Abstract
Background
Risk stratification for patients with differentiated thyroid cancer (DTC) is based primarily on pathologic tumor characteristics. Accurate preoperative prognostication could allow for more informed initial surgical recommendations, particularly among patients at a higher risk for distant metastasis (DM). The objective of this study was to characterize the genetic profile of DTC with DM and to validate a molecular‐based risk stratification.
Methods
A case‐control study design was used to analyze patients who had DTC with DM (n = 62) and a propensity matched cohort of patients who had DTC without DM after at least 5 years of follow‐up using the ThyroSeq version 3 targeted next‐generation sequencing assay. The results were classified into high‐risk, intermediate‐risk, and low‐risk of aggressive disease.
Results
Most patients who had DTC with DM (66%) had a late‐hit mutation in TERT, TP53, or PIK3CA. After propensity matching by age, tumor size, and sex, the high‐risk molecular profile had strong association with DM (high‐risk vs intermediate‐risk: odds ratio, 25.1; 95% CI, 3.07‐204.4; P < .001; high‐risk vs low‐risk: odds ratio, 122.5; 95% CI, 14.5‐1038.4; P < .001). Overall, molecular risk categories were associated with DM risk, with a concordance index of 0.836 (95% CI, 0.759‐0.913), which remained consistent after internal validation. Within the range of 5% to 10% of DM observed in DTC, the expected probability of DM would be 0.2% to 0.4% for the low‐risk molecular profile, 4.7% to 9.4% for the intermediate‐risk molecular profile, and 19.3% to 33.5% for the high‐risk molecular profile.
Conclusions
In this matched case‐control study, genetic profiling using an available molecular assay provided accurate and robust risk stratification for DM in patients with DTC. The availability of preoperative prognostication may allow tailoring treatment for patients with DTC.
Keywords: distant metastasis, molecular genetics, molecular profile, thyroid cancer
Short abstract
In this matched case‐control study, comprehensive genetic profiling provides accurate and robust risk stratification for distant metastasis in patients with differentiated thyroid cancer. The availability of preoperative prognostication may allow for molecular‐directed treatment recommendations to tailor care for these patients.
Introduction
More than 50,000 new cases of differentiated thyroid cancer (DTC) are diagnosed yearly in the United States, but most new cases will not result in disease‐specific mortality. 1 Current management guidelines use algorithms that allow for treatment and surveillance de‐escalation; however, such guidelines rely on accurate identification of the 5% to 10% of cases that can be associated with aggressive disease, either at presentation or diagnosed at subsequent follow‐up. 2 , 3 Histologic features are prognostically useful. However, if risk assessment of disease aggressiveness could occur preoperatively, then clinicians could provide precise recommendations for the appropriate extent of initial surgery while triaging selected patients to active surveillance protocols. 4
It is known that the initiation of thyroid cancer involves several early or primary driver mutations, of which the most common are BRAF V600E and RAS. BRAF V600E mutations typically occur in the classic or tall cell variant of papillary thyroid carcinoma (PTC); they were initially described as being associated with lymph node metastasis and recurrence but lack specificity on their own as an accurate prognostic marker. 5 , 6 Mutations in the RAS family of genes (including the HRAS, KRAS, and NRAS genes), commonly found in the follicular variant of PTC and in follicular thyroid carcinoma (FTC), are typically associated with more indolent disease. 7 , 8 A subset of thyroid cancers have fusions involving RET, NTRK, ALK, BRAF, PPARG, and other oncogenes as primary driver events, which in fact activate signaling pathways similar to those activated by either BRAF V600E or RAS mutations. 6 In Hurthle cell cancers, chromosomal copy number alterations are the most common finding and may represent a primary driver event. 9
The development of more aggressive and de‐differentiated thyroid tumors is believed to be associated with additional late‐hit driver mutations. 6 , 10 The most common and well studied of those are TERT promoter mutations, which have been associated with recurrence and mortality, particularly when they co‐occur with a primary BRAF or RAS mutation. 11 , 12 , 13 TERT promoter mutations may be a prognostic marker even among patients with thyroid cancer who are considered to already have disease at high risk for recurrence according to American Thyroid Association (ATA) stratification. 13 Additional late mutations that may also serve as prognostic markers include mutations in TP53 and genes in the PI3K/AKT/mTOR pathway, such as PIK3CA and AKT1. 14 , 15 , 16 More recently, PLEKHS1 promoter mutations have also been identified with distant metastasis (DM) in some thyroid cancers. 17 However, many molecular studies have included poorly differentiated or anaplastic cancers, which by definition are aggressive histologic variants, and other studies have limited molecular analysis to only a few known genetic markers of poor prognosis. 18 , 19
Aggressive DTC is challenging to define. Although tumor recurrence in the neck is an important prognostic metric, whether recurrence is caused by tumor biology or incomplete initial treatment is sometimes unclear. However, when DTC is associated with DM, 5‐year relative survival decreases from 99.9% to 55%. 1 In addition, DTC at high risk or with known DM should be treated with initial total thyroidectomy and radioactive iodine, as recommended by current guidelines. 2 , 3 The objective of our current study was to characterize the genetic profile of DTC associated with DM and validate the molecular‐based risk stratification of DTC using a matched case‐control study design.
Materials and Methods
Patient Cohorts
After we obtained Institutional Review Board approval, unselected patients with DTC and DM (cases) who were seen for multidisciplinary follow‐up after January 2008 at a single institution were identified, and clinical, radiographic, and pathologic data were retrieved from existing medical records. Histopathology slides were reviewed to confirm a diagnosis of metastatic DTC. All patients who had poorly differentiated or anaplastic thyroid cancer were excluded. Patients underwent thyroidectomy with or without lymphadenectomy and received radioactive iodine ablation according to standard management algorithms. 2 DM required pathologic confirmation and/or characteristic extracervical avidity on a whole‐body scan after the administration of iodine‐131. For the matched cohort (controls), consecutive patients were identified as those who had DTC and ≥5 years of follow‐up without evidence of DM.
Molecular Analysis and Risk Groups
Nucleic acids were extracted from formalin‐fixed, paraffin‐embedded tissues obtained from surgically excised tumor samples and were tested using the ThyroSeq version 3 (TSv3) targeted next‐generation sequencing assay (University of Pittsburgh Medical Center), as previously described. 20 The test analyzes 112 genes for point mutations, fusions, DNA copy number alterations (CNAs), and gene expression alterations of either BRAF V600E type or RAS type. Detected molecular alterations were classified into 5 groups (high‐risk, BRAF‐like, RAS‐like, CNAs, and gene expression alterations), as reported by Steward et al, 21 and were further categorized into low‐risk, intermediate‐risk, and high‐risk of aggressive disease molecular risk groups (MRGs). The risk stratification was based on associations between molecular alterations and the probability of disease aggressiveness reported by us and others. 2 , 5 , 7 , 13 , 22 Specifically, the low‐risk MRG included RAS and RAS‐like alterations present as the only event. The intermediate‐risk MRG included BRAF V600E, other BRAF‐like alterations, and CNA. The high‐risk profile included the presence of an early mutation and a late‐hit mutation, including TERT, TP53, AKT1, and PIK3CA. 21
Statistical Analysis
Propensity matching was used for the case‐control analysis and was based on age at diagnosis, tumor size, and sex, 3 factors known preoperatively that influence thyroid cancer prognosis. Ten caliper‐matched (ie, with similar variables) controls (DTC without DM) were selected at random, from which 1 match was selected using the minimum Mahalanobis distance. The process was repeated until each case had a propensity‐matched control. For each individual risk factor, odds ratios were computed with 95% CIs. A multivariate logistic regression model for the risk of DM was constructed using Akaike's information criteria for variable selection. Candidate variables included age, sex, nodule size, individual molecular alteration, and the MRG. The final model was presented as the predicted probability of DM and summarized using the concordance index (C‐index) to quantify the area under the receiver operating characteristic curve and R2 to assess the proportion of variation explained by the regression model. Variable selection and the C‐index were internally cross‐validated with 200 bootstrap samples. To check model calibration, we used bootstrap resampling of another 200 samples to calculate the bias and corrected mean absolute error of prediction. By using the Bayes theorem, the posterior probability of DM was computed to identify the observed proportions of patients with DM in each of the 3 MRG. Predicted probabilities were then computed for selected prevalence scenarios from 0.0 to 0.2 to identify the estimated population‐level proportion of patients with DM according to the MRG.
Results
Molecular Profiles of DTC With DM
In total, 62 patients who had DTC with DM were identified and, within this cohort, the mean age was 60.5 ± 16.1 years, most patients were men (55%), and the majority had papillary thyroid cancer (PTC) (87%) (Table 1). Synchronous metastasis (DM diagnosed within 6 months of initial diagnosis) was identified in 53% of patients, and the remaining patients had metachronous metastasis diagnosed at a median of 35 months (interquartile range, 103 months). There were no significant differences in age at diagnosis, sex, histologic thyroid cancer type, mean tumor size, or location of metastasis between patients with synchronous versus metachronous metastasis (Table 1). Median survival was longer for patients who had metachronous metastasis (151 vs 77 months).
TABLE 1.
Patient Demographics and Molecular Characteristics of Differentiated Thyroid Cancer With Distant Metastasis
| Variable | No. (%) | P | ||
|---|---|---|---|---|
| All | Synchronous Metastasis | Metachronous Metastasis | ||
| No. of patients | 62 (100) | 33 (53) | 29 (47) | |
| Age at initial surgery: Mean ± SD, y | 60.5 ± 16.1 | 62.2 ± 16.3 | 58.7 ± 15.9 | .4 |
| No. of men | 34 (55) | 22 (67) | 12 (41) | .07 |
| Cancer type | ||||
| Papillary | 54 (87) | 29 (88) | 25 (86) | 1.0 |
| Follicular | 2 (3) | 2 (6) | 0 (0) | |
| Oncocytic | 6 (10) | 2 (6) | 4 (14) | |
| Tumor size: Mean ± SD, cm | 3.6 ± 2.4 | 3.4 ± 2.5 | 3.9 ± 2.2 | .4 |
| Metastatic location | ||||
| Bone | 8 (13) | 5 (15) | 3 (10) | .5 |
| Lung | 39 (63) | 22 (67) | 17 (59) | |
| >1 | 12 (19) | 4 (12) | 8 (28) | |
| Other | 3 (5) | 2 (6) | 1 (3) | |
| Survival: Median [IQR], mo | 92 [82] | 77 [31] | 151 [160] | — |
| No. of deaths | 31 (50) | 18 (55) | 13 (45) | .6 |
| Primary molecular alteration | .8 | |||
| BRAF V600E | 27 (44) | 16 (48) | 11 (38) | |
| RAS | 8 (13) | 5 (15) | 3 (10) | |
| NTRK fusion | 5 (8) | 2 (6) | 3 (10) | |
| RET fusion | 7 (11) | 4 (12) | 3 (10) | |
| Copy number alterations | 12 (19) | 4 (12) | 8 (28) | |
| Other | 3 (5) | 2 (6) | 1 (3) | |
| Late secondary mutation | 41 (66) | 21 (64) | 20 (69) | .8 |
| Molecular risk group | ||||
| Low | 1 (1.6) | 0 (0) | 1 (3) | .9 |
| Intermediate | 20 (32) | 11 (33) | 8 (28) | |
| High | 41 (66) | 21 (64) | 20 (69) | |
Abbreviation: IQR, interquartile range
The most common primary molecular alteration in these tumors was BRAF V600E, which was identified in 44% of DTCs with DM (Table 1). CNAs and RAS mutations were also frequent and were identified in 19% and 13% of patients, respectively. Gene fusions were identified in 14 patients (23%), and the most common were RET (11%) and NTRK (8%) fusions. The distribution of primary molecular alterations did not differ between patients who had DTC with synchronous versus metachronous metastasis. The majority of DTC with DM (66%) had late‐hit mutations in TERT, TP53, or PIK3CA (Table 1). Although most BRAF V600E‐mutated and RAS‐mutated tumors had late‐hit mutations (89% and 75%, respectively), these were identified in smaller proportions of metastatic DTCs with NTRK (20%) and RET (43%) fusions (Fig. 1). Overall, 41 patients (66%) who had DTC with DM were in the high‐risk MRG, 20 (32%) were in the intermediate‐risk MRG, and only 1 (1.6%) had a low‐risk molecular profile. The latter was a patient who had an isolated NRAS Q61K mutation in a 2‐cm, encapsulated follicular variant PTC and iodine‐avid lung metastasis.
Figure 1.
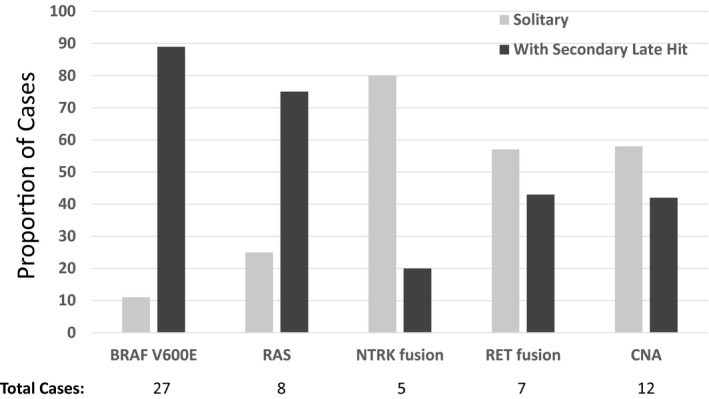
The proportions of patients who had differentiated thyroid cancer with distant metastasis and late secondary hits are illustrated. CNA indicates copy number alterations.
Results of the Matched Case‐Control Study
To estimate the relative risk of DM associated with specific genetic alterations and with the MRG classification, a consecutive cohort of 225 patients with DTC who had ≥5 years of follow‐up without DM were identified from a previously characterized group of patients. 22 Patients with DM were older at diagnosis, had larger tumors, and were more likely to be men (Table 2). To account for this heterogeneity, 53 patients who had DTC with DM were propensity matched to 55 patients who had DTC without DM; and, after matching, the 3 factors that distinguished patients who had DTC with DM from those without DM (age, tumor size, and sex) were no longer significantly different (Table 2). The molecular profiles of the propensity‐matched cohorts obtained using the TSv3 targeted next‐generation sequencing assay are illustrated in Figure 2. DM was associated with the presence of TERT mutation (odds ratio, 11.4; 95% CI, 4.5‐29.3; P < .001) or a late‐hit mutation (odds ratio, 11.4; 95% CI, 4.5‐29.3; P < .001) (Table 3).
TABLE 2.
Propensity Matching of Patients Who Had Differentiated Thyroid Cancer With and Without Distant Metastasis
| Prematch | DTC With DM, N = 62 | DTC Without DM, N = 225 | P |
|---|---|---|---|
| Age at diagnosis: Mean ± SD, y | 60.5 ± 16.1 | 48.8 ± 15.2 | <.001 |
| Tumor size: Mean ± SD, cm | 3.6 ± 2.4 | 2.1 ± 1.6 | <.001 |
| No. of men (%) | 34 (55.0) | 48 (21.0) | <.001 |
| Postmatch | DTC With DM, N = 53 | DTC Without DM, N = 55 | P |
|---|---|---|---|
| Age at diagnosis: Mean ± SD, y | 60.4 ± 15.8 | 56.7 ± 14.1 | .20 |
| Tumor size: Mean ± SD, cm | 3.6 ± 2.4 | 3.2 ± 2.0 | .34 |
| No. of men (%) | 29 (55.0) | 29 (53.0) | 1.00 |
Abbreviations: DM, distant metastasis; DTC, differentiated thyroid cancer.
Figure 2.
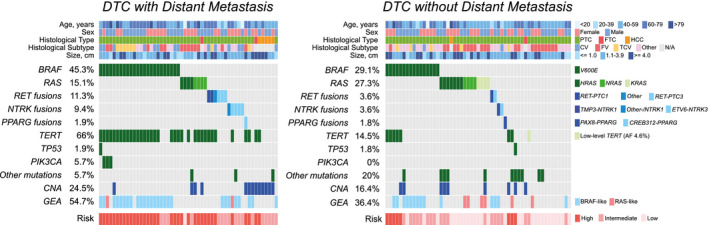
Molecular profiles and features of differentiated thyroid cancer (DTC) with and without distant metastasis are illustrated in the current matched case‐control cohort. CNA indicates copy number alteration; CV, classic variant; FTC, follicular thyroid carcinoma; FV, follicular variant; GEA, gene expression alteration; HCC, Hurthle cell carcinoma; N/A, not applicable; PTC, papillary thyroid carcinoma; TCV, tall cell variant.
TABLE 3.
Distribution of Molecular Alterations in Patients With Distant Metastasis (Cases) Compared With Propensity‐Matched Controls
| Molecular Alteration | No. (%) | OR | 95% CI | Adjusted P | |
|---|---|---|---|---|---|
| Cases, N = 53 | Controls, N = 55 | ||||
| Molecular risk group | |||||
| Low | 1 (2) | 28 (51) | — a | ||
| Intermediate | 17 (32) | 19 (35) | — b | ||
| High | 35 (66) | 8 (15) | |||
| TERT | 35 (66) | 8 (15) | 11.42 | 4.46‐29.27 | <.0001 |
| Late secondary hits: TERT, TP53, PIK3CA | 35 (66) | 8 (15) | 11.42 | 4.46‐29.27 | <.0001 |
| Gene expression analysis | 29 (55) | 20 (36) | 2.11 | 0.98‐4.57 | .12 |
| BRAF V600E | 24 (45) | 16 (29) | 2.02 | 0.91‐4.46 | .1454 |
| RAS | 8 (15) | 15 (27) | 0.47 | 0.18‐1.24 | .1795 |
| RET fusions | 6 (11) | 2 (4) | 3.38 | 0.65‐17.58 | .1795 |
| NTRK fusions | 5 (9) | 2 (4) | 2.76 | 0.51‐14.90 | .2416 |
| Copy number alterations | 13 (25) | 9 (16) | 1.66 | 0.64‐4.29 | .2914 |
Abbreviation: OR, odds ratio.
The OR for the high‐risk group relative to the intermediate‐risk group was 25.1 (95% CI, 3.07‐204.4; P < .001).
The OR for the high‐risk group relative to the low‐risk group was 122.5 (95% CI, 14.5‐1038.4; P < .001).
Among the patients who had DTC with DM assessed after propensity matching to those who had DTC without DM, the high‐risk MRG had a strong association with DM (odds ratio of high‐risk relative to intermediate‐risk MRG: 25.1; 95% CI, 3.07‐204.4; P < .001; odds ratio of high‐risk relative to low‐risk MRG: 122.5; 95% CI, 14.5‐1038.4; P < .001). Although most patients who had DTC with DM (66%) were in the high‐risk MRG, there was 1 patient in the low‐risk MRG. Conversely, most patients who had DTC without DM (51%) were in the low‐risk MRG, with 8 (15%) in the high‐risk MRG (Fig. 2, Table 3). Of note, on longer follow‐up collected after selecting the control group, 1 of the 8 patients who had DTC without DM in the high‐risk MRG developed DM >8 years after initial diagnosis and thus was initially misclassified. Among the 36 patients who had DTC in the intermediate‐risk MRG, 47% were associated with DM, and the other 53% were not (Table 3).
Estimation of Clinical Probability of DM in MRGs
The C‐index demonstrated a strong correlation between MRG and DM (0.836; 95% CI, 0.759‐0.913); and, after internal validation with 200 bootstrap samples, the C‐index remained high (0.834), with a slight decrease in the R2 from 0.483 to 0.469. Compared with observed values, the mean absolute error estimate from 200 bootstrap samples was 0.023, and the line of agreement between observed and bias‐corrected predictive values was essentially coincident with the diagonal. These results suggest that, in a generalized population of patients with DTC, the association of the molecular‐based risk stratification to DTC with DM would be expected to be nearly as accurate as that for the analyzed propensity‐matched cohorts.
For each MRG, the predicted prevalence of DM in DTC populations with different baseline risks of DM was derived using the probability of DM in the study cohort and the expected population‐based prevalence of DM in patients with DTC (Table 4, Fig. 3). Within the 5% to 10% prevalence of DM expected in most populations of patients with DTC, 1 the probability of DM would be from 0.2% to 0.4% in the low‐risk MRG, from 4.7% to 9.4% in the intermediate‐risk MRG, and from 19.3% to 33.5% in the high‐risk MRG (Table 4) (Fig. 3).
TABLE 4.
Estimated Associated Risk for Distant Metastasis by Prevalence of Metastasis and Molecular Risk Group
| Prevalence of DM, % | Molecular Risk Group, % | ||
|---|---|---|---|
| Low | Intermediate | High | |
| 1.0 | 0.04 | 0.9 | 4.4 |
| 5.0 | 0.2 | 4.7 | 19.3 |
| 10.0 | 0.4 | 9.4 | 33.5 |
| 15.0 | 0.6 | 14.1 | 44.9 |
| 20.0 | 0.9 | 18.8 | 53.2 |
Abbreviation: DM, distant metastasis.
Figure 3.
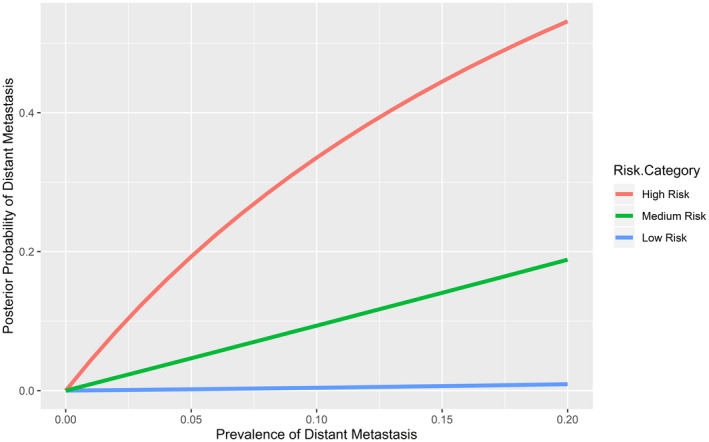
Prevalence‐adjusted predicted probability of distant metastasis associated with molecular risk group is illustrated.
Discussion
DM is an unequivocal marker of aggressive DTC, and the accurate preoperative identification of patients with at‐risk disease can affect initial management recommendations. The most common risk stratification system currently used to guide patient management is the ATA risk stratification, which uses primarily histopathologic findings to predict the risk of structural recurrence and incorporates only TERT and BRAF V600E mutations as molecular risk modifiers. In the current propensity‐matched case‐control study, we demonstrated that a molecular‐based, TSv3‐derived classification, which can readily be applied to preoperative biopsy, was able to accurately stratify the risk of DM.
Unlike prior studies describing molecular profiles of aggressive thyroid cancer, we used a commercially available molecular testing panel that is already commonly used for further assessment of cytologically indeterminate nodules. 21 In a consecutive series of 1510 DTCs, we previously demonstrated the prognostic implications associated with a small subset of the genetic changes that were detected in the current study. 22 In short‐term follow‐up, disease‐specific recurrence was associated with 15% of BRAF V600E‐positive or RET/PTC 1 and 3 fusion‐positive DTCs but was rarely seen in RAS‐positive or PAX8/PPARG‐positive cancers. With the current expanded panel, which included TERT and other markers of aggressive thyroid cancer, we were able to validate the molecular profile associated with aggressive DTC and further stratify patients into low‐risk, intermediate‐risk, and high‐risk groups with robust discrimination (C‐index, 0.836). Importantly, the probability of DM in each MRG observed in this study was similar to the risk of structural disease recurrence expected for the ATA risk groups. 2
Although most of the 62 patients who had DTC with DM had either BRAF V600E or RAS as a primary driver mutation, an associated late‐hit mutation, most commonly TERT, was also detected in 66%. In meta‐analysis, concurrent BRAF V600E with a TERT mutation was associated with thyroid cancer in older patients, in men, and in cancers at advanced TNM stage (with lymph node metastasis and DM). 18 Even after case matching for age, sex, and tumor size, we observed the association of concurrent TERT mutations with DM. However, although a TERT mutation alone has been associated with aggressive disease, in our case cohort, we did not observe any isolated TERT mutations, and all cases had an additional genetic alteration, including BRAF V600E (n = 24), RAS (n = 6), and CNA (n = 11). Of note, 8 controls without DM within 5 years of diagnosis also were in the high‐risk MRG; however, 1 patient with BRAF V600E and a TERT mutation developed lung metastasis, which was diagnosed >5 years after initial surgery. Therefore, continued surveillance of patients with a high‐risk molecular profile would be prudent.
For tumors in the intermediate‐risk MRG, the likelihood of DM had a rate between that of the low‐risk and high‐risk groups. There were no additional molecular features that further risk stratified this cohort, including the type of primary driver mutation. However, the exclusion of a low‐risk MRG is still prognostically valuable and is information that may be used to guide the extent of surgery and surveillance. Further studies to identify clinical or histologic features that may refine risk stratification of tumors with an intermediate‐risk molecular profile could be useful. In contrast, only 1 patient with DM in the current study had a low‐risk molecular profile with an isolated RAS mutation. Analysis in an independent cohort may provide additional confirmation, but our internal validation of the MRGs demonstrated likely excellent reproducibility if applied to a generalized DTC population.
We did not propensity match by histologic subtype; however, histologic subtype is not prognostic information that is known preoperatively and thus cannot be used pragmatically in preoperative decision making. Furthermore, studies over the last decade have indicated that the encapsulated follicular variant of papillary carcinoma shares more biologic and clinical characteristics with follicular carcinoma than with classic papillary carcinoma, suggesting that separating these 2 types of DTC may have diminished clinical impact. 6 , 23 , 24 There may be differences in the molecular mechanisms of oncocytic carcinoma compared with papillary or follicular cancer, as the former is dominated by widespread chromosomal CNA. 9 Indeed, in the 6 oncocytic carcinomas with DM in the current study, 5 had solitary CNA (considered intermediate‐risk), whereas 1 was high‐risk with concurrent TERT mutation and CNA. However, CNA were identified in all 3 DTC types in this study, pointing toward overlapping genetic mechanisms between Hurthle cell carcinoma and other types of DTC.
In addition to patient age, tumor size, and sex, other clinical and histologic factors have been associated with DM in DTC. In a recent National Cancer Database study, lymph node metastasis, minimal or gross extrathyroidal extension, lymphovascular invasion, tumor size, and histology were all risk factors for DM. 25 In another single‐institution study, the incidence of lateral lymph node metastasis was higher in patients who had DM compared with those who did not (70% vs 52%). 26 Although lymph node metastasis can be a marker of aggressive disease, in thyroid cancer, its diagnosis often occurs after histologic evaluation, and the presence of lymph node metastasis lacks specificity for aggressive biologic behavior. For example, the most recent 8th edition of the American Joint Committee on Cancer staging manual downstaged patients with lymph node disease compared with the previous 7th edition 27 ; and, in a single‐institution, multivariable analysis comparing the 2 editions, the presence of either central or lateral lymph node metastasis indeed was not associated with diminished disease‐specific survival. 28 Therefore, because lymph node metastases are often diagnosed definitively only after histologic assessment and data on the prognostic impact of lymph node disease in thyroid cancer vary, the presence of lymph node metastasis was not included as a variable in the propensity matching. Future studies to assess whether lymph node status may be of additive value to molecular risk stratification would be important.
The absence of DM after 5 years of follow‐up was the inclusion criteria for the matched cohort, yet DM can be identified >10 years after initial diagnosis. The availability and integrity of tissue for molecular testing from original histology >10 years after diagnosis in large part led to this constraint. This limitation likely led to potential misclassification of some metastatic DTCs as control, nonmetastatic cases. Indeed, we observed that 1 of the 8 patients who had DTC with a high‐risk molecular profile in the no‐DM group developed lung metastasis on longer follow‐up. Therefore, considering a 10‐year window, the high‐risk molecular profile of this misclassified case further supports the robustness of the MRG. Regardless, it is unlikely that this limitation affected the <0.5% risk of DM associated with a low‐risk molecular signature expected in DTC with a population‐based prevalence of DM at 10%.
Preoperative risk stratification using the tumor's molecular profile could be 1 of the few available clinical parameters that identify DTC with aggressive biology and higher risk for developing DM, which may be useful to determine the extent of initial thyroidectomy. Although lymph node metastasis is another clinical parameter that may be associated with aggressive disease and affects the extent of surgery, it can typically be detected with standard preoperative ultrasound or, occasionally, intraoperatively. 3 Knowing the absolute risk of DM for each MRG may be useful to guide preoperative clinical decisions. For example, in recent Surveillance, Epidemiology, and End Results data, the frequency of DM at presentation in conventional PTC is approximately 3%. 1 If a high‐risk profile is detected preoperatively, the DM risk increases to 10%, and initial total thyroidectomy may be a consideration. Conversely, a preoperatively detected low‐risk molecular profile would be associated with an exceedingly low 0.12% risk of DM, and thyroid lobectomy would be an oncologically appropriate option. Prospective studies are ongoing to assess whether the molecular profile can accurately guide the extent of initial surgery.
In summary, the results of this matched case‐control study demonstrate that the molecular profile can robustly and quite accurately stratify the risk of aggressive DTC, defined as DM. The preoperative detection of DTC at high risk for DM may help to inform the extent of initial surgical treatment and identify patients who may be candidates for more effective therapeutic trials. Furthermore, patients with low‐risk DTC may be candidates for de‐escalated care. We observed no clinical or molecular features that could distinguish patients with synchronous DM from those with metachronous DM, and extended surveillance would be prudent for all patients who have DTC with a high‐risk molecular profile. Molecular testing can further optimize prognostication for patients with DTC and help clinicians to guide clinical management.
Funding Support
This work was supported by a Specialized Programs of Research Excellence (SPORE) grant (P50 CA097190‐15) from the National Institutes of Health.
Conflict of Interest Disclosures
Esra Karslioglu‐French reports personal fees from Horizon Therapeutics, outside the submitted work. Dan P. Zandberg reports institutional research support from Merck, Bristol Myers Squibb, AstraZeneca, Aduro, Macrogenics, GlaxoSmithKline, Lilly, and Astellas, outside the submitted work; and personal fees from Blueprint Medicines, outside the submitted work. Robert L. Ferris reports research funding from Astra‐Zeneca/MedImmune, Bristol Myers Squibb, Merck, Novasenta Consulting, and Tesaro, outside the submitted work; personal fees from Aduro Biotech, Bristol Myers Squibb, EMD Serano, MacroGenics Inc, Merck, Novasenta Consulting, personal fees from Numab Therapeutics AG, personal fees Pfizer, Sanofi, and Zymeworks Inc, outside the submitted work; and owns stock in Novasenta Consulting. Marina N. Nikiforova reports personal fees from Sonic Healthcare USA, outside the submitted work, and has a patent and copyrights with royalties paid related to ThyroSeq from the University of Pittsburgh. Yuri E. Nikiforov reports personal fees from Sonic Healthcare USA, outside the submitted work, and has a patent and copyrights with royalties paid related to ThyroSeq from the University of Pittsburgh. The remaining authors made no disclosures.
Author Contributions
Linwah Yip: Conceptualization and study design, data acquisition and interpretation, writing–original draft, and writing–review and editing. William E. Gooding: Study design and methodology, formal data analysis and data interpretation, and writing–review and editing. Alyaksandr Nikitski: Data curation, data analysis and interpretation, and writing–review and editing. Abigail I. Wald: Data curation, methodology, project administration, and writing–review and editing. Sally E. Carty: Data acquisition, data analysis and interpretation, and writing–review and editing. Esra Karslioglu‐French: Data acquisition, data analysis and interpretation, and writing–review and editing. Raja R. Seethala: Data acquisition, data analysis and interpretation, and writing–review and editing. Dan P. Zandberg: Data acquisition, data analysis and interpretation, and writing–review and editing. Robert L. Ferris: Data acquisition, data analysis and interpretation, and writing–review and editing. Marina N. Nikiforova: Conceptualization, data curation, data acquisition, data analysis and interpretation, and writing–review and editing. Yuri E. Nikiforov: Conceptualization and study design, data curation, data acquisition and interpretation, writing–original draft, and writing–review and editing.
Yip L, Gooding WE, Nikitski A, Wald AI, Carty SE, Karslioglu‐French E, Seethala RR, Zandberg DP, Ferris RL, Nikiforova MN, Nikiforov YE. Risk assessment for distant metastasis in differentiated thyroid cancer using molecular profiling: A matched case‐control study. Cancer. 2021. 10.1002/cncr.33421
References
- 1. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review (CSR), 1975‐2017. Based on November 2019 SEER data submission, posted to the SEER website, April 2020. National Cancer Institute; 2020. [Google Scholar]
- 2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel KN, Yip L, Lubitz CC, et al. The American Association of Endocrine Surgeons guidelines for the definitive surgical management of thyroid disease in adults. Ann Surg. 2020;271:e21‐e93. [DOI] [PubMed] [Google Scholar]
- 4. Carty SE, Ohori NP, Hilko DA, et al. The clinical utility of molecular testing in the management of thyroid follicular neoplasms (Bethesda IV nodules). Ann Surg. 2020;272:621‐627. [DOI] [PubMed] [Google Scholar]
- 5. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cancer Genome Atlas Research Network . Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel SG, Carty SE, McCoy KL, et al. Preoperative detection of RAS mutation may guide extent of thyroidectomy. Surgery. 2017;161:168‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ravella L, Lopez J, Descotes F, et al. Preoperative role of RAS or BRAF K601E in the guidance of surgery for indeterminate thyroid nodules. World J Surg. 2020;44:2264‐2271. [DOI] [PubMed] [Google Scholar]
- 9. Ganly I, Makarov V, Deraje S, et al. Integrated genomic analysis of Hurthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell. 2018;34:256‐270.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126:1052‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xing M, Liu R, Liu X, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32:2718‐2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melo M, da Rocha AG, Vinagre J, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2014;99:E754‐E765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song YS, Lim JA, Choi H, et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer. 2016;122:1370‐1379. [DOI] [PubMed] [Google Scholar]
- 14. Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375:1054‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sohn SY, Park WY, Shin HT, et al. Highly concordant key genetic alterations in primary tumors and matched distant metastases in differentiated thyroid cancer. Thyroid. 2016;26:672‐682. [DOI] [PubMed] [Google Scholar]
- 16. Song E, Song DE, Ahn J, et al. Genetic profile of advanced thyroid cancers in relation to distant metastasis. Endocr Relat Cancer. 2020;27:285‐293. [DOI] [PubMed] [Google Scholar]
- 17. Jung CK, Jung SH, Jeon S, et al. Risk stratification using a novel genetic classifier including PLEKHS1 promoter mutations for differentiated thyroid cancer with distant metastasis. Thyroid. 2020;30:1589‐1600. [DOI] [PubMed] [Google Scholar]
- 18. Moon S, Song YS, Kim YA, et al. Effects of coexistent BRAF(V600E) and TERT promoter mutations on poor clinical outcomes in papillary thyroid cancer: a meta‐analysis. Thyroid. 2017;27:651‐660. [DOI] [PubMed] [Google Scholar]
- 19. Shen X, Liu R, Xing M. A six‐genotype genetic prognostic model for papillary thyroid cancer. Endocr Relat Cancer. 2017;24:41‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nikiforova MN, Mercurio S, Wald AI, et al. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. 2018;124:1682‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steward DL, Carty SE, Sippel RS, et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: a prospective blinded multicenter study. JAMA Oncol. 2019;5:204‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yip L, Nikiforova MN, Yoo JY, et al. Tumor genotype determines phenotype and disease‐related outcomes in thyroid cancer: a study of 1510 patients. Ann Surg. 2015;262:519‐525; discussion 524‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoo SK, Lee S, Kim SJ, et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016;12:e1006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asa SL, Giordano TJ, LiVolsi VA. Implications of the TCGA genomic characterization of papillary thyroid carcinoma for thyroid pathology: does follicular variant papillary thyroid carcinoma exist? Thyroid. 2015;25:1‐2. [DOI] [PubMed] [Google Scholar]
- 25. Al‐Qurayshi Z, Sullivan CB, Pagedar N, Lee GS, Tufano R, Kandil E. Prevalence and risk of metastatic thyroid cancers and management outcomes: a national perspective. Laryngoscope. 2021;131:237‐244. [DOI] [PubMed] [Google Scholar]
- 26. Barbosa MP, Momesso D, Bulzico DA, et al. Metastatic lymph node characteristics as predictors of recurrence/persistence in the neck and distant metastases in differentiated thyroid cancer. Arch Endocrinol Metab. 2017;61:584‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2018;68:55‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tam S, Boonsripitayanon M, Amit M, et al. Survival in differentiated thyroid cancer: comparing the AJCC Cancer Staging seventh and eighth editions. Thyroid. 2018;28:1301‐1310. [DOI] [PubMed] [Google Scholar]


