Abstract
Objective
Many women with breast cancer hesitate to raise sexual concerns clinically. We evaluated a multimedia intervention to facilitate BC patients’ communication about sexual/menopausal health, called Starting the Conversation (STC).
Methods
Female BC patients (N=144) were randomly assigned to either STC [20-minute video, workbook, and resource guide] or control [resource guide only]. Audio-recorded dialogue from patients’ next oncology clinic encounter was coded for patients’ sexual health communication. Self-report surveys assessed patients’ beliefs about sexual health communication, self-efficacy for clinical interactions, sexual function/activity, anxiety/depression symptoms, and quality of life at baseline, post-intervention, and 2-month follow-up. T-tests or mixed-effects logistic regression compared study arms.
Results
Women in the STC arm were more likely to raise the topic of sexual health [51%; OR=2.62(1.02, 6.69), p=.04] and ask a sexual health question [40%; OR=2.85(1.27, 6.38), p=.01] during their clinic encounter than those in the control arm (30% and 19% for raise and ask, respectively). At follow-up, women in the STC arm showed greater improvements in sexual health communication self-efficacy (p=.009) and in anxiety symptoms (p=.03), and more women were sexually active at follow-up, compared to the control arm (OR=1.5, 70% vs. 46%, p=.04).
Conclusions
The STC intervention facilitated women’s clinical communication about sexual health and reduced women’s anxiety, possibly due to increased confidence in expressing their medical needs. Helpful information gained from clinical discussions could have improved women’s willingness or ability to engage in sexual activity. Future studies should identify aspects of the clinical encounter most critical to improving women’s sexual outcomes.
Keywords: Cancer, Oncology, Breast Cancer, Patient-Provider Communication, Sexuality, Sexual Function, Psycho-Oncology
Background
Over 90% of women diagnosed with breast cancer (BC) survive 5 years beyond their diagnosis,1 making quality of life (QOL) concerns such as sexuality highly significant. Up to 70% of BC survivors report sexual function disturbances,2 including vaginal dryness, loss of libido, and decreased sexual activity.3–5 BC surgery commonly leads to body image distress associated with worse sexual functioning4, 6 and disruption in survivors’ intimate relationships.7 Although nearly all treatments given to BC patients can lead to negative sexual sequelae, those that reduce or eliminate estrogen (e.g., aromatase inhibitors, chemotherapy, ovarian suppression) cause especially distressing sexual and menopausal symptoms.8
Guidelines recommend that sexual function be included as part of routine care for patients diagnosed with breast and other cancers,9, 10 and most BC patients endorse wanting sexual health information.11, 12 Further, effective treatment options for sexual problems are increasingly available,13 suggesting that clinical discussions of sexual concerns could be fruitful. Yet only one-third of BC patients report receiving information about sexual side effects of their treatments,14, 15 indicating that sexual health needs are unaddressed for many.
Whereas BC clinicians may assume their patients will raise the topic of sexual health,16 this is generally found not to be the case, even for patients reporting sexual problems.17, 18 In fact, despite clinicians’ own purported discomfort in communicating about sexual health,16, 19 clinical discussions of sexual issues for BC patients are usually initiated by the clinician.18 Patients’ reluctance to discuss sexual health can stem from knowledge and skills gaps and from unhelpful beliefs (e.g., that raising the topic will cause an undue burden for their clinician).16 Interventions that can equip BC patients to discuss sexual health with their clinicians are thus critically needed.
The objective of the present study was to evaluate a multimedia intervention called Starting the Conversation (STC), aimed at facilitating BC patients’ clinical communication about sexual health, in a randomized controlled trial. The intervention and trial were guided by principles of social cognitive theory,20 including the importance of individuals’ self-efficacy (confidence in successfully accomplishing a task), and outcome expectancies (belief that accomplishing a task will lead to desired outcomes). We hypothesized that the STC intervention would show significant effects on women’s beliefs about sexual health communication (self-efficacy and outcome expectancies), and on their communication about sexual health during post-intervention clinic visits. We also assessed intervention effects on women’s self-efficacy for clinical interactions, perceived barriers to sexual health communication, sexual function and activity, psychological distress (anxiety and depression symptoms), and health-related QOL.
Methods
Study Design and Setting
Women with a BC diagnosis were recruited at a comprehensive cancer center in Philadelphia until the target sample size was achieved and randomly assigned 1:1 (unblinded) to receive either the STC intervention (video, workbook, and resource guide) or control (resource guide only) using computer-generated block randomization (block size 6), with stratification by metastatic disease and education (< high school diploma vs. greater). The study biostatistician (EH) generated the randomization sequence and the study manager (KS) manually assigned patients to conditions using the sequence. Women completed self-report surveys immediately after enrolling through online consent (baseline), at an in-person clinic encounter with their BC clinician several weeks after receiving the intervention materials (post-intervention), and at 2-month follow-up. Post-intervention clinic encounters with BC clinicians were audio recorded and the dialogue analyzed for patients’ sexual health communication. The study protocol was approved by the Fox Chase Institutional Review Board (IRB Protocol #14–833) and was entered prospectively on clinicaltrials.gov (entry #NCT03624972).
Participants
Adult women with any stage BC were eligible if they were undergoing active treatment or had completed treatment within 10 years, were being seen in follow-up, and agreed to have their clinic encounter audio recorded. Women were ineligible if they could not speak English, had an ECOG score21 > 2, or had overt cognitive dysfunction, psychiatric disturbance, or severe physical or mental illness. Medical oncology clinicians (physicians, advanced practice clinicians) consented to their patients’ encounters being audio recorded and received standardized educational materials on BC-related sexual issues prior to patient enrollment.22
Starting the Conversation (STC) Intervention Arm
The STC intervention consisted of a 20-minute video slideshow with narration accessible via smartphone, computer or tablet, and an accompanying 5-page workbook providing information and skills training for communicating with providers about sexual concerns (see Table 1). In addition, they also received a 2-page resource guide consisting of information about institutional and external resources on menopausal and sexual health (see Supplemental File). Intervention content was informed by focus groups with BC patients and clinicians on patients’ knowledge and skills gaps and intervention preferences16 and guided by principles of social cognitive theory,20 which emphasized building self-efficacy and enhancing outcome expectancies through learning skills for effectively discussing sexual health.
Table 1.
Skill-Building Exercises in the Starting the Conversation (STC) Intervention
| Exercise | Title | Learning Objective(s) | Activities |
|---|---|---|---|
| 1 | Practice Using the SEA Model (be Specific; Explain why the concern is important; Ask a question) | Recognize and structure effective ways of expressing concerns using SEA model |
|
| 2 | Identify Your Current Problems/Concerns | Identify and prioritize concerns using checklist of sexual/menopausal concerns |
|
| 3 | Plan and Practice My Communication | Plan and practice intended communication for clinic visit |
|
Control Arm
Women in the control arm received only the resource guide (described above).
Procedures
After being identified from clinicians’ clinic schedules, women were sent informational letters, and called by the study research assistant for screening. Interested patients were sent a link to online consent forms for self-enrollment. All participants were sent the resource guide several weeks prior to an upcoming clinic visit with instructions to read it prior to their visit. In addition, women in the STC intervention arm were sent via email a link to the video and instructions to watch the video and complete the accompanying workbook. Participants received a reminder call close to their scheduled visit. Patients received $20 gift cards for completing each study survey. Clinicians received gift cards worth $50.
Measures
Primary Outcomes
Clinical Communication Behaviors.
Patients’ clinical communication about sexual health [defined as sexual activity, function (desire, arousal, orgasm, pain/discomfort), or relationships, general sexual concerns, or body image] was assessed as the proportion of post-training clinic encounters in which the patient (1) asked a question pertaining to sexual health (coded “yes” if ≥ 1 question about sexual health during clinic encounter and “no” otherwise), and (2) raised the topic of sexual health (coded “yes” if the first discussion of sexual health was raised by patient and coded “no” if no one raised the topic of sexual health). These data were obtained from audio recorded, transcribed, and coded clinical encounters according to standardized code definitions. Trained research assistants blinded to patients’ conditions coded the data; inter-rater reliability exceeded a pre-specified threshold of ≥ .60.
Self-efficacy and Outcome Expectancies.
Two items assessed patients’ self-efficacy (confidence) for communicating with their breast cancer clinician about sexual health concerns in terms of either talking (item 1) or asking (item 2) about sexual health. Five items assessed the belief that discussing sexual health with her BC clinician would lead to positive outcomes (e.g., “find a solution to a problem”). Response options used an 11-point scale (0=not at all confident/not at all to 10=extremely confident/very much). Mean scores were used. Self-efficacy and outcome expectancy items were developed according to social cognitive theory guidelines20, 23 with input from a trans-disciplinary team, were used successfully in similar studies,22 and had excellent reliability (Cronbach’s alphas ≥ .97).
Secondary Outcomes
General Efficacy for Clinical Interactions.
The 5-item version of the Perceived Efficacy in Patient–Physician Interactions scale (PEPPI-5),24 assessed patients’ general self-efficacy for interactions with health care providers (modified to specify the BC clinician), such as having key health-related questions answered. Response options are scored on a 6-point scale (1=not at all confident, 5=very confident) and a total score was used. The scale had very good reliability (Cronbach’s alpha=.89).
Barriers to Sexual Health Communication.
Thirteen items adapted from a prior published study25 assessed women’s perceived barriers to discussing sexual health with their BC provider,26 including embarrassment/discomfort, lack of skills, or provider perceptions. Response options used a 6-point scale (1=strongly disagree, 5=strongly agree). A total score was used. The scale had very good reliability (Cronbach’s alpha=.88).
Sexual Function and Activity.
Sexual function was assessed using the Lubrication, Vaginal Discomfort, Satisfaction, and Interest Domain scores from the PROMIS SexFS Brief Profile Version 2.0.27 Higher scores for all domains except Vaginal Discomfort signify better function. Scores are converted to a T-score metric where a score of 50 equates to the mean of the U.S. population of sexually active adults, with a standard deviation of 10. This measure has been used successfully in women with BC.28, 29 The PROMIS sexual activity screener item assessed any sexual activity (partnered or unpartnered; examples are “masturbation, oral sex, and intercourse”) within the past 30 days. Women who are not sexually active complete only the Interest items;27 women endorsing this item complete all items.
Psychological Distress and Health-Related Quality of Life (HRQOL).
Psychological distress was measured by the Hospital Anxiety and Depression Scale anxiety (HADS-A) and depressive symptoms (HADS-D) subscales.30 Both subscales had good reliability (Cronbach’s alpha=.86 and .83, respectively). HRQOL was assessed using the widely used Functional Assessment of Cancer Therapy – Breast Cancer Short Form (FACT-B)31 total score, which includes items assessing physical and functional well-being, and breast cancer-specific symptoms. This measure had good reliability (Cronbach’s alpha=.89).
Clinical and Socio-demographic Data
Clinical data were obtained through chart review. Socio-demographic information was obtained through the baseline surveys.
Statistical Analyses
Women in the study arms were compared on demographics at baseline using t-tests and Chi-square tests to assess balance and on outcome measures using an intent-to-treat approach. Linear mixed models tested for the effects of the interventions on self-efficacy and outcome expectancies over time, accounting for the longitudinal/clustered nature of the data using hierarchical random effects to account for assessment times within patients within providers. Box-Cox power transformations were used to reduce skewness. To assess intervention effects at follow-up, we included an interaction between time and treatment; statistical significance would indicate that the intervention influenced self-efficacy and outcome expectancies at follow-up. Disease stage and education were controlled. As a sensitivity analysis (to confirm effects in light of right-skewed outcomes), we used ordinal mixed effect logistic regression models (for integer responses), with clustering at the patient level. Fisher’s exact tests tested the hypothesis that patient communication behaviors differed for the intervention and control arms. Then, we performed mixed effects logistic regression models with random intercepts at the provider level. For raising the topic, only informative encounters (i.e., in which the clinician did not raise the topic) were included.
Secondary Analyses
We examined whether the STC intervention affected differences from baseline to follow-up for secondary patient-reported communication outcomes using paired t-tests and used hierarchical linear mixed models to determine the effect of the study arm on these measures, accounting for the two levels of clustering. Hierarchical logistic regression models like those used for primary outcomes examined the effect of study arm on sexual activity at follow-up.
Sample Size Calculations
Sample size was determined based on the primary clinical communication behavior outcomes. With a sample size of 128 audio recordings, we estimated having 80% power with 5% 2-sided Type-I error to detect a difference of 23% between the study arms for asking and of 30% for raising the topic. For raising the topic, we anticipated that 90 of the 128 clinic encounters would be informative (i.e., clinician would not raise the topic).
Results
Recruitment and Retention
The CONSORT flow diagram is shown in Figure 1. From June 2018 through July 2019, 531 women were sent introductory letters, 177 of whom were screened eligible and approached to participate, and of these, 146 consented (88%). Women (N=144) were randomized (73 to intervention; 71 to control), with clinic recordings obtained from 127 women (88%; 63 in intervention arm; 64 women in control arm). Post-intervention and 2-month follow-up surveys were obtained from 142 (99%) and 140 women (97%), respectively. Across both study arms, most participants reporting using study materials (see Figure 1), with particularly high rates seen for using the video (88%) and the workbook (85%). Data collected ended in October, 2019.
Figure 1.
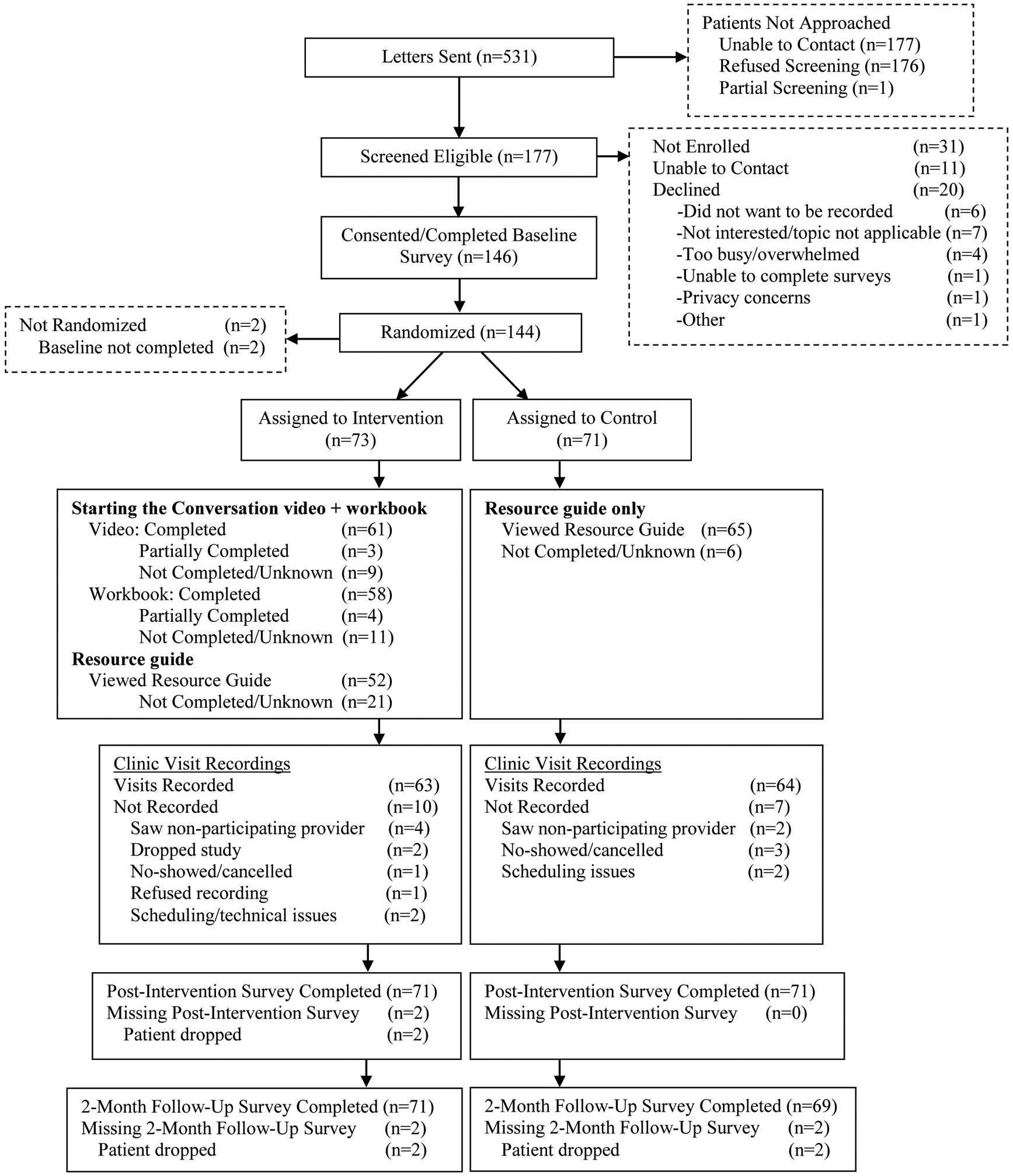
CONSORT Diagram
Sample Characteristics
Patient characteristics are shown in Table 2. Overall, most women in the sample identified as White (67%) or Black/African-American (27%), and most were partnered, and highly educated. Most patients were diagnosed with non-metastatic BC and had been treated through surgery, endocrine therapy, chemotherapy and radiation; few had immunotherapy or ovarian suppression. Most women were currently taking either aromatase inhibitors or tamoxifen. Study arms were well balanced on baseline demographics and self-report outcomes (all p values>.16). Of the 9 participating clinicians (M age=38; SD=9; 4 medical oncologists; 4 nurse practitioners; 1 physician assistant), 6 were female (67%), 5 were Caucasian (56%), and 4 were Asian (44%). Two thirds of clinicians (67%) had fewer than 5 years in practice, and the remainder had at least 5 years in practice.
Table 2.
Participant Characteristics by Study Arm
| STC (N=73) | Control (N=71) | |
|---|---|---|
| Characteristic | Mean(SD) | |
| Age, y | 55.8(11.2) | 56.1(10.9) |
| Time since diagnosis, m | 43.8(42.6) | 56.6(64.4) |
| n(%) | ||
| Race/Ethnicity | ||
| White/Caucasian | 43(58.9) | 53(74.6) |
| Black/African American | 26(35.6) | 13(18.3) |
| More than one race | 1(1.4) | 3(4.2) |
| Other/Unknown | 3(4.1) | 2(2.8) |
| Hispanic/Latina | 3(4.1) | 3(4.2) |
| Relationship Status | ||
| In romantic relationship | 51(69.9) | 46(64.8) |
| Education | ||
| Some high school | 2(2.7) | 2(2.8) |
| High school/GED | 12(16.4) | 12(16.9) |
| Some college | 22(30.1) | 17(23.9) |
| Completed college/Graduate school | 37(50.7) | 40(56.3) |
| Employment Status | ||
| Full/Part-time | 40(54.8) | 39(54.9) |
| Retired | 15(20.5) | 18(25.4) |
| Unemployed/disability/other | 18(24.7) | 14(19.6) |
| Sexual Orientation | ||
| Heterosexual/straight | 70(95.9) | 70(98.6) |
| Lesbian/homosexual | 2(2.7) | 1(1.4) |
| Bisexual | 1(1.4) | 0(0) |
| Menopausal Status | ||
| Pre-menopausal/peri-menopausal | 20(27.4) | 18(25.4) |
| Post-m enopaus al | 53(72.6) | 53(74.6) |
| Disease Stage | ||
| I | 31(42.5) | 35(49.3) |
| II | 23(31.5) | 18(25.4) |
| III | 8(11.0) | 8(11.2) |
| IV | 11(15.1) | 10(14.1) |
| Surgery | 70(95.9) | 68(95.8) |
| Breast conserving | 40(54.8) | 43(60.6) |
| Mastectomy with reconstruction | 23(31.5) | 23(32.4) |
| Mastectomy without reconstruction | 7(9.6) | 2(2.8) |
| Chemotherapy | 51(69.9) | 44(62.0) |
| Current | 12(16.4) | 11(15.5) |
| Endocrine Therapy | 50(68.5) | 57(80.3) |
| Current tamoxifen | 14(19.2) | 8(11.3) |
| Current aromatase inhibitors | 34(46.6) | 40(56.3) |
| Ovarian Suppression | 4(5.5) | 4(5.6) |
| Current | 3(4.1) | 3(4.2) |
| Radiation Therapy | 44(60.3) | 48(67.6) |
| Current | 2(2.7) | 1(1.4) |
| Immunotherapy | 22(30.1) | 26(36.6) |
| Current | 13(17.8) | 10(14.1) |
Primary Analyses
Clinical Communication Behaviors.
Nearly two-thirds of the 127 clinic encounters (n=75; 59%) included discussion of sexual health (55% raised by clinician; 45% raised by patient). Within the informative encounters (i.e., clinician did not raise topic; N=39 intervention encounters; N=47 control encounters), women in the intervention arm had significantly greater odds of raising the topic [51%; OR=2.62(1.02, 6.69), p=.04] compared to those in the control arm (30%). Within all 127 encounters (N=63 intervention encounters; N=64 control encounters), women in the intervention arm had significantly greater odds of asking a question about sexual health [40%; OR=2.85(1.27, 6.38), p=.01], compared to those in the control arm (19%).
Self-Efficacy and Outcome Expectancies.
As shown in Table 3, compared to women in the control arm, those in the intervention arm reported greater mean self-efficacy at both post-intervention (p=.02) and 2-month follow-up (p=.009). For outcome expectancies, there were no significant differences between the study arms.
Table 3.
Self-Report Outcomes at Baseline and 2-Month Follow-Up and Regression Results
| Mean(SD) | Coefficient | SE | P value | ||
|---|---|---|---|---|---|
| STC(N=73) | Control(N=71) | ||||
| Primary Outcomes | |||||
| Self-Efficacy | |||||
| Baseline | 7.4(2.6) | 7.7(2.4) | |||
| Post-Intervention | 8.8(2.1) | 8.5(1.8) | 0.68 | 0.37 | .03 |
| Follow-up | 8.2(2.2) | 7.8(2.0) | 0.84 | 0.37 | .009 |
| Outcome Expectancies | |||||
| Baseline | 7.4(2.5) | 7.6(2.3) | |||
| Post-Intervention | 8.4(2.0) | 8.2(2.2) | 0.51 | 0.38 | .20 |
| Follow-up | 7.8(2.2) | 7.8(2.1) | 0.22 | 0.38 | .55 |
| Secondary Outcomes | |||||
| General Clinical Self-Efficacy | |||||
| Baseline | 21.1(3.4) | 21.6(3.4) | |||
| Follow-up | 22.2(3.0) | 21.6(3.5) | 1.06 | 0.56 | .07 |
| Barriers to Sexual Health Communication | |||||
| Baseline | 30.5(9.9) | 29.6(8.9) | |||
| Follow-up | 27.8(9.9) | 29.0(9.0) | −2.19 | 1.54 | .16 |
| Anxiety Symptoms (HADS-A) | |||||
| Baseline | 7.5(4.6) | 7.3(4.2) | |||
| Follow-up | 6.6(4.9) | 7.3(4.2) | −0.97 | 0.46 | .04 |
| Depressive Symptoms (HADS-D) | |||||
| Baseline | 5.3(4.0) | 4.4(3.4) | |||
| Follow-up | 4.6(3.7) | 4.4(3.5) | −0.06 | 0.09 | .48 |
| QOL (FACT-B) | |||||
| Baseline | 60.8(16.7) | 61.9(14.3) | |||
| Follow-up | 61.8(18.2) | 62.6(13.9) | 0.69 | 1.52 | .67 |
| Vaginal Lubrication | |||||
| Baseline | 41.6(8.1) | 42.8(10.1) | |||
| Follow-up | 41.6(9.7) | 42.2(8.5) | 2.04 | 1.69 | .23 |
| Vaginal Discomfort | |||||
| Baseline | 58.0(11.1) | 56.1(10.7) | |||
| Follow-up | 58.4(11.1) | 55.8(11.8) | −1.13 | 1.97 | .57 |
| Sexual Satisfaction | |||||
| Baseline | 46.0(9.1) | 46.3(8.1) | |||
| Follow-up | 44.7(7.3) | 46.3(8.5) | −0.05 | 1.61 | .98 |
| Sexual Interest | |||||
| Baseline | 36.6(11.9) | 35.6(9.9) | |||
| Follow-up | 37.8(11.2) | 35.7(9.7) | 0.78 | 1.47 | .60 |
| n(%) | |||||
| Sexually Active | |||||
| Baseline | 41/73(56.2) | 35/70(50.0) | |||
| Follow-up | 50/71(70.4) | 32/69(46.4) | 1.51 | 0.74 | .04 |
Note: Analyses included all 144 patients except for sexual outcomes; data was available from 86–95 sexually active patients on these outcomes.
Secondary Analyses
As shown in Table 3, at follow-up, more women in the intervention arm were sexually active (70%) than in the control arm (46%; OR=8.25 [CI: 1.83, 37.14]; p=.04). Effects were also significant for anxiety, such that at 2-month follow-up, women in the intervention arm experienced significant reductions in anxiety, relative to those in the control arm (p=.04). No other significant effects on secondary outcomes were found.
Discussion
We found that the Starting the Conversation (STC) intervention was feasible and effective at facilitating BC patients’ communication about sexual health during clinic encounters. These findings are consistent with prior studies showing that patient-focused interventions can encourage active clinical communication by cancer patients32, 33 and are notable in light of the intervention’s brevity; it consisted of a 20-minute educational video and four-page workbook plus resources. Further, most participants reported using the materials regardless of study arm, indicating high intervention acceptability, and suggesting that intervention effects were due to specific intervention content rather than receiving materials about sexuality. Efforts to improve clinical discussion of sexual health in cancer populations have largely targeted clinicians rather than patients’,22, 34, 35 adding to the significance of these findings.
Women in the STC arm also saw significant improvements in their self-efficacy for discussing sexual health with their clinicians. Consistent with social cognitive theory,36 this finding suggests that confidence for discussing sexual concerns likely improves alongside increases in actual communication about this subject. Women receiving STC also reported decreased anxiety at follow-up compared to those receiving resources only, possibly due to greater confidence in effectively expressing concerns to their clinicians. Previous communication prompt interventions for patients with cancer have shown mixed findings for effects on anxiety,32 making it important that the present findings are replicated.
With regard to sexual outcomes, significantly more women in the STC arm were sexually active at follow-up than in the control arm. Helpful information obtained during encounters with clinicians could have affected women’s willingness or ability to engage in sexual activity, although precise reasons for this effect and the specific types of sexual activity that increased are not known. However, the intervention did not have significant effects on women’s sexual function, possibly due to the physiological nature of sexual difficulties often seen for many breast cancer survivors. Sexual dysfunction was not an eligibility criterion, which may have compromised our ability to see significant effects on this outcome. Most women in this sample (~70%) scored below the normative means for sexual function, however.27, 28 Future studies should examine intervention effects on sexual function (both for inactive/active women) and activity and on specific types of sexual activities. Future studies might also screen for sexual dysfunction, and identify aspects of clinical sexual health communication that are most effective in addressing women’s sexual concerns.
Study Limitations
The present study had several key strengths including strong participation and retention, which may enhance generalizability of findings, and use of audio recorded clinic dialogue to assess intervention effects on women’s clinical communication about sexual health. However, the study also had several limitations including a relatively short follow-up time period and limited participation by women from racial/ethnic groups other than White or African American/Black, or who identify as sexual minority. Future studies should examine whether intervention effects are sustained over a longer time frame and across multiple clinic visits, and consider the needs of sexual health needs of women from underrepresented groups.
Clinical Implications
Despite these limitations, findings from the study have important clinical implications. First, they demonstrate that a relatively brief sexual health communication intervention can yield positive effects on BC patients’ communication about sexual health issues during a routine clinical encounter and increase overall rates of discussion of sexual health, thereby making the findings highly relevant to clinical practice. Third, given that BC clinicians often rely on their patients to drive conversations about sexual health,16 increased engagement by patients around this topic could influence clinicians’ communication, as well, although this will need to be examined empirically. Finally, the findings offer evidence of potential broader-ranging health benefits of addressing sexual health, such as on patients’ psychological well-being, and could thus further incentivize BC clinicians to discuss sexual health with their patients.
Conclusions
Sexual health is a consistently unmet health need for women with BC. Yet even when experiencing sexual concerns, many women with BC are reluctant to raise this topic with their clinicians. Patient-focused interventions hold promise for integrating sexual health into cancer care for women with cancer.
Supplementary Material
Acknowledgments:
Jennifer B. Reese, PhD was supported by a Mentored Research Scholar Grant, MRSG-14-031-CPPB from the American Cancer Society and P30CA006927 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/pon.5613.
Conflicts of Interest: None.
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Howlader N, Noone AM, Krapcho M et al. SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. [Google Scholar]
- 2.Panjari M, Bell RJ, Davis SR. Sexual Function after Breast Cancer. J Sex Med 2011; 8:294–302. [DOI] [PubMed] [Google Scholar]
- 3.Safarinejad MR, Shafiei N, Safarinejad S. Quality of life and sexual functioning in young women with early-stage breast cancer 1 year after lumpectomy. Psychooncology 2013; 22:1242–1248. [DOI] [PubMed] [Google Scholar]
- 4.Boquiren VM, Esplen MJ, Wong J et al. Sexual functioning in breast cancer survivors experiencing body image disturbance. Psychooncology 2016; 25:66–76. [DOI] [PubMed] [Google Scholar]
- 5.Lee M, Kim YH, Jeon MJ. Risk factors for negative impacts on sexual activity and function in younger breast cancer survivors. Psycho-oncology 2015; 24:1097–1103. [DOI] [PubMed] [Google Scholar]
- 6.Pesek S, Onstad M, Fogarty S et al. Sexual Function After Breast Cancer Surgery. Gynecol Oncol 2015; 139:588. [Google Scholar]
- 7.Reese JB, Porter LS, Casale KE et al. Adapting a couple-based intimacy enhancement intervention to breast cancer: A developmental study. Health Psychol 2016; 35:1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumgart J, Nilsson K, Evers AS et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause 2013; 20:162–168. [DOI] [PubMed] [Google Scholar]
- 9.Runowicz CD, Leach CR, Henry NL et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin 2016; 66:43–73. [DOI] [PubMed] [Google Scholar]
- 10.Denlinger CS, Sanft T, Baker KS et al. Survivorship, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017; 15:1140–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill EK, Sandbo S, Abramsohn E et al. Assessing gynecologic and breast cancer survivors’ sexual health care needs. Cancer 2011; 117:2643–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Den Ouden MEM, Pelgrum-Keurhorst MN, Uitdehaag MJ, De Vocht HM. Intimacy and sexuality in women with breast cancer: professional guidance needed. Breast Cancer 2019; 26:326–332. [DOI] [PubMed] [Google Scholar]
- 13.Carter J, Lacchetti C, Andersen BL et al. Interventions to Address Sexual Problems in People With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Adaptation of Cancer Care Ontario Guideline. J Clin Oncol 2017; 36:492–511. [DOI] [PubMed] [Google Scholar]
- 14.Flynn KE, Reese JB, Jeffery DD et al. Patient experiences with communication about sex during and after treatment for cancer. Psychooncology 2012; 21:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reese JB, Sorice K, Beach MC et al. Patient-provider communication about sexual concerns in cancer: a systematic review. J Cancer Surviv 2017; 11:175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reese JB, Beach MC, Smith KC et al. Effective patient-provider communication about sexual concerns in breast cancer: A qualitative study. Support Care Cancer 2017; 25:3199–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor S, Harley C, Takeuchi E et al. Detecting and Discussing Sexual Problems during Chemotherapy for Breast Cancer. The Breast Journal 2013; 19:566–567. [DOI] [PubMed] [Google Scholar]
- 18.Reese JB, Sorice K, Lepore SJ et al. Patient-Clinician Communication about Sexual Health in Breast Cancer: A Mixed-Methods Analysis of Clinic Dialogue. Patient Educ Couns 2019; 102:436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sporn NJ, Smith KB, Pirl WF et al. Sexual health communication between cancer survivors and providers: how frequently does it occur and which providers are preferred? Psychooncology 2014. [DOI] [PubMed] [Google Scholar]
- 20.Bandura A Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, NJ: Prentice Hall, Inc. 1986. [Google Scholar]
- 21.Oken MM, Creech RH, Tormey DC et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–655. [PubMed] [Google Scholar]
- 22.Reese JB, Lepore SJ, Daly MB et al. A brief intervention to enhance breast cancer clinicians’ communication about sexual health: Feasibility, acceptability, and preliminary outcomes. Psychooncology 2019; 28:872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandura A Guide for Constructing Self-Efficacy Scales. In Pajares F, Urdan T (eds): Self-Efficacy Beliefs of Adolescents, Edition Greenwich, CT: IAP-Information Age Publishing Inc. 2006; 307–338. [Google Scholar]
- 24.ten Klooster PM, Oostveen JCM, Zandbelt LC et al. Further validation of the 5-item Perceived Efficacy in Patient–Physician Interactions (PEPPI-5) scale in patients with osteoarthritis. Patient Educ Couns 2012; 87:125–130. [DOI] [PubMed] [Google Scholar]
- 25.Hordern A, Grainger M, Hegarty S et al. Discussing sexuality in the clinical setting: The impact of a brief training program for oncology health professionals to enhance communication about sexuality. Asia-Pac J Clin Oncol 2009; 5:270–277. [Google Scholar]
- 26.Zimmaro LA, Lepore SJ, Beach MC, Reese JB. Patients’ Perceived Barriers to Discussing Sexual Health with Breast Cancer Healthcare Providers. Psychooncology 2020; 29:1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinfurt KP, Lin L, Bruner DW et al. Development and Initial Validation of the PROMIS® Sexual Function and Satisfaction Measures Version 2.0. J Sex Med 2015; 12:1961–1974. [DOI] [PubMed] [Google Scholar]
- 28.Reese JB, Sorice KA, Pollard W et al. Understanding sexual help-seeking for women with breast cancer: What distinguishes women who seek help from those who do not? J Sex Med 2020; 17:1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ljungman L, Ahlgren J, Petersson L-M et al. Sexual dysfunction and reproductive concerns in young women with breast cancer: Type, prevalence, and predictors of problems. Psychooncology 2018; 27:2770–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica 1983; 67:361–370. [DOI] [PubMed] [Google Scholar]
- 31.Brady MJ, Cella DF, Mo F et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 1997; 15:974–986. [DOI] [PubMed] [Google Scholar]
- 32.Dimoska A, Tattersall MHN, Butow PN et al. Can a “prompt list” empower cancer patients to ask relevant questions? Cancer 2008; 113:225–237. [DOI] [PubMed] [Google Scholar]
- 33.Henselmans I, de Haes HCJM, Smets EMA. Enhancing patient participation in oncology consultations: a best evidence synthesis of patient-targeted interventions. Psychooncology 2013; 22:961–977. [DOI] [PubMed] [Google Scholar]
- 34.Wang LY, Pierdomenico A, Lefkowitz A, Brandt R. Female Sexual Health Training for Oncology Providers: New Applications. Sexual medicine 2015; 3:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winterling J, Lampic C, Wettergren L. Fex-Talk: a Short Educational Intervention Intended to Enhance Nurses’ Readiness to Discuss Fertility and Sexuality with Cancer Patients. J Cancer Educ 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bandura A Health promotion from the perspective of social cognitive theory. Psychol Health 1998; 13:623–649. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


