Abstract
There is a rapidly growing body of literature supporting the notion that differential oxidative metabolism in cancer versus normal cells represents a metabolic frailty that can be exploited to open a therapeutic window into cancer therapy. These cancer cell-specific metabolic frailties may be amenable to manipulation with non-toxic small molecule redox active compounds traditionally thought to be antioxidants. In this review we describe the potential mechanisms and clinical applicability in cancer therapy of four small molecule redox active agents: melatonin, vitamin E, selenium, and vitamin C. Each has shown the potential to have pro-oxidant effects in cancer cells while retaining antioxidant activity in normal cells. This dichotomy can be exploited to improve responses to radiation and chemotherapy by opening a therapeutic window based on a testable biochemical rationale amenable to confirmation with biomarker studies during clinical trials. Thus, the unique pro-oxidant/antioxidant properties of melatonin, vitamin E, selenium, and vitamin C have the potential to act as effective adjuvants to traditional cancer therapies, thereby improving cancer patient outcomes.
Keywords: Cancer therapy, Antioxidant supplementation, Ascorbate
1. Introduction
Metabolic alterations associated with neoplastic transformation and malignant progression are recognized as emergent hallmarks of cancer [1]. Recent theoretical constructs connecting cancer development to aging and malignant progression argue that oxidative metabolic abnormalities in dividing cells undergoing neoplastic transformation lead to acceleration of genomic instability and thereby drive malignant progression [[2], [3], [4]]. These models propose that genetic instability associated with carcinogenesis may be driven by perturbations in mitochondrial oxidative metabolism. Dysfunctional mitochondrial oxidative metabolism often leads to a “build-up” of electrons at sites capable of mediating one-electron reduction of O2, leading to an increase in the steady-state levels of intracellular reactive oxygen species (ROS) that are believed to contribute to initiation, promotion, and progression of the malignant phenotype.
Cancer cells are generally thought to have increased levels of ROS, e.g. superoxide and hydrogen peroxide, compared to their normal cell counterparts [[5], [6], [7], [8], [9]]. Increased levels of ROS significantly contribute to: genomic instability, inability to perform differentiated function, immortalization, uncontrolled cellular proliferation, and the progression to the malignant state [2,3,[5], [6], [7], [10], [11]]. The altered metabolism of tumor cells was first reported by Otto Warburg in 1927 [12].
The Warburg effect describes the increased glucose uptake of tumor tissue relative to adjacent normal tissues. This increased glucose uptake may be utilized in the pentose phosphate pathway to generate reducing equivalents (i.e., NADPH). Transketolase is a critical pentose phosphate pathway mediator necessary for regenerating NADPH. Recently, transketolase has been shown to be critical in regulating cancer cell redox metabolism in hepatocellular carcinoma cells [13]. In this model system, transketolase inhibition led to increased oxidative distress and enhanced sensitivity to sorafenib. By driving the detoxification of hydroperoxides via the glutathione peroxidases and peroxiredoxins, increased NADPH can help mitigate the consequences of increased levels of superoxide (O2•−) and hydrogen peroxide (H2O2) in cancer cells under proliferating conditions [6,14]. Given the prooxidant-propelled metabolic alterations in cancer cells, relative to normal cells, we hypothesize that redox active small molecules that can selectively act as pro-oxidants in cancer cells may provide a novel, biochemical adjuvant to cancer therapy while simultaneously protecting normal tissues by functioning in an antioxidant capacity. Here we examine four redox active small molecules (i.e., melatonin, vitamin E forms, selenium, and vitamin C) that are well tolerated at pharmacological levels in vivo and have shown significant promise as adjuvants to cancer therapies (Table 1).
Table 1.
Clinical trials investigating traditional antioxidants as adjuvants to cancer therapy.
| Cancer Type | Treatment | Phase | Trial Identifiera |
|---|---|---|---|
| Breast | Melatonin 3 mg | Early Phase 1 | NCT01805089 |
| Non-small Cell Lung | Dietary supplement: 20 mg melatonin vs. placebo | Phase 3 | NCT00668707 |
| Head and Neck | Dietary supplement: 40 mg melatonin vs. matched placebo | Phase 2 | NCT02430298 |
| Gastrointestinal and Lung | Dietary supplement: 20 mg melatonin vs. matched placebo | Phase 3 | NCT00513357 |
| Prostate | Dietary supplement: 400 IU vitamin E vs. placebo | Phase 3 | NCT00809458 |
| Head and Neck | Dietary supplement: 1000 mg vitamin E for 7 weeks | Phase 2 | NCT02397486 |
| Pancreatic | Dietary supplement: vitamin E δ-tocotrienol supplied as 100 mg, 200 mg, and 400 mg capsules | Phase 1 | NCT00985777 |
| Breast | Dietary supplement: tocotrienol 200 mg twice a day | Phase 2 | NCT04496492 |
| Soft Tissue Sarcoma | Intravenous high dose ascorbate (75 g) in combination with radiotherapy | Phase 1/2 | NCT03508726 |
| Pancreatic | Intravenous high dose ascorbate (75 g) in combination with radiation and chemotherapy | Phase 2 | NCT02905578 |
| Ovarian | Dietary Supplement: Oral ascorbate in combination with chemotherapy | Phase 2 | NCT00228319 |
| Bladder | Intravenous ascorbate in combination with chemotherapy | Phase 2 | NCT04046094 |
| Glioblastoma | Intravenous ascorbate (87.5 g) in combination with radiation and chemotherapy | Phase 2 | NCT02344355 |
| Renal Cell Carcinoma | Oral selenomethionine (4 mg) in combination with axitinib (5 mg) | Phase 1/2 | NCT02535533 |
ClinicalTrials.gov Identifier at http://clinicaltrials.gov as accessed 2020.11.05.
2. Melatonin
Melatonin (N-acetyl-5-methoxytryptamine) has been suggested to be a compound capable of preventing and treating several cancer types by suppressing pathways associated with the hallmarks of cancer, including the regulation of pro-survival signaling and tumor metabolism, the inhibition of angiogenesis and metastasis, and the induction of epigenetic alterations in oncogenes and tumor suppressors. Studies have also suggested that melatonin can reduce normal tissue injury associated with radiation and chemotherapy treatment, as well as enhance the efficacy of chemotherapy, thereby improving cancer survival rates [15].
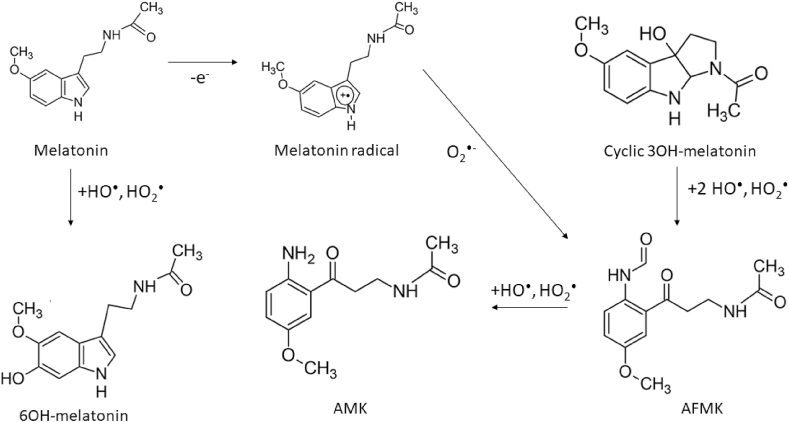
Melatonin is a hormone secreted by the pineal gland known for its role in regulating circadian rhythms [16] and oxidative metabolism [17]. Melatonin secretion is regulated by the duration of light exposure; darkness is associated with high melatonin secretion. In addition to regulating circadian rhythms, melatonin exhibits anti-inflammatory, antioxidant, and oncostatic properties [16,17]. Once in circulation, melatonin is taken into cells via G-coupled receptors, melatonin receptors 1 and 2, which have picomolar and nanomolar binding affinities, respectively [18]. Intracellularly, melatonin accumulates in the mitochondria, where it can scavenge ROS [19] and regulate N-ribosyldihydronicotinamide (NRH):quinone oxidoreductase 2 (NQO2) and therefore modulate levels of ROS [17,20]. Melatonin is thought to chemically interact with various forms of ROS and reactive nitrogen species (RNS) to produce three main chemical products: N(1)-acetyl-N(2)-formyl-5-methoxykynurenin (AFMK), N(1)-acetyl-5-methoxykynurenin (AMK), and hydroxymelatonin (HO-melatonin) (Fig. 1) [21,22]. The generation of AFMK and HO-melatonin is dependent on the concentration of O2. AFMK is the primary product in aerated solutions (84%), while HO-melatonin is prevalent in low O2 environments (86%). Because human tissues are typically at 4–5% O2, HO-melatonin is the most physiologically relevant intermediate of melatonin [23]. For this reason, 3OH-melatonin is considered a potential biomarker of in vivo ROS levels [24]. It is thought that AMK, AMFK, and cyclic 3HO-melatonin are better HO• and HO2• scavengers than melatonin [25,26]. In addition to its ability to act as a free radical scavenger, melatonin may also chemically react with redox active metals contained within the cell to blunt the catalysis of oxidation reactions (e.g. Fenton reactions of iron). Melatonin has been shown to mitigate the activity of the heme containing protein, myeloperoxidase [27]. Melatonin has also been shown to mitigate both estradiol-induced, oxidative DNA damage (e.g. 8-oxoguanine) and iron-catalyzed lipid peroxidation [28,29]. Along with iron, there is also evidence of melatonin being able to decrease copper-mediated DNA damage in vivo [30]. Thus, it may be well reasoned to consider melatonin as a potential metal chelator. Due to its apparent antioxidant capacity and potential to interact with catalytic metals, melatonin is currently being investigated as a supplement for both cancer prevention and treatment.
Fig. 1.
Schematic of melatonin and associated metabolites reactions with reactive oxygen species [22].
2.1. Anti-cancer effects of melatonin
Increased blood melatonin levels initiate homeostatic metabolic rhythm signals to enter a state of rest. Light exposure at adverse times can disrupt circadian rhythms and may contribute to the development, promotion, and progression of cancer [31]. Artificial light exposure during normal circadian rest cycles is associated with reduced melatonin secretion and an increased risk of breast cancer [32]. Reduced secretion of melatonin may lead to an increase in reproductive hormone, including estradiol. Increased levels of estradiol are associated with an elevated risk of breast cancer development [33]. In support of this, in vitro experiments performed in estrogen receptor-positive MCF-7 human breast cancer cells demonstrated that melatonin, at a physiological concentration (1 nM) and in the presence of estradiol, inhibits cell proliferation, increases the expression of p53 and p21WAF1 proteins, and counteracts the stimulatory effect of estradiol on cell invasiveness. Melatonin's effect on invasion and metastasis is partly mediated by a melatonin-induced increased expression of the cell surface adhesion proteins E-cadherin and beta(1)-integrin [34]. Physiological concentrations of melatonin have been shown to decrease the growth rate of breast cancer cells by increased expression of p21/WAF1 along with p53 [34]. Increased expression of p53 promotes genetic stability and makes cells less prone to tumor formation [35]. Melatonin has also been shown to be pro-apoptotic in colorectal cancer cells when given in combination with a somatostatin analog [36].
Melatonin has been shown to enhance the expression of the antioxidant enzymes catalase, superoxide dismutase, and glutathione reductase [37,38]. Activation of this antioxidant network has been shown to reduce intracellular ROS levels and promote cellular differentiation in prostate cancer cells [5,10,39,40]. While many researchers attribute the anticancer effects of melatonin to its antioxidant function, there is some suggestion that it may function as a pro-oxidant in cancer cells [41,42] by selectively disrupting mitochondrial and metal ion metabolism. In human leukemia Jurkat cells, high concentrations of melatonin (10–1000 μM) led to Fas–induced apoptosis via ROS generation [43]. Rodogna et al. showed that 1 mM melatonin induced ROS production as early as 1 min following exposure with ROS and persisting up to 6 h post-treatment [44]. High-doses (1–10 mM) of melatonin elevate ROS within 15 min in HepG2 cells [45]. The pro-oxidant nature of high-dose melatonin has been shown to promote apoptosis via caspase activation and can be counteracted by N-acetyl-l-cysteine, Trolox, PEG-catalase, and glutathione [46]. The fundamental pro-oxidant mechanism of high-dose melatonin is still poorly understood. MT1 and MT2 receptors are not believed to be central to the pro-oxidant role of melatonin, because MT1/2 antagonists could not mitigate melatonin-mediated ROS production in U937 cells [44].
While the role that MT1/2 plays in carcinogensis is unclear, it appears there may be differential expression amongst tumors. The melatonin receptor, MT2, has recently been shown to have differential expression among lung cancer subtypes, with higher MT2 expression being associated with a more favorable prognosis [47]. This suggests the potential utility of melatonin agonists, such as ramelteon or agomelatine, to function as anti-neoplastic agents. Both ramelteon and agomelatine are promising because they have greater bioavailability than melatonin itself [48]. The plasma half-life of agomelatine is ≈ 2 h [49] and ≈ 1–2 h for ramelteon [50,51]. While primarily for use as treatments for insomnia and depression, ramelteon and agomelatine have garnered interest as cancer therapeutics [48]. Despite their theoretical clinical promise, the anti-cancer potential of both ramelteon and agomelatine need to be investigated.
The pro-oxidant nature of melatonin may be partly due to disruptions in the electron transport chain (ETC), a hypothesis based on the preferential accumulation of melatonin in the mitochondria [19,52]. In human mesangial cells and mouse kidney mitochondria, high doses of melatonin disrupt complex III of the ETC, leading to increased superoxide formation [53,54]. High-dose melatonin has also been shown to increase nNOS expression transiently in vitro, resulting in a decrease in oxidative phosphorylation and mitochondrial membrane potential [55,56]. The definitive role of the ETC disruptions by melatonin has yet to be elucidated but shows great promise since it is well known that cancer cells have significantly elevated levels of ROS relative to normal cells [3,5,40].
2.2. Normal tissue protection
In addition to its potential for enhancing cancer therapy, melatonin may also protect normal tissues from cancer therapy–associated toxicity by assuming a role as a donor antioxidant at higher doses, inducing NQO2, which is able to reduce quinones and semiquinones to hydroquinones, and/or acting as a chelator of redox active metal ions [17,20,42]. The chemotherapy agent, adriamycin, is associated with significant heart and liver toxicity via oxidative damage. In 2008, a study of adult male rats given a single dose of 10 mg kg−1 adriamycin had significant increases in creatine kinase, lactic dehydrogenase, and aminotransferases, circulating iron, ferritin, and transferrin [57]. These elevations correlated with a decrease in liver and heart glutathione and glutathione-S-transferase activity, along with increases in lipid peroxidation, protein oxidation, catalase activity, and glutathione peroxidase activity. The supplementation of melatonin to adriamycin-therapy returned hepatic and cardiac function markers, circulating iron markers, and TBARS and protein carbonyl levels to baseline levels. Glutathione and glutathione peroxidase activity in the heart and liver also returned to baseline levels following the addition of melatonin to Adriamycin. This study presents an example of the potential novelty of melatonin supplementation during cancer therapy to mitigate normal tissue injuries.
Melatonin has also demonstrated significant activity as a radioprotector [58], protecting against radiation-induced DNA damage [59]. More recently, the combination of melatonin and vitamin C reduced DNA damage in peripheral blood samples [60]. In this study, 15 healthy volunteers were given an oral dose of 300 mg melatonin and 300 mg vitamin C with peripheral blood being collected 1 h, 2 h, and 3 h following supplementation. The blood was irradiated with 200 cGy of 6 MV photons and assessed for nuclear fragmentation. Maximum protection occurred at 1 h following oral supplementation. In addition, melatonin recently has been shown to mitigate radiation-induced lung fibrosis in a preclinical animal model [61]. Male C57BL/6 mice were treated with 1 mg melatonin daily for 7 days following a single dose of 15 Gy to the lungs. Mice that received melatonin had significantly reduced oxidative stress markers and macrophage infiltration in the lungs following radiation.
Further support for the hypothesis that melatonin may mitigate cancer therapy–associated toxicity arises from its ability to protect brain tissue from neurotoxins [62]. Melatonin protects the brain from oxidative injuries from methamphetamine [[63], [64], [65], [66]], neural lipid peroxidation associated with aminolevulinic acid accumulation [67,68], and mitochondrially produced O2•- from rotenone [69]. The extensive literature on melatonin's ability to protect CNS structures from a wide variety of neurotoxins suggests that melatonin may also protect against neurotoxicity following cancer therapy.
2.3. Clinical relevance
The ideal dosage for melatonin as a potential cancer preventative or cancer therapy adjuvant has yet to be determined. Oral doses of melatonin ranging from 20 to 40 mg given daily, gradually throughout the day, have been well tolerated [[70], [71], [72]]. Di Bella has reported that supraphysiological doses delivered intravenously up to a maximum dose of 1 g have limited side effects [73]. The primary side effect reported with 1 g given intravenously was drowsiness, usually at the beginning of the treatment [16]. A recent review of the clinical pharmacokinetics of melatonin documented the time to maximum serum concentrations being approximately 50 min [74]. Both the oral and intravenous half-life of melatonin is approximately 45 min (28–126 min) [74]. Oral bioavailability of melatonin is low with significant individual variability (9–33%) [74]. A separate review recognized that intranasal melatonin has substantially higher bioavailability of approximately 55–94% [75].
There are a limited number of completed clinical studies investigating melatonin's utility as an adjuvant to cancer therapy (Table 2). The Di Bella Method (DBM) [73] suggests that melatonin is clinically useful for the enhancement of cancer therapy while mitigating the high levels of toxicity. Note that this method also includes various other antioxidants and immunomodulators such as vitamin E, C, and D3, interleukin 2, and retinoids [16,76,77]. Retrospective clinical studies using the DBM have shown its potential to improve the therapeutic outcomes of patients with breast and head and neck cancer [[60], [61], [62],68]. In 2005, Mills et al. published a meta-analysis of all randomized, controlled clinical trials utilizing melatonin in cancer patients with solid tumors [78]. By surveying electronic databases, they found 10 randomized, controlled clinical trials utilizing melatonin from 1992 to 2003 including a total of 643 patients. From each of these studies, the authors concluded that melatonin reduced the risk of death at 1 year (relative risk = 0.66, 95% confidence interval: 0.59–0.73) with no severe adverse events.
Table 2.
Recently completed clinical trials utilizing melatonin as an adjuvant therapy.
| Cancer Type | Dosage | Combination of Drugs | Results | References |
|---|---|---|---|---|
| Metastatic Colorectal Cancer | melatonin (40 mg/day orally) | low-dose subcutaneous interleukin-2 | Significantly increased 1-year survival rate of patients | [83] |
| Metastatic Breast Cancer | melatonin (20 mg/day) orally starting 7 days before tamoxifen | Tamoxifen | Partial response in 4/14 (28.5%) patients, improved anxiety in most patients and did not enhance the toxicity of tamoxifen. Serum levels of IGF-1 were decreased by the combination therapy. | [81] |
| Metastatic NSCL Cancer | melatonin (20 mg/day) orally in the evening | cisplatin and etoposide | Improved overall tumor response rate and 5-year survival, with better tolerance to chemotherapy | [80] |
| Breast Cancer | Di Bella Method | somatostatin, retinoids, vitamin D3, and low dose of cyclophosphamide | Positively correlated with survival and tumor response | [77] |
A more recent meta-analysis of melatonin in combination with chemotherapy, radiotherapy, or both was published in 2012 by Wang et al. [79]. This study evaluated 8 randomized, controlled clinical trials from 1992 to 2011 using an oral dosage of 20 mg daily in patients with solid tumors. They found that melatonin significantly increased both complete and partial remission (16.5% and 32.6%, respectively), increased the 1-year survival rate from 28.4% to 52.2% with a relative risk of 1.9 (95% confidence interval: 1.28–2.83), and decreased radiotherapy-related side effects including thrombocytopenia (19.7% vs. 2.2%, relative risk = 0.13), neurotoxicity (15.2% vs. 2.5%, relative risk = 0.19), and fatigue (49.1% vs. 17.2%, relative risk = 0.37). All reported effects were independent of cancer type and no severe adverse events associated with melatonin were reported. Despite the great promise for the use of melatonin as an adjuvant to cancer therapy, further investigation into the utility of melatonin, both mechanistically and translationally, is warranted. In prospective clinical trials, oral melatonin has been shown to enhance the effectiveness of traditional chemotherapy approaches, such as cisplatin and etoposide [80], tamoxifen [81], and irinotecan [82].
In addition to its potential ability to enhance cancer therapy, supplementation with melatonin during treatment may improve patient quality of life. A double-blind, placebo-controlled, randomized clinical trial showed that patients receiving 6 mg oral melatonin daily had a lower risk of developing depressive symptoms [84]. This trial also showed that melatonin supplementation significantly improved sleep efficiency by reducing waking episodes after sleep onset [85]. A separate randomized, placebo-controlled trial reported significant increases in subjective sleep quality [86]. Another placebo-controlled crossover trial suggested that 20 mg melatonin daily improved fatigue in patients with advanced cancer [87]. These data suggest that melatonin has potential to mitigate negative side effects often experienced by cancer patients throughout the course of treatment and further enhances the notion that melatonin may mitigate normal tissue injuries as well.
3. Vitamin E
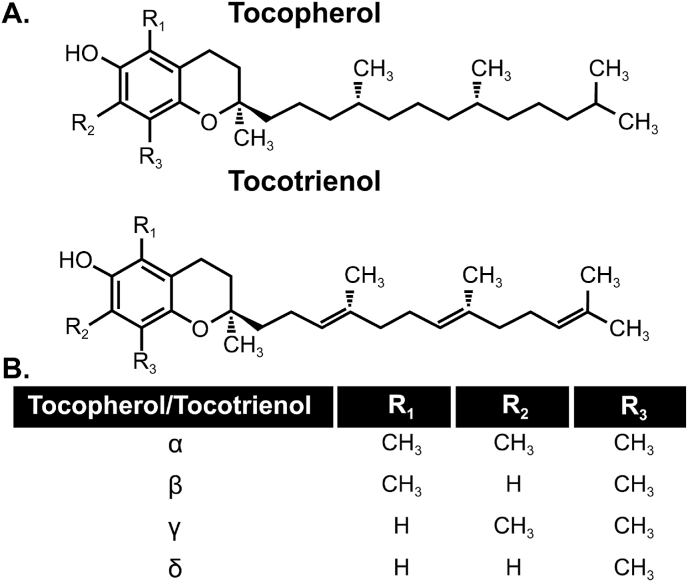
Nuts, plant seeds, and plant oils are rich in vitamin E and selenium [88]. There are several natural forms of vitamin E, namely, α-,β-,γ-,δ – tocopherol (TOH) and tocotrienol (TE-OH) [89] and have been suggested to have both antioxidant effects in normal tissues as well as pro-oxidant effects in cancer cells [90]. Structurally, tocopherol and tocotrienols isoforms are similar but tocopherols have a saturated side chain while tocotrienols have 3 double bonds on the side chain (Fig. 2). The combination of phenolic group on the chromanol ring and the phytol (or phytol-like) tail allows all vitamin E isoforms to be highly lipophilic antioxidants that can readily donate a hydrogen atom to scavenge lipid peroxide radicals in lipid regions of membranes as well as low density lipoprotein [91].
Fig. 2.
Chemical structure of Vitamin E. A. Structural comparison of tocopherol and tocotrienol forms. B. Side chain moieties of individual tocopherols and tocotrienols.
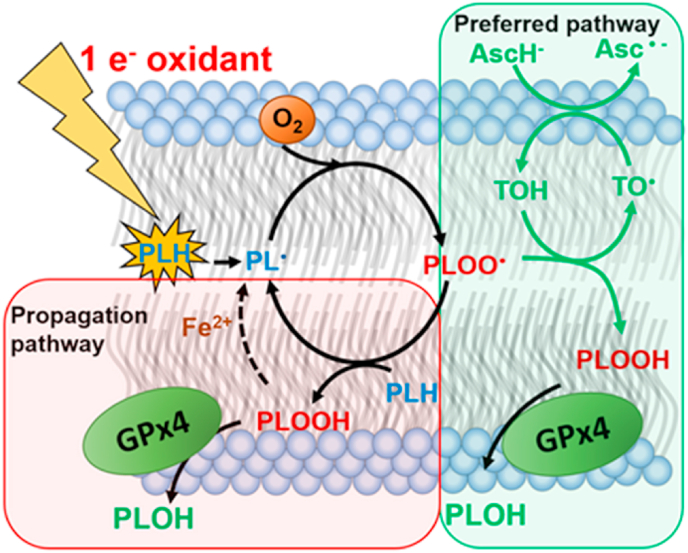
Lipid peroxidation chain reactions can be initiated by 1-electron oxidants (Fig. 3). This results in the formation of carbon-centered lipid radicals (PL•), which upon reaction with oxygen to form a phospholipid peroxyl radicals (PLOO•) [[92], [93], [94]]. Peroxyl radicals can then initiate new chains of free radical-mediated oxidations resulting in the formation of phospholipid hydroperoxides (PLOOH). However, there is a more biochemical energetically favorable way to produce the PLOOH [94]. The rate constant of inter-lipid propagation reactions is relatively low (k ≈ 10 M −1 s−1) [95]. Vitamin E can outcompete the propagation reaction with a much higher rate constant (k ≈ 8 × 104 M −1 s−1) [96] to also form PLOOH while preventing further propagation. The residual vitamin E radical (TO•) is then recycled by vitamin C via a water-lipid interface interaction [94,97,98]. The vitamin C (Asc•-) radical dismutes, forming AscH− and dehydroascorbate [94]. DHA is relatively harmless due to its rapid reduction back to AscH−.
Fig. 3.
The antioxidant triad, vitamins C, E and selenium cooperatively terminate lipid peroxidation. Oxidation of phospholipids can be initiated by 1-electron oxidants. Upon oxygenation, a phospholipid peroxyl radical, PLOO•, is formed. This species can be protonated through two pathways to form PLOOH. The first pathway, in green, utilizes vitamin C and E as antioxidants thus, protecting adjacent phospholipids to form PLOOH. The second pathway abstracts a hydrogen atom of another phospholipid to form PLOOH. However, this creates a new lipid radical PL•, and thus propagates lipid peroxidation. As shown, both pathways create PLOOH, but the antioxidant pathway is preferred, because this does not reinitiate lipid peroxidation reactions. The formed PLOOH is a substrate for GPx4 that detoxifies it to an alcohol, terminating lipid peroxidation. If GPx4 activity is blunted, PLOOH can initiate chain-branching lipid peroxidation reactions when ferrous iron is available. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Collaboratively with vitamins E and C, the metalloid selenium (Se), when incorporated in glutathione peroxidase-4 (GPx4; associated with the lipid bilayers) and glutathione peroxidase-1 (GPx1; in the mitochondrial matrix and cytosol) assist the termination of lipid peroxidation [99]. GPx4 detoxifies PLOOH and converts it to its corresponding alcohol while GPx1 reacts with both H2O2 and ROOH to form either H2O or ROH. If the hydrogen atom transfer of vitamin E to a lipid peroxyl radical is too slow, perhaps due to low levels of TOH, PLOO• will abstract a hydrogen atom from a neighboring phospholipid, thereby propagating the chain reactions of lipid peroxidation. In addition, insufficient GPx4 activity can increase the steady-state level of PLOOH, providing the potential for additional free radical oxidations.
Low GPx4 activity can be caused by selenium deficiency, which can be overcome by supplementation of Se [99]. During Se-deficiency or inhibition of GPx4, the higher steady-state level of PLOOH could lead to more reactions of ferrous iron through a Fenton-like reaction with PLOOH, leading to PLO•, which initiates new chain-branching free radical reactions [[100], [101], [102]]. Therefore, the synergy of vitamin E and selenium are thought to be novel partners that can prevent these free radical-mediated oxidations that could lead to cancer or be used in the treatment of cancer.
While GPx4 appears to play a major role in the Se-dependent antioxidant effect of lipid peroxidation, other Se-dependent enzymes should not be overlooked. A different isoform in the GPx family, GPx1, also has Se at its active site. GPx1 reduces hydrogen peroxide as well as organic hydroperoxides in the cytosolic fraction of cells. Cytosolic hydroperoxides consist of lipid sections that were cleaved of oxidized phospholipids by phospholipase A2 [103]. Like GPx1, the 2-Cys members of the peroxiredoxin family (prxd1-5) can also act on these organic hydroperoxides [103,104]. In contrast to GPx4 termination of oxidation within the lipid bilayer, both GPx1 and the 2-Cys peroxiredoxins detoxify downstream products of lipid oxidation. The peroxiredoxins overlap with the glutaredoxins in the antioxidant recycling system, as described by Ng et al. [105]. Both, the peroxiredoxins and the glutaredoxins receive electrons from the pentose phosphate pathway, and thus NADPH. The flow of electrons occurs via the reductase/glutaredoxin pathway (GR/GRX), and the Se-dependent thioredoxin/thioredoxin reductase (Trx/TrxR) pathway [[106], [107], [108], [109]]. This shows how Se-dependent enzymes, besides GPx4, can contribute to the termination of lipid peroxidation.
3.1. Cancer prevention
Vitamin E has been well investigated as an antioxidant supplement for cancer prevention. In 1994, a randomized, double-blind, placebo-controlled trial examined vitamin E's ability to reduce the incidence of lung cancer in male smokers (the study also tested beta-carotene and a combination of vitamin E and beta-carotene, 87). The trial found no difference in the incidence of lung cancer [110]. Similarly, a study of Finnish male smokers who consumed vitamin E supplements for five to eight years failed to show a significant decrease in the incidence of pancreatic carcinoma [111]. This same cohort was also analyzed for the incidence of urinary tract cancer (urothelial cancer and renal cell cancer) and again showed no beneficial effect of vitamin E on cancer initiation [112]. In contrast, the same Finnish male smokers did show a modest preventative effect of colorectal cancer risk with long-term supplementation of vitamin E [112]. Likewise, a study conducted between 1992 and 2004 that recruited healthy US women and randomly assigned them to receive vitamin E or placebo reported no overall benefit for cancer prevention [113]. The SELECT trial was a randomized, placebo-controlled trial of vitamin E supplementation for 7–12 years ability to prevent prostate cancer in healthy men (the study also investigated selenium supplementation, or a combination of vitamin E and selenium, 91). This trial failed to show a benefit in prostate cancer incidence [114]. Recent reviews have suggested that supplementation of vitamin E and selenium may only be effective in cancer prevention when the subjects are deficient but not when are sufficient in these nutrients [115,116]. Based on these results, the hope of using supplemental vitamin E as a means of cancer prevention has waned.
3.2. Anticancer mechanisms of vitamin E
The biochemical anticancer pro-oxidant effects of vitamin E have been extensively studied and are thought to be mediated by tocopheroxyl/tocotrienal-radical formation from mitochondrial metabolism in cancer cells [89,90,[117], [118], [119]]. The enhanced efficacy of γ-TE-OH may be partly explained by its increased accumulation in cancer cells compared to the tocopherols [120]. Tocotrienols have also been shown to accumulate selectively in cancer cells relative to normal cells following oral administration [96,97]. The tocotrienols γ-TOH, δ-TOH, γ-TE-OH, and δ-TE-OH have all been shown to be effective in inducing growth arrest, apoptosis, autophagy, and endoplasmic reticulum stress in cancer cells [[121], [122], [123], [124], [125]]. The tocotrienols γ-TE-OH and δ-TE-OH, specifically, are believed to be much stronger anticancer agents than the tocopherols, with an IC50 range of 10–20 μM, as compared to ≥25–50 μM [95,99,100] — all of which are thought to be more potent anticancer agents than α-TOH. By direct comparison, 50 μM α-TOH failed to produce any reduction in viable PC-3 cells, while an equivalent dose of γ-TOH reduced cell viability by approximately 50% [126]. As for γ-TE-OH, it has been shown to be capable of slowing cell growth and activating apoptosis in both prostate and breast cancer cells [95,100,102,103]. This is thought to be related to the mitochondrial electron transport chain II targeting of redox metabolic differences between cancer versus normal cells [129,130].
In both prostate and breast tumor cells, γ-TE-OH induces apoptosis via caspase activation by suppressing Id1 and NF-kB via activation of the proapoptotic JNK pathway [95,102]. Other antiproliferative and cell stress–associated pathways affected by γ-TOH and γ-TE-OH include PPAR-γ upregulation [[127], [128], [131]], inhibition of P13K-mediated AKT phosphorylation [95,102,105], increased caspase 8/9 activation, and expression of the autophagy marker LC3II [96,102,106]. A possible biochemical mechanism for the anticancer effects of vitamin E forms may be the modulation of sphingolipids. Persistent accumulation of sphingolipids in cancer cells is known to induce apoptosis and inhibit cell growth [[132], [133], [134]]. Both γ-TOH and γ-TE-OH elevate dihydrosphingosine, dihydroceramides, and ceramides in prostate [126], breast [135], colon [136], and pancreatic [137] cancers. One mechanism of sphingolipid accumulation occurs through dihydroceramide desaturase inhibition during de novo synthesis of sphingolipids, potentially leading to enhanced sphingomyelin hydrolysis [137]. Blocking de novo synthesis of sphingolipids can reverse the anticancer effects of both γ-TOH and γ-TE-OH [126,135,137]. Inhibition of sphingolipid synthesis prohibits γ-TOH and γ-TE-OH apoptotic induction via endoplasmic reticulum stress–induced activation of the JNK/CHOP/DR5 pathway [135]. Taken together these data suggest that vitamin E isoforms interact with sphingolipids to enhance cellular stress and promote cancer cell death.
3.3. Vitamin E as a potential adjuvant therapy
While the various forms of vitamin E are structurally similar, they greatly vary in bioavailability. Of the eight isoforms of vitamin E, the most readily available in plasma and tissues is α-tocopherol [138]. Serum concentrations of α-tocopherol range from 20 to 50 μM and have a half-life of approximately 30 h but is dependent on the α-tocopherol transfer protein expression [139,140]. The other vitamin E isoforms have a 4–8 times shorter half-life and much lower serum concentrations [139]. With supplementation, γ-tocopherol has a serum concentration of 10–30 μM [141,142]. An oral dose of 300 mg α-, γ-, and δ-tocotrienol has been shown to have a plasma half–life of 4.4, 4.3, and 2.3 h, respectively [143]. Although most cancer prevention trials utilizing vitamin E were unsuccessful, there is growing interest in the utility of vitamin E forms as adjuvants to cancer therapy (Table 3). In a phase I pharmacokinetic study, δ-TE-OH was given to patients in an oral dose range of 200–1600 mg daily [144]. Serum concentrations up to 18 μM δ-TE-OH were achieved and were sufficient to activate caspase–3 in dysplastic and malignant tissues [144]. At this dose level, no dose-limiting toxicities were observed [144]. There is concern of increased mortality associated with high dose (>400 mg) vitamin E due to cardiovascular complications [145]. A meta-analysis from 2012 of 19 independent clinical trials found there was a significant increase in the all-cause mortality rate (39 per 10,000 persons) for high dose vitamin E (>400 mg) compared to low dose supplementation (16 per 10,000 persons) [146]. The increased mortality associated with high dose vitamin E may be primarily as a result of cardiometabolic effects. In 2005, a randomized, double-blind, placebo-controlled trial evaluating the effects of high dose vitamin E supplementation (400 mg daily) on patients at risk for cardiovascular disease was completed [147]. The long-term study followed patients (n = 3994) for a median of 7 years. The results of this trial showed that patients that received a 400 mg vitamin E supplement were at risk for cardiovascular disease (relative risk = 1.13; 95% CI, 1.01–1.26; P = .03) and hospitalization associated with cardiovascular disease (relative risk = 1.21; 95% CI, 1.00–1.47; P = .045). Despite showing minimal toxicities in cancer related trials, there may be significant long-term effects associated with chronic, high dose vitamin E supplementation that warrant consideration.
Table 3.
Recently completed clinical trials utilizing vitamin E forms as adjuvant therapy.
| Cancer Type | Dosage | Results | References |
|---|---|---|---|
| Pancreatic Cancer | δTE at escalation doses of 200–3200 mg/d for 2 weeks before surgery | δTE is generally safe and induced apoptosis in dysplastic or malignant tissues from pancreas | [144] |
| Colorectal Cancer | γTmTs for 1 or 2 weeks | Bioavailability, plasma F2-isoprostane, inflammation markers | NCT00905918 |
| Ovarian Cancer | Cabazitaxel (25 mg/m2) vs. tocotrienol (300 mg × 3); 3 months | Survival rate and cancer progression | NCT02560337 |
| Lung Cancer | Tocotrienol, 300 mg × 3 plus standard chemotherapy | Disease progression–free survival | NCT02644252 |
One form of vitamin E often considered as a cancer therapy is the tocotrienol-rich fraction (TRF) extracted from palm oil [89]. TRFs have been shown to have an antitumor effect in breast cancer [148] and lung cancer [149] in preclinical animal models. A double-blind, placebo-controlled trial utilizing oral TRFs (200 mg or placebo control) in combination with tamoxifen (20 mg daily) in stage I/II estrogen receptor positive breast cancer found a 5-year disease-free survival rate of 86.7% compared to 83.3% in the control group [150]. The risk of mortality due to breast cancer was found to be significantly reduced by TRF supplementation (hazard ratio = 0.4, 95% confidence interval = 0.08–2.05) [150].
In preclinical studies, tocotrienols have shown promise as a potential adjuvant to radiation therapy. Kumar et al. showed that when nude mice with PC3 tumor xenografts were given 400 mg kg−1 γ-TE-OH 24 h before a single dose of 12 Gy radiation, there was a 40% reduction in tumor volume compared to reduction by radiation alone [151]. The addition of γ-TE-OH was shown to have no toxic effects in the rectum and was slightly protective of the liver [151]. Unfortunately, the authors did find that the addition of γ-TE-OH appeared to sensitize the kidneys to lipid peroxidation along with the tumor [151].
3.4. Normal tissue protection by vitamin E
Tocotrienols may also protect normal tissues from radiation-induced damage. Mice receiving a single dose of 200 mg kg−1 γ-TE-OH before 8 Gy total body irradiation (TBI) exposure were able to protect hematopoietic stem cells and progenitor cells compared to mice receiving radiation alone [152]. A single dose γ-TE-OH (400 mg kg−1 given subcutaneously 24 h before 9 Gy TBI) was able to improve post-radiation survival, decreased radiation-induced vascular oxidative stress, and protected mice from gastrointestinal injury [153]. Similar to γ-TE-OH, a single dose of δ-TE-OH (400 mg kg−1 given subcutaneously before radiation) protected 100% of mice from TBI-induced death measured 30 days after radiation [154]. Subcutaneous δ-TE (75–100 mg kg−1) given to mice before 12 Gy TBI protected the animals from TBI-induced gastrointestinal damage, as evidenced by an increased number of jejunal crypt cells present 30 days post-radiation relative to radiation alone [154].
4. Selenium as a cancer therapeutic
Redox-active inorganic Se-compounds, such as sodium selenite, are known to be pro-oxidant at high doses [[155], [156], [157]]. The associated increase in formation of ROS may tip the redox status of cancer cells and thus, killing the cells. Cells in healthy tissues can cope with these fluxes of oxidative stress, while cancer cells are at the limit of their ability to control the oxidative distress, thus killing them. A variety of oxidative damages of redox-active selenium compounds have been reported: sodium selenite was shown to induce single-strand DNA breaks [[158], [159], [160], [161], [162]], as well as oxidative DNA lesions e.g. 8-hydroxydeoxyguanosine [163]. As a result, apoptosis can be readily observed in cells treated with single digit μM concentrations of sodium selenite. Additionally, a different study showed inhibition of neoplastic growth of HeLa cells upon treatment with selenite (5 μM). In contrast, Se-dl-cystine, a non-redox active selenium compound needed a much higher concentration (100 μM) to show a similar anti-tumor response [164]. This last effect is an appropriate example of how understanding and selecting the right Se-compound for each study important. Generally, organic selenium compounds, e.g. selenomethionine, methyl-selenocysteine or Se-dl-cystine are less toxic at higher concentrations. Because these compounds are not redox active, they do not generate ROS readily. An extensive overview on selenium compounds for cancer treatment are provided in Ref. [165].
Zakharia et al. employ a different strategy to utilize selenium in cancer therapy. Where inorganic sources are typically used to induce oxidative distress, this clinical trial (NCT02535533) uses high dose seleno-l-methionine (SLM), a well tolerated, organic source of Se [166,167]. Preclinical work suggests that high-dose SLM results in downregulation of hypoxia induced factor 1α and 2α (HIFs) and downstream vascular endothelial growth factor (VEGF) and associated oncogenic miRNA 155 and 210, which results in reduced vascular permeability and improved drug delivery into the tumor upon treatment with high-dose SLM [[168], [169], [170], [171], [172], [173], [174], [175]]. These preclinical data provided the rationale for the use of a defined dose and schedule of SLM in sequential combination with standard of care axitinib as second line and beyond in metastatic clear cell renal cell carcinoma. Patients were treated with high doses of SLM, as high as 4 mg, twice daily for 2 weeks then once a day in combination with axitinib, without a dose limiting toxicity (DLT), and yet with promising efficacy signal [176]. A limitation of this study is that SLM can be incorporated into proteins as a replacement of methionine, especially in plasma [177]. This has the potential to limit the amount of Se that reach target organs or tumor tissues.
4.1. Normal tissue protection by selenium
Selenium can also be used as an adjuvant for chemotherapy and radiation therapy [178,179]. Muecke et al., suggested that Se could restore selenoenzyme activity in Se-deficient patients that are under treatment. This would alleviate the side-effects of the treatment. In their phase 3 clinical trial, Se successfully reduced radiation therapy induced side effects while not impacting the efficacy of the antitumor effect of the therapy. In 2014, a follow-up research article was published where an increased 10-year survival rate was reported [179]. Because this study was conducted with selenium deficient subjects, it is likely that the se-dependent antioxidant enzymatic activities were restored, as is shown in cell culture systems [180]. Other mechanisms have not been identified, since these relatively low doses of sodium selenite do not exhibit any toxicity in humans. While the biochemical of this incorporation is not well understood, there are suggestions that selenium supplementation is capable of protecting normal tissues against oxidative distress associated with therapy [181].
5. Vitamin C, ascorbate
Since its discovery in 1933, vitamin C (ascorbic acid, AscH−) has been biologically implicated in a variety of applications [182,183]. Vitamin C is a ketolactone with two ionizable hydroxyl groups at the second and third carbons. The hydroxyl groups have pKa's of 4.2 and 11.6, making the ascorbate monoanion (AscH−) the most prevalent species at pH of 7.4 [183]. AscH− acts as a reducing agent and donor antioxidant that can undergo two consecutive one-electron oxidations to produce the ascorbate radical (Asc•-) and dehydroascorbic acid (DHA), respectively. The ability to undergo redox reactions makes vitamin C a potent antioxidant and pro-oxidant, depending on concentration and environment. Primates and domestic guinea pigs are the only species, including plants, that cannot synthesize vitamin C. This phenomenon is due to an inability to convert l-gluconolactone to l-ascorbic acid and contributes to humans' need to consume vitamin C through dietary supplementation [184].
5.1. Cancer prevention
Because of vitamin C's ability to act as a potent antioxidant, vitamin C oral supplements were speculated to prevent cancer initiation. In 2004, researchers reported on the efficacy of 120 mg vitamin C supplementation for the prevention of cancer incidence, describing a randomized, double-blind, placebo-controlled primary prevention trial of 13,017 French adults. After a median time of 7.5 years, the subjects taking vitamin C supplements had lower total cancer incidence compared to controls [185]. Conversely, this same cohort had a higher incidence of skin cancer [186]. Another study, its results published in 2006, monitored 29,361 men for up to 8 years and found no evidence that vitamin C supplementation reduced the incidence of prostate cancer [187]. Additionally, a 2015 meta-analysis of seven randomized trials in which participants received either vitamin C or placebo did not show any evidence to support oral vitamin C supplementation prevents esophageal and gastric cancers [188]. However, these studies we not stratified according to circulating levels of ascorbate and therefor might not reflect what supplementation would do in a setting where the subjects were deficient.
5.2. Anticancer effects: ascorbate, a pro-oxidant
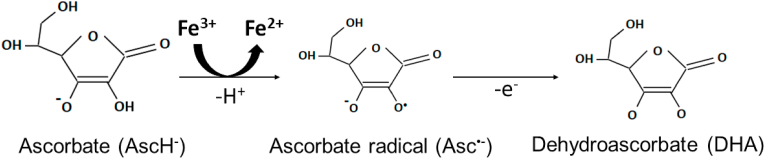
The anticancer mechanisms of ascorbate have been well elucidated in comparison to other redox active small molecules. Ascorbate oxidation produces hydrogen peroxide (H2O2) and has long been proposed to enhance H2O2-mediated tumor cell killing [[189], [190], [191], [192]]. Ascorbate oxidation occurs more readily in the presence of catalytic metals, including redox active iron, where ascorbate can act as a one-electron reducing agent and thereby reduce ferric (Fe3+) to ferrous (Fe2+) while producing an ascorbate radical (Equation (1), Fig. 4). The dysfunctional mitochondrial ETC of cancer cells are more prone to increased one electron reductions of O2 to form superoxide (O2●-) formation, which in turn increases catalytically active iron [193]. Thus, catalytically active iron is central to pharmacological ascorbate toxicity, as evidenced by the ability for desferrioxamine, an iron chelator, to mitigate cancer cell sensitivity to ascorbate [193,194].
| (1) |
Fig. 4.
Schematic oxidation of ascorbate. Ascorbate oxidation is enhanced by the presence of catalytically active ferric iron (Fe3+) that can readily accept an electron to be reduced to ferrous iron (Fe2+).
In the presence of oxygen, the ferrous iron resulting from the reaction with ascorbate can be oxidized to generate superoxide (Equation (2)).
| (2) |
The superoxide radical can then be dismuted by superoxide dismutase (SOD) to produce H2O2 and O2 (Equation (3)).
| (3) |
H2O2 is the central cytotoxic product of high-dose ascorbate. This large flux of H2O2 generated after administration of pharmacological ascorbate (intravenous, typically tens of grams ascorbate) is then able to re-reduce ferric iron formed in reactions 2 and 4, setting up a detrimental redox cycle. The oxidants formed by the oxidation of pharmacological ascorbate can facilitate the release of even more iron into the labile iron from iron sulfur cluster-containing proteins (e.g, aconitase).
The H2O2 produced is also able to rapidly interact with catalytically active ferrous iron in classical Fenton chemistry (Equation (4)) to produce the highly reactive hydroxyl radical (HO•) and cause further cellular damage.
| (4) |
This mechanism of H2O2-mediated cytotoxicity is supported by the ability of catalase to decrease the amount of iron in catalytically active iron pools, and to mitigate the toxicity of pharmacological ascorbate by the removal of the H2O2 formed (193,195). Across multiple tumor cell lines, the ED50 of pharmacological ascorbate is directly correlated with intracellular catalase activity, whereas catalase inhibition with 3-amino-1,2,4-triazole enhanced ascorbate cancer cell killing [195]. These data all point to ascorbate being a pro-oxidant by the formation of high fluxes of H2O2 upon its oxidation. The failure of high-dose ascorbate to show cytotoxic effects may be related to enhanced H2O2 removal in untransformed cells via catalase and especially peroxiredoxins [[195], [196], [197], [198], [199], [200], [201]].
5.3. Pharmacological ascorbate therapy
In the 1970s, the potential utility of supraphysiological doses of ascorbate (pharmacological ascorbate; achieving mM plasma ascorbate concentrations) given intravenously was established in multiple trials that showed the safety and efficacy of the treatment with various terminal cancer patients [[202], [203], [204], [205]]. In contrast, two randomized, double-blind clinical trials with high-dose oral ascorbate failed to show a clinical benefit relative to placebo [206,207]. These failures significantly reduced interest in using pharmacologic ascorbate as an anticancer agent.
Subsequent studies showed that oral ascorbate administration does not achieve the mM plasma ascorbate concentrations necessary to provide anticancer effects. Following an oral dose of 200 mg, the plasma ascorbate concentration reaches ≈80 μM [[208], [209]]. Following oral administration, ascorbate concentrations saturate at ≈ 220 μM at doses ≥1000 mg [144,145]. Compare these to an intravenous ascorbate dose of 50 g, which can achieve plasma concentrations of 13.4 mM [210]. In a pilot clinical study, a 10 g intravenous dose of ascorbate reached an average plasma concentration of 1.1 mM [211]. Therefore, we currently think the most optimal way to effectively deliver pharmacological doses of ascorbate is intravenously.
Since determining efficacious delivery approaches, the field has seen a resurgence in interest for evaluating the potential anticancer capabilities of pharmacological ascorbate (Table 4). In 2004, Riordan et al. performed a pilot study of pharmacological ascorbate in late-stage cancer patients diagnosed with renal cell carcinoma, colorectal cancer, pancreatic cancer, non-Hodgkin's lymphoma, and breast cancer [147,148]. This study showed that gram dose intravenous ascorbate was safe and efficacious. The most common adverse events included nausea, edema, and dry mouth/skin. A 2008 phase I clinical trial using single-agent pharmacological ascorbate showed no significant toxicity but also failed to show any treatment responses [213]. The study concluded that although pharmacological ascorbate may be well tolerated, pharmacological ascorbate may be more beneficial as an adjuvant therapy rather than a single agent [213]. In 2014, a phase I clinical trial of 14 stage IV pancreatic cancer patients combined pharmacological ascorbate with gemcitabine to assess safety and efficacy [214]. The average overall survival was 15 ± 2 months, and the average time to progression was 26 ± 7 weeks as compared to 6 months and 9 weeks, historically [214]. Also in 2014, a phase 1 clinical trial in ovarian cancer showed that pharmacological ascorbate enhanced chemosensitivity in murine models and protected against carboplatin and paclitaxel chemotherapy-associated toxicity in human subjects [215]. A recent phase I clinical trial of newly diagnosed glioblastoma patients assessed the potential efficacy of pharmacological ascorbate with radiation and temozolomide [216]. This study reported a median overall survival of 18 months and a progression-free survival of 9.4 months, compared to the historical median overall survival of 14.6 months and progression-free survival of 6 months [217]. A phase 1 clinical trial in locally advanced pancreatic cancer that combined pharmacological ascorbate with therapeutic ionizing radiation and gemcitabine chemotherapy demonstrated a significant improvement in progression-free overall survival from 4.6 months to 13.7 months, relative to patients receiving radiation and gemcitabine alone [218].
Table 4.
Recently completed clinical trials using pharmacological ascorbate as an adjuvant therapy.
| Cancer Type | Dosage | Combination of Drugs | Results | References |
|---|---|---|---|---|
| Refractory Multiple Myeloma | 1 g ascorbate on days 1, 4, 8, and 11 of a 21-day cycle for a maximum of 8 cycles IV | Ascorbate, Bortezomib and Arsenic Trioxide | Objective responses were observed in 27% of patients (2 partial and 4 minor). | [219] |
| Lymphoid Malignancies, Relapsed and Refractory | 1000 mg ascorbate for 5 days during week 1 followed by twice weekly during weeks 2–6 IV | Ascorbate, Arsenic Trioxide | Overall median survival was 7.6 months 6% complete and partial response |
[220] |
| Advanced Stage Non-small Cell Lung Cancer | 1 cycle is 21 days: IV pharmacological ascorbate (two 75 g infusions per week, up to 4 cycles) | Ascorbate, Carboplatin, paclitaxel, and ascorbate | Imaging confirmed partial responses to therapy (n = 4), stable disease (n = 9), and disease progression (n = 1). | [193] |
| Glioblastoma | Ascorbate (from 15 to 125 g, 3 times per week for 7 weeks). Ascorbate (2 times per week, dose escalation until 20 mM plasma concentration, around ≈85 g infusion). |
Ascorbate with radiation and temozolomide | Progression-free survival 13.3 months; average overall survival 21.5 months | [193,216] |
| Late-stage Terminal Cancer Patients | 150–710 mg/kg/day for up to 8 weeks | Ascorbate only | One patient had stable disease and continued treatment for 48 weeks. IV vitamin C was considered safe |
[212] |
Aside from the enhancement of cancer therapy, in a preclinical model pharmacological ascorbate (4 g kg−1delivered intraperitoneally) was shown to mitigate radiation- and chemotherapy-induced intestinal damage [221]. Similarly, pharmacological doses of ascorbate in C57Bl/6NHsd mice prevented radiation-induced (15 Gy/1 fx) alopecia and achromotrichia [221]. These exciting observations suggest that in addition to sensitizing cancer cells to radiation and chemotherapy, pharmacological ascorbate may also ameliorate radiation-induced side effects.
6. Conclusions
The promising, preliminary translational results summarized above have led to clinical studies of the aforementioned small redox active molecules based on fundamental differences in oxidative metabolism between cancer versus normal tissues. There are still many unknown mechanistic details needed to better understand the electron movement and relationships between anti-cancer and normal tissue effects of these molecules. However, these molecules are safe and well-tolerated in humans at supraphysiological levels and are therefore excellent candidates for interventional studies. Of particular interest is the ability of redox active small molecules to function as antioxidants in normal untransformed cells while simultaneously functioning as cytotoxic pro-oxidants in neoplastic cells that can potentially both sensitize cancers to conventional therapies while protecting normal tissues opening new therapeutic windows of opportunity for improving clinical outcomes. A foundational example of this is pharmacological ascorbate, which takes advantage of dysfunctional mitochondria and increased labile iron pools in cancer cells, compared to normal cells, to generate cytotoxic fluxes of H2O2. This biphasic functionality may allow the field of cancer biology to further understand the underlying differences in electron movement in cancer cells relative to their normal cell counterparts. Due to their antioxidant/pro-oxidant potential, appropriate administration of melatonin, vitamin E, selenium, and vitamin C may offer novel, alternative strategies to enhance traditional cancer therapies, that take advantage of fundamental differences in oxidative metabolism between tumor and normal tissue.
Funding
This work was supported by NIH grants T32 CA078586, P01 CA217797, R01 CA169046, R01 CA182804, and the Gateway for Cancer Research grant G-17-1500. Core facilities were supported in part by the Carver College of Medicine and the Holden Comprehensive Cancer center, NIH P30 CA086862.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
The ESR Facility at The University of Iowa provided invaluable support. The content is solely the responsibility of the authors and does not represent views of the National Institutes of Health.
References
- 1.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metabol. 2016 Jan 12;23(1):27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y., Dean A.E., Horikoshi N., Heer C., Spitz D.R., Gius D. Emerging evidence for targeting mitochondrial metabolic dysfunction in cancer therapy. J. Clin. Invest. 2018 Aug 31;128(9):3682–3691. doi: 10.1172/JCI120844. 2018/08/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spitz D.R. Manipulations of redox metabolism for enhancing radiation therapy responses: a historical perspective and novel hypothesis. Semin. Radiat. Oncol. 2019 Jan;29(1):1–5. doi: 10.1016/j.semradonc.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petronek M.S., Spitz D.R., Buettner G.R., Allen B.G. Linking cancer metabolic dysfunction and genetic instability through the lens of iron metabolism. Cancers. 2019 Jul 30;11(8):1077. doi: 10.3390/cancers11081077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberley L.W., Buettner G.R. Role of superoxide dismutase in cancer: a review. Canc. Res. 1979 Apr 1;39(4):1141. [PubMed] [Google Scholar]
- 6.Aykin-Burns N., Ahmad I.M., Zhu Y., Oberley L.W., Spitz D.R. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem. J. 2009 Feb 15;418(1):29–37. doi: 10.1042/BJ20081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liou G.-Y., Storz P. Reactive oxygen species in cancer. Free Radic. Res. 2010 May;44(5):479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misra S., Boylan M., Selvam A., Spallholz J.E., Björnstedt M. Redox-active selenium compounds--from toxicity and cell death to cancer treatment. Nutrients. 2015 May;7(5):3536–3556. doi: 10.3390/nu7053536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buettner G.R. Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anti Canc. Agents Med. Chem. 2011 May;11(4):341–346. doi: 10.2174/187152011795677544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberley L.W., Oberley T.D., Buettner G.R. Cell differentiation, aging and cancer: the possible roles of superoxide and superoxide dismutases. Med. Hypotheses. 1980 Mar 1;6(3):249–268. doi: 10.1016/0306-9877(80)90123-1. [DOI] [PubMed] [Google Scholar]
- 11.Oberley L.W., Oberley T.D., Buettner G.R. Cell division in normal and transformed cells: the possible role of superoxide and hydrogen peroxide. Med. Hypotheses. 1981 Jan 1;7(1):21–42. doi: 10.1016/0306-9877(81)90018-9. [DOI] [PubMed] [Google Scholar]
- 12.Warburg O., Wind F., Negelein E. The metabolism OF tumors IN the body. J. Gen. Physiol. 1927 Mar 7;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu I.M.-J., Lai R.K.-H., Lin S.-H., Tse A.P.-W., Chiu D.K.-C., Koh H.-Y. Transketolase counteracts oxidative stress to drive cancer development. Proc. Natl. Acad. Sci. U.S.A. 2016 Feb 9;113(6):E725. doi: 10.1073/pnas.1508779113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuttle S.W., Varnes M.E., Mitchell J.B., Biaglow J.E. Sensitivity to chemical oxidants and radiation in CHO cell lines deficient in oxidative pentose cycle activity. Int. J. Radiat. Oncol. Biol. Phys. 1992 Jan 1;22(4):671–675. doi: 10.1016/0360-3016(92)90500-h. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Li S., Zhou Y., Meng X., Zhang J.-J., Xu D.-P. Melatonin for the prevention and treatment of cancer. Oncotarget. 2017 Jun 13;8(24):39896–39921. doi: 10.18632/oncotarget.16379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Bella G., Mascia F., Gualano L., Di Bella L. Melatonin anticancer effects: review. Int. J. Mol. Sci. 2013 Jan 24;14(2):2410–2430. doi: 10.3390/ijms14022410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Méndez I., Vázquez-Martínez O., Hernández-Muñoz R., Valente-Godínez H., Díaz-Muñoz M. Redox regulation and pro-oxidant reactions in the physiology of circadian systems. Biochimie. 2016 May;124:178–186. doi: 10.1016/j.biochi.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Legros C., Devavry S., Caignard S., Tessier C., Delagrange P., Ouvry C. Melatonin MT₁ and MT₂ receptors display different molecular pharmacologies only in the G-protein coupled state. Br. J. Pharmacol. 2014 Jan;171(1):186–201. doi: 10.1111/bph.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venegas C., García J.A., Escames G., Ortiz F., López A., Doerrier C. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012 Mar 1;52(2):217–227. doi: 10.1111/j.1600-079X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 20.Janda E., Nepveu F., Calamini B., Ferry G., Boutin J.A. Molecular pharmacology of NRH:quinone oxidoreductase 2: a detoxifying enzyme acting as an undercover toxifying enzyme. Mol. Pharmacol. 2020 Nov 1;98(5):620. doi: 10.1124/molpharm.120.000105. [DOI] [PubMed] [Google Scholar]
- 21.Bonnefont-Rousselot D., Collin F., Jore D., Gardès-Albert M. Reaction mechanism of melatonin oxidation by reactive oxygen species in vitro. J. Pineal Res. 2011 Apr 1;50(3):328–335. doi: 10.1111/j.1600-079X.2010.00847.x. [DOI] [PubMed] [Google Scholar]
- 22.Johns J.R., Platts J.A. Theoretical insight into the antioxidant properties of melatonin and derivatives. Org. Biomol. Chem. 2014;12(39):7820–7827. doi: 10.1039/c4ob01396d. [DOI] [PubMed] [Google Scholar]
- 23.McKeown S.R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 2014 Mar;87(1035) doi: 10.1259/bjr.20130676. 20130676–20130676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan D.-X., Manchester L.C., Reiter R.J., Plummer B.F., Hardies L.J., Weintraub S.T. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: a biomarker of in vivo hydroxyl radical generation. Biochem. Biophys. Res. Commun. 1998 Dec 30;253(3):614–620. doi: 10.1006/bbrc.1998.9826. [DOI] [PubMed] [Google Scholar]
- 25.Galano A., Tan D.X., Reiter R.J. On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J. Pineal Res. 2013 Apr 1;54(3):245–257. doi: 10.1111/jpi.12010. [DOI] [PubMed] [Google Scholar]
- 26.Tan D.-X., Hardeland R., Manchester L., Galano A., Reiter R. Cyclic-3-hydroxymelatonin (C3HOM), A potent antioxidant, scavenges free radicals and suppresses oxidative reactions. Curr. Med. Chem. 2013 Nov 28:21. doi: 10.2174/0929867321666131129113146. [DOI] [PubMed] [Google Scholar]
- 27.Galijasevic S., Abdulhamid I., Abu-Soud H.M. Melatonin is a potent inhibitor for myeloperoxidase. Biochemistry. 2008 Feb 1;47(8):2668–2677. doi: 10.1021/bi702016q. [DOI] [PubMed] [Google Scholar]
- 28.Karbownik M., Reiter R.J., Burkhardt S., Gitto E., Tan D.-X., Lewiñski A. Melatonin attenuates estradiol-induced oxidative damage to DNA: relevance for cancer prevention. Exp. Biol. Med. 2001 Jul 1;226(7):707–712. doi: 10.1177/153537020222600718. [DOI] [PubMed] [Google Scholar]
- 29.Karbownik M., Lewiński A. Melatonin reduces fenton reaction-induced lipid peroxidation in porcine thyroid tissue. J. Cell. Biochem. 2003 Nov 1;90(4):806–811. doi: 10.1002/jcb.10689. [DOI] [PubMed] [Google Scholar]
- 30.Wang J., Wang X., He Y., Jia L., Yang C.S., Reiter R.J. Antioxidant and pro-oxidant activities of melatonin in the presence of copper and polyphenols in vitro and in vivo. Cells. 2019 Aug 15;8(8):903. doi: 10.3390/cells8080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens R.G., Brainard G.C., Blask D.E., Lockley S.W., Motta M.E. Breast cancer and circadian disruption from electric lighting in the modern world. CA A Cancer J. Clin. 2014;64(3):207–218. doi: 10.3322/caac.21218. 2013/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang W.-S., Deng Q., Fan W.-Y., Wang W.-Y., Wang X. Light exposure at night, sleep duration, melatonin, and breast cancer: a dose–response analysis of observational studies. Eur. J. Canc. Prevent. 2014;23(4) doi: 10.1097/CEJ.0000000000000030. https://journals.lww.com/eurjcancerprev/Fulltext/2014/07000/Light_exposure_at_night,_sleep_duration,.6.aspx [DOI] [PubMed] [Google Scholar]
- 33.Stevens R.G. Electric power use and breast cancer: a hypothesis. Am. J. Epidemiol. 1987 Apr 1;125(4):556–561. doi: 10.1093/oxfordjournals.aje.a114569. [DOI] [PubMed] [Google Scholar]
- 34.Mediavilla M.D., Cos S., Sánchez-Barceló E.J. Melatonin increases p53 and p21WAFl expression in MCF-7 human breast cancer cells in vitro. Life Sci. 1999 Jun 18;65(4):415–420. doi: 10.1016/s0024-3205(99)00262-3. [DOI] [PubMed] [Google Scholar]
- 35.Schernhammer E.S., Schulmeister K. Melatonin and cancer risk: does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br. J. Canc. 2004 Mar 8;90(5):941–943. doi: 10.1038/sj.bjc.6601626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mełen-Mucha G., Winczyk K., Pawlikowski M. Somatostatin analogue octreotide and melatonin inhibit bromodeoxyuridine incorporation into cell nuclei and enhance apoptosis in the transplantable murine colon 38 cancer. Anticanc. Res. 1998;18(5A):3615–3619. [PubMed] [Google Scholar]
- 37.Kadoma Y., Fujisawa S. Radical-scavenging activity of melatonin, either alone or in combination with vitamin E, ascorbate or 2-mercaptoethanol as Co-antioxidants, using the induction period method. In vivo (athens, Greece) 2010 Nov 30;25:49–53. [PubMed] [Google Scholar]
- 38.Sánchez-Sánchez A.M., Martín V., García-Santos G., Rodríguez-Blanco J., Casado-Zapico S., Suarez-Garnacho S. Intracellular redox state as determinant for melatonin antiproliferative vs cytotoxic effects in cancer cells. 2011 Nov 1;45(11–12):1333–1341. doi: 10.3109/10715762.2011.623700. null. [DOI] [PubMed] [Google Scholar]
- 39.Sainz R.M., Mayo J.C., Tan D., León J., Manchester L., Reiter R.J. Melatonin reduces prostate cancer cell growth leading to neuroendocrine differentiation via a receptor and PKA independent mechanism. Prostate. 2005 Apr 1;63(1):29–43. doi: 10.1002/pros.20155. [DOI] [PubMed] [Google Scholar]
- 40.Aykin-Burns N., Ahmad I.M., Zhu Y., Oberley L.W., Spitz D.R. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem. J. 2009 Feb 15;418(1):29–37. doi: 10.1042/BJ20081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H.-M., Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014 Sep 1;57(2):131–146. doi: 10.1111/jpi.12162. [DOI] [PubMed] [Google Scholar]
- 42.Chul Won Y., Kim S., Lee J., sang hun L. Melatonin promotes apoptosis of colorectal cancer cells via superoxide-mediated ER stress by inhibiting cellular prion protein expression. Anticancer Res. 2018 Jul 1;38:3951–3960. doi: 10.21873/anticanres.12681. [DOI] [PubMed] [Google Scholar]
- 43.Wölfler A., Caluba H.-C., Abuja P.M., Dohr G., Schauenstein K., Liebmann P.M. Prooxidant activity of melatonin promotes fas-induced cell death in human leukemic Jurkat cells. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2001 Aug 3;502(3):127–131. doi: 10.1016/s0014-5793(01)02680-1. [DOI] [PubMed] [Google Scholar]
- 44.Radogna F., Paternoster L., De Nicola M., Cerella C., Ammendola S., Bedini A. Rapid and transient stimulation of intracellular reactive oxygen species by melatonin in normal and tumor leukocytes. Toxicol. Appl. Pharmacol. 2009 Aug 15;239(1):37–45. doi: 10.1016/j.taap.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Osseni R.A., Rat P., Bogdan A., Warnet J.-M., Touitou Y. Evidence of prooxidant and antioxidant action of melatonin on human liver cell line HepG2. Life Sci. 2000 Dec 15;68(4):387–399. doi: 10.1016/s0024-3205(00)00955-3. [DOI] [PubMed] [Google Scholar]
- 46.Bejarano I., Espino J., Barriga C., Reiter R.J., Pariente J.A., Rodríguez A.B. Pro-oxidant effect of melatonin in tumour leucocytes: relation with its cytotoxic and pro-apoptotic effects. Basic Clin. Pharmacol. Toxicol. 2011 Jan 1;108(1):14–20. doi: 10.1111/j.1742-7843.2010.00619.x. [DOI] [PubMed] [Google Scholar]
- 47.Jablonska K., Nowinska K., Piotrowska A., Partynska A., Katnik E., Pawelczyk K. Prognostic impact of melatonin receptors MT1 and MT2 in non-small cell lung cancer (NSCLC) Cancers. 2019 Jul 17;11(7):1001. doi: 10.3390/cancers11071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kast R.E. Agomelatine or ramelteon as treatment adjuncts in glioblastoma and other M1- or M2-expressing cancers. Contemp. Oncol. 2015;19(2):157–162. doi: 10.5114/wo.2015.51421. 2015/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardeland R. New approaches in the management of insomnia: weighing the advantages of prolonged-release melatonin and synthetic melatoninergic agonists. Neuropsychiatric Dis. Treat. 2009;5:341–354. doi: 10.2147/ndt.s4234. 2009/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greenblatt D.J., Harmatz J.S., Karim A. Age and gender effects on the pharmacokinetics and pharmacodynamics of ramelteon, a hypnotic agent acting via melatonin receptors MT1 and MT2. J. Clin. Pharmacol. 2007 Apr;47(4):485–496. doi: 10.1177/0091270006298602. [DOI] [PubMed] [Google Scholar]
- 51.Karim A., Tolbert D., Cao C. Disposition kinetics and tolerance of escalating single doses of ramelteon, a high-affinity MT1 and MT2 melatonin receptor agonist indicated for treatment of insomnia. J. Clin. Pharmacol. 2006 Feb;46(2):140–148. doi: 10.1177/0091270005283461. [DOI] [PubMed] [Google Scholar]
- 52.López A., García J.A., Escames G., Venegas C., Ortiz F., López L.C. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J. Pineal Res. 2009 Mar 1;46(2):188–198. doi: 10.1111/j.1600-079X.2008.00647.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang B., Liu yi, Luo Y., Niu W., Li Z.-C. Alteration of serotonin 2C receptor expression in the aorta and the pulmonary artery in rats exposed to hypoxia. Chin. J. Physiol. 2009 Jan 1;51:338–347. [PubMed] [Google Scholar]
- 54.Zhang H.-M., Zhang Y., Zhang B.-X. The role of mitochondrial complex III in melatonin-induced ROS production in cultured mesangial cells. J. Pineal Res. 2011 Jan;50(1):78–82. doi: 10.1111/j.1600-079X.2010.00815.x. 2010/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarti P., Magnifico M.C., Altieri F., Mastronicola D., Arese M. New evidence for cross talk between melatonin and mitochondria mediated by a circadian-compatible interaction with nitric oxide. Int. J. Mol. Sci. 2013 May 28;14(6):11259–11276. doi: 10.3390/ijms140611259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arese M., Magnifico M.C., Mastronicola D., Altieri F., Grillo C., Tjj Blanck. Nanomolar melatonin enhances nNOS expression and controls HaCaT-cells bioenergetics. IUBMB Life. 2012 Mar 1;64(3):251–258. doi: 10.1002/iub.603. [DOI] [PubMed] [Google Scholar]
- 57.Othman A.I., El-Missiry M.A., Amer M.A., Arafa M. Melatonin controls oxidative stress and modulates iron, ferritin, and transferrin levels in adriamycin treated rats. Life Sci. 2008 Oct 10;83(15):563–568. doi: 10.1016/j.lfs.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 58.Amini P., Mirtavoos-Mahyari H., Motevaseli E., Almirtah D., Musa A., Cheki M. Mechanisms for radioprotection by melatonin; can it be used as a radiation countermeasure? Curr. Mol. Pharmacol. 2018 Aug 2:12. doi: 10.2174/1874467211666180802164449. [DOI] [PubMed] [Google Scholar]
- 59.Vijayalaxmi Reiter R., Herman T., Meltz M. Melatonin reduces gamma radiation-induced primary DNA damage in human blood lymphocytes. Mutat. Res. 1998 Feb;397(2):203–208. doi: 10.1016/s0027-5107(97)00211-x. [DOI] [PubMed] [Google Scholar]
- 60.Rostami A., Moosavi S.A., Dianat Moghadam H., Bolookat E.R. Micronuclei assessment of the radioprotective effects of melatonin and vitamin C in human lymphocytes. Cell J. 2016;18(1):46–51. doi: 10.22074/cellj.2016.3986. 2016/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu X., Ji H., Wang Y., Gu C., Gu W., Hu L. Melatonin Alleviates Radiation-Induced Lung Injury via Regulation of miR-30e/NLRP3 Axis. Oxid. Med. Cell. Longev. 2019:4087298. doi: 10.1155/2019/4087298. 2019 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reiter R.J., Manchester L.C., Tan D.-X. Neurotoxins: free radical mechanisms and melatonin protection. Curr. Neuropharmacol. 2010 Sep;8(3):194–210. doi: 10.2174/157015910792246236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirata H., Ladenheim B., Rothman R.B., Epstein C., Cadet J.L. Methamphetamine-induced serotonin neurotoxicity is mediated by superoxide radicals. Brain Res. 1995 Apr 24;677(2):345–347. doi: 10.1016/0006-8993(95)00218-f. [DOI] [PubMed] [Google Scholar]
- 64.Ali S., Martin J., Black M., Itzhak Y. Neuroprotective role of melatonin in methamphetamine- and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity. Ann. N. Y. Acad. Sci. 1999;890:119. doi: 10.1111/j.1749-6632.1999.tb07986.x. [DOI] [PubMed] [Google Scholar]
- 65.Tocharus J., Khonthun C., Chongthammakun S., Govitrapong P. Melatonin attenuates methamphetamine-induced overexpression of pro-inflammatory cytokines in microglial cell lines. J. Pineal Res. 2010 May;48(4):347–352. doi: 10.1111/j.1600-079X.2010.00761.x. [DOI] [PubMed] [Google Scholar]
- 66.Hirata H., Asanuma M., Cadet J. Melatonin attenuates methamphetamine-induced toxic effects on dopamine and serotonin terminals in mouse brain. Synapse (New York, NY) 1998 Oct;30(2):150–155. doi: 10.1002/(SICI)1098-2396(199810)30:2<150::AID-SYN4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 67.Princ F.G., Maxit A.G., Cardalda C., Batlle A., Ana Juknat A. In vivo protection by melatonin against δ-aminolevulinic acid-induced oxidative damage and its antioxidant effect on the activity of haem enzymes. J. Pineal Res. 1998 Jan 1;24(1):1–8. doi: 10.1111/j.1600-079x.1998.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 68.Demasi M., Penatti C.A.A., Delucia R., Bechara E.J.H. The prooxidant effect of 5-aminolevulinic acid in the brain tissue of rats: implications in neuropsychiatric manifestations in porphyrias. Free Radic. Biol. Med. 1996 Jan 1;20(3):291–299. doi: 10.1016/0891-5849(95)02035-7. [DOI] [PubMed] [Google Scholar]
- 69.Lin C.-H., Huang J.-Y., Ching C.-H., Chuang J.-I. Melatonin reduces the neuronal loss, downregulation of dopamine transporter, and upregulation of D2 receptor in rotenone-induced parkinsonian rats. J. Pineal Res. 2008 Mar 1;44(2):205–213. doi: 10.1111/j.1600-079X.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 70.Di Bella G. Complete objective response to biological therapy of plurifocal breast carcinoma. Neuroendocrinol. Lett. 2009 Jan 1;29:857–866. [PubMed] [Google Scholar]
- 71.Di Bella G., Colori B. The Di Bella Method (DBM) improved survival, objective response and performance status in a retrospective observational clinical study on 23 tumours of the head and neck. Neuroendocrinol. Lett. 2012 Jan 1;33:249–256. [PubMed] [Google Scholar]
- 72.Di Bella G., Colori B., Toscano R. Complete objective response, stable for 5 years, with the Di Bella Method, of multiple-metastatic carcinoma of the breast. Neuroendocrinol. Lett. 2017 Dec;38(6):401–407. [PubMed] [Google Scholar]
- 73.Di Bella G. The Di Bella method (DBM) Neuroendocrinol. Lett. 2010 Sep 1;31(Suppl 1):1–42. [PubMed] [Google Scholar]
- 74.Harpsøe N.G., Andersen L.P.H., Gögenur I., Rosenberg J. Clinical pharmacokinetics of melatonin: a systematic review. Eur. J. Clin. Pharmacol. 2015 Aug 1;71(8):901–909. doi: 10.1007/s00228-015-1873-4. [DOI] [PubMed] [Google Scholar]
- 75.Zetner D., Andersen L., Rosenberg J. Pharmacokinetics of alternative administration routes of melatonin: a systematic review. Drug Res. 2015 Oct 30:66. doi: 10.1055/s-0035-1565083. [DOI] [PubMed] [Google Scholar]
- 76.García-Mauriño S., Pozo D., Carrillo-Vico A., Calvo J.R., Guerrero J.M. Melatonin activates Th1 lymphocytes by increasing IL-12 production. Life Sci. 1999 Oct 8;65(20):2143–2150. doi: 10.1016/s0024-3205(99)00479-8. [DOI] [PubMed] [Google Scholar]
- 77.Di Bella G., Mascia F., Ricchi A., Colori B. Evaluation of the safety and efficacy of the first-line treatment with somatostatin combined with melatonin, retinoids, vitamin D3, and low doses of cyclophosphamide in 20 cases of breast cancer: a preliminary report. Neuroendocrinol. Lett. 2013;34(7):660–668. [PubMed] [Google Scholar]
- 78.Mills E., Wu P., Seely D., Guyatt G. Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta-analysis. J. Pineal Res. 2005 Nov 1;39(4):360–366. doi: 10.1111/j.1600-079X.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y., Jin B., Ai F., Duan C., Lu Y., Dong T. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta-analysis of randomized controlled trials. Canc. Chemother. Pharmacol. 2012 May 1;69(5):1213–1220. doi: 10.1007/s00280-012-1828-8. [DOI] [PubMed] [Google Scholar]
- 80.Lissoni P., Chilelli M., Villa S., Cerizza L., Tancini G. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: a randomized trial. J. Pineal Res. 2003 Aug 1;35(1):12–15. doi: 10.1034/j.1600-079x.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 81.Lissoni P., Barni S., Meregalli S., Fossati V., Cazzaniga M., Esposti D. Modulation of cancer endocrine therapy by melatonin: a phase II study of tamoxifen plus melatonin in metastatic breast cancer patients progressing under tamoxifen alone. Br. J. Canc. 1995 Apr;71(4):854–856. doi: 10.1038/bjc.1995.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cerea G., Vaghi M., Ardizzoia A., Villa S., Bucovec R., Mengo S. Biomodulation of cancer chemotherapy for metastatic colorectal cancer: a randomized study of weekly low-dose irinotecan alone versus irinotecan plus the oncostatic pineal hormone melatonin in metastatic colorectal cancer patients progressing on 5-fluorouracil-containing combinations. Anticanc. Res. 2003 Mar 1;23:1951–1954. [PubMed] [Google Scholar]
- 83.Barni S., Lissoni P., Cazzaniga M., Ardizzoia A., Meregalli S., Fossati V. A randomized study of low-dose subcutaneous interleukin-2 plus melatonin versus supportive care alone in metastatic colorectal cancer patients progressing under 5-fluorouracil and folates. Oncology. 1995;52(3):243–245. doi: 10.1159/000227465. [DOI] [PubMed] [Google Scholar]
- 84.Hansen M.V., Andersen L.T., Madsen M.T., Hageman I., Rasmussen L.S., Bokmand S. Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Breast Canc. Res. Treat. 2014 Jun 1;145(3):683–695. doi: 10.1007/s10549-014-2962-2. [DOI] [PubMed] [Google Scholar]
- 85.Madsen M.T., Hansen M.V., Andersen L.T., Hageman I., Rasmussen L.S., Bokmand S. Effect of melatonin on sleep in the perioperative period after breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Journal Clincal Sleep Medicine. 2016 Feb;12(2):225–233. doi: 10.5664/jcsm.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen W.Y., Giobbie-Hurder A., Gantman K., Savoie J., Scheib R., Parker L.M. A randomized, placebo-controlled trial of melatonin on breast cancer survivors: impact on sleep, mood, and hot flashes. Breast Canc. Res. Treat. 2014 Jun 1;145(2):381–388. doi: 10.1007/s10549-014-2944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lund Rasmussen C., Klee Olsen M., Thit Johnsen A., Aagaard Petersen M., Lindholm H., Andersen L. Effects of melatonin on physical fatigue and other symptoms in patients with advanced cancer receiving palliative care: a double-blind placebo-controlled crossover trial. Cancer. 2015 Oct 15;121(20):3727–3736. doi: 10.1002/cncr.29563. [DOI] [PubMed] [Google Scholar]
- 88.McLaughlin P.J., Weihrauch J.L. Vitamin E contents of foods. J. Am. Diet Assoc. 1979 Jan 1:75. [PubMed] [Google Scholar]
- 89.Jiang Q. Natural forms of vitamin E as effective agents for cancer prevention and therapy. Advances in Nutrition. 2017 Nov 1;8(6):850–867. doi: 10.3945/an.117.016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aykin-Burns N., Pathak R., Boerma M., Kim T., Hauer-Jensen M. Utilization of vitamin E analogs to protect normal tissues while enhancing antitumor effects. Semin. Radiat. Oncol. 2019 Jan;29(1):55–61. doi: 10.1016/j.semradonc.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014 Jul;72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035. 2014/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yin H., Xu L., Porter N.A. Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 2011 Oct;111(10):5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 93.Pratt D.A., Tallman K.A., Porter N.A. Free radical oxidation of polyunsaturated lipids: new mechanistic insights and the development of peroxyl radical clocks. Accounts Chem. Res. 2011 Jun;44(6):458–467. doi: 10.1021/ar200024c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buettner G. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993 Feb;300(2):535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 95.Fridovich I. The biology of oxygen radicals. Science (New York, NY) 1978 Sep;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 96.Ogura Y., Yamazaki I. Steady-state kinetics of the catalase reaction in the presence of cyanide. J. Biochem. 1983 Aug;94(2):403–408. doi: 10.1093/oxfordjournals.jbchem.a134369. [DOI] [PubMed] [Google Scholar]
- 97.Atkinson J., Epand R.F., Epand R.M. Tocopherols and tocotrienols in membranes: a critical review. Free Radic. Biol. Med. 2008 Mar;44(5):739–764. doi: 10.1016/j.freeradbiomed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 98.Krumova K., Friedland S., Cosa G. How lipid unsaturation, peroxyl radical partitioning, and chromanol lipophilic tail affect the antioxidant activity of α-tocopherol: direct visualization via high-throughput fluorescence studies conducted with fluorogenic α-tocopherol analogues. J. Am. Chem. Soc. 2012 Jun 20;134(24):10102–10113. doi: 10.1021/ja301680m. [DOI] [PubMed] [Google Scholar]
- 99.Stolwijk J.M., Falls-Hubert K.C., Searby C.C., Wagner B.A., Buettner G.R. Simultaneous detection of the enzyme activities of GPx1 and GPx4 guide optimization of selenium in cell biological experiments. Redox Biol. 2020 May;32 doi: 10.1016/j.redox.2020.101518. 2020/03/29. 101518–101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wilcox A., Marnett L. Polyunsaturated fatty acid alkoxyl radicals exist as carbon-centered epoxyallylic radicals: a key step in hydroperoxide-amplified lipid peroxidation. Chem. Res. Toxicol. 1993;6(4):413–416. doi: 10.1021/tx00034a003. [DOI] [PubMed] [Google Scholar]
- 101.Girotti A. Mechanisms of lipid peroxidation. J. Free Radic. Biol. Med. 1985;1(2):87–95. doi: 10.1016/0748-5514(85)90011-x. [DOI] [PubMed] [Google Scholar]
- 102.Qian S., Wang H., Schafer F., Buettner G. EPR detection of lipid-derived free radicals from PUFA, LDL, and cell oxidations. Free Radic. Biol. Med. 2000 Sep;29(6):568–579. doi: 10.1016/s0891-5849(00)00407-x. [DOI] [PubMed] [Google Scholar]
- 103.Rhee S., Kang S., Chang T., Jeong W., Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001 Jul;52(1–2):35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- 104.Poole L.B., Hall A., Nelson K.J. Overview of peroxiredoxins in oxidant defense and redox regulation. Curr. Protoc. Toxicol. 2011 Aug doi: 10.1002/0471140856.tx0709s49. (Chapter 7):Unit7.9-Unit7.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ng C., Schafer F., Buettner G., Rodgers V. The rate of cellular hydrogen peroxide removal shows dependency on GSH: mathematical insight into in vivo H2O2 and GPx concentrations. Free Radic. Res. 2007 Nov 1;41:1201–1211. doi: 10.1080/10715760701625075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sevanian A., Kim E. Phospholiphase A2 dependent release of fatty acids from peroxidized membranes. J. Free Radic. Biol. Med. 1985 Jan 1;1(4):263–271. doi: 10.1016/0748-5514(85)90130-8. [DOI] [PubMed] [Google Scholar]
- 107.Fisher A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017 Mar;617:68–83. doi: 10.1016/j.abb.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu G., Feinstein S.I., Wang Y., Dodia C., Fisher D., Yu K. Comparison of glutathione peroxidase 1 and peroxiredoxin 6 in protection against oxidative stress in the mouse lung. Free Radic. Biol. Med. 2010 Oct;49(7):1172–1181. doi: 10.1016/j.freeradbiomed.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ralat L.A., Manevich Y., Fisher A.B., Colman R.F. Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase π with activity changes in both enzymes. Biochemistry. 2006 Jan 1;45(2):360–372. doi: 10.1021/bi0520737. [DOI] [PubMed] [Google Scholar]
- 110.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994 Apr 14;330(15):1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 111.Rautalahti M., Virtamo J., Taylor P., Heinonen O., Albanes D., Haukka J. The effects of supplementation with α‐tocopherol and β‐carotene on the incidence and mortality of carcinoma of the pancreas in a randomized, controlled trial. Cancer. 1999 Aug 1;86:37–42. [PubMed] [Google Scholar]
- 112.Virtamo J., Edwards B.K., Virtanen M., Taylor P.R., Malila N., Albanes D. Effects of supplemental alpha-tocopherol and beta-carotene on urinary tract cancer: incidence and mortality in a controlled trial (Finland) Canc. Causes Contr. 2000 Dec 1;11(10):933–939. doi: 10.1023/a:1026546803917. [DOI] [PubMed] [Google Scholar]
- 113.Lee I.-M., Cook N.R., Gaziano J.M., Gordon D., Ridker P.M., Manson J.E. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. J. Am. Med. Assoc. 2005 Jul;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 114.Lippman S.M., Klein E.A., Goodman P.J., Lucia M.S., Thompson I.M., Ford L.G. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin E cancer prevention trial (SELECT) J. Am. Med. Assoc. 2009 Jan 7;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wilson K.M., Mucci L.A. Diet and lifestyle in prostate cancer. In: Dehm S.M., Tindall D.J., editors. Prostate Cancer: Cellular and Genetic Mechanisms of Disease Development and Progression [Internet] Springer International Publishing; Cham: 2019. pp. 1–27. Available from: [DOI] [Google Scholar]
- 116.Fred Gey K. Vitamins E plus C and interacting conutrients required for optimal health. Biofactors. 1998 Jan 1;7(1‐2):113–174. doi: 10.1002/biof.5520070115. [DOI] [PubMed] [Google Scholar]
- 117.Ju J., Picinich S.C., Yang Z., Zhao Y., Suh N., Kong A.-N. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010 Apr 1;31(4):533–542. doi: 10.1093/carcin/bgp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ling M.T., Luk S.U., Al-Ejeh F., Khanna K.K. Tocotrienol as a potential anticancer agent. Carcinogenesis. 2012 Feb 1;33(2):233–239. doi: 10.1093/carcin/bgr261. [DOI] [PubMed] [Google Scholar]
- 119.Kannappan R., Gupta S.C., Kim J.H., Aggarwal B.B. Tocotrienols fight cancer by targeting multiple cell signaling pathways. Genes Nutr. 2012 Jan 1;7(1):43–52. doi: 10.1007/s12263-011-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shah S.J., Sylvester P.W. γ-Tocotrienol inhibits neoplastic mammary epithelial cell proliferation by decreasing akt and nuclear factor κB activity. Exp. Biol. Med. 2005 Apr 1;230(4):235–241. doi: 10.1177/153537020523000402. [DOI] [PubMed] [Google Scholar]
- 121.Husain K., Francois R.A., Hutchinson S.Z., Neuger A.M., Lush R., Coppola D. Vitamin E delta-tocotrienol levels in tumor and pancreatic tissue of mice after oral administration. Pharmacology. 2009;83(3):157–163. doi: 10.1159/000190792. 2009/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hiura Y., Tachibana H., Arakawa R., Aoyama N., Okabe M., Sakai M. Specific accumulation of γ- and δ-tocotrienols in tumor and their antitumor effect in vivo. J. Nutr. Biochem. 2009 Aug 1;20(8):607–613. doi: 10.1016/j.jnutbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 123.Wali V.B., Bachawal S.V., Sylvester P.W. Endoplasmic reticulum stress mediates γ-tocotrienol-induced apoptosis in mammary tumor cells. Apoptosis. 2009 Nov 1;14(11):1366–1377. doi: 10.1007/s10495-009-0406-y. [DOI] [PubMed] [Google Scholar]
- 124.Shah S., Sylvester P.W. Tocotrienol-induced caspase-8 activation is unrelated to death receptor apoptotic signaling in neoplastic mammary epithelial cells. Exp. Biol. Med. 2004 Sep 1;229(8):745–755. doi: 10.1177/153537020422900806. [DOI] [PubMed] [Google Scholar]
- 125.Jiang Q., Rao X., Kim C.Y., Freiser H., Zhang Q., Jiang Z. Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. Int. J. Canc. 2012 Feb 1;130(3):685–693. doi: 10.1002/ijc.26054. 2011/05/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang Q., Wong J., Fyrst H., Saba J.D., Ames B.N. γ-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc. Natl. Acad. Sci. U. S. A. 2004 Dec 21;101(51):17825. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yap W.N., Chang P.N., Han H.Y., Lee D.T.W., Ling M.T., Wong Y.C. γ-Tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways. Br. J. Canc. 2008 Dec 1;99(11):1832–1841. doi: 10.1038/sj.bjc.6604763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yap W.N., Zaiden N., Tan Y.L., Ngoh C.P., Zhang X.W., Wong Y.C. Id1, inhibitor of differentiation, is a key protein mediating anti-tumor responses of gamma-tocotrienol in breast cancer cells. Canc. Lett. 2010 May 28;291(2):187–199. doi: 10.1016/j.canlet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 129.Ralph S.J., Moreno-Sánchez R., Neuzil J., Rodríguez-Enríquez S. Inhibitors of succinate: quinone reductase/Complex II regulate production of mitochondrial reactive oxygen species and protect normal cells from ischemic damage but induce specific cancer cell death. Pharmaceut. Res. 2011 Nov;28(11) doi: 10.1007/s11095-011-0566-7. 2695–2730. [DOI] [PubMed] [Google Scholar]
- 130.Yan B., Stantic M., Zobalova R., Bezawork-Geleta A., Stapelberg M., Stursa J. Mitochondrially targeted vitamin E succinate efficiently kills breast tumour-initiating cells in a complex II-dependent manner. BMC Canc. 2015 May 13;15 doi: 10.1186/s12885-015-1394-7. 401–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Campbell S.E., Stone W.L., Lee S., Whaley S., Yang H., Qui M. Comparative effects of RRR-alpha- and RRR-gamma-tocopherol on proliferation and apoptosis in human colon cancer cell lines. BMC Canc. 2006 Jan 17;6(1):13. doi: 10.1186/1471-2407-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sylvester P., Shah S., Samant G. Intracellular signaling mechanisms mediating the antiproliferative and apoptotic effects of γ-tocotrienol in neoplastic mammary epithelial cells. J. Plant Physiol. 2005 Aug 1;162:803–810. doi: 10.1016/j.jplph.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 133.Park S.K., Sanders B.G., Kline K. Tocotrienols induce apoptosis in breast cancer cell lines via an endoplasmic reticulum stress-dependent increase in extrinsic death receptor signaling. Breast Canc. Res. Treat. 2010 Nov;124(2):361–375. doi: 10.1007/s10549-010-0786-2. [DOI] [PubMed] [Google Scholar]
- 134.Ryland L.K., Fox T.E., Liu X., Loughran T.P., Kester M. Dysregulation of sphingolipid metabolism in cancer. 2011 Jan 15;11(2):138–149. doi: 10.4161/cbt.11.2.14624. null. [DOI] [PubMed] [Google Scholar]
- 135.Gopalan A., Yu W., Jiang Q., Jang Y., Sanders B.G., Kline K. Involvement of de novo ceramide synthesis in gamma-tocopherol and gamma-tocotrienol-induced apoptosis in human breast cancer cells. Mol. Nutr. Food Res. 2012 Dec 1;56(12):1803–1811. doi: 10.1002/mnfr.201200350. [DOI] [PubMed] [Google Scholar]
- 136.Jang Y., Park N.-Y., Rostgaard-Hansen A.L., Huang J., Jiang Q. Vitamin E metabolite 13′-carboxychromanols inhibit pro-inflammatory enzymes, induce apoptosis and autophagy in human cancer cells by modulating sphingolipids and suppress colon tumor development in mice. Free Radic. Biol. Med. 2016 Jun 1;95:190–199. doi: 10.1016/j.freeradbiomed.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jang Y., Rao X., Jiang Q. Gamma-tocotrienol profoundly alters sphingolipids in cancer cells by inhibition of dihydroceramide desaturase and possibly activation of sphingolipid hydrolysis during prolonged treatment. J. Nutr. Biochem. 2017 Aug 1;46:49–56. doi: 10.1016/j.jnutbio.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brigelius-Flohé R., Traber M.G. Vitamin E: function and metabolism. Faseb. J. 1999 Jul 1;13(10):1145–1155. [PubMed] [Google Scholar]
- 139.Traber M.G., Leonard S.W., Bobe G., Fu X., Saltzman E., Grusak M.A. α-Tocopherol disappearance rates from plasma depend on lipid concentrations: studies using deuterium-labeled collard greens in younger and older adults. Am. J. Clin. Nutr. 2015 Apr;101(4):752–759. doi: 10.3945/ajcn.114.100966. 2015/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lim Y., Traber M.G. Alpha-tocopherol transfer protein (alpha-TTP): insights from alpha-tocopherol transfer protein knockout mice. Nutr. Res. Pract. 2007;1(4):247–253. doi: 10.4162/nrp.2007.1.4.247. 2007/12/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Burbank A.J., Duran C.G., Almond M., Wells H., Jenkins S., Jiang Q. A short course of gamma-tocopherol mitigates LPS-induced inflammatory responses in humans ex vivo. J. Allergy Clin. Immunol. 2017 Oct 1;140(4):1179–1181.e4. doi: 10.1016/j.jaci.2017.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wiser J., Alexis N.E., Jiang Q., Wu W., Robinette C., Roubey R. In vivo γ-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic. Biol. Med. 2008 Jul 1;45(1):40–49. doi: 10.1016/j.freeradbiomed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yap S.P., Yuen K.H., Wong J.W. Pharmacokinetics and bioavailability of α-, γ- and δ-tocotrienols under different food status. J. Pharm. Pharmacol. 2001 Jan 1;53(1):67–71. doi: 10.1211/0022357011775208. [DOI] [PubMed] [Google Scholar]
- 144.Springett G.M., Husain K., Neuger A., Centeno B., Chen D.-T., Hutchinson T.Z. A phase I safety, pharmacokinetic, and pharmacodynamic presurgical trial of vitamin E δ-tocotrienol in patients with pancreatic ductal neoplasia. EBioMedicine. 2015 Dec 1;2(12):1987–1995. doi: 10.1016/j.ebiom.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brigelius-Flohé R. Adverse effects of vitamin E by induction of drug metabolism. Genes Nutr. 2007 Dec;2(3):249–256. doi: 10.1007/s12263-007-0055-0. 2007/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miller E., Pastor-Barriuso R., Dalal D., Riemersma R., Appel L., Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005 Jan 4;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 147.The H.O.P.E., HOPE-TOO Trial Investigators Effects of long-term vitamin E supplementation on cardiovascular events and CancerA randomized controlled trial. J. Am. Med. Assoc. 2005 Mar 16;293(11):1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 148.Nesaretnam K., Ambra R., Selvaduray K.R., Radhakrishnan A., Reimann K., Razak G. Tocotrienol-rich fraction from palm oil affects gene expression in tumors resulting from MCF-7 cell inoculation in athymic mice. Lipids. 2004 May 1;39(5):459. doi: 10.1007/s11745-004-1251-1. [DOI] [PubMed] [Google Scholar]
- 149.Wada S., Satomi Y., Murakoshi M., Noguchi N., Yoshikawa T., Nishino H. Tumor suppressive effects of tocotrienol in vivo and in vitro. Canc. Lett. 2005 Nov 18;229(2):181–191. doi: 10.1016/j.canlet.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 150.Nesaretnam K., Selvaduray K.R., Abdul Razak G., Veerasenan S.D., Gomez P.A. Effectiveness of tocotrienol-rich fraction combined with tamoxifen in the management of women with early breast cancer: a pilot clinical trial. Breast Canc. Res. 2010 Oct 8;12(5):R81. doi: 10.1186/bcr2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kumar K.S., Raghavan M., Hieber K., Ege C., Mog S., Parra N. Preferential radiation sensitization of prostate cancer in nude mice by nutraceutical antioxidant γ-tocotrienol. Life Sci. 2006 Mar 27;78(18):2099–2104. doi: 10.1016/j.lfs.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 152.Kulkarni S., Ghosh S.P., Satyamitra M., Mog S., Hieber K., Romanyukha L. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat. Res. 2010 Mar 9;173(6):738–747. doi: 10.1667/RR1824.1. [DOI] [PubMed] [Google Scholar]
- 153.Berbée M., Fu Q., Boerma M., Wang J., Kumar K.S., Hauer-Jensen M. γ-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat. Res. 2009 May 1;171(5):596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li X.H., Ghosh S.P., Ha C.T., Fu D., Elliott T.B., Bolduc D.L. Delta-tocotrienol protects mice from radiation-induced gastrointestinal injury. Radiat. Res. 2013 Dec;180(6):649–657. doi: 10.1667/RR13398.1. [DOI] [PubMed] [Google Scholar]
- 155.Lin Y., Spallholz J.E. Generation of reactive oxygen species from the reaction of selenium compounds with thiols and mammary tumor cells. Biochem. Pharmacol. 1993 Jan 26;45(2):429–437. [PubMed] [Google Scholar]
- 156.Seko Y., Imura N. Active oxygen generation as a possible mechanism of selenium toxicity. Biomed. Environ. Sci.: BES (Biomed. Environ. Sci.) 1997 Oct 1;10:333–339. [PubMed] [Google Scholar]
- 157.Lafin J.T., Sarsour E.H., Kalen A.L., Wagner B.A., Buettner G.R., Goswami P.C. Methylseleninic acid induces lipid peroxidation and radiation sensitivity in head and neck cancer cells. Int. J. Mol. Sci. 2019 Jan 8;20(1):225. doi: 10.3390/ijms20010225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lu J., Kaeck M., Jiang C., Wilson A., Thompson H. Selenite induction of DNA strand breaks and apoptosis in mouse leukemic L1210 cells. Biochem. Pharmacol. 1994 Apr;47(9):1531–1535. doi: 10.1016/0006-2952(94)90528-2. [DOI] [PubMed] [Google Scholar]
- 159.Yan L., Frenkel G. Inhibition of cell attachment by selenite. Canc. Res. 1992 Oct;52(20):5803–5807. [PubMed] [Google Scholar]
- 160.Garberg P., Ståhl A., Warholm M., Högberg J. Studies of the role of DNA fragmentation in selenium toxicity. Biochem. Pharmacol. 1988 Sep;37(18):3401–3406. doi: 10.1016/0006-2952(88)90688-0. [DOI] [PubMed] [Google Scholar]
- 161.Peyroche G., Saveanu C., Dauplais M., Lazard M., Beuneu F., Decourty L. Sodium selenide toxicity is mediated by O2-dependent DNA breaks. PloS One. 2012;7(5) doi: 10.1371/journal.pone.0036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wycherly B.J., Moak M.A., Christensen M.J. High dietary intake of sodium selenite induces oxidative DNA damage in rat liver. Nutr. Canc. 2004;48(1):78–83. doi: 10.1207/s15327914nc4801_11. [DOI] [PubMed] [Google Scholar]
- 163.Stewart M., Spallholz J., Neldner K., Pence B. Selenium compounds have disparate abilities to impose oxidative stress and induce apoptosis. Free Radic. Biol. Med. 1999 Jan;26(1–2):42–48. doi: 10.1016/s0891-5849(98)00147-6. [DOI] [PubMed] [Google Scholar]
- 164.Wallenberg M., Misra S., Wasik A.M., Marzano C., Björnstedt M., Gandin V. Selenium induces a multi-targeted cell death process in addition to ROS formation. J. Cell Mol. Med. 2014 Apr;18(4):671–684. doi: 10.1111/jcmm.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stolwijk J.M., Garje R., Sieren J.C., Buettner G.R., Zakharia Y. Understanding the redox biology of selenium in the search of targeted cancer therapies. Antioxidants (Basel) 2020 May;9(5) doi: 10.3390/antiox9050420. http://europepmc.org/abstract/MED/32414091 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Garje R., An J.J., Sanchez K., Greco A., Stolwijk J., Devor E. Current landscape and the potential role of hypoxia-inducible factors and selenium in clear cell renal cell carcinoma treatment. Int. J. Mol. Sci. 2018 Dec;19(12) doi: 10.3390/ijms19123834. http://europepmc.org/abstract/MED/30513765 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Garje R., Brown J.A., Nepple K.G., Dahmoush L., Bellizzi A., Bonner J. Preliminary results of phase I clinical trial of high doses of seleno-L-methionine (SLM) in sequential combination with axitinib in previously treated and relapsed clear cell renal cell carcinoma (ccRCC) patients. J. Clin. Orthod. 2019 Feb 26;37(7_suppl) 660–660. [Google Scholar]
- 168.Bhattacharya A., Seshadri M., Oven S.D., Tóth K., Vaughan M.M., Rustum Y.M. Tumor vascular maturation and improved drug delivery induced by methylselenocysteine leads to therapeutic synergy with anticancer drugs. Clin. Canc. Res.: Off. J. Am. Assoc. Canc. Res. 2008 Jun;14(12):3926–3932. doi: 10.1158/1078-0432.CCR-08-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cao S., Durrani F.A., Rustum Y.M. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin. Canc. Res.: Off. J. Am. Assoc. Canc. Res. 2004 Apr;10(7):2561–2569. doi: 10.1158/1078-0432.ccr-03-0268. [DOI] [PubMed] [Google Scholar]
- 170.Bhattacharya A., Tóth K., Sen A., Seshadri M., Cao S., Durrani F.A. Inhibition of colon cancer growth by methylselenocysteine-induced angiogenic chemomodulation is influenced by histologic characteristics of the tumor. Clin. Colorectal Canc. 2009 Jul;8(3):155–162. doi: 10.3816/CCC.2009.n.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tóth K., Chintala S., Rustum Y.M. Constitutive expression of HIF-α plays a major role in generation of clear-cell phenotype in human primary and metastatic renal carcinoma. Appl. Immunohistochem. Mol. Morphol.: Appl. Immunohistochem. Mol. Morphol. AIMM. 2014 Oct;22(9):642–647. doi: 10.1097/PAI.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chintala S., Najrana T., Toth K., Cao S., Durrani F.A., Pili R. Prolyl hydroxylase 2 dependent and Von-Hippel-Lindau independent degradation of Hypoxia-inducible factor 1 and 2 alpha by selenium in clear cell renal cell carcinoma leads to tumor growth inhibition. BMC Canc. 2012 Jul 17;12 doi: 10.1186/1471-2407-12-293. 293–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chintala S., Tóth K., Cao S., Durrani F.A., Vaughan M.M., Jensen R.L. Se-methylselenocysteine sensitizes hypoxic tumor cells to irinotecan by targeting hypoxia-inducible factor 1alpha. Canc. Chemother. Pharmacol. 2010 Oct;66(5):899–911. doi: 10.1007/s00280-009-1238-8. 2010/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cho H., Du X., Rizzi J.P., Liberzon E., Chakraborty A.A., Gao W. On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models. Nature. 2016 Nov 3;539(7627):107–111. doi: 10.1038/nature19795. 2016/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen W., Hill H., Christie A., Kim M.S., Holloman E., Pavia-Jimenez A. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016 Nov;539(7627):112–117. doi: 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zakharia* Yousef, Garje Rohan G., Brown James, Nepple Kenneth, Andrew Bellizzi, Bonner Jaime PD39-02 RESULTS OF phase 1 clinical trial OF high doses OF seleno-l-methionine (SLM) IN sequential combination with axitinib IN previously treated and relapsed clear cell renal carcinoma (ccrcc) patients. J. Urol. 2020 Apr 1;203(Supplement 4) e808–e808. [Google Scholar]
- 177.Burk R., Hill K., Motley A. Plasma selenium in specific and non-specific forms. Biofactors. 2001;14(1–4):107–114. doi: 10.1002/biof.5520140115. [DOI] [PubMed] [Google Scholar]
- 178.Muecke R., Schomburg L., Glatzel M., Berndt-Skorka R., Baaske D., Reichl B. Multicenter, phase 3 trial comparing selenium supplementation with observation in gynecologic radiation oncology. Int. J. Radiat. Oncol. Biol. Phys. 2010 Nov 1;78(3):828–835. doi: 10.1016/j.ijrobp.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 179.Muecke R., Micke O., Schomburg L., Glatzel M., Reichl B., Kisters K. Multicenter, phase III trial comparing selenium supplementation with observation in gynecologic radiation oncology: follow-up analysis of the survival data 6 Years after cessation of randomization. Integr. Canc. Ther. 2014 Jul 11;13(6):463–467. doi: 10.1177/1534735414541963. [DOI] [PubMed] [Google Scholar]
- 180.Stolwijk J.M., Falls-Hubert K.C., Searby C.C., Wagner B.A., Buettner G.R. Simultaneous detection of the enzyme activities of GPx1 and GPx4 guide optimization of selenium in cell biological experiments. Redox Biol. 2020 May;32 doi: 10.1016/j.redox.2020.101518. 2020/03/29. 101518–101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hondal R., Motley A., Hill K., Burk R. Failure of selenomethionine residues in albumin and immunoglobulin G to protect against peroxynitrite. Arch. Biochem. Biophys. 1999 Nov;371(1):29–34. doi: 10.1006/abbi.1999.1435. [DOI] [PubMed] [Google Scholar]
- 182.Svirbely J.L., Szent-Györgyi A. The chemical nature of vitamin C. Biochem. J. 1933;27(1):279–285. [PMC free article] [PubMed] [Google Scholar]
- 183.Du J., Cullen J.J., Buettner G.R. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta Rev. Canc. 2012 Dec 1;1826(2):443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Burns J.J. Missing step in man, monkey and Guinea pig required for the biosynthesis of L-ascorbic acid. Nature. 1957 Sep 1;180(4585) doi: 10.1038/180553a0. 553–553. [DOI] [PubMed] [Google Scholar]
- 185.Hercberg S., Galan P., Preziosi P., Bertrais S., Mennen L., Malvy D. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch. Intern. Med. 2004 Nov;164(21):2335–2342. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 186.Hercberg S., Ezzedine K., Guinot C., Preziosi P., Galan P., Bertrais S. Antioxidant supplementation increases the risk of skin cancers in women but not in men. J. Nutr. 2007 Sep 1;137(9):2098–2105. doi: 10.1093/jn/137.9.2098. [DOI] [PubMed] [Google Scholar]
- 187.Kirsh V.A., Hayes R.B., Mayne S.T., Chatterjee N., Subar A.F., Dixon L.B. Supplemental and dietary vitamin E, β-carotene, and vitamin C intakes and prostate cancer risk. JNCI: J. Natl. Cancer Inst. 2006 Feb 15;98(4):245–254. doi: 10.1093/jnci/djj050. [DOI] [PubMed] [Google Scholar]
- 188.Lee B., Oh S.-W., Myung S.-K. Efficacy of vitamin C supplements in prevention of cancer: a meta-analysis of randomized controlled trials. Kor. J. Fam. Med. 2015 Nov;36(6):278–285. doi: 10.4082/kjfm.2015.36.6.278. 2015/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chen Q., Espey M.G., Krishna M.C., Mitchell J.B., Corpe C.P., Buettner G.R. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. U. S. A. 2005 Sep 20;102(38):13604–13609. doi: 10.1073/pnas.0506390102. 2005/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Du J., Martin S.M., Levine M., Wagner B.A., Buettner G.R., Wang S. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin. Canc. Res. 2010 Jan 15;16(2):509–520. doi: 10.1158/1078-0432.CCR-09-1713. 2010/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen Q., Espey M.G., Sun A.Y., Lee J.-H., Krishna M.C., Shacter E. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid <em>in vivo</em>. Proc. Natl. Acad. Sci. U.S.A. 2007 May 22;104(21):8749. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen Q., Espey M.G., Sun A.Y., Pooput C., Kirk K.L., Krishna M.C. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. U. S. A. 2008 Aug 12;105(32):11105–11109. doi: 10.1073/pnas.0804226105. 2008/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schoenfeld J.D., Sibenaller Z.A., Mapuskar K.A., Wagner B.A., Cramer-Morales K.L., Furqan M. O(2)(⋅-) and H(2)O(2)-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Canc. Cell. 2017 Apr 10;31(4):487–500.e8. doi: 10.1016/j.ccell.2017.02.018. 2017/03/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schoenfeld J.D., Sibenaller Z.A., Mapuskar K.A., Bradley M.D., Wagner B.A., Buettner G.R. Redox active metals and H2O2 mediate the increased efficacy of pharmacological ascorbate in combination with gemcitabine or radiation in pre-clinical sarcoma models. Redox Biol. 2018 Apr 1;14:417–422. doi: 10.1016/j.redox.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Doskey C.M., Buranasudja V., Wagner B.A., Wilkes J.G., Du J., Cullen J.J. Tumor cells have decreased ability to metabolize H2O2: implications for pharmacological ascorbate in cancer therapy. Redox Biol. 2016 Dec;10:274–284. doi: 10.1016/j.redox.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nicolussi A., D’inzeo S., Capalbo C., Giannini G., Coppa A. The role of peroxiredoxins in cancer (Review) Mol. Clin. Oncol. 2017 Feb 1;6(2):139–153. doi: 10.3892/mco.2017.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yanagawa T., Ishikawa T., Ishii T., Tabuchi K., Iwasa S., Bannai S. Peroxiredoxin I expression in human thyroid tumors. Canc. Lett. 1999 Oct 18;145(1):127–132. doi: 10.1016/s0304-3835(99)00243-8. [DOI] [PubMed] [Google Scholar]
- 198.Mishra M., Jiang H., Wu L., Chawsheen H.A., Wei Q. The sulfiredoxin-peroxiredoxin (Srx-Prx) axis in cell signal transduction and cancer development. Canc. Lett. 2015 Oct 1;366(2):150–159. doi: 10.1016/j.canlet.2015.07.002. 2015/07/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lee D.J., Kang D.H., Choi M., Choi Y.J., Lee J.Y., Park J.H. Peroxiredoxin-2 represses melanoma metastasis by increasing E-cadherin/β-catenin complexes in adherens junctions. Canc. Res. 2013 Aug 1;73(15):4744. doi: 10.1158/0008-5472.CAN-12-4226. [DOI] [PubMed] [Google Scholar]
- 200.Fernandez-Ranvier G.G., Weng J., Yeh R.-F., Shibru D., Khafnashar E., Chung K.-W. Candidate diagnostic markers and tumor suppressor genes for adrenocortical carcinoma by expression profile of genes on chromosome 11q13. World J. Surg. 2008 May 1;32(5):873–881. doi: 10.1007/s00268-008-9521-0. [DOI] [PubMed] [Google Scholar]
- 201.Nicolussi A., D'Inzeo S., Mincione G., Buffone A., Marcantonio Di, Carmela M., Cotellese R. PRDX1 and PRDX6 are repressed in papillary thyroid carcinomas via BRAF V600E-dependent and -independent mechanisms. Int. J. Oncol. 2014 Feb 1;44(2):548–556. doi: 10.3892/ijo.2013.2208. [DOI] [PubMed] [Google Scholar]
- 202.Cameron E., Campbell A. The orthomolecular treatment of cancer II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem. Biol. Interact. 1974 Oct 1;9(4):285–315. doi: 10.1016/0009-2797(74)90019-2. [DOI] [PubMed] [Google Scholar]
- 203.Cameron E., Campbell A., Jack T. The orthomolecular treatment of cancer: III. Reticulum cell sarcoma: double complete regression induced by high-dose ascorbic acid therapy. Chem. Biol. Interact. 1975 Nov 1;11(5):387–393. doi: 10.1016/0009-2797(75)90007-1. [DOI] [PubMed] [Google Scholar]
- 204.Cameron E., Pauling L. Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. U. S. A. 1976 Oct;73(10):3685–3689. doi: 10.1073/pnas.73.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cameron E., Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. U. S. A. 1978 Sep;75(9):4538–4542. doi: 10.1073/pnas.75.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Creagan E., Moertel C., O'Fallon J., Schutt A., O'Connell M., Rubin J. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N. Engl. J. Med. 1979 Sep;301(13):687–690. doi: 10.1056/NEJM197909273011303. [DOI] [PubMed] [Google Scholar]
- 207.Moertel C.G., Fleming T.R., Creagan E.T., Rubin J., O'Connell M.J., Ames M.M. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had No prior chemotherapy. N. Engl. J. Med. 1985 Jan 17;312(3):137–141. doi: 10.1056/NEJM198501173120301. [DOI] [PubMed] [Google Scholar]
- 208.Graumlich J., Ludden T., Conry-Cantilena C., Cantilena L., Wang Y., Levine M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharmaceut. Res. 1997 Sep;14(9):1133–1139. doi: 10.1023/a:1012186203165. [DOI] [PubMed] [Google Scholar]
- 209.Levine M., Conry-Cantilena C., Wang Y., Welch R., Washko P., Dhariwal K. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. U. S. A. 1996 Apr;93(8):3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Padayatty S.J., Sun H., Wang Y., Riordan H.D., Hewitt S.M., Katz A. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann. Intern. Med. 2004 Apr;140(7):533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 211.Riordan H.D., Riordan N.H., Jackson J.A., Casciari J.J., Hunninghake R., González M.J. Intravenous vitamin C as a chemotherapy agent: a report on clinical cases. Puert. Rico Health Sci. J. 2004 Jun;23(2):115–118. [PubMed] [Google Scholar]
- 212.Riordan H.D., Casciari J.J., González M.J., Riordan N.H., Miranda-Massari J.R., Taylor P. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. Puert. Rico Health Sci. J. 2005 Dec;24(4):269–276. [PubMed] [Google Scholar]
- 213.Hoffer L., Levine M., Assouline S., Melnychuk D., Padayatty S., Rosadiuk K. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol.: Off. J. Eur. Soc. Med. Oncol. 2008 Nov;19(11):1969–1974. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- 214.Welsh J., Wagner B., van’t Erve T., Zehr P., Berg D., Halfdanarson T. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Canc. Chemother. Pharmacol. 2013 Mar;71(3):765–775. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ma Y., Chapman J., Levine M., Polireddy K., Drisko J., Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 2014 Feb 5;6(222):222ra18. doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 216.Allen B.G., Bodeker K.L., Smith M.C., Monga V., Sandhu S., Hohl R. First-in-Human phase I clinical trial of pharmacologic ascorbate combined with radiation and temozolomide for newly diagnosed glioblastoma. Clin. Canc. Res. 2019 Nov 15;25(22):6590. doi: 10.1158/1078-0432.CCR-19-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J.B. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005 Mar 10;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 218.Alexander M.S., Wilkes J.G., Schroeder S.R., Buettner G.R., Wagner B.A., Du J. Pharmacologic ascorbate reduces radiation-induced normal tissue toxicity and enhances tumor radiosensitization in pancreatic cancer. Canc. Res. 2018 Dec 15;78(24):6838–6851. doi: 10.1158/0008-5472.CAN-18-1680. 2018/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Berenson J.R., Matous J., Swift R.A., Mapes R., Morrison B., Yeh H.S. A phase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin. Canc. Res.: Off. J. Am. Assoc. Canc. Res. 2007 Mar;13(6):1762–1768. doi: 10.1158/1078-0432.CCR-06-1812. [DOI] [PubMed] [Google Scholar]
- 220.Chang J., Voorhees P., Kolesar J., Ahuja H., Sanchez F., Rodriguez G. Phase II study of arsenic trioxide and ascorbic acid for relapsed or refractory lymphoid malignancies: a Wisconsin Oncology Network study. Hematol. Oncol. 2009 Mar;27(1):11–16. doi: 10.1002/hon.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Schoenfeld J.D., Alexander M.S., Waldron T.J., Sibenaller Z.A., Spitz D.R., Buettner G.R. Pharmacological ascorbate as a means of sensitizing cancer cells to radio-chemotherapy while protecting normal tissue. Semin. Radiat. Oncol. 2019 Jan 1;29(1):25–32. doi: 10.1016/j.semradonc.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]