Abstract
Background:
Autologous hematopoietic stem cell transplantation (ASCT) is a standard treatment for multiple myeloma (MM). Consensus guidelines recommend collecting sufficient stem cells in case there is need for stem cell boost for delayed/poor engraftment or for future second ASCT. However, collecting and storing backup stem cells in all patients requires significant resources and cost, and the rates of backup stem cell utilization are not well studied.
Objective:
To examine the utilization of backup stem cells (BSCs) in patients with MM undergoing ASCT.
Study Design:
Patients with MM aged ≥18 years old who underwent first ASCT at our institution from January 2010 through December 2015 and collected sufficient stem cells for at least two transplants were included in this single-center retrospective study. This timeframe was selected to allow for adequate follow-up.
Results:
A total of 393 patients were included. The median age was 58 years (range 25–73). After a median follow-up of 6 years, the median PFS of the cohort was 3 years. 61% (n = 240) of patients progressed or relapsed. Chemotherapy based mobilization was used in almost all patients (98%). The median total CD34+ cells collected was 18.2 × 106/kg (range 3.4–112.4). A median of 5.7 × 106 CD34+ cells/kg (range 1.8–41.9) were infused during the first ASCT and a median of 10.1 × 106 CD34+ cells/kg (range 1.5–104.5) were cryopreserved for future use. 6.9% (n = 27) of patients utilized backup stem cells, with 2.3% (n = 10) using them for stem cell boost, 4.6% (n = 18) for a second salvage ASCT, including 1 patient for both stem cell boost and second ASCT. Rates of backup stem cell use amongst patients aged <60, 60–69, and ≥70 years were 7.8%, 5.7%, and 5.9%, respectively. There was a trend toward higher rates of backup stem cell utilization for second ASCT in patients who were younger, had suboptimal disease control at time of first ASCT, and longer PFS. The median dose of stem cell boost given was 5.6 × 106 CD34+ cells/kg (range 1.9–20). The median time from stem cell boost to neutrophil, hemoglobin, and platelet engraftment was 4 (range 2–11), 15 (range 4–34), and 12 (range 0–34) days, respectively. Lower CD34+ dose and older age at time of ASCT predicted need for stem cell boost.
Conclusions:
With new salvage therapies for relapsed MM, the rates of second ASCT are very low. The low rates of utilization suggest that institutional policies regarding universal BSC collection and long-term storage should be reassessed and individualized. However, need for stem cell boost in 2.3% of patients may present a challenge to that.
Keywords: multiple myeloma, autologous stem cell transplantation, non-engraftment, delayed engraftment, salvage transplantation, mobilization, cryopreservation
Introduction
Autologous stem cell transplant (ASCT) is a standard of care treatment for eligible patients with multiple myeloma (MM).1 Patients typically receive induction therapy with chemotherapy regimens consisting of immunomodulatory agents, proteasome inhibitors, and anti-CD38 monoclonal antibodies.1,2 Consolidative ASCT after induction therapy has been shown to improve progression free survival,1,3 with some studies also showing an overall survival (OS) benefit.4–11 Most institutional and consensus guidelines recommend that at time of first ASCT, enough hematopoietic stem cells (HSC) should be collected to perform two stem cell transplants, with a minimum goal of 2 million CD34+ cells/kg.12–14 The back-up stem cells may be used for second transplants or less commonly for stem cell boosts in case of non-engraftment or delayed engraftment. However, with newer therapy options conferring increased OS rates, patients and physicians may opt to delay first ASCT.15 Moreover, patients who undergo ASCT in their late 60s or 70s are unlikely to be candidates for second transplants later in the disease course.15 Collecting enough stem cells to perform two transplants requires considerable resources, including additional days of stem cell collection and long-term cryopreservation.
Back up stem cells may also be used for stem cell boost without additional conditioning in case of poor graft function or delayed engraftment. Delayed engraftment or poor graft function is a rare, life-threatening post-transplantation complication associated with infections, bleeding, and secondary iron overload due to transfusions. However, while the treatment of poor graft function with stem cell boost has been established in allogeneic stem cell transplantation16–20, it has not been well studied in ASCT for treatment of diseases such as MM.
There is limited data on the utilization of backup stem cells for stem cell boost or second ASCT in patients with multiple myeloma.21,22 It is unclear if backup stem cells should be continue to be collected in all patients, or in a select group of patients who are at risk of potential poor graft function and who may be candidates for a second transplant in the future. Understanding the utilization of backup stem cells can identify specific groups of patients for whom backup stem cells should be collected and subsequently optimize healthcare resource utilization. In this study, we evaluated the utilization of backup stem cells for stem cell boost and second ASCT in these patients. For patients requiring stem cell boost, we examine the incidence and risk factors for PGF and describe engraftment and cell count recovery following stem cell boost.
Methods
Study Population
Patients with MM aged ≥18 years old who underwent first ASCT at our institution from January 2010 through December 2015 and who collected sufficient HSCs for two transplants to allow for stem cell boost or second ASCT were included in this study. Sufficient HSCs was defined as at least 1.5 × 10 cells/kg remaining after first ASCT. Forty-seven patients were excluded because they did not collect sufficient HSCs. Patients receiving tandem transplants (n = 3) were excluded. Data on demographics, disease characteristics, response, progression, total number of stem cells collected, number of apheresis days, mobilization regimen, and use of backup stem cells were collected. This retrospective study was approved by the Institutional Review Board of Stanford University.
Definitions
Patients were risk stratified by the International Staging System23 and presence of high-risk cytogenetics on FISH, defined as presence of deletion 17p/monosomy 17, t(4;14), t(14;16), t(14;20), or gain 1q.24 Response to therapy prior to first ASCT was defined by the International Myeloma Working Group consensus criteria (PR = partial response, VGPR = very good partial response, CR = complete response, sCR = stringent complete response, SD = stable disease, PD = progressive disease).25
Second ASCT was defined as infusion of HSCs after administration of high dose therapy in patients with MM who had progressed after a prior line of therapy, including first ASCT. Date of engraftment for each cell line was defined as follows: for neutrophils, the first of three consecutive days where the absolute neutrophil count (ANC) is higher than 500 cells/mm3 (0.5 K/uL); for hemoglobin, the first day where the hemoglobin is greater than 8 g/dL without red blood cell transfusion in the last 7 days; for platelets, the first day where the platelet count is greater than 20 K/uL without platelet transfusion in the last seven days (must be confirmed by repeat value). All patients received G-CSF 5 mcg/kg post-transplant until neutrophil engraftment. Stem cell boost was defined as infusion of HSCs for delayed engraftment or non-engraftment following first ASCT, with the goal of improving blood count recovery. Delayed engraftment was defined as failure to engraft at least one cell line within the expected time frame after ASCT; non-engraftment was defined as failure to engraft any cell lines within the expected time frame. The expected time frame and decision to administer boost were at the discretion of the treating physician. Progression-free survival (PFS) was defined as time from transplant to progression or death.
Mobilization protocol
Mobilization protocol is described in detail under supplementary methods. Chemotherapy based mobilization occurred using cyclophosphamide 4 g/m2 alone or cyclophosphamide followed by etoposide 2 g/m2 (sequential cycles) with G-CSF 10 mcg/kg/day starting the day after chemotherapy. GCSF-based mobilization was done using G-CSF 10 mcg/kg/day for 4 days and plerixafor was added on day 4 if day 4 CD34+ count was less than 10/microL.
Across different mobilization regimens, the goal of collection was at least 2 million CD34+ cells/kg for planned transplant, with additional collection of 2 million CD34+ cells/kg preferred for back-up.
Statistical analysis
Statistical analysis was performed using JMP Pro 15 (SAS). Chi-Square and Fischer Exact tests were used to carry out univariate analysis for categorical variables and Wilcoxon Rank Sum/Kruskal Wallis for continuous variables. Survival Analysis was carried out using the Kaplan-Meier method and the log-rank test was used to compare survival curves.
Results
Characteristics of study population
A total of 393 patients were included (Table 1). The median age at first ASCT was 58 years and 63% (n = 246) of patients were male. 15% (n = 60) of patients had high-risk cytogenetics at diagnosis. 49% (n = 194) of patients received one line of induction therapy before first ASCT; 39% (n = 154) received doublet regimens and 55% (n = 216) received triplet regimens. Maintenance was administered in 28% (n = 110) of patients after first ASCT. After a median follow-up of 6 years since transplant, the median PFS of the cohort was 3 years and median overall survival was 9 years. 61% (n = 240) of patients experienced progression or relapse by last follow-up.
Table 1.
Patient demographics and stem cell collection characteristics
| Characteristic | % (n), N = 393 | |
|---|---|---|
| Age at ASCT, median, years (range) | 58 (25–73) | |
| Male | 63 (246) | |
| Race | Hispanic or Latino | 14 (55) |
| Asian | 11 (45) | |
| Black | 6 (25) | |
| Other | 1 (4) | |
| Pacific Islander | 1 (3) | |
| Unknown | 17 (66) | |
| White | 64 (250) | |
| ISS stage | 1 | 34 (133) |
| 2 | 22 (85) | |
| 3 | 18 (69) | |
| N/A | 27 (106) | |
| High-risk cytogenetics1 (N/A in n = 45) | 15 (60) | |
| Lines of chemotherapy before first ASCT | 1 | 49 (194) |
| 2 | 32 (125) | |
| 3 | 11 (42) | |
| >3 | 8 (32) | |
| Induction regimen | Doublet | 39 (154) |
| Triplet | 55 (216) | |
| Quadruplet | 2 (6) | |
| Other | 4 (17) | |
| Maintenance | Yes | 28 (110) |
| No | 72 (283) | |
| Mobilization method | Cyclophosphamide | 40 (159) |
| Cyclophosphamide + etoposide | 57 (225) | |
| G-CSF-based | 2 (8) | |
| D-PACE | 0.3 (1) | |
| Relapse after first ASCT | 61 (240) | |
| Median (range) | ||
| Number of apheresis sessions | 2 (1–8) | |
| Total CD34+ cells collected (x106 cells/kg) | 18.2 (3.4–112.4) | |
| CD34+ cells used in first ASCT (x106 cells/kg) | 5.7 (1.8–41.9) | |
| CD34+ cells remaining after first ASCT (x106 cells/kg) | 10.1 (1.5–104.5) | |
Table Abbreviations: ISS = International Staging System; ASCT = autologous stem cell transplant; G-CSF = granulocyte colony-stimulating factor; D-PACE = dexamethasone, cisplatin, doxorubicin, cyclophosphamide, etoposide
High-risk cytogenetics were defined as deletion 17p/monosomy 17, t(4;14), t(14;16), t(14;20), or gain 1q
Characteristics of HSC collection
98% of patients (n = 385) underwent chemotherapy-based mobilization. 40% (159) underwent cyclophosphamide based mobilization, 57% (n = 225) underwent sequential cyclophosphamide and etoposide based mobilizations, and 1 patient underwent dexamethasone, cisplatin, doxorubicin, cyclophosphamide, and etoposide (D-PACE) based mobilization. The median number of apheresis sessions for the cohort was 2 (range 1–8), with 85% of patients undergoing 1 or 2 apheresis sessions. The median total CD34+ cells collected was 18.2 × 106/kg (range 3.4–112.4). A median of 5.7 × 106 CD34+ cells/kg (range 1.8–41.9) were infused during the first ASCT and a median of 10.1 × 106 CD34+ cells/kg (range 1.5–104.5) were cryopreserved for future use.
Utilization of backup stem cells
Overall, 6.9% (n = 27) of patients utilized backup stem cells, with 2.5% (n = 10) using them for stem cell boost for delayed or non-engraftment, 4.6% (n = 18) for a second salvage ASCT, including one patient who underwent both stem cell boost and second ASCT (Table 2). Rates of backup stem cell use (n = stem cell boost and second salvage ASCT) amongst patients aged <60, 60–69, and ≥70 years were 7.8% (5 and 13), 5.8% (4 and 5), and 5.9% (1 and 0), respectively (Figure 1). Patients with ISS stage 1, 2, and 3 disease utilized backup stem cells for stem cell boost at rates of 1.5% (n = 2), 3.5% (n = 3), and 2.9% (n = 2), respectively, compared to rates of 6.0% (8), 4.7% (n = 4), and 4.3% (n = 3) for second ASCT. 3.3% (n = 2) of patients with high-risk cytogenetics used backup stem cells (1 for boost and 1 for second ASCT); no patients with deletion 17p used backup stem cells. Depth of response prior to first ASCT inversely correlated with backup stem cell use for second salvage ASCT, with 1.7% of patients in CR pre-transplant compared to 11.1% patients with stable disease.
Table 2.
Use of backup stem cells for second ASCT and stem cell boost
| Characteristic | % using backup stem cells for second ASCT (n) | % using backup stem cells for stem cell boost (n) | |
|---|---|---|---|
| Overall | 4.8 (19) | 2.5 (10) | |
| Age, years | <60 | 5.9 (13) | 2.3 (5) |
| 60–70 | 3.2 (5) | 2.5 (4) | |
| >70 | 0 (0) | 5.9 (1) | |
| Cytogenetics | Standard risk | 5.6 (16) | 2.4 (7) |
| High risk | 1.7 (1) | 1.7 (1) | |
| ISS stage | 1 | 6.0 (8) | 1.5 (2) |
| 2 | 4.7 (4) | 3.5 (3) | |
| 3 | 2.9 (2) | 2.9 (2) | |
| Duration of first remission in relapsed patients (n = 240) | <2 years | 4.8 (6) | 2.4 (3) |
| 2–3 years | 4.8 (2) | 0 (0) | |
| ≥3 years | 13.9 (10) | 4.2 (3) | |
| Response status prior to first ASCT | SD | 16.7 (3) | 5.6 (1) |
| VGPR | 5.9 (7) | 1.7 (2) | |
| PR | 3.8 (7) | 2.7 (5) | |
| CR | 1.7 (1) | 3.4 (2) | |
| Stringent CR | 0 (0) | 0 (0) | |
| PD | 0 (0) | 0 (0) | |
Table abbreviations: SD = stable disease, VGPR = very good partial response; PR = partial response; CR = complete response; PD = progressive disease
Figure 1.
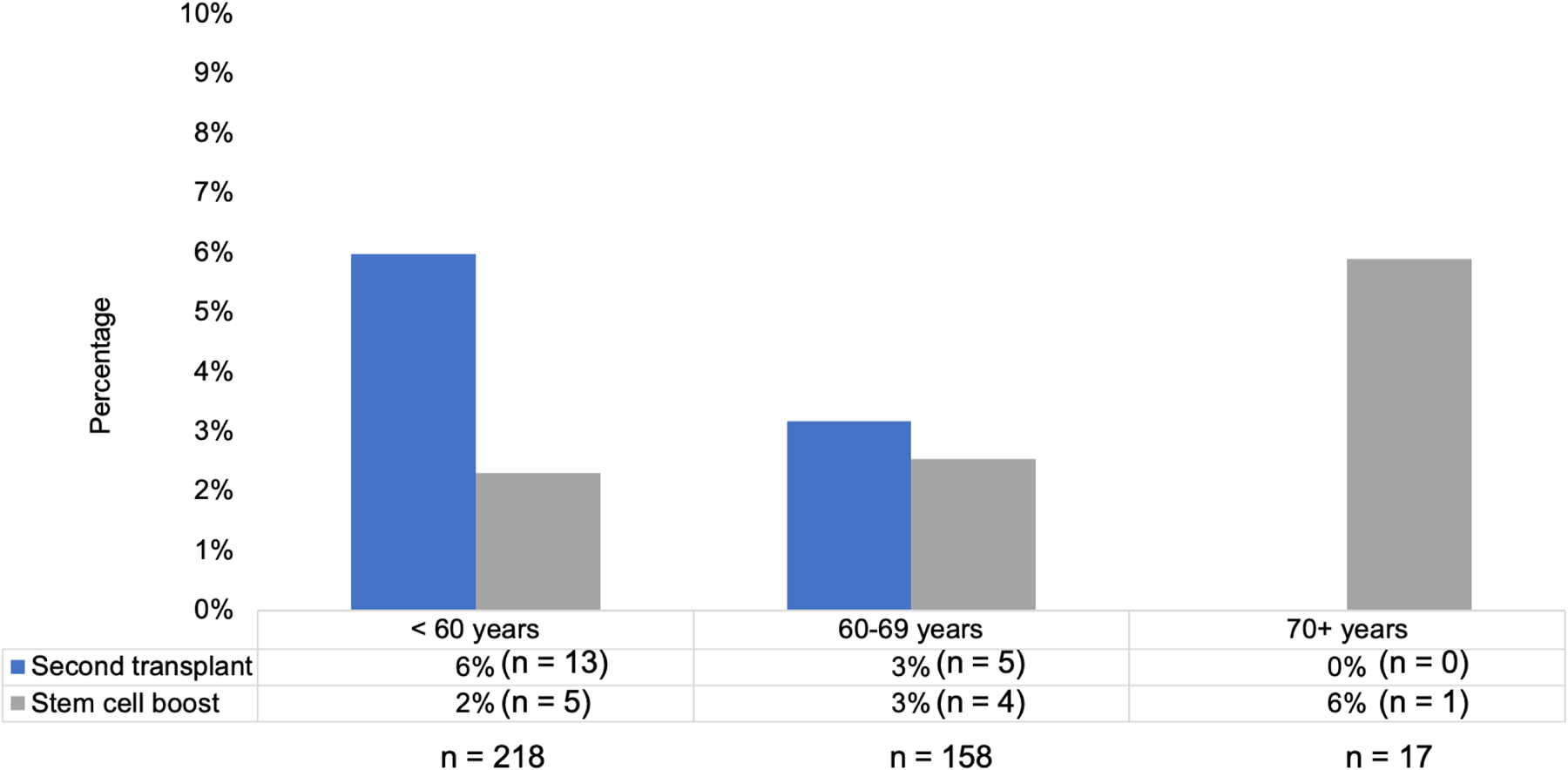
Rates of backup stem cell use by age for second transplant and stem cell boost
Second transplant in relapsed patients
Amongst the 240 patients experiencing a relapse, over a median follow up time of 3.5 years since relapse, 7.5% (n = 18) patients used backup stem cells for a second transplant (Figure 2). Within this subset, rates of backup stem cell utilization for second transplant based on duration of response after first transplant (progression-free survival) of <2, 2–3, and ≥3 years were 4.7% (n = 6), 4.8% (n = 2), and 13.9% (n = 10), respectively.
Figure 2.
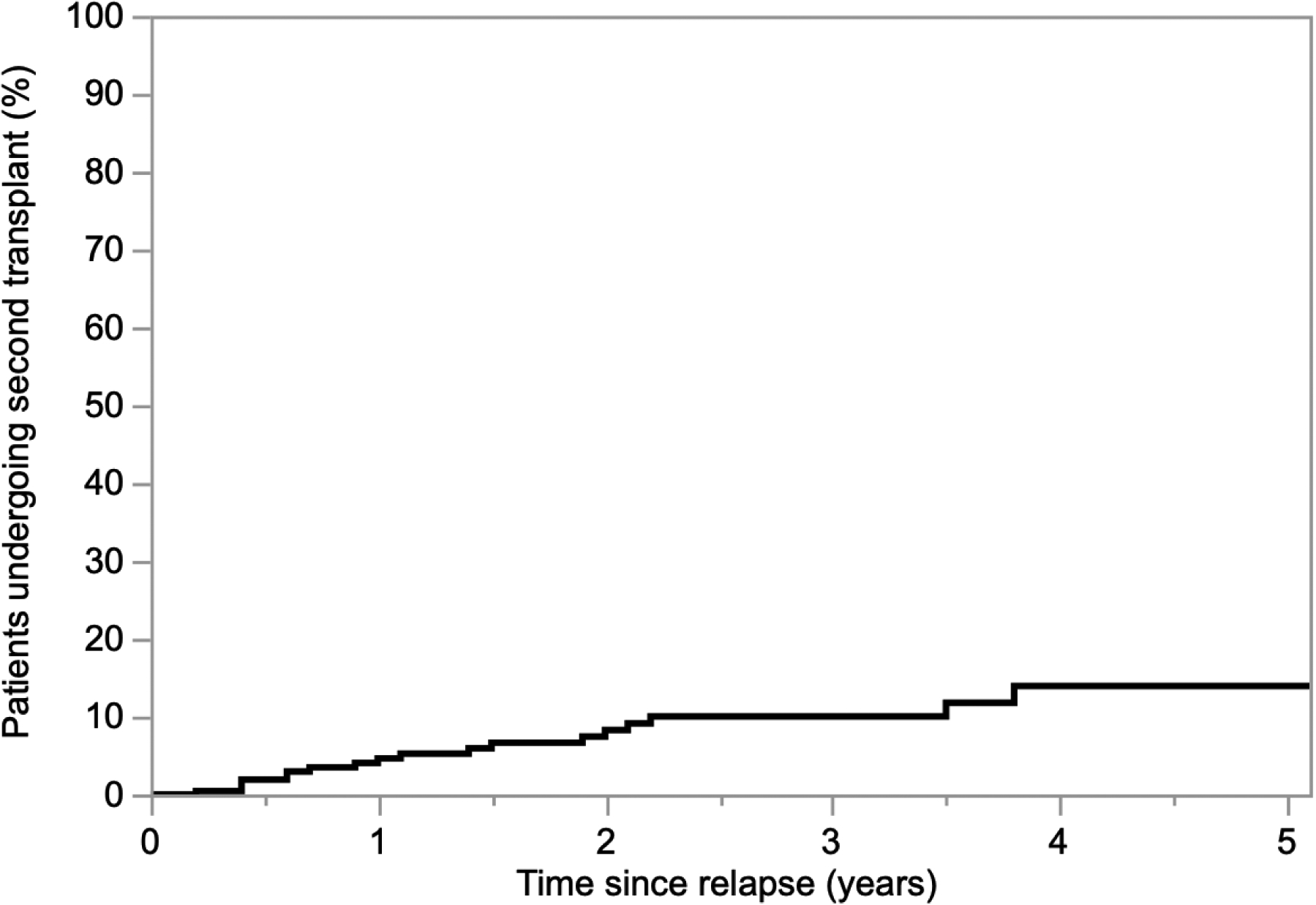
Cumulative use of backup stem cells for second transplant following relapse
Characteristics of patients who utilized backup stem cells for stem cell boost
Ten patients needed cryopreserved stem cells for stem cell boost, including one patient who underwent two stem cell boosts (Table 3). The median age of these patients was 62 years (range 47–72). The median dose of stem cells infused at the time of transplant was 3.1 × 106 CD34+ cells/kg (range 1.8–18). Median dose of stem cells given at the time of stem cell boost was 5.6 × 106 CD34+ cells/kg (range 1.9–20). Boost was given at a median of 35 days after ASCT (range 17–81). Boost was given for non-engraftment of all cell lines in 4 patients, isolated non-engraftment of platelets in 5 patients, and non-engraftment of both red blood cells and platelets in 1 patient. One patient required stem cell boost for new pancytopenia of unknown etiology 81 days after initial ASCT. No patients required boost for isolated neutrophil non-engraftment. In all cases of non- or delayed engraftment, patients engrafted successfully after stem cell boost (Figure 2).The median time for neutrophil, hemoglobin, and platelet engraftment after boost was 4 (range 2–11), 15 (range 4–34), and 12 (range 0–34) days, respectively.
Table 3.
Characteristics of stem cell boost population (n = 10, including one patient who required two stem cell boosts)
| Characteristic | Median (range) | ||
|---|---|---|---|
| Age, years | 62 (47–72) | ||
| Response status prior to first ASCT | SD | 1 | |
| PR | 5 | ||
| CR/VGPR | 4 | ||
| CD34+ dose at ASCT (x 106/kg) | 3.1 (1.8–18) | ||
| Day of stem cell boost after ASCT | 35 (17–81) | ||
| Stem cell boost dose (CD34+ cells × 106/kg) | 5.6 (1.9–20) | ||
| Day of engraftment following stem cell boost | Neutrophil | 4 (2–11) | |
| Hemoglobin | 15 (4–34) | ||
| Platelet | 12 (0–34) | ||
| Number of patients | |||
| Indication for boost | Delayed engraftment | 6 | |
| Non-engraftment | 4 (including 1 patient who required second boost for delayed engraftment) | ||
| New pancytopenia | 1 | ||
Table abbreviations: ASCT = autologous stem cell transplant; SD = stable disease, VGPR = very good partial response; PR = partial response; CR = complete response; PD = progressive disease
Predictors of delayed/non-engraftment requiring stem cell boost were lower pre-transplant platelet count (p = 0.003), particularly fewer than 100 × 103/L (27% vs. 1.8%, p = 0.002) and lower CD34+ dose at time of transplant (p = 0.05), particularly fewer than 2.5 × 106 cells/kg given (9.3% vs. 1.7%, p = 0.02). Patients older than 65 years tended to be more likely to require boost (5.8% vs. 1.8%, p = 0.08). Pre-transplant white blood cell count, absolute neutrophil count, and hemoglobin, both when analyzed as continuous variables and discrete variables with cutoffs, were not associated with need for stem cell boost. Response status prior to transplant was not associated with need for boost (at least partial response vs. not, p = 0.53; very good partial response vs. not, p = 0.79). Given the very few events of delayed engraftment requiring stem cell boost, we did not attempt a multivariate analysis.
Discussion
In this study, we found that rates of backup stem cell utilization were very low, ranging from 2.3% for stem cell boost for delayed/non-engraftment and 4.6% for second ASCT in the entire cohort. In the sub-group experiencing relapse, rates of second transplant were 7.5% Patients who were younger, had suboptimal disease control at time of first ASCT, but longer PFS following transplant were most likely to undergo second ASCT, though these results did not reach statistical significance and should be interpreted with caution. The most common indications for stem cell boost were non-engraftment of all cell lines and isolated non-engraftment of platelets. All patients who received stem cell boost ultimately engrafted successfully, with platelets having the longest engraftment time.
Second ASCT has been shown to increase OS in patients with relapsed or refractory MM compared to chemotherapy.5–8 In particular, the Myeloma X and GMMG trials demonstrated that salvage ASCT had superior OS and PFS compared to continuous cyclophosphamide in relapsed patients who had remission ≥18 months and lenalidomide/dexamethasone in relapsed patients who had remission ≥12 months, respectively.5,6 However, in the Myeloma X trial, 30% of patients could not undergo second ASCT due to insufficient collection at initial ASCT.6 Consensus guidelines therefore recommend collecting enough stem cells for two transplants, and consideration of second salvage ASCT for relapsed patients with long remission after first ASCT, with minimum PFS after first ASCT being at least of ≥18 months.14,26 As a result of newer therapeutic options at relapse and some patients delaying second ASCT until progression, second transplants are no longer routinely performed.14 Tandem transplants are also less common now with results of the STAMINA trial not showing a benefit for tandem transplants.27 In our study, 60% of patients relapsed with current follow-up, with only 7.5% of these patients going on receive second ASCT. Our 4.6% overall rate of backup stem cell use for second ASCT are lower than other studies (Phipps et al., 19% and Chhabra et al., 12.0%).21,22 Similar to prior studies, predictors of second ASCT in our cohort were younger age, poorer disease control at time of first ASCT, and longer PFS after transplant, indicative of chemo-responsiveness to high-dose therapy.7,28,29
In our cohort, ten patients used backup stem cells for stem cell boost due to poor graft function. Decision to use backup stem cells for delayed/non-engraftment was at the decision of the treating physician as there are no consensus guidelines for this rare complication. Engraftment times following stem cell boost in our study were similar to or shorter than prior reported engraftment times following initial ASCT.30–34 A previous study evaluating poor graft function in patients with various hematogenous malignancies undergoing autologous stem cell transplant found that predictors of poor graft function included higher weight at time of transplant, Caucasian ethnicity, receipt of maintenance therapy, transfusion dependence, pretransplant platelet count, and lower infused CD34+ dose.35 The incidence of delayed engraftment in multiple myeloma was 10% in this study, with 2.3% requiring stem cell boost.35 In our study examining specifically MM patients undergoing autologous stem cell transplant, we found that lower infused CD34+ dose and older age predicted use of stem cell boost, whereas pre-transplant response status was not associated with poor graft function.
Our stem cell boost rate of 2.3% is similar to the 2% rate reported in a recent study by Chhabra et al. examining backup stem cell utilization at their institution.22 They found that only 9% of backup stem cells were used for stem cell boost (2%) or second ASCT (7%), with an estimated mean additional cost of HSC collection of $10,795 per patient.22 These findings led to revision of their institutional HSC collection targets, in particular decreasing the collection target for patients ≥70 years of age.22 While our low rates of utilization suggest that institutional policies regarding universal backup stem cell collection and long-term storage should be reassessed and individualized, the need for stem cell boost for poor graft function presents a challenge. As demonstrated in this study, it is crucial to have backup stem cells available to treat life-threatening poor graft function/true non-engraftment. For example, if we had decreased collection targets for patients ≥70 years of age, our 72-year-old patient would not have had sufficient cells for stem cell boost for platelet non-engraftment.
Our findings raise the question of the benefit of continuing to collect adequate stem cells for two transplants in all patients. As discussed above, some patients can derive a significant benefit from a second salvage transplant and stem cell boost can preempt significant morbidity in case of the rare, but difficult to predict and catastrophic complication of poor graft function. The disadvantages are that the collection of additional stem cells often requires additional days of apheresis and resources for long-term storage of cryopreserved cells. After consideration of all factors, including potential benefits and resource, we have continued to maintain a sufficient HSC collection goal at our institution in case of need for second ASCT or stem cell boost if it can be easily collected. A potential approach is to discard cryopreserved HSCs for patients who are older than 75, have poor performance status, uncontrolled comorbidities, or other contraindications.36 This approach has not been utilized at our institution and it may be difficult to keep track of dynamic health status of patients with cryopreserved HSCs. As cellular immunotherapy such as CAR-T cell therapy emerges as a treatment option for myeloma, backup stem cells may play a role in management of cytopenias following CAR-T therapy. Patients can have delayed immune reconstitution following CAR-T therapy.37,38 In a subset of patients, cytopenias may be prolonged and severe enough to require extended growth factor and transfusion support, although the underlying mechanism remains unclear. Infusion of stored stem cells could have a future role in expediting count recovery. However, there is no clear data on the success rate of using stem cell boost in this situation. Ongoing innovation in stem cell mobilization methods with newer drugs may further decrease the side-effects, time taken and resources needed for stem cell mobilization.39 Such methods are presently being studied in ongoing trials in patients with myeloma and in healthy volunteers. (NCT04552743; NCT03932864).40,41
The main limitation of this study is its single-center design. Transplant centers often have different HSC collection targets and protocols for mobilization, apheresis, cryopreservation, and storage, which can affect the number of HSC available for backup. Center-specific practices may also lead to variable rates of second ASCT and stem cell boost. We also observed a very low number of patients utilizing backup stem cells for boost, which limits our ability to examine predictors of poor graft function in this cohort. In addition, nearly all patients in this study (98%) underwent chemomobilization, which has been replaced by GCSF +/− plerixafor as the standard initial mobilization strategy at most centers in the United States.42 While chemomobilization results in higher collection yields and fewer days of apheresis compared to growth factors, it also leads to increased risk of complications such as neutropenic fever.43–45 Therefore, American Society for Blood and Marrow Transplantation guidelines recommend G-CSF and plerixafor based mobilization for the majority of patients with myeloma.45 While chemotherapy based mobilization was our institutional standard during the study period, we have recently transitioned to G-CSF+/−plerixafor based mobilization for the majority of patients. We selected our study cohort to be patients who underwent first ASCT between 2010 and 2015 in order to allow for sufficient follow up to capture backup stem cell utilization for second ASCT. Lastly, we did not calculate the financial implications additional sessions of apheresis needed to collect additional cells or costs for long-term storage of unused backup stem cells, as Chhabra et al. did at their institution.22 As it has been many years since our cohort underwent first transplantation, this data are not readily available. As chemomobilization is also no longer standard of practice, financial costs associated with mobilization would not be clinically relevant.
In conclusion, we show that rates of backup stem cell utilization for second ASCT and stem cell boost in patients with multiple myeloma undergoing autologous transplant are very low. However, the survival benefit of second ASCT in select patients and particularly need for stem cell boost for poor graft function may necessitate continuing to collect and store backup stem cells in all patients. Future studies should evaluate factors which may a-priori help identify patients who are at risk of poor graft function or who may benefit from a second transplant.
Supplementary Material
Highlights.
Rates of backup stem cell use in MM patients are very low (<10%)
Almost all patients who utilized backup stem cells were <70 years old
The need for stem cell boost is a limiting factor for reducing HSC collection goals
FUNDING:
SS was supported by: KL2TR003143, KL2 Mentored Career Development Program, Stanford Clinical Translational Science Award Program
Financial disclosure statement:
AR: One-time ad hoc scientific advisory boards for Nohla and for Kaleido, both in 2018. Medical expert witness work for U.S. Department of Justice. Brother works for Johnson & Johnson. SS: Consultancy: Janssen. Research Funding: Janssen, Allogene, Magenta Therapeutics.
References
- 1.Attal M et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med 376, 1311–1320 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voorhees PM et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 136, 936–945 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benboubker L et al. Lenalidomide and Dexamethasone in Transplant-Ineligible Patients with Myeloma. N. Engl. J. Med 371, 906–917 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Rajkumar SV Multiple myeloma: 2018 update on diagnosis, risk-stratification, and management. Am. J. Hematol 93, 1091–1110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldschmidt H et al. Salvage autologous transplant and lenalidomide maintenance vs. lenalidomide/dexamethasone for relapsed multiple myeloma: the randomized GMMG phase III trial ReLApsE. Leukemia (2020). doi: 10.1038/s41375-020-0948-0 [DOI] [PubMed] [Google Scholar]
- 6.Cook G et al. High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI Myeloma X Relapse [Intensive trial]): A randomised, open-label,. Lancet Oncol. 15, 874–885 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Hagen PA & Stiff P The Role of Salvage Second Autologous Hematopoietic Cell Transplantation in Relapsed Multiple Myeloma. Biology of Blood and Marrow Transplantation 25, e98–e107 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Cook G et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol. 3, e340–e351 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Gay F et al. Survival Analysis of Newly Diagnosed Transplant-Eligible Multiple Myeloma Patients in the Randomized Forte Trial. Blood 136, 35–37 (2020). [Google Scholar]
- 10.Perrot A et al. Early Versus Late Autologous Stem Cell Transplant in Newly Diagnosed Multiple Myeloma: Long-Term Follow-up Analysis of the IFM 2009 Trial. Blood 136, 39–39 (2020). [Google Scholar]
- 11.Cavo M et al. Upfront Autologous Hematopoietic Stem-Cell Transplantation Improves Overall Survival in Comparison with Bortezomib-Based Intensification Therapy in Newly Diagnosed Multiple Myeloma: Long-Term Follow-up Analysis of the Randomized Phase 3 EMN02/HO95 Study. Blood 136, 37–38 (2020). [Google Scholar]
- 12.Giralt S et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: Consensus guidelines and recommendations. Biology of Blood and Marrow Transplantation 20, 295–308 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Giralt S et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100). Leukemia 23, 1904–1912 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Gertz MA & Dingli D How we manage autologous stem cell transplantation for patients with multiple myeloma. Blood 124, 882–890 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Hamed R, Bazarbachi AH, Malard F, Harousseau JL & Mohty M Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer Journal 9, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mainardi C et al. CD34+ selected stem cell boosts can improve poor graft function after paediatric allogeneic stem cell transplantation. Br. J. Haematol 180, 90–99 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi S et al. Optimizing peripheral blood stem cells transplantation outcome through amend relapse and graft failure: a review of current literature. Exp Hematol Oncol 6, 24 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milone G et al. CD34+ selected haematopoietic stem cell (HSC) not preceded by any immunosuppressive therapy as effective treatment for graft failure (multiple letters) [1]. Bone Marrow Transplantation 35, 521–522 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Klyuchnikov E et al. CD34+-Selected stem cell boost without further conditioning for poor graft function after allogeneic stem cell transplantation in patients with hematological malignancies. Biol. Blood Marrow Transplant 20, 382–386 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Haen SP et al. Poor graft function can be durably and safely improved by CD34+-selected stem cell boosts after allogeneic unrelated matched or mismatched hematopoietic cell transplantation. J. Cancer Res. Clin. Oncol 141, 2241–2251 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phipps C et al. Utilization of stored autologous PBSCs to support second autologous transplantation in multiple myeloma patients in the era of novel agent therapy. Bone Marrow Transplant. 50, 663–667 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Chhabra S et al. Utilization and cost implications of hematopoietic progenitor cells stored for a future salvage autologous transplantation or stem cell boost in myeloma patients. Biol. Blood Marrow Transplant 0, (2020). [DOI] [PubMed] [Google Scholar]
- 23.Greipp PR et al. International staging system for multiple myeloma. J. Clin. Oncol 23, 3412–20 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Sonneveld P et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 127, 2955–2962 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. The Lancet Oncology 17, e328–e346 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Giralt S et al. American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group Consensus Conference on Salvage Hematopoietic Cell Tra. Biology of Blood and Marrow Transplantation 21, 2039–2051 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stadtmauer EA et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: Results of the BMT CTN 0702 trial. J. Clin. Oncol 37, 589–597 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michaelis LC et al. Salvage Second Hematopoietic Cell Transplantation in Myeloma. Biol. Blood Marrow Transplant 19, 760–766 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruutu T et al. Second allogeneic transplantation for relapse of malignant disease: Retrospective analysis of outcome and predictive factors by the EBMT. Bone Marrow Transplant. 50, 1542–1550 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Lemoli RM et al. Engraftment, clinical, and molecular follow-up of patients with multiple myeloma who were reinfused with highly purified CD34+ cells to support single or tandem high-dose chemotherapy. Blood 95, 2234–2239 (2000). [PubMed] [Google Scholar]
- 31.Kumar S et al. Delayed Platelet Engraftment and Outcome of Stem Cell Transplant for Multiple Myeloma: Possible Microenvironment Effect?. Blood 110, 939–939 (2007). [Google Scholar]
- 32.Kulkarni U et al. Use of Non-Cryopreserved Peripheral Blood Stem Cells Is Associated with Adequate Engraftment in Patients with Multiple Myeloma Undergoing an Autologous Transplant. Biol. Blood Marrow Transplant 24, e31–e35 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Wallington-Beddoe CT, Gottlieb DJ, Garvin F, Antonenas V & Sartor MM Failure to achieve a threshold bose of CD34+CD110+ progenitor cells in the graft predicts delayed platelet engraftment after autologous stem cell transplantation for multiple myeloma. Biol. Blood Marrow Transplant 15, 1386–1393 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Maymani H et al. Comparison of Outcomes of Allogeneic Hematopoietic Cell Transplantation for Multiple Myeloma Using Three Different Conditioning Regimens. Biol. Blood Marrow Transplant 25, 1039–1044 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutfi F et al. Clinical predictors of delayed engraftment in autologous hematopoietic cell transplant recipients. Hematol. Oncol. Stem Cell Ther 13, 23–31 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Booth GS, Gehrie EA, Jagasia MH, Shaw BE & Savani BN When Can You Discard Stem Cells? Biology of Blood and Marrow Transplantation 21, 2033 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Wang Y et al. Kinetics of Immune Reconstitution after CD19 CAR-T Cell Therapy in ALL Patients. Blood 134, 1301–1301 (2019). [Google Scholar]
- 38.Baird JH et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv. 5, 143–155 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiPersio JF et al. Rapid and Robust Mobilization of CD34+ HSCs without G-CSF Following Administration of Mgta-145 Alone or in Combination with Plerixafor. Blood 134, 1961–1961 (2019). [Google Scholar]
- 40.MGTA-145 + Plerixafor in the Mobilization of Hematopoietic Stem Cells for Autologous Transplantation in Multiple Myeloma - Full Text View - ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT04552743. (Accessed: 16th November 2020)
- 41.Study Assessing Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of MGTA-145 in Healthy Volunteers as a Single Agent or in Combination With Plerixafor - Full Text View - ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT03932864?term=MGTA-145&draw=2&rank=2. (Accessed: 16th November 2020)
- 42.Dipersio JF et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. (2009). doi: 10.1182/blood-2008-08-174946 [DOI] [PubMed]
- 43.Hamadani M et al. Intermediate-Dose versus Low-Dose Cyclophosphamide and Granulocyte Colony-Stimulating Factor for Peripheral Blood Stem Cell Mobilization in Patients with Multiple Myeloma Treated with Novel Induction Therapies. Biol. Blood Marrow Transplant 18, 1128–1135 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Wood WA et al. Chemomobilization with etoposide is highly effective in patients with multiple myeloma and overcomes the effects of age and prior therapy. Biol. Blood Marrow Transplant 17, 141–146 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Duong HK et al. Peripheral Blood Progenitor Cell Mobilization for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant 20, 1262–1273 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


