Abstract
Purpose:
The purpose of this study was to compare the cholesteryl ester (CE) profiles expressed from human meibomian gland epithelial cells (HMGECs) in response to rosiglitazone-induced differentiation to that of normal human meibum.
Methods:
HMGECs were cultured with rosiglitazone (vehicle control, 20 μM, or 50 μM) and fetal bovine serum (FBS, 2% or 10%) for 2 days or 5 days. Following culture, lipid extracts were processed and analyzed by ESI-MSMSALL in positive ion mode. CEs were identified using both LipidView 1.2 and PeakView 2.2 (SCIEX, Framingham, MA) and compared to literature reports of CEs in normal human meibum.
Results:
There were 34 CEs with carbon number ranging from 14 to 34 detected from HMGECs. Across all conditions, HMGECs provided a CE profile that was 14.0% saturated, 60.6% monounsaturated, and 25.4% polyunsaturated. Culturing with 50 μM rosiglitazone and 2% FBS for 2 days resulted in the greatest number of upregulated saturated and monounsaturated CEs and downregulated polyunsaturated CEs. Five CEs were identified as being the most responsive to 50 μM rosiglitazone: CE 24:1, CE 28:1, CE 26:1, CE 18:1, and CE 22:1.
Conclusion:
Although differences in the CE profile exist between meibum and HMGECs, rosiglitazone promotes upregulation of highly expressed meibum-relevant CEs and shifts the saturation level toward a more meibum-like profile. The use of rosiglitazone as a differentiating agent is recommended in HMGEC research, and analysis by ESI-MSMSALL is encouraged to differentiate meibum-relevant CEs from other nonpolar distractors detected by vital stains.
Keywords: cholesteryl esters, human meibomian gland epithelial cells, mass spectrometry, meibomian gland, peroxisome proliferator activator receptor-γ (PPARγ), rosiglitazone
I. Introduction
Human meibomian gland epithelial cells (HMGECs) were first isolated, immortalized, and described in 2010.[1] HMGECs have been shown to accumulate lipids, express genes for enzymes involved in lipogenesis, and respond to androgen exposure.[1] Despite these similarities to the meibomian gland’s function in vivo, there have also been reports of scarce lipid production or aberrant lipid profiles that have prompted additional investigation into differentiating conditions.[2] Serum, azithromycin, omega-3 and −6 fatty acids, and brimonidine have all demonstrated the ability to promote differentiation and lipid production from HMGECs.[3–8] More recently, though, rosiglitazone, a peroxisome proliferator activator receptor-γ (PPARγ) agonist, has also been proposed as a differentiating agent.[9, 10]
PPARγ is a ligand-activated nuclear receptor that has been shown to regulate differentiation and lipid production in both adipocytes and sebocytes.[11, 12] PPARγ has many natural and synthetic ligands, including polyunsaturated fatty acids, select prostanoids, and the thiazolidinediones (TZD) commonly used as insulin sensitizers during anti-diabetic therapy.[13] In 2016, Jester et al. reported that PPARγ regulates differentiation and lipid synthesis in primary and immortalized mouse meibocytes.[9] Later, Kim et al. found that rosiglitazone, a TZD derivative and synthetic yet highly specific PPARγ agonist, also increases lipogenic gene expression and lipogenesis in a human model using HMGECs.[10] In the latter paper, lipid production was quantified by LipidTox staining—an efficient method for visualizing nonpolar lipid production when only a gestalt measure is necessary. While LipidTox does show specificity for nonpolar lipids, it lacks discrimination among different nonpolar lipid classes and species.[2] For this level of resolution, mass spectrometry can be used to more fully interrogate aspects of the HMGEC lipidome.
The lipidome of normal human meibum, however, has already been well characterized. Comprised of >90% nonpolar lipids, human meibum consists primarily of wax esters (WEs, 48 ± 4% of total lipid pool) and cholesteryl esters (CEs, 40 ± 2% of total lipid pool) with smaller contributions by triacylglycerols, diacylglycerols, and others.[14] For CEs specifically, there have been 28 to 58 different species detected in meibum (depending on analytical methods) with hydrocarbon chain lengths varying from 16 to 36.[14, 15] Of note, very long-chain CEs (carbon number [nc] 20 to 25) and ultra long-chain CEs (nc ≥ 26) are characteristic of meibum and sebum.[16] However, those CEs with nc ≥ 26 are absent from other lipid sources, such as plasma and epithelial cells.[17, 18] Previous reports have been in agreement that polyunsaturated CEs are of low abundance in meibum, while saturated and monounsaturated CEs are of high abundance.[14, 15, 19, 20] Taken together, the signature lipid profile of normal human meibum is predominantly nonpolar, high in CEs (and WEs), diverse in CE chain length (16 to 36), highly abundant in saturated and monounsaturated CEs, and highly abundant in CEs with nc 22 to 26.[15]
In this study, the effects of serum, rosiglitazone, and culture duration were evaluated on the lipidomic profile produced by HMGECs. We hypothesized that increased concentrations of rosiglitazone would positively influence HMGEC differentiation as determined by the upregulation of meibum-relevant CEs (saturated and monounsaturated CEs with carbon number 16 to 32).
II. Materials and Methods
A. Cell culture and lipid extraction
Immortalized HMGECs (passages 25 and 26) were maintained until 80% confluence in keratinocyte serum-free media (KSFM) with 5 ng/ml epidermal growth factor (EGF) and 50 μg/ml bovine pituitary extract (BPE). HMGECs were differentiated at a density of one million cells per 6-cm glass petri dish in Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F12 (1:1) containing 10 ng/ml EGF, fetal bovine serum (FBS, 2% or 10%), and rosiglitazone (20 μM or 50 μM, Cayman Chemical, Ann Arbor, MI), the concentrations of these latter two additives were guided by the foundational work of Jester et al. and Kim et al. performed in the same or similar cell culture systems.[9, 10] Rosiglitazone was dissolved in sterile-filtered dimethyl sulfoxide (DMSO, Hybri-Max™, Sigma-Aldrich, St. Louis, MO) as a vehicle and was added to the culture media as the last step before exposure to HMGECs. The concentration of DMSO was maintained at 0.5% across all conditions. All experiments consisted of two experimental replicates and two technical replicates.
After two days or five days, the culture media was withdrawn, and the cells were washed twice with molecular biology-grade water. A modified Folch technique was used for lipid extraction.[21, 22] Specifically, 3 mL of pre-mixed and pre-chilled chloroform-methanol (2:1 v/v at −20°C) was added directly to the adhered cells. The growth surface was scraped with a sterile stainless-steel scraper, and the resulting solution was transferred to a glass vial. Ammonium acetate in molecular biology-grade water (50 mM, 0.75 mL) was added to the samples, creating a final ratio of chloroform-methanol-water of 8:4:3, consistent with the Folch technique. The emulsion was agitated on ice at 350 rpm for 20 minutes then centrifuged at 1600 × g for 5 minutes to promote phase separation. The lower, nonpolar phase was withdrawn and kept for mass spectrometric analysis. All steps involving organic solvents were performed with glass, polytetrafluoroethylene (PTFE), or stainless steel.
B. Analysis by mass spectrometry
All samples were directly infused into a Triple TOF 5600 mass spectrometer (SCIEX, Framingham, MA) with electrospray ionization by isocratic flow at 7 μl/min. MS and MS/MSALL spectra were acquired in positive ion mode. MS/MSALL analysis was performed with the SWATH technology. Product ion MS/MS spectra for all precursor ions were acquired between 200 m/z and 1,200 m/z at every one Dalton step over six minutes. Ionization spray voltage was 5.5 kV, collision energy per step was 35 eV, and collision energy spread was 15 eV. The acquired MS and MS/MSALL data were processed and lipid species identified using LipidView 1.2 (SCIEX, Framingham, MA). Spectra were viewed and interpreted using PeakView 2.2 (SCIEX, Framingham, MA).
C. Data analysis
CEs were the predominant focus of this study. CEs are a class of lipids that consist of a cholesterol moiety esterified to a fatty acid. CEs that have no double bonds in the fatty acid are termed “saturated” CEs, as every carbon is fully saturated with hydrogens. CEs with one double bond are termed “monounsaturated” and with two or more double bonds are termed “polyunsaturated.” The labeling convention for CEs is nc:db, where nc and db are the number of carbons and double bonds, respectively, in the fatty acyl chain. For example, CE 18:1 is a monounsaturated cholesteryl ester with a fatty acyl group consisting of 18 carbons and one double bond. To be included in the analysis, a given CE species had to be present in all replicates of at least one condition. Each analyzed CE was normalized to the sum intensity and reported as percent of the overall CE pool. One-way ANOVA with Tukey post-hoc testing (SPSS v26, Armonk, NY) was used to compare means between all conditions when tests of normality (Kolmogorov-Smirnov) and homogeneity of variance (Levene’s Test) were satisfied. If Levene’s Test was violated, then Games-Howell post-hoc testing was used. If Kolmogorov-Smirnov testing was violated, then the non-parametric Kruskal Wallis test was used. A significance threshold was set at 0.05.
III. Results
A. Description of the CE profile across all conditions
There were 68 CEs detected across all samples from all conditions; however, only 34 met the criteria for inclusion in the analysis. Across all conditions, the chain length varied from 14 to 34, and the most abundant CE was CE 18:1 (Figure 1). Very long-chain (20 ≤ nc ≤ 25) and ultra long-chain CEs (nc ≥ 26) were detected from all conditions and comprised 30.6% (SD = 1.6%) of the overall CE pool. Of the 34 included CEs, seven were saturated, 12 were monounsaturated, and 15 were polyunsaturated. Monounsaturated CEs were the most abundant (60.6%, SD = 10.6%), followed by polyunsaturated (25.4%, SD = 2.2%) and saturated (14.0%, SD = 3.1%).
Figure 1.
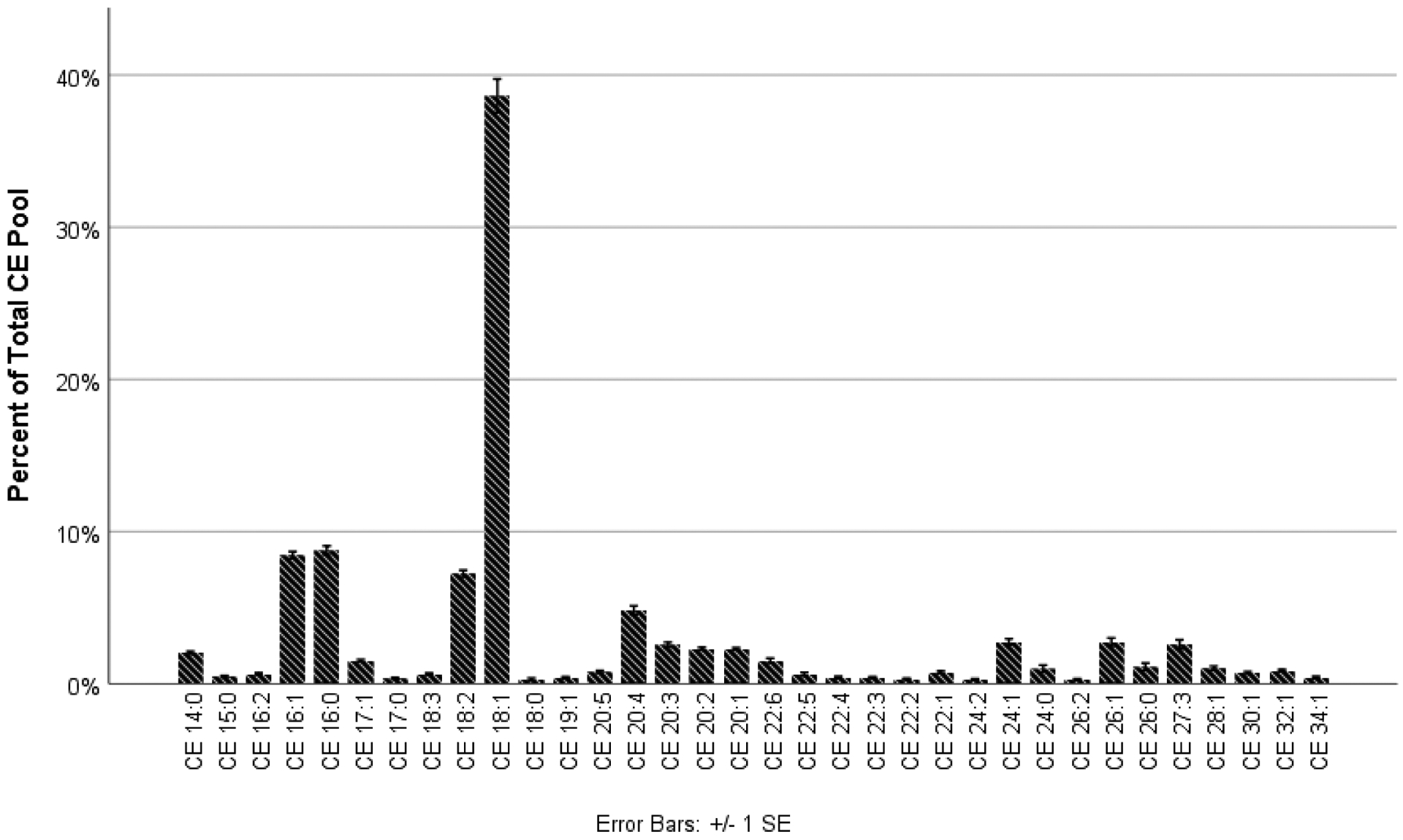
A diverse CE profile, rich in monounsaturated CEs, was quantified across all conditions in this study. CE 18:1 dominated the CE pool.
B. Comparison of the CE profile across culture conditions
HMGECs were differentiated in culture conditions containing FBS (2% or 10%) and rosiglitazone (vehicle control, 20 μM, or 50 μM) for two days or five days. Culturing with the three concentrations of rosiglitazone and 10% FBS for two days yielded significant differences among seven of the 34 CEs (Table 1). The remaining 27 CEs showed no differences (Figure 2). Of the seven that were significantly different, one was saturated (CE 18:0), three were monounsaturated (CE 26:1, CE 24:1, and CE 28:1), and three were polyunsaturated (CE 22:6, CE 16:2, and CE 22:4). For all seven CEs, culturing with 50 μM rosiglitazone increased expression of the saturated and monounsaturated CEs but decreased expression of the polyunsaturated CEs. Extending the culture duration from two days to five days did not alter expression of any CEs (Figure 3).
Table 1.
Effect of Rosiglitazone
| Cholesteryl Esters (CEs) | 10-DMSO-2d (vehicle control) | 10-20-2d | 10-50-2d | |||
|---|---|---|---|---|---|---|
| CE 18:0 | 0.28 | 0.16 | 0.30 | 0.22 | 1.76 | 0.48 |
| p = 0.037 | ||||||
| CE 24:1 | 2.16 | 0.18 | 1.96 | 0.22 | 3.91 | 0.36 |
| p = 0.009 | ||||||
| p = 0.002 | ||||||
| CE 26:1 | 2.08 | 0.47 | 2.05 | 0.26 | 4.20 | 0.56 |
| p = 0.004 | ||||||
| p = 0.003 | ||||||
| CE 28:1 | 0.52 | 0.30 | 0.67 | 0.24 | 1.93 | 0.13 |
| p = 0.003 | ||||||
| p = 0.012 | ||||||
| CE 16:2 | 0.97 | 0.22 | 0.81 | 0.17 | 0.13 | 0.08 |
| p = 0.043 | ||||||
| CE 22:6 | 3.03 | 0.19 | 3.77 | 0.22 | 1.57 | 0.29 |
| p = 0.001 | ||||||
| p < 0.001 | ||||||
| CE 22:4 | 0.95 | 0.25 | 1.41 | 0.21 | 0.19 | 0.22 |
| p = 0.048 | ||||||
| p < 0.001 | ||||||
HMGECs were cultured with a vehicle control (DMSO) or rosiglitazone (20 μM or 50 μM) and 10% FBS for two days. Culture conditions are abbreviated as FBS % – Rosiglitazone (μM) – Duration. Only the cholesteryl esters (CEs) that reached statistical significance are displayed in the table. CEs are listed with two numbers. The two numbers denote the number of carbons and double bonds in the acyl chains, respectively.
Figure 2.
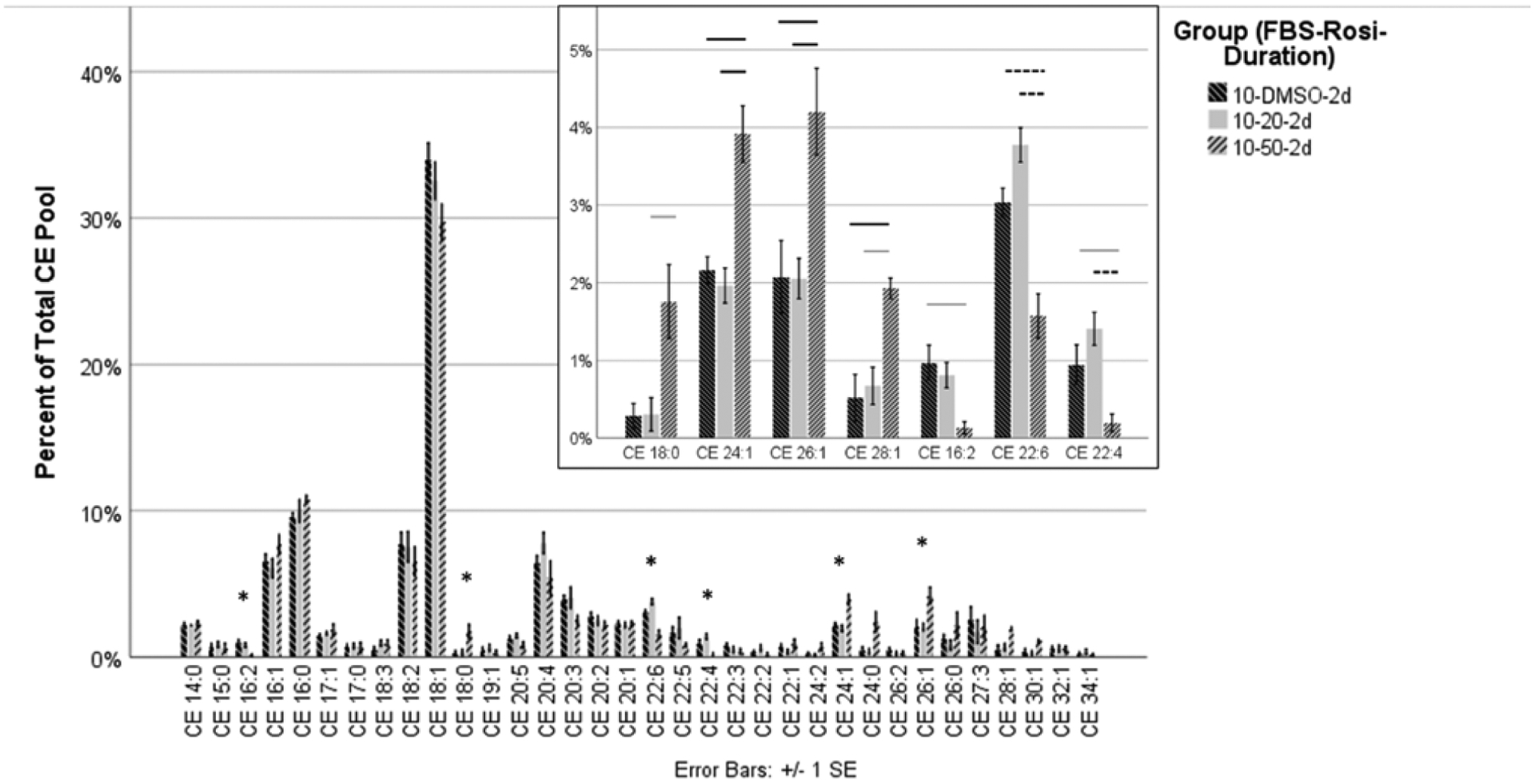
HMGECs were cultured with a vehicle control (DMSO) or rosiglitazone (20 μM or 50 μM) and FBS (2% or 10%) for two days or five days. All conditions were analyzed together as described in the section titled Materials and Methods. To ensure readability, the data are displayed across three different figures (Figures 2–4). Depicted here are the results of varying rosiglitazone levels (vehicle, 20 μM, and 50 μM) with 10% FBS for two days. There was a significant change in seven CEs following culture with 50 μM rosiglitazone (inset). The order of the CEs has been rearranged in the inset to separate saturated, monounsaturated, and polyunsaturated CEs. One saturated and three monounsaturated CEs were upregulated with 50 μM rosiglitazone, and two polyunsaturated CEs were downregulated—a pattern consistent with the CE profile in normal human meibum.
* denotes statistical significance
Gray bar denotes significance at p ≤ 0.05
Black bar denotes significance at p ≤ 0.01
Dashed line denotes significance at p ≤ 0.001
Figure 3.
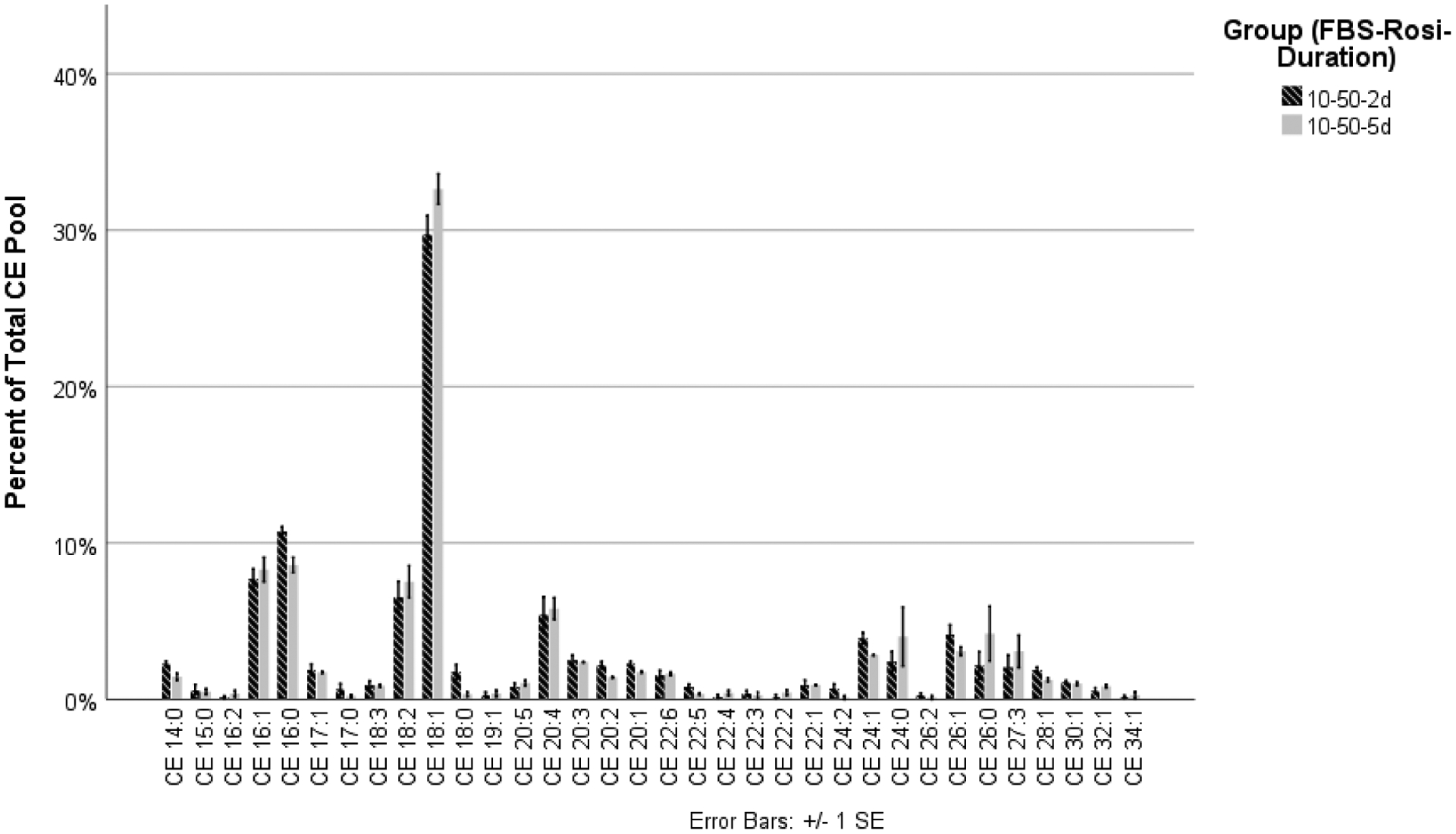
HMGECs were cultured with a vehicle control (DMSO) or rosiglitazone (20 μM or 50 μM) and FBS (2% or 10%) for two days or five days. All conditions were analyzed together as described in the section titled Materials and Methods. To ensure readability, the data are displayed across three different figures (Figures 2–4). Depicted here are the results of the two culture durations (two days and five days) with the most favorable rosiglitazone concentration (50 μM, Figure 2) and 10% FBS. Extending the culture duration of HMGECs in 10% FBS and 50 μM rosiglitazone from two days to five days revealed no changes in relative CE expression.
Reducing the serum concentration from 10% to 2% in the presence of 50 μM rosiglitazone for two days yielded significant differences among six of the 34 CEs (Table 2, Figure 4). The remaining 28 were unchanged. Of the six that were significantly different, one was saturated (CE 26:0), two were monounsaturated (CE 16:1 and CE 26:1), and three were polyunsaturated (CE 18:3, CE 22:6, and CE 22:5). Culturing with 2% FBS yielded higher abundance of monounsaturated CEs and lower abundance of polyunsaturated CEs. Culturing with 10% FBS, however, yielded a higher abundance of the saturated CE 26:0.
Table 2.
Effect of Serum Concentration
| 10-50-2d | 2-50-2d | |||
|---|---|---|---|---|
| CE 26:0 | 2.17 | 0.89 | 0.28 | 0.28 |
| p = 0.023 | ||||
| CE 16:1 | 7.70 | 0.64 | 10.70 | 0.22 |
| p = 0.004 | ||||
| CE 26:1 | 4.20 | 0.56 | 7.60 | 0.58 |
| p < 0.001 | ||||
| CE 18:3 | 0.95 | 0.23 | 0.00 | 0.00 |
| p = 0.025 | ||||
| CE 22:6 | 1.57 | 0.29 | 0.42 | 0.42 |
| p = 0.02 | ||||
| CE 22:5 | 0.83 | 0.14 | 0.14 | 0.14 |
| p = 0.033 | ||||
The decrease of 10% FBS to 2% FBS in the presence of 50 μM rosiglitazone after two days of culture resulted in a significant change in six cholesteryl esters (CEs). Only the CEs that reached statistical significance are displayed in the table. Culture conditions are abbreviated as FBS % – Rosiglitazone (μM) – Duration. CEs are listed with two numbers. The two numbers denote the number of carbons and double bonds in the acyl chains, respectively.
Figure 4.
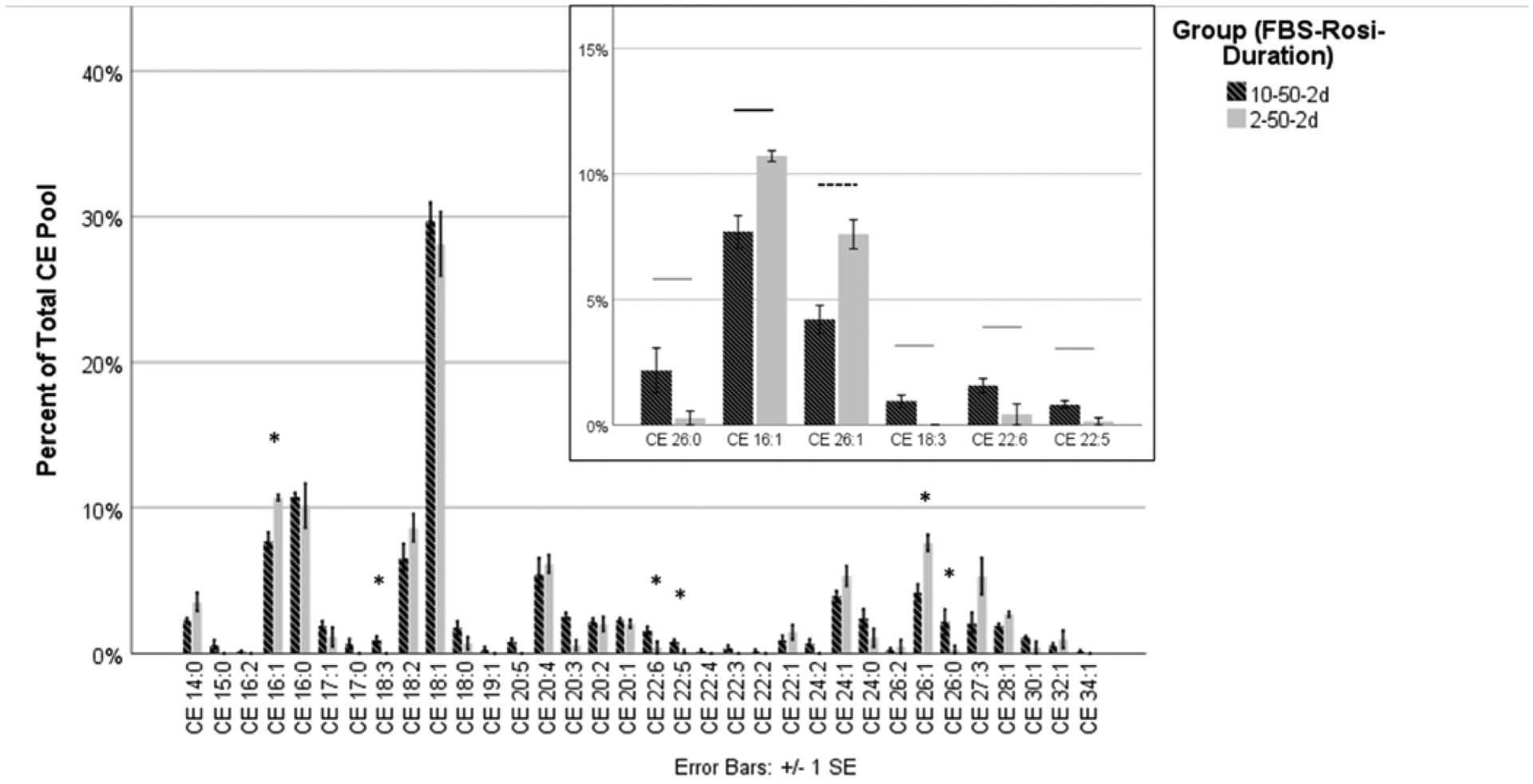
HMGECs were cultured with a vehicle control (DMSO) or rosiglitazone (20 μM or 50 μM) and FBS (2% or 10%) for two days or five days. All conditions were analyzed together as described in the section titled Materials and Methods. To ensure readability, the data are displayed across three different figures (Figures 2–4). Depicted here are the results of the two different FBS concentrations (2% or 10%) with the most favorable rosiglitazone concentration (50 μM, Figure 2) and culture duration (two days, Figure 3). The decrease of 10% FBS to 2% FBS in the presence of 50 μM rosiglitazone after two days of culture resulted in a significant change in six CEs (inset). The order of the CEs has been rearranged in the inset to separate saturated, monounsaturated, and polyunsaturated CEs. Two monounsaturated CEs were upregulated with 2% FBS, while one saturated and three monounsaturated CEs were downregulated. The CE profile of normal human meibum is high in saturated and monounsaturated CEs and low in polyunsaturated CEs.
* denotes statistical significance
Gray bar denotes significance at p ≤ 0.05
Black bar denotes significance at p ≤ 0.01
Dashed line denotes significance at p ≤ 0.001
C. Comparison of optimized culture conditions to literature reports of normal human meibum
To evaluate the performance of each condition, the number of CEs that favored each condition was summed and compared (Table 3). For this comparison, “favored” is defined to mean that a saturated or monounsatured CE was maximally increased for a given condition or a polyunsatured CE was maximally decreased for a given condition. Further examples and elaboration are provided in the caption of Table 3. Of the 34 total CEs that met the criteria for analysis, 10 had no significant differences between any of the conditions. Of the remaining 24, 10 CEs favored 50 μM rosiglitazone with 2% FBS for 2 days (denoted as 2-50-2d [FBS-rosiglitazone-duration]), meaning that saturated and monosaturated CEs were maximized while polyunsaturated CEs were minimized. The remaining 14 CEs favored a mix of seven different conditions, with no other condition being favored by more than three CEs. Culturing with 2-50-2d ultimately yielded the most favorable profile—as defined by the patterns observed in normal human meibum—for 82.4% of the CEs. All other conditions varied between 55.9% to 79.4%.
Table 3.
Summary Table
| Condition (FBS % - Rosi μM – Duration) | Number of CEs with no significant difference across all conditions | Number of CEs where given condition is superior to all other conditions | Number of CEs where given condition is equivalent to the superior condition (p > 0.05) | Number of CEs where given condition is inferior to the superior condition (p < 0.05) | Total Number of CEs | Percentage of CEs that responded optimally to each condition |
|---|---|---|---|---|---|---|
| 2-DMSO-2d | 10 | 2 | 14 | 8 | 34 | 76.5% |
| 2-DMSO-5d | 10 | 2 | 11 | 11 | 34 | 67.7% |
| 2-20-2d | 10 | 0 | 16 | 8 | 34 | 76.5% |
| 2-20-5d | 10 | 0 | 14 | 10 | 34 | 70.6% |
| 2-50-2d | 10 | 10 | 8 | 6 | 34 | 82.4% |
| 2-50-5d | 10 | 3 | 14 | 7 | 34 | 79.4% |
| 10-DMSO-2d | 10 | 0 | 9 | 15 | 34 | 55.9% |
| 10-DMSO-5d | 10 | 1 | 8 | 15 | 34 | 55.9% |
| 10-20-2d | 10 | 0 | 11 | 13 | 34 | 61.8% |
| 10-20-5d | 10 | 1 | 8 | 15 | 34 | 55.9% |
| 10-50-2d | 10 | 2 | 14 | 8 | 34 | 76.5% |
| 10-50-5d | 10 | 3 | 11 | 10 | 34 | 70.6% |
Each culture condition was evaluated to determine the number of cholesteryl esters (CEs) that responded optimally. Culture conditions are abbreviated as FBS % – Rosiglitazone (μM) – Duration. CEs are listed with two numbers. The two numbers denote the number of carbons and double bonds in the acyl chains, respectively. n = 4 for each culture condition.
A total of 34 CEs, as shown in Column E, met the criteria for analysis in this study. Column A shows that 10 CEs did not vary across all twelve culture conditions. The definition of a “superior” condition in Column B is dependent on a given CE’s saturation level. For saturated and monounsaturated CEs, a condition was deemed superior if it yielded the highest expression relative to the other conditions. For polyunsaturated CEs, a condition was considered superior if it yielded the lowest expression relative to the other conditions. If a given condition was not the “superior” condition, then one of two other outcomes were possible: it could either have been statistically equivalent to the superior condition (Column C) or statistically inferior to the superior condition (Column D). The final column represents the percentage of CEs that responded optimally to each condition. Culturing with 50 uM rosiglitazone and 2% FBS for 2 days optimized 82.4% of CEs and was considered superior for 10 of 34 (29.4%) of CEs.
D. Most responsive CEs to rosiglitazone supplementation
To determine which CEs were most responsive to rosiglitazone treatment, correlation coefficients were calculated (Table 4). Thirteen CEs showed a significant correlation with respect to rosiglitazone. Three of the thirteen were saturated, six were monounsaturated, and four were polyunsaturated. The following CEs all had statistically significant correlation coefficients at or above 0.50, which is considered the minimum for a moderately high correlation:[23] CE 24:1 (ρ = +0.70), CE 28:1 (ρ = +0.69), CE 26:1 (ρ = +0.66), CE 18:1 (ρ = −0.59), and CE 22:1 (ρ = +0.50).
Table 4.
Correlation Coefficients Between Cholesteryl Esters and Rosiglitazone
| Cholesteryl Ester (CE) | Correlation Coefficient (ρ) | Significance (p-value) | Cholesteryl Ester (CE) | Correlation Coefficient (ρ) | Significance (p-value) |
|---|---|---|---|---|---|
| CE 14:0 | 0.127 | 0.389 | CE 22:6 | −0.094 | 0.525 |
| CE 15:0 | −0.230 | 0.116 | CE 22:5 | −0.089 | 0.549 |
| CE 16:2 | −0.469 | 0.001 | CE 22:4 | −0.209 | 0.154 |
| CE 16:1 | 0.273 | 0.061 | CE 22:3 | −0.077 | 0.602 |
| CE 16:0 | 0.337 | 0.019 | CE 22:2 | −0.086 | 0.561 |
| CE 17:1 | −0.011 | 0.941 | CE 22:1 | 0.500 | <0.001 |
| CE 17:0 | −0.063 | 0.670 | CE 24:2 | 0.227 | 0.122 |
| CE 18:3 | −0.011 | 0.940 | CE 24:1 | 0.698 | <0.001 |
| CE 18:2 | −0.041 | 0.785 | CE 24:0 | 0.453 | 0.001 |
| CE 18:1 | −0.589 | <0.001 | CE 26:2 | 0.168 | 0.253 |
| CE 18:0 | 0.426 | 0.003 | CE 26:1 | 0.656 | <0.001 |
| CE 19:1 | −0.187 | 0.203 | CE 26:0 | 0.130 | 0.378 |
| CE 20:5 | −0.295 | 0.042 | CE 27:3 | 0.026 | 0.862 |
| CE 20:4 | 0.133 | 0.369 | CE 28:1 | 0.694 | <0.001 |
| CE 20:3 | −0.300 | 0.038 | CE 30:1 | 0.245 | 0.093 |
| CE 20:2 | −0.346 | 0.016 | CE 32:1 | −0.085 | 0.566 |
| CE 20:1 | −0.022 | 0.881 | CE 34:1 | −0.384 | 0.007 |
Spearman’s correlation coefficients were calculated between each cholesteryl ester (CE) and rosiglitazone. CEs are listed with two numbers. The two numbers denote the number of carbons and double bonds in the acyl chains, respectively. All correlations are positive unless otherwise denoted. Thirteen CEs (bolded) reached statistical significance. Five of these CEs had moderate to high correlations: CE 24:1, CE 28:1, CE 26:1, CE 18:1, and CE 22:1. n = 48 for each CE.
IV. Discussion
This report demonstrates that HMGECs readily express cholesteryl esters, including very long-chain and ultra long-chain CEs that are characteristic of human meibum.[15] It also establishes culture conditions that promote a better preclinical model of meibomian gland physiology relative to serum-induced differentiation alone.[3] When supplemented with rosiglitazone, a PPARγ agonist, HMGECs modulate their CE expression profile to better mirror the saturation profile of normal human meibum: upregulation of select saturated and monounsaturated CEs and downregulation of select polyunsaturated CEs.[14, 19] The duration of culture provides little influence on the CE profile; however, reducing the serum concentration to 2% further refines CE expression toward that which is observed in vivo. Together, these findings suggest that rosiglitazone imparts significant effects on the differentiation of and lipid expression by HMGECs, which carries implications for future culturing conditions and potentially therapeutic benefits.
Cholesteryl esters, particularly with respect to saturation level, are attractive targets in HMGEC research. At the ocular surface, CEs are less abundant than WEs with a molar ratio recently reported as 0.49:1 (CE:WE) in normals.[24] In MGD, however, CE expression decreases and the molar ratio drops to 0.33:1.[24, 25] Functionally, we are still learning what roles CEs play in the tear film. Large differences in CE expression have been observed in subsets of normal meibum donors who have stable tear films, suggesting that large variations can be tolerated without an impact on tear dynamics.[24, 26] CEs, therefore, are unlikely to confer stability to tears at the ocular surface. Instead, tear film CEs have unique properties that allow them to be powerful modulators of phase transition temperatures and, thus, meibum viscosity.[27] Partly owing to its five-fold increase in saturation relative to wax esters,[14] reductions in CEs may inversely affect the melting point of meibum, thereby increasing its viscosity—findings that have been reported clinically, biochemically, and biophysically in patients with MGD.[28, 29] Beyond these effects on the tear film, CEs may serve biochemical and immunological roles also. There is substantial evidence that cholesterol can modulate the function of transient receptor potential (TRP) ion channels[30] and that certain CEs (such as CE 18:2 and CE 20:4) may impart antimicrobial effects in mucosal fluids.[31] The observation that CEs of all saturation levels are responsive to the agonistic effects on PPARγ by rosiglitazone reinforces their adequacy as a meaningful outcome parameter in HMGEC research.
Although all CE saturation levels varied with rosiglitazone, five specific monounsaturated CEs emerged as being the most responsive: CE 24:1, CE 28:1, CE 26:1, CE 18:1, and CE 22:1. The expression of all five of these CEs stratified with respect to rosiglitazone concentration. The longer-chain CEs (24:1, 28:1, 26:1, and 22:1) were all positively correlated, demonstrating higher expression in response to the highest concentration of rosiglitazone. Conversely, CE 18:1 demonstrated a negative correlation and therefore showed lower expression in response to the highest concentration of rosiglitazone. In general, these patterns are mostly consistent with the work of Jester et al. and Kim et al.[9, 10] Using LipidTox staining of all nonpolar lipids, Jester et al. found that nonpolar lipid production in mouse meibocytes increased in a dose-dependent manner from 0 to 50 μM rosiglitazone.[9] In HMGECs, again stained with LipidTox, Kim et al. found a linear, dose-dependent increase in nonpolar lipid production from 0 to 30 μM; however, beyond this concentration, lipid production began to decrease.[10] Neither of these studies sought to describe the relationship between rosiglitazone and the production of specific nonpolar lipid classes, nonetheless specific CE species. Our work shows that although all CEs are nonpolar, differential expression is observed among specific CEs in response to rosiglitazone (Table 2). The use of a vital stain like LipidTox can mask some of these effects; therefore, the use of analytical methods that can provide molecular data is recommended for greatest resolution.
Of further interest, the four CEs with the strongest positive correlation to rosiglitazone (CE 24:1, CE 28:1, CE 26:1, and CE 22:1) are among the 10 most highly expressed CEs in normal human meibum, comprising up to 24.5% of the overall CE pool.[15] Further, CE 24:1, the CE manifesting the highest responsivity to rosiglitazone, is also the most highly abundant CE in meibum. In our study, as well as others,[32] CE 18:1 is expressed disproportionately high in HMGECs relative to meibum. Expression approaching 40% was detected across all conditions in this study, but this value decreased to 28.1% under our optimized conditions: 50 μM rosiglitazone and 2% serum for two days. Thus, a negative correlation between CE 18:1 and rosiglitazone is consistent with a shift toward a more meibum-like profile, considering that CE 18.1 was reported to be 5.5% of all CEs in a recent study of meibum.[15] These findings are also consistent with other reports.[14, 19] Rosiglitazone’s role in promoting production of meibum-relevant lipids from HMGECs further supports its use as a differentiating agent and builds upon the strong foundational work by Jester et al. and Kim et al.[9, 10, 33]
Beyond rosiglitazone, the effects of serum concentration were also investigated in this study. Early reports described serum as a powerful differentiating agent on HMGECs.[1, 3] In 2014, Sullivan and colleagues cultured HMGECs in serum-free media (SFM) or serum-containing media (SCM, 10%) for fourteen days and detected altered expression of 2,789 genes, many involved in cell differentiation and lipid metabolism.[3] Separated and analyzed by liquid chromatography-mass spectrometry (LC-MS), lipid extracts from HMGECs exposed to SCM showed high levels of phospholipid production, which represented 58.7% of the total lipid pool.[3] Forty-seven species of WEs (0.92% of the total lipid pool) and fourteen species of CEs (1.97% of the total lipid pool) were also identified; however, for CEs, the acyl chain length only varied from 14 to 24, and the CEs that were reported to be most responsive to serum supplementation were highly polyunsaturated (CE 20:3, CE 20:4, CE 22:4).[3] This described lipidome—high expression of phospholipids, upregulation of polyunsaturated CEs, and expression of shorter-chain CEs—bears little resemblance to human meibum, which is believed to have a very low pool of phospholipids (0.006%).[34] Further skepticism of serum-induced differentiation was reported in 2015 when Hampel and colleagues discovered a significant increase in cytokeratins after serum supplementation.[32] Hyperkeratinization is thought to be a core mechanism in MGD[28] and thus may be a better indicator of pathology, rather than a sign of differentiation. Recently, Kim et al. found that HMGECs treated with 10% SCM showed little nonpolar lipid production by LipidTox staining and appeared to be equivalent to the production observed by SFM.[10] Our results seem to be in agreement with the latter two reports and suggest that a lower concentration of serum—or perhaps none at all—may be more favorable when part of differentiating conditions.
Culture duration was the third factor that was varied in our study. Most reports that have investigated HMGEC lipid production have ranged in culture duration from between one and fourteen days. One study found that in serum-treated HMGECs, lipid production was greatest at the one-day time point when monitored at intervals up to 14 days.[32] For the rosiglitazone studies on meibocytes, differentiating conditions were maintained from one to seven days.[9, 10] In mouse meibocytes, a time-dependent increase in lipid production was observed with rosiglitazone-induced differentiation. The greatest increase was detected between three (about two-fold increase versus control) and five days (about five-fold increase versus control).[9] In HMGECs, there was a gradual increase in lipid droplet formation visualized between two days and six days.[10] In our studies, a significant effect due to culture duration was not found. Extending differentiating conditions from two days to five days provided no effect on CE expression. One reason that we may not have detected a time-dependent effect associated with rosiglitazone is that we quantified relative expression, rather than absolute expression, of each CE. Therefore, if there was a uniform increase in CE production between two and five days, this effect would have been masked by our normalization methods. Similarly, Hampel et al. reported little change in relative expression of meibum-relevant lipids, such as WEs, CEs, and TAGs, between one day and three days.[2, 32] For future HMGEC research, the decision to pursue a given culture duration should be determined by analytical methods. If vital stains and fluorescent microscopy will be used, then absolute expression may be needed for optimal thresholding procedures, and a longer duration should be considered. If greater resolution is needed to identify specific lipid subclasses or species, then a sensitive technique like mass spectrometry may be needed, and a shorter culture duration would provide greater efficiency and reduce use of consumables.
Previous reports have raised concern about the adequacy of the HMGEC line’s ability to replicate the lipid profile of normal human meibum, primarily due to very abundant expression of the polar phospholipids.[2] Having focused our analyses on cholesteryl esters, our experiments were designed and our methods optimized to identify this specific subclass, rather than the lipidome as a whole. Despite this, our observations support that phospholipids, at least with our mass spectrometry acquisition parameters, continue to be the highest lipid class produced by HMGECs (data not shown). Further comparison of any classes other than CEs is outside of the scope of this study. However, our CE profile from HMGECs can be compared with and contrasted against literature reports of the CE pool in normal human meibum.
A recent report by Chen and Nichols comprehensively defined the meibum lipidome, including CEs.[15] Depending on the mass spectrometry methodology, normal human meibum has been reported to express between 28 and 58 different CEs, ranging in chain length from 16 to 36 carbons.[15] By comparison, we identified 34 unique CEs that met our criteria for inclusion. The carbon numbers ranged from 14 to 34, aligning well with the diversity and chain lengths observed in meibum. The contribution of saturated, monounsaturated, and polyunsaturated CEs to the overall CE pool in meibum is 48.8%, 44.8%, and 6.3%, respectively.[15] In our study of HMGECs, when cultured with 2% FBS and 50 μM rosiglitazone for two days, we found 15.8%, 60.5%, and 23.7%. While the monounsaturated CE content was relatively similar, we found more polyunsaturated CEs, at the expense of saturated CEs, from HMGECs. There were further differences in the top five most abundant CEs. Meibum shows high levels of CE 24:1, CE 25:0, CE 24:0, CE 26:0, and CE 22:1 (in descending order, ranging from 9.6% to 6.0% of the total CE pool).[15] From our studies of HMGECs, high expression was detected for CE 18:1, CE 16:1, CE 16:0, CE 18:2, and CE 26:1 (ranging from 28.1% to 7.6% of the total CE pool). The four most abundant CEs from HMGECs all have shorter acyl chains, a finding that is poorly aligned with meibum CEs. These discrepancies in the CE lipidome between normal human meibum and HMGECs emphasizes the importance of using rosiglitazone as a differentiating agent. As previously stated, CE 24:1, CE 28:1, CE 26:1, and CE 22:1 were the CEs that demonstrated the greatest increase with rosiglitazone supplementation. All four of these are among the ten most prominent CEs in meibum, accounting for 24.5% of the total CE pool. This comparison further highlights the importance of using mass spectrometry to analyze lipid production, as vital stains, such as LipidTox, cannot differentiate the relevant CEs from the less relevant ones. Taken together, variations do indeed exist in the CE lipidomes between meibum and HMGECs, but differentiation by rosiglitazone increases the most meibum-relevant CEs toward a more meibum-like profile.
As with all research utilizing immortalized cell culture systems, this study has a few limitations. First, HMGECs have been genetically manipulated to proliferate indefinitely, likely preventing these cells from reaching a terminally differentiated mature or hypermature state.[2] Despite this manipulation, some of the original work on HMGECs revealed no karyotype differences until passage 35.[1] We used passages 25 and 26 in this work to maintain high confidence in the results and limit concerns related to genetic drift. Secondly, some may argue against studying CEs from HMGECs, given that others have previously reported low CE production relative to that found in human meibum.[3, 32] In our opinion, however, expecting a high degree of similarity in the total lipidomes between immortalized HMGECs and human meibum is unrealistic. Meibocytes are not the only cells believed to contribute to meibum composition; in vivo, ductal epithelium is thought to recycle phospholipids from the meibocyte cell membranes.[3, 35] In meibocyte cell culture systems, however, no other cells or tissues exist to influence the final lipid composition; therefore all membrane phospholipids contribute to the final lipidome, which further dilutes the total CE component. The presence of phospholipids and the awareness that HMGECs do not reach a mature or hypermature state of differentiation likely contributes to the overall lower abundance of CEs from HMGECs. Despite these shortcomings, HMGECs remain a useful tool in meibomian gland research, permitting the opportunity to test hypotheses that are too preliminary, too difficult to control, or too controversial to test in human subjects. We argue—and provide evidence in this report—that assessing for changes in well-selected target outcome variables in response to a challenge or intervention is the ideal approach for applying HMGECs in a research setting. Here, we show that after 48 hours of exposure to a known meibogenic compound, HMGECs modify their CE profile to be more similar to human meibum, specifically upregulating select saturated and monounsatured CEs and downregulating polyunsaturated CEs. This discovery strongly favors the use of rosiglitazone as a differentiating agent in future HMGEC research and builds upon previous hypotheses that rosiglitazone may have therapeutic potential in restoring an abnormal lipid profile in patients with meibomian gland dysfunction.[9, 10, 33]
V. Conclusion
This study reveals that cholesteryl esters are responsive to PPARγ agonism by rosiglitazone and that they are attractive outcome parameters in HMGEC research. Rosiglitazone positively influences HMGEC differentiation as detected by the upregulation of select saturated and monounsaturated cholesteryl esters and the downregulation of select polyunsaturated cholesteryl esters. Culturing HMGECs with 50 μM rosiglitazone and 2% serum for two days yields a CE profile that is more similar to that of human meibum than when culturing with serum alone. We further report that four CEs that are among the highest expressed in human meibum are also the most sensitive to rosiglitazone supplementation: CE 24:1, CE 28:1, CE 26:1, and CE 22:1. In conclusion, we concur with previous researchers who have proposed rosiglitazone as a differentiating agent for HMGECs[9, 10, 33] and advocate for the use of mass spectrometry to focus on specific CE species that are the most relevant to human meibum.
Acknowledgments:
The authors would like to thank Dr. David Sullivan (Schepens Eye Research Institute) for providing the human meibomian gland epithelial cells.
Funding: This work was supported by the Office of Research Infrastructure Programs of the National Institutes of Health under S10 RR027822-01. Career developmental support for the first author was provided by the National Eye Institute under K23 EY028629-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest to disclose.
References
- [1].Liu S, Hatton MP, Khandelwal P, Sullivan DA. Culture, immortalization, and characterization of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:3993–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hampel U, Garreis F. The human meibomian gland epithelial cell line as a model to study meibomian gland dysfunction. Exp Eye Res. 2017;163:46–52. [DOI] [PubMed] [Google Scholar]
- [3].Sullivan DA, Liu Y, Kam WR, Ding J, Green KM, Shaffer SA, et al. Serum-induced differentiation of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2014;55:3866–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu Y, Kam WR, Ding J, Sullivan DA. Effect of azithromycin on lipid accumulation in immortalized human meibomian gland epithelial cells. JAMA Ophthalmology. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu Y, Kam WR, Ding J, Sullivan DA. One man’s poison is another man’s meat: using azithromycin-induced phospholipidosis to promote ocular surface health. Toxicology. 2014;320:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu Y, Kam WR, Sullivan DA. Influence of Omega 3 and 6 Fatty Acids on Human Meibomian Gland Epithelial Cells. Cornea. 2016;35:1122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hampel U, Kruger M, Kunnen C, Garreis F, Willcox M, Paulsen F. In vitro effects of docosahexaenoic and eicosapentaenoic acid on human meibomian gland epithelial cells. Exp Eye Res. 2015;140:139–48. [DOI] [PubMed] [Google Scholar]
- [8].Han X, Liu Y, Kam WR, Sullivan DA. Effect of brimonidine, an alpha2 adrenergic agonist, on human meibomian gland epithelial cells. Exp Eye Res. 2018;170:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jester JV, Potma E, Brown DJ. PPARgamma Regulates Mouse Meibocyte Differentiation and Lipid Synthesis. Ocul Surf. 2016;14:484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim SW, Xie Y, Nguyen PQ, Bui VT, Huynh K, Kang JS, et al. PPARgamma regulates meibocyte differentiation and lipid synthesis of cultured human meibomian gland epithelial cells (hMGEC). Ocul Surf. 2018;16:463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Janani C, Ranjitha Kumari BD. PPAR gamma gene--a review. Diabetes Metab Syndr. 2015;9:46–50. [DOI] [PubMed] [Google Scholar]
- [12].Dozsa A, Dezso B, Toth BI, Bacsi A, Poliska S, Camera E, et al. PPARgamma-mediated and arachidonic acid-dependent signaling is involved in differentiation and lipid production of human sebocytes. J Invest Dermatol. 2014;134:910–20. [DOI] [PubMed] [Google Scholar]
- [13].Ferre P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes. 2004;53 Suppl 1:S43–50. [DOI] [PubMed] [Google Scholar]
- [14].Chen J, Green KB, Nichols KK. Quantitative profiling of major neutral lipid classes in human meibum by direct infusion electrospray ionization mass spectrometry. Invest Ophthalmol Vis Sci. 2013;54:5730–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen J, Nichols KK. Comprehensive shotgun lipidomics of human meibomian gland secretions using MS/MS(all) with successive switching between acquisition polarity modes. J Lipid Res. 2018;59:2223–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sassa T, Kihara A. Metabolism of very long-chain Fatty acids: genes and pathophysiology. Biomolecules & therapeutics. 2014;22:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sampaio JL, Gerl MJ, Klose C, Ejsing CS, Beug H, Simons K, et al. Membrane lipidome of an epithelial cell line. Proc Natl Acad Sci U S A. 2011;108:1903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Butovich IA. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J Lipid Res. 2009;50:501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Butovich IA. Fatty acid composition of cholesteryl esters of human meibomian gland secretions. Steroids. 2010;75:726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- [22].Ziemanski JF, Chen J, Nichols KK. Evaluation of Cell Harvesting Techniques to Optimize Lipidomic Analysis from Human Meibomian Gland Epithelial Cells in Culture. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24:69–71. [PMC free article] [PubMed] [Google Scholar]
- [24].Borchman D, Ramasubramanian A, Foulks GN. Human Meibum Cholesteryl and Wax Ester Variability With Age, Sex, and Meibomian Gland Dysfunction. Invest Ophthalmol Vis Sci. 2019;60:2286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shrestha RK, Borchman D, Foulks GN, Yappert MC, Milliner SE. Analysis of the composition of lipid in human meibum from normal infants, children, adolescents, adults, and adults with meibomian gland dysfunction using (1)H-NMR spectroscopy. Invest Ophthalmol Vis Sci. 2011;52:7350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Eftimov P, Yokoi N, Tonchev V, Nencheva Y, Georgiev GA. Surface properties and exponential stress relaxations of mammalian meibum films. Eur Biophys J. 2017;46:129–40. [DOI] [PubMed] [Google Scholar]
- [27].Borchman D, Yappert MC, Milliner SE, Duran D, Cox GW, Smith RJ, et al. 13C and 1H NMR ester region resonance assignments and the composition of human infant and child meibum. Exp Eye Res. 2013;112:151–9. [DOI] [PubMed] [Google Scholar]
- [28].Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Green-Church KB, Butovich I, Willcox M, Borchman D, Paulsen F, Barabino S, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52:1979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ciardo M, Ferror-Montiel A. Lipids as central modulators of sensory TRP channels. Biochim Biophys Acta. 2017;1859:1615–28. [DOI] [PubMed] [Google Scholar]
- [31].Do T, Moshkani S, Castillo P, Anunta S, Pogosyan A, Cheung A, et al. Lipids including cholesteryl linoleate and cholesteryl arachidonate contribute to the inherent antibacterial activity of human nasal fluid. J Immunol. 2009;181:4177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hampel U, Schroder A, Mitchell T, Brown S, Snikeris P, Garreis F, et al. Serum-induced keratinization processes in an immortalized human meibomian gland epithelial cell line. PLoS One. 2015;10:e0128096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim SW, Brown DJ, Jester JV. Transcriptome analysis after PPARgamma activation in human meibomian gland epithelial cells (hMGEC). Ocul Surf. 2019;17:809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Brown SH, Kunnen CM, Duchoslav E, Dolla NK, Kelso MJ, Papas EB, et al. A comparison of patient matched meibum and tear lipidomes. Invest Ophthalmol Vis Sci. 2013;54:7417–24. [DOI] [PubMed] [Google Scholar]
- [35].Chen J, Green-Church KB, Nichols KK. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Invest Ophthalmol Vis Sci. 2010;51:6220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


