Abstract
Background:
Among boys with X-Linked adrenoleukodystrophy, a subset will develop childhood cerebral adrenoleukodystrophy (CCALD). CCALD is typically lethal without hematopoietic stem cell transplant before or soon after symptom onset. We sought to establish evidence-based guidelines detailing the neuroimaging surveillance of boys with neurologically asymptomatic adrenoleukodystrophy.
Methods:
To establish the most frequent age and diagnostic neuroimaging modality for CCALD, we completed a meta-analysis of relevant studies published between January 1, 1970 and September 10, 2019. We used the consensus development conference method to incorporate the resulting data into guidelines to inform the timing and techniques for neuroimaging surveillance. Final guideline agreement was defined as >80% consensus.
Results:
One hundred twenty-three studies met inclusion criteria yielding 1285 patients. The overall mean age of CCALD diagnosis is 7.91 years old. The median age of CCALD diagnosis calculated from individual patient data is 7.0 years old (IQR: 6.0–9.5, n = 349). Ninety percent of patients were diagnosed between 3 and 12. Conventional MRI was most frequently reported, comprised most often of T2-weighted and contrast-enhanced T1-weighted MRI. The expert panel achieved 95.7% consensus on the following surveillance parameters: (a) Obtain an MRI between 12 and 18 months old. (b) Obtain a second MRI 1 year after baseline. (c) Between 3 and 12 years old, obtain a contrast-enhanced MRI every 6 months. (d) After 12 years, obtain an annual MRI.
Conclusion:
Boys with adrenoleukodystrophy identified early in life should be monitored with serial brain MRIs during the period of highest risk for conversion to CCALD.
Keywords: adrenoleukodystrophy, childhood, cerebral, MRI, newborn screening, imaging, surveillance
1 |. INTRODUCTION
X-linked adrenoleukodystrophy (ALD) is caused by mutations in the ABCD1 gene. Mutations lead to an accumulation of very long chain fatty acids (VLCFA) in plasma, adrenal glands, testes, and the central nervous system.1 Multiple phenotypes emerge as a consequence with no established genotype-phenotype relationship.2 About 35% of affected males will develop childhood cerebral ALD (CCALD).3–5 Lesions most often originate in the splenium of the corpus callosum and, over the ensuing months, progressively spread into the adjacent white matter.6,7 Disease severity is assessed on MRI using a semi-quantitative scoring system, most commonly referred to as the “Loes Score.”8 Contrast-enhanced MRI has been shown to have a high predictive value for CCALD. 85% to 90% will progress due to inflammatory cerebral demyelination with blood brain barrier breakdown, as evidenced by perilesional contrast enhancement on MRI, followed by a rapid phase of severe, irreversible neurological injury.3,9 Hematopoietic stem cell transplantation (HSCT) is a therapeutic option for boys with active CCALD.9,10 Conversely, 10% to 21% of CCALD patients will undergo spontaneous arrest of disease, without evidence of blood brain barrier breakdown, and the lesion does not contrast-enhance.3,11–15 These individuals should not receive HSCT due to the morbidity and mortality associated with HSCT, and its failure to modify the disease course.14,16
Historically, it may have taken years for patients with CCALD to be correctly diagnosed.17 In the absence of presymptomatic neuroimaging surveillance, CCALD lesions grow silently for months or even years before they are large enough to cause overt clinical symptoms. ALD boys who presented with CCALD as their first symptom translated to large CCALD lesions on MRI (Figure 1E,F), continued accumulation of neurologic deficits, and poor HSCT outcomes.3,18–21 Importantly, several studies have demonstrated that HSCT can arrest CCALD progression if performed early, when the MRI is abnormal but neurological symptoms are not yet apparent.2,9–11,22–24 In addition, a recent clinical trial of autologous hematopoietic stem-cell gene therapy in boys with early-stage CCALD has produced encouraging results, and may soon become an additional treatment approach.25,26
FIGURE 1.
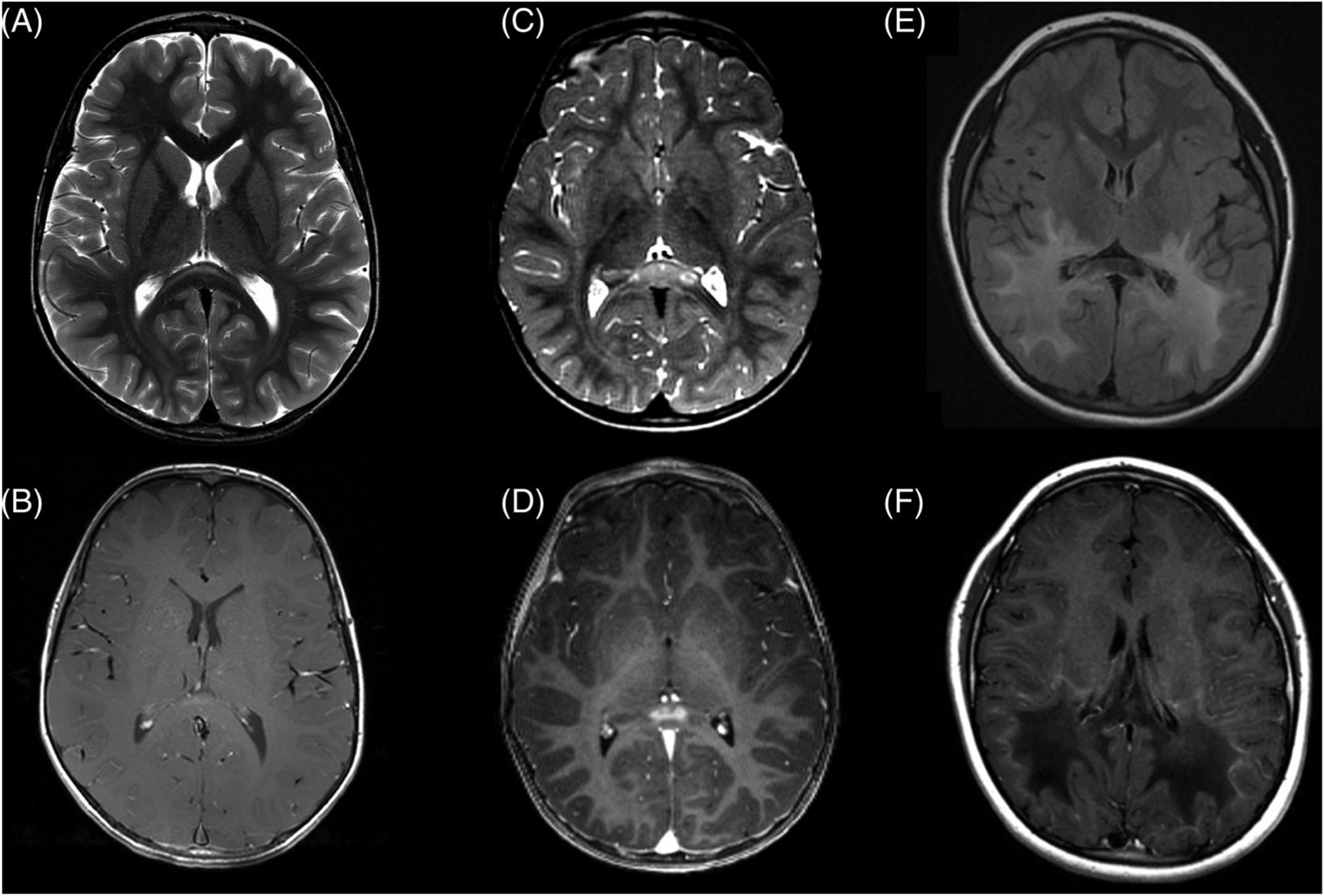
Early and late-stage demyelinating lesions in childhood cerebral adrenoleukodystrophy. (a) Very early CCALD lesion in the splenium of the corpus callosum, T2-MRI. (b) Corresponding post-contrast T1-MRI. (c) More prominent early lesion with (d) clear lesional contrast-enhancement. The goal of surveillance is to identify and treat patients at this stage of disease. (e) Advanced parieto-occipital lesion crossing the splenium of the corpus callosum, T2-FLAIR, with (f) a garland of “leading edge” contrast enhancement on T1-post contrast MRI. Patients are often symptomatic at this stage
Given the limited window of time to intervene in CCALD, ALD newborn screening (NBS) was recently added to the Recommended Uniform Screening Panel.27–29 Universal screening, using an assay that measures VLCFA levels in dried blood spots, is now underway in a growing number of US states and countries.30,31 There is now an opportunity to monitor for CCALD from birth.27 The primary aim of ALD NBS is the early detection and timely treatment of progressive CCALD in the window prior to symptom onset.7,20,32,33 HSCT is most effective when instituted while patients are asymptomatic and actively inflammatory cerebral lesions are small (Figure 1C,D).7,10,20,23–25,34
The newborn screening experience has largely borne out earlier ALD prevalence estimates of 1:15 000 live births, although some regions may have higher rates of disease.27,35 As states across the US begin to screen for ALD, more patients will be detected who will need reliable disease monitoring. Multiple group and institution-specific evidence-based surveillance practices have been published.27,36–39 While generally similar, they vary in timing, duration, sequence selection, and age to initiate MRI screening. The primary objective of this guideline is to provide pediatricians and child neurologists with a meta-analysis-based, generalizable, high-sensitivity imaging surveillance protocol to detect early-stage brain lesions in neurologically asymptomatic boys with ALD.
2 |. MATERIALS AND METHODS
2.1 |. Participants
A multicenter, multidisciplinary workgroup of clinicians and practitioners with expertise in ALD (with representation from neurology, stem cell transplant oncology, genetics, endocrinology, and leukodystrophy care coordination), a NBS laboratory director, ALD research experts, and a patient advocacy representative was assembled. The workgroup spanned nine U.S. academic centers, one based in the Netherlands, a U.S. state department of health, and a patient advocacy group. A systematic review and meta-analysis were performed by members of the workgroup (B.T., H.Y., E.M., F.E., A.F.) under the guidance of two institutional librarians (C.P. and M.D.). Using the consensus development conference methodology,40,41 the group convened during five in-person meetings and participated in three conference calls from January 2018 to January 2020 in order to develop and reach consensus on guidelines for the imaging surveillance of neurologically asymptomatic boys with ALD.
2.2 |. Systematic review and meta-analysis
To establish the age range of patients in need of imaging surveillance, we performed a systematic review of the literature, followed by a meta-analysis. We sought to answer two questions: Question 1 (Q1) What is the earliest age (and range) that patients were historically diagnosed with CCALD? and Question 2 (Q2) Of the studies identified in Q1, what imaging modality was most used to diagnose CCALD?
PRISMA and Cochrane Handbook guidelines were followed.42,43 Librarian C.P. developed the search strategy after consulting with the research team. The search was applied to PubMed via NCBI, Embase via Elsevier, the Cochrane Library via Wiley, Scopus, Web of Science via Clarivate Analytics, CINAHL Plus via EBSCOhost, and Clinicaltrials.gov for studies published between January 1, 1970 and September 10, 2019 (eFile 1). Controlled vocabulary, such as Medical Subject Headings (MeSH) and Emtree terms, when appropriate, were used in combination with keywords for the condition of ALD.
Studies included for analysis met the following criteria: (a) report at least one case of CCALD, where childhood is defined as 0–18 years of age, (b) “adrenoleukodystrophy” is present in the title, (c) “cerebral” is present in the title, abstract, or body of the text, (d) number of patients with CCALD in the study was reported, (e) CCALD diagnosis, defined as reported age and/or range of diagnosis (made by first brain imaging findings and/or neurological deficit), or first presentation of neurological symptoms in the setting of a delayed diagnosis. Exclusion criteria were: (a) publications without a patient ≤18 years old, (b) studies focused solely on other aspects of ALD (eg, neonatal ALD, adults with ALD, adrenomyeloneuropathy, women with ALD, endocrine function in ALD, reproduction in ALD), (c) review articles, (d) non-peer-reviewed studies, (e) non-human studies, (f) studies that do not explicitly report age and/or age range of first symptom or diagnosis (eg, listing age and/or range at time of enrollment to a large cohort study, or age and/or range of ages of treatment).
Titles and abstracts were examined initially by two independent reviewers (H.Y. and E.M.) against the inclusion/exclusion criteria, and irrelevant publications were removed. The full text of the remaining articles were retrieved by librarian M.D. The full text articles were organized using a Mendeley desktop reference manager (Version 1.17.13). The two reviewers then independently scored the publications for admission into the systematic review. Discrepancies in scoring were resolved by re-review of the full text article, and agreement on inclusion between reviewers. As recommended, bias was limited by varying rater expertise.43
To address Q2, we analyzed the final set studies included for the systematic review above. We identified the subset of articles relevant to imaging in CCALD by excluding any articles without mention of a diagnostic imaging modality.
2.3 |. Statistical analysis
Individual patient data were extracted from case reports, and where available, studies with more than one subject. When individual data were not available, the number of patients with CCALD, mean and/or median age, SD and/or range were extracted. Overall mean age of CCALD diagnosis was calculated from the extracted data. A sub-analysis of the individually extracted subjects was also performed. Normally distributed data are expressed as mean and SD (mean ± SD). Skewed data are expressed as median, IQR, and range. We report the frequency of differing imaging modalities and sequences used to diagnose CCALD in Q2.
2.4 |. Guideline development
Results from the systematic review and meta-analysis were integrated into the guideline development. We aimed to establish evidence-based best practices, specifically: (a) the age range of patients who should undergo surveillance, (b) imaging intervals, and (c) the MRI sequences to be included. Additional clinical expertise and experience-based recommendations addressing need for sedation, adrenal insufficiency in the setting of a sedated imaging procedure, and physician-patient partnerships are reported. The consensus process took place over three conference calls and five biannual meetings, alternating between the Hunter’s Hope Annual Medical Symposium and the Annual Aidan Jack Seeger Foundation ALD Standards of Care meeting, between January 2018 and January 2020. Iterations of the surveillance protocol were presented and revised each time the group convened. Suggestions for additional discussion points were collected and added to subsequent agendas. Data requests made by participants for specific discussion questions were fulfilled by BT, EM and AF. Final consensus for all recommendations was achieved by a vote. Consensus was defined as >80% agreement within the workgroup. We adhered to standards for reporting, rating the level of evidence, development, and grading the strength of recommendations put forth in clinical practice guidelines derived from a systematic review of the literature.44–47
3 |. RESULTS
3.1 |. Systematic review and meta-analysis
5888 individual records were screened, and 324 underwent full text analysis (Figure 2). After review by the two raters, 123 studies were included in the meta-analysis (eFile 2). 201 studies were removed (eFile 3) based on inclusion/exclusion criteria. The following case data were parsed from these studies and included as incidence of CCALD: Age of diagnosis of CCALD (defined by first brain imaging findings and/or neurological deficit), or age of first reported neurological sign or symptom if the formal diagnosis of CCALD was delayed.
FIGURE 2.
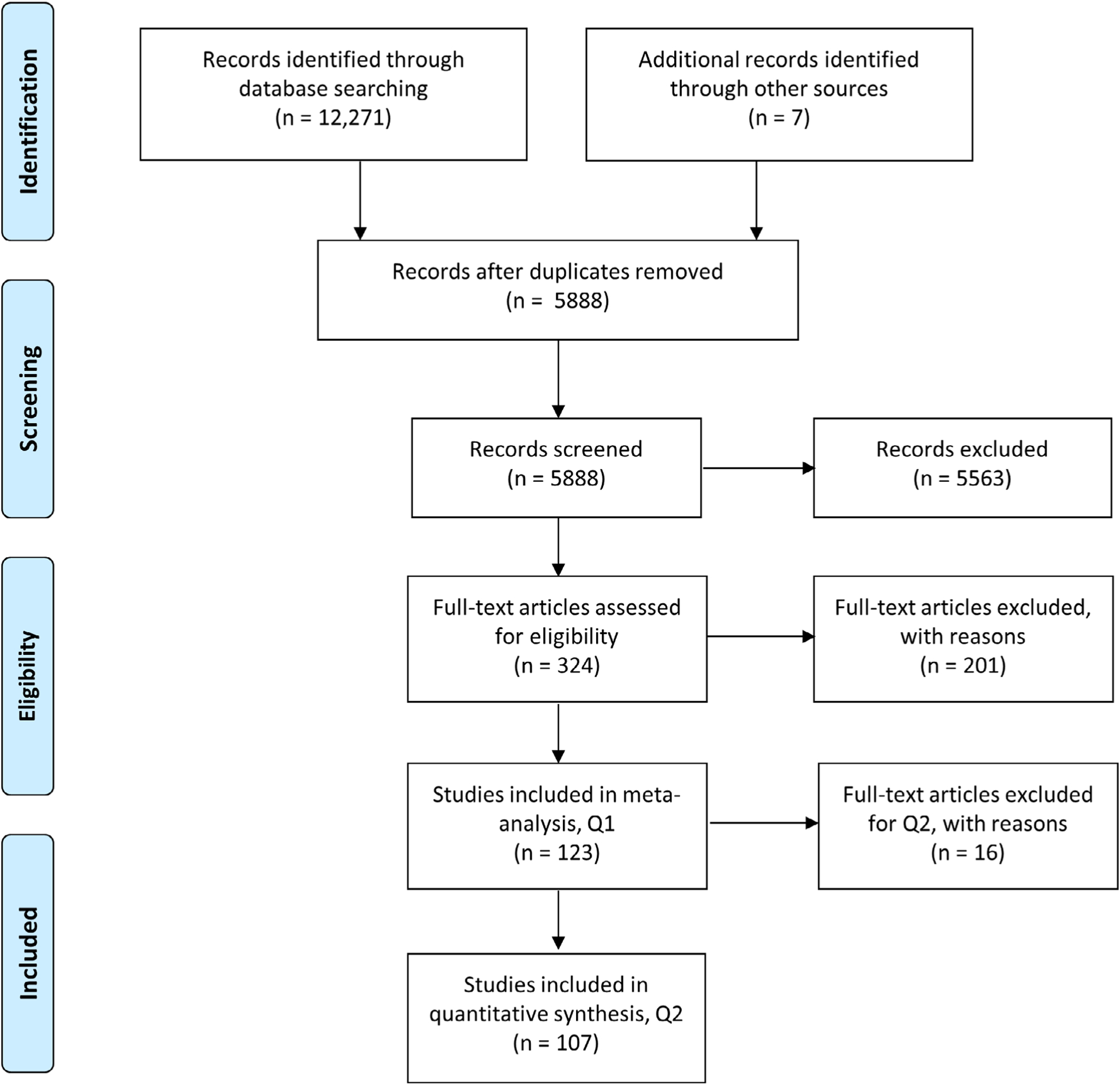
PRISMA flow diagram
1285 total cases of CCALD, age range 0 to 18 years old, were identified from all 123 studies. Mean age was extracted from 13 studies (n = 823). Individual patient data was extracted from 105 studies (n = 349 cases). 4 studies reported range of age only (n = 91). One study reported a median age only (n = 22). The latter five studies were not included in the overall mean calculation due to insufficient numerical information necessary for the calculation. Therefore, the overall mean was calculated from 1172 cases extracted from 118 studies.
3.1.1 |. Question 1: What is the age and range of CCALD diagnosis?
The overall mean age of CCALD diagnosis was calculated from 1172 cases extracted from 118 studies. The overall mean age of patients diagnosed with CCALD is 7.91 years old.
Of the 349 individual patients with data, the median age of CCALD diagnosis is 7.0 years old (IQR: 6.0–9.5). 90.0% of the individual cases identified were diagnosed between 3 and 12 years old inclusive (Figure 3). Thirty patients were diagnosed over the age of 12 years (8.6% of cases). Five patients were identified between the ages of 1 and 3 years old (1.4% of cases, (Table S1). No patients developed CCALD under the age of 1.
FIGURE 3.
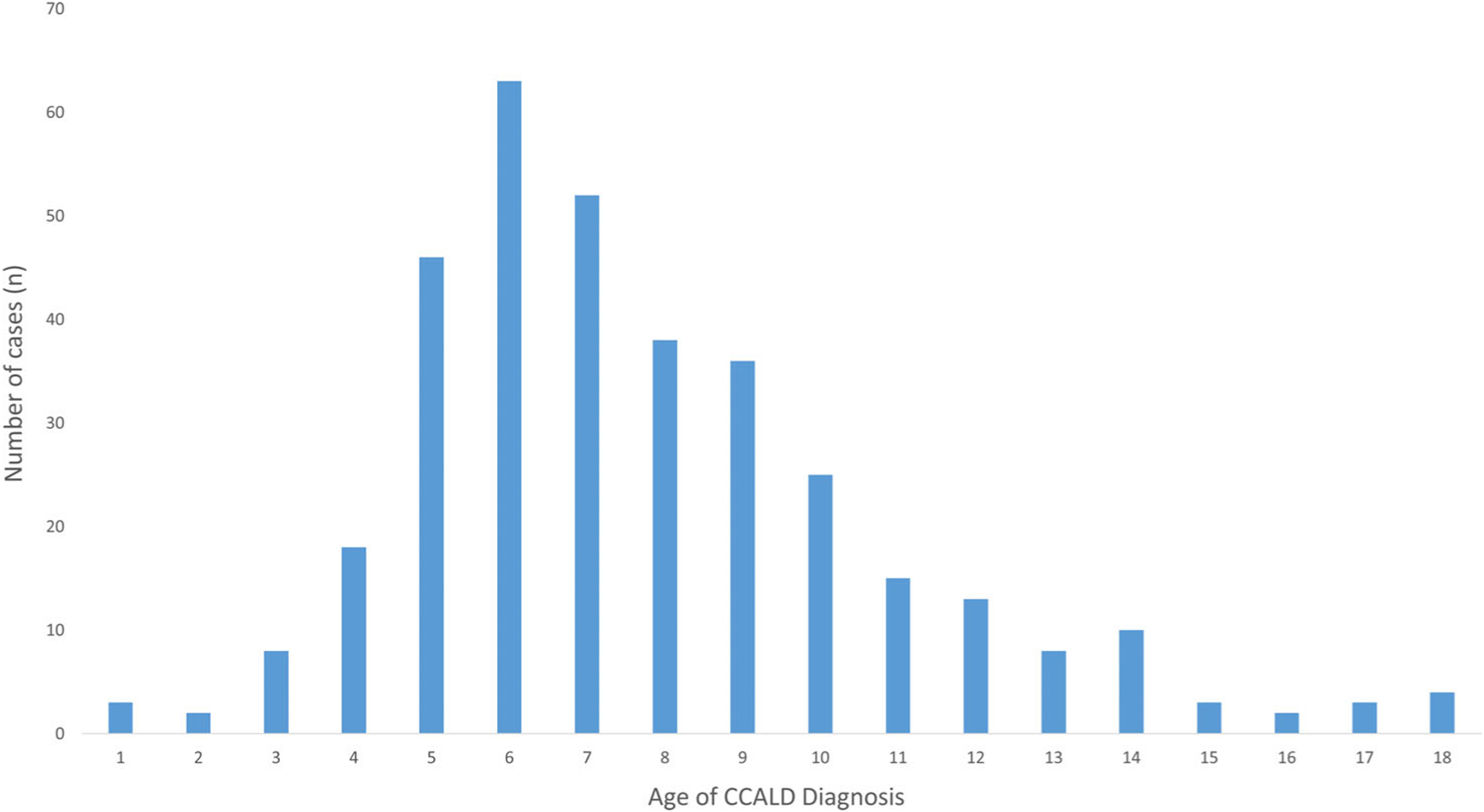
Age of CCALD diagnosis calculated from the sub-analysis of individual patient data. Age of CCALD diagnosis was extracted from the sub-analysis of 349 individually reported cases. The median age of diagnosis is 7.0 years old (IQR: 6.0–9.5). 90.0% of the cases diagnosed are between 3 and 12 years of age, inclusive
3.1.2 |. Question 2: Of the studies identified in Q1, what imaging modality was most used to diagnose CCALD?
One hundred and seven of the 123 texts met inclusion to answer Q2. Conventional MRI was reported in 94 of the 107 studies (87.9%), followed by CT (22.4%), MR Spectroscopy (14.0%), Positron Emission Tomography (3.7%), and Magnetization Transfer Contrast Imaging (0.9%). Some studies reported multiple modalities per patient.
Sixty-eight of the 94 MRI studies reported specific diagnostic sequences. T2-weighted MRI was reported in 44 of the 68 studies (64.7%). Fluid Attenuated Inversion Recovery (T2 FLAIR) was reported in 27 (39.7%). T1-weighted contrast-enhanced MRI (paired pre- and post-contrast sequences) was reported in 32 studies (47.1%). Non-contrast-enhanced T1-weighted MRI only was reported in 13 studies (19.1%). Diffusion-weighted imaging (DWI) was reported once (0.5%). Multiple sequences were often used per patient.
3.2 |. General recommendations
All neurologically asymptomatic boys with ALD should undergo routine MRI screening for radiographic evidence of CCALD. Boys should begin a surveillance program as early as possible. All MRIs, especially with suspect or confirmed findings, should be reviewed by a physician with expertise in ALD. Family education and physician-family partnerships should be emphasized to help prevent deviation from MR surveillance or loss to follow up. Given the currently evolving clinical experience of prospectively following asymptomatic patients identified on NBS, we recommend re-review, refinement, and upgrade in the recommendations within the next 5 to 10 years. This will allow for sufficient time to pass, and thereby a sufficient number of asymptomatic boys to be prospectively identified by screening. We expect that with MR surveillance the age and distribution of CCALD diagnosis to be younger than those resulted from our meta-analysis.
3.3 |. Surveillance guidelines
Based on the resultant age distribution of CCALD (Figure 3), we reached consensus that the first MRI scan should be performed between 12 and 18 months old (Table 1). Contrast is not recommended at this time point because the likelihood of developing an active, inflammatory brain lesion is sufficiently low.3 The second MRI scan should be performed 12 months after the first scan (between 24 and 30 months old). Similarly, contrast is not recommended at that time point because the likelihood of developing an active, inflammatory brain lesion remains low.3 The results do not support obtaining an MRI in patients less than 12 months old.
TABLE 1.
MRI surveillance recommendations
| Age | MRI frequency | Contrast administration recommended?a |
|---|---|---|
| 1–1.5 years old | Once | No |
| 2–2.5 years old | Once | No |
| 3–12 years old | Every 6 months | Yes |
| 12+ years old | Yearly | Lesion positive? Yes Lesion negative? No |
If at any time a lesion appears the patient should be urgently referred to an ALD Specialist or Expert Center.
The risk of CCALD (first reported finding or diagnosis) peaks between the ages of 3 and 12 years old, accounting for 90% of the individual cases uncovered by the sub-analysis of individual patient data. Therefore, during this period we recommend more intensive screening; we recommend completing a contrast-enhanced brain MRI every 6 months. Although there are theoretical risks with repeated administration of gadolinium, we recommend administering contrast as the likelihood of developing an active brain lesion is highest in this age group. Strategies to limit the exposure of patients to repeated contrast (eg, “real-time MRI reading”) may be employed here where available.
The risk for CCALD declines during teenage years (Figure 3). In literature, these lesions have been shown to tend to progress more slowly.7 Therefore, an MRI scan can be performed annually after the age of 12. Contrast is not recommended from this time point onwards unless there is evidence of a lesion on a previous MRI, or there is clinical concern (eg, new neurological symptoms).
Early-stage CCALD lesions are characterized by small T2 hyperintensities centered most often within in the genu or splenium of the corpus callosum. Less often they appear in the corticospinal tracts or cerebellar white matter.7 Very early lesions may not exhibit clear gadolinium enhancement; alternatively a subset of early lesions may self-arrest15,21 (Figure 1A,B). If an early-stage lesion is detected, an urgent referral should be made to a physician or center with expertise in monitoring and treating CCALD. An MRI should be repeated in 3 months to re-assess for gadolinium enhancement whose presence indicates active CCALD, and is the indication for HSCT (Table 1). If a more prominent, clearly gadolinium-enhancing, early CCALD lesion is detected, the patient should be urgently triaged for stem cell transplant (Figure 1C,D).
3.4 |. Sequence selection
The resultant frequencies of each diagnostic imaging sequence were used to inform the imaging recommendations. More specifically, the analysis provided evidence to support the use of standard T1/T2-weighted MRI and refute the primary use of other modalities and sequences including diffusion-based imaging or MR Spectroscopy. We recommend standard clinical MRI sequences be acquired, which should include an axial T2-weighted and/or FLAIR sequence for surveillance across all age groups. In addition, axial T1-weighted pre- and post-contrast sequences should be acquired in patients between 3 and 12 years old, and in those patients with a cerebral lesion on previous imaging (Table 1). We do not recommend advanced imaging modalities or sequences as part of the routine clinical surveillance protocol. Additional, advanced imaging protocols should only be performed in specific cases upon expert recommendation or as part of a research endeavor. Research-based sequences should only be performed as part of an IRB-approved study.
3.5 |. Final consensus
We achieved 95.7% consensus by vote from all 23 authors on the final version of the surveillance guidelines: 60.9% (n = 14) strongly agree, 34.8% (n = 8) agree, 4.3% (n = 1) remained neutral. There were no disagreements.
3.6 |. Level of evidence and grade of recommendation
The data derived from the systematic review and meta-analysis of case and cohort studies in a rare neurological disease qualifies for Level II Evidence. There are no prospective randomized control treatment trials in CCALD. The surveillance guidelines, therefore, qualify as Grade B practice recommendations.
4 |. ADDITIONAL CONSIDERATIONS
4.1 |. Anesthesia for MRI sedation
Presymptomatic lesions are small and require optimal spatial resolution on MRI. Therefore, limiting patient movement during MRI scans to minimize motion artifacts increases the sensitivity of lesion detection. This is especially important in detecting subtle white matter changes characteristic of early-stage CCALD (Figure 1A).7 Until children are able to lie still for the duration of the MRI, we recommend general anesthesia for the duration of the scan. Given that the majority of affected boys are cognitively normal during surveillance, child life specialists and behavioral psychology services should be employed to decrease the need for anesthesia.
4.2 |. Adrenal insufficiency
Most patients with ALD will develop adrenal insufficiency, with about half becoming affected before the age of 10.4 Boys with ALD should be routinely monitored by an endocrinologist.27 Specific guidelines for the management of adrenal insufficiency in ALD have been published.48 Importantly, patients should be evaluated for the presence of adrenal insufficiency, and recommendations for stress dose steroids should be made prior to MRIs which require sedation.
5 |. DISCUSSION
Pursuant to the opportunity to monitor boys from birth for CCALD, we offer the first meta-analysis-based surveillance guidelines designed to detect early-stage cerebral disease in patients with ALD. The overall goal of the guidelines are to expedite the diagnosis and timely treatment of CCALD.2,7,9–11,20,22–24,32,33 The systematic review and meta-analysis revealed patients were diagnosed or presented with their first neurological symptom prior to diagnosis at a mean age of 7.9 years old. Our result independently verifies the peak incidence of CCALD calculated by Hugo Moser from his original cohort of 372 patients.3 A T2-based MR imaging protocol was used most often to diagnose cerebral disease, followed by contrast-enhanced T1 MRI.
Within the consensus protocol, a data request was made towards highlighting CCALD cases under the age of three: Notably, five patients between 1 and 3 years old were diagnosed with CCALD indicating that while rare, CCALD can occur in this window.49–53 Some of the patients in this age group presented atypically. Rather than presenting with radiographic disease only, they were diagnosed with CCALD during or after another neurological event, including head trauma50 and seizure51,53 (Table S1). These are in addition to one case of a boy reported to have an abnormal MRI at 1.7 years old (this study did not pass inclusion criteria for the meta-analysis),6 and one boy who presented with neurologically symptomatic CCALD 2 weeks after his third birthday indicating his disease likely began prior to 3 years of age (personal communication).
We recommend an initial MRI be obtained between 12 and 18 months old to evaluate for disease, and also to serve as a comparator in the event the patient has an abnormal MRI at a later date. The first MRI serving as a baseline comparator to the 24-month second MRI was the first consideration that drove the consensus recommendation of beginning surveillance at 1 year, despite the rarity of CCALD in this age group (1.4%). A second consideration was the historic temporal delay in pre-newborn screening CCALD cases, between clinically silent lesion growth and first reported symptom. The initiation age was a focus of debate during the consensus discussion protocols, and despite the variety in clinical practice, the recommendation achieved consensus (96.7% agree or strongly agree). The recommended age of first MRI may benefit most from emergent newborn screening case data and experience gained over the next 5 to 10 years. We therefore recommend re-review of this guideline, specifically of the first age of MRI. Importantly, no patients presented under the age of 1. Therefore, we do not recommend that MRIs be obtained in patients less than 12 months old.
Beginning at 3 years of age we recommend contrast-enhanced MRI screening every 6 months until the age of 12. If applied, this portion of the surveillance protocol captures 90.0% of the individually identified cases of CCALD from our study. If at any time an early-stage lesion appears the patient should be urgently referred to the closest center with expertise in the diagnosis, monitoring, and treatment of CCALD. That referral should be expedited if patients are identified with significant cerebral involvement or the patient is neurologically symptomatic. Ideally, all patient MRIs should be reviewed by a physician with expertise in the diagnosis of CCALD.
Two additional clinical considerations are relevant to an ALD MRI surveillance protocol. The first are the potential risks associated with repeated exposures to anesthesia. While experimental data supports the neurotoxic effects of exposing the developing brain in rodents and non-human primates to general anesthesia,54,55 recent evidence offers some reassurance against major neurocognitive deficits among children with single and multiple exposures to general anesthesia in early childhood.56,57 Due to the morbidity and mortality associated with CCALD, including its early neurocognitive effects,24 and because subtle white matter lesions may be lost due to movement artifact, we recommend MRI sedation as needed to successfully complete MRI surveillance. Because most boys with ALD will develop adrenal insufficiency in childhood, a plan for adrenal replacement should be recommended by an endocrinologist prior to sedation. Specific guidelines for the management of adrenal insufficiency in ALD have been published.48
Second, patients will undergo repeated exposures to gadolinium-based contrast agents The FDA safety communication on gadolinium-based contrast agents (GBCAs) acknowledges that GBCAs may be retained in the body months to years after exposure (https://www.fda.gov/media/109825/download). However, to date they have not been linked to disease in patients with normal kidney function. Therefore, in accordance with the FDA safety communication recommendations, the consensus recommends the repeated use of GBCAs for surveillance in patients with normal kidney function.
Strategies exist to minimize MRI duration, duration of anesthesia, and to mitigate exposure to GBCAs. Some centers offer MRI sensitization programs for young children in an effort to forego sedation. If available, institutions will use the “Child Life” staff to make the medical experience for pediatric patients less intimidating. Centers with experience in the early radiographic diagnosis of CCALD may employ “real-time reading”: a neuroradiologist or neurologist will interpret the MRI while the patient is in the scanner and only administer contrast if a potential lesion is seen. This is an important strategy which can be used to limit gadolinium exposure in boys undergoing surveillance.
Limitations of the systematic review and meta-analysis include the heterogeneity in patient symptomatology at the time of diagnosis; patients often came to medical attention when symptomatic and were diagnosed later than radiographic disease-onset. This likely shifted the overall mean to an age older than true disease-onset. Importantly, this highlights the need for early monitoring. We did not include data reporting the age of CCALD treatment (including large trials), as these studies did not report age of CCALD diagnosis. Additionally, treatment has often been in advanced disease, and would therefore be non-informative for an early diagnostic protocol. Given the rarity of ALD, and the relatively small number of expert physicians who see these patients and contribute to research, it is plausible that some patients may have participated in more than one study included in the meta-analysis. We expect this bias to be reduced given the majority of studies analyzed were case reports (n = 1), and there were no exclusions based on country of publication or primary language. Lastly, our recommendations on the frequency of MRI scans were not drawn from the meta-analysis due to lack of reporting in the primary articles. Therefore, the recommendation was guided by a previous protocol,27 and based on new research which quantified the rate of cerebral disease progression as a function of lesion distribution and age.6,7
Boys with ALD identified early in life should be vigilantly monitored with serial brain MRIs during the period of highest risk for conversion to CCALD. These guidelines provide a practical, high-sensitivity MRI screening protocol designed to detect early-stage brain lesions. Prospective monitoring will fundamentally change the clinical approach to disease, and as a welcome by-product, provide a better understanding of the development and natural history of CCALD. As clinical experience is gained from prospectively monitoring patients, the guidelines should be iteratively optimized over time.
Supplementary Material
Synopsis.
These guidelines provide a practical, evidence-based, high-sensitivity MRI screening protocol designed to detect early-stage brain lesions in neurologically asymptomatic boys with X-linked adrenoleukodystrophy.
ACKNOWLEDGMENTS
To the families who participated in the generation of these guidelines, we thank you. We acknowledge the Aidan Jack Seeger Foundation (AJSF) for assembling the AJSF Neurology Workgroup. We thank the AJSF, Hunter’s Hope and the Leukodystrophy Care Network for providing multiple opportunities for the authors to convene in-person, generate, and refine the guidelines. This work is dedicated to the memory of Hugo W. Moser, MD. Funding was received from National Institutes of Health for activities related to this project (A.F. NICHD U54HD079123, E.J.M. K12NS066274) and from Brian’s Hope (B.T., A.F.).
Funding information
National Institute of Child Health and Human Development, U54HD079123, National Institute of Neurological Disorders and Stroke, K12NS066274; Brian’s Hope, National Institutes of Health
Abbreviations:
- ALDX
lined adrenoleukodystrophy
- CCALD
childhood cerebral adrenoleukodystrophy
- GBCA
gadolinium-based contrast agents
- HSCT
hematopoietic stem cell transplant
- NBS
newborn screening
- VLCFA
very long chain fatty acids
Footnotes
CONFLICT OF INTEREST
E. S. is the Founder of the Aidan Jack Seeger Foundation (AJSF). The AJSF provided support for in-person meetings during which these guidelines were generated, presented, and revised. The other authors have no financial relationships or conflicts of interest relevant to this article to disclose.
INFORMED CONSENT AND ETHICS APPROVAL
This study was conducted using published, publicly available data. No human subjects were enrolled. This study is therefore exempt from Ethics/Institutional Review Board approval and informed consent was not applicable.
ANIMAL RIGHTS
This article does not contain any studies with human or animal subjects performed by the any of the authors.
DATA AVAILABILITY STATEMENT
Following publication, any data not published within this article will be shared by request from any qualified investigator.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Moser AB, Kreiter N, Bezman L, et al. Plasma very long chain fatty acids in 3,000 peroxisome disease patients and 29,000 controls. Ann Neurol. 1999;45(1):100–110. . [DOI] [PubMed] [Google Scholar]
- 2.Moser HW, Moser AB, Smith KD, et al. Adrenoleukodystrophy: phenotypic variability and implications for therapy. J Inherit Metab Dis. 1992;15(4):645–664. 10.1007/BF01799621. [DOI] [PubMed] [Google Scholar]
- 3.Moser HW, Loes DJ, Melhem ER, et al. X-linked adrenoleukodystrophy: overview and prognosis as a function of age and brain magnetic resonance imaging abnormality. A study involving 372 patients. Neuropediatrics. 2000;31(5):227–239. 10.1055/s-2000-9236. [DOI] [PubMed] [Google Scholar]
- 4.Huffnagel IC, Laheji FK, Aziz-Bose R, et al. The natural history of adrenal insufficiency in X-linked adrenoleukodystrophy: an international collaboration. J Clin Endocrinol Metab. 2019;104 (1):118–126. 10.1210/jc.2018-01307. [DOI] [PubMed] [Google Scholar]
- 5.Bezman L, Moser HW. Incidence of X-linked adrenoleukodystrophy and the relative frequency of its phenotypes. Am J Med Genet. 1998;76:415–419. [PubMed] [Google Scholar]
- 6.Loes DJ, Fatemi A, Melhem ER, et al. Analysis of MRI patterns aids prediction of progression in X-linked adrenoleukodystrophy. Neurology. 2003;61(3):369–374. 10.1212/01.WNL.0000079050.91337.83. [DOI] [PubMed] [Google Scholar]
- 7.Liberato AP, Mallack EJ, Aziz-Bose R, et al. MRI brain lesions in asymptomatic boys with X-linked adrenoleukodystrophy. Neurology. 2019;92(15):e1698–e1708. 10.1212/WNL.0000000000007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loes DJ, Hite S, Moser H, et al. Adrenoleukodystrophy: a scoring method for brain MR observations. Am J Neuroradiol. 1994;15(9):1761–1766. 10.1016/j.rcl.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raymond GV, Aubourg P, Paker A, et al. Survival and functional outcomes in boys with cerebral adrenoleukodystrophy with and without hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018;25(3):538–548. 10.1016/j.bbmt.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 10.Peters C, Charnas LR, Tan Y, et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104 (3):881–888. 10.1182/blood-2003-10-3402. [DOI] [PubMed] [Google Scholar]
- 11.Korenke GC, Pouwels PJ, Frahm J, et al. Arrested cerebral adrenoleukodystrophy: a clinical and proton magnetic resonance spectroscopy study in three patients. Pediatr Neurol. 1996;15(2):103–107. [DOI] [PubMed] [Google Scholar]
- 12.Melhem ER, Loes DJ, Georgiadis C, et al. X-linked adrenoleukodystrophy: the role of contrast-enhanced MR imaging in predicting disease progression. AJNR Am J Neuroradiol. 2000; 21(5):839–844. http://www.ajnr.org.ezproxy.med.cornell.edu/content/ajnr/21/5/839.full.pdf. Accessed September 10, 2017. [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JE, Armour EA, Heshmati A, et al. Pearls & Oy-sters: adolescent-onset adrenomyeloneuropathy and arrested cerebral adrenoleukodystrophy. Neurology. 2019;93(2):81–84. 10.1212/WNL.0000000000007755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Geel BM, Poll-The BT, Verrips A, Boelens JJ, Kemp S, Engelen M. Hematopoietic cell transplantation does not prevent myelopathy in X-linked adrenoleukodystrophy: a retrospective study. J Inherit Metab Dis. 2015;38(2):359–361. 10.1007/s10545-014-9797-1. [DOI] [PubMed] [Google Scholar]
- 15.Mallack EJ, van de Stadt S, Caruso PA, et al. Clinical and radiographic course of arrested cerebral adrenoleukodystrophy. Neurology. 2020;94(24):e2499–e2507. 10.1212/wnl.0000000000009626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henig I, Zuckerman T. Hematopoietic stem cell transplantation—50 years of evolution and future perspectives. Rambam Maimonides Med J. 2014;5(4):e0028. 10.5041/rmmj.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Geel BM, Assies J, Haverkort EB, et al. Delay in diagnosis of X-linked adrenoleukodystrophy. Clin Neurol Neurosurg. 1993;95(2):115–120. [DOI] [PubMed] [Google Scholar]
- 18.Beckmann NB, Miller WP, Dietrich MS, Orchard PJ. Quality of life among boys with adrenoleukodystrophy following hematopoietic stem cell transplant. Child Neuropsychol. 2018;24(7):986–998. 10.1080/09297049.2017.1380176. [DOI] [PubMed] [Google Scholar]
- 19.Ashwal S, Michelson D, Plawner L, Dobyns WB. Practice parameter: diagnostic assessment of the child with report of the quality standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Am Acad Neurol. 2006;67:1542–1550. 10.1212/Wnl.0b013e3181d5e057. [DOI] [PubMed] [Google Scholar]
- 20.Mahmood A, Raymond GV, Dubey P, Peters C, Moser HW. Survival analysis of haematopoietic cell transplantation for childhood cerebral X-linked adrenoleukodystrophy: a comparison study. Lancet Neurol. 2007;6(8):687–692. 10.1016/S1474-4422(07)70177-1. [DOI] [PubMed] [Google Scholar]
- 21.Pierpont EI, Nascene DR, Shanley R, et al. Neurocognitive benchmarks following transplant for emerging cerebral adrenoleukodystrophy. Neurology. 2020;95(5):e591–e600. 10.1212/WNL.0000000000009929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Geel BM, Bezman L, Loes DJ, Moser HW, Raymond GV. Evolution of phenotypes in adult male patients with X-linked adrenoleukodystrophy. Ann Neurol. 2001;49(2):186–194. . [DOI] [PubMed] [Google Scholar]
- 23.Shapiro E, Krivit W, Lockman L, et al. Long-term effect of bone-marrow transplantation for childhood-onset cerebral X-linked adrenoleukodystrophy. Lancet (Lond, Engl). 2000;356 (9231):713–718. 10.1016/S0140-6736(00)02629-5. [DOI] [PubMed] [Google Scholar]
- 24.Pierpont EI, Eisengart JB, Shanley R, et al. Neurocognitive trajectory of boys who received a hematopoietic stem cell transplant at an early stage of childhood cerebral adrenoleukodystrophy. JAMA Neurol. 2017;74(6):710–717. 10.1001/jamaneurol.2017.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eichler F, Duncan C, Musolino PL, et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N Engl J Med. 2017;377(17):1630–1638. 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallack EJ, Turk B, Yan H, Eichler FS. The landscape of hematopoietic stem cell transplant and gene therapy for X-linked adrenoleukodystrophy. Curr Treat Options Neurol. 2019;21(12): 61. 10.1007/s11940-019-0605-y. [DOI] [PubMed] [Google Scholar]
- 27.Vogel BHH, Bradley SEE, Adams DJJ, et al. Newborn screening for X-linked adrenoleukodystrophy in New York state: diagnostic protocol, surveillance protocol and treatment guidelines. Mol Genet Metab. 2015;114(4):599–603. 10.1016/j.ymgme.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Salzman A. Adrenoleukodystrophy patient perspective: turning despair into a gene therapy breakthrough. Hum Gene Ther. 2011;22(6):647–648. 10.1089/hum.2011.2503. [DOI] [PubMed] [Google Scholar]
- 29.Salzman R. Venture philanthropy and gene therapy: lessons from adrenoleukodystrophy. Hum Gene Ther. 2016;27(1):14–18. 10.1089/hum.2015.29016.rsa. [DOI] [PubMed] [Google Scholar]
- 30.Turgeon CT, Moser AB, Morkrid L, et al. Streamlined determination of lysophosphatidylcholines in dried blood spots for newborn screening of X-linked adrenoleukodystrophy. Mol Genet Metab. 2015;114:46–50. 10.1016/j.ymgme.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Moser AB, Jones RO, Hubbard WC, et al. Newborn screening for X-linked adrenoleukodystrophy. Int J Neonatal Screen. 2016;2(4):15. 10.3390/ijns2040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemper AR, Brosco J, Comeau AM, et al. Newborn screening for X-linked adrenoleukodystrophy: evidence summary and advisory committee recommendation. Genet Med. 2017;19(1): 121–126. 10.1038/gim.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aubourg P, Sellier N, Chaussain JL, Kalifa G. MRI detects cerebral involvement in neurologically asymptomatic patients with adrenoleukodystrophy. Neurology. 1989;39(12):1619–1621. http://www.ncbi.nlm.nih.gov/pubmed/2586779. Accessed March 1, 2019. [DOI] [PubMed] [Google Scholar]
- 34.Miller WP, Rothman SM, Nascene D, et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood. 2011;118(7):1971–1978. 10.1182/blood-2011-01-329235. [DOI] [PubMed] [Google Scholar]
- 35.Wiens K, Berry SA, Choi H, et al. A report on state-wide implementation of newborn screening for X-linked adrenoleukodystrophy. Am J Med Genet Pt A. 2019;179(7):ajmg.a.61171. 10.1002/ajmg.a.61171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjelloun FZM, Kriouile Y, Cheillan D, Daoud-Tetouani H, Chabraoui L. Management of X-linked adrenoleukodystrophy in Morocco: actual situation. BMC Res Notes. 2017;10:567. 10.1186/s13104-017-2902-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimozawa N, Honda A, Kajiwara N, et al. X-linked adrenoleukodystrophy: diagnostic and follow-up system in Japan. J Hum Genet. 2011;56:106–109. 10.1038/jhg.2010.139. [DOI] [PubMed] [Google Scholar]
- 38.Engelen M, Kemp S, De Visser M, et al. X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis. 2012;7(1):1–14. 10.1186/1750-1172-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renaud DL, Khan S. Development of a multidisciplinary programme for the treatment of X-linked adrenoleukodystrophy. Paediatr Child Health (Oxf). 2009;19:S217–S219. 10.1016/j.paed.2009.08.003. [DOI] [Google Scholar]
- 40.Murphy MK, Sanderson C, Black NA, et al. Consensus Development Methods, and Their Use in Clinical Guideline Development. Vol 2.; 1998. www.hta.ac.uk/htacd.htm. Accessed November 25, 2019. [PubMed] [Google Scholar]
- 41.Black N, Murphy M, Lamping D, et al. Consensus development methods: a review of best practice in creating clinical guidelines. J Health Serv Res Policy. 1999;4(4):236–248. 10.1177/135581969900400410. [DOI] [PubMed] [Google Scholar]
- 42.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furlan AD, Malmivaara A, Chou R, et al. 2015 updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine (Phila Pa 1976). 2015;40(21):1660–1673. 10.1097/BRS.0000000000001061. [DOI] [PubMed] [Google Scholar]
- 44.Shaneyfelt TM, Mayo-Smith MF, Rothwangl J. Are guidelines following guidelines? JAMA. 1999;281(20):1900–1905. 10.1001/jama.281.20.1900. [DOI] [PubMed] [Google Scholar]
- 45.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 46.Richards D. GRADING – levels of evidence. Evid Based Dent. 2009;10(1):24–25. 10.1038/sj.ebd.6400636. [DOI] [PubMed] [Google Scholar]
- 47.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–310. 10.1097/PRS.0b013e318219c171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Regelmann MO, Kamboj MK, Miller BS, et al. Adrenoleukodystrophy: guidance for adrenal surveillance in males identified by newborn screen. J Clin Endocrinol Metab. 2018;103(11): 4324–4331. 10.1210/jc.2018-00920. [DOI] [PubMed] [Google Scholar]
- 49.Jiang MY, Cai YN, Liang CL, et al. Clinical, biochemical, neuroimaging and molecular findings of X-linked adrenoleukodystrophy patients in South China. Metab Brain Dis. 2015;30:1439–1444. 10.1007/s11011-015-9717-6. [DOI] [PubMed] [Google Scholar]
- 50.Okamura K, Watanabe T, Onishi T, et al. Successful allogeneic unrelated bone marrow transplantation using reduced-intensity conditioning for the treatment of X-linked adrenoleukodystrophy in a one-yr-old boy. Pediatr Transpl. 2009;13: 130–133. 10.1111/j.1399-3046.2008.00962.x. [DOI] [PubMed] [Google Scholar]
- 51.Marsh WW, Hurst DL. Variable phenotypes in a family kindred with adrenoleukodystrophy. Pediatr Neurol. 1991;7(1):50–52. 10.1016/0887-8994(91)90106-U. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes JF, Bonfim C, Kerbauy FR, et al. Haploidentical bone marrow transplantation with post transplant cyclophosphamide for patients with X-linked adrenoleukodystrophy: a suitable choice in an urgent situation. Bone Marrow Transplant. 2018;53(4):392–399. 10.1038/s41409-017-0015-2. [DOI] [PubMed] [Google Scholar]
- 53.Kaga M, Furushima W, Inagaki M, Nakamura M. Early neuropsychological signs of childhood adrenoleukodystrophy (ALD). Brain Dev. 2009;31:558–561. 10.1016/j.braindev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Paule MG, Li M, Allen RR, et al. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33(2):220–230. 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23(3):876–882. http://www.ncbi.nlm.nih.gov/pubmed/12574416. Accessed August 20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun LS, Li G, Miller TLK, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA – J Am Med Assoc. 2016;315(21):2312–2320. 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warner DO, Zaccariello MJ, Katusic SK, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia. Anesthesiology. 2018;129(1): 89–105. 10.1097/ALN.0000000000002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


