Abstract
The clinical outcome of advanced-stage Extranodal NK/T cell lymphoma (ENKTL) patients using conventional chemotherapy is extremely poor. The aim of this study was to investigate the outcomes of advanced-stage ENKTL patients treated with non-anthracycline-based chemotherapy followed by upfront autologous stem cell transplant (ASCT). From 8 institutions, 27 patients were recruited from February 2016 to May 2019. Patients were treated with 4 cycles of VIDL induction chemotherapy. Patients who achieved complete response (CR) or partial response (PR) underwent upfront ASCT. This study is registered with clinicaltrial.gov, # NCT02544425. Twenty patients (74.1%) completed 4 cycles of VIDL induction. The overall response rate of VIDL was 74.1%, including 17 (63.0%) with CR and 3 (11.1%) with PR. Primary toxicity of the induction regimen was grade 3 or 4 neutropenia, and no treatment-related mortality was reported. Seventeen patients proceeded with upfront ASCT, and 9 patients relapsed after ASCT, among whom, 4 was central nervous system (CNS) relapse. The median duration of response was 15.2 months (95% CI, 6.3–24.1 months). This study suggested that VIDL induction chemotherapy followed by upfront ASCT is feasible and effective for the treatment of advanced-stage ENKTL. However, CNS relapse prevention is needed in the treatment of advanced-stage ENKTL.
Subject terms: T-cell lymphoma, Phase II trials
Extranodal NK/T cell lymphoma (ENKTL) is a rare subtype of non-Hodgkin’s lymphoma. Patients with advanced-stage ENKTL have particularly poor responses to chemotherapy, and almost all eventually relapse after treatment. Nevertheless, evidence-based standard therapy has not been established because of the rarity of the disease and lack of prospective trials [1]. Recently, we reported that phase II study involving concurrent chemoradiotherapy followed by VIDL is an effective and tolerable treatment strategy for localized ENKTL with acceptable toxicity [2]. Therefore, this phase II trial investigated that clinical outcome of four cycles of VIDL induction chemotherapy followed by upfront autologous stem cell transplantation (ASCT) for the treatment of advanced-stage ENKTL.
Patients with newly diagnosed advanced-stage ENTKL who are eligible for ASCT were included. Patients with aggressive NK/T cell leukemia, and those with central nervous system (CNS) involvement of NK/T cell lymphoma or with signs of spinal cord compression were excluded. All pathologic specimens were reviewed and re-classified by central review in accordance with the WHO criteria for pathologic diagnosis [3]—positive immunohistochemical expression of cytoplasmic CD3, CD56, and cytotoxic molecules and positive EBV in situ hybridization results. The overall response rate (ORR) of patients with advanced-stage NK/T cell lymphoma treated with an anthracycline-based combination chemotherapeutic regimen was estimated to be 10–20% [4]. Aiming to improve the ORR by 45%, binomial analysis for non-inferiority was conducted, and therefore 24 eligible patients were required. This study was approved by both the Protocol Review Committee and Institutional Review Board of each participating institution, in accordance with the Declaration of Helsinki. All patients provided written informed consent before enrollment. (clinicaltrial.gov, # NCT02544425).
Patients were treated with 4 cycles of VIDL regimen (etoposide (100 mg/m2), ifosfamide (1200 mg/m2), and dexamethasone (40 mg) for 3 days, followed by intramuscular injection of L-asparaginase (4000 IU/m2) every other day from day 8 to 20, repeated every 4 weeks), and the patients who achieved complete response (CR) or partial response (PR) after induction chemotherapy were assigned to high-dose chemotherapy and upfront ASCT. Peripheral blood stem cell mobilization and collection were conducted according to each participating institution’s policy. Patients were administrated a high-dose therapy using either busulfan, cyclophosphamide, and etoposide (BuCyE) or busulfan, melphalan, and etoposide (BuMelE) before ASCT.
Response assessment was performed within 3–4 weeks after completion of the second and fourth cycle of VIDL, and after completion of ASCT and whenever disease progression was suspected using blood EBV DNA titer and a CT scan of initially involved sites. A PET/CT scan was mandatory at the time of diagnosis, after the fourth cycle of VIDL, and after completion of ASCT. Responses were assessed according to the revised International Workshop Criteria [5]. Toxicity was assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 for every VIDL cycle and ASCT period.
From 9 independent institutions, 27 patients with advanced-stage ENTKL were recruited from February 2016 to May 2019. The median age was 55 years (range, 30–64 years). Five patients (18.5%) were classified as Ann arbor stage III, and 22 (81.5%) as stage IV. Twenty-five patients (92.6%) were assigned to high risk according to prognostic index for natural killer cell lymphoma (PINK), and 25 patients (92.6%) were assigned to high risk according to prognostic index for natural killer cell lymphoma–EBV, which incorporated EBV DNA data to PINK [6]. All patients were newly diagnosed and did not receive any treatment prior to study enrollment. All patients except one had positive EBV DNA titer quantification at diagnosis. The median EBV DNA titer was 16,844 copies/mL (range 0–3,066,623).
Twenty patients (74.1%) completed four courses of VIDL, and five patients (18.5%) progressed after 2 cycles of VIDL. One patient (3.7%) withdrew consent after 1 cycle of VIDL. Finally, 17 patients (63.0%) proceeded to upfront ASCT after the completion of 4 cycles of VIDL chemotherapy. All 17 patients were administered chemo-mobilization using etoposide and G-CSF. Two patients used plerixafor, however there was no case of collection failure. For the conditioning regimen before ASCT, 15 patients (88.2%) received BuCyE and 2 patients (11.8%) received BuMelE. The median infused CD34+ cell count was 7.0 × 106 CD34+/kg (range, 2.6–26.5 × 106 CD34+/kg). There were no cases of engraftment failure.
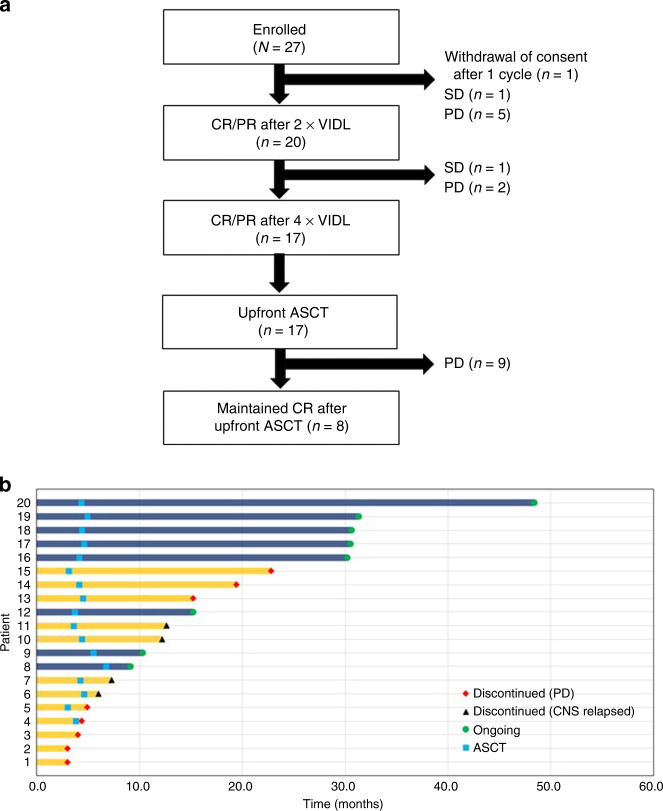
Among the 27 eligible patients, CR was achieved in 17 patients (63.0%), PR in 3 patients (11.1%), stable disease (SD) in 1 patient (3.7%), progressive disease (PD) in 5 patients (18.5%), and non-measurable in 1 patient (3.7%). The ORR was 74.1% (95% CI 57.6–90.6%). After a median follow-up duration of 31.2 months (range, 9.8–49.4 months), 18 patients (66.7%) experienced disease progression or relapse. Nine patients progressed before ASCT and nine patients relapsed after ASCT. The flowing chart of this study cohort is described in Fig. 1A. Among the 18 patients who underwent disease progression or relapse, four experienced only CNS relapses without systemic involvement of lymphoma. Median DOR was 15.2 months (95% CI, 6.3–24.1 months), and eight patients (29.6%) are still in CR (Fig. 1B). The median PFS was 13.2 months (95% CI, 5.7–20.7 months), and median OS was 27.0 months (95% CI, 7.8–46.2 months). Some transplanted patients could achieve long-term disease free survival compare to previous studies [7, 8].
Fig. 1. Flowing chart of the study cohort showing the numbers and types of treatment failures and responding patients throughout the different stages of the protocoled treatment algorithm.
VIDL etoposide, ifosfamide, dexamethasone, L-asparginase; CR complete remission, PR partial remission, SD stable disease; PD progressive disease, ASCT autologous stem cell transplantation (A) and duration of response (DOR) of the patients who achieved complete or partial response (B).
During a total of 92 cycles of VIDL chemotherapy, the most common hematologic toxicity was neutropenia (26.1%), followed by anemia (16.3%) and thrombocytopenia (15.2%). Among the non-hematologic toxicities, nausea (15.2%) was the most common adverse event and anorexia (14.1%), infection (7.6%) followed. There was a case of an L-asparaginase-induced grade 2 thromboembolic event and of an L-asparaginase induced grade 3 urticaria. L-asparaginase was discontinued after these events and the patients improved after medical treatment. There were no cases of pancreatitis associated with L-asparaginase. The most common toxicity during upfront ASCT period was febrile neutropenia (Grade 1–2, 29.4%; Grade 3–4, 47.1%). Infection occurred in nine patients, but all were treated successfully with broad-spectrum antibiotics. There was no incidence of hepatic veno-occlusive disease nor treatment-related mortality.
This study evaluated the efficacy and toxicity of VIDL induction treatment followed by upfront ASCT for newly diagnosed advanced-stage ENKTL. The efficacy and survival outcome of the VIDL induction is not superior than the previous regimens such as SMILE or DDGP, which was recently reported by Chinese group [9]. However, VIDL induction chemotherapy was meaningful in that it presented relatively tolerable and effective option to treating patients with advanced-stage ENKTL and suggested the promising result of upfront ASCT as a consolidation.
Among the 18 patients with disease relapse or progression, four recurred at the CNS. In a previous study involving 208 patients with NK/T cell lymphoma, Kim et al. reported a significant association between advanced-stage and lymph node involvement with CNS disease in ENKTL [10]. It is presumed that the cause of the relatively high incidence of CNS relapse in this study was because of the absence of an effective agent for the CNS, such as methotrexate.
In conclusion, VIDL induction chemotherapy followed by upfront ASCT is effective with acceptable responses and manageable toxicities in patients with advanced-stage ENKTL. However, a high CNS recurrence rate suggests that CNS prophylaxis should be considered for the treatment of advanced-stage ENKTL.
Author contributions
Study concepts and design: D-HY, WSK. Data acquisition: All of the authors. Data analysis and interpretation: G-YS, DHY, D-HY, and WSK. Manuscript preparation: G-YS, DHY, D-HY, and WSK. Manuscript review: All of the authors.
Availability of data
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ga-Young Song, Dok Hyun Yoon, Deok-Hwan Yang, Won Seog Kim
Contributor Information
Deok-Hwan Yang, Email: drydh1685@hotmail.com.
Won Seog Kim, Email: wskimsmc@skku.edu.
References
- 1.Yhim HY, Kim JS, Mun YC, Moon JH, Chae YS, Park Y, et al. Clinical outcomes and prognostic factors of up-front autologous stem cell transplantation in patients with extranodal natural killer/t cell lymphoma. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2015;21:1597–604. doi: 10.1016/j.bbmt.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Kim SJ, Yang DH, Kim JS, Kwak JY, Eom HS, Hong DS, et al. Concurrent chemoradiotherapy followed by L-asparaginase-containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08-01 phase II study. Ann Hematol. 2014;93:1895–901. doi: 10.1007/s00277-014-2137-6. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe ES. Pathology and genetics of tumours of haematopoietic and lymphoid tissues, vol. 3. IARC, 2001.
- 4.Kohrt H, Advani R. Extranodal natural killer/T-cell lymphoma: current concepts in biology and treatment. Leuk lymphoma. 2009;50:1773–84. doi: 10.3109/10428190903186502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Yoon DH, Jaccard A, Chng WJ, Lim ST, Hong H, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016;17:389–400. doi: 10.1016/s1470-2045(15)00533-1. [DOI] [PubMed] [Google Scholar]
- 7.Au WY, Lie AKW, Liang R, Kwong YL, Yau CC, Cheung MMC, et al. Autologous stem cell transplantation for nasal NK/T-cell lymphoma: a progress report on its value. Ann Oncol. 2003;14:1673–6. doi: 10.1093/annonc/mdg458. [DOI] [PubMed] [Google Scholar]
- 8.Fox CP, Boumendil A, Schmitz N, Finel H, Luan JJ, Sucak G, et al. High-dose therapy and autologous stem cell transplantation for extra-nodal NK/T lymphoma in patients from the Western hemisphere: a study from the European Society for Blood and Marrow Transplantation. Leuk Lymphoma. 2015;56:3295–300. doi: 10.3109/10428194.2015.1037764. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Cui Y, Sun Z, Zhang L, Li L, Wang X, et al. DDGP versus SMILE in newly diagnosed advanced natural killer/T-cell lymphoma: a randomized controlled, multicenter, open-label study in China. Clin Cancer Res: Off J Am Assoc Cancer Res. 2016;22:5223–8. doi: 10.1158/1078-0432.ccr-16-0153. [DOI] [PubMed] [Google Scholar]
- 10.Kim SJ, Oh SY, Hong JY, Chang MH, Lee DH, Huh J, et al. When do we need central nervous system prophylaxis in patients with extranodal NK/T-cell lymphoma, nasal type? Ann Oncol. 2010;21:1058–63. doi: 10.1093/annonc/mdp412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.