metalloproteinase-7; MPO, myeloperoxidase; N, number; NO, nitric oxide; PON-3, paraoxonase 3; ROS, reactive oxygen species; sNOX2-dp, soluble Nox2-derived peptide; SOD, Superoxide dismutase; TAC, total antioxidant capacity; TBARS, Thiobarbituric acid reactive substances; tGSH, total glutathione; TNF-α, tumor necrosis factor alpha; TRAIL-R2, tumor necrosis factor-related apoptosis inducing ligand receptor 2; UPAR, urokinase plasminogen activator surface receptor; 8-OH-dG, 8-hydroxy-2-deoxyguanosine.
In observational studies, adherence to the Mediterranean dietary pattern compared to reference diets (i.e., habitual or Western diet) was associated with significant differences in levels of oxidative stress biomarkers in 6 out of 9 studies with no differences reported in 3 out of 9 studies [[30], [31], [32],[38], [39], [40], [41], [42],44]. Levels were lower for the soluble Nox2-derived peptide (sNox2-dp) [30,44] and F2-Isoprostane [40] and higher for fluorescent oxidation product_320 (FlOP_320), FlOP_360 [41] and total anti-oxidant capacity (TAC) [42]. Contradicting results for oxidized low-density lipoprotein (ox-LDL) were reported in two observational studies, with one study showing elevated levels following Mediterranean diet enriched with higher intakes of fish and shellfish [39], whereas the second study reported lower ox-LDL levels related to consumption of traditional Mediterranean diet [42].
In intervention studies, significantly different biomarker levels in Mediterranean diet groups compared to control groups/baseline levels were reported in 7 out of 8 studies [[45], [46], [47],[49], [50], [51], [52],54]. Following Mediterranean diet intervention led to significantly reduced levels of a wide range of biomarkers reflecting different aspects of oxidative stress, including biomarkers of lipid peroxidation (F2-isoprostanes, ox-LDL, malondialdehyde (MDA), thiobarbituric acid reactive substances (TBARS)), oxidative DNA damage (8-hydroxydeoxyguanosine (8-OH-dG)) [45,46,51,54], reactive metabolic products and byproducts (methylglyoxal and N (6)-carboxymethyllysine (CML)) [47,50], and biomarkers representing endogenous immune-inflammatory activation as sources of oxidative stress (myeloperoxidase (MPO) activity) [46,52]. Conversely, higher levels were observed for of biomarkers of antioxidant defense and ROS detoxification such as superoxide dismutase (SOD) activity and protein level, catalase activity, xanthine oxidase activity [56]. Moreover, in addition to oxidative stress biomarkers, adherence to Mediterranean diet was related to lower concentrations of pro-inflammatory biomarkers - CRP, IL-6, TNF-α, serum amyloid A and homocysteine in observational studies [40,42]. Consistently, in intervention studies, the results for CRP, IL-6 and TNF-α were further confirmed and additionally increased levels of anti-inflammatory biomarkers – IL-10 and adiponectin were demonstrated [45,46,50].
Vegetarian dietary patterns were evaluated in six observational studies and in one intervention study. Compared to reference diets consumption of vegetarian diet was associated with significant differences in levels of oxidative stress biomarkers in 4 out of 6 observational studies. Levels were lower for oxidative stress biomarkers reflecting lipid peroxidation and immune-inflammatory activation as sources of oxidative stress MDA and MPO [35,36,43], whereas levels for biomarkers of antioxidant defense and ROS detoxification (ferric reducing ability of plasma (FRAP), TAC, nitrite, SOD protein level, glutathione (GSH) peroxidase protein level) were higher [36,37,43]. In the intervention study, reduced levels of L-derived ROS and TBARS were additionally reported [51]. One observational study explored adherence to vegan diet in relation to oxidative stress biomarkers and showed that the levels of TBARS and nitrite were elevated compared to an omnivorous diet [37]. The DASH diet was evaluated in one observational and two intervention studies. Unexpectedly, in the observational study, DASH diet was positively, albeit weakly, associated with FlOP_320 and FlOP_360 [41], whereas lower levels of MDA, TBARS and CRP and higher levels of nitric oxide (NO) and GSH were seen in the intervention studies [55,57]. Adherence to the USDA HEI diet and the paleolithic diet were shown to be associated (also weakly) with higher levels of FLOP_320 and FLOP_360 [41] and lower levels of F2-isoprostane and CRP [40], respectively.
Finally, results from observational studies have reported that consumption of Western/fast-food diets was associated with elevated levels of a range of biomarkers reflecting different aspects of oxidative stress (e.g., MDA, heme oxygenase-1 levels, paraoxonase 3 (PON-3)) and inflammation (e.g., resistin, tumor necrosis factor-related apoptosis inducing ligand receptor 2 (TRAIL-R2), urokinase plasminogen activator surface receptor (UPAR), interleukin-2 receptor subunit alpha (IL2RA)) and with decreased levels of biomarkers reflecting antioxidant defense (e.g., TAC, MPO) [29,32,33,35,37]. The direction of association in Western and fast-food diets differed for MPO and interleukin-1 receptor type 1 (IL1RT1), showing to be elevated in a Western diet pattern while decreased in a fast-food pattern [35]. In the same study, galectin-4 (Gal-4) was inversely associated with both Western and fast-food patterns [35].
3.6. Risk of bias and study quality assessment
The study quality and risk of bias assessments for each observational and intervention study included in the systematic review are presented in Supplementary Tables 8 and 9, respectively. The total assessment points ranged from 4.5. to 12 for observational studies (median = 9.75) and from 4 to 11.5 for intervention studies (median = 7). Among the 16 evaluated observational studies, 8 studies scored as high quality, 6 as moderate quality and one as low quality. Among the 13 included intervention studies, one study scored as high quality, 8 as moderate quality and 4 as low quality.
4. Discussion
This systematic review provides a comprehensive summary and evaluation of the recent evidence from human studies on the association between dietary patterns and biomarkers of oxidative stress and inflammation. Overall results from both observational and intervention studies indicated an inverse association between plant-based diets - the Mediterranean and DASH diet - and oxidative stress and proinflammatory biomarkers. In addition, the vegetarian diet, the USDA HEI diet and the paleolithic diet were associated with lower levels of oxidative stress and inflammation, whereas Western and fast-food diets were positively associated with oxidative stress and inflammation biomarkers based on evidence from observational studies. To our knowledge this is the first systematic review to provide an updated summary and evaluation of various dietary patterns and biomarkers of oxidative stress and inflammation depicting novel trends in human research within the last few years.
4.1. Dietary patterns and oxidative stress biomarkers
The studies in the current systematic review explored a number of dietary patterns among which the most commonly assessed was the Mediterranean diet. This may not be surprising due to the increased popularity of the health beneficial properties of the Mediterranean diet and its components in the recent years. Greater adherence to the Mediterranean diet was consistently associated with a lower risk of cardiovascular disease, diabetes, cancer and neurodegenerative diseases [81,82], as well as with reduced overall mortality [83]. To explain these favorable associations, the potential ability of the Mediterranean diet to decrease oxidative stress due to its high antioxidant capacity emerged as one of the leading candidate hypotheses [[84], [85], [86], [87]].
The Mediterranean diet is characterized by high intakes of fruit, vegetables, cereals, legumes, nuts, and seeds; a low-to-moderate intake of dairy products, fish, poultry and wine; and low intakes of red meat and eggs; with olive oil used as a main source of fat [88]. The synergistic effects of the various plant-based foods with antioxidant potential may explain why the overall quality of the diet could be more valuable compared to single food components. In this vein, the observational studies that explored associations between Mediterranean diet and oxidative stress biomarkers provided promising results reporting inverse associations for circulating levels of ox-LDL [89] and MDA [90] and positive associations with biomarkers that reflect antioxidant defense mechanisms such as SOD and glutathione peroxidase activity [90], and plasma ratio of reduced to oxidized glutathione (GSH/GSSG ratio) [91]. However, intervention trials yielded inconsistent results with some studies showing that Mediterranean diet was associated with decreased blood levels of MDA, TBARS [92], ox-LDL [93] and urine levels of F2-isoprostane and 8-oxo-dG [94], others have reported no change in oxidative stress biomarkers such as blood MDA [95] and TBARS [96] levels, and urinary F2-isoprostanes [97]. A previous systematic review that summarized results from four intervention studies published up to 2012 further concluded that the evidence was inconsistent [25]. Potential explanations of these inconsistencies could include the differences in study designs, the lack of standard definition of Mediterranean diet and the variety of biomarkers and analytical techniques used for their measurement. Compared to previous work, the current systematic review included a larger number of studies of both observational (n = 6) and intervention design (n = 8). Consistently, negative associations between Mediterranean diet and blood biomarkers of lipid peroxidation were shown in both observational and intervention studies for F2-isoprostanes [39,40,54] and in intervention studies for MDA (measured via TBARS or as a proxy from TBARS) [46,51]. Both, F2-isoprostanes and MDA are considered to be among the most reliable available markers to assess oxidative stress [98]. Both higher levels of F2-isoprostanes, a lipid peroxidation product of arachidonic acid, and MDA, a reactive aldehyde derived from lipid peroxidation of various polyunsaturated fatty acids that can form DNA adducts [[60], [61], [62], [99]], have shown to be associated with several chronic diseases, e.g., cardiovascular diseases, type 2 diabetes, neurodegenerative diseases and cancer [60,100].
The oxidation of lipids, particularly of LDL cholesterol, has been long suggested to predispose the atherosclerotic lesion formation [101,102]. Thus, ox-LDL has been associated with several cardio-metabolic diseases, including cardiovascular diseases and type 2 diabetes [103], however, it is important to note that ox-LDL is a non-specific measure of oxidative stress [60]. Overall, the extent of lipid peroxidation largely depends on the generation of oxygen free radicals, the presence of lipid substrates, and the activity of antioxidants [99]. Thus, increased consumption of dietary antioxidants through diet high in fruits and vegetables and low in saturated fat, total fat, and cholesterol such as the Mediterranean diet may protect against oxidative stress-mediated lipid peroxidation via decreasing lipid substrate available for peroxidation and increasing the concentration of antioxidants [104]. In fact, the current review has shown that Mediterranean diet has been associated with increased TAC in an observational study [42], while showing to increase SOD and catalase activity (enzymes involved in the detoxification of ROS) and decrease the activity of xanthine oxidase [56] and MPO [46] in intervention studies. The antioxidant properties of the Mediterranean diet itself might also be involved in mechanisms of ROS detoxification [56]. Furthermore, the current review suggested that Mediterranean diet leads to a decrease in 8-OH-dG levels, one of the best known and widely used biomarker of oxidative DNA damage [46]. 8-OH-dG is a oxidative DNA base lesion that is increased in oxidative stress conditions and that, if not repaired, can lead to GC → TA transversion – a point mutation [105]. The interaction of ROS with DNA along with of membrane lipid peroxidation products such as MDA lead to formation of numerous DNA adducts [99]. It is well known that DNA stability is essential for the maintenance of normal cell functions and the damaged DNA promotes a spectrum of acute and chronic disorders [106,107]. These results are in line with a recent review of human intervention studies (n = 8) on the effect of Mediterranean diet on markers of DNA damage and DNA repair that suggested that intervention with Mediterranean diet alone or in combination with bioactive-rich foods is protective against DNA damage [108]. One plausible mechanism explaining this effect may involve the balance between polyunsaturated fatty acids (PUFA) and saturated fatty acids (SFA) [108]. For example, inverse associations have been reported between monounsaturated fatty acids (MUFAs) and DNA damage, whereas positive associations were seen between intake of SFAs and DNA damage [109].
Our review also suggested that the DASH diet is associated with lower levels of oxidative stress biomarkers. The DASH diet is characterized by consumption of high amount of fruit and vegetables, low sodium intake and low-fat milk and dairy products [20]. This type of diet not only effectively reduced blood pressure in intervention studies [110], but was also shown to lead to a lower risk of chronic disease incidence and mortality [111,112]. Previous research has shown inconclusive results for the association between this type of diet and oxidative stress biomarkers [25]. Despite we could only identify a few studies on the DASH diet, the results from intervention studies support its potential to reduce oxidative stress and inflammation levels. In particular, the DASH diet was shown to lower biomarkers of lipid peroxidation, i.e., MDA and TBARS [55,57], and to increase antioxidant status biomarkers, i.e., GSH levels. Similar to the Mediterranean diet, the DASH diet contains plant-based foods that are rich in antioxidants which regular intakes could favor a better balance between cellular oxidant and antioxidant systems and support the regulation of the oxidizing (redox) mechanisms in health and disease states [113]. Furthermore, results suggested that dietary intervention based on the DASH diet led to increased levels of nitric oxide, known as a potent vasodilator [55]. This may be reasonable since this type of diet is composed by many vegetables with high nitrate content that can especially promote the formation of nitric oxide and protect against the initiation of salt-induced hypertension and associated cardio-vascular complications [113]. This finding may be of special interest in designing prevention strategies in high-risk populations characterized by high salt intakes [114]. Our review included studies that evaluated different types of commonly assessed plant-based diets such as different vegetarian diets, USDA HEI diet and the Paleolithic diet [34,37,41]. However, these were mainly explored in observational research setting and no intervention studies were available to evaluate effects of these diets in modulating oxidative stress biomarkers.
In addition to favorable dietary patterns, our review identified a number of observational studies that explored unhealthy dietary patterns classified as ‘Western diet’ and ‘fast-food diet’ in relation to oxidative stress biomarkers [29,32,33,[35], [36], [37]]. Empirically derived dietary patterns characterized by low consumption of fruits, vegetables, legumes and fiber-rich foods, but with high amounts of refined grains, sugar-sweetened beverages, red and processed meat, were associated with higher levels of biomarkers of lipid peroxidation, i.e., MDA [36] and lipoprotein-associated phospholipase A2 [32] and lower levels of biomarkers of antioxidant defense, i.e., TAC [36] and MPO protein levels [35]. Western diets have been associated with obesity and metabolic dysfunction [115]. Adipose tissue is an active endocrine organ releasing a variety of biologically active molecules known as adipokines. The prolonged adipokine secretion in obesity leads to chronic low-grade inflammation and oxidative stress, thereby potentially predisposing chronic disease development [116]. However, due to the cross-sectional study designs employed so far, causal inference on the link between Western diet and oxidative stress mediators is limited. To better understand the potentially detrimental role of Western diets in modulating various aspects of oxidative stress and inflammation, further studies with repeated assessment of dietary patterns and measurements of changes in biomarker concentrations over time are highly warranted.
4.2. Dietary patterns and inflammatory biomarkers
Having the close interdependence between oxidative stress and inflammation, the present systematic review also included studies that simultaneously assessed dietary patterns in relation to inflammatory biomarkers. It is known that inflammatory cells can produce large amounts of ROS as part of an immunological defense mechanism to protect human organisms against invading pathogens [117]. In line with previously discussed improvement in oxidative stress markers, results from observational studies [[29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]] and intervention studies [[45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]] collectively suggested a corresponding reduction in levels of common biomarkers of inflammation and immune response – CRP, IL-6 and TNF-α as well as favorable modulation of specific adipokines – visfatin and adiponectin following Mediterranean and DASH diets [40,42,45,46,50,55]. These results extend on our previous work on a systematic review and meta-analysis of intervention trials that evaluated different types of plant-based diets which revealed that the Mediterranean diet and the DASH led to the largest reduction in inflammatory biomarkers among different evaluated diet types [118]. These associations could be partially accounted for by the array of phytochemicals and other compounds present in plant‐based diets i.e. carotenoids and flavonoids that may directly or indirectly modulate inflammatory and immunological processes [119].
4.3. Strengths and limitations
Strengths of the present study include the application of state-of-the-art approaches of conducting systematic search process conducted by several investigators, the variety of dietary patterns and spectrum of biomarkers that allowed making a comprehensive overview of research evidence published on this topic. The review is also based on most recently published studies that allow depicting novel tendencies in research.
Limitations of the present study also warrant consideration. Due to the large heterogeneity of evaluated biomarkers used in studies as proxies of oxidative stress and inflammation and the various analytical techniques used for measuring biomarker concentrations, the quantification of effect size for individual biomarkers by means of meta-analysis was not feasible in this systematic review. The numerous biomarkers of oxidative stress can bear own advantages and limitations, including the lack of tissue and signaling pathway specificity in target tissues [120]. Therefore, some of the non-significant results may be explained by inability of measured biomarkers to reflect a true change rather than by the inability of diet to modulate oxidative stress levels. The various biomarkers represent different pathways and possibly complementing pathways, therefore assessing single biomarkers may not be representative of the level of oxidative stress, however so far none of the studies attempted to evaluate a combination of multiple oxidative stress biomarkers as endpoint in their analyses.
The observational studies had cross-sectional design which does not allow making inferences on the causal effects of dietary patterns in modulating oxidative stress and inflammation and may be influenced by potential bias and confounding. Despite of the prospective study design and randomization that address these limitations of observational studies, the quality of intervention studies ranged mostly from low to moderate and the majority of studies included low number of participants and were of short duration. Differences in the study populations and methods used to evaluate the adherence or compliance to dietary patterns may have also contributed to differences in reported associations with biomarkers.
4.4. Implications for future research
Approaches for reducing the generation of oxidative stress are increasingly deemed important in chronic disease risk prevention, especially in adult and older age, when most of the endogenous antioxidant defense systems fail to offer appropriate protection against elevated oxidative stress and the human organism is exposed to increased ROS formation in ageing cells. While intervention trials on synthetic antioxidants failed to support the beneficial effects of these compounds in preventing age-related diseases [15], the current results suggest that following a balanced plant-based diet, such as the Mediterranean diet, may represent an important alternative to targeted disease prevention. Thus, a healthy adult person may not need additional vitamin and mineral supplements if he follows a balanced food pattern that includes diversity of plant sources. However, to further strengthen this evidence a number of gaps in research are still to be filled.
Our review revealed that studies used to employ a variety of biomarkers and analytical techniques for their quantification with often poorly reported biomarker measurement information. Although numerous analytical methods for assessing oxidative stress have been developed in the recent years, so far there have not been studies to validate biomarker use in epidemiological research in a systematic manner [60]. This largely restricts validity of conclusions regarding observed effects particularly in a prospective study setting where biomarkers serve as endpoints and assessment of exposure-related long-term effects are of major interest. Reliability assessment studies, measurements of panels of biomarkers and improved reporting of biomarker measurements could prove useful in improving the quality of the studies and the understanding of how diets might affect different aspects of oxidative stress and inflammatory pathways.
Individual genetic variation is another factor to be taken into account in future research when studying the effect of diet on biomarkers of oxidative stress. Genetic variation in antioxidant enzymes may influence the susceptibility of oxidative stress and may be a potential modifier when studying the responses to dietary interventions rich in antioxidants [121]. As an example, nutrigenetic studies suggested that fruit and vegetable consumption from diet can modify the association between polymorphisms in genes coding antioxidant enzymes and breast cancer [122]. So far, few studies have utilized biomarkers of oxidative stress in understanding the link between diet, genetic variation and diseases associated with oxidative stress [121].
With regards to dietary pattern evaluation, most of the studies used a priori dietary indices and no study was identified to apply innovative approaches in nutritional epidemiological data modeling of developing a posteriori patterns, i.e., identifying patterns that explain largest variation in oxidative stress biomarkers. In observational studies, future investigation should therefore include methodological studies based on population cohorts to identify dietary patterns best suited to reduce oxidative stress levels. Further methodological modeling is also needed to determine whether specific dietary components or combinations of components could play more protective role than others research. In this context, novel statistical approaches for complex data modeling, incl. bioinformatics and machine learning techniques could be employed to establish novel food and biomarker combinations. Prospective cohort studies with available biosample collections could be designed to explore mediating effects of biomarkers of oxidative stress and inflammation on observed associations with incident disease outcomes. For example, based on data from a large prospective cohort study we previously reported that the association between obesity and colorectal cancer was partly mediated by biomarkers of oxidative stress and inflammation [123]. Additionally, studies with repeated measurements over longer periods of time in healthy individuals will be valuable to assess whether dietary patterns limit the onset of chronic diseases, when corrected for confounding factors such as environment and lifestyle.
In intervention research, future randomized trials should include larger and longer trials conducted in heterogeneous populations to assess adherence, efficacy, and effect of dietary patterns on a range of oxidative stress and inflammatory biomarkers assessed as individual and combined exposure. In addition, randomized trials are also warranted to assess the relative effectiveness of specific dietary patterns, i.e., Mediterranean diet compared with other healthy diets, i.e., the DASH or healthy diet. Finally, further work from well conducted randomized trials is needed to identify novel dietary patterns with high antioxidant potential (potentially generated by observational studies) able to counteract systemic as well as mitochondrial-derived oxidative stress, enhance the endogenous antioxidant defenses, and alleviate symptoms or prevent complications of oxidative-stress associated diseases.
5. Conclusion
In conclusion, the current systematic review of observational and intervention studies suggested that the plant-based diets, including the Mediterranean and DASH diet, bear potential in reducing concentrations of various biomarkers of oxidative stress and inflammation. These findings are consistent with observed beneficial effects of plant-based dietary patterns on age-related pathologies. Nevertheless, due to the modest quality of evidence, future well-designed dietary trials using validated biomarkers are needed to corroborate the evidence highlighting the beneficial effects of dietary patterns on oxidative stress and inflammation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Tom Heinze (Institute of Nutritional Sciences, University of Potsdam) for his assistance with literature search, data extraction and evaluation of study quality.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.101869.
Author contributions
Conceptualisation, KA (Krasimira Aleksandrova); writing—original draft preparation, KA (Krasimira Aleksandrova); writing—review and editing, KA, LK, CER (Krasimira Aleksandrova, Liselot Koelman, Caue Egea Rodrigues); supervision, KA (Krasimira Aleksandrova). All authors have read and agreed to the published version of the manuscript.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sohal R.S., Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273(5271):59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Höhn A. Happily (n)ever after: aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biology. 2017;11:482–501. doi: 10.1016/j.redox.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liguori I. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw P.X., Werstuck G., Chen Y. Oxidative stress and aging diseases. Oxid Med Cell Longev. 2014;2014 doi: 10.1155/2014/569146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gladyshev V.N. The free radical theory of aging is dead. Long live the damage theory! Antioxidants Redox Signal. 2014;20(4):727–731. doi: 10.1089/ars.2013.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hald A., Lotharius J. Oxidative stress and inflammation in Parkinson's disease: is there a causal link? Exp. Neurol. 2005;193(2):279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Mittal M. Reactive oxygen species in inflammation and tissue injury. Antioxidants Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojas-Gutierrez E. Alzheimer's disease and metabolic syndrome: a link from oxidative stress and inflammation to neurodegeneration. Synapse. 2017;71(10) doi: 10.1002/syn.21990. [DOI] [PubMed] [Google Scholar]
- 9.Signorelli S.S., Katsiki N. Oxidative stress and inflammation: their role in the pathogenesis of peripheral artery disease with or without type 2 diabetes mellitus. Curr. Vasc. Pharmacol. 2018;16(6):547–554. doi: 10.2174/1570161115666170731165121. [DOI] [PubMed] [Google Scholar]
- 10.Garcia N., Zazueta C., Aguilera-Aguirre L. Oxidative stress and inflammation in cardiovascular disease. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/5853238. 5853238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Almeida A. Unveiling the role of inflammation and oxidative stress on age-related cardiovascular diseases. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/1954398. 1954398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez-Satta M. Relevance of oxidative stress and inflammation in frailty based on human studies and mouse models. Aging (Albany NY) 2020;12(10):9982–9999. doi: 10.18632/aging.103295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masoudkabir F., Sarrafzadegan N. The interplay of endothelial dysfunction, cardiovascular disease, and cancer: what we should know beyond inflammation and oxidative stress. Eur J Prev Cardiol. 2020;27(19):2075–2076. doi: 10.1177/2047487319895415. [DOI] [PubMed] [Google Scholar]
- 14.Fortmann S.P. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013;159(12):824–834. doi: 10.7326/0003-4819-159-12-201312170-00729. [DOI] [PubMed] [Google Scholar]
- 15.Schwingshackl L. Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a systematic review and meta-analysis of primary prevention trials. Adv Nutr. 2017;8(1):27–39. doi: 10.3945/an.116.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cespedes E.M., Hu F.B. Dietary patterns: from nutritional epidemiologic analysis to national guidelines. Am. J. Clin. Nutr. 2015;101(5):899–900. doi: 10.3945/ajcn.115.110213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu F.B. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Tapsell L.C. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr. 2016;7(3):445–454. doi: 10.3945/an.115.011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy E.T. The healthy eating index: design and applications. J. Am. Diet Assoc. 1995;95(10):1103–1108. doi: 10.1016/S0002-8223(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 20.The DASH diet Dietary approaches to stop hypertension. Lippincott's Prim. Care Pract. 1998;2(5):536–538. [PubMed] [Google Scholar]
- 21.Park Y.M. Mediterranean diet, Dietary Approaches to Stop Hypertension (DASH) style diet, and metabolic health in U.S. adults. Clin. Nutr. 2017;36(5):1301–1309. doi: 10.1016/j.clnu.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Kant A.K. Dietary patterns: biomarkers and chronic disease risk. Appl. Physiol. Nutr. Metabol. 2010;35(2):199–206. doi: 10.1139/H10-005. [DOI] [PubMed] [Google Scholar]
- 23.Schulze M.B. Food based dietary patterns and chronic disease prevention. BMJ. 2018;361:k2396. doi: 10.1136/bmj.k2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis K.K., Go Y.M., Jones D.P. Redox systems biology of nutrition and oxidative stress. J. Nutr. 2019;149(4):553–565. doi: 10.1093/jn/nxy306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vetrani C. Nutrition and oxidative stress: a systematic review of human studies. Int. J. Food Sci. Nutr. 2013;64(3):312–326. doi: 10.3109/09637486.2012.738651. [DOI] [PubMed] [Google Scholar]
- 26.Moher D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 27.Schwingshackl L. Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Advances in nutrition (Bethesda, Md. 2016;7(6):994–1004. doi: 10.3945/an.116.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wirsching J. Development and reliability assessment of a new quality appraisal tool for cross-sectional studies using biomarker data (BIOCROSS) BMC Med. Res. Methodol. 2018;18(1) doi: 10.1186/s12874-018-0583-x. 122-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cinegaglia N. Association of omnivorous and vegetarian diets with antioxidant defense mechanisms in men. Journal of the American Heart Association. 2020;9(12) doi: 10.1161/JAHA.119.015576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baratta F. Poor adherence to mediterranean diet and serum lipopolysaccharide are associated with oxidative stress in patients with non-alcoholic fatty liver disease. Nutrients. 2020;12(6) doi: 10.3390/nu12061732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruggeri R.M. 2020. INFLUENCE OF DIETARY HABITS ON OXIDATIVE STRESS MARKERS IN HASHIMOTO'S THYROIDITIS. Thyroid. [DOI] [PubMed] [Google Scholar]
- 32.Seyedi S.H.S. The relationship between dietary patterns and lipoprotein-associated phospholipase A2 levels in adults with cardiovascular risk factors: tehran Lipid and Glucose Study. J. Res. Med. Sci. 2020;25:3. doi: 10.4103/jrms.JRMS_256_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abashzadeh K. Unhealthy dietary patterns are related to low ceruloplasmin in female nurses. BMJ Mil Health. 2020;166(5):307–311. doi: 10.1136/jramc-2019-001157. [DOI] [PubMed] [Google Scholar]
- 34.Crowe-White K.M. Dietary quality assessed by the HEI-2010 and biomarkers of cardiometabolic disease: an exploratory analysis. J. Am. Coll. Nutr. 2019;38(7):640–647. doi: 10.1080/07315724.2019.1580168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemming E.W. Dietary pattern specific protein biomarkers for cardiovascular disease: a cross‐sectional study in 2 independent cohorts. Journal of the American Heart Association. 2019;8(11) doi: 10.1161/JAHA.118.011860. p. e011860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirmiran P. Effect of dietary patterns on oxidative stress in Patiants with metabolic syndrome: tehran Lipid and Glucose Study. Caspian J Intern Med. 2018;9(4):376–385. doi: 10.22088/cjim.9.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanacore D. Effect of restriction vegan diet's on muscle mass, oxidative status, and myocytes differentiation: a pilot study. J. Cell. Physiol. 2018;233(12):9345–9353. doi: 10.1002/jcp.26427. [DOI] [PubMed] [Google Scholar]
- 38.Kakkoura M.G. Mediterranean diet–gene interactions: a targeted metabolomics study in Greek-Cypriot women. Mol. Nutr. Food Res. 2017;61(4) doi: 10.1002/mnfr.201600558. [DOI] [PubMed] [Google Scholar]
- 39.Aranda N. Consumption of seafood and its estimated heavy metals are associated with lipid profile and oxidative lipid damage on healthy adults from a Spanish Mediterranean area: a cross-sectional study. Environ. Res. 2017;156:644–651. doi: 10.1016/j.envres.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 40.Whalen K.A. Paleolithic and mediterranean diet pattern scores are inversely associated with biomarkers of inflammation and oxidative balance in adults. J. Nutr. 2016;146(6):1217–1226. doi: 10.3945/jn.115.224048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung S. Healthy dietary patterns and oxidative stress as measured by fluorescent oxidation products in nurses' health study. Nutrients. 2016;8(9) doi: 10.3390/nu8090587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koloverou E. Adherence to Mediterranean diet and 10-year incidence (2002–2012) of diabetes: correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes Metabol. Res. Rev. 2016;32(1):73–81. doi: 10.1002/dmrr.2672. [DOI] [PubMed] [Google Scholar]
- 43.Xie Z. Effects of a fruit-vegetable dietary pattern on oxidative stress and genetic damage in coke oven workers: a cross-sectional study. Environ. Health. 2015;14(1):40. doi: 10.1186/s12940-015-0028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pastori D. Does mediterranean diet reduce cardiovascular events and oxidative stress in atrial fibrillation? Antioxidants Redox Signal. 2015;23(8):682–687. doi: 10.1089/ars.2015.6326. [DOI] [PubMed] [Google Scholar]
- 45.Kaliora A.C. The effectiveness of mediterranean diet in nonalcoholic fatty liver disease clinical course: an intervention study. J. Med. Food. 2019;22(7):729–740. doi: 10.1089/jmf.2018.0020. [DOI] [PubMed] [Google Scholar]
- 46.Luisi M.L.E. Effect of mediterranean diet enriched in high quality extra virgin olive oil on oxidative stress, inflammation and gut microbiota in obese and normal weight Adult subjects. Front. Pharmacol. 2019;10:1366. doi: 10.3389/fphar.2019.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodríguez J.M. Reduction of serum advanced glycation end-products with a low calorie Mediterranean diet. Nutr. Hosp. 2015;31(6):2511–2517. doi: 10.3305/nh.2015.31.6.8936. [DOI] [PubMed] [Google Scholar]
- 48.Bloomer R.J., Gunnels T.A., Schriefer J.M. Comparison of a restricted and unrestricted vegan diet plan with a restricted omnivorous diet plan on health-specific measures. Healthcare (Basel) 2015;3(3):544–555. doi: 10.3390/healthcare3030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parcina M. No short-term effects of calorie-controlled Mediterranean or fast food dietary interventions on established biomarkers of vascular or metabolic risk in healthy individuals. Nutr Res Pract. 2015;9(2):165–173. doi: 10.4162/nrp.2015.9.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yubero-Serrano E.M. Mediterranean diet and endothelial function in patients with coronary heart disease: an analysis of the CORDIOPREV randomized controlled trial. PLoS Med. 2020;17(9) doi: 10.1371/journal.pmed.1003282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sofi F. Low-calorie vegetarian versus mediterranean diets for reducing body weight and improving cardiovascular risk profile: CARDIVEG study (cardiovascular prevention with vegetarian diet) Circulation. 2018;137(11):1103–1113. doi: 10.1161/CIRCULATIONAHA.117.030088. [DOI] [PubMed] [Google Scholar]
- 52.Jaacks L.M. Pilot randomized controlled trial of a Mediterranean diet or diet supplemented with fish oil, walnuts, and grape juice in overweight or obese US adults. BMC Nutr. 2018;4:26. doi: 10.1186/s40795-018-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim Y., Keogh J.B., Clifton P.M. Effects of two different dietary patterns on inflammatory markers, advanced glycation end products and lipids in subjects without type 2 diabetes: a randomised crossover study. Nutrients. 2017;9(4) doi: 10.3390/nu9040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis C.R. A mediterranean diet reduces F(2)-isoprostanes and triglycerides among older Australian men and women after 6 months. J. Nutr. 2017;147(7):1348–1355. doi: 10.3945/jn.117.248419. [DOI] [PubMed] [Google Scholar]
- 55.Razavi Zade M. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: a randomized clinical trial. Liver Int. 2016;36(4):563–571. doi: 10.1111/liv.12990. [DOI] [PubMed] [Google Scholar]
- 56.Sureda A. Mediterranean diets supplemented with virgin olive oil and nuts enhance plasmatic antioxidant capabilities and decrease xanthine oxidase activity in people with metabolic syndrome: the PREDIMED study. Mol. Nutr. Food Res. 2016;60(12):2654–2664. doi: 10.1002/mnfr.201600450. [DOI] [PubMed] [Google Scholar]
- 57.Choi S.H., Choi-Kwon S. The effects of the DASH diet education program with omega-3 fatty acid supplementation on metabolic syndrome parameters in elderly women with abdominal obesity. Nutr Res Pract. 2015;9(2):150–157. doi: 10.4162/nrp.2015.9.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forrester S.J. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122(6):877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adams L., Franco M.C., Estevez A.G. Reactive nitrogen species in cellular signaling. Exp. Biol. Med. 2015;240(6):711–717. doi: 10.1177/1535370215581314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frijhoff J. Clinical relevance of biomarkers of oxidative stress. Antioxidants Redox Signal. 2015;23(14):1144–1170. doi: 10.1089/ars.2015.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moselhy H.F. A specific, accurate, and sensitive measure of total plasma malondialdehyde by HPLC. J. Lipid Res. 2013;54(3):852–858. doi: 10.1194/jlr.D032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito F., Sono Y., Ito T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants. 2019;8(3):72. doi: 10.3390/antiox8030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allaman I., Bélanger M., Magistretti P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015;9(23) doi: 10.3389/fnins.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine. 2018;54(4):287–293. [Google Scholar]
- 65.Forman H.J., Zhang H., Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Aspect. Med. 2009;30(1–2):1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tinkov A.A. The role of the thioredoxin/thioredoxin reductase system in the metabolic syndrome: towards a possible prognostic marker? Cell. Mol. Life Sci. 2018;75(9):1567–1586. doi: 10.1007/s00018-018-2745-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Priyanka K., Singh S., Gill K. Paraoxonase 3: structure and its role in pathophysiology of coronary artery disease. Biomolecules. 2019;9(12):817. doi: 10.3390/biom9120817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Araujo J., Zhang M., Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 2012;3(119) doi: 10.3389/fphar.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaggini M., Sabatino L., Vassalle C. Conventional and innovative methods to assess oxidative stress biomarkers in the clinical cardiovascular setting. Biotechniques. 2020;68(4):223–231. doi: 10.2144/btn-2019-0138. [DOI] [PubMed] [Google Scholar]
- 70.Pulli B. Measuring myeloperoxidase activity in biological samples. PloS One. 2013;8(7) doi: 10.1371/journal.pone.0067976. e67976-e67976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pulli B. Measuring myeloperoxidase activity in biological samples. PloS One. 2013;8(7) doi: 10.1371/journal.pone.0067976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cristani M. Circulating advanced oxidation protein products as oxidative stress biomarkers and progression mediators in pathological conditions related to inflammation and immune dysregulation. Curr. Med. Chem. 2016;23(34):3862–3882. doi: 10.2174/0929867323666160902154748. [DOI] [PubMed] [Google Scholar]
- 73.Rastogi R. NOX activation by subunit interaction and underlying mechanisms in disease. Front. Cell. Neurosci. 2017;10(301) doi: 10.3389/fncel.2016.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Del Ben M. NOX2-generated oxidative stress is associated with severity of ultrasound liver steatosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2014;14 doi: 10.1186/1471-230X-14-81. 81-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tellis C.C., Tselepis A.D. Τhe role of lipoprotein-associated phospholipase A2 in atherosclerosis may depend on its lipoprotein carrier in plasma. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2009;1791(5):327–338. doi: 10.1016/j.bbalip.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 76.Sproston N.R., Ashworth J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor perspectives in biology. 2014;6(10) doi: 10.1101/cshperspect.a016295. a016295-a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalliolias G.D., Ivashkiv L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016;12(1):49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saraiva M., O'Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10(3):170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 80.Lago F. Adipokines as emerging mediators of immune response and inflammation. Nat. Clin. Pract. Rheumatol. 2007;3(12):716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 81.Galbete C. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: an umbrella review of meta-analyses. Eur. J. Epidemiol. 2018;33(10):909–931. doi: 10.1007/s10654-018-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dinu M. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018;72(1):30–43. doi: 10.1038/ejcn.2017.58. [DOI] [PubMed] [Google Scholar]
- 83.Soltani S. Adherence to the mediterranean diet in relation to all-cause mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Adv Nutr. 2019;10(6):1029–1039. doi: 10.1093/advances/nmz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koloverou E. Adherence to Mediterranean diet and 10-year incidence (2002-2012) of diabetes: correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes Metab Res Rev. 2016;32(1):73–81. doi: 10.1002/dmrr.2672. [DOI] [PubMed] [Google Scholar]
- 85.Visioli F., Galli C. The role of antioxidants in the Mediterranean diet. Lipids. 2001;36(Suppl):S49–S52. doi: 10.1007/s11745-001-0682-z. [DOI] [PubMed] [Google Scholar]
- 86.Vassalle C. Antioxidants in the diet and cognitive function: which role for the mediterranean life-style? J Prev Alzheimers Dis. 2017;4(1):58–64. doi: 10.14283/jpad.2016.109. [DOI] [PubMed] [Google Scholar]
- 87.Martinez-Gonzalez M.A., Estruch R. Mediterranean diet, antioxidants and cancer: the need for randomized trials. Eur. J. Canc. Prev. 2004;13(4):327–335. doi: 10.1097/01.cej.0000137512.71845.bf. [DOI] [PubMed] [Google Scholar]
- 88.Martinez-Gonzalez M.A., Trichopoulou A. Observational epidemiology, lifestyle, and health: the paradigm of the mediterranean diet. Am. J. Health Promot. 2020;34(8):948–950. doi: 10.1177/0890117120960580c. [DOI] [PubMed] [Google Scholar]
- 89.Panagiotakos D.B. Status and management of blood lipids in Greek adults and their relation to socio-demographic, lifestyle and dietary factors: the ATTICA Study. Blood lipids distribution in Greece. Atherosclerosis. 2004;173(2):353–361. doi: 10.1016/j.atherosclerosis.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 90.Azzini E. Mediterranean diet effect: an Italian picture. Nutr. J. 2011;10:125. doi: 10.1186/1475-2891-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dai J. Association between adherence to the Mediterranean diet and oxidative stress. Am. J. Clin. Nutr. 2008;88(5):1364–1370. doi: 10.3945/ajcn.2008.26528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stachowska E. Elements of Mediterranean diet improve oxidative status in blood of kidney graft recipients. Br. J. Nutr. 2005;93(3):345–352. doi: 10.1079/bjn20051374. [DOI] [PubMed] [Google Scholar]
- 93.Fito M. Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch. Intern. Med. 2007;167(11):1195–1203. doi: 10.1001/archinte.167.11.1195. [DOI] [PubMed] [Google Scholar]
- 94.Mitjavila M.T. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clin. Nutr. 2013;32(2):172–178. doi: 10.1016/j.clnu.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 95.Hagfors L. Antioxidant intake, plasma antioxidants and oxidative stress in a randomized, controlled, parallel, Mediterranean dietary intervention study on patients with rheumatoid arthritis. Nutr. J. 2003;2:5. doi: 10.1186/1475-2891-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Papandreou C. Effect of Mediterranean diet on lipid peroxidation marker TBARS in obese patients with OSAHS under CPAP treatment: a randomised trial. Sleep Breath. 2012;16(3):873–879. doi: 10.1007/s11325-011-0589-7. [DOI] [PubMed] [Google Scholar]
- 97.Ambring A. Effects of a Mediterranean-inspired diet on blood lipids, vascular function and oxidative stress in healthy subjects. Clin. Sci. (Lond.) 2004;106(5):519–525. doi: 10.1042/CS20030315. [DOI] [PubMed] [Google Scholar]
- 98.Grune T., Berger M.M. Markers of oxidative stress in ICU clinical settings: present and future. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10(6) doi: 10.1097/MCO.0b013e3282f0c97c. [DOI] [PubMed] [Google Scholar]
- 99.Samoylenko A. Nutritional countermeasures targeting reactive oxygen species in cancer: from mechanisms to biomarkers and clinical evidence. Antioxidants Redox Signal. 2013;19(17):2157–2196. doi: 10.1089/ars.2012.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van 't Erve T.J. Classifying oxidative stress by F2-isoprostane levels across human diseases: a meta-analysis. Redox Biology. 2017;12:582–599. doi: 10.1016/j.redox.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diaz M.N. Antioxidants and atherosclerotic heart disease. N. Engl. J. Med. 1997;337(6):408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 102.Tribble D.L., Frank E. Dietary antioxidants, cancer, and atherosclerotic heart disease. West. J. Med. 1994;161(6):605–612. [PMC free article] [PubMed] [Google Scholar]
- 103.Trpkovic A. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit. Rev. Clin. Lab Sci. 2015;52(2):70–85. doi: 10.3109/10408363.2014.992063. [DOI] [PubMed] [Google Scholar]
- 104.Miller E.R., 3rd, Appel L.J., Risby T.H. Effect of dietary patterns on measures of lipid peroxidation: results from a randomized clinical trial. Circulation. 1998;98(22):2390–2395. doi: 10.1161/01.cir.98.22.2390. [DOI] [PubMed] [Google Scholar]
- 105.Evans M.D., Dizdaroglu M., Cooke M.S. Oxidative DNA damage and disease: induction, repair and significance. Mutat. Res. 2004;567(1):1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 106.Ribezzo F., Shiloh Y., Schumacher B. Systemic DNA damage responses in aging and diseases. Semin. Canc. Biol. 2016;37–38:26–35. doi: 10.1016/j.semcancer.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nelson B.C., Dizdaroglu M. Implications of DNA damage and DNA repair on human diseases. Mutagenesis. 2020;35(1):1–3. doi: 10.1093/mutage/gez048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Del Bo C. Overview of human intervention studies evaluating the impact of the mediterranean diet on markers of DNA damage. Nutrients. 2019;11(2) doi: 10.3390/nu11020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bishop K.S. An investigation into the association between DNA damage and dietary fatty acid in men with prostate cancer. Nutrients. 2015;7(1):405–422. doi: 10.3390/nu7010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Filippou C.D. Dietary approaches to stop hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11(5):1150–1160. doi: 10.1093/advances/nmaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soltani S. Adherence to the dietary approaches to stop hypertension (DASH) diet in relation to all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Nutr. J. 2020;19(1):37. doi: 10.1186/s12937-020-00554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ali Mohsenpour M. Adherence to dietary approaches to stop hypertension (DASH)-Style diet and the risk of cancer: a systematic review and meta-analysis of cohort studies. J. Am. Coll. Nutr. 2019;38(6):513–525. doi: 10.1080/07315724.2018.1554460. [DOI] [PubMed] [Google Scholar]
- 113.Mills C.E. It is rocket science - why dietary nitrate is hard to 'beet'! Part II: further mechanisms and therapeutic potential of the nitrate-nitrite-NO pathway. Br. J. Clin. Pharmacol. 2017;83(1):140–151. doi: 10.1111/bcp.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kurtz T.W. Functional foods for augmenting nitric oxide activity and reducing the risk for salt-induced hypertension and cardiovascular disease in Japan. J. Cardiol. 2018;72(1):42–49. doi: 10.1016/j.jjcc.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marseglia L. Oxidative stress in obesity: a critical component in human diseases. Int. J. Mol. Sci. 2014;16(1):378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kopp W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab Syndr Obes. 2019;12:2221–2236. doi: 10.2147/DMSO.S216791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tan B.L. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front. Pharmacol. 2018;9(1162) doi: 10.3389/fphar.2018.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eichelmann F. Effect of plant-based diets on obesity-related inflammatory profiles: a systematic review and meta-analysis of intervention trials. Obes. Rev. 2016;17(11):1067–1079. doi: 10.1111/obr.12439. [DOI] [PubMed] [Google Scholar]
- 119.Watzl B. Anti-inflammatory effects of plant-based foods and of their constituents. Int. J. Vitam. Nutr. Res. 2008;78(6):293–298. doi: 10.1024/0300-9831.78.6.293. [DOI] [PubMed] [Google Scholar]
- 120.Therond P. Biomarkers of oxidative stress: an analytical approach. Curr. Opin. Clin. Nutr. Metab. Care. 2000;3(5):373–384. doi: 10.1097/00075197-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 121.Da Costa L.A., Badawi A., El-Sohemy A. Nutrigenetics and modulation of oxidative stress. Ann. Nutr. Metab. 2012;60(Suppl 3):27–36. doi: 10.1159/000337311. [DOI] [PubMed] [Google Scholar]
- 122.Chen Y., Pei J. Possible risk modifications in the association between MnSOD Ala-9 Val polymorphism and breast cancer risk: subgroup analysis and evidence-based sample size calculation for a future trial. Breast Canc. Res. Treat. 2011;125(2):495–504. doi: 10.1007/s10549-010-0978-9. [DOI] [PubMed] [Google Scholar]
- 123.Aleksandrova K. Biomarker patterns of inflammatory and metabolic pathways are associated with risk of colorectal cancer: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Eur. J. Epidemiol. 2014;29(4):261–275. doi: 10.1007/s10654-014-9901-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.