Abstract
Introduction:
Quality improvement efforts have focused on reducing interstage mortality for infants with hypoplastic left heart syndrome (HLHS). In 1/2016 two publications reported that use of digoxin was associated with reduced interstage mortality. The degree to which these findings have affected real world practice has not been evaluated.
Methods:
The discharge medications of neonates with HLHS undergoing Norwood operation between 1/2007 and 12/2018 at Pediatric Health Information Systems Database hospitals were studied. Mixed effects models were calculated to evaluate the hypothesis that the likelihood of digoxin prescription increased after 1/2016, adjusting for measurable confounders with furosemide and aspirin prescription measured as falsification tests. Inter-hospital practice variation was measured using the median odds ratio.
Results:
Over the study period 6,091 subjects from 45 hospitals were included. After adjusting for measurable covariates, discharge after 1/2016 was associated with increased odds of receiving digoxin (OR: 3.9, p<0.001). No association was seen between date of discharge and furosemide (p=0.26) or aspirin (p=0.12). Prior to 1/2016, the likelihood of receiving digoxin was decreasing (OR: 0.9 per year, p<0.001) while after 1/2016 the rate has increased (OR: 1.4 per year, p<0.001). However, there remains significant inter-hospital variation in the likelihood of receiving digoxin even after adjusting for known confounders (median odds ratio=3.5, p<0.0001).
Conclusion:
Following publication of studies describing an association between digoxin and improved interstage survival, the likelihood of receiving digoxin at discharge increased without similar changes for furosemide or aspirin. Despite concerted efforts to standardize interstage care, inter-hospital variation in pharmacotherapy in this vulnerable population persists.
Keywords: Outcomes, pharmacoepidemiology, health services research, pediatrics, pediatric cardiology
Introduction:
The interstage period between Norwood operation and superior cavopulmonary connection operation has been identified as a period of high risk for infants with hypoplastic left heart syndrome (HLHS) and anatomic variants with historical series reporting mortality rates between 10–19%1–10. As a result, improving survival in this period has been the focus of concerted local2–6,8,11 and national quality improvement efforts12,13. In January 2016, two publications separately reported that use of digoxin during the interstage period was associated with decreased interstage mortality14,15, after being presented at the American College of Cardiology and American Heart Association Scientific sessions in 2015. Contemporaneous studies reported that digoxin was being used in a minority of Norwood patients16,17, suggesting that digoxin prescription might be a modifiable factor with the potential to improve transplant-free survival through the interstage period.
To our knowledge, the effect of these findings on real world practice has not been studied. Therefore, we sought to study trends in the prescription of digoxin at discharge in neonates with HLHS after Norwood operation using data from the Pediatric Health Information Systems (PHIS) Database, hypothesizing that the likelihood of prescription would increase after the publication of these studies. We also sought to determine whether significant inter-hospital variation in the use of digoxin remained after adjusting for measurable confounders, hypothesizing that practice variation in prescription of digoxin would have decreased in parallel in this patient population.
METHODS:
Data source:
The PHIS database contains administrative data from inpatient, emergency department, ambulatory surgery, and observation encounters from 45 not-for-profit, tertiary care pediatric hospitals in the United States. These hospitals are affiliated with the Children’s Hospital Association (CHA) (Overland Park, KS). Data quality and reliability are assured through a joint effort between CHA and participating hospitals. Participating hospitals provide discharge/encounter data including demographics, diagnoses, and procedures as well as utilization data (e.g. pharmacy products, radiologic studies, and laboratory studies). Data are de-identified at the time of data submission and are subject to a number of reliability and validity checks.
Study Population:
We studied infants (age at admission <30 days) with HLHS who underwent Norwood operation at a PHIS hospital between 1/1/2007 and 12/31/2018. Subjects were identified using International Classification of Diseases version 9 and 10 (ICD-9 and ICD-10) codes. The study spanned periods in which both diagnosis code systems were used. ICD-9 codes for diagnoses and procedures were identified and converted to ICD-10 codes using United States Center for Medicare and Medicaid Services ICD-9 to ICD-10 crosswalk (cms.gov). Manual review of these tables was performed to ensure accuracy. As the focus of the study was on the use of digoxin in the interstage period, subjects who died prior to discharge or underwent orthotopic heart transplant during their Norwood hospitalization were excluded from analysis. Hospitals reporting five or fewer subjects either prior to or after 1/2016 were excluded to avoid including centers with extremely low Norwood volumes.
Study Measures:
Data were extracted from the PHIS database by direct query using ICD-9/10 codes for diagnoses and procedures and Clinical Transaction Codes (CTC) for pharmaceutical products. The primary exposure for this study was the date of discharge. The goal of the study was to capture discharge prescription of digoxin. PHIS records data from hospital-based encounters and does not record discharge prescriptions. As a surrogate for a discharge prescription we sought to capture medications that subjects were receiving immediately prior to discharge. However, during design of the study we recognized that some discharge medications might not be administered and/or recorded on the day of discharge. To optimize sensitivity, the primary outcome was defined as digoxin ordered in the three days prior to discharge. Digoxin ordered on the day of discharge was also recorded for a pre-planned sensitivity analysis. We acknowledge that this does not account for changes in prescription after discharge, but at a minimum provides a best estimate of the intended treatment plan of the team at the hospital where the patient underwent their Norwood operation. We did not expect that the likelihood of prescribing aspirin or furosemide would have changed over the study period. Therefore, analogous data about their prescription patterns were also collected for use in falsification tests.
Data about a broad range of potential covariates were collected. During the design of the study, we suspected that documented arrhythmia would be associated with increased likelihood of digoxin use, as would factors suggestive of clinical instability (e.g. previous cardiac arrest, extracorporeal membrane oxygenation, discharge receiving opiate medications and prolonged length of stay)18. Not all factors associated with digoxin use are available in the current data set (atrioventricular valve regurgitation or ventricular dysfunction)14. Other demographic (sex, race, insurance payer) and clinical (genetic syndrome and non-cardiac medical conditions) factors were also considered as potentially influential. Non-cardiac medical conditions were grouped by type as described previously19. A limitation of these codes is that it is not possible to determine which of these conditions were present before Norwood operation and which occurred after, but because the influence of these conditions on likelihood of receiving digoxin is not related to their timing this distinction was less important in this case. The type of Norwood operation (Blalock-Taussig shunt vs. right ventricle to pulmonary artery conduit vs. hybrid) was also considered as a potential factor influencing digoxin prescription, but this was not well delineated in the database and so could not be used.
Statistical Analysis
The characteristics of the study population were described using standard descriptive statistics. Continuous variables were expressed as mean ± standard deviation or median (interquartile range (IQR) and range) as appropriate. Categorical data are expressed as counts and percentages.
Studying changes in likelihood of receiving digoxin was our primary aim. As noted, the two studies14,15 that highlighted the potential benefits of digoxin in the interstage period were published in 1/2016 but were presented in March and November of 2015 at the American College of Cardiology and American Heart Association Scientific Sessions. We chose 1/2016 as an inflection for analysis, recognizing that early dissemination was a possibility that would (if anything) bias our results towards the null. We chose (prior to analysis) to keep this pre-specified threshold regardless of subsequent observations, which would potentially bias our results towards the null. The observed rate of digoxin prescription per year of discharge was calculated. The likelihood of discharge prescription prior to 1/1/2016 was compared to the likelihood after that date using a chi-square test. The same analysis was applied to furosemide and aspirin. A key source of potential bias in studies comparing two different periods is changes in the case-mix of the study population between the compared times. Differences in the distribution of possible covariates were compared between subjects discharged before and after 1/2016 using Student’s t-test, Wilcoxon rank sum, and Chi-square tests.
To address any potential bias from differences in the distribution of these covariates, multivariable models were calculated. The degree to which covariates influenced the likelihood of receiving digoxin (or other medications) was not known. We sought to identify pertinent covariates empirically, and so evaluated the association between each covariate and the receipt of digoxin. To avoid bias, a liberal threshold (p≤0.2) was used to determine which factors would be included in subsequent models20. Mixed effects models (using generalized linear models with logit link) including these covariates as fixed effects and clustering by hospital were performed. Two main models were calculated 1) separating the cohort into those discharged before and after 1/2016 and 2) evaluating the rate of change in likelihood before and after 1/2016. The latter analysis is an extension of a difference-in-difference analysis (controlling for secular trends in behavior preceding the change). An interrupted time series methodology, specifically including an adoption period during which changes in practice were ignored, was not performed because prior to analysis it was not clear how long dissemination and adoption of new practices would take in an era characterized by potentially rapid dissemination of research findings (electronic communication and prevalent quality improvement efforts). Omitting an adoption period also has the potential to bias our results towards the null.
Measuring variation in practice between centers in the sample was a pre-identified secondary goal. To do so, we utilized the mixed effects model to calculate the median odds ratio for treatment with digoxin. The median odds ratio (MOR) is a means of quantifying the magnitude of variation in practice between hospitals in a sample and has been used in multiple studies of adult21–24 and pediatric25–29 cardiac patients. It represents the relative odds that a single hypothetical patient would receive different care (i.e. digoxin or not) at two randomly selected hospitals in the sample30. An MOR>1.2 is considered of significant magnitude, reflecting meaningful practice variation30.
Several pre-specified sensitivity analyses were performed to measure whether choices made in the design phase inadvertently introduced important systematic error(s). First, analyses were repeated with receipt of a medication within 1 day of discharge as the outcome. Second, an analysis was performed excluding hospitals in which a disproportionate number of the potential subjects were from either the period before or after 1/2016. The average proportion of cases after 2016 was 28% with a standard deviation of 11%. We excluded hospitals where the proportion of cases after 2016 were more than two standard deviations from this average (either <6% or >50%). Ultimately, this excluded two centers (both of which had a disproportionately large proportion of cases after 1/2016). Third, an analysis was performed restricted to subjects in which no arrhythmia was recorded to account for bias(es) introduced by changes in coding of arrhythmia or in the treatment of dysrhythmia in this population. One post-hoc sensitivity was performed. We had not excluded subjects kept in hospital through a second stage operation. To evaluate whether this introduced bias, we repeated our primary models excluding subjects whose LOS was >90 days. Because of concerns raised that the prevalence of comorbid conditions was influenced by the conversion from ICD-9 to ICD-10 coding, a post-hoc sensitivity analysis was performed of the primary model with comorbid non-cardiac disease removed from the model.
A post-hoc secondary analysis was performed to evaluate the response of individual hospitals over the study period. The likelihood of receiving digoxin was calculated for each hospital for the period before 1/1/2016 and after and depicted. The change in likelihood was calculated using the sign rank test. A second post-hoc analysis was performed to evaluate whether Norwood operation annual volume was associated with likelihood of discharge prescription of digoxin. Norwood volume was incorporated in the primary model as either a continuous count or by dividing the study population into four groups based on natural cutpoints in Norwood volume base on visual inspection their histogram31,32.
Missing data were generally infrequent (<1% for most variables). However, data for race were missing for a significant number of patients, which is typical for studies using administrative data25,28,32–36. To mitigate potential bias, a separate categorical variable for “missing race” was generated. Otherwise cases with missing data were excluded by case restriction, and no imputation was applied, since the benefit for addressing these rare instances was minimal. The primary analyses were pre-specified, and other analyses should be considered exploratory. No formal adjustment for multiple comparisons was made.
All data analysis was performed using Stata MP 13 (Statacorp, College Station, TX). The threshold for statistical significance was p<0.05.
RESULTS
Study population:
Initial query of the PHIS database generated a study cohort of 6,154 subjects from 51 hospitals. Based on exclusion criteria, 63 subjects from 6 hospitals were excluded leaving 6,091 subjects from 45 hospitals in the analytic cohort. The population was 62% male and 54% non-Hispanic white with 40% receiving commercial insurance and 49% receiving Medicaid (Supplementary Table 1).
Dividing the study population between those discharged before 1/2016 and those after, there were several significant differences (Table 1). Notably the proportion of subjects with neurologic conditions (p=0.01), renal conditions (p<0.001), gastrointestinal conditions (p<0.001), metabolic disorder (p=0.001), and technology dependence (p<0.001) were all higher in subjects discharged in or after 2016 than those discharged before. At the same time, the prevalence of genetic syndromes was significantly less in those discharged in or after 2016 (p=0.02). The proportion who received ECMO was also higher (11% vs. 8% p<0.001). Total and post-operative length of stays were greater in those discharged in or after 2016 (p<0.001 for both).
TABLE 1:
Study cohort
| 1/2007–12/2015 (n=4559) | 1/2016–12/2018 (n=1526) | p | |
|---|---|---|---|
| Male sex | 62% (2821) | 61% (932) | 0.81 |
| Race | <0.001 | ||
| Non-Hispanic white | 54% (2477) | 54% (820) | |
| Hispanic white | 12% (542) | 11% (167) | |
| Non-Hispanic black | 11% (479) | 12% (190) | |
| Other Hispanic | 7% (310) | 7% (118) | |
| Other or more than one race | 4% (163) | 1% (20) | |
| Missing | 13% (588) | 14% (211) | |
| Payer | <0.001 | ||
| Commercial | 39% (1795) | 41% (625) | |
| Medicaid | 48% (2191) | 53% (805) | |
| Other government | 5% (214) | 3% (46) | |
| Other | 7% (312) | 2% (27) | |
| Missing | 1% (47) | 2% (23) | |
| Prematurity (<37 weeks GA) | 9% (414) | 11% (163) | 0.07 |
| Prematurity | 0.11 | ||
| <=26 weeks | 0.1% (4) | 0.1% (2) | |
| 27–30 | 0.3% (15) | 0.4% (6) | |
| 31–34 | 3% (134) | 3% (53) | |
| 35–37 | 6% (261) | 7% (100) | |
| Missing GA but preterm | 0% (0) | 0.1% (2) | |
| Term | 91% (4145) | 89% (1363) | |
| Genetic syndrome | 4% (174) | 3% (39) | 0.02 |
| Neurological condition | 6% (260) | 8% (115) | 0.01 |
| Respiratory condition | 7% (333) | 8% (124) | 0.29 |
| Renal condition | 12% (548) | 22% (340) | <0.001 |
| Gastrointestinal condition | 21% (964) | 26% (396) | <0.001 |
| Hematological condition | 2% (99) | 2% (35) | 0.78 |
| Metabolic disorder | 4% (191) | 6% (97) | 0.001 |
| Technology dependent | 27% (1239) | 34% (512) | <0.001 |
| Arrhythmia | 25% (1136) | 22% (340) | 0.04 |
| ECMO during hospitalization | 8% (362) | 11% (166) | <0.001 |
| Total LOS | Median: 31 (IQR: 20–52) | Median 36.5 (IQR: 23–65) | <0.0001 |
| Preoperative LOS | Median: 5 (IQR: 3–7) | Median 5 (IQR: 2–7) | 0.02 |
| Post operative LOS | Median 25 (IQR: 15–45) | Median 32 (IQR: 18–59) | <0.0001 |
| Digoxin | 23% (1039) | 43% (651) | <0.001 |
| Aspirin | 60% (2728) | 59% (903) | 0.65 |
| Furosemide | 71% (3258) | 73% (1114) | 0.25 |
| Opiate | 2% (110) | 2% (33) | 0.58 |
Abbreviations: ECMO extracorporeal membrane oxygenation, GA gestational age, LOS length of stay
Observed trends in use of digoxin:
The proportion of subjects receiving digoxin at discharge was significantly higher in those discharged on or after 1/2016 compared to those discharged earlier (43% vs.23%, p<0.01). There was no significant difference in the likelihood of receiving aspirin (p=0.65) or furosemide (p=0.25) over the same period. The percentage of subjects receiving digoxin, furosemide, and aspirin is depicted in Figure 1. In sensitivity analyses no significant differences in these associations were observed.
Figure 1: Discharge prescription of digoxin, furosemide, and aspirin in neonates with hypoplastic left heart syndrome at PHIS hospitals 2007–2018.
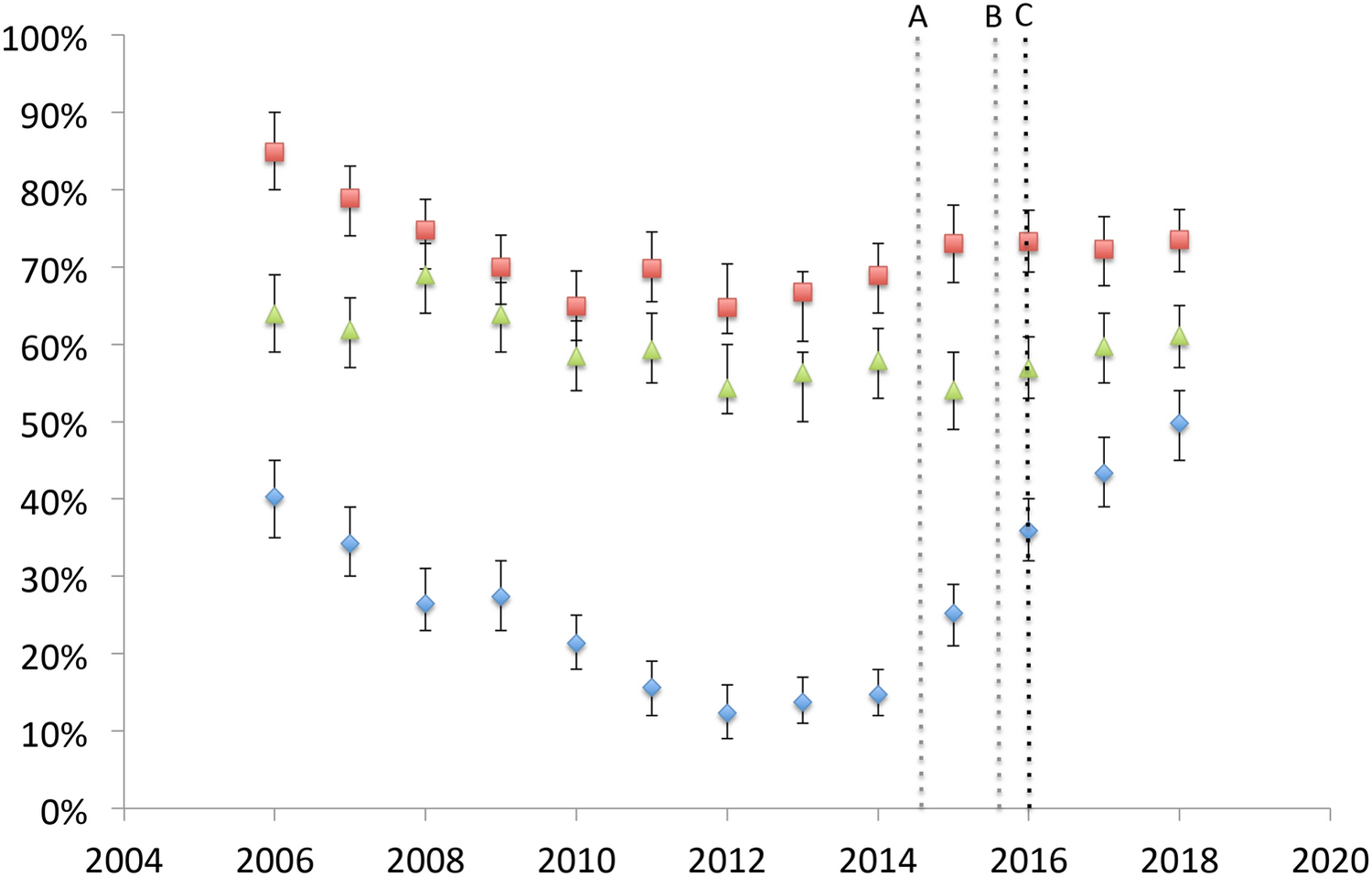
The observed likelihood of receiving digoxin (blue diamond) along with furosemide (red square) and aspirin (green triangle) in each year of the study period are depicted. 95% confidence intervals are depicted in brackets. Hashed lines denote the date of publication of abstracts (A: American College of Cardiology, B American Heart Association) and manuscripts (C).
Adjusted analyses of the use of digoxin
Bivariable screening of factors was performed (Table 2), and a mixed effects model for the likelihood of digoxin was calculated (Table 3). Discharge on or after 1/2016 was associated with increased odds of receiving digoxin (OR: 3.83, 95% CI: 3.29–4.48, p<0.001). No such association was seen for either furosemide (p=0.33, Supplementary Table 2) or aspirin (p=0.06, Supplementary Table 3). As planned a second model was also performed evaluating trends in digoxin usage over time before and after 1/2016 (Table 4). Prior to 2016, the odds of receiving digoxin were lower with each year (OR: 0.94 per year, 95% CI: 0.91–0.96, p<0.001). After 2016, the trend reversed and increasing time was associated with larger odds (OR: 2.38 per year, 95% CI: 2.14–2.64, p<0.001). In sensitivity analyses, restricting analysis to medications received within 1 day of discharge, likelihood of receiving aspirin was significantly less likely after 2016 than before (OR: 0.85, 95% CI: 0.72–1.00, p=0.04). No other changes in the observed associations were seen in other specified sensitivity analyses (data not shown).
Table 2:
Factors associated with digoxin use
| OR | 95% CI | p | |
|---|---|---|---|
| Discharge ≥1/1/2016 | 2.52 | 2.22–2.85 | <0.001 |
| Male sex | 1.18 | 1.05–1.32 | 0.005 |
| Race | |||
| Non-Hispanic white | 1 | n/a | n/a |
| Hispanic white | 0.84 | 0.70–1.00 | 0.06 |
| Non-Hispanic black | 1.08 | 0.90–1.29 | 0.40 |
| Other Hispanic | 0.80 | 0.64–1.02 | 0.07 |
| Other or more than one race | 1.41 | 1.04–1.93 | 0.03 |
| Missing | 0.68 | 0.57–0.82 | <0.001 |
| Payer | |||
| Commercial | 1 | n/a | n/a |
| Medicaid | 0.95 | 0.85–1.07 | 0.44 |
| Other government | 0.72 | 0.54–0.98 | 0.04 |
| Other | 0.54 | 0.40–0.72 | <0.001 |
| Missing | 0.40 | 0.20–0.79 | 0.008 |
| Prematurity (<37 weeks GA) | 0.69 | 0.56–0.85 | <0.001 |
| Genetic syndrome | 0.54 | 0.37–0.77 | 0.001 |
| Neurological condition | 0.79 | 0.61–1.01 | 0.06 |
| Respiratory condition | 0.95 | 0,77–1.18 | 0.67 |
| Renal condition | 0.69 | 0.58–0.82 | <0.001 |
| Gastrointestinal condition | 1.23 | 1.08–1.40 | 0.002 |
| Hematological condition | 0.99 | 0.68–1.45 | 0.97 |
| Metabolic disorder | 0.96 | 0.74–1.26 | 0.79 |
| Technology dependent | 1.16 | 1.03–1.32 | 0.01 |
| Arrhythmia | 1.57 | 1.38–1.78 | <0.001 |
| ECMO during hospitalization | 1.49 | 1.23–1.80 | <0.001 |
| Cardiac arrest during hospitalization | 0.78 | 0.53–1.15 | 0.20 |
| Total LOS > median (32 days) | 1.55 | 1.38–1.73 | <0.001 |
| Aspirin | 2.64 | 2.33–3.00 | <0.001 |
| Furosemide | 3.42 | 2.93–4.00 | <0.001 |
| Opiate | 0.66 | 0.43–0.99 | 0.04 |
Abbreviations: ECMO extracorporeal membrane oxygenation, GA gestational age, LOS length of stay
Table 3:
Multivariable mixed effect model for likelihood of digoxin
| OR | 95% CI | p | |
|---|---|---|---|
| Discharge ≥1/2016 | 3.83 | 3.29–4.48 | <0.001 |
| Male sex | 1.16 | 1.01–1.33 | 0.04 |
| Race | |||
| Non-Hispanic white | 1 | n/a | n/a |
| Hispanic white | 1.43 | 1.13–1.82 | 0.003 |
| Non-Hispanic black | 1.09 | 0.87–1.37 | 0.47 |
| Other Hispanic | 0.93 | 0.69–1.25 | 0.64 |
| Other or more than one race | 1.81 | 1.24–2.64 | 0.002 |
| Missing | 0.79 | 0.63–0.99 | 0.05 |
| Payer | |||
| Commercial | 1 | n/a | n/a |
| Medicaid | 0.97 | 0.84–1.13 | 0.72 |
| Other government | 0.82 | 0.58–1.16 | 0.26 |
| Other | 0.77 | 0.54–1.08 | 0.13 |
| Missing | 0.67 | 0.31–1.43 | 0.30 |
| Prematurity (<37 weeks GA) | 0.74 | 0.58–0.95 | 0.02 |
| Genetic syndrome | 0.80 | 0.52–1.22 | 0.30 |
| Neurological condition | 0.65 | 0.48–0.87 | 0.003 |
| Renal condition | 0.75 | 0.60–0.95 | 0.02 |
| Gastrointestinal condition | 0.83 | 0.66–1.06 | 0.13 |
| Technology dependent | 1.34 | 1.06–1.68 | 0.01 |
| Arrhythmia | 1.42 | 1.22–1.65 | <0.001 |
| ECMO during hospitalization | 0.92 | 0.72–1.18 | 0.53 |
| Total LOS > median (32 days) | 1.48 | 1.27–1.73 | <0.001 |
| Aspirin | 2.71 | 2.27–3.24 | <0.001 |
| Furosemide | 3.31 | 2.68–4.08 | <0.001 |
| Opiate | 0.71 | 0.44–1.13 | 0.15 |
Abbreviations: ECMO extracorporeal membrane oxygenation, GA gestational age, LOS length of stay
Table 4:
Multivariable mixed effect model for likelihood of digoxin with non-linear association for time
| OR | 95% CI | p | |
|---|---|---|---|
| Per year prior to 1/1/2016 | 0.94 | 0.91–0.96 | <0.001 |
| Per year on or after 1/1/2016 | 2.38 | 2.14–2.64 | <0.001 |
| Male sex | 1.14 | 0.99–1.31 | 0.06 |
| Race | |||
| Non-Hispanic white | 1 | n/a | n/a |
| Hispanic white | 1.28 | 1.01–1.63 | 0.04 |
| Non-Hispanic black | 1.05 | 0.84–1.32 | 0.67 |
| Other Hispanic | 0.95 | 0.71–1.28 | 0.76 |
| Other or more than one race | 1.58 | 1.08–2.31 | 0.02 |
| Missing | 0.80 | 0.64–1.00 | 0.05 |
| Payer | |||
| Commercial | 1 | n/a | n/a |
| Medicaid | 0.98 | 0.85–1.14 | 0.83 |
| Other government | 0.80 | 0.57–1.12 | 0.20 |
| Other | 0.64 | 0.45–0.91 | 0.01 |
| Missing | 0.49 | 0.22–1.08 | 0.08 |
| Prematurity (<37 weeks GA) | 0.73 | 0.57–0.94 | 0.01 |
| Genetic syndrome | 0.77 | 0.50–1.18 | 0.23 |
| Neurological condition | 0.70 | 0.53–0.94 | 0.02 |
| Renal condition | 0.76 | 0.60–0.95 | 0.02 |
| Gastrointestinal condition | 0.84 | 0.66–1.06 | 0.15 |
| Technology dependent | 1.29 | 1.02–1.61 | 0.03 |
| Arrhythmia | 1.38 | 1.19–1.61 | <0.001 |
| ECMO during hospitalization | 0.94 | 0.74–1.21 | 0.65 |
| Total LOS > median (32 days) | 1.58 | 1.35–1.85 | <0.001 |
| Aspirin | 2.51 | 2.10–2.99 | <0.001 |
| Furosemide | 3.39 | 2.74–4.18 | <0.001 |
| Opiate | 0.66 | 0.41–1.06 | 0.08 |
Median odds ratio: 3.49 (95% CI: 4.05–2.96 p<0.001)
Abbreviations: ECMO extracorporeal membrane oxygenation, GA gestational age, LOS length of stay
In the primary model (Table 3), several subject-level factors were associated with the likelihood of receiving digoxin. Known arrhythmia was associated with increased odds of receiving digoxin (OR: 1.42, p<0.001). Prolonged length of stay (OR: 1.48, p<0.001), technology dependence (OR: 1.34, p=0.01), receipt of aspirin (OR: 2.71, p<0.001), and receipt of furosemide (OR: 3.31, p<0.001) were also associated with odds of receiving digoxin. Prematurity (OR: 0.74, p=0.02), renal conditions (OR: 0.75, p=0.02), and neurological conditions (OR: 0.65, p=0.003) were associated with decreased odds of receiving digoxin. Though point estimates changed, the associations were not significantly different in the second model. In sensitivity analyses, in which subjects with LOS>90 were restricted, no changes were seen in the main effects (data not shown).
Practice variation:
Before 1/2016, the median hospital in the sample discharged 17% of subjects with digoxin (IQR: 7–27%, range: 0–75%), and after 1/2016, the median hospital discharged 39% of subjects with digoxin (IQR: 16–62%, range: 0–78%, Figure 2A). At the hospital level, there was a significant increase in the likelihood of being discharged with digoxin when comparing the time periods (sign-rank test p<0.0001). Expressed a different way, prior to 1/2016, 7% of hospitals discharged >60% of Norwood survivors with digoxin, while after 1/2016, 31% of hospitals discharged >60% of subjects with digoxin (Figure 2B, p=0.005). However, the response was not uniform (Figure 3) as 71% (32/45) of hospitals demonstrated increased utilization of digoxin, with 22% (10/45) decreasing their utilization of digoxin (median change was −14% IQR: −21 to −9%). This is reflected in the observation that 33% of hospitals still discharge <20% of subjects with digoxin (Figure 2B).
Figure 2: Use of digoxin by hospital.
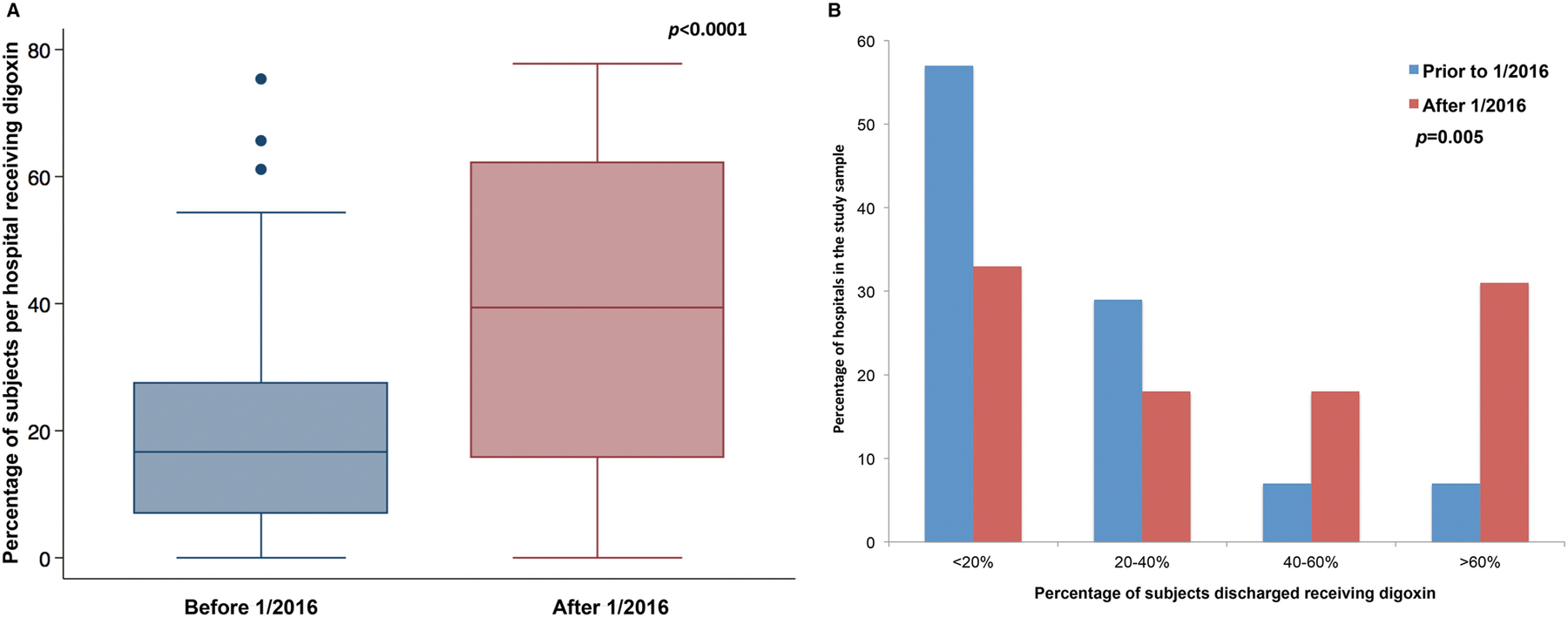
A. Box and whiskers plot of the use of digoxin at hospitals in the study sample. The use of digoxin at the center level increased significantly (Sign rank test p<0.0001)
B. Bar graph depicts the hospitals-level distribution of discharge with digoxin after Norwood operation before (blue) and after (red) 1/1/2016. (Fisher’s exact test p=0.005)
Figure 3: Hospital level variation in the use of digoxin.
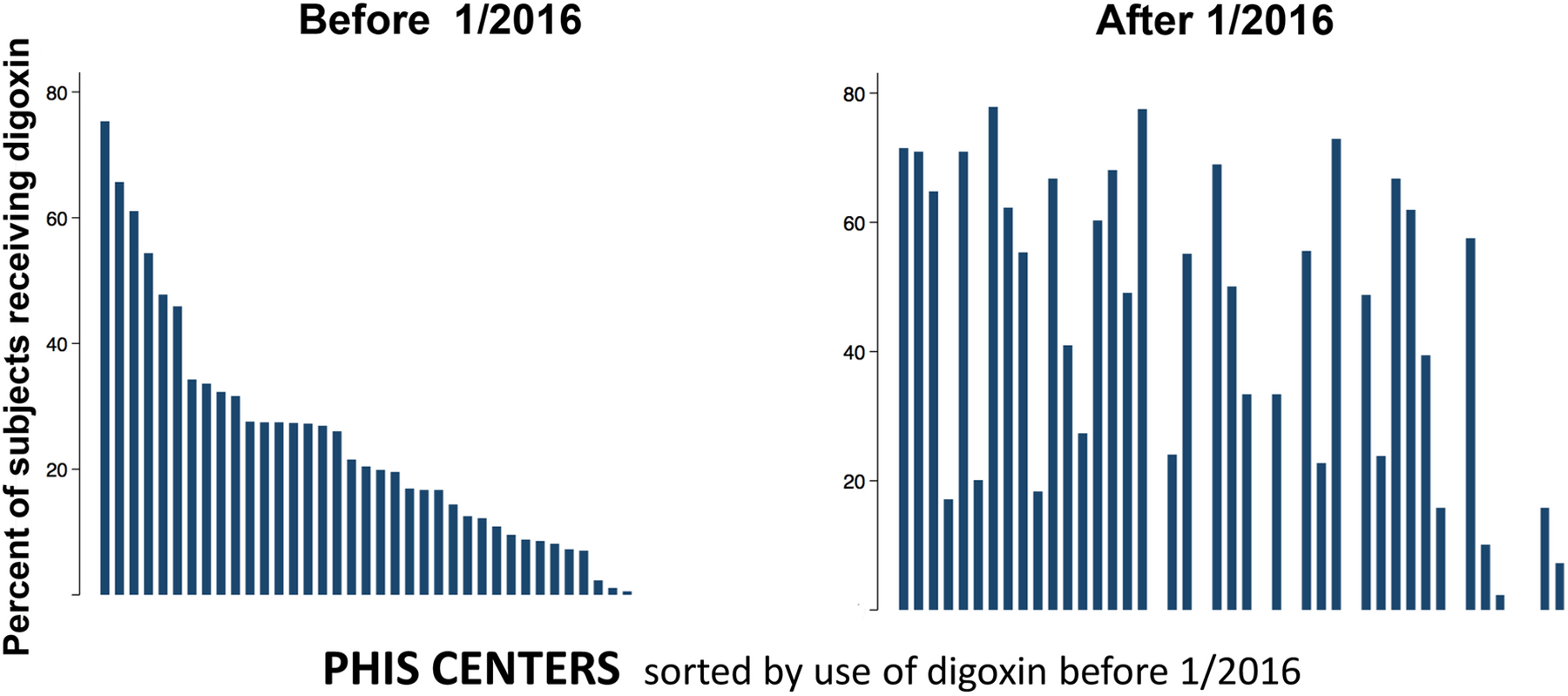
Bar plot of the percentage of subjects receiving digoxin at each hospital before 1/2016 (Left panel) or after 1/2015 (Right panel). Hospitals are sorted by their pre- 1/2016 utilization of digoxin in both panels. Use of digoxin increased at 71% (30/45) of PHIS hospitals, but also decreased at 22% of hospitals (10/45).
Across the entire study period, in adjusted analyses, there is evidence of large magnitude practice variation (primary model: MOR: 3.56, 95% CI: 2.48–4.70 and secondary model: MOR: 3.50, 95% CI: 2.96–4.05). The magnitude of practice variation was not significantly different before (MOR: 4.00 9% CI: 2.58–5.57) and after 1/2016 (MOR: 5.01 95% CI: 2.90–7.50). In post-hoc secondary analyses, procedural volume (expressed either as a continuous variable or dividing the study sample into four ordinal groups) was not significantly associated with the odds of receiving digoxin at discharge (data not shown).
DISCUSSION
This observational study of practices at a number of US primary pediatric hospitals demonstrates that the use of digoxin for patients with HLHS at the time of discharge from their Norwood hospitalization was decreasing prior to 2016 and that the trend reversed in 2016 and that utilizations has continued in a significant proportion of neonates with HLHS. This period coincided temporally with the publication of two papers14,15 demonstrating an association between digoxin usage and improved interstage outcomes. These trends were significant in both the observed proportion of cases in each era and in multivariable analyses adjusting for measurable confounders. Though in this design causality is challenging to ascertain, falsification tests to evaluate whether parallel trends could be seen in the prescription of either of two other common cardiac medications did not demonstrate similar trends, supporting that the observed associations were not due to changes in recording prescriptions or other secular trends.
The current analysis also evaluated the variation in the use of digoxin between hospitals. There was large-scale variation in practice that was not explained by differences in the case-mix at different hospitals. Moreover, the magnitude of this variation was not significantly different before and after 2016. Though digoxin was used more frequently in the later era, its adoption was not uniform between hospitals, suggesting that there are unresolved issues regarding the use of digoxin in this population. Moreover, despite bridging a period of intense interest in collaboration across centers and quality improvement efforts, practice variation remained large both before and after 2016.
HLHS and anatomic variants continue to represent a challenge in pediatric/congenital cardiology with 5-year transplant-free survival of 60–70%37–42 and the consumption of resources out of proportion to its prevalence43. The interstage period between the Norwood operation and superior cavopulmonary connection has been identified as a period with a high risk of mortality, with historical series reporting rates between 10–19%1–10. Single-center studies have demonstrated impressive reductions in mortality after the introduction of intensive home monitoring programs2,4,6,8,11. However, results in multicenter series have been more modest44, underscoring the public health benefit of measures with the potential to further reduce interstage mortality. The observation that use of digoxin, a pharmaceutical that was at the time only used in a minority of Norwood patients16,17, was associated with reduced interstage mortality in two separate studies14,15 would seem to be a powerful inducement to change practice.
The current study demonstrated a significant increase in the use of digoxin at discharge after Norwood operation after 2016. Utilization is far from universal, and though most centers increased their use, a minority did not. The current study is not designed to explore the general and center-specific reasons for this. The effect of digoxin on survival has not been evaluated in a prospective study, and the results were not replicable in a cohort of Norwood patients that included non-HLHS patients17. There is uncertainty regarding the mechanism that confers a benefit for digoxin (negative chronotropy, positive inotropy, diuresis, or some other mechanism). Tied to this, it is unclear whether all Norwood survivors would benefit from digoxin or whether the benefits are restricted to a subset of subjects with specific anatomic or physiologic traits. Moreover, digoxin has a narrow therapeutic index (difference between an effective and toxic drug level). There have also been concerns that in the original studies, the benefit associated with digoxin use may have been partially contaminated by the presence of unmeasured confounding45. How to address these questions remains unsettled.
Utilization of digoxin is highly variable between centers even after accounting for measurable confounders with the odds of a hypothetical patient receiving digoxin varying by a factor of 3.5 between two randomly selected hospitals in the sample. The current study cannot explore why digoxin prescription practices differ between study centers. The decision-making to prescribe digoxin for individual patients, by individual physicians, and for centers is complicated to evaluate and likely requires data that would be best collected in a prospective fashion. Large magnitude heterogeneity of practice demonstrates that there is equipoise for a potential clinical trial and/or sufficient variability in practice for an observational study (assuming a data source with sufficient clinical detail to address questions of unmeasured confounding was available). It was surprising in an era in which many operative centers are engaged in collaborative quality improvement initiatives that the magnitude of practice variation did not appear to change in the face of evidence supporting the use of digoxin.
An additional observation was that the cohort of subjects in the more recent era demonstrates an increased proportion of several factors associated with increased severity of illness (renal, gastrointestinal, neurological, technology dependence, and metabolic conditions). It is possible that this is an artifact of the transition from ICD-9 to ICD-10 coding systems. The ICD-10 system contains a much larger number of codes and it is possible that this expansion could result in a higher sensitivity for non-cardiac diagnoses. At the same time, this trend is consistent with the observation that the preoperative case complexity for cardiac surgical patients has increased over time37,46. A sensitivity analysis was performed to evaluate whether including these factors in models biased estimates of the treatment in the later period, which did not demonstrate evidence of bias. As noted previously, it is not possible to differentiate between preoperative conditions and morbidity that followed the operation. It is a reasonable hypothesis that increased prevalence of non-cardiac conditions present before or after surgery would be associated with increased use of digoxin. However, the current models adjust for the presence of these factors mitigating bias introduced by any historical changes in case-mix. Also, in these models the association between individual factors and the likelihood of receiving digoxin is not homogenous. For instance, a subject that is technology-dependent (e.g. has a feeding tube) is more likely to receive digoxin, while subjects with renal or neurological disorders were less likely. The association between medical complexity and the receipt of digoxin is therefore not simple and is deserving of further attention.
There are several additional limitations to this study. Though care was taken to mitigate bias and confounding, an observational study of trends in the use of a therapy over time cannot “prove” a causal relationship. Administrative datasets have imperfect access to clinical data, specifically granular data (degrees of ventricular dysfunction or atrioventricular valve regurgitation), which might lead to unmeasured confounding. As noted, current inpatient medications are used in this study as a surrogate for discharge medications, and changes that might occur in medications in the outpatient setting cannot be addressed in this dataset. The study includes the years in which ICD-9 transitioned to ICD-10. This affects identification of cases (by diagnosis and procedure). However, since the outcome of interest in the study is coded using PHIS drug codes, this change does not introduce bias directly. Race data were missing in a significant minority of subjects, which is consistent with previous studies in this database. Missing race was associated with increased likelihood of digoxin receipt. We cannot ascertain the etiology of this association in the current study. Because PHIS is a database of inpatient and observation encounters, the association between digoxin prescription and interstage mortality could not be evaluated. Though the number of subjects who ultimately underwent second stage palliations can be identified, this number would be a low-bound estimate since subjects who undergo subsequent operations at a different center would be lost to follow up in the PHIS database.
CONCLUSION:
The use of digoxin increased in the period following publication of data supporting its potential benefit during the interstage period. However, digoxin is still used in a minority of patients, and, independent of case-mix, there is significant variation in practice between hospitals. Determining which patients benefit from digoxin and improving the application of this therapy are both important goals for ongoing research.
Supplementary Material
Funding:
Dr. O’Byrne (K23 HL130420-01) receives funding from the National Heart Lung and Blood Institute. The funding agency had no role in the planning or execution of the study, nor did they edit the manuscript as presented. The manuscript represents the opinions of the authors alone.
Footnotes
DECLARATIONS
Conflicts: No financial conflicts of interest to disclose.
Ethics Approval: The manuscript describes an analysis of a de-identified administrative dataset. The Institutional Review Board at our institution has ruled that all analyses from this database are exempt from review.
Availability of data and code: The data analyzed is proprietary and cannot be shared under the conditions of our data use agreement. Analytic code will be shared on request provided the requestor pledges that it will be used for academic purposes and appropriate citation is applied.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCE:
- 1.Alsoufi B, McCracken C, Kochilas LK, Clabby M, Kanter K. Factors Associated With Interstage Mortality Following Neonatal Single Ventricle Palliation. World J Pediatr Cong Heart Surg 2018;9:616–623. [DOI] [PubMed] [Google Scholar]
- 2.Hansen JH, Furck AK, Petko C, Buchholz-Berdau R, Voges I, Scheewe J, Rickers C, Kramer H-H. Use of surveillance criteria reduces interstage mortality after the Norwood operation for hypoplastic left heart syndrome. Eur J Cardiothorac Surg 2012;41:1013–1018. [DOI] [PubMed] [Google Scholar]
- 3.Ghanayem NS, Hoffman GM, Mussatto KA, Cava JR, Frommelt PC, Rudd NA, Steltzer MM, Bevandic SM, Frisbee SS, Jaquiss RDB, Litwin SB, Tweddell JS. Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg 2003;126:1367–1377. [DOI] [PubMed] [Google Scholar]
- 4.Castellanos DA, Herrington C, Adler S, Haas K, Ram Kumar S, Kung GC. Home Monitoring Program Reduces Mortality in High-Risk Sociodemographic Single-Ventricle Patients. Pediatr Cardiol 2016;37:1575–1580. [DOI] [PubMed] [Google Scholar]
- 5.Furck AK, Uebing A, Hansen JH, Scheewe J, Jung O, Fischer G, Rickers C, Holland-Letz T, Kramer H-H. Outcome of the Norwood operation in patients with hypoplastic left heart syndrome: a 12-year single-center survey. J Thorac Cardiovasc Surg 2010;139:359–365. [DOI] [PubMed] [Google Scholar]
- 6.Gardner MM, Mercer-Rosa L, Faerber J, DiLorenzo MP, Bates KE, Stagg A, Natarajan SS, Szwast A, Fuller S, Mascio CE, Fleck D, Torowicz DL, Giglia TM, Rome JJ, Ravishankar C. Association of a Home Monitoring Program With Interstage and Stage 2 Outcomes. J Amer Heart Assoc 2019;8:e010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hehir DA, Dominguez TE, Ballweg JA, Ravishankar C, Marino BS, Bird GL, Nicolson SC, Spray TL, Gaynor JW, Tabbutt S. Risk factors for interstage death after stage 1 reconstruction of hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg 2008;136:94–9– 99.e1–3. [DOI] [PubMed] [Google Scholar]
- 8.Petit CJ, Fraser CD, Mattamal R, Slesnick TC, Cephus CE, Ocampo EC. The impact of a dedicated single-ventricle home-monitoring program on interstage somatic growth, interstage attrition, and 1-year survival. J Thorac Cardiovasc Surg 2011;142:1358–1366. [DOI] [PubMed] [Google Scholar]
- 9.Simsic JM, Bradley SM, Stroud MR, Atz AM. Risk factors for interstage death after the Norwood procedure. Pediatric Cardiology. 2005;26:400–403. [DOI] [PubMed] [Google Scholar]
- 10.Taylor LC, Burke B, Donohue JE, Yu S, Hirsch-Romano JC, Ohye RG, Goldberg CS. Risk Factors for Interstage Mortality Following the Norwood Procedure: Impact of Sociodemographic Factors. Pediatr Cardiol 2016;37:68–75. [DOI] [PubMed] [Google Scholar]
- 11.Rudd NA, Frommelt MA, Tweddell JS, Hehir DA, Mussatto KA, Frontier KD, Slicker JA, Bartz PJ, Ghanayem NS. Improving interstage survival after Norwood operation: outcomes from 10 years of home monitoring. J Thorac Cardiovasc Surg 2014;148:1540–1547. [DOI] [PubMed] [Google Scholar]
- 12.Clauss SB, Anderson JB, Lannon C, Lihn S, Beekman RH, Kugler JD, Martin GR. Quality improvement through collaboration: the National Pediatric Quality improvement Collaborative initiative. Curr Opin Pediatr 2015;27:555–562. [DOI] [PubMed] [Google Scholar]
- 13.Anderson JB, Beekman RH, Kugler JD, Rosenthal GL, Jenkins KJ, Klitzner TS, Martin GR, Neish SR, Brown DW, Mangeot C, King E, Peterson LE, Provost L, Lannon C, National Pediatric Cardiology Quality Improvement Collaborative. Improvement in Interstage Survival in a National Pediatric Cardiology Learning Network. Circ Cardiovasc Qual Outcomes 2015;8:428–436. [DOI] [PubMed] [Google Scholar]
- 14.Brown DW, Mangeot C, Anderson JB, Peterson LE, King EC, Lihn SL, Neish SR, Fleishman C, Phelps C, Hanke S, Beekman RH III, Lannon CM, the National Pediatric Cardiology Quality Improvement Collaborative. Digoxin Use Is Associated With Reduced Interstage Mortality in Patients With No History of Arrhythmia After Stage I Palliation for Single Ventricle Heart Disease. J Amer Heart Assoc 2016;5:85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oster ME, Kelleman M, McCracken C, Ohye RG, Mahle WT. Association of Digoxin With Interstage Mortality: Results From the Pediatric Heart Network Single Ventricle Reconstruction Trial Public Use Dataset. J Amer Heart Assoc 2016;5:I82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghelani SJ, Spurney CF, Martin GR, Cross RR. Impact of pharmacotherapy on interstage mortality and weight gain in children with single ventricle. Congenit Heart Dis 2013;8:219–227. [DOI] [PubMed] [Google Scholar]
- 17.Truong DT, Menon SC, Lambert LM, Burch PT, Sheng X, Minich LL, Williams RV. Digoxin Use in Infants with Single Ventricle Physiology:. Pediatr Cardiol 2018;39:1200–1209. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed H, Anderson JB, Bates KE, Fleishman CE, Natarajan S, Ghanayem NS, Sleeper LA, Lannon CM, Brown DW, National Pediatric Cardiology Quality Improvement Collaborative. Development of a validated risk score for interstage death or transplant after stage I palliation for single-ventricle heart disease. J Thorac Cardiovasc Surg 2019; [DOI] [PubMed] [Google Scholar]
- 19.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatr 2001;107:1–5. [DOI] [PubMed] [Google Scholar]
- 20.Sun G-W, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. Journal of Clinical Epidemiology. 1996;49:907–916. [DOI] [PubMed] [Google Scholar]
- 21.Hira RS, Kennedy K, Jneid H, Alam M, Basra SS, Petersen LA, Ballantyne CM, Nambi V, Chan PS, Virani SS. Frequency and practice-level variation in inappropriate and nonrecommended prasugrel prescribing: insights from the NCDR PINNACLE registry. J Am Coll Cardiol 2014;63:2876–2877. [DOI] [PubMed] [Google Scholar]
- 22.Chan PS, Maddox TM, Tang F, Spinler S, Spertus JA. Practice-level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR PINNACLE program). Am J Cardiol 2011;108:1136–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maddox TM, Chan PS, Spertus JA, Tang F, Jones P, Ho PM, Bradley SM, Tsai TT, Bhatt DL, Peterson PN. Variations in coronary artery disease secondary prevention prescriptions among outpatient cardiology practices: insights from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol 2014;63:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson PN, Chan PS, Spertus JA, Tang F, Jones PG, Ezekowitz JA, Allen LA, Masoudi FA, Maddox TM. Practice-level variation in use of recommended medications among outpatients with heart failure: Insights from the NCDR PINNACLE program. Circ Heart Fail 2013;6:1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Byrne ML, Shinohara RT, Grant EK, Kanter JP, Gillespie MJ, Dori Y, Rome JJ, Glatz AC. Increasing propensity to pursue operative closure of atrial septal defects following changes in the instructions for use of the Amplatzer Septal Occluder device: An observational study using data from the Pediatric Health Information Systems database. Am Heart J [Internet]. 2017;192:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Byrne ML, Kennedy KF, Rome JJ, Glatz AC. Variation in practice patterns in device closure of atrial septal defects and patent ductus arteriosus: An analysis of data from the IMproving Pediatric and Adult Congenital Treatment (IMPACT) registry. Am Heart J 2018;196:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glatz AC, Kennedy KF, Rome JJ, O’Byrne ML. Variations in Practice Patterns and Consistency With Published Guidelines for Balloon Aortic and Pulmonary Valvuloplasty. JACC Cardiovasc Interv 2018;11:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Byrne ML, Glatz AC, Mercer-Rosa L, Gillespie MJ, Dori Y, Goldmuntz E, Kawut S, Rome JJ. Trends in pulmonary valve replacement in children and adults with tetralogy of fallot. Am J Cardiol [Internet]. 2015;115:118–124. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0002914914019389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Byrne ML, Millenson ME, Grady CB, Huang J, Bamat NA, Munson DA, Song L, Dori Y, Gillespie MJ, Rome JJ, Glatz AC. Trends in transcatheter and operative closure of patent ductus arteriosus in neonatal intensive care units. Am Heart J 2019;217:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. AmJEpidemiol 2005;161:81–88. [DOI] [PubMed] [Google Scholar]
- 31.Jayaram N, Spertus JA, O’Byrne ML, Chan PS, Kennedy KF, Bergersen L, Glatz AC. Relationship between hospital procedure volume and complications following congenital cardiac catheterization: A report from the IMproving Pediatric and Adult Congenital Treatment (IMPACT) registry. Am Heart J [Internet]. 2017;183:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Byrne ML, Glatz AC, Shinohara RT, Jayaram N, Gillespie MJ, Dori Y, Rome JJ, Kawut S. Effect of center catheterization volume on risk of catastrophic adverse event after cardiac catheterization in children. Am Heart J 2015;169:823–832.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Byrne ML, Glatz AC, Song L, Griffis HM, Millenson ME, Gillespie MJ, Dori Y, DeWitt AG, Mascio CE, Rome JJ. Association Between Variation in Preoperative Care Before Arterial Switch Operation and Outcomes in Patients With Transposition of the Great Arteries. Circulation. 2018;138:2119–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of Transcatheter and Operative Pulmonary Valve Replacement (from the Pediatric Health Information Systems Database). Am J Cardiol 2016;117:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Byrne ML, Glatz AC, Faerber JA, Seshadri R, Millenson ME, Mi L, Shinohara RT, Dori Y, Gillespie MJ, Rome JJ, Kawut SM, Groeneveld PW. Interhospital Variation in the Costs of Pediatric/Congenital Cardiac Catheterization Laboratory Procedures: Analysis of Data From the Pediatric Health Information Systems Database. J Amer Heart Assoc [Internet]. 2019;8:e011543. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=31023121&retmode=ref&cmd=prlinks [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Byrne ML, Glatz AC, Hanna BD, Shinohara RT, Gillespie MJ, Dori Y, Rome JJ, Kawut SM. Predictors of Catastrophic Adverse Outcomes in Children With Pulmonary Hypertension Undergoing Cardiac Catheterization: A Multi-Institutional Analysis From the Pediatric Health Information Systems Database. J Am Coll Cardiol [Internet]. 2015;66:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mascio CE, Irons ML, Ittenbach RF, Gaynor JW, Fuller SM, Kaplinski M, Kennedy AT, Steven JM, Nicolson SC, Spray TL. Thirty years and 1663 consecutive Norwood procedures: Has survival plateaued? J Thorac Cardiovasc Surg 2019;158:220–229. [DOI] [PubMed] [Google Scholar]
- 38.Poh CL, d’Udekem Y. Life After Surviving Fontan Surgery: A Meta-Analysis of the Incidence and Predictors of Late Death. Heart, Lung and Circulation. 2018;27:552–559. [DOI] [PubMed] [Google Scholar]
- 39.Allen KY, Downing TE, Glatz AC, Rogers LS, Ravishankar C, Rychik J, Fuller S, Montenegro LM, Steven JM, Spray TL, Nicolson SC, Gaynor JW, Goldberg DJ. Effect of Fontan-Associated Morbidities on Survival With Intact Fontan Circulation. Am J Cardiol 2017;119:1866–1871. [DOI] [PubMed] [Google Scholar]
- 40.Newburger JW, Sleeper LA, Frommelt PC, Pearson GD, Mahle WT, Chen S, Dunbar-Masterson C, Mital S, Williams IA, Ghanayem NS, Goldberg CS, Jacobs JP, Krawczeski CD, Lewis AB, Pasquali SK, Pizarro C, Gruber PJ, Atz AM, Khaikin S, Gaynor JW, Ohye RG, Pediatric Heart Network Investigators. Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial. Circulation. 2014;129:2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao JY, Phan K, Ayer J, Celermajer DS, Winlaw DS. Long term survival of hypoplastic left heart syndrome infants: Meta-analysis comparing outcomes from the modified Blalock-Taussig shunt and the right ventricle to pulmonary artery shunt. Int J Cardiol 2018;254:107–116. [DOI] [PubMed] [Google Scholar]
- 42.Menon SC, Keenan HT, Weng HYC, Lambert LM, Burch PT, Edwards R, Spackman A, Korgenski KE, Tani LY. Outcome and Resource Utilization of Infants Born With Hypoplastic Left Heart Syndrome in the Intermountain West. Am J Cardiol 2012;110:720–727. [DOI] [PubMed] [Google Scholar]
- 43.Keren R, Luan X, Localio R, Hall M, McLeod L, Dai D, Srivastava R, Pediatric Research in Inpatient Settings (PRIS) Network. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med 2012;166:1155–1164. [DOI] [PubMed] [Google Scholar]
- 44.Cross RR, Harahsheh AS, McCarter R, Martin GR, National Pediatric Cardiology Quality Improvement Collaborative. Identified mortality risk factors associated with presentation, initial hospitalisation, and interstage period for the Norwood operation in a multi-centre registry:. Cardiol Young 2014;24:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Hare GF. Perspective. Digoxin for interstage single ventricle patients: What could possibly go wrong? Congenit Heart Dis 2019;14:321–323. [DOI] [PubMed] [Google Scholar]
- 46.Brown KL, Crowe S, Franklin R, McLean A, Cunningham D, Barron D, Tsang V, Pagel C, Utley M. Trends in 30-day mortality rate and case mix for paediatric cardiac surgery in the UK between 2000 and 2010. Open Heart 2015;2:e000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


